Chemosensing Properties of Coumarin Derivatives: Promising Agents with Diverse Pharmacological Properties, Docking and DFT Investigation
Abstract
:1. Introduction
2. Experimental Section
2.1. General Information
2.2. 3-Acetyl-4-hydroxycoumarine
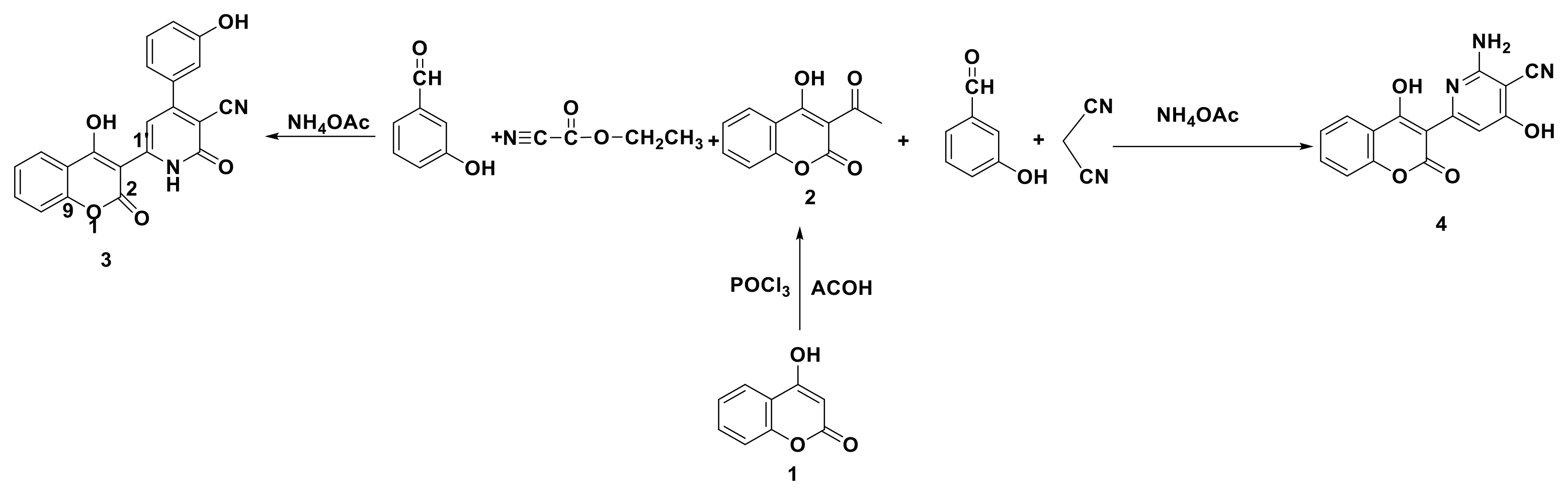
2.3. General Procedure for the Synthesis of 4-aryl-1,2-dihydro-6-(4-hydroxy-2-oxo-2H-chromen-3-yl)-2-oxopyridin-3-carbonitrile 3
2.4. Preparation of 2-amino-4-hydroxy-6-(4-hydroxy-2-oxo-2H-chromen-3-yl)nicotinonitrile 4
2.5. Antibacterial Activity
2.6. Antioxidant Activity
2.7. Hydroxyl Radical Scavenging Assay
2.8. Anti-Inflammatory Activity
2.9. Photochemical Quantum Yields
2.10. Fluorescence Quantum Yields
2.11. Computational Details
2.12. Molecular Docking
2.13. Preparation of PVA/DHCOC Nanocomposite Films
3. Results and Discussion
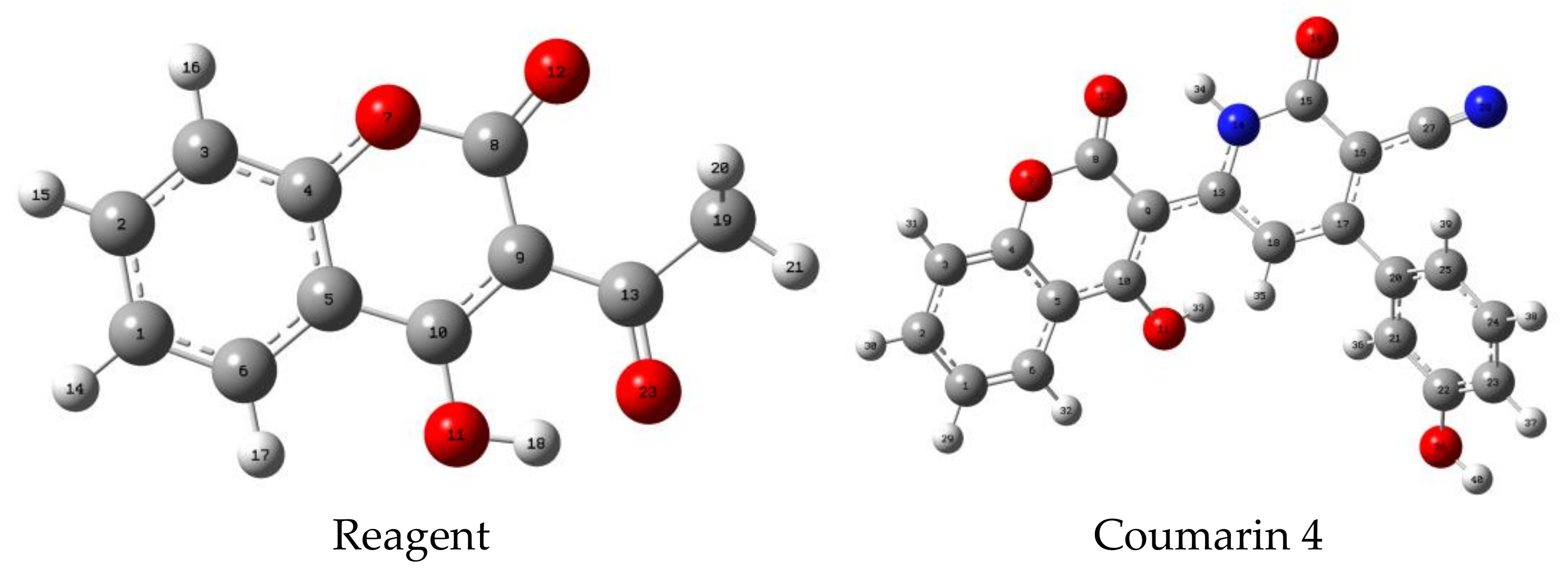
3.1. Theoretical Results
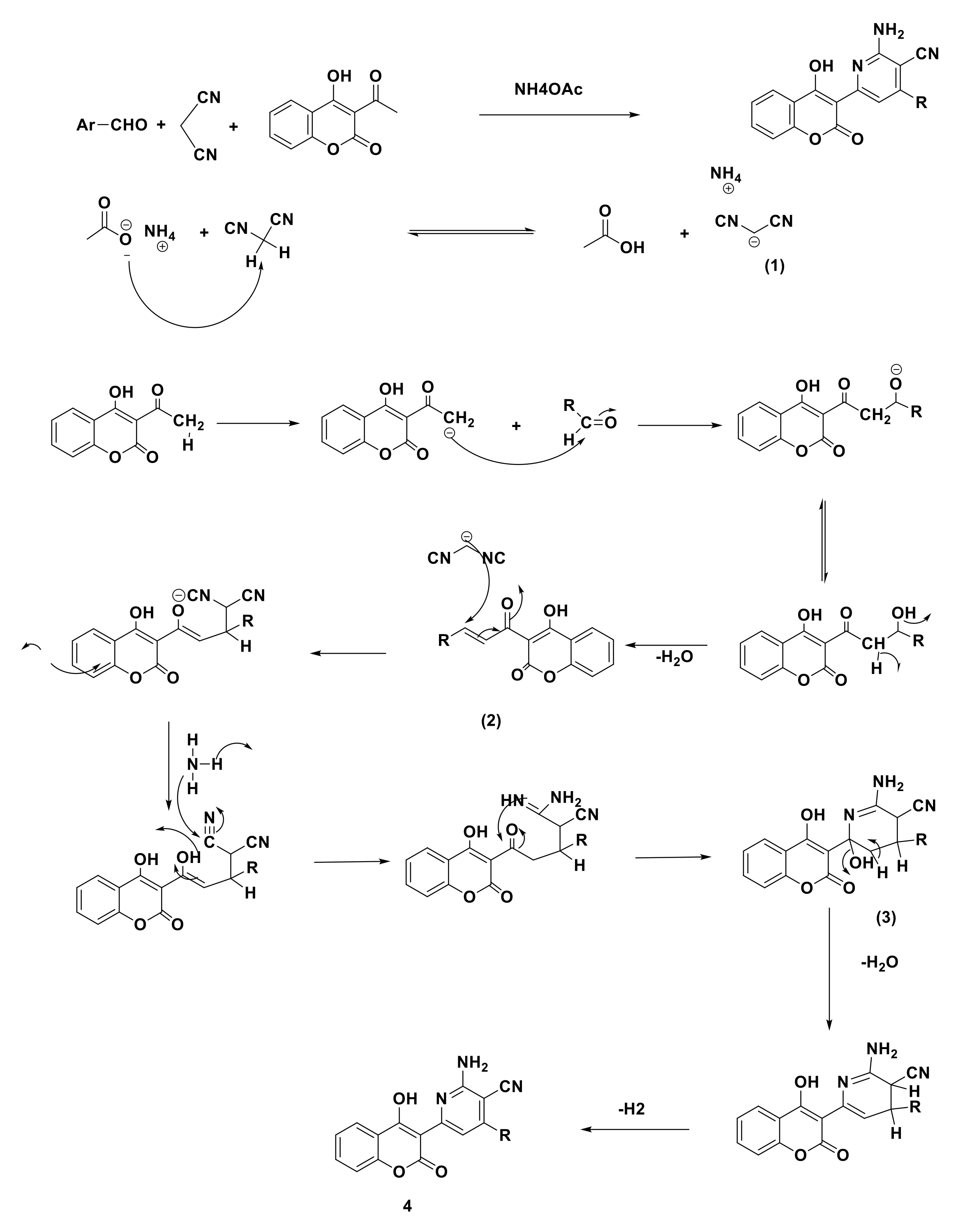
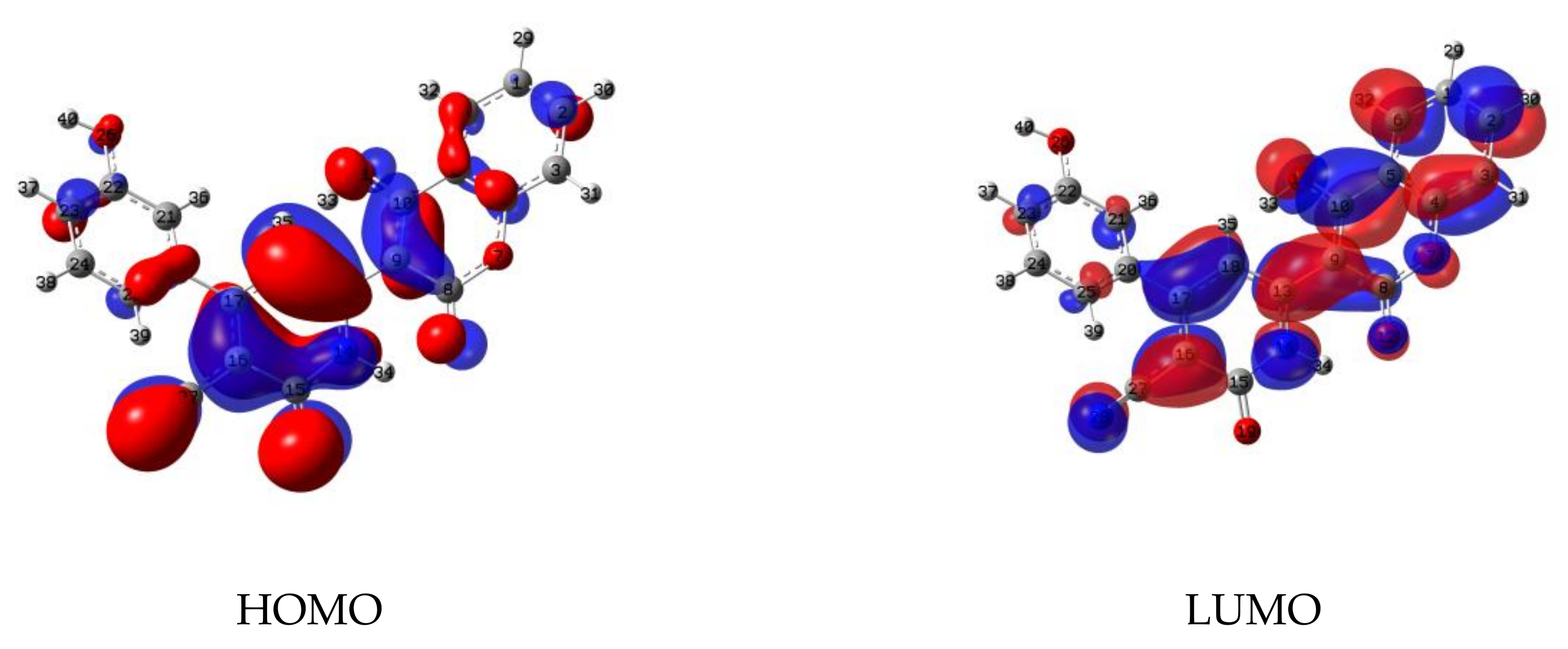
3.1.1. Modeling of the Product
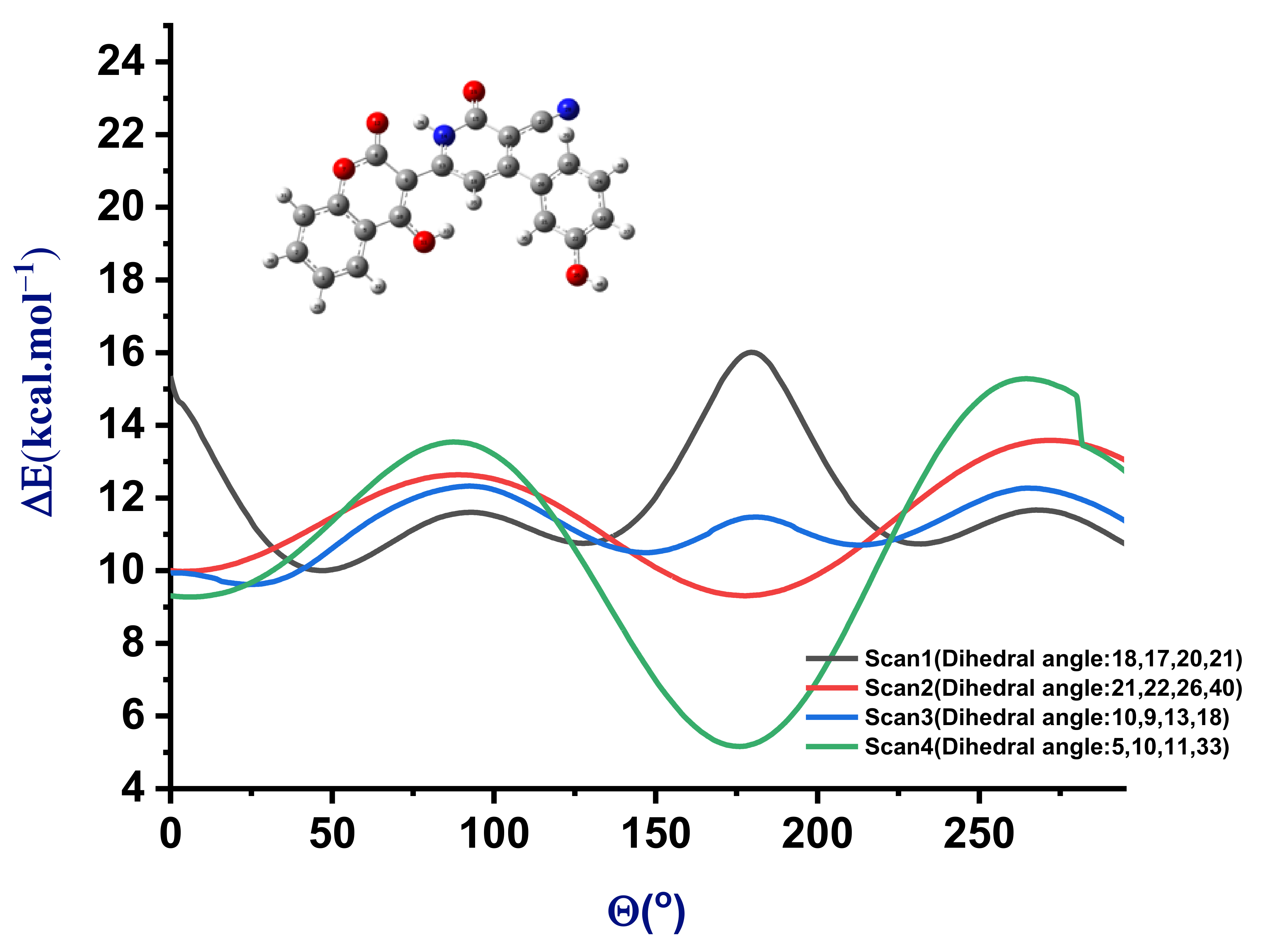
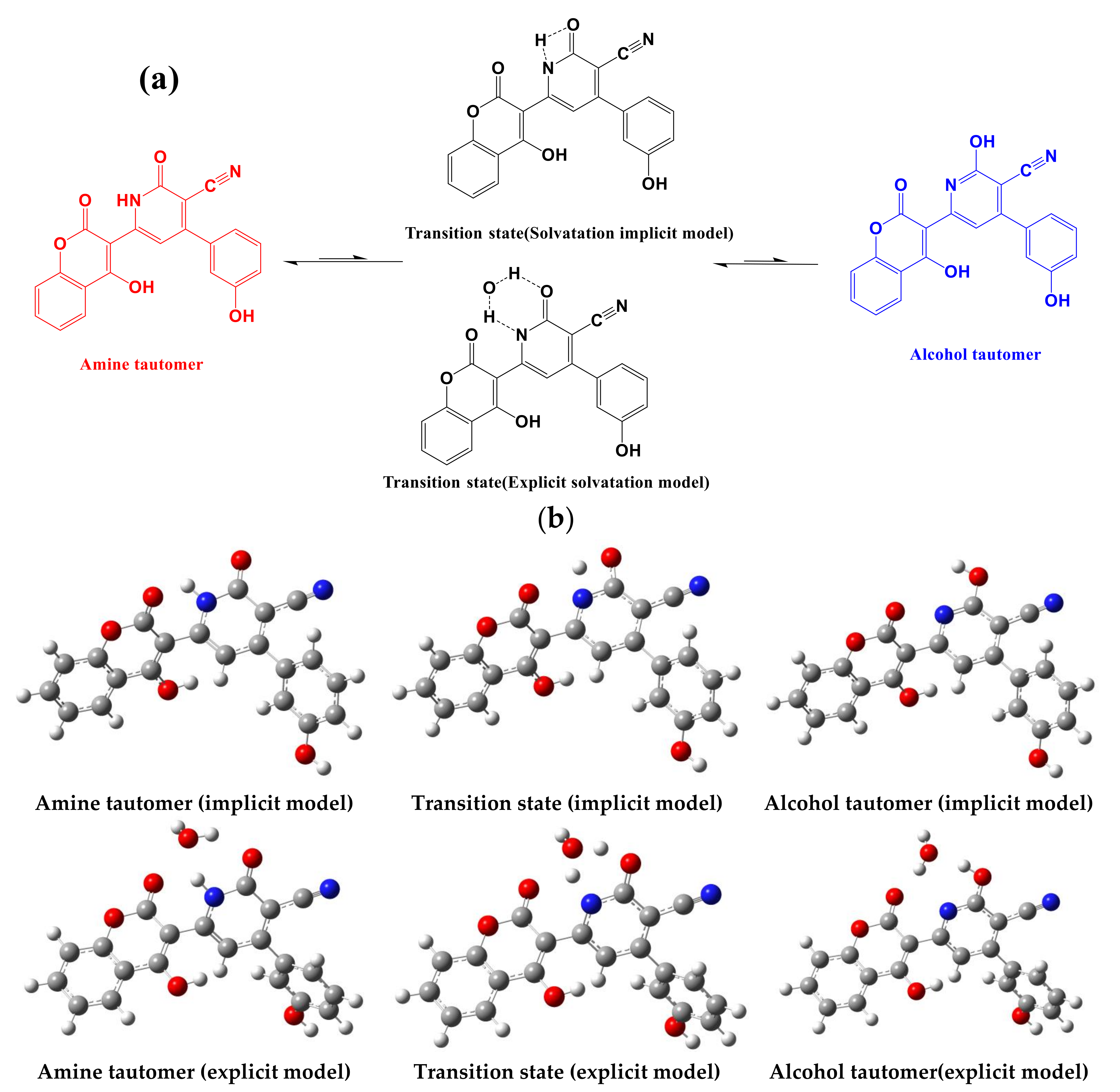
3.1.2. Study of the Tautomeric Equilibrium (Imine↔Amine)
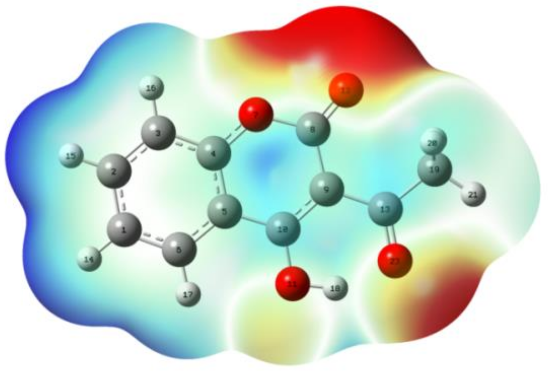
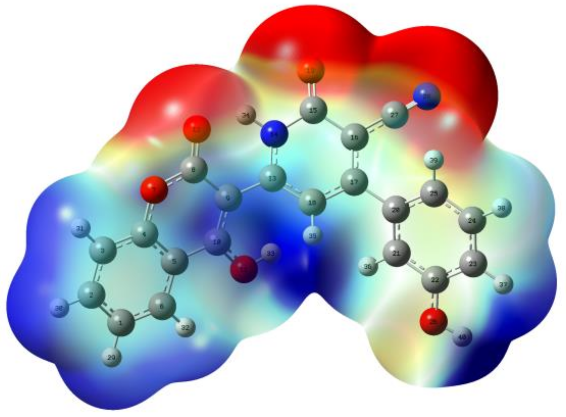
3.1.3. Mapped Electrostatic Potential Surface (MEPs) Analysis
3.2. UV Calculations
3.2.1. TD-DFT Absorption UV Spectra Analysis
3.2.2. TD-DFT Fluorescence UV Spectra Analysis
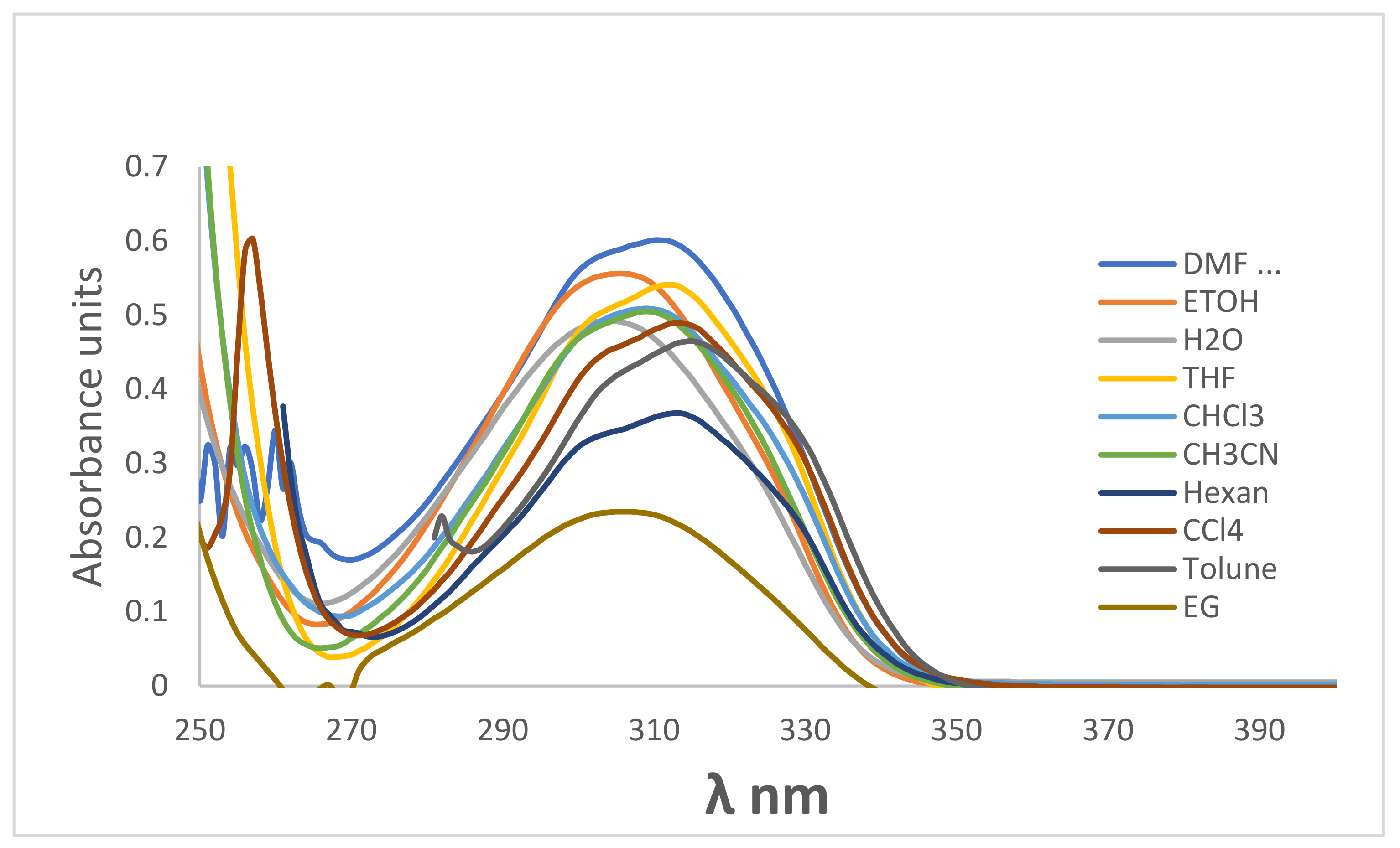
3.3. UV-Visible and Fluorescence Spectra of 2-amino-4-hydroxy-6-(4-hydroxy-2-oxo-2H-chromen-3-yl)nicotinonitrile4
3.4. Photostability
3.5. Effect of Viscosity of the Medium
3.6. Biological Activities
3.6.1. Antibacterial Activities
3.6.2. Antioxidant Activity
3.6.3. Anti-Inflammatory Activity
3.6.4. Antiproliferative Activity
3.7. Docking Result Analysis
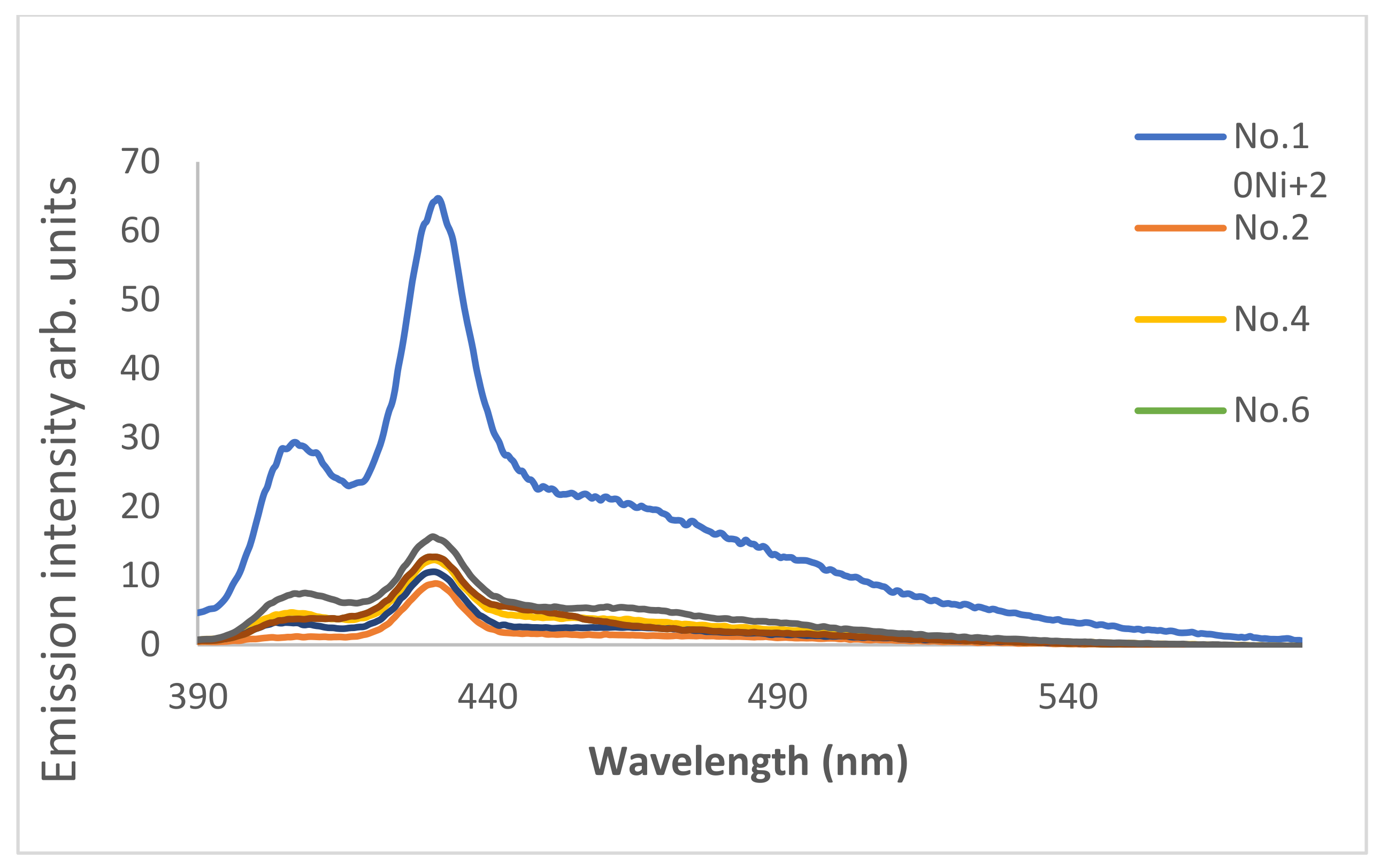
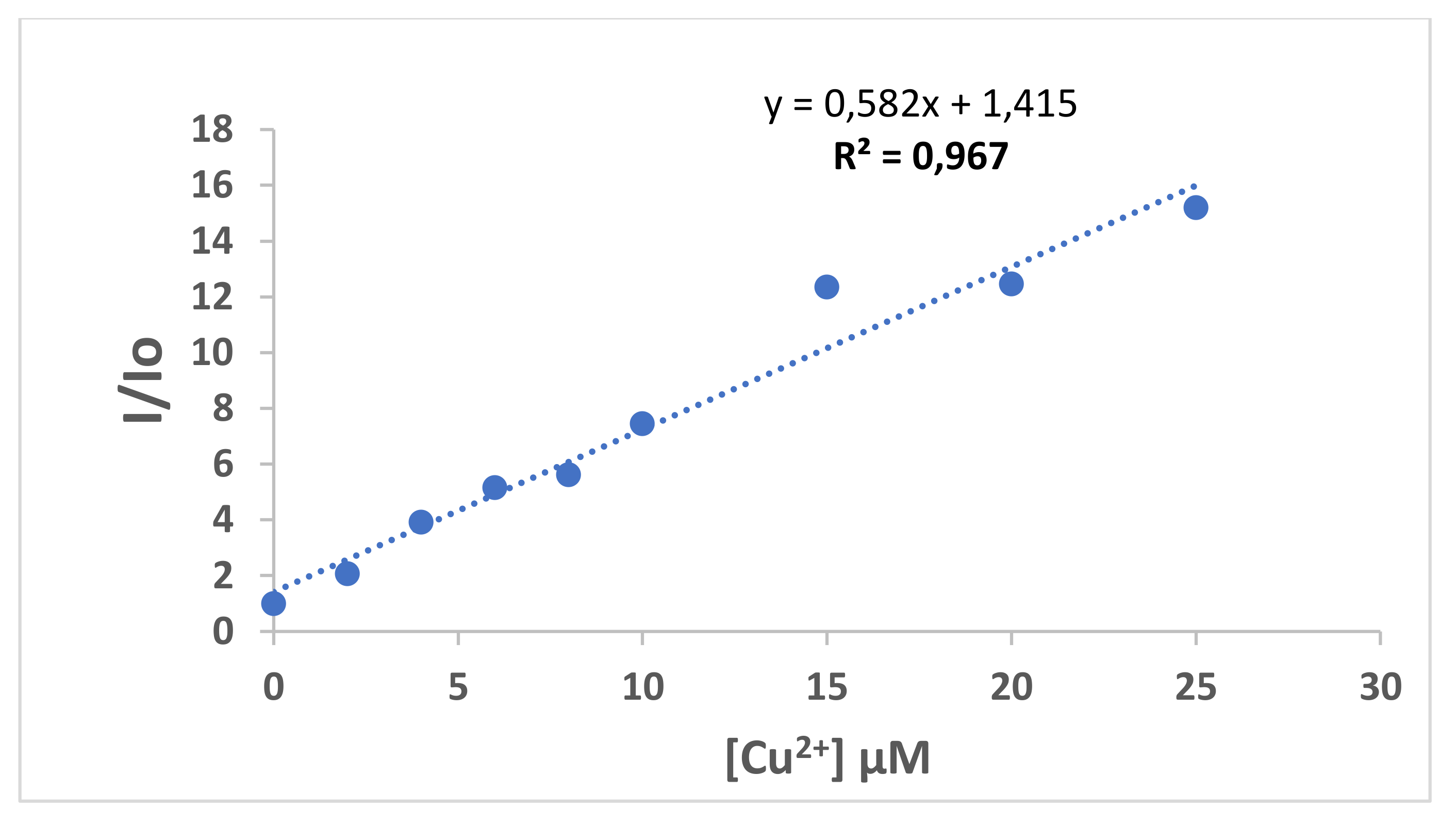
3.8. Coumarin 4 as a Chemosensor for Cu2+ and Ni+2 Ions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability Statement
Abbreviations
References
- Slimani, I.; Hamdi, N.; Al-Hazmy, S.M.; Alhagri, I.A.; Ebeid, E.-Z.M.; Okba, E.A. Synthesis and Spectral Characterisation of (E)-3-(3-(4 (Dimethylamino)Phenyl)Acrylo-yl)-4-Hydroxy-2H-Chromen-2-One and Their Antibacterial Activity and Acetylcholinesterase Inhibition. J. Chem. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Mangasuli, S.N.; Hosamani, K.M.; Devarajegowda, H.C.; Kurjogi, M.M.; Joshi, S.D. Synthesis of coumarin-theophylline hybrids as a new class of anti-tubercular and anti-microbial agents. Eur. J. Med. Chem. 2018, 146, 747–756. [Google Scholar] [CrossRef]
- Kasperkiewicz, K.; Ponczek, M.B.; Owczarek, J.; Guga, P.; Budzisz, E. Antagonists of Vitamin K—Popular Coumarin Drugs and New Synthetic and Natural Coumarin Derivatives. Molecules 2020, 25, 1465. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, J.; Liu, Y.; Zeng, Y.; Wu, G. A Review on Anti-Tumor Mechanisms of Coumarins. Front. Oncol. 2020, 10, 2720. [Google Scholar] [CrossRef] [PubMed]
- Akkol, E.K.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Galloway, W.; Isidro-Llobet, A.; Spring, D. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun. 2010, 1, 80. [Google Scholar] [CrossRef] [PubMed]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Parveen, F.; Patra, T.; Upadhyayula, S. A structure–activity relationship study using DFT analysis of Bronsted–Lewis acidic ionic liquids and synergistic effect of dual acidity in one-pot conversion of glucose to value-added chemicals. New J. Chem. 2017, 42, 1423–1430. [Google Scholar] [CrossRef]
- Casida, M.E. Time-Dependent Density Functional Response Theory for Molecules. In Recent Advances in Density Functional Methods; Chong, D.P., Ed.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 1995; Volume 1. [Google Scholar]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Tozer, D.J.; Handy, N.C. Improving virtual Kohn–Sham orbitals and eigenvalues: Application to excitation energies and static polarizabilities. J. Chem. Phys. 1998, 109, 10180–10189. [Google Scholar] [CrossRef]
