Abstract
The [1,2,3]triazin-4(3H)-one ring is a synthetically important molecular platform for a variety of chemical transformations. Despite this, currently, there has been little research on the reaction of the thermal opening of the [1,2,3]triazin-4(3H)-one nucleus. In this work, we describe the synthetic potential of anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-trione in the reaction of the thermal opening of a cycle following the [4+2]-cycloaddition reaction with a number of pyridine derivatives and quinoline. It is shown that this method is effective for the synthesis of the 6H-naphtho[2,3-H]pyrido(quinolino)[2,1-b]quinazoline-6,9,14-trione system. We also investigate the influence of the position of substituents in the structure of pyridine on the formation characteristics of the target products.
1. Introduction
For over 150 years, the 9,10-anthracenedione (9,10-anthraquinone) tricarbocyclic system has been one of the key quinone molecular platforms. Due to the features of reactivity and molecular affinity, 9,10-anthracenediones are characterized by a powerful synthetic, applied, and pharmacological potential [1,2,3]. Over time, a large amount of research has been accumulated on methods for obtaining and transforming this type of compound [1,2,3]. Thus, 9,10-anthracenediones occupy a prominent place in the production of a number of dyes, various reagents for analytical needs, organic chemosensors, and catalysts [1,2,3]. Gradually, 9,10-dioxoanthracene derivatives have enriched the arsenal of biologically active substances, among which compounds with antitumor [4], antiprotozoal [5], antiviral, antimicrobial, and anti-inflammatory [6] effects have been found. On the basis of 9,10-anthracenediones, antitumor drugs (mitoxantrone and ametantrone) have been developed, which are widely used in chemotherapy practice worldwide [4]. A number of 9,10-anthracenedione derivatives are in phase II and III clinical studies [7].
Condensed heteryl derivatives of 9,10-anthracenedione are structural elements of many industrially important pigments and disperse dyes [8]. In particular, 9,10-anthracenedione derivatives have been condensed with anthrimidecarbazole, imidazole, oxazole, thiazole, phthaloylacridine, and pyrazine rings [9]. In addition, the literature contains information related to annulation of the 9,10-anthracenedione structure using triazole [10], pyrazole, and pyridazine nuclei [11,12,13].
In previous work, we synthesized anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-triones as important biologically active compounds [14]. Moreover, anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-triones are interesting substrates for further chemical transformations and for the study of the reactivity of 9,10-anthracenedione derivatives.
1,2,3-Benzotriazin-4(3H)-one and its derivatives [15], and also some azolo[1,2,3]triazin-4(3H)-ones [16] are the most studied objects in the synthetic transformations of the [1,2,3]triazin-4(3H)-one ring. The thermolysis reaction of a 1,2,3-benzotriazin-4(3H)-one nucleus is one of the interesting and, at the same time, less studied transformations [17,18,19,20,21,22]. In the reaction of the thermal opening of the cycle, 1,2,3-benzotriazin-4(3H)-ones serve as precursors in the generating process of corresponding iminoketene. The mechanism of iminoketene formation through an azetidinone intermediate has been experimentally confirmed using isotopic 15N-labels [17,23,24]. At the same time, despite the knowledge of annelated [1,2,3]triazine-(3H)-4-ones with carbo- and azole-containing cyclic systems [15,16], the reaction of iminoketene with such electron-depleted systems as pyridine is practically unknown. The only exception is the work by [25], which describes the ring-opening–annulation reaction of imidazolo[1,2,3]triazin-4-ones in the presence of pyridine, which results in the formation of pyrido[1,2-a]purinone as a valuable object for studying fluorescence in biological systems.
Today, there is no information regarding the in situ preparation of the 1-imino-2-ketene-9,10-anthracenedione intermediate that can serve as a convenient heterodiene model object in the hetero-Diels–Alder reaction. Transformations with such iminoketene participation are also unknown in the literature. In turn, pyridine can be used as a convenient dienophile in the [4+2]-cycloaddition reaction. Therefore, the purpose of this work is to study the ring-opening–annulation reaction of anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-trione 1 in the presence of pyridines.
2. Results and Discussion
In the context of an extended study of reactivity of 9,10-anthracenedione derivatives [26,27,28,29,30,31,32], in this work, we investigated the behavior of the [1,2,3]triazine-3(H)-4-one fragment of the previously synthesized anthratriazinetrione 1 [14] in the reaction of the thermal opening of the cycle. Pyridine was used as a model dienophile in the possible [4+2]-cycloaddition reaction (Scheme 1) with in situ-generated 9,10-dioxoanthracene iminoketene (heterodiene) in various molar ratios and the presence of a number of solvents (Table 1). Atmospheric oxygen was used as a ”green” oxidant in the reaction.
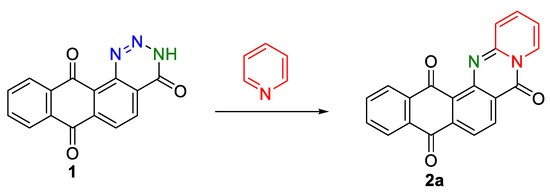
Scheme 1.
Interaction of anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-trione 1 with pyridine.

Table 1.
Optimization of reaction conditions of anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-trione 1 with pyridine.
On the basis of these results (Table 1), the use of pyridine as a reagent and solvent turned out to be highly effective. The temperature regime in the range of 110–115 °C was determined by the temperature at the beginning of the thermal decomposition of anthratriazinone 1. It could be observed by releasing nitrogen bubbles from the reaction mixture. The duration of the reaction time was determined by TLC. The six-hour heating mode with atmospheric oxygen bubbling turned out to be optimal with complete conversion into the target product 2a.
The optimized conditions and a number of substituted pyridines, namely 3-methylpyridine, 4-methylpyridine, 3,5-dimethylpyridine, and quinoline, used as a reagent and reaction medium, allowed 6H-naphtho[2,3-h]pyrido[2,1-b]quinazoline-6,9,14-triones 2a–e and the first representative of the 6H-naphtho[2,3-h]quinolino[2,1-b]quinazoline-6,9,14-trione system 2f to be obtained with yields of 75–90% (Scheme 2).
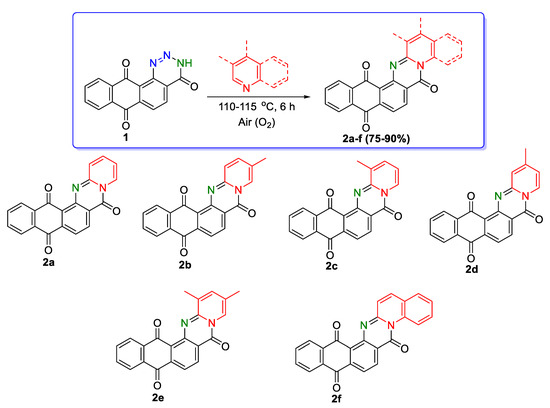
Scheme 2.
Synthesis of 6H-naphtho[2,3-h]pyrido[2,1-b]quinazoline-6,9,14-triones 2a–e and 6H-naphtho[2,3-h]quinolino[2,1-b]quinazoline-6,9,14-trione 2f.
It should be noted that compound 2a has been described in the literature [33], and it has been obtained from 2-carbomethoxy-1-chloro-9,10-anthracenedione and 2-aminopyridine in the presence of copper(II) acetate in boiling ethylene glycol diacetate at 186–187 °C with a very low yield (9%). Attempts have been unsuccessful to synthesize 2a via the interaction of 2-chloropyridine with 1-amino-9,10-dioxo-9,10-dihydroanthracene-2-carboxylic acid or its methyl ester under long-term boiling (up to 30 h) in acetonitrile or toluene in the presence of K2CO3 or CuI [34,35], as well as at 110 °C in DMF.
It was established that a mixture of two isomeric 6H-naphtho[2,3-h]pyrido[2,1-b]quinazoline-6,9,14-triones 2b and 2c (Scheme 1) was formed in the ratio 2:1, when 3-methylpyridine was used as a reagent. Compounds 2b and 2c were not possible to separate either by crystallization or chromatography methods. In the 1H NMR spectrum, the singlet of three protons of the methyl group of the major isomer 2b was fixed at 3.99 ppm, and for the minor isomer 2c was slightly shifted towards the strong field to 3.69 ppm. In the 13C NMR spectrum, the signal of the carbon atom of the methyl group of dominant isomer 2b was recorded at 15.58 ppm, and for 2c at 17.30 ppm.
It was found that pyridines with a chlorine atom or a methyl group in the second position of the ring (2-chloropyridine, 2,3-dimethylpyridine) reacted with anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-trione 1, forming the corresponding dihydrogenated cyclic compounds 3a,b (Scheme 3) as the final products. In the case of 2,6-dimethylpyridine, no reaction occurred.
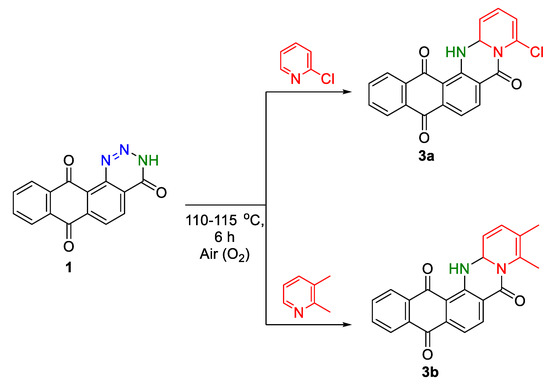
Scheme 3.
Interaction of anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-trione 1 with 2-chloro- and 2,3-dimethylpyridines.
An attempt with long-term air bubbling (up to 18 h) through the reaction mixture in the case of 2-chloropyridine or 2,3-dimethylpyridine did not contribute to obtaining oxidized forms. The isolation of dehydrogenated products 3a,b in the individual state indicates the stepwise course of the process, due to the influence of the +I induction effect of the chlorine atom and the methyl group, located in position 4 of the pentacyclic system. Such influence significantly affects the ability of the products to undergo further oxidation.
The formation of products 3a,b is confirmed by the presence in the IR spectra of the corresponding characteristic absorption bands of valence vibrations of the secondary amino group –NH– in the range of 3264–3328 cm−1. The singlet proton signal of the NH group in 1H NMR spectra for 3b is recorded at 10.15 ppm, whereas this signal for 3a appears at 10.41 ppm, respectively.
In the 13C NMR spectrum of compound 3b in DMSO-d6 solution, the carbon atom signal of the –NH-CH–N– fragment of the dihydropyrimidine ring resonates at 73.29 ppm. Moreover, corresponding peaks of molecular ions of derivatives 3a,b are present in the LC-MS spectra.
Taking into consideration the data on the mechanism of thermolysis of 1,2,3-benzotriazin-4(3H)-one [17,23,24], the probable mechanism of thermolysis of anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-trione 1 in the presence of pyridines is realized through an azetidinone intermediate with the formation of iminoketene via intermediates A–D (Scheme 4). In the first stage, the elimination of nitrogen molecules occurs with the formation of azetidinone A, via the ring-opening rearrangements into iminoketene B. The carbonyl group of the intermediate B undergoes a nucleophilic attack by the nitrogen atom of pyridine with the formation of pyridine salt C. Next, the nucleophilic iminoketene nitrogen attacks the carbon atom in the α-position of the pyridine ring with future ring closure. This conducts to a dihydrogenated intermediate product D, whose following oxidation by air leads to the formation of the target pentacyclic anthrapyridinopyrimidinone product 2. Furthermore, the isolation of intermediate type D compounds 3 in the individual state is a weighty confirmation of the passing of the reaction.
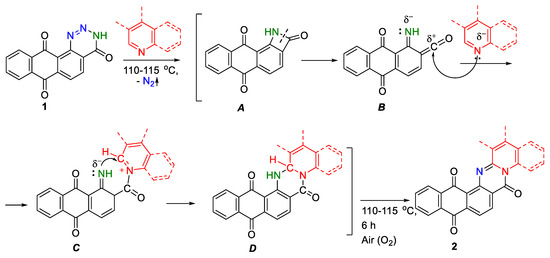
Scheme 4.
A probable mechanism for the formation of anthrapyridinopyrimidinones 2.
During the thermal opening of the triazinone ring 1 in the presence of 4-methylpyridine, in addition to the target annelated product 2d, a by-product formation with a mass of m/z 434 [M + 1] (LC-MS) was unexpectedly determined in the reaction mixture. In the 1H NMR spectrum of the by-product as compared with derivative 2d, in addition to the signals of aromatic protons and the methyl group, a doublet of doublets of two protons was recorded at 4.01 ppm. The 13C NMR spectrum of the by-product showed the absence of one of the signals of the carbonyl carbons of the 9,10-anthracenedione ring and the appearance of two new signals of carbon atoms at 74.78 and 53.04 ppm, respectively. To establish and confirm the structure of the isolated by-product, a single-crystal structural study was carried out. The X-ray study showed the unexpected formation of compound 4 (Figure 1), due to the addition of the methyl group of 4-methylpyridine to the carbonyl group of the anthraquinone fragment (Scheme 5).
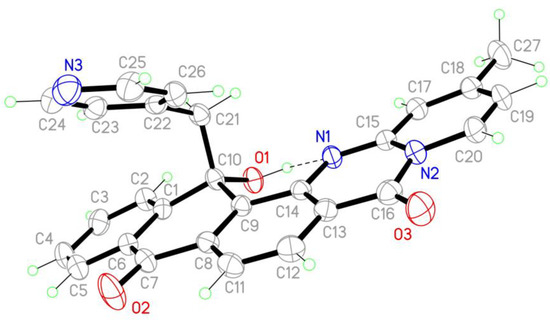
Figure 1.
Molecular structure of compound 4. Ellipsoids are drawn at 50% probability level (CCDC 2192784).
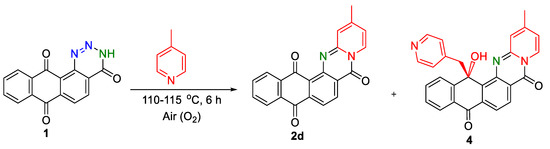
Scheme 5.
An interaction of anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-trione 1 with 4-methylpyridine.
In molecule 4, the main polycyclic core of the molecule is bent at line C7-C10 atoms and twisted at line N1-C16. Thus, the C1-C6 atoms of the phenyl ring and the atoms of the polycyclic N1N2C8-C20 system mean planes make a dihedral angle of 7.7(1)°. The maximal deviation of atoms from the plane in the twisted N1N2C8-C20 system reaches 0.080 Å. In the twisted N1N2C8–C20 fragment, the dihedral angles between the planes of the C8C9C11-C14 atoms (rms deviation of fitted atoms is 0.0067) and the N2C15C17-C20 atoms (rms deviation is 0.0113) are 5.9(1)°. The C1C6-C10 cycle is non-planer and has a half-boat conformation, therefore, the C1C6–C9 atoms lie in the plane with rms deviation of fitted atoms 0.0249, and the C10 atom deviates from this plane for 0.280(3) Å. The bond distances of C10-C1 and C10-C9 are 1.519(3) and 1.522(3) Å, respectively, which are common values for Csp3 atoms connected to the aromatic system. The N1-C15 bond length is 1.324(2) Å, which is longer than the standard value typical for the C=N double bond (1.28 Å).
Bond distances C7-C6 and C7-C8, as well as C13-C16, are in the range 1.450–1.488(3) Å, which are a bit shorter than the standard value typical for single C-C bonds due to conjugation between C=O groups with the aryl ring C1-C6 and C8C9C11-C14. Similar shortening was observed for bond lengths N1-C14, N2-C15, N2-C16, and N2-C20, which were found to be in the range 1.380–1.428(3) Å. These values are shorter than standard C-N bonds and longer than C=N (1.45 and 1.28 Å, respectively), and the rest of the carbon-carbon bond lengths in the main fragment are in the range 1.338–1.433(3) Å, which are also intermediate between single and double carbon-carbon bond values due to conjugation in the system. In molecules, the intramolecular O1-H1–N1 hydrogen bond was found with the following parameters: O-H 0.94(3) O···N 2.657(2) Å, OHN 147(3)°.
It is noteworthy that the addition of C-nucleophiles to the carbonyl groups of 9,10-anthracenedione has been represented by a limited number of examples. In particular, the addition has been described in the literature only by reactions in the presence of strong bases, such as Grignard reagents [36], alkyl and aryl lithium [37], alkali-metal acetelenides [38], and dimethylsulfonium methylide [39]. In turn, C-addition of the methyl group of 4-methylpyridine to the carbonyl group with the formation of a methylene bridge occurs in the presence of polyacids [40], Lewis acids [41], strong bases (butyllithium or lithium diisopropylamide [42,43,44]), as well as tetrabutylammonium fluoride under conditions of microwave irradiation [45]. A feature of the found reaction is the possibility of forming a new sp3-hybridized hydroxy-functionalized center based on the anthraquinone fragment. It is crucial that the process of addition is implemented with the participation of the low nucleophilic methyl group of 4-methylpyridine, as well as 4-methylpyridine itself as a weak alkaline medium in the absence of specific catalysts.
Hence, we tried to determine the moment of the addition, which could be before the start of thermolysis, during its course, or after the formation of the annelated product 2d. For this purpose, two independent experiments were conducted: heating of anthratriazinone 1 with 4-methylpyridine at 70 °C for 6 h, and heating of anthrapyridinopyrimidinone 2d with 4-methylpyridine at 115 °C for 6 h. In both cases, product 4 was not detected by TLC or LC-MS. The latter indicates its formation in the process of thermolysis. Taking into consideration these results and the above Scheme 3, we proposed a mechanism for the formation of by-product 4 in the reaction of anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-trione 1 with 4-methylpyridine through intermediates A–C (Scheme 6).
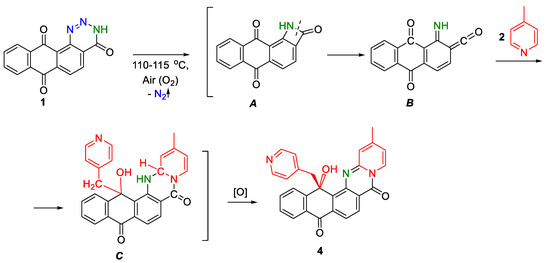
Scheme 6.
A probable mechanism of formation for compound 4.
Thus, the results show the possibility of using pyridine (quinoline) as a dienophile in the hetero-Diels–Alder reaction with the in situ-generated 1-iminoanthraquinone-2-ketene as a model heterodyne. Moreover, a convenient and effective method to obtain anthrapyrido[1,2-a]pyrimidin-4-one systems was proposed.
3. Materials and Methods
General Information
Melting points were measured in open glass capillaries using a Buchi B-540 melting point apparatus and were uncorrected. The elemental analysis was performed on a PerkinElmer 2400 CHN analyzer, and the results were found to be in good agreement with the calculated values. The 1H NMR spectra and 13C NMR spectra in DMSO-d6 and CF3COOD were recorded on a Varian Mercury-400 spectrometer with TMS as an internal standard. Mass spectra were recorded on an Agilent 1100 Series G1956B LC/MSD SL LCMS system, using electrospray ionization at atmospheric pressure (70 eV). The individuality of the obtained compounds was controlled by the TLC method on Silufol UV-254 plates in the solvent system acetonitrile/benzene/trifluoroacetic acid, 3:1:0.1. The X-ray structural analysis of a single crystal of compound 4 was performed at 173 K on a Bruker Smart Apex II diffractometer operating in the ω scans mode. The intensity data were collected within the θmax ≤ 26.4° using Mo-Kα radiation (λ = 0.71078 Å). The intensities of 21,514 reflections were collected (4234 unique reflections, Rmerg = 0.0534). The structure was solved by direct methods and refined by the full-matrix least-squares technique using the Bruker SHELXTL program package [46]. All chemicals were of reagent grade and used without further purification. The solvents were purified according to the standard procedures [47]. Anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-trione 1 was prepared using the method in [14].
General procedure for the preparation of anthrapirido[1,2-a]pyrimidin-4-ones 2a–f, 3a,b. To 30 mL of the corresponding pyridine, in a round-bottom flask equipped with a reflux condenser, was added 0.5 g of anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-trione 1 at room temperature followed by stirring. The reaction mixture was heated to 110–115 °C for 1 h. Then, air was bubbled into the reaction mixture for 6 h, while maintaining the indicated temperature regime. After, the reaction mass was cooled, the excess of the corresponding pyridine was distilled off in a vacuum. The residue was suspended in 100 mL of water, and the precipitate was filtered off, washed with water, and dried.
6H-Naphtho[2,3-h]pyrido[2,1-b]quinazoline-6,9,14-trione 2a. Yield 75%; m.p. > 300 °C (336–338.5 °C в [33]). 1H-NMR (CF3COOD): δ = 9.48 (d, 1H, J = 7.0 Hz, CHar), 9.12 (d, 1H, J = 8.4 Hz, CHar), 8.76–8.65 (m, 2H, CHar), 8.54 (l, 1H, J = 7.4 Hz, CHar), 8.50 (d, 1H, J = 6.7 Hz, CHar), 8.42 (d, 1H, J = 8.9 Hz, CHar), 8.13–8.07 (m, 2H, CHar), 7.89 (t, 1H, J = 6.9 Hz, CHar). 13C-NMR (CF3COOD): δ = 187.08, 183.01, 154.42 (C=O), 147.46, 147.02, 140.21, 138.20, 136.34 (2C), 132.75, 132.12, 130.05, 128.01, 127.97, 127.79, 124.91, 119.69, 118.38, 118.34, 117.41 (Car). LC-MS (70 eV): m/z = 327 [M + 1] (100%). Anal. Calcd. for C20H10N2O3: C, 73.62; H, 3.09; N, 8.59. Found: C 73.68; H 3.04; N 8.63.
Mixture of 3-methyl-6H-naphtho[2,3-h]pyrido[2,1-b]quinazoline-6,9,14-trione 2b тa 1-methyl-6H-naphtho[2,3-h]pyrido[2,1-b]quinazoline-6,9,14-trione 2c: Yield 75%; m.p. = 223–228 °C. 1H-NMR (CF3COOD): δ = 10.32 (t, 0.65 H, J = 6.5 Hz, CHmajor), 10.28–10.20 (m, 0.35 H, CHminor), 10.09–10.01 (m, 1H, CHmajor), 9.71–9.60 (m, 1.3 H, CHmixture), 9.59–9.53 (m, 0.7 H, CHmajor), 9.51–9.41 (m, 2.5 H, CHmixture), 9.32–9.23 (m, 0.35 H, CHminor), 9.06–9.02 (m, 1.7 H, CHmixture), 8.75 (q, 0.7 H, J = 5.9 Hz, CHmajor), 3.99 (s, 2H, CH3major), 3.69 (s, 1H, CH3minor). 13C-NMR (CF3COOD): δ = 188.18 (C=Omajor), 187.98 (C=Ominor), 183.96 (C=O, 2Cmajor+minor), 155.49 (C=Omajor), 155.24 (C=Ominor), 150.64 (Cmajor), 147.48 (Cmajor, 2C), 147.09 (Cminor, 2C), 146.16 (Cminor), 141.02 (Cminor), 140.94 (Cmajor), 138.95 (Cminor), 138.85 (Cmajor), 137.19 (Cmajor), 137.16 (Cmajor), 137.06 (Cminor, 2C), 133.63 (Cmajor), 133.55 (Cminor), 132.98 (Cmajor), 132.96 (Cmajor), 132.91 (Cminor, 2C), 128.97 (Cmajor), 128.88 (Cmajor, 2C), 128.74 (Cminor), 128.37 (Cminor), 128.31 (Cminor), 125.80 (Cmajor), 125.51 (Cminor), 119.91 (Cmajor), 119.06 (Cminor), 119.04 (Cmajor), 118.50 (Cminor), 118.01 (Cminor), 117.86 (Cmajor), 17.30 (CH3minor), 15.57 (CH3major). LC-MS (70 eV): m/z = 341 [M + 1] (64%), 341 [M + 1] (36%). Anal. Calcd. for C21H12N2O3: C, 74.11; H, 3.55; N, 8.23. Found: C, 74.16; H, 3.50; N, 8.27.
2-Methyl-6H-naphtho[2,3-h]pyrido[2,1-b]quinazoline-6,9,14-trione 2d: Yield 65%. m.p. > 300 °C. 1H-NMR (CF3COOD): δ = 10.30–10.24 (m, 1H, CHar), 10.04 (d, 1H, J = 9.0 Hz, CHar), 9.64 (d, 1H, J = 8.0 Hz, CHar), 9.50–9.41 (m, 2H, CHar), 9.12–9.08 (m, 1H, CHar), 9.07–9.02 (m, 2H, CHar), 8.68–8.62 (m, 1H, CHar), 3.84 (s, 3H, CH3). 13C-NMR (CF3COOD): δ = 187.97, 184.09, 164.55 (C=O), 155.36, 147.06, 141.09, 139.36, 137.26, 137.14, 133.62, 132.91, 130.05, 129.99, 128.80, 128.74, 125.50, 122.74, 119.12, 118.09, 117.33 (Car), 22.10 (CH3). LC-MS (70 eV): m/z = 341 [M + 1] (100%). Anal. Calcd. for C21H12N2O3: C, 74.11; H, 3.55; N, 8.23. Found, %: C, 74.15; H, 3.61; N, 8.28.
1,3-Dimethyl-6H-naphtho[2,3-h]pyrido[2,1-b]quinazoline-6,9,14-trione 2e: Yield 75%; m.p. = 293–294 °C. 1H-NMR (CF3COOD): δ = 10.12–10.08 (m, 1H, CHar), 10.01 (d, 1H, J = 8.3 Hz, CHar), 9.62 (d, 1H, J = 8.3 Hz, CHar), 9.53 (d, 1H, J = 6.3 Hz, CHar), 9.41 (d, 1H, J = 6.4 Hz, CHar), 9.35–9.30 (m, 1H, CHar), 9.04–8.99 (m, 2H, CHar), 3.93 (s, 3H, CH3), 3.63 (s, 3H, CH3). 13C-NMR (CF3COOD): δ = 188.22, 184.11, 155.54 (C=O), 150.00, 145.47, 140.94, 138.84, 137.20 (2C), 137.12, 133.70, 133.00, 132.35, 128.91, 128.89, 127.67, 126.65, 125.56, 119.02, 117.76 (Car), 17.33, 15.47 (CH3). LC-MS (70 eV): m/z = 355 [M + 1] (100%). Anal. Calcd. for C22H14N2O3: C, 74.57; H, 3.98; N, 7.91. Found: C, 74.65; H, 3.93; N, 7.99.
6H-Naphtho[2,3-h]quinolino[2,1-b]quinazoline-6,9,14-trione 2f: Yield 90%; m.p. = 262–263 °C. 1H-NMR (CF3COOD): δ = 10.72–10.64 (m, 1H, CHar), 10.08 (t, 1H, J = 7.5 Hz, CHar), 9.86–9.78 (m, 1H, CHar), 9.71 (t, 1H, J = 7.5 Hz, CHar), 9.53–9.49 (m, 1H, CHar), 9.48–9.44 (m, 1H, CHar), 9.22–9.13 (m, 2H, CHar), 9.08–8.94 (m, 4H, CHar). 13C-NMR (CF3COOD): δ = 188.05, 183.97, 159.14 (C=O), 150.81, 149.90, 140.29, 137.39, 137.23, 137.18, 137.08, 136.08, 135.09, 133.60, 133.13, 131.59, 130.83, 129.02, 128.84, 126.79, 126.14, 122.33, 121.99, 119.21, 114.73 (Car). LC-MS (70 eV): m/z = 377 [M + 1] (100%). Anal. Calcd. for C24H12N2O3: C, 76.59; H, 3.21; N, 7.44. Found: C, 76.65; H, 3.25; N, 7.41.
4-Chloro-15,15a-dihydro-6H-naphtho[2,3-h]pyrido[2,1-b]quinazoline-6,9,14-trione 3a: Yield 68%; m.p. > 300 °C. 1H-NMR (CF3COOD): δ = 10.41 (s, 1H, NH), 9.79–9.72 (m, 1H, CHar), 9.49–9.44 (m, 2H, CHar), 9.42–9.35 (m, 3H, CHar), 9.05–8.95 (m, 4H, CHar). 13C-NMR (CF3COOD): δ = 186.85, 183.71, 169.75 (C=O), 139.17, 138.66, 137.53, 136.46, 136.30, 135.93, 133.42, 133.17, 132.21, 128.27, 128.24, 128.00, 126.20, 124.93, 123.09, 122.78 (Car), 76.62 (CH). IR, cm−1: 3328 (NH), 1736, 1664, 1624 (C=O), 712 (Cl). LC-MS (70 eV): m/z = 363 [M + 1] (100%). Anal. Calcd. for C20H11ClN2O3: C, 66.22; H, 3.06; Cl, 9.77; N, 7.72. Found: C, 66.27; H, 3.01; Cl, 9.79; N, 7.76.
3,4-Dimethyl-15,15a-dihydro-6H-naphtho[2,3-h]pyrido[2,1-b]quinazoline-6,9,14-trione 3b: Yield 70%; m.p. = 203–204 °C. 1H-NMR (DMSO-d6): δ = 10.15 (s, 1H, NH), 8.29 (d, 1H, J = 7.9 Hz, CHar), 8.26–8.22 (m, 1H, CHar), 8.19–8.16 (m, 1H, CHar), 7.93 (pd, 2H, J = 7.3, 1.6 Hz, CHar), 7.64 (d, 1H, J = 7.9 Hz, CHar), 7.26 (d, 1H, J = 7.7 Hz, CH), 6.00 (dt, 1H, J = 5.7, 1.3 Hz, CH), 5.63 (dd, 1H, J = 7.8, 5.8 Hz, CH), 2.22 (s, 3H, CH3), 1.45 (s, 3H, CH3). 13C-NMR (DMSO-d6): δ = 185.70, 182.56, 158.85 (C=O), 145.96, 138.06, 135.43, 135.30, 134.84, 134.14, 132.94, 131.06, 127.15, 127.09, 121.21, 119.19, 118.68, 116.63 (Car), 115.67, 107.80, 73.29 (CH), 23.66, 16.93 (CH3). IR, cm−1: 3264 (NH), 1712, 1660, 1612 (C=O). LC-MS (70 eV): m/z = 357 [M + 1] (100%). Anal. Calcd. for C22H16N2O3: C, 74.15; H, 4.53; N, 7.86. Found: C, 74.19; H, 4.48; N, 7.81.
14-Hydroxy-2-methyl-14-(pyridin-4-ylmethyl)-6H-naphtho[2,3-h]pyrido[2,1-b]quinazoline-6,9(14H)-dione 4: It was separated from compound 2d by chromatography on silica gel (eluent—ethyl acetate: hexane: triethylamine, 1:1:0.01). Yield 12%; m.p. = 228–230 °C. 1H-NMR (CF3COOD): δ = 9.31 (d, J = 6.8 Hz, 1H, CHar), 8.86 (d, J = 8.5 Hz, 1H, CHar), 8.50 (s, 3H, CHar), 8.27 (d, J = 8.3 Hz, 1H, CHar), 8.12 (s, 1H, CHar), 7.96 (d, J = 7.6 Hz, 2H, CHar), 7.79 (t, J = 7.5 Hz, 1H, CHar), 7.66 (d, J = 6.9 Hz, 1H, CHar), 7.16 (s, 2H, CHar), 4.01 (dd, J = 74.8, 13.0 Hz, 2H, CH2), 2.87 (s, 3H, CH3). 13C-NMR (DMSO-d6): δ = 181.68, 158.27 (C=O), 149.15, 149.02, 148.26, 146.58 (2C), 144.01, 138.06, 134.87, 134.57, 134.07, 129.50, 128.28, 126.86, 126.57, 126.02, 125.31, 125.01 (2C), 123.33, 119.90, 118.47, 117.29 (Car), 74.78 (C-OH), 53.04 (CH2), 20.93 (CH3). LC-MS (70 eV): m/z = 434 [M + 1] (100%). Anal. Calcd. for C27H19N3O3: C, 74.81; H, 4.42; N, 9.69. Found: C, 74.85; H, 4.39; N, 9.71.
Crystal data: Monoclinic, space group P21/c, a = 8.7345(3), b = 25.1641(9), c = 9.8241(4) Å, β = 106.547(2), V = 2069.87(13) Å3, Z = 4, dc = 1.391, µ 0.092mm−1, F(000) 904, crystal size ca. 0.06 x 0.32 x 0.5mm. All CH hydrogen atoms were placed at calculated positions and refined as a ‘riding’ model. The OH hydrogen atom was found in DF synthesis and refined isotropically. Convergence was obtained at R1 = 0.0575 and wR2 = 0.1383 for 2787 observed reflections with I ≥ 2σ(I); R1 = 0.0926 and wR2 = 0.1587, GOF = 1.026 for independent reflections, 303 parameters, the largest and minimal peaks in the final difference map 0.27 and –0.22 e/Å3.
The crystallographic data for the structure 4 in this paper have been deposited at Cambridge Crystallographic Data Centre as supplementary publication numbers CCDC 2192784. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB21EZ, UK, (fax +44-(0)1223-336033 or e-mail deposit@ccdc.cam.ac.uk).
4. Conclusions
As a result of the conducted research, we proposed an effective method to obtain the anthrapyrido[1,2-a]pyrimidin-4-one systems 2 and 3. In the first stage, the method includes the thermal elimination of molecular nitrogen from the triazine nucleus of anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-trione 1. In the second stage, the in situ-generated 1-iminoanthraquinone-2-ketene reacts with the pyridine or quinoline nucleus via an [4+2]-cycloaddition reaction. The effect of substituents in the pyridine ring on the formation of annelated products was studied. In particular, it was determined that the interaction of 2-chloropyridine or 2,3-dimethylpyridine with anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-trione 1 gave the dihydrogenated cyclic compounds. It was found that the reaction of compound 1 with 4-methylpyridine was accompanied by the formation of an unexpected by-product 4 in addition to the target compound 2d. The structure of compound 4 was determined by X-ray diffraction analysis. The isolation of by-product 4 showed the formation of a new sp3-hybridized hydroxy-functionalized center based on the carbonyl group of anthracenyl cycle with the participation of the low nucleophilic methyl group of 4-methylpyridine, as well as 4-methylpyridine itself as an alkaline medium in the absence of any catalysts. In addition, we proposed a probable mechanism of the thermal ring-opening–annulation reaction of anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-trione in the presence of pyridines (quinoline) with the formation of 6H-naphtho[2, 3-h]pyrido[2,1-b]quinazoline-6,9,14-trione and 6H-naphtho[2,3-h]quinolino[2,1-b]quinazoline-6,9,14-trione systems.
Author Contributions
V.Z., synthesis of the compounds, characterization of all the obtained compounds; M.S., conceptualization and supervision of synthesis, interpretation of the results, writing of original draft; E.R., X-ray structure determination and description; M.V., discussion of the experimental data of the chemical part. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the manuscript and from the corresponding author (M.S.) upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 2a-f, 3a,b are available from the authors.
References
- Bien, H.-S.; Stawitz, J.; Wunderlich, K. Anthraquinone Dyes and Intermediates. In Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Kellersohn, T., Ed.; Wiley-VCH Verlag Gmbh: Weinheim, Germany, 2003; pp. 513–578. [Google Scholar]
- Gouda, M.A.; Berghot, M.A.; Shoeip, A.; Elattar, K.M.; Khalil, A.E.-G.M. Chemistry of 2-aminoanthraquinones. Turk. J. Chem. 2010, 34, 651–709. [Google Scholar] [CrossRef]
- Fouillaud, M.; Caro, Y.; Venkatachalam, M.; Grondin, I.; Dufossé, L. Anthraquinones. In Phenolic Compounds in Food Characterization and Analysis; Nollet, L.M.L., Gutiérrez-Uribe, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 130–170. [Google Scholar]
- Tikhomirov, A.S.; Shtil, A.A.; Shchekotikhin, A.E. Advances in the discovery of anthraquinone-based anticancer agents. Recent Pat. Anti-Cancer Drug Discov. 2018, 13, 159–183. [Google Scholar] [CrossRef]
- Winkelmann, E.; Raether, W. Chemotherapeutically active anthraquinones. I. Aminoanthraquinones. Arzneimittelforschung 1979, 29, 1504–1509. [Google Scholar] [PubMed]
- Malik, E.M.; Muller, C.E. Anthraquinones as pharmacological tools and drugs. Med. Res. Rev. 2016, 36, 705–748. [Google Scholar] [CrossRef] [PubMed]
- Thomson Reuters Integrity Database. Available online:https://integrity.clarivate.com/integrity/xmlxsl/ (accessed on 14 December 2019).
- Gorelik, M.V.; Puchkova, V.V.; Khan, I.G. Method for Obtaining 4-Substituted 1, 2-Anthraquinimidazolines. USSR Patent 499260, 15 January 1976. [Google Scholar]
- Bergmann, U.; Hoch, H.; Reinhold, K. Process and Alkyl Benzoate Solvents for the Preparation of (Benzoylamino)anthraquinone Compounds. European Patent EP653416, 17 May 1995. [Google Scholar]
- Bulgakova, N.A.; Gornostaev, L.M. Cyclization of 1-Aryl-3-[4-aryl(cyclohexyl)amino-9,10-dioxo-1-anthryl]triazenes to 3-Aryl-5-aryl(cyclohexyl)aminoanthra[1,2-d][1,2,3]triazole-6,11-diones. Russ. J. Org. Chem. 2001, 37, 1351–1352. [Google Scholar] [CrossRef]
- Stepanov, A.A.; Gornostaev, L.M.; Vasilevsky, S.F.; Arnold, E.V.; Mamatyuk, V.I.; Fadeev, D.S.; Gold, B.; Alabugin, I.V. Chameleonic reactivity of vicinal diazonium salt of acetylenyl-9,10-anthraquinones: Synthetic application toward two heterocyclic targets. J. Org. Chem. 2011, 76, 8737–8748. [Google Scholar] [CrossRef]
- Fedenok, L.G.; Barabanov, I.I.; Zolnikova, N.A.; Bashurova, V.S.; Bogdanchikov, G.A. Mechanism and synthesis potentialities of the cyclization of vic-(alkynyl)arenediazonium salts. Chem. Sustain. Dev. 2011, 19, 647–651. [Google Scholar] [CrossRef]
- Fedenok, L.G.; Barabanov, I.I.; Bashurova, V.S.; Bogdanchikov, G.A. Mechanism of the heterocyclization of vic-alkynylanthra- and vic-alkynylnaphthoquinone diazonium salts. Tetrahedron 2004, 60, 2137–2145. [Google Scholar] [CrossRef]
- Zvarych, V.; Stasevych, M.; Novikov, V.; Rusanov, E.; Vovk, M.; Szweda, P.; Grecka, K.; Milewski, S. Anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-triones as a new class of antistaphylococcal agents: Synthesis and biological evaluation. Molecules 2019, 24, 4581. [Google Scholar] [CrossRef] [PubMed]
- Khalid, Z.; Ahmad, H.A.; Munawar, M.A.; Khan, M.; Gul, S. 1,2,3-Benzotriazin-4(3H)-ones: Synthesis, reactions and applications. Heterocycles 2017, 94, 3–54. [Google Scholar] [CrossRef]
- Gurenko, A.O. Dominance of Pirazolo[3,4-d][1,2,3]triazine-4-One in and Products of Their Transformation. Ph.D Thesis, Institute of Bioorganic Chemistry and Naphthochemistry, Kyyv, Ukraine, 2015. [Google Scholar]
- Crabtree, H.E.; Smalley, R.K.; Suschitzky, H. Thermolytic reactions of benzotriazinone and isatoic anhydride. J. Chem. Soc. Sect. C Org Chem. 1968, 2730–2733. [Google Scholar] [CrossRef]
- Smalley, R.K.; Suschitzky, H.; Tanner, E.M. Thermolysis of isatoic anhydride and benzotriazinone. Tetrahedron Lett. 1966, 29, 3465–3469. [Google Scholar] [CrossRef]
- Gescher, A.; Stevens, M.F.G.; Turnbull, C.P. Triazines and related products. Part 18. Decomposition of 1,2,3-benzotriazines and related triazenes with sodium azide in acetic acid: A convenient route to azidoarenes. J. Chem. Soc. Perkin Trans. 1977, 2, 103–106. [Google Scholar] [CrossRef]
- Paterson, T.M.; Smalley, R.K.; Suschitzky, H.; Barker, A.J. 1,2,3-Benzotriazin-4-ones and related systems. Part 6. Thermal and photolytic decomposition of 3-arylideneamino-, 3-imidoyl-, and 3-heteroaryl-1,2,3-benzotriazin-4-ones. J. Chem. Soc. Perkin Trans. 1980, 1, 633–638. [Google Scholar] [CrossRef]
- Barker, A.J.; McC Paterson, T.; Smalley, R.K.; Suschitzky, H. 1,2,3-Benzotriazin-4(3H)-ones and related systems. Part 5. Thermolysis of 3-aryl- and 3-alkenyl-1,2,3-benzotriazin-4(3H)-ones. J. Chem. Soc. Perkin Trans. 1979, 1, 2203–2208. [Google Scholar] [CrossRef]
- Murray, A.W.; Vaughan, K. Thermolysis of 1,2,3-benzotriazin-4(3H)-one. J. Chem. Soc. Sect. C Org. Chem. 1970, 15, 2070–2074. [Google Scholar] [CrossRef]
- Ege, G. 7-Methyl-4-phenyl-8H-1,3,5,6-dithiadiazocin-2-one, an Eight-Membered Heterocycle with Chiral Conformation. Angew. Chem. Int. Ed. 1967, 6, 629–630. [Google Scholar] [CrossRef]
- Burgess, E.M.; Milne, G. N-Phenylbenzoazetinone. Tetrahedron Lett. 1966, 1, 93–96. [Google Scholar] [CrossRef]
- Krawczyk, S.H.; Townsend, L.B. Synthesis of a pyrido[1,2-a]purine nucleoside by a novel ring cleavage-annulation reaction of 3-β-D-ribofuranosylimidazo-[4,5-d]-triazin-4-one. Tetrahedron Lett. 1991, 32, 5693–5696. [Google Scholar] [CrossRef]
- Zvarych, V.I.; Stasevych, M.V.; Lunin, V.V.; Vovk, M.V.; Novikov, V.P. Synthesis of (1H-pyrrol-1-yl)anthracene-9,10-diones. Chem. Heterocycl. Compd. 2016, 52, 421–423. [Google Scholar] [CrossRef]
- Strobel, T.; Schmidt, Y.; Linnenbrink, A.; Luzhetskyy, A.; Luzhetska, M.; Taguchi, T.; Brötz, E.; Paululat, T.; Stasevych, M.; Stanko, O.; et al. Tracking Down Biotransformation to the Genetic Level: Identification of a Highly Flexible Glycosyltransferase from Saccharothrix espanaensis. Appl. Environ. Microbiol. 2013, 79, 5224–5232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stasevich, M.V.; Zvarich, V.I.; Novikov, V.P.; Zagorodnya, S.D.; Povnitsa, O.Y.; Chaika, M.A.; Nesterkina, M.V.; Kravchenko, I.A.; Druzhilovskiy, D.S.; Poroikov, V.V. 9,10-Anthraquinone Dithiocarbamates as Potential Pharmaceutical Substances with Pleiotropic Actions: Computerized Prediction of Biological Activity and Experimental Validation. Pharm. Chem. J. 2020, 53, 905–913. [Google Scholar] [CrossRef]
- Stasevych, M.; Zvarych, V.; Musyanovych, R.; Novikov, V.; Vovk, M. Synthesis of N-Benzoyl-N’-(9,10-Dioxo-9,10-Dihydroanthacen-1-yl) Thioureas and Quantum-Chemical Analysis of the Reaction Passing. Chem. Chem. Technol. 2014, 8, 135–140. [Google Scholar] [CrossRef]
- Stasevych, M.V.; Zvarych, V.I.; Novikov, V.P. Study of the Antifungal Action of the Lacquer Based on the GABA Derivative of 2-Chloro-N-(9,10-Dioxo-9,10-Dihydroanthracen-1-yl)Acetamide. Biointerface Res. Appl. Chem. 2021, 11, 8818–8824. [Google Scholar] [CrossRef]
- Stasevych, M.V.; Zvarych, V.I.; Novikov, V.P.; Vovk, M.V. Synthesis and Study of Antimicrobial Activity of 2-Dithiocarbamate-N-(9,10-Dioxo-9,10-Dihydroanthracenyl)Acetamides. Biointerface Res. Appl. Chem. 2021, 11, 7725–7734. [Google Scholar] [CrossRef]
- Stasevych, M.; Zvarych, V.; Lunin, V.; Khomyak, S.; Vovk, M.; Novikov, V. Synthesis of pyrazole and tetrazole derivatives of 9,10-anthraquinonylhydrazones. Chem. Heterocycl. Compd. 2017, 53, 927–929. [Google Scholar] [CrossRef]
- Mosby, W.L.; Berry, W.L. Products of nucleophilic displacement reactions in the anthraquinone series. Tetrahedron 1960, 8, 107–115. [Google Scholar] [CrossRef]
- Hostyn, S.; Van Baelen, G.; Lemière, G.L.; Maes, B.U. Synthesis of α-Carbolines Starting from 2,3-Dichloropyridines and Substituted Anilines. Adv. Synth. Catal. 2008, 350, 2653–2660. [Google Scholar] [CrossRef]
- Li, J.; Li, G. Tetradentate and Octahedral Metal Complexes Containing Naphthyridinocarbazole and Its Analogues. US Patent US20160359125 A1, 8 December 2016. [Google Scholar]
- Cohen, D.; Hewitt, L.; Millar, I.T. Reaction of anthraquinone with some alkylmagnesium halides. Configuration and conformation of 9,10-diethyl- and 9,10-di-n-propyl-anthracene-9,10-diols. J. Chem. Soc. (C) 1969, 17, 2266–2269. [Google Scholar] [CrossRef]
- Konieczny, M.; Harvey, R.G. Reductive methylation of polycyclic aromatic quinones. J. Org. Chem. 1980, 45, 1308–1310. [Google Scholar] [CrossRef]
- Chumbalov, T.K.; Muzychkina, R.A.; Nazarov, V.D. Study of the interaction reaction of anthraquinone with acetylene. Russ. J. Org. Chem. 1970, 6, 1752–1758. [Google Scholar]
- Hanswilli, B.; Von Schwamberger, E. Verfahren zur Herstellung von Anthranol-(9)-bzw. Anthron-(9)-aldehyden-(10). DE 1232567. 19 January 1967. [Google Scholar]
- Hao, S.-H.; Zhang, X.-Y.; Dong, D.-Q.; Wang, Z.-L. Alumina-supported heteropoly acid: An efficient catalyst for the synthesis of azaarene substituted 3-hydroxy-2-oxindole derivatives via C(sp3)-H bond functionalization. Chin. Chem. Lett. 2015, 26, 599–602. [Google Scholar] [CrossRef]
- Vuppalapati, S.V.N.; Lee, Y.R. Iodine-catalyzed efficient synthesis of aza-arene substituted 3-hydroxy-2-oxindole derivatives through sp3 C-H functionalization. Tetrahedron 2012, 68, 8286–8292. [Google Scholar] [CrossRef]
- Labrie, F.; Gauthier, S.; Cloutier, J.; Mailhot, J.; Potvin, S.; Dion, S.; Sancéau, J.-Y. Preparation of 17α-Substituted Steroids as Systemic Antiandrogens and Selective Androgen Receptor Modulators, World Intellectual Property Organization. WO2008124922 A1, 23 October 2008. [Google Scholar]
- Anderson, D.R.; Volkmann, R.A.; Menniti, F.S. Selective Octahydrocyclopenta[c]Pyrroles as Negative Modulators of NR2B and Their Preparation, World Intellectual Property Organization. WO 2015048507 A1, 2 April 2015. [Google Scholar]
- Adam, J.G.; Andrieux, J.; Plat, M. Acid-catalyzed decomposition of tertiary benzocyclobutenic azides. Novel method for synthesis of an indole nucleus by ring expansion. Tetrahedron 1985, 41, 399–407. [Google Scholar] [CrossRef]
- Kumari, K.; Allam, B.K.; Singh, K.N. A simple and sustainable tetrabutylammonium fluoride (TBAF)-catalyzed synthesis of azaarene-substituted 3-hydroxy-2-oxindoles through sp3 C-H functionalization. RSC Adv. 2014, 4, 19789–19793. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C. Purification of Laboratory Chemicals, 5th ed.; Elsevier: Oxford, UK, 2003. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).