Phytochemical Characterization, Anti-Oxidant, Anti-Enzymatic and Cytotoxic Effects of Artemisia verlotiorum Lamotte Extracts: A New Source of Bioactive Agents
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Profile
2.2. HPLC-ESI-MSn Analysis
2.3. Quantification of Phytochemicals
2.4. Antioxidant Activity
2.5. Anti-Tyrosinase, Anti-Cholinesterase, and Anti-Glucosidase Activities
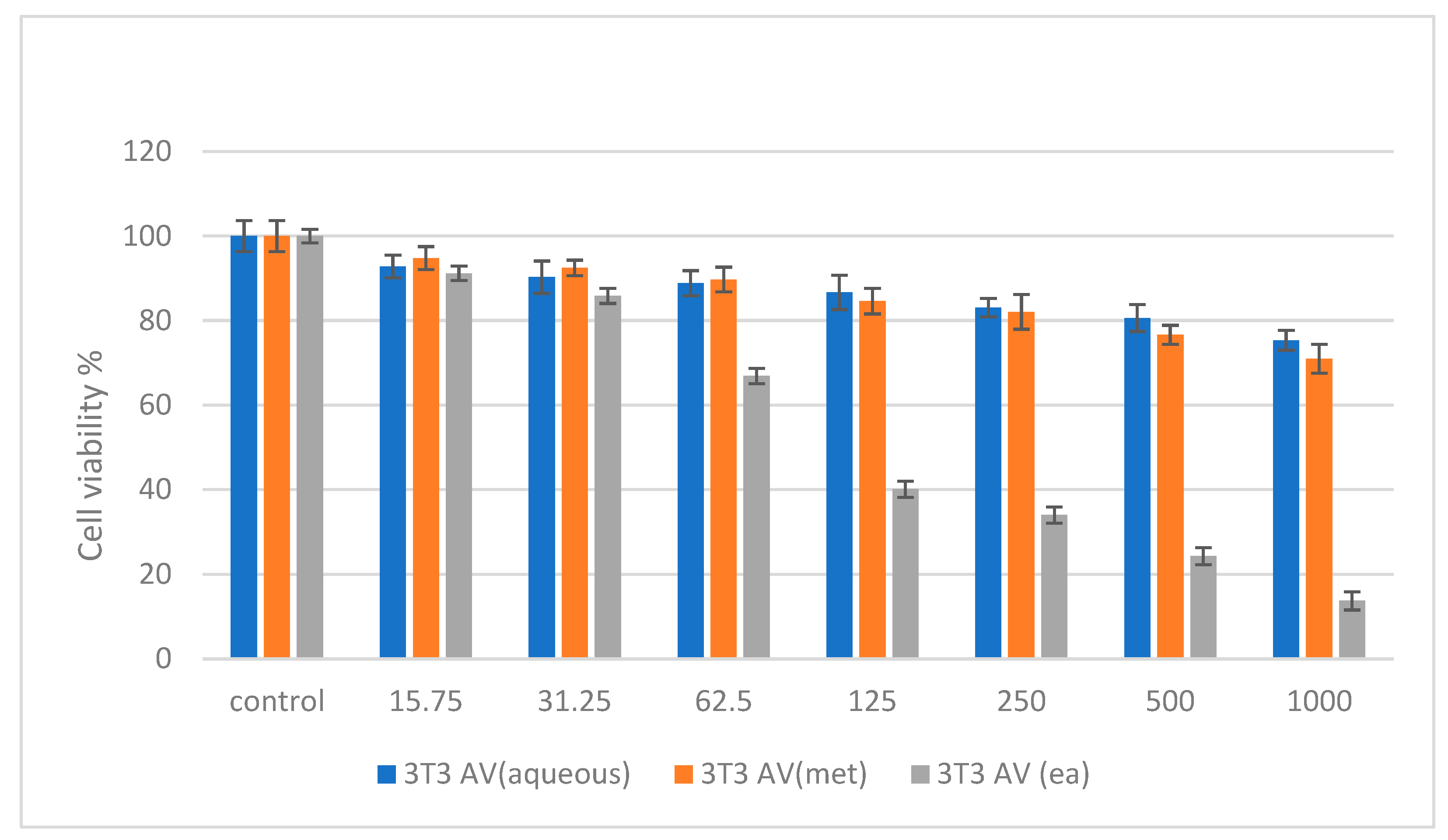
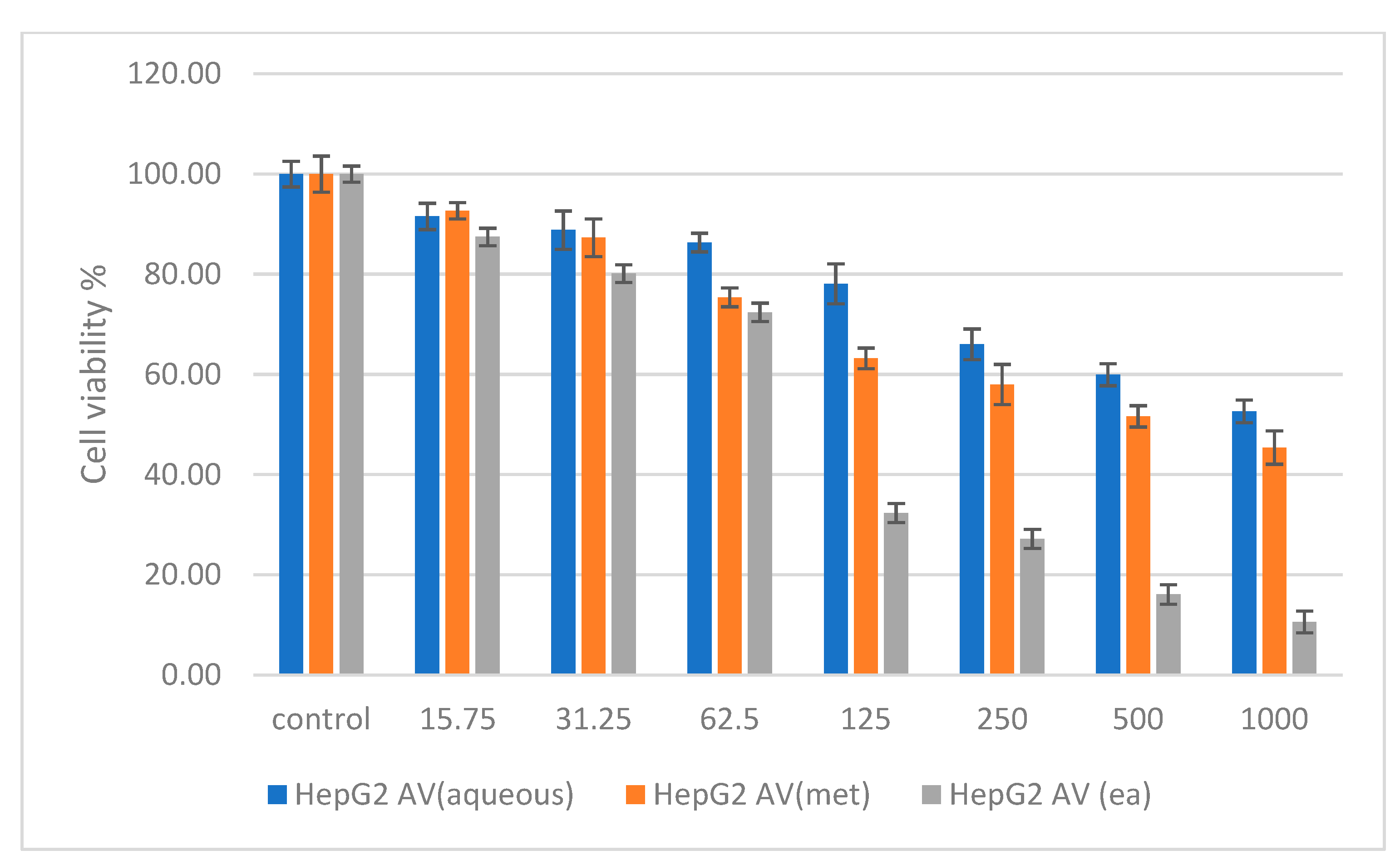
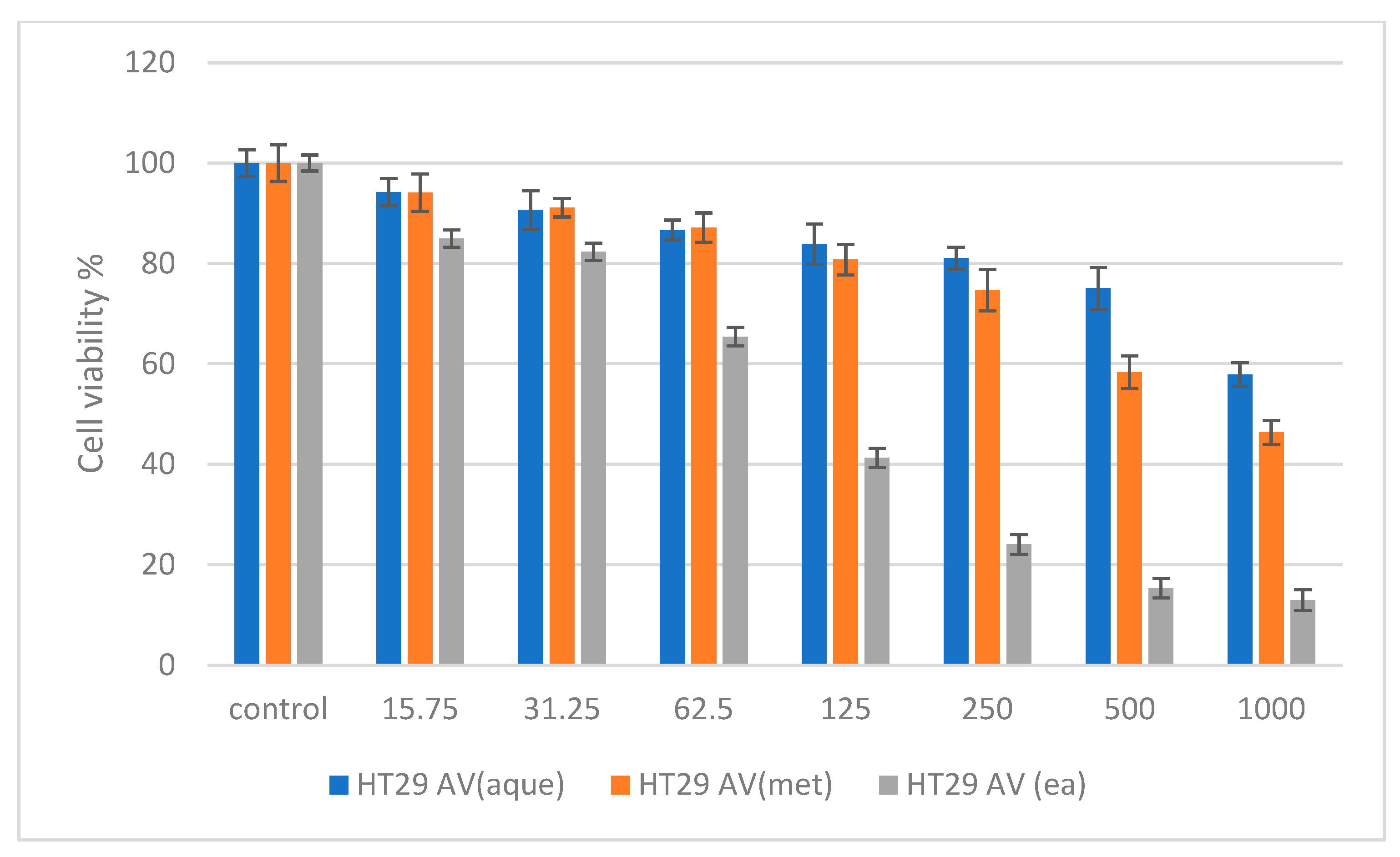
2.6. Cytotoxicity Studies on Normal (NIH 3T3) and Cancer (HepG2 and HT 29) Cell Lines
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.1.1. Extraction of Phytochemicals
4.1.2. Phytochemical Composition
4.1.3. HPLC Analysis
4.1.4. Instrumentation for the HPLC Analysis
4.1.5. Antioxidant and Enzyme Inhibitory Assays
4.1.6. Cell Viability Assay
4.1.7. Cytotoxicity Studies on Normal (NIH 3T3) and Cancer (HepG2 and HT 29) Cell Lines
4.1.8. Statistical Analyses
5. Conclusions
6. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Garcia, S. Pandemics and Traditional Plant-Based Remedies. A Historical-Botanical Review in the Era of COVID19. Front. Plant Sci. 2020, 11, 571042. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Chandran, R.; Abrahamse, H. Identifying Plant-Based Natural Medicine against Oxidative Stress and Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2020, 2020, 8648742. [Google Scholar] [CrossRef] [PubMed]