- Hua, C.-J.; Zhang, K.; Xin, M.; Ying, T.; Gao, J.-R.; Jia, J.-H.; Li, Y.-J. High quantum yield and pH sensitive fluorescence dyes based on coumarin derivatives: Fluorescence characteristics and theoretical study. RSC Adv. 2016, 6, 49221–49227. [Google Scholar] [CrossRef]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Warrier, S.B.; Kharkar, P.S. A coumarin based chemosensor for selective determination of Cu (II) ions based on fluorescence quenching. J. Lumin 2018, 199, 407–415. [Google Scholar] [CrossRef]
- Inaba, Y.; Nakamura, M.; Zuguchi, M.; Chida, K. Development of Novel Real-Time Radiation Systems Using 4-Channel Sensors. Sensors 2020, 20, 2741. [Google Scholar] [CrossRef]
- Vo, P.P.; Doan, H.N.; Kinashi, K.; Sakai, W.; Tsutsumi, N.; Huynh, D.P. X-ray Visualization and Quantification Using Fibrous Color Dosimeter Based on Leuco Dye. Appl. Sci. 2020, 10, 3798. [Google Scholar] [CrossRef]
- Diffey, B. The Early Days of Personal Solar Ultraviolet Dosimetry. Atmosphere 2020, 11, 125. [Google Scholar] [CrossRef]
- Kržanović, N.; Stanković, K.; Živanović, M.; Đaletić, M.; Ciraj-Bjelac, O. Development and testing of a low cost radiation protection instrument based on an energy compensated Geiger-Müller tube. Radiat. Phys. Chem. 2019, 164, 108358. [Google Scholar] [CrossRef]
- Murray, C.K.; Hospenthal, D.R. Broth Microdilution Susceptibility Testing for Leptospira spp. Antimicrob Agents Chemother. 2004, 48, 1548–1552. [Google Scholar] [CrossRef]
- Jelali, H.; Al Nasr, I.S.; Koko, W.S.; Khan, T.A.; Deniau, E.; Sauthier, M.; Alresheedi, F.; Hamdi, N. Synthesis, characterization and in vitro bioactivity studies of isoindolin-1-3-phosophonate compounds. J. Heterocycl. Chem. 2021, 59, 493–506. [Google Scholar] [CrossRef]
- Güven, K.; Yücel, E.; Cetintaş, F. Antimicrobial Activities of Fruits of Crataegus and Pyrus. Species. Pharm. Biol. 2006, 44, 79–83. [Google Scholar] [CrossRef]
- Sellem, I.; Kaaniche, F.; Chakchouk-Mtibaa, A.; Mellouli, L. Anti-oxidant, antimicrobial and anti-acetylcholinesterase activities of organic extracts from aerial parts of three Tunisian plants and correlation with polyphenols and flavonoids contents. Bangladesh J. Pharmacol. 2016, 11, 531–544. [Google Scholar] [CrossRef]
- Kirby, A.J.; Schmidt, R.J. The antioxidant activity of Chinese herbs for eczema and of placebo herbs—I. J. Ethnopharmacol. 1997, 56, 103–108. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Yarasir, M.N.; Kandaz, M.; Senkal, B.F.; Koca, A.; Salih, B. Metal-ion sensing and aggregation studies on reactive phthalocyanines bearing soft-metal receptor moieties; synthesis, spectroscopy and electrochemistry. Polyhedron 2007, 26, 5235–5242. [Google Scholar] [CrossRef]
- Shinde, U.A.; Kulkarni, K.R.; Phadke, A.S.; Nair, A.M.; Mungantiwar, A.A.; Dikshit, V.J.; Saraf, M.N. Mast cell stabilizing and lipoxygenase inhibitory activity of Cedrus deodara (Roxb.) Loud. wood oil. Ind. J. Exp. Biol. 1999, 371, 258–261. [Google Scholar]
- El-Daly, S.A.; Al-Hazmy, A.S.M.; Ebeid, E.M.; Bhasikuttan, A.C.; Palit, D.K.; Sapre, A.A.V.; Mittal, J.P. Spectral, Acid−Base, and Laser Characteristics of 1,4-Bis[β-(2-quinolyl)vinyl]benzene (BQVB). J. Phys. Chem. 1996, 100, 9732–9737. [Google Scholar] [CrossRef]
- Mnasri, A.; Mejri, A.; Al-Hazmy, S.M.; Arfaoui, Y.; Özdemir, I.; Gürbüz, N.; Hamdi, N. Silver– N -heterocyclic carbene complexes-catalyzed multicomponent reactions: Synthesis, spectroscopic characterization, density functional theory calculations, and antibacterial study. Arch. Pharm. 2021, 354, e2100111. [Google Scholar] [CrossRef]
- Millam, J.M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J.W.; Martin, R.L.; Morokuma, K.; Farkas, O.; Foresman, J.B.; Gaussian, Inc. GaussView 5.0, Wallingford, CT, USA. 2016. Available online: https://gaussian.com/citation/ (accessed on 11 March 2022).
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Zouaghi, M.O.; Doggui, M.Y.; Arfaoui, Y. Regio- and stereoselectivity of the [3 + 2] cycloaddition of nitrones with methyl-acetophenone: A DFT investigation. J. Mol. Graph. Model. 2021, 107, 107960. [Google Scholar] [CrossRef] [PubMed]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2 1993, 799–805. [Google Scholar] [CrossRef]
- Andzelm, J.; Kölmel, C.; Klamt, A. Incorporation of solvent effects into density functional calculations of molecular energies and geometries. J. Chem. Phys. 1995, 103, 9312–9320. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Zouaghi, M.O.; Arfaoui, Y.; Champagne, B. Density functional theory investigation of the electronic and optical properties of metallo-phthalocyanine derivatives. Opt. Mater. 2021, 120, 111315. [Google Scholar] [CrossRef]
- Zutterman, F.; Liégeois, V.; Champagne, B. Simulation of the UV/Visible Absorption Spectra of Fluorescent Protein Chromophore Models. ChemPhotoChem 2017, 1, 281–296. [Google Scholar] [CrossRef]
- Tao, A.; Huang, Y.; Shinohara, Y.; Caylor, M.L.; Pashikanti, S.; Xu, D. ezCADD: A Rapid 2D/3 D Visualization-Enabled Web Modeling Environment for Democratizing Computer-Aided Drug Design. J. Chem. Inf. Model. 2018, 59, 18–24. [Google Scholar] [CrossRef]
- Schmidtke, P.; Le Guilloux, V.; Maupetit, J.; Tuffery, P. fpocket: Online tools for protein ensemble pocket detection and tracking. Nucleic Acids Res. 2010, 38, W582–W589. [Google Scholar] [CrossRef]
- Koes, D.R.; Baumgartner, M.P.; Camacho C., J. Lessons Learned in Empirical Scoring with smina from the CSAR 2011 Benchmarking Exercise. J. Chem. Inf. Model. 2013, 53, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Alashrah, S.; El-Ghoul, Y.; Almutairi, F.M.; Omer, M.A.A. Development, Characterization and Valuable Use of Novel Dosimeter Film Based on PVA Polymer Doped Nitro Blue Tetrazolium Dye and AgNO3 for the Accurate Detection of Low X-ray Doses. Polymers 2021, 13, 3140. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, N.; Ben Hsouna, A.; Bruneau, C.; Medyouni, R.; Al-Ayed, A.S.; Soleiman, H.A.; Zaghrouba, F. Synthesis of Novel Antibacterial Metal Free And Metallophthalocyanines Appending With Four Peripheral Coumarin Derivatives And Their Separation Of Structural Isomers. Heterocycles 2013, 87, 2283. [Google Scholar] [CrossRef]
- Jelali, H.; Chakchouk-Mtibaa, A.; Baklouti, L.; Bilel, H.; Bathich, Y.; Mellouli, L.; Hamdi, N. Development of New Multicomponent Reactions in Eco-Friendly Media-Greener Reaction and Expeditious Synthesis of Novel Bioactive Benzylpyranocoumarins. J. Chem. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Dlala, N.A.; Bouazizi, Y.; Ghalla, H.; Hamdi, N. DFT Calculations and Molecular Docking Studies on a Chromene Derivative. J. Chem. 2021, 2021, 1–17. [Google Scholar] [CrossRef]
- Dholakia, V.N.; Parekh, M.G.; Trivedi, N.K. Improved and rapid synthesis of new coumarinyl chalcone derivatives and their antiviral activity. Aust. J. Chem. 1968, 22, 345–2347. [Google Scholar]
- Hamdi, M.; Sakellariou, R.; Speziale, V. Synthesis of N-substituted α-amino acids from 4-hydroxycoumarin. J. Heterocycl. Chem. 1992, 29, 1817. [Google Scholar] [CrossRef]
- Hamdi, M.; Granier, P.; Sakellariou, R.; Spéziale, V. Reaction of amines on 3-ureidomethylenecoumarins. A new route to N-(methylene-4-oxocoumarinyl)amines. J. Heterocycl. Chem. 1993, 30, 1155–1157. [Google Scholar] [CrossRef]
- Steinführer, T.; Hantschmann, A.; Pietsch, M.; Weißenfels, M. Heterocyclisch [c]-anellierte cumarine aus 4-azido-3-cumarincarbaldehyden. Liebigs Ann. Chem. 1992, 1992, 23–28. [Google Scholar] [CrossRef]
- Hasanein, A.A.; Senior, S.A. DFT calculations of amine-imine tautomerism in some pyrimidine derivatives and their 1:1 and 1:2 complexes with water. Int. J. Quantum Chem. 2011, 111, 3993–4010. [Google Scholar] [CrossRef]
- Makhloufi-Chebli, M.; Hamdi, S.M.; Rabahi, A.; Silva, A.M.; Hamdi, M. Estimation of ground- and excited-state dipole moments of 3-acetoacetyl-coumarin derivatives from a solvatochromic shift method based on the solvent polarity parameter. J. Mol. Liq. 2013, 181, 89–96. [Google Scholar] [CrossRef]
- A El-Daly, S.; Abdel-Kader, M.H.; Issa, R.M.; A El-Sherbini, E.-S. Influence of solvent polarity and medium acidity on the UV–Vis spectral behavior of 1-methyl-4-[4-amino-styryl] pyridinum iodide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc 2002, 59, 405–411. [Google Scholar] [CrossRef]
- Slimani, I.; Şahin-Bölükbaşı, S.; Ulu, M.; Evren, E.; Gürbüz, N.; Özdemir, I.; Hamdi, N.; Özdemir, I. Rhodium(i) N-heterocyclic carbene complexes: Synthesis and cytotoxic properties. New J. Chem. 2021, 45, 5176–5183. [Google Scholar] [CrossRef]
- Patil, N.R.; Melavanki, R.M.; Muttannavar, V.T.; Vaijayanthimala, S.; Naik, L.R.; Kadadevarmath, J.S. Solvent effects on the dipole moments and photo physical properties of laser dye. Indian J. Pure Appl. Phys. 2018, 56, 749–754. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zhang, Y.; Liu, L. Fluorescence lifetimes and quantum yields of ten rhodamine derivatives: Structural effect on emission mechanism in different solvents. J. Lumin. 2013, 145, 448–453. [Google Scholar] [CrossRef]
- Azim, S.; Al-Hazmy, S.; Ebeid, E.; El-Daly, S. A new coumarin laser dye 3-(benzothiazol-2-yl)-7-hydroxycoumarin. Opt. Laser Technol. 2005, 37, 245–249. [Google Scholar] [CrossRef]
- Al-Ragehey, A.S.J.M. Effect Of Solvent Polarity On The Quantum Yield Of (C17H19N3). J. Multidiscip. Eng. Sci. Technol. 2016, 2, 561–564. [Google Scholar]
- Hoche, J.; Schulz, A.; Dietrich, L.M.; Humeniuk, A.; Stolte, M.; Schmidt, D.; Brixner, T.; Würthner, F.; Mitric, R. The origin of the solvent dependence of fluorescence quantum yields in dipolar merocyanine dyes. Chem. Sci. 2019, 10, 11013–11022. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, E.; Lima, J.C.; Parola, A.J.; Branco, P.S. Incorporation of Coumarin-Based Fluorescent Monomers into Co-Oligomeric Molecules. Polymers 2018, 10, 396. [Google Scholar] [CrossRef] [PubMed]
- Loutfy, R.O.; Arnold, B.A. Effect of viscosity and temperature on torsional relaxation of molecular rotors. J. Phys. Chem. 1982, 86, 4205–4211. [Google Scholar] [CrossRef]
- Law, K. Fluorescence probe for microenvironments: Anomalous viscosity dependence of the fluorescence quantum yield of p-N,N-dialkylaminobenzylidenemalononitrile in 1-alkanols. Chem. Phys. Lett. 1980, 75, 545–549. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mardinia, F.; Azimi, N.; Qomi, M.; Balali, E. Prediction of chalcone derivative cytotoxicity activity against MCF-7 human breast cancer cell by Monte Carlo method. J. Mol. Struct. 2018, 1181, 305–311. [Google Scholar] [CrossRef]
- Gangadevi, V.; Muthumary, J. Preliminary studies on cytotoxic effect of fungal taxol on cancer cell lines. Afr. J. Biotechnol. 2007, 6, 1382–1386. [Google Scholar]
- Thusyanthan, J.; Wickramaratne, N.S.; Senathilake, K.S.; Rajagopalan, U.; Tennekoon, K.H.; Thabrew, I.; Samarakoon, S.R. Cytotoxicity against Human Hepatocellular Carcinoma (HepG2) Cells and Anti-Oxidant Activity of Selected Endemic or Medicinal Plants in Sri Lanka. Adv. Pharmacol. Pharm. Sci 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Żamojć, K.; Wiczk, W.; Zaborowski, B.; Jacewicz, D.; Chmurzyński, L. Analysis of Fluorescence Quenching of Coumarin Derivatives by 4-Hydroxy-TEMPO in Aqueous Solution. J. Fluoresc. 2013, 24, 713–718. [Google Scholar] [CrossRef] [Green Version]


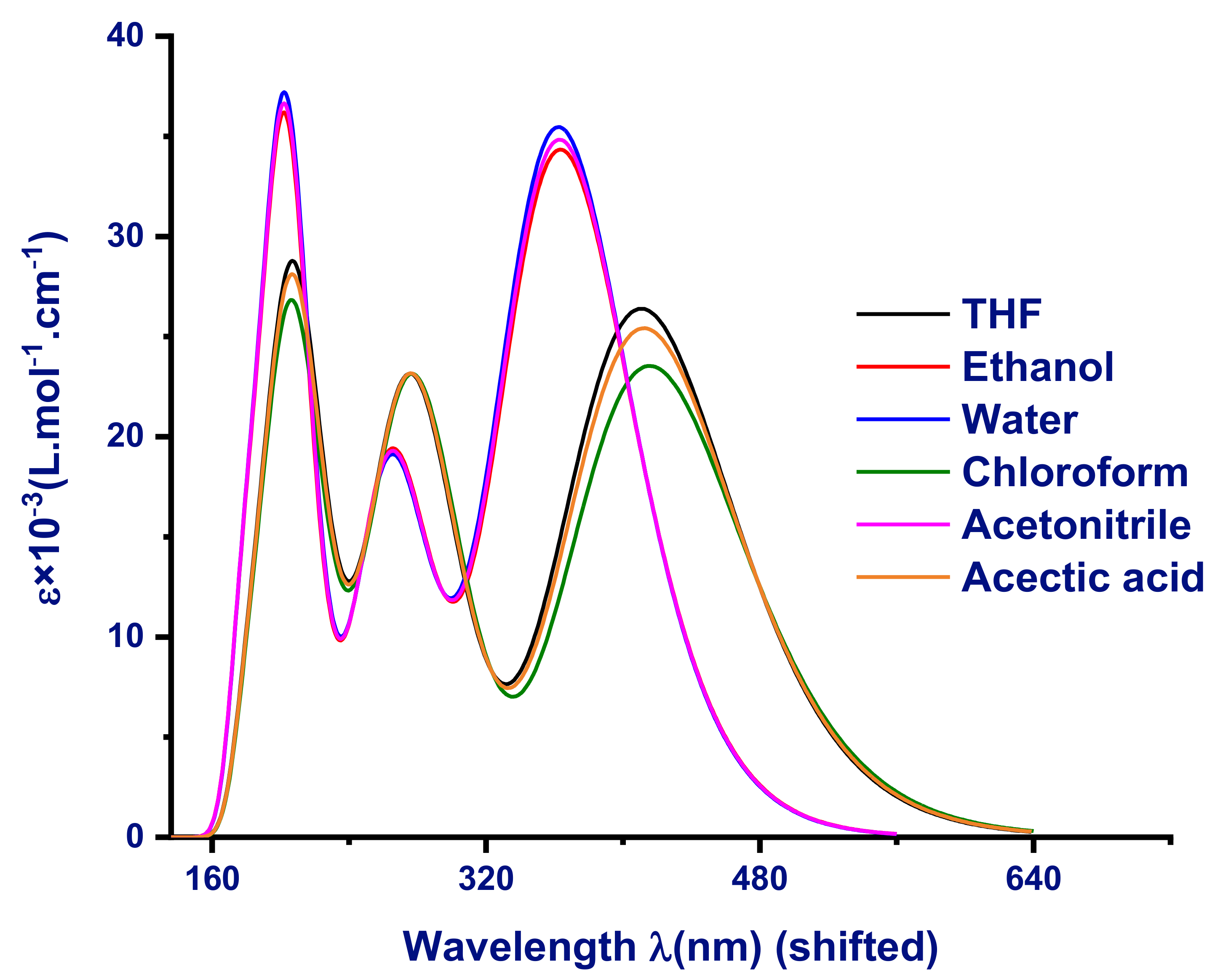
 CCl4,
CCl4,  n-heptane,
n-heptane,  ethanol,
ethanol,  CHCl3,
CHCl3,  THF,
THF,  formamide.
formamide.
 CCl4,
CCl4,  n-heptane,
n-heptane,  ethanol,
ethanol,  CHCl3,
CHCl3,  THF,
THF,  formamide.
formamide.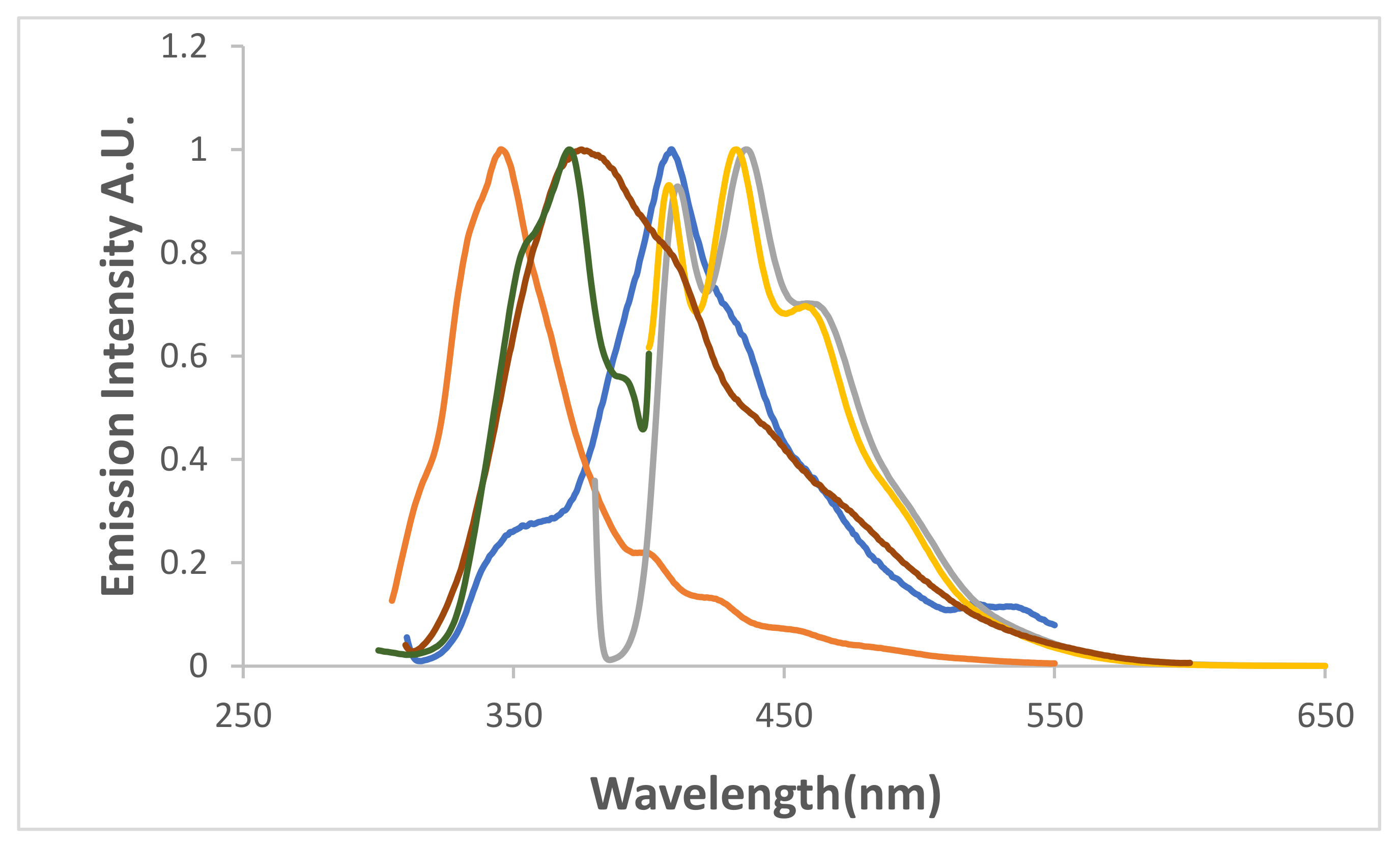
 THF,
THF,  CH3CN, and
CH3CN, and  CHCl3.
CHCl3.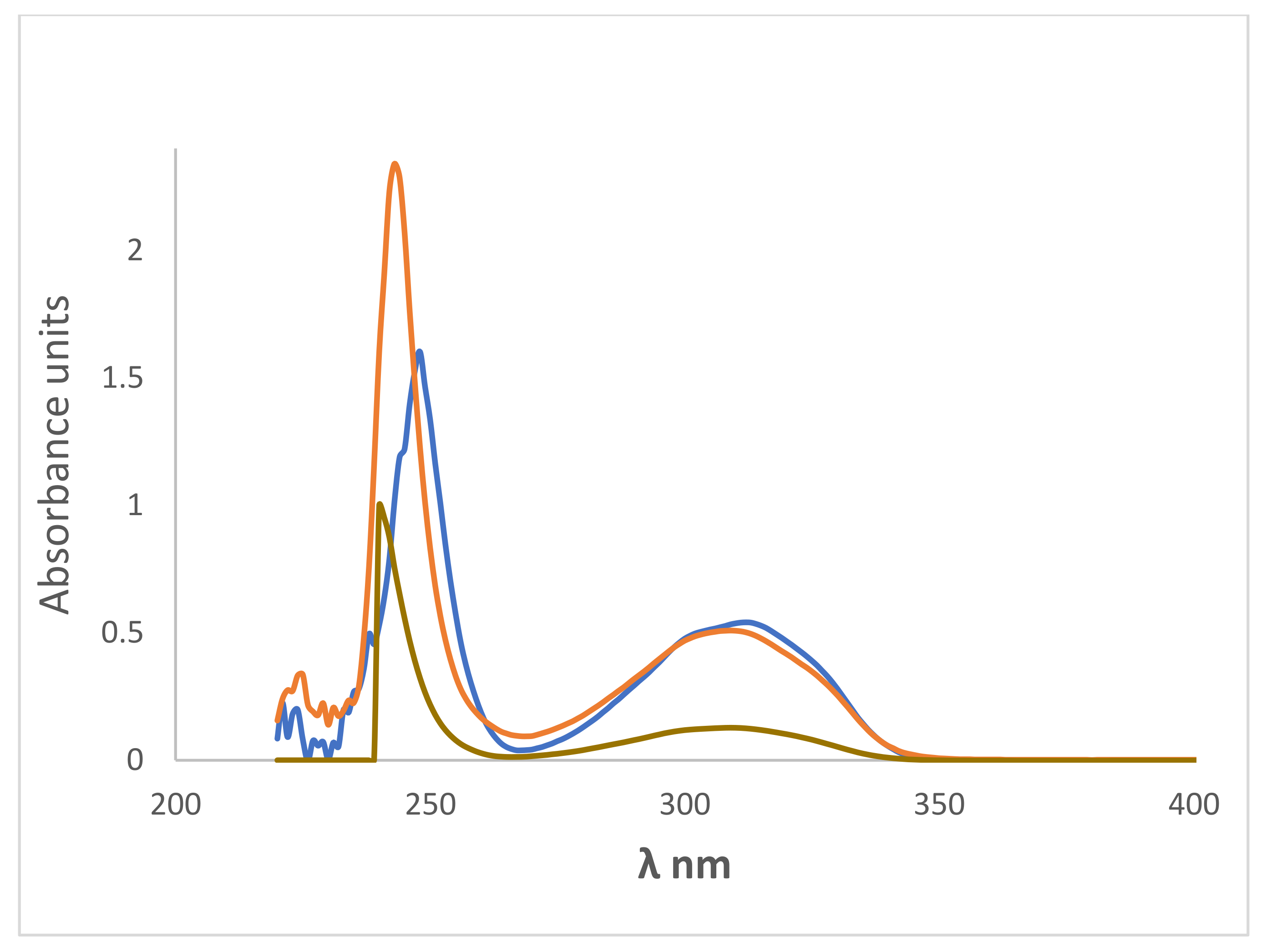
 CH3CN and
CH3CN and  CCl4 solvents.
CCl4 solvents.
 CH3CN and
CH3CN and  CCl4 solvents.
CCl4 solvents.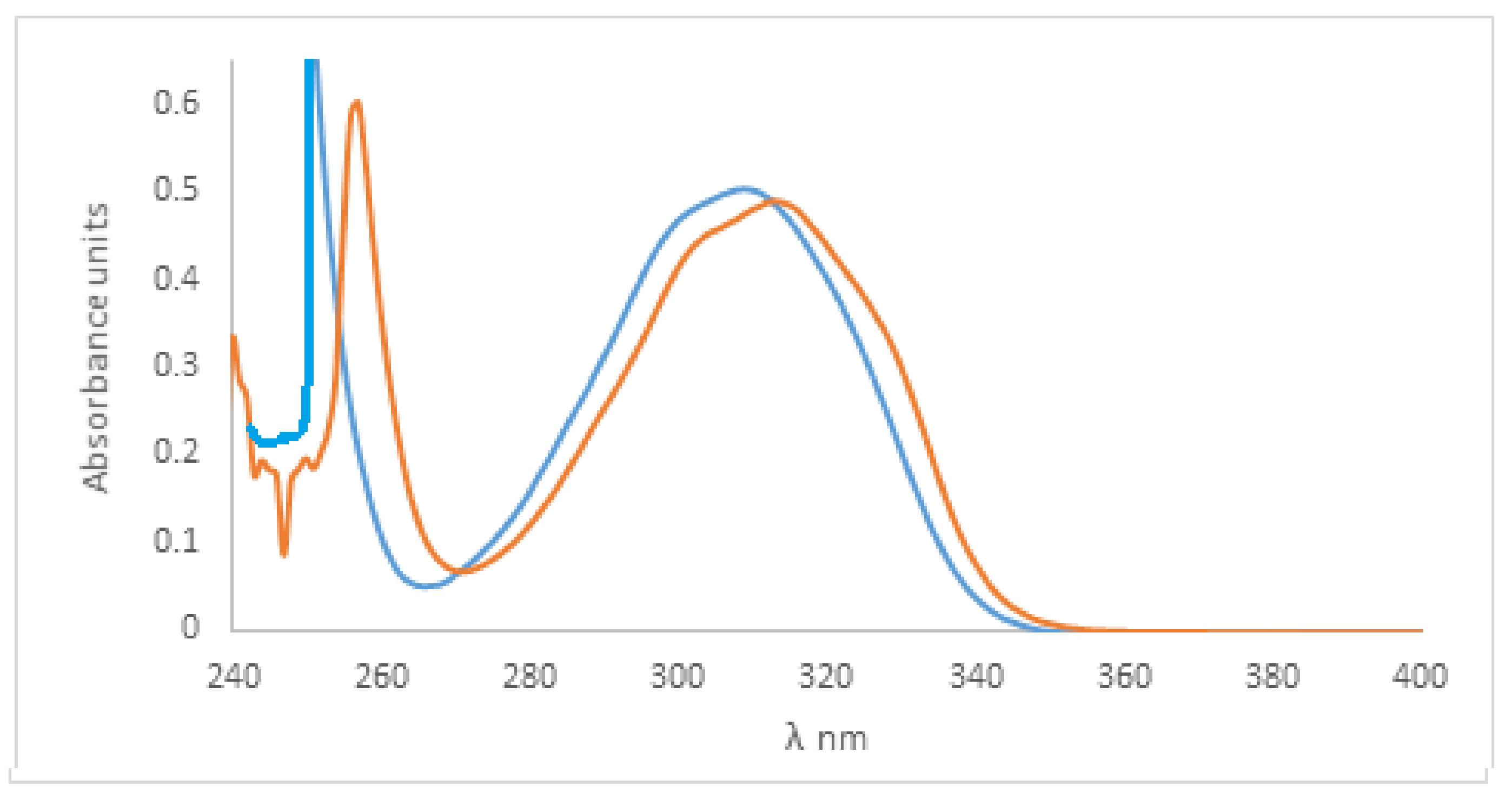
 CHCl3,
CHCl3,  ethanol and
ethanol and  6.8 × 10−5 M of 2-amino-4-hydroxy-6-(4-hydroxy-2-oxo-2H-chromen-3-yl)nicotinonitrile 4-doped PVA thin film, thickness 100 μm (λab max at 243, 307 nm, λab max at 238, 307 nm and λab max at 238, 307 nm, respectively).
6.8 × 10−5 M of 2-amino-4-hydroxy-6-(4-hydroxy-2-oxo-2H-chromen-3-yl)nicotinonitrile 4-doped PVA thin film, thickness 100 μm (λab max at 243, 307 nm, λab max at 238, 307 nm and λab max at 238, 307 nm, respectively).
 CHCl3,
CHCl3,  ethanol and
ethanol and  6.8 × 10−5 M of 2-amino-4-hydroxy-6-(4-hydroxy-2-oxo-2H-chromen-3-yl)nicotinonitrile 4-doped PVA thin film, thickness 100 μm (λab max at 243, 307 nm, λab max at 238, 307 nm and λab max at 238, 307 nm, respectively).
6.8 × 10−5 M of 2-amino-4-hydroxy-6-(4-hydroxy-2-oxo-2H-chromen-3-yl)nicotinonitrile 4-doped PVA thin film, thickness 100 μm (λab max at 243, 307 nm, λab max at 238, 307 nm and λab max at 238, 307 nm, respectively).
 toluene,
toluene,  CHCl3,
CHCl3,  ethanol,
ethanol,  pentanol and
pentanol and  formamide, λex = 376 nm.
formamide, λex = 376 nm.
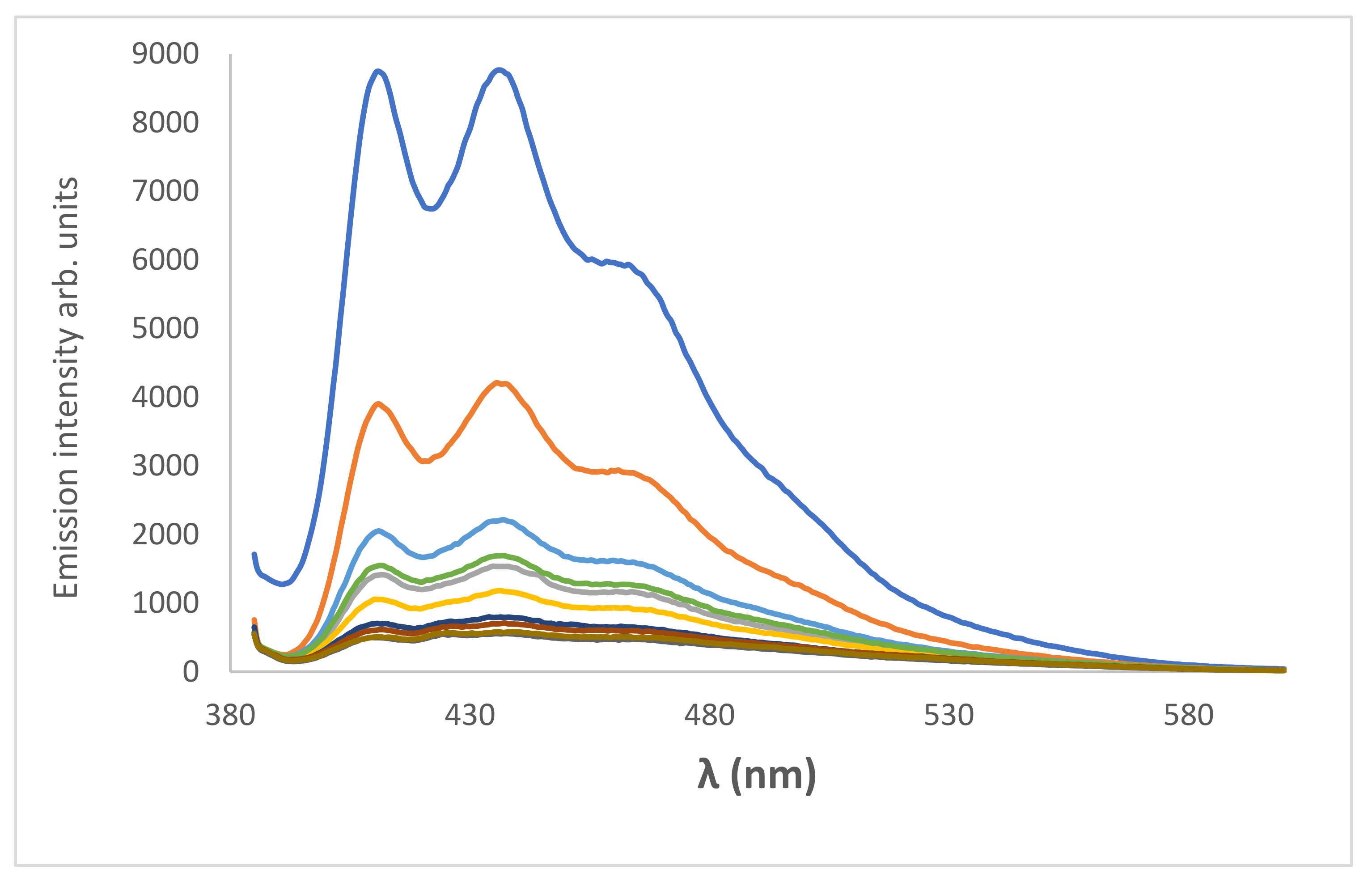
 toluene,
toluene,  CHCl3,
CHCl3,  ethanol,
ethanol,  pentanol and
pentanol and  formamide, λex = 376 nm.
formamide, λex = 376 nm.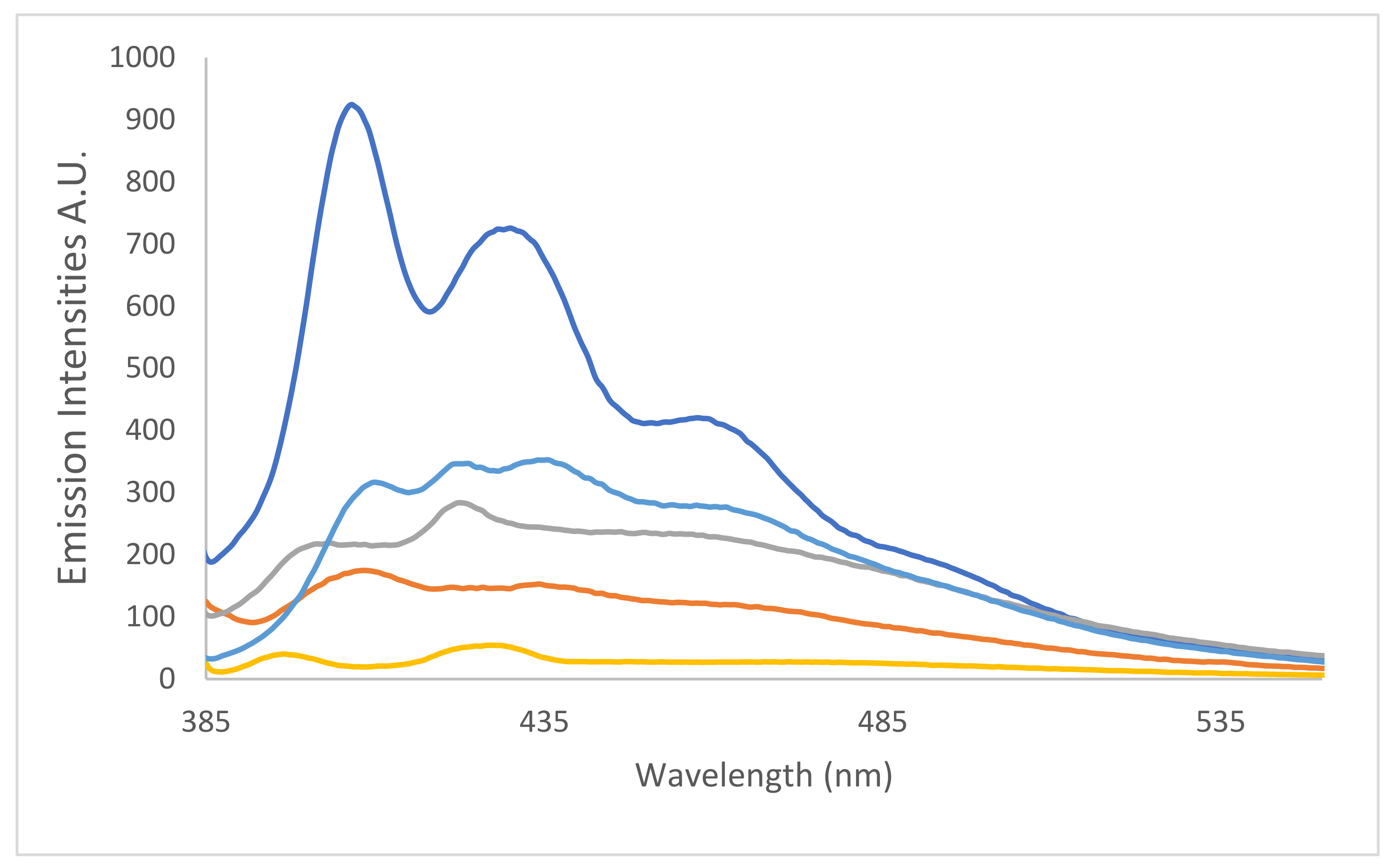
 fresh and
fresh and  irradiated for 71 min, λex = 376 nm.
irradiated for 71 min, λex = 376 nm.
 fresh and
fresh and  irradiated for 71 min, λex = 376 nm.
irradiated for 71 min, λex = 376 nm.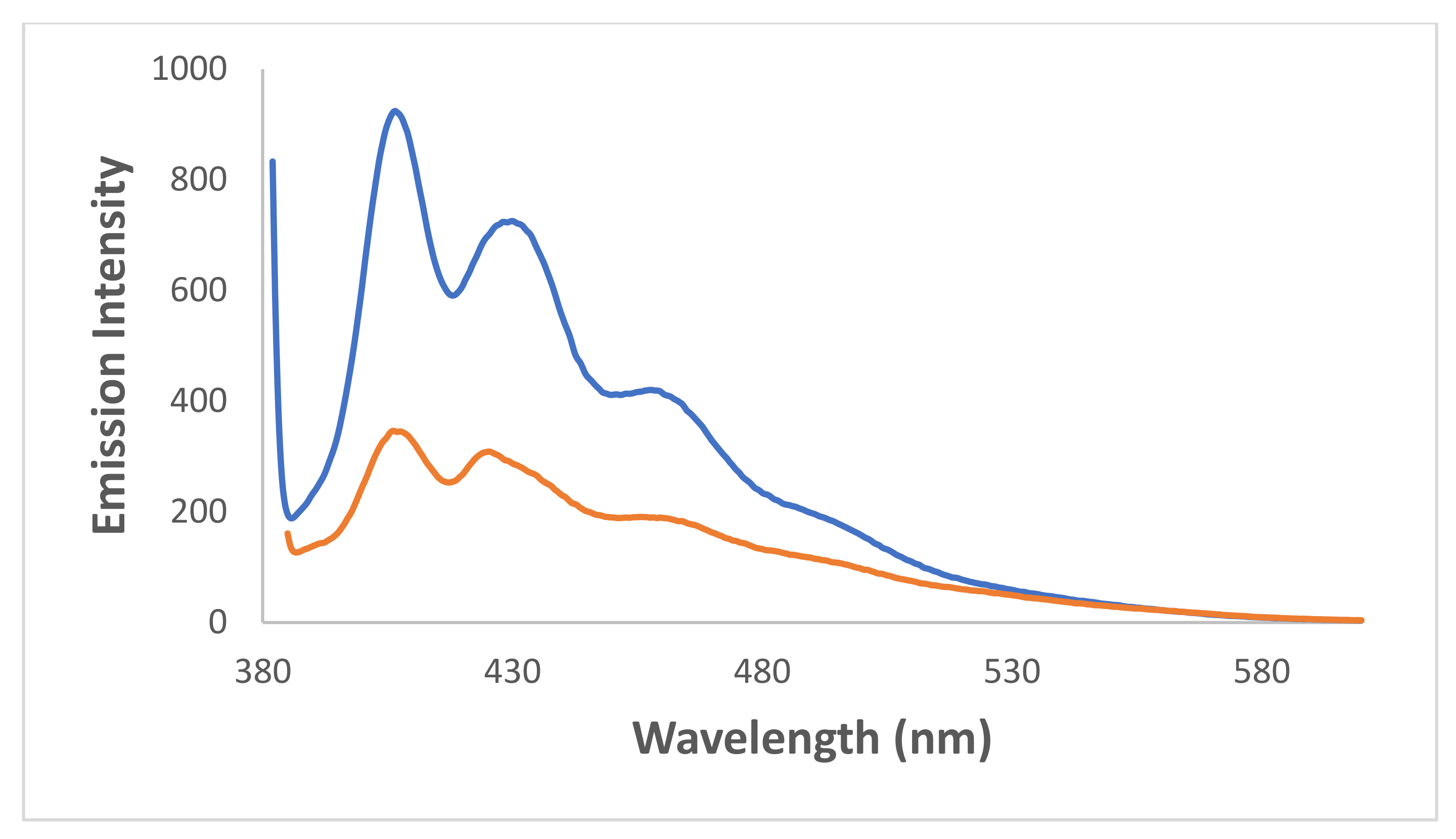
 fresh,
fresh,  alkalized using NaOH, and
alkalized using NaOH, and  acidified using H2SO4.
acidified using H2SO4.
 fresh,
fresh,  alkalized using NaOH, and
alkalized using NaOH, and  acidified using H2SO4.
acidified using H2SO4.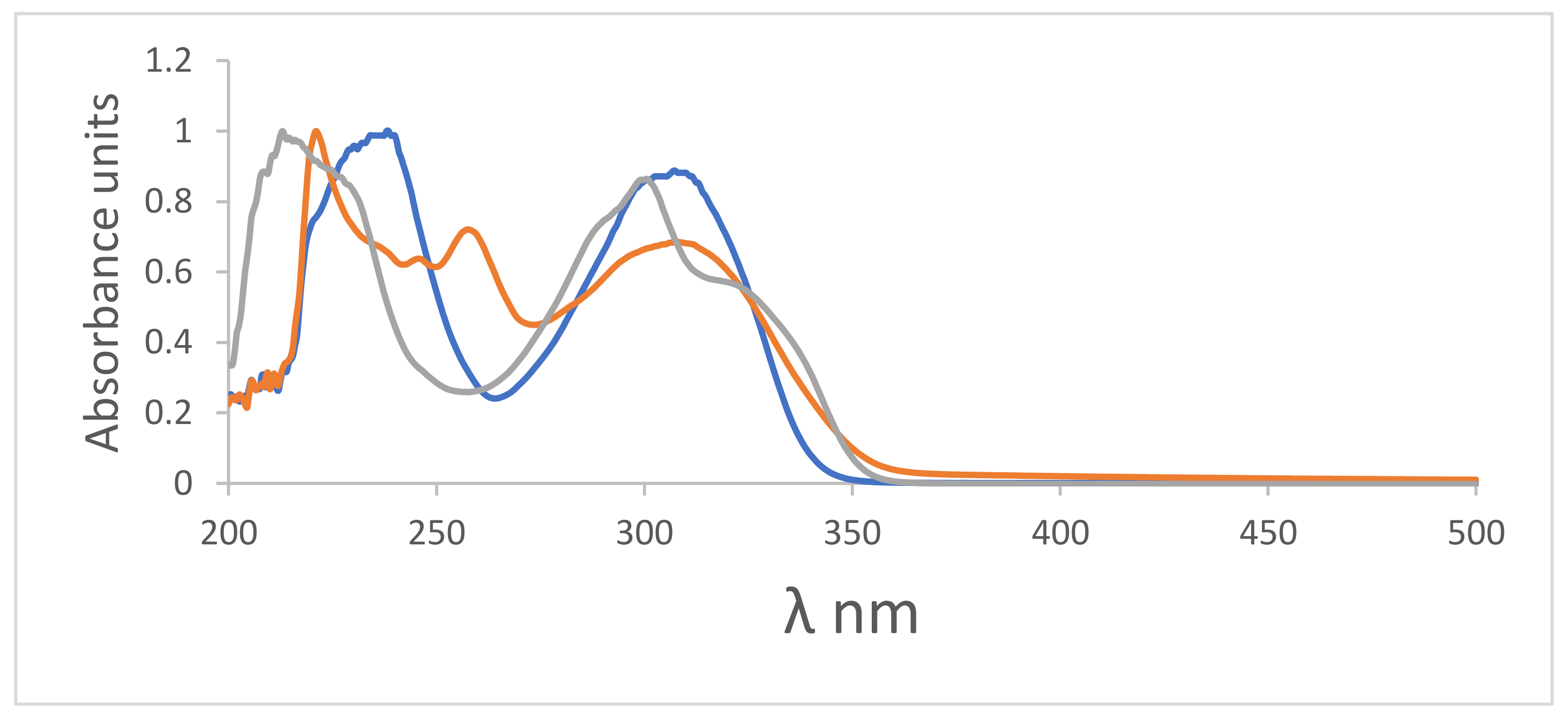
 fresh λem, max 406 nm, alkalized usingNaOH
fresh λem, max 406 nm, alkalized usingNaOH  λem, max 404 nm, and acidified using H2SO4
λem, max 404 nm, and acidified using H2SO4  λem, max 387 nm.
λem, max 387 nm.
 fresh λem, max 406 nm, alkalized usingNaOH
fresh λem, max 406 nm, alkalized usingNaOH  λem, max 404 nm, and acidified using H2SO4
λem, max 404 nm, and acidified using H2SO4  λem, max 387 nm.
λem, max 387 nm.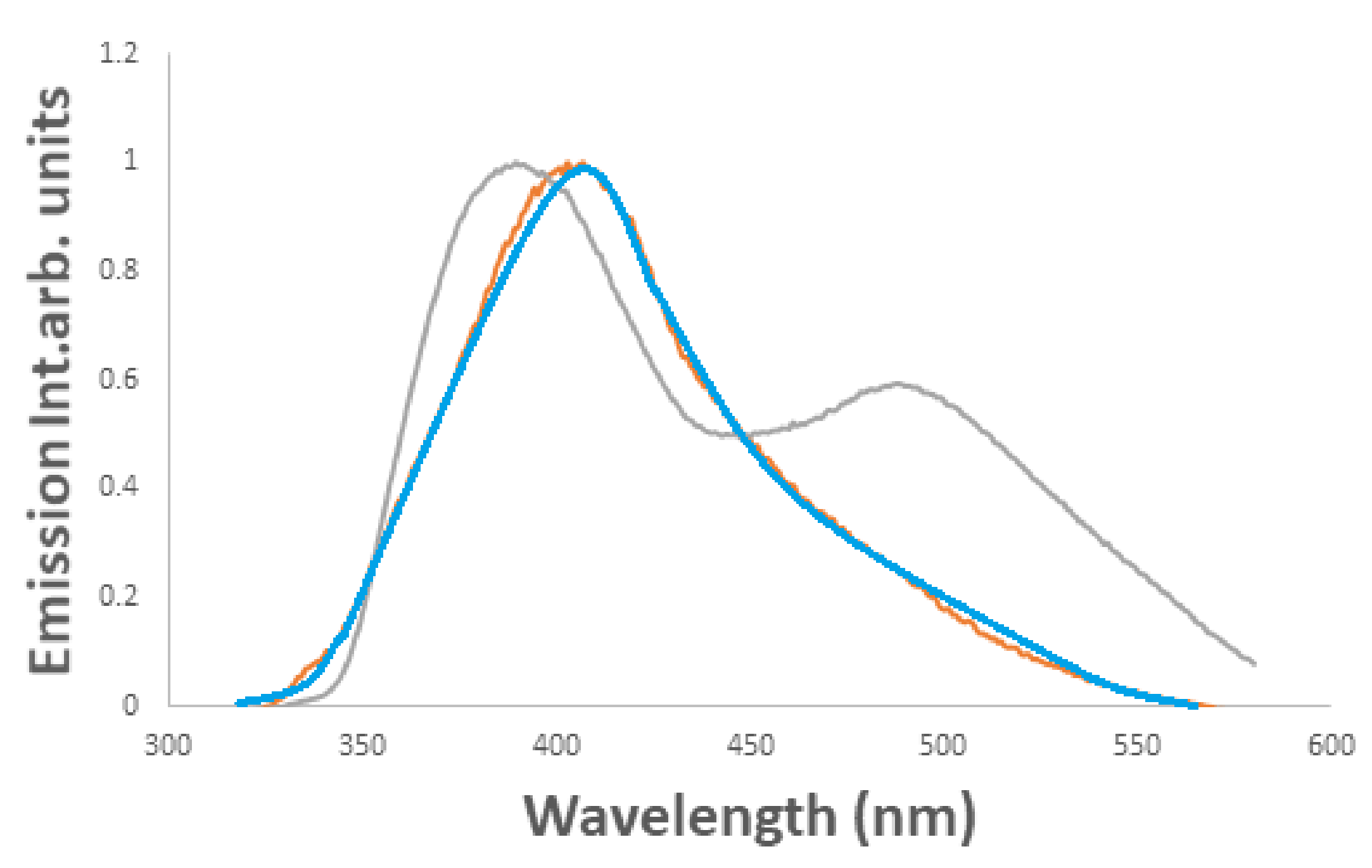

| Reaction Medium | ||
| Gas phase | 36.00 | −6.86 |
| Explicit model | 14.18 | −1.65 |
| Implicit model | 35.87 | −7.72 |
 | ||
|---|---|---|
| Site | ||
| C8 | −20.71 | |
| O11 | −8.38 | |
| O12 | −31.38 | |
| C13 | 8.60 | |
| H18 | 10.04 | |
| H20 | 5.44 | |
| H21 | 14.12 | |
| H22 | 2.61 | |
| O23 | −23.33 | |
 | ||
|---|---|---|
| Site | ||
| C8 | 17.18 | |
| O10 | 16.47 | |
| O12 | −28.29 | |
| N14 | 15.49 | |
| C15 | 4.72 | |
| O19 | −42.57 | |
| C22 | 1.60 | |
| O26 | −11.28 | |
| C27 | 2.55 | |
| N28 | −37.98 | |
| H33 | 33.29 | |
| H34 | 11.43 | |
| H40 | 47.24 | |
| Solvent | (%) | ε() | |||
|---|---|---|---|---|---|
| THF | 309 | 3.21 | H→L (58%) | 26,736 | 0.65 |
| 3.24 | H-1→L (57%) | ||||
| 249 | 3.80 | H-2→L (94%) | - | - | |
| 4.04 | H-1→L + 1 (66%) | ||||
| 4.10 | H-4→L (62%) | ||||
| 202 | 4.60 | H-2→L + 1 (88%) | - | - | |
| 4.71 | H-3→L + 1 (84%) | ||||
| 4.89 | H→L + 2 (41%) | ||||
| 181 | 5.40 | H-8→L (41%) | - | - | |
| 5.71 | H-4→L + 2 (24%) | ||||
| Ethanol | 306 | 3.21 | H-1→L (82%) | 27,407 | 0.67 |
| 3.24 | H→L (82%) | ||||
| 249 | 3.89 | H-3→L (86%) | - | - | |
| 4.05 | H-1→L + 1 (84%) | ||||
| 4.10 | H-4→L (64%) | ||||
| 201 | 4.58 | H-2→L + 1 (89%) | - | - | |
| 4.91 | H-1→L + 2 (33%) | ||||
| H→L + 2 (28%) | |||||
| 5.07 | H-6→L (39%) | ||||
| 181 | 5.41 | H→L (82%) | - | - | |
| 5.69 | H→L (82%) | ||||
| Water | 306 | 3.21 | H-1→L (88%) | 27,437 | 0.67 |
| 3.25 | H→L (88%) | ||||
| 249 | 3.89 | H-3→L (88%) | - | - | |
| 4.05 | H-1→L (84%) | ||||
| 4.10 | H-4→L (63%) | ||||
| 201 | 4.58 | H-2→L + 1 (89%) | - | - | |
| 4.73 | H-2→L + 2 (49%) | ||||
| 4.91 | H-3→L + 1 (85%) | ||||
| 181 | 5.42 | H-8→L (52%) | - | - | |
| 5.69 | H-4→L + 2 (31%) | ||||
| Chloroform | 310 | 3.19 | H→L (83%) | 0.60 | |
| 249 | 3.80 | H-2→L (91%) | - | - | |
| 3.92 | H-3→L (57%) | - | - | ||
| 4.04 | H-1→L + 1 (88%) | - | - | ||
| 4.16 | H-5→L (59%) | - | - | ||
| 204 | 4.62 | H-2→L + 1 (86%) | - | - | |
| 4.71 | H-3→L + 1 (86%) | - | - | ||
| 4.88 | H→L + 2 (37%) | - | - | ||
| H-7→L (26%) | |||||
| 5.38 | H-6→L + 1 (28%) | - | - | ||
| H-7→L + 1 (24%) | |||||
| Acetonitrile | 306 | 3.21 | H-1→L (86%) | 26,188 | 0.67 |
| 3.25 | H→L (86%) | ||||
| 349 | 3.89 | H-3→L (87%) | - | - | |
| 4.05 | H-1→L + 1 (84%) | ||||
| 4.10 | H-4→L (64%) | ||||
| 201 | 4.58 | H-2→L + 1 (89%) | - | - | |
| 4.73 | H-3→L + 1 (85%) | ||||
| 4.91 | H-1→L + 2 (40%) | ||||
| 5.07 | H-6→L (40%) | ||||
| 181 | 5.42 | H-8→L (51%) | - | - | |
| 5.70 | H-4→L + 2 (29%) | ||||
| Acetic acid | 309 | 3.21 | H→L (64%) | 26,137 | 0.54 |
| 249 | 3.80 | H-2→L (93%) | - | - | |
| 203 | 4.61 | H-2→L + 1 (87%) | - | - | |
| 4.89 | H→L + 2 (41%) | ||||
| 181 | 5.39 | H-8→L (38%) | - | - | |
| 5.72 | H-4→L + 2 (26%) |
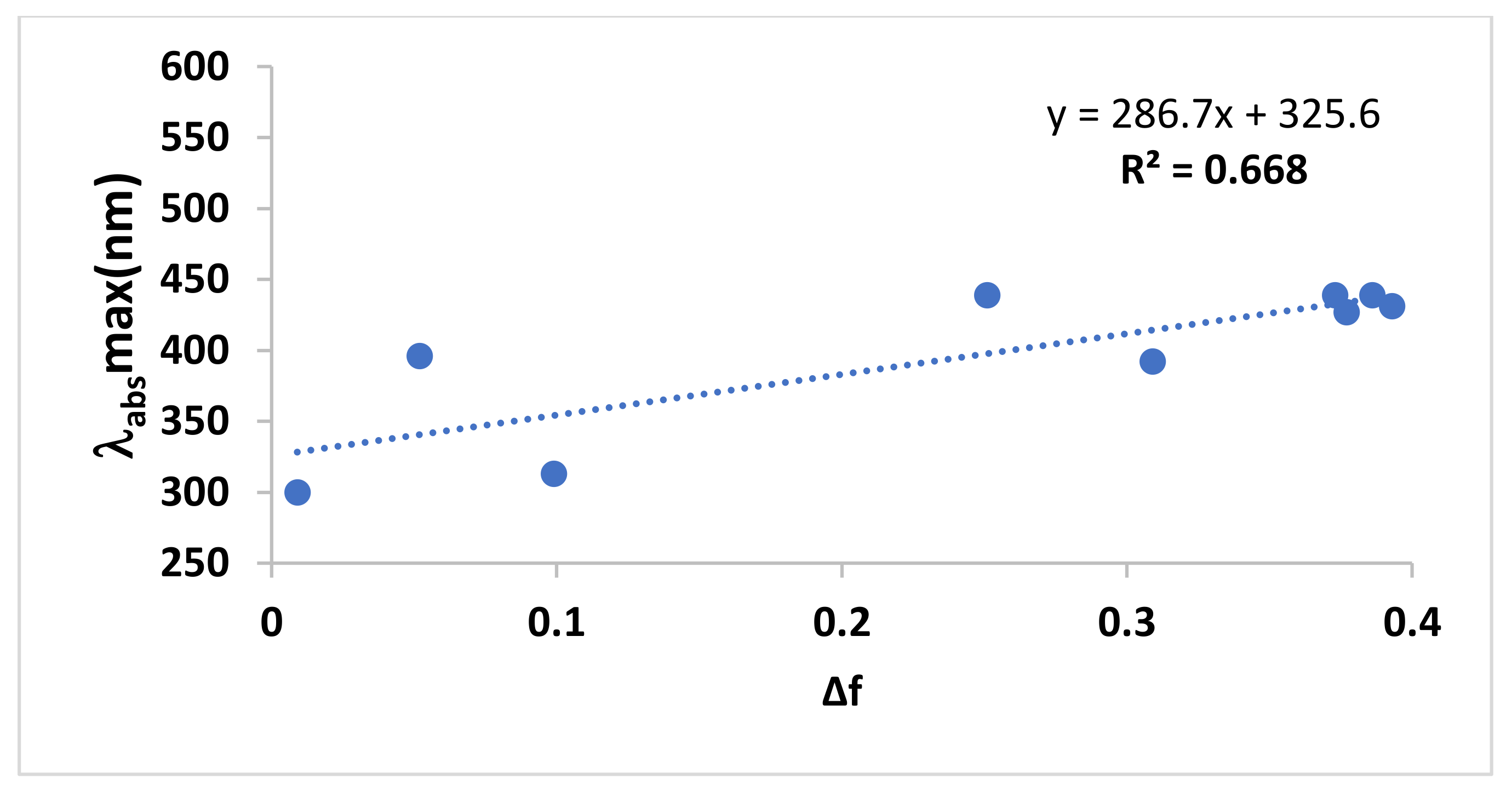
| Solvent | ||
|---|---|---|
| THF | 350 | 3.54 |
| Ethanol | 328 | 3.78 |
| Water | 326 | 3.81 |
| Chloroform | 354 | 3.50 |
| Acetonitrile | 326 | 3.81 |
| Aceticacid | 350 | 3.54 |
| Solvent | ||
|---|---|---|
| THF | 320 | 0.34 |
| Ethanol | 0.37 | |
| Water | 0.37 | |
| Chloroform | 0.33 | |
| Acetonitrile | 0.37 | |
| Acetic acid | 0.33 |
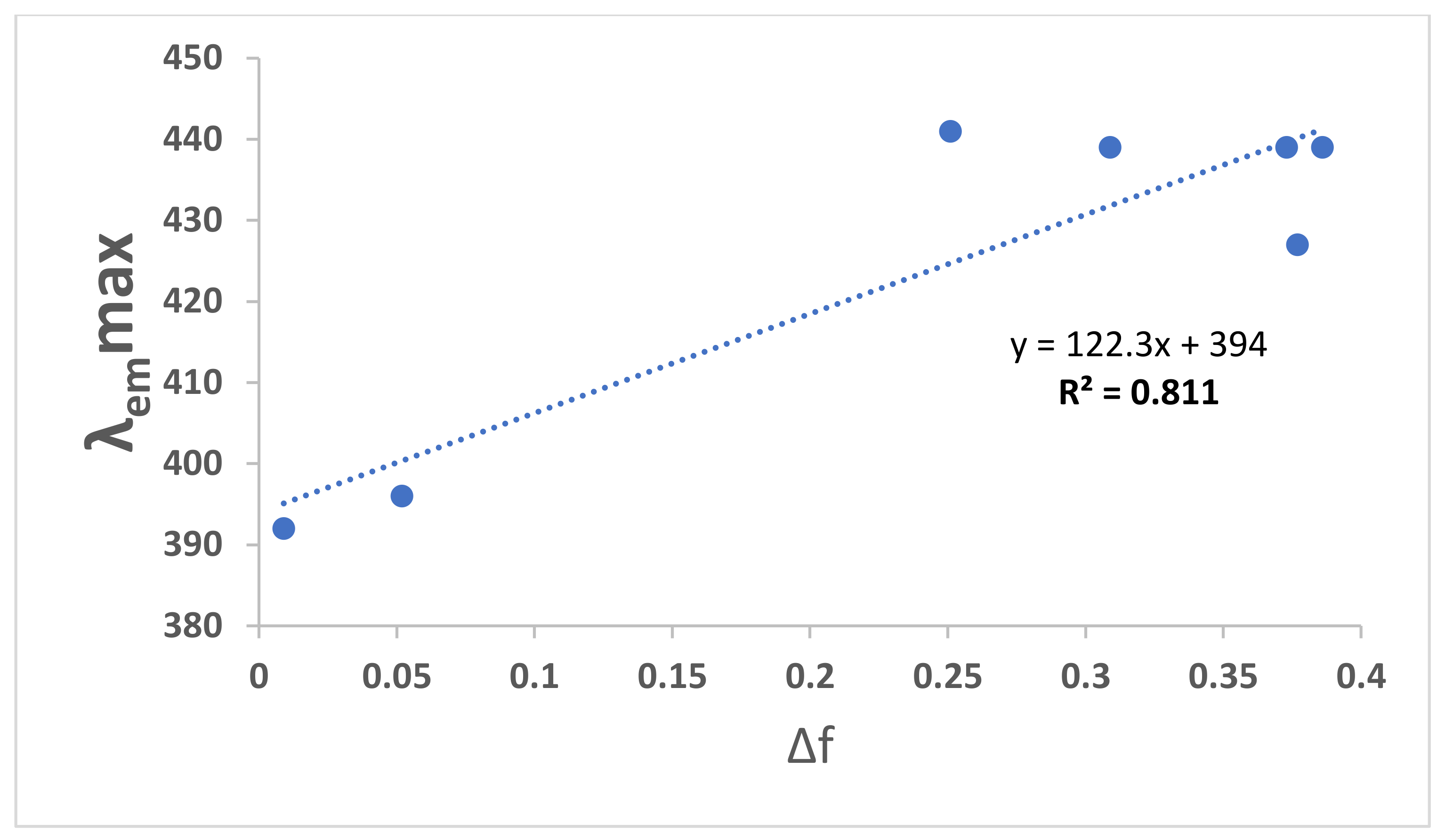
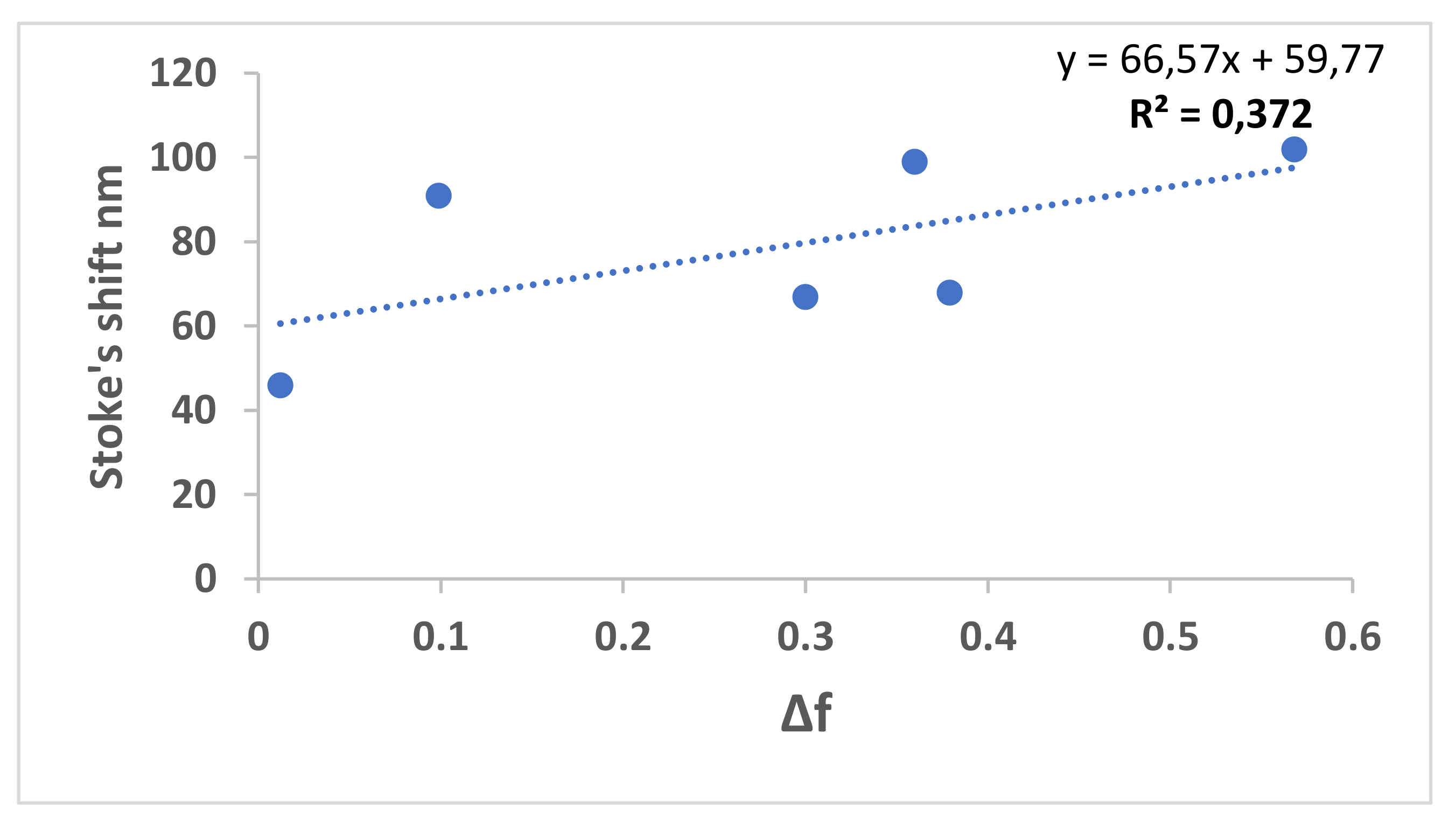
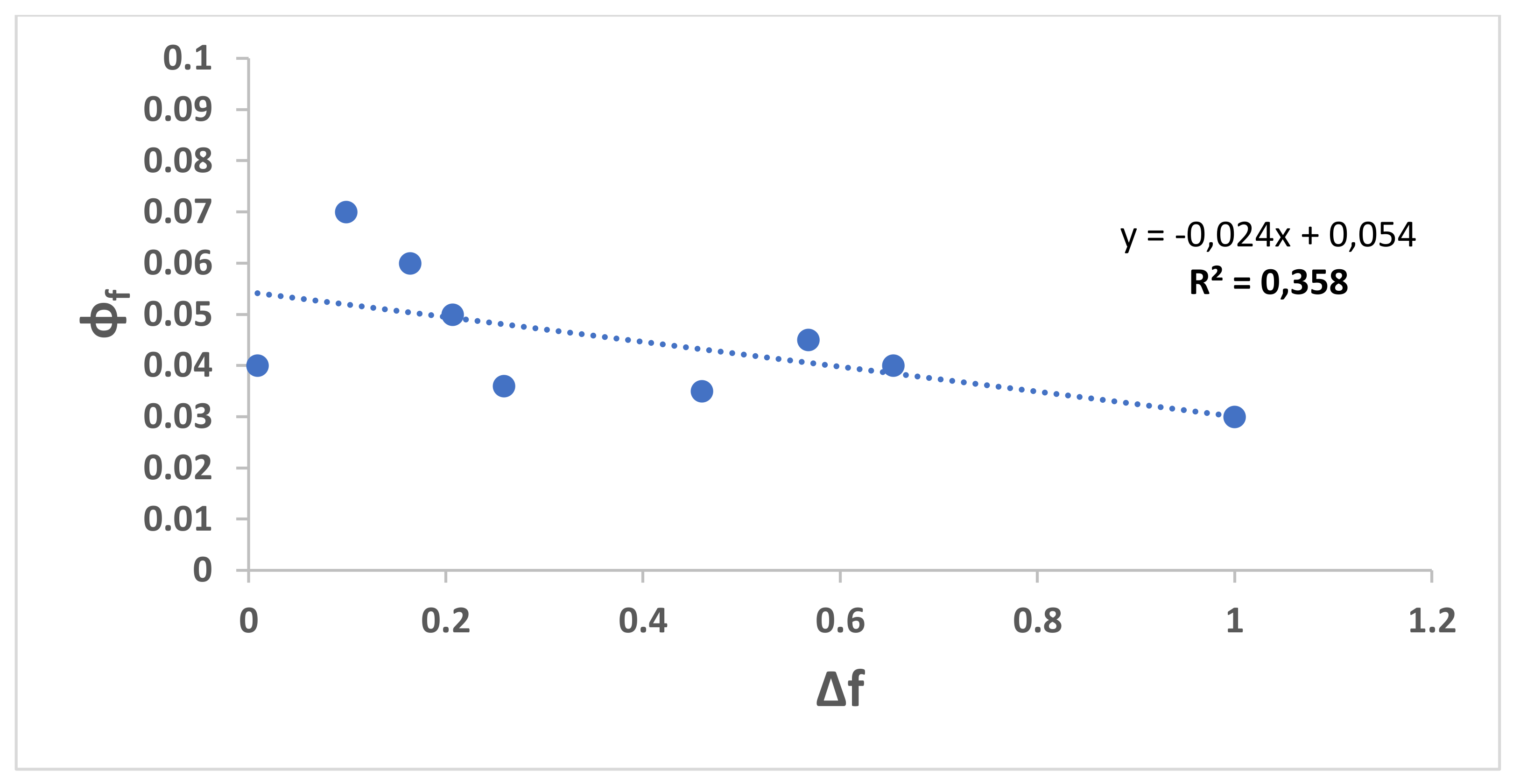
| Solvents | λabs, max (nm) | λem, max (nm) | λex, max (nm) | Δf | ɸc | ɸf | Stock’s Shift |
|---|---|---|---|---|---|---|---|
| N-heptane | 300 | 346 | 300 | 0.012 | 46 | ||
| CCl4 | 316 | 433 | 396 | 0.052 | 0.002 | 117 | |
| Toluene | 310 | 407 | 313 | 0.099 | 0.07 | 91 | |
| Hexane | 313 | 425 | 392 | 0.009 | 0.04 | ||
| 1,4 Dioxane | 314 | 407 | 312 | 0.164 | 0.06 | ||
| THF | 313 | 344.5 | 258 | 0.207 | 31 | ||
| CHCl3 | 309 | 436 | 376 | 0.259 | 127 | ||
| CH2Cl2 | 312 | 409 | 311 | 0.269 | |||
| EG | 309 | 376 | 350 307 | 0.30 | 67 | ||
| C4H9Cl | 311 | 431 | 310 | ||||
| DMF | 311 | 409 | 306 | 0.386 | |||
| CH3CN | 307 | 404 | 308 | 0.460 | |||
| EtOH | 307 | 372 | 306 | 0.654 | 0.003 | 68 | |
| Glycerol | 310 | 411 | 365 | 0.812 | |||
| Pentanol | 307 | 410 | 307 | 0.568 | 102 | ||
| H2O | 306 | 405 | 306 | 1 | 99 | ||
| Dye-doped PVA | 309 | 433 | 0.001 | ||||
| SDS in aqueous solution | 307 | 406 |
| Microorganisms Product | Micrococcusluteus LB14110 | Listeria monocytogenes ATCC 19117 | Salmonella Typhimurium ATCC 14028 | Staphylococcus Aureus ATCC 6538 | Pseudomonas aeruginosa | Candida albicans |
|---|---|---|---|---|---|---|
| 2 | 28 | 25 | 24 | 26 | 22 | 23 |
| 3 | 27 | 24 | 23 | 24 | 23 | 22 |
| 4 | 26 | 22 | 27 | 30 | 30 | 30 |
| AMC | 25 | 22 | 20 | 23 | 20 | 22 |
| Microorganism Indicator | Compound | MIC (mg/mL) |
|---|---|---|
| Listeria monocytogenes ATCC 19117 | 2 | 0.524 |
| 3 | 0.038 | |
| 4 | 0.413 | |
| SalmonellaTyphimurium ATCC 14028 | 2 | 1.26 |
| 3 | 1.25 | |
| 4 | 0.039 | |
| Micrococcus luteus LB14110 | 2 | 0.624 |
| 3 | 1.51 | |
| 4 | ||
| Ampicillin | 0.037 |
| EC50 in µg mL−1/ Compounds | DPPH | ABTS |
|---|---|---|
| 2 | 48.07 | 32.21 |
| 3 | 47.08 | 31.23 |
| 4 | 49.07 | 30.22 |
| BHT | 31.25 | 17.38 |
| Compounds | IC50 Values in µM | |
|---|---|---|
| Lipoxygenase Inhibition Assay | PLA2 Inhibition Assay | |
| 2 | 8.2 | 1105 |
| 3 | 7.3 | 1004 |
| 4 | 8.5 | 1110 |
| Indomethacin | 8.0 | - |
| Aristolochic acid | 8.1 | 25.0 |
| IC50 | Compounds | |
|---|---|---|
| HepG-2 | HCT-116 | |
| 11.75 | 7.76 | 2 |
| 17.45 | 13.56 | 3 |
| 20.65 | 15.36 | 4 |
| 6.05 | 3.83 | Vinblastine Standard |
| Protein | PDB ID | PDB DOI | Full Name |
|---|---|---|---|
| Lipoxygenase | 6N2W | 10.2210/pdb6 NRW/pdb | Crystal structure of Dpr1 IG1 bound to DIP-eta IG1 |
| 4NRE | 10.2210/pdb4NRE/pdb | The structure of human 15-lipoxygenase-2 with a substrate mimic | |
| PLA2 | 1TH6 | 10.2210/pdb1TH6/pdb | Crystal structure of phospholipase A2 in complex with atropine at 1.23 A resolution |
| 2QU9 | 10.2210/pdb2QU9/pdb | Crystal structure of the complex of group II phospholipase A2 with eugenol | |
| 4DBK | 10.2210/pdb4DBK/pdb | Crystal structure of porcine pancreatic phospholipase A2 complexed with berberine | |
| HepG-2 | 2W3L | 10.2210/pdb2W3L/pdb | Crystal structure of Chimeric Bcl2-xL and Phenyl Tetrahydroisoquinoline Amide Complex |
| HCT-116 | 1YWN | 10.2210/pdb1YWN/pdb | Vegfr2 in complex with a novel 4-amino-furo[2,3-d]pyrimidine |
| Anti-Inflammatory Activity | ||||
|---|---|---|---|---|
| Protein | Resolution | n | Volume | Eb |
| 6N2W (Lipoxygenase) | 2.40 | 34 | 1155 | −10.3 |
| 4NRE (Lipoxygenase) | 2.63 | 33 | 2171 | −8.3 |
| 1TH6 (PLA2) | 1.23 | 6 | 1247 | −9.0 |
| 2QU9 (PLA2) | 2.08 | 5 | 670 | −8.9 |
| 4DBK (PLA2) | 2.30 | 9 | 650 | −8.8 |
| Antiproliferative activity | ||||
| 2W3L (HepG-2) | 2.10 | 7 | 534 | −8.8 |
| 1YWN (HCT-116) | 1.71 | 18 | 1096 | −8.7 |
| Protein | (a) | (b) |
|---|---|---|
| 6N2W | 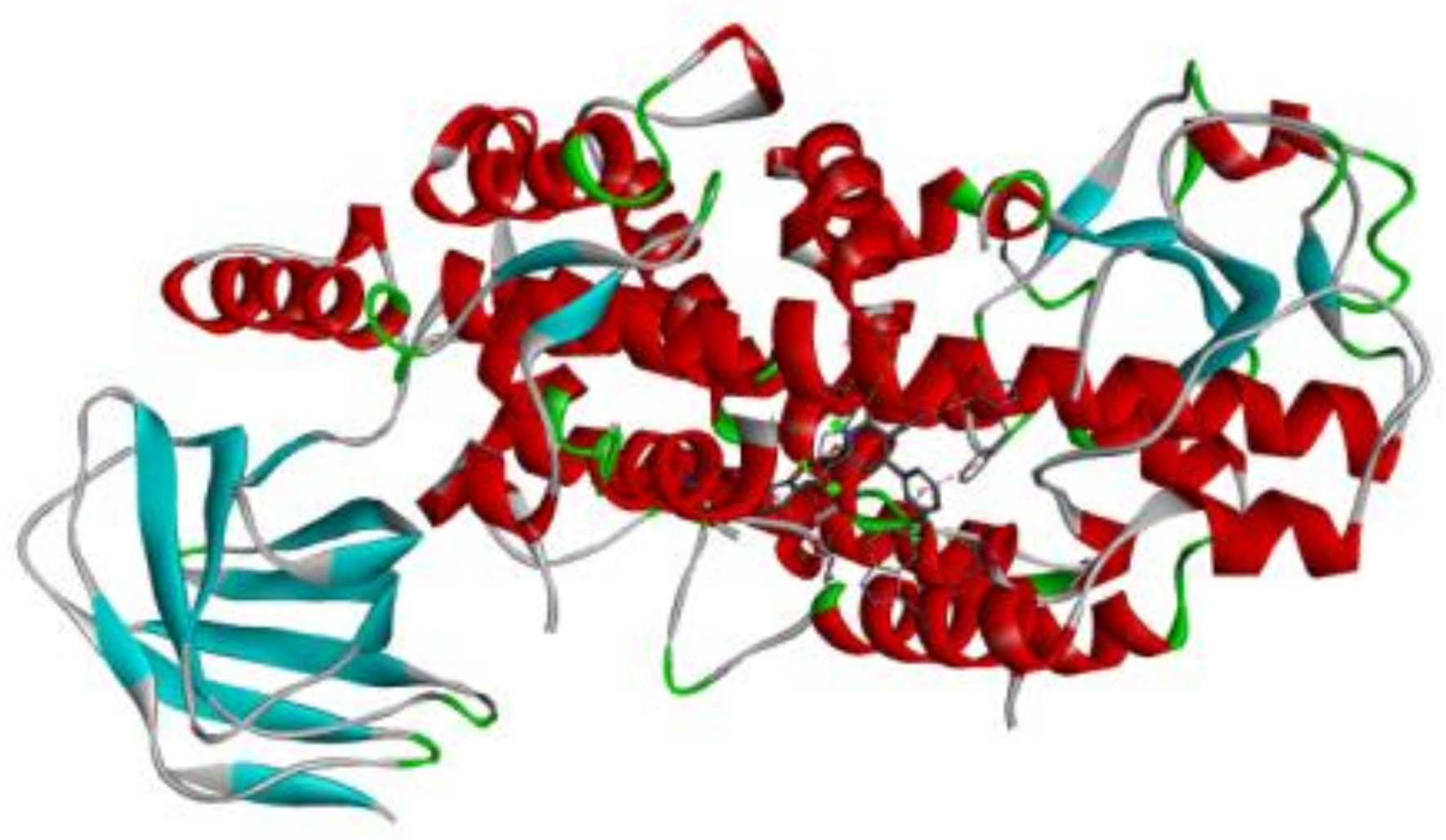 | 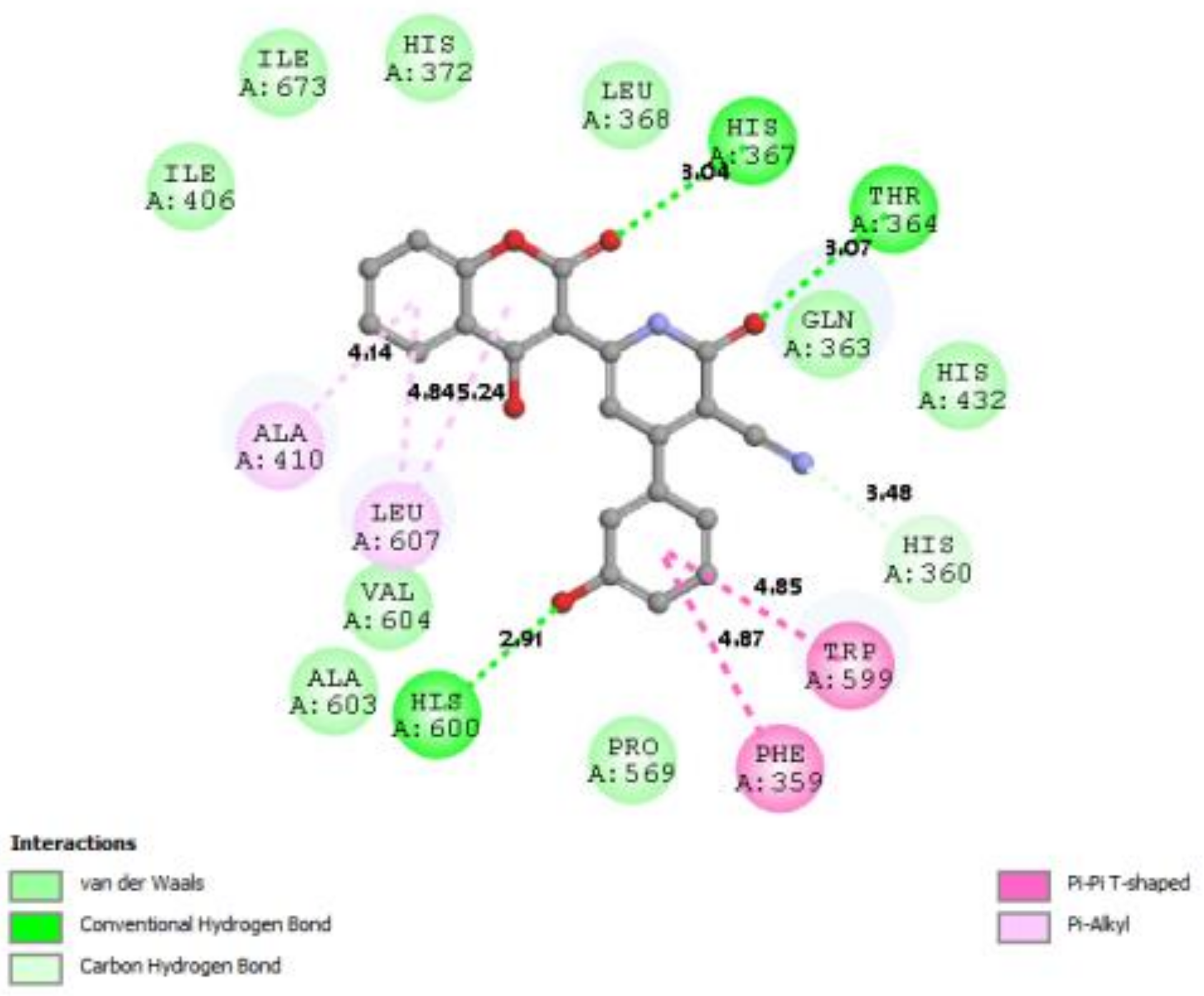 |
| 4NRE |  | 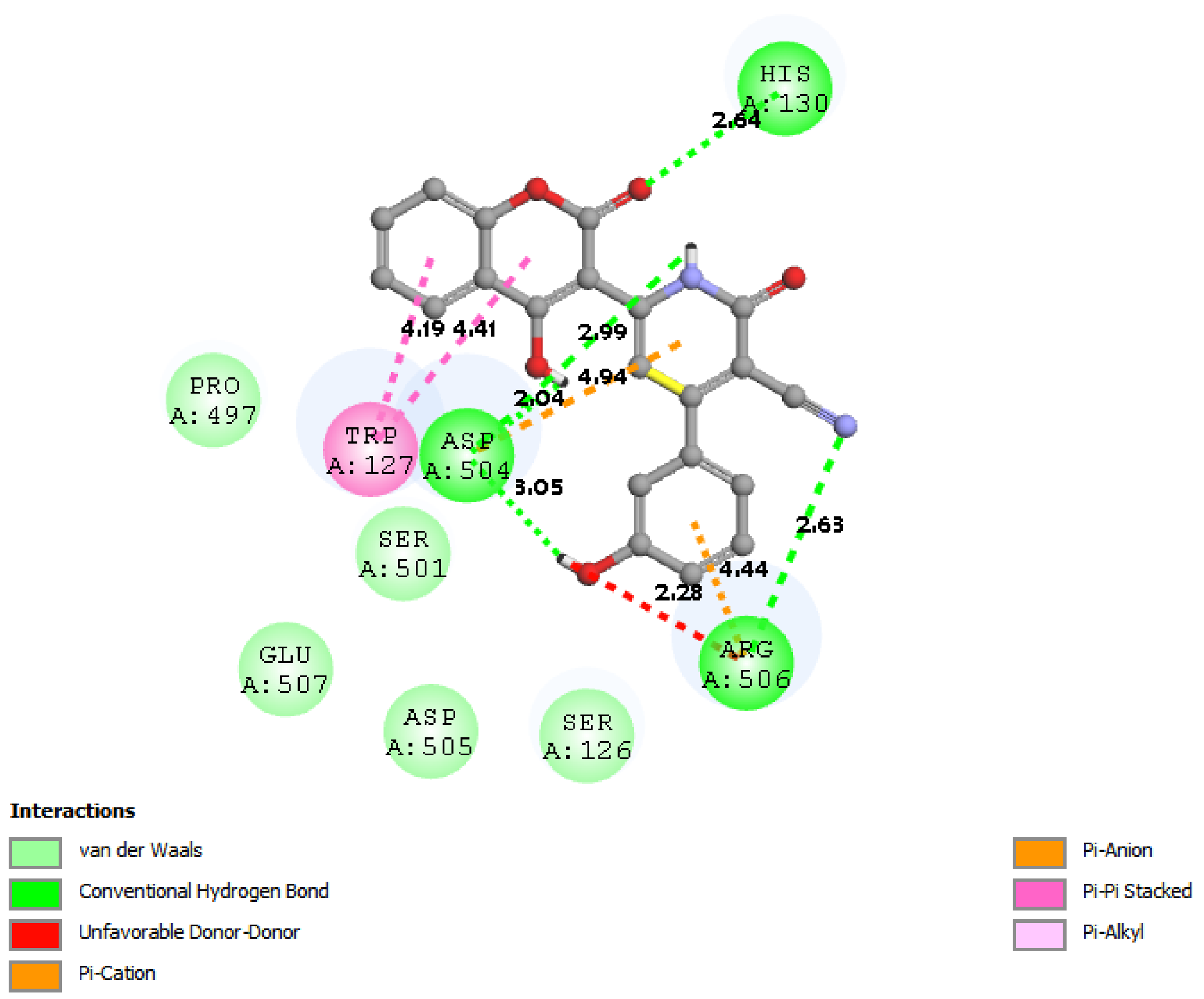 |
| 1TH6 |  |  |
| 2QU9 | 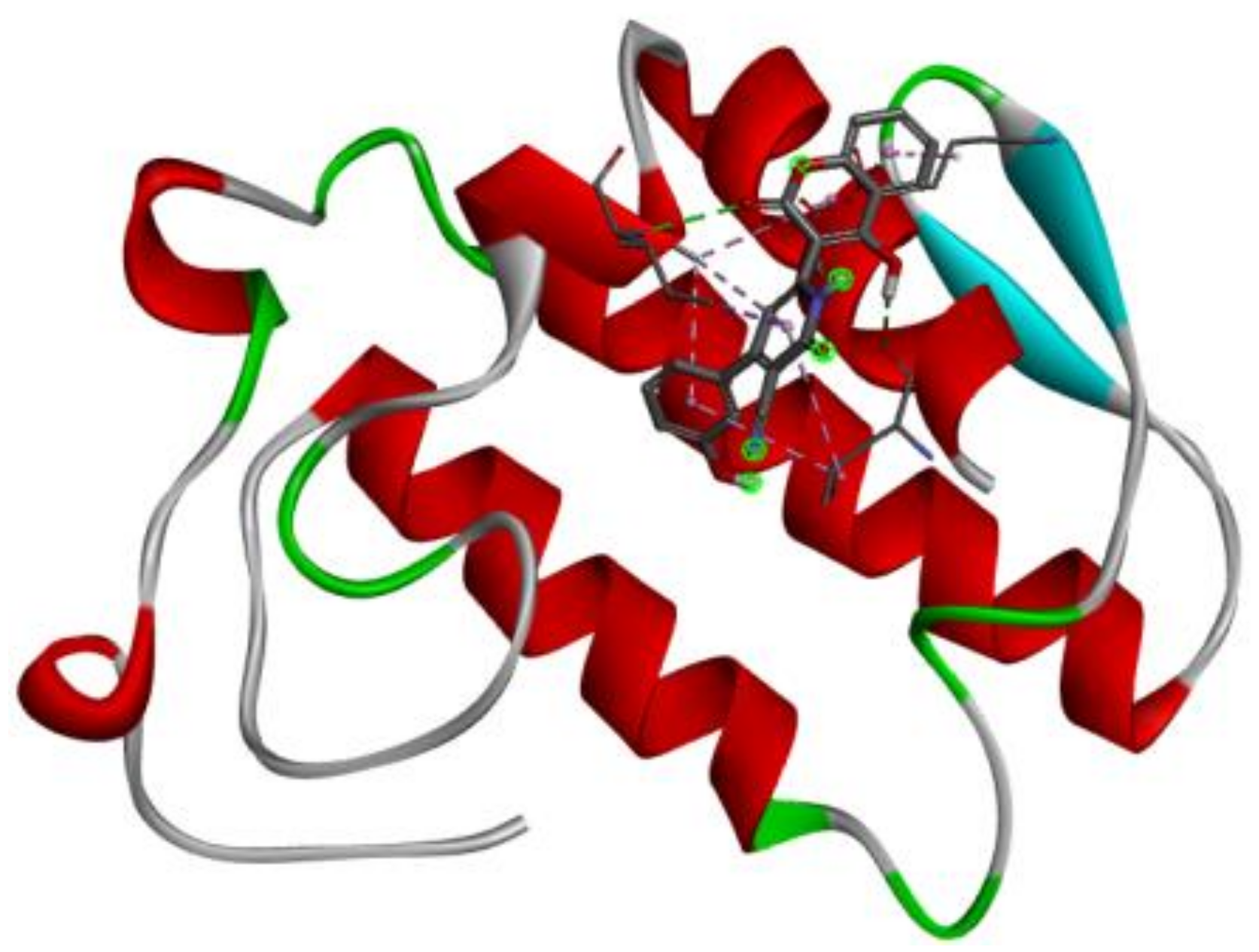 |  |
| 4DBK |  | 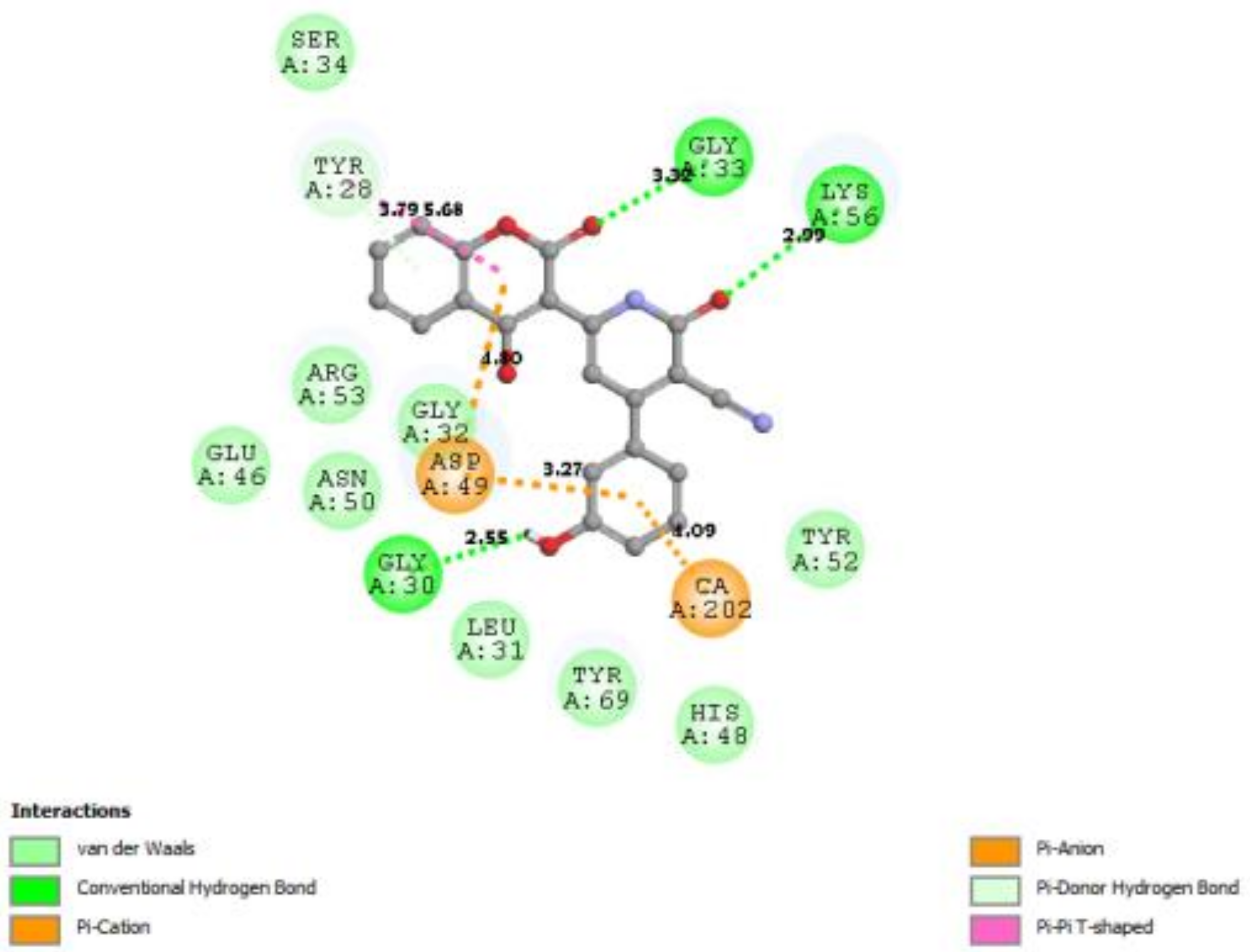 |
| 2W3L | 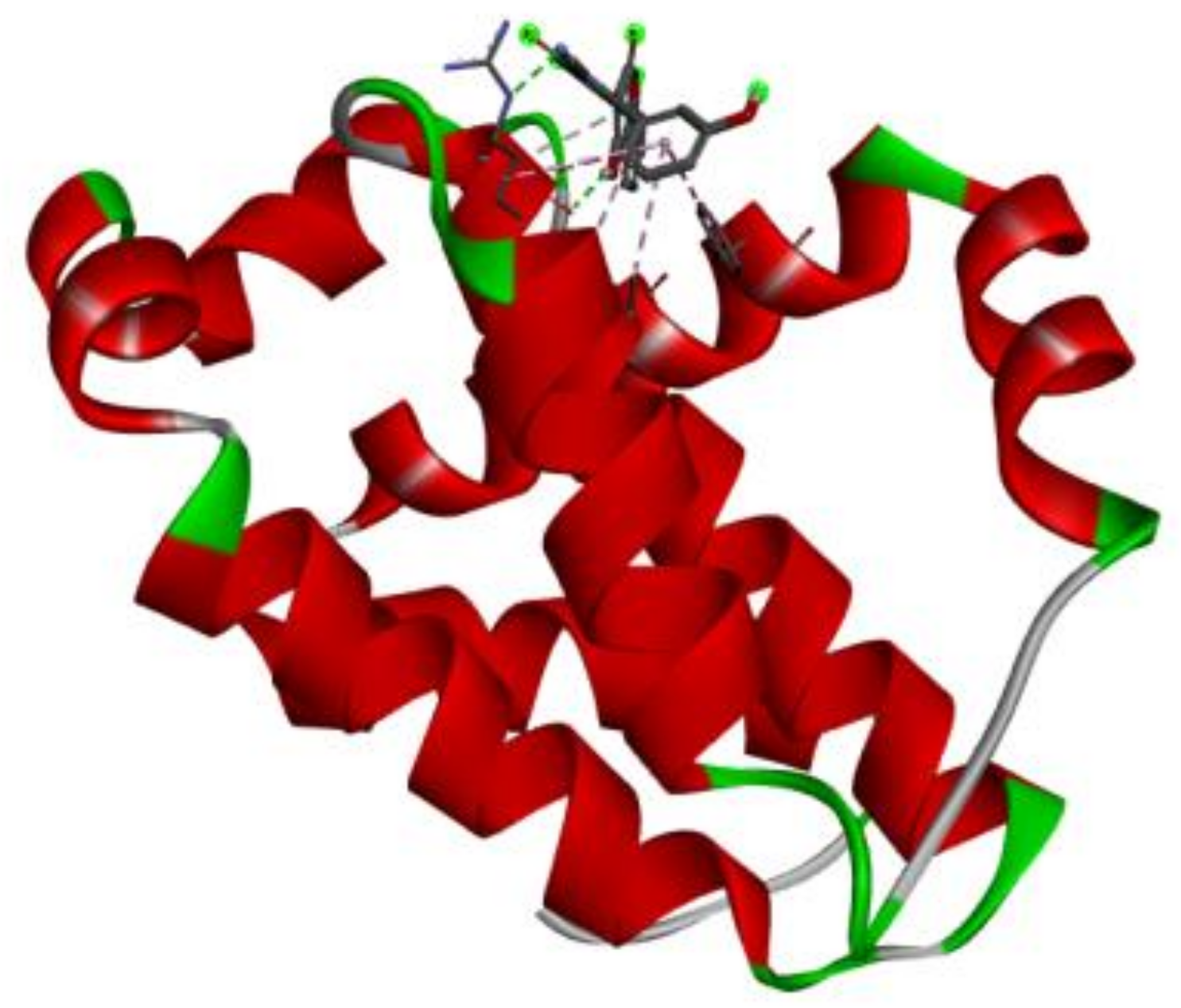 | 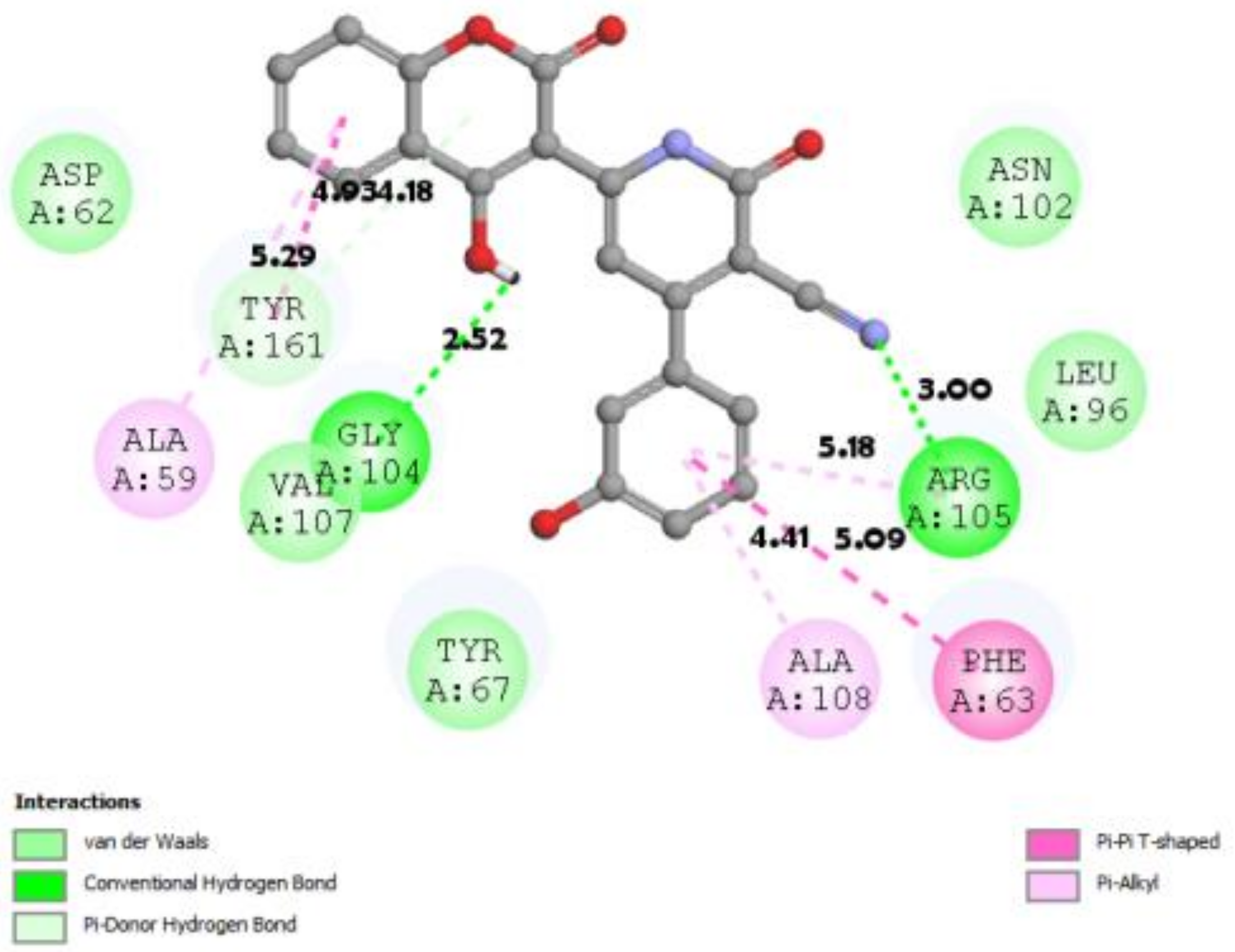 |
| 1YWN |  | 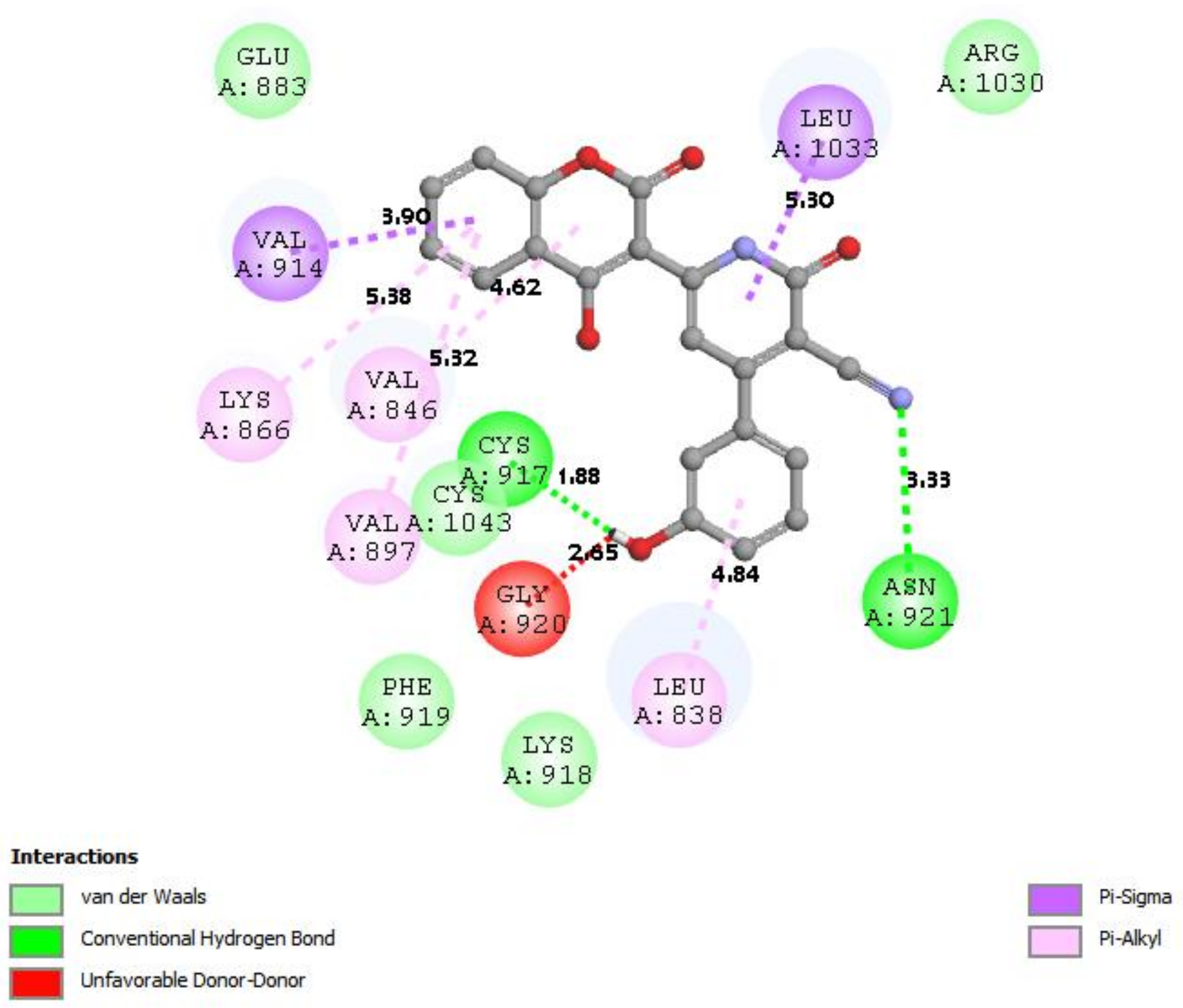 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hazmy, S.M.; Zouaghi, M.O.; Al-Johani, J.N.; Arfaoui, Y.; Al-Ashwal, R.; Hammami, B.; Alhagri, I.A.; Alhemiary, N.A.; Hamdi, N. Chemosensing Properties of Coumarin Derivatives: Promising Agents with Diverse Pharmacological Properties, Docking and DFT Investigation. Molecules 2022, 27, 5921. https://doi.org/10.3390/molecules27185921
Al-Hazmy SM, Zouaghi MO, Al-Johani JN, Arfaoui Y, Al-Ashwal R, Hammami B, Alhagri IA, Alhemiary NA, Hamdi N. Chemosensing Properties of Coumarin Derivatives: Promising Agents with Diverse Pharmacological Properties, Docking and DFT Investigation. Molecules. 2022; 27(18):5921. https://doi.org/10.3390/molecules27185921
Chicago/Turabian StyleAl-Hazmy, Sadeq M., Mohamed Oussama Zouaghi, Jamal N. Al-Johani, Youssef Arfaoui, Rania Al-Ashwal, Bechir Hammami, Ibrahim A. Alhagri, Nabil A. Alhemiary, and Naceur Hamdi. 2022. "Chemosensing Properties of Coumarin Derivatives: Promising Agents with Diverse Pharmacological Properties, Docking and DFT Investigation" Molecules 27, no. 18: 5921. https://doi.org/10.3390/molecules27185921
APA StyleAl-Hazmy, S. M., Zouaghi, M. O., Al-Johani, J. N., Arfaoui, Y., Al-Ashwal, R., Hammami, B., Alhagri, I. A., Alhemiary, N. A., & Hamdi, N. (2022). Chemosensing Properties of Coumarin Derivatives: Promising Agents with Diverse Pharmacological Properties, Docking and DFT Investigation. Molecules, 27(18), 5921. https://doi.org/10.3390/molecules27185921





