Comparative Quantification of the Phenolic Compounds, Piperine Content, and Total Polyphenols along with the Antioxidant Activities in the Piper trichostachyon and P. nigrum
Abstract
:1. Introduction
2. Results
2.1. Estimation of the TPC
2.2. Estimation of the TF
2.3. Antioxidant Activities
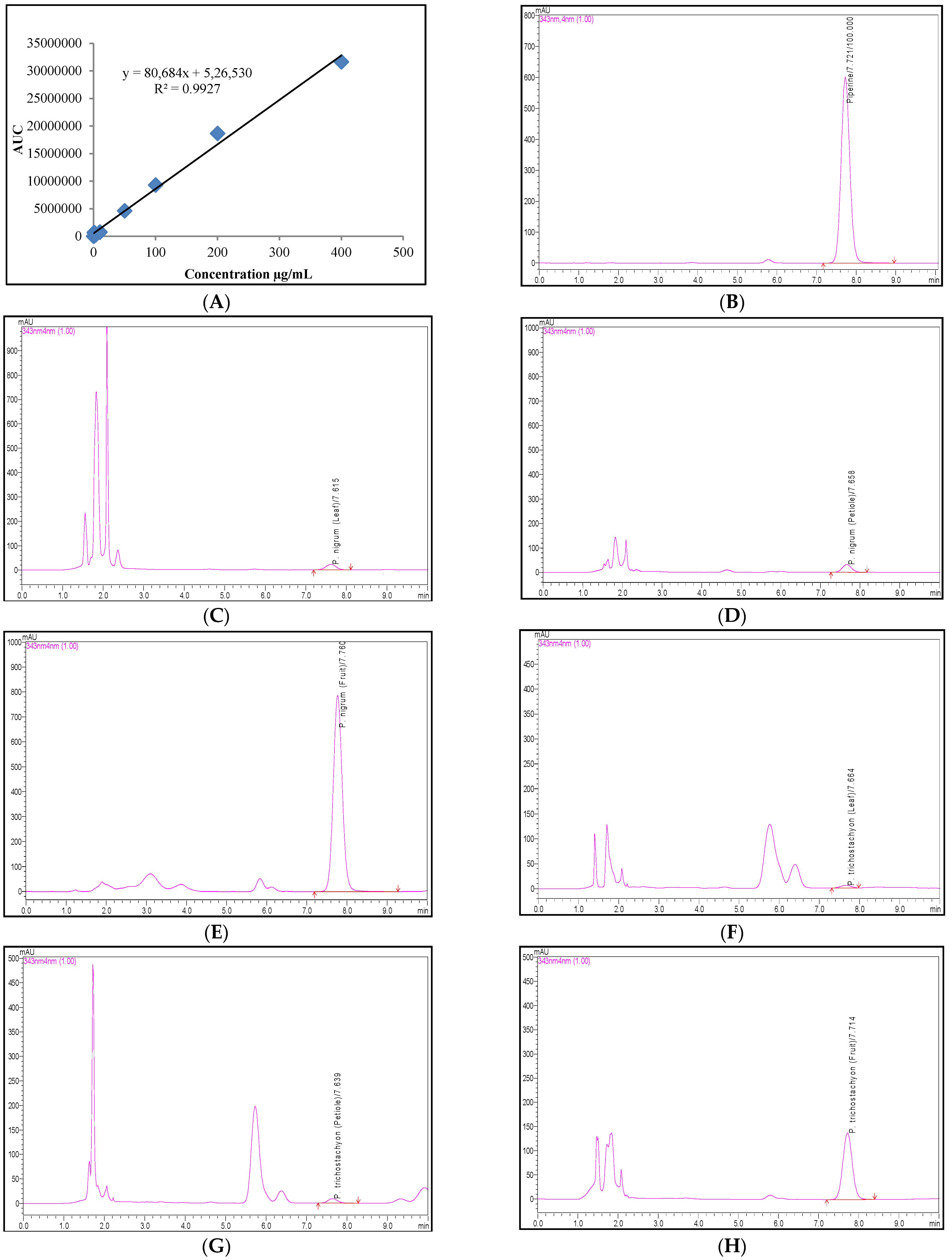
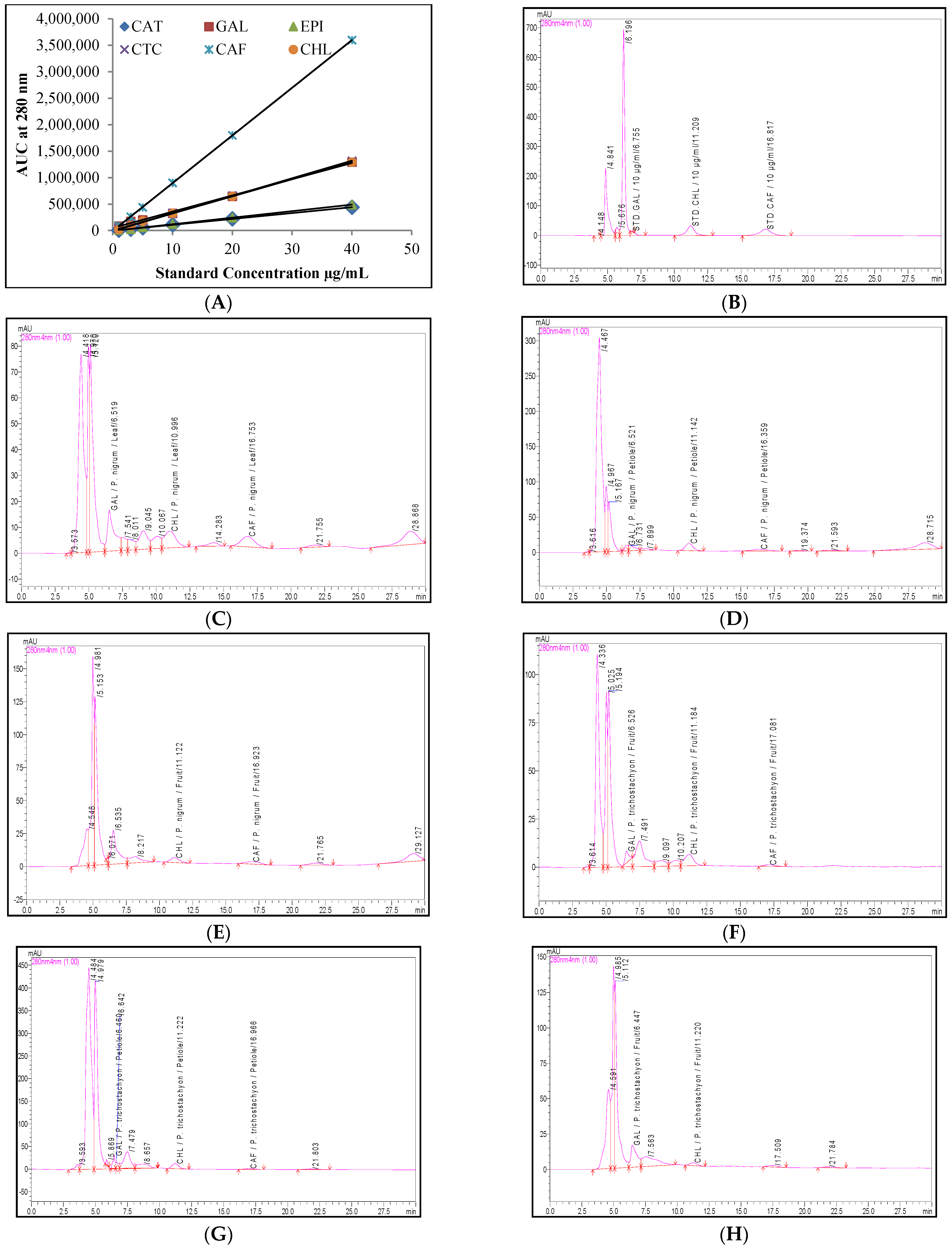
2.4. Quantification of the Phenolic Compounds and Piperine Using the RP-UFLC Method
3. Discussion
4. Materials and Methods
4.1. Standards and Chemical Reagents
4.2. Plant Material
4.3. Sample Extraction
4.4. Estimation of the Total Phenolic Content (TPC)
4.5. Estimation of the Total Flavonoid (TF)
4.6. Antioxidant Activities
4.6.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Assay
4.6.2. Ferric Reducing Antioxidant Power (FRAP) Assay
4.7. Quantification of the Phenolic Compounds and Piperine Using the RP-UFLC Method
4.7.1. Instrumentation and Conditions
4.7.2. System Suitability
4.8. Statistical Analysis

5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Punekar, S.; Lakshminarasimhan, P. Flora of Anashi National Park, Western Ghats-Karnataka, 1st ed.; Bioshperes Publications: Pune, India, 2011. [Google Scholar]
- Gamble, J.S. Flora of the Presidency of Madras, Volume 2; Bishen Sing Mahendra Pal Sing: Dehra Dun, India, 1925. [Google Scholar]
- Sen, S.; Dayanandan, S.; Davis, T.; Ganesan, R.; Jagadish, M.; Mathew, P.; Ravikanth, G. Origin and evolution of the genus Piper in Peninsular India. Mol. Phylogen. Evol. 2019, 138, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, P.N.; Nirmal Babu, K.; Sasikumar, B.; Krishnamurthy, K.S. Botany and crop improvement of black pepper. In Black Pepper: Piper Nigrum; Ravindran, P.N., Ed.; Overseas Publishers Association: Amsterdam, The Netherlands, 2000; pp. 25–146. [Google Scholar]
- Srinivasan, K. Black Pepper and its Pungent Principle-Piperine: A Review of Diverse Physiological Effects. Crit. Rev. Food Sci. Nutr. 2007, 47, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Damanhouri, Z.A.; Ahmad, A. A review on therapeutic potential of Piper nigrum L. (Black Pepper): The King of Spices. Med. Aromat. Plants 2014, 3, 161. [Google Scholar] [CrossRef]
- Upadhya, V. Ethnomedicobotany and Development of Quality Control Parameters for Selected Medicinal Plants of Belgaum Region. Ph.D. Thesis, KLE University, Belagavi, Karnataka, India, 2014. [Google Scholar]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, U.; Saji, K.V.; Jayarajan, S.K.; Parthasarathy, V.A. Biodiversity of Piper in South India—Application of GIS and cluster analysis. Curr. Sci. 2006, 91, 652–658. [Google Scholar]
- Ravindran, P.N. Studies on Black Pepper and Some of Its Wild Relatives. Ph. D. Thesis, University of Calicut, Kozhikode, Kerala, India, 1991. [Google Scholar]
- Upadhya, V.; Pai, S.R.; Ankad, G.; Hegde, H.V. Pharmacognostic screening of piper trichostachyon fruits and its comparative analysis with Piper nigrum using chromatographic techniques. Pharmacogn. Mag. 2016, 12, S152–S158. [Google Scholar] [CrossRef]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef]
- Magaña-Barajas, E.; Buitimea-Cantúa, G.V.; Hernández-Morales, A.; Torres-Pelayo, V.D.R.; Vázquez-Martínez, J.; Buitimea-Cantúa, N.E. In vitro α-amylase and α-glucosidase enzyme inhibition and antioxidant activity by capsaicin and piperine from Capsicum chinense and Piper nigrum fruits. J. Environ. Sci. Health Part B 2021, 56, 282–291. [Google Scholar] [CrossRef]
- Smilkov, K.; Ackova, D.G.; Cvetkovski, A.; Ruskovska, T.; Vidovic, B.; Atalay, M. Piperine: Old spice and new nutraceutical? Curr. Pharm. Des. 2019, 25, 1729–1739. [Google Scholar] [CrossRef]
- Tiwari, A.; Mahadik, K.R.; Gabhe, S.Y. Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Med. Drug Discov. 2020, 7, 100027. [Google Scholar] [CrossRef]
- Parmar, V.S.; Jain, S.C.; Bisht, K.S.; Jain, R.; Taneja, P.; Jha, A.; Tyagi, O.D.; Prasad, A.K.; Wengel, J.; Olsen, C.; et al. Phytochemistry of the genus Piper. Phytochemistry 1997, 46, 597–673. [Google Scholar] [CrossRef]
- Koul, I.B.; Kapil, A. Evaluation of the Liver Protective Potential of Piperine, an Active Principle of Black and Long Peppers. Planta Med. 1993, 59, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Stojanović-Radić, Z.; Pejčić, M.; Dimitrijević, M.; Aleksić, A.; Anil Kumar, N.V.; Salehi, B.; Cho, C.W.; Sharifi-Rad, J. Piperine-A Major Principle of Black Pepper: A review of its bioactivity and studies. Appl. Sci. 2019, 9, 4270. [Google Scholar] [CrossRef]
- Singh, J.; Dhar, K.; Atal, C. Studies on the genus piper-IX. Structure of trichostachine, an alkaloid from piper trichostachyon. Tetrahedron Lett. 1969, 10, 4975–4978. [Google Scholar] [CrossRef]
- Vladimir-Knežević, S.; Blažeković, B.; Bival Štefan, M.; Babac, M. Plant Polyphenols as Antioxidants Influencing the Human Health. In Phytochemicals as Nutraceuticals—Global Approaches to Their Role in Nutrition and Health; Rao, V., Ed.; InTech: Rijeka, Croatia, 2012; pp. 155–180. [Google Scholar]
- Ashadevi, S.; Umasankar, M.E.; Babu, S. A comparative study of antioxidant properties in common Indian spices. Int. Res. J. Pharma. 2012, 3, 465–468. [Google Scholar]
- Nahak, G.; Sahu, R.K. Phytochemical evaluation and antioxidant activity of Piper cubeba and Piper nigrum. J. Appl. Pharma. Sci. 2011, 1, 153–157. [Google Scholar]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Pradhan, K.; Variyar, P.; Bandekar, J. Antimicrobial Activity of Novel Phenolic Compounds from Green Pepper (Piper nigrum L.). LWT-Food Sci. Technol. 1999, 32, 121–123. [Google Scholar] [CrossRef]
- Prasad, M.P.; Sushant, S.; Babhulkar, A. Phytochemical Analysis and Antioxidant potential of Piper species and its Molecular Characterization by RAPD Markers. Int. J. Fundam. Appl. Sci. 2012, 1, 71–73. [Google Scholar]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, J.; Famous, E.; Pan, S.; Peng, X.; Tian, J. Antioxidant, hepatoprotective and antifungal activities of black pepper (Piper nigrum L.) essential oil. Food Chem. 2021, 346, 128845. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, K.; Saravana, P.S.; Payal, H.; Mohammed, S.P.; Williams, B. Antioxidant potential of pepper (Piper nigrum Linn.) leaves and its antimicrobial potential against some pathogenic microbes. Indian J. Nat. Prod. Resour. 2012, 3, 570–577. [Google Scholar]
- Gülçin, I. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int. J. Food Sci. Nutr. 2005, 56, 491–499. [Google Scholar] [CrossRef]
- Zarai, Z.; Boujelbene, E.; Ben Salem, N.; Gargouri, Y.; Sayari, A. Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT-Food Sci. Technol. 2013, 50, 634–641. [Google Scholar] [CrossRef]
- Nakatani, N.; Inatani, R.; Ohta, H.; Nishioka, A. Chemical constituents of peppers (Piper spp.) and application to food preservation: Naturally occurring antioxidative compounds. Environ. Health Perspect. 1986, 67, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.M.; Puniani, E.; Jensen, H.; Livesey, J.F.; Poveda, L.; Sánchez-Vindas, P.; Durst, T.; Arnason, J.T. Analysis of Piperaceae Germplasm by HPLC and LCMS: A Method for Isolating and Identifying Unsaturated Amides from Piper spp. Extracts. J. Agric. Food Chem. 2005, 53, 1907–1913. [Google Scholar] [CrossRef]
- Qin, B.; Yang, K.; Cao, R. Synthesis and Antioxidative Activity of Piperine Derivatives Containing Phenolic Hydroxyl. J. Chem. 2020, 2020. [Google Scholar] [CrossRef]
- Manayi, A.; Nabavi, S.M.; Setzer, W.N.; Jafari, S. Piperine as a Potential Anti-cancer Agent: A Review on Preclinical Studies. Curr. Med. Chem. 2018, 25, 4918–4928. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Dias, R.J.; Ghorpade, V.S.; Mali, K.K. Freeze dried multicomponent inclusion complexes of piperine with cyclodextrin and hydrophilic polymers: Physicochemical characterization and In vivo anti-inflammatory activity. Res. J. Pharm. Technol. 2020, 13, 4916–4924. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Chonpathompikunlert, P.; Muchimapura, S.; Priprem, A.; Tankamnerdthai, O. Piperine, the potential functional food for mood and cognitive disorders. Food Chem. Toxicol. 2008, 46, 3106–3110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, Q.; Song, M.; Xiao, J.; Cao, Y.; Huang, Q.; Ho, C.-T.; Lu, M. A review on the bioavailability, bio-efficacies and novel delivery systems for piperine. Food Funct. 2021, 12, 8867–8881. [Google Scholar] [CrossRef] [PubMed]
- Koul, S.; Taneja, S.; Pushpangadan, P.; Dhar, K. Lignans of Piper trichostachyon. Phytochemistry 1988, 27, 1479–1482. [Google Scholar] [CrossRef]
- Ravindran, P.N.; Nirmal Babu, K. Chemotaxonomy of South Indian Piper. J. Spices Aromat. Crops. 1994, 3, 6–13. [Google Scholar]
- Upadhya, V.; Pai, S.R.; Sharma, A.K.; Hegde, H.V.; Kholkute, S.D.; Joshi, R.K. Compound Specific Extraction of Camptothecin from Nothapodytes nimmoniana and Piperine from Piper nigrum Using Accelerated Solvent Extractor (ASE). J. Anal. Methods Chem. 2014, 2014. [Google Scholar] [CrossRef]
- Nirmal Babu, K.; Naik, V.G.; Ravindran, P.N. Two new taxa of Piper (Piperacease) from Kerala, India with a note on their origin and inter relationship. J. Spices Aromat. Crops. 1993, 2, 26–33. [Google Scholar]
- Ankad, G.; Upadhya, V.; Pai, S.R.; Nimbalkar, M.S.; Hegde, H.V.; Joshi, R.K.; Kholkute, S.D. Evaluating Nothapodytes nimmoniana population from three localities of Western Ghats using camptothecin as phytochemical marker and selection of elites using a new-content range chart method. Pharmacogn. Mag. 2015, 11, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Pawar, N.; Pai, S.; Nimbalkar, M.; Dixit, G. RP-HPLC analysis of phenolic antioxidant compound 6-gingerol from different ginger cultivars. Food Chem. 2011, 126, 1330–1336. [Google Scholar] [CrossRef]
- Luximon-Ramma, A.; Bahorun, T.; Soobrattee, A.M.; Aruoma, O.I. Antioxidant activities of phenolic, proanthocyanidin and flavonoid components in extracts of Cassia fistula. J. Agric. Food Chem. 2002, 50, 5042–5047. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Species | Part | TPC mg/g | TF mg/g | DPPH µM | FRAP µM |
|---|---|---|---|---|---|
| P. nigrum | Leaf | 6.26 ± 0.31 | 5.61 ± 0.28 | 496.50 ± 24.82 | 151.00 ± 07.55 |
| Petiole | 7.50 ± 0.38 | 1.89 ± 0.09 | 366.00 ± 18.33 | 300.50 ± 15.03 | |
| Fruit | 6.71 ± 0.34 | 63.11 ± 3.16 | 85.50 ± 4.28 | 116.50 ± 05.83 | |
| P. trichostachyon | Leaf | 7.28 ± 0.36 | 5.14 ± 0.26 | 117.00 ± 5.85 | 181.00 ± 09.05 |
| Petiole | 13.01 ± 0.65 | 3.42 ± 0.17 | 641.00 ± 32.05 | 474.50 ± 23.73 | |
| Fruit | 11.13 ± 0.56 | 2.19 ± 0.11 | 176.50 ± 8.83 | 181.00 ± 09.05 |
| Parameters | Units/Abb | Alkaloid | Phenolics | |||||
|---|---|---|---|---|---|---|---|---|
| PIP | CAT | GAL | EPI | CTC | CAF | CHL | ||
| Dissolved in | mg/mL | MeOH | MeOH | MeOH | MeOH | MeOH | MeOH | MeOH |
| Concentration | µg/mL | 0.01–400 | 1–40 | 1–40 | 1–40 | 1–40 | 1–40 | 1–40 |
| Total levels | -- | 8 | 6 | 6 | 6 | 6 | 6 | 6 |
| Linearity equation | -- | y = 8068x + 5265 | y = 11,117x − 2913 | y = 30,739x + 47,889 | y = 12,349x − 4444 | y = 33,185x − 8782 | y = 90,138x − 7086 | y = 32,301x − 498.7 |
| R2 | -- | 0.992 | 0.999 | 0.997 | 0.999 | 0.999 | 0.999 | 0.999 |
| LOD | ng/mL | 120.00 ± 60.00 | 17.80 ± 0.00 | 82.40 ± 0.02 | 40.92 ± 0.02 | 21.31 ± 0.00 | 5.00 ± 0.02 | 36.90 ± 0.03 |
| LOQ | ng/mL | 350.00 ± 20.00 | 54.00 ± 0.01 | 249.70 ± 0.09 | 124.01 ± 0.05 | 64.60 ± 0.02 | 15.16 ± 0.01 | 111.97 ± 0.04 |
| Retention time | min | 7.769 ± 0.090 | 11.919 ± 0.146 | 6.208 ± 0.040 | 16.056 ± 0.158 | 12.455 ± 0.037 | 15.918 ± 0.056 | 11.670 ± 0.245 |
| RT RSD | % | 1.161 | 1.224 | 0.643 | 0.982 | 0.297 | 0.351 | 2.100 |
| Theoretical Plates | N | 5320.8 ± 376.8 | 5112.8 ± 398.9 | 6684.3 ± 241.1 | 4796.7 ± 299.1 | 5467.4 ± 331.5 | 4610.1 ± 254.6 | 2305.1 ± 397.9 |
| Tailing factor | Tf | 1.103 ± 0.010 | 0.897 ± 0.106 | 1.049 ± 0.024 | 0.803 ± 0.029 | 1.107 ± 0.044 | 0.950 ± 0.040 | 1.205 ± 0.071 |
| Plant | Parts | Piperine (mg/100 g) | Phenolic Compounds Contents (mg/100 g) | |||||
|---|---|---|---|---|---|---|---|---|
| CAF | GAL | CAT | CTC | CHL | EPI | |||
| P. nigrum | Leaf | 2.58 ± 0.13 | 1.10 ± 0.06 | 5.64 ± 0.28 | ND | ND | 4.08 ± 0.20 | ND |
| Petiole | 3.32 ± 0.17 | 0.75 ± 0.04 | 1.05 ± 0.05 | ND | ND | 4.39 ± 0.22 | ND | |
| Fruit | 1555.49 ± 77.77 | 0.56 ± 0.03 | ND | ND | ND | 1.78 ± 0.09 | ND | |
| P. trichostachyon | Leaf | 0.60 ± 0.03 | 0.21 ± 0.01 | 0.74 ± 0.04 | ND | ND | 2.97 ± 0.15 | ND |
| Petiole | 0.97 ± 0.05 | 0.39 ± 0.02 | 3.11 ± 0.16 | ND | ND | 4.24 ± 0.21 | ND | |
| Fruit | 14.35 ± 0.72 | ND | 5.91 ± 0.30 | 0.26 ± 0.01 | ND | 0.84 ± 0.04 | ND | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Khayri, J.M.; Upadhya, V.; Pai, S.R.; Naik, P.M.; Al-Mssallem, M.Q.; Alessa, F.M. Comparative Quantification of the Phenolic Compounds, Piperine Content, and Total Polyphenols along with the Antioxidant Activities in the Piper trichostachyon and P. nigrum. Molecules 2022, 27, 5965. https://doi.org/10.3390/molecules27185965
Al-Khayri JM, Upadhya V, Pai SR, Naik PM, Al-Mssallem MQ, Alessa FM. Comparative Quantification of the Phenolic Compounds, Piperine Content, and Total Polyphenols along with the Antioxidant Activities in the Piper trichostachyon and P. nigrum. Molecules. 2022; 27(18):5965. https://doi.org/10.3390/molecules27185965
Chicago/Turabian StyleAl-Khayri, Jameel Mohammed, Vinayak Upadhya, Sandeep Ramachandra Pai, Poornananda Madhava Naik, Muneera Qassim Al-Mssallem, and Fatima Mohammed Alessa. 2022. "Comparative Quantification of the Phenolic Compounds, Piperine Content, and Total Polyphenols along with the Antioxidant Activities in the Piper trichostachyon and P. nigrum" Molecules 27, no. 18: 5965. https://doi.org/10.3390/molecules27185965
APA StyleAl-Khayri, J. M., Upadhya, V., Pai, S. R., Naik, P. M., Al-Mssallem, M. Q., & Alessa, F. M. (2022). Comparative Quantification of the Phenolic Compounds, Piperine Content, and Total Polyphenols along with the Antioxidant Activities in the Piper trichostachyon and P. nigrum. Molecules, 27(18), 5965. https://doi.org/10.3390/molecules27185965