- Setzer, M.C.; Setzer, W.N.; Jackes, B.R.; Gentry, G.A.; Moriarity, D.M. The Medicinal Value of Tropical Rainforest Plants from Paluma, North Queensland, Australia. Pharm. Biol. 2001, 39, 67–78. [Google Scholar] [CrossRef]
- McRae, J.; Palombo, E.; Harding, I.; Crawford, R. Environmental Engineering Research Event Antimicrobial Activity of Traditional Medicinal Plants. In Proceedings of the 7th Annual EERE Conference, Victoria, Australia, December 2003; pp. 225–233. [Google Scholar]
- Murillo-Alvarez, J.I.; Encarnación, D.R.; Franzblau, S. Antimicrobial and Cytotoxic Activity of Some Medicinal Plants from Baja California Sur (Mexico). Pharm. Biol. 2001, 39, 445–449. [Google Scholar] [CrossRef]
- Suroowan, S.; Pynee, K.; Mahomoodally, M. A comprehensive review of ethnopharmacologically important medicinal plant species from Mauritius. S. Afr. J. Bot. 2019, 122, 189–213. [Google Scholar] [CrossRef]
- De Souza, L.F.B.; Laughinghouse, H.D., IV; Pastori, T.; Tedesco, M.; Kuhn, A.W.; Canto-Dorow, T.S.D.; Tedesco, S.B. Genotoxic potential of aqueous extracts of Artemisia verlotiorum on the cell cycle of Allium cepa. Int. J. Environ. Sci. 2010, 67, 871–877. [Google Scholar]
- Gurib-Fakim, A. Volatile constituents of the leaf oil of Artemisia verlotiorum Lamotte and Ambrosia tenuifolia Sprengel (Syn.: Artemisia psilostachya auct. non L.). J. Essent. Oil Res. 1996, 8, 559–561. [Google Scholar] [CrossRef]
- Martinotti, E.; Calderone, V.; Breschi, M.C.; Bandini, P.; Cioni, P.L. Pharmacological action of aqueous crude extracts of Artemisia verlotorum Lamotte (Compositae). Phytother. Res. 1997, 11, 612–614. [Google Scholar] [CrossRef]
- Mootoosamy, A.; Mahomoodally, M.F. Ethnomedicinal application of native remedies used against diabetes and related complications in Mauritius. J. Ethnopharmacol. 2014, 151, 413–444. [Google Scholar] [CrossRef]
- Gerontakos, S.E.; Casteleijn, D.; Shikov, A.N.; Wardle, J. Focus: Plant-based Medicine and Pharmacology: A Critical Review to Identify the Domains Used to Measure the Effect and Outcome of Adaptogenic Herbal Medicines. Yale J. Biol. Med. 2020, 93, 327. [Google Scholar] [PubMed]
- Ganzera, M.; Sturm, S. Recent advances on HPLC/MS in medicinal plant analysis—An update covering 2011–2016. J. Pharm. Biomed. 2018, 147, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Mbemya, G.T.; Vieira, L.A.; Canafistula, F.G.; Pessoa, O.D.L.; Rodrigues, A.P.R. Reports on in vivo and in vitro contribution of medicinal plants to improve the female reproductive function. Reprodução Clim. 2017, 32, 109–119. [Google Scholar] [CrossRef]
- Ruiz, A.; Mardones, C.; Vergara, C.; Hermosín-Gutiérrez, I.; von Baer, D.; Hinrichsen, P.; Rodriguez, R.; Arribillaga, D.; Dominguez, E. Analysis of hydroxycinnamic acids derivatives in calafate (Berberis microphylla G. Forst) berries by liquid chromatography with photodiode array and mass spectrometry detection. J. Chromatogr. A 2013, 1281, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Vidal, J.; Ruiz-Riaguas, A.; Fernández-de Córdova, M.; Ortega-Barrales, P.; Llorent-Martínez, E. Phenolic profile and antioxidant activity of Jasonia glutinosa herbal tea. Influence of simulated gastrointestinal in vitro digestion. Food Chem. 2019, 287, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MS n identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef]
- Van Hoyweghen, L.; De Bosscher, K.; Haegeman, G.; Deforce, D.; Heyerick, A. In vitro inhibition of the transcription factor NF-κB and cyclooxygenase by Bamboo extracts. Phytother. Res. 2014, 28, 224–230. [Google Scholar] [CrossRef]
- Islam, M.N.; Ishita, I.J.; Jung, H.A.; Choi, J.S. Vicenin 2 isolated from Artemisia capillaris exhibited potent anti-glycation properties. Food Chem. Toxicol. 2014, 69, 55–62. [Google Scholar] [CrossRef]
- Seo, H.-C.; Suzuki, M.; Ohnishi-Kameyama, M.; Oh, M.-J.; Kim, H.-R.; Kim, J.-H.; Nagata, T. Extraction and identification of antioxidant components from Artemisia capillaris herba. Plant Foods Hum. Nutr. 2003, 58, 1–12. [Google Scholar] [CrossRef]
- Sinan, K.I.; Dall’Acqua, S.; Ferrarese, I.; Mollica, A.; Stefanucci, A.; Glamočlija, J.; Sokovic, M.; Nenadić, M.; Aktumsek, A.; Zengin, G. LC-MS based analysis and biological properties of Pseudocedrela kotschyi (Schweinf.) Harms extracts: A valuable source of antioxidant, antifungal, and antibacterial compounds. Antioxidants 2021, 10, 1570. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Boligon, A.; Athayde, M. Importance of HPLC in analysis of plants extracts. Austin Chromatogr. 2014, 1, 2. [Google Scholar]
- Martin, M.; Guiochon, G. Effects of high pressure in liquid chromatography. J. Chromatogr. A 2005, 1090, 16–38. [Google Scholar] [CrossRef]
- Loel, D.A. Use of acid cleanser in endodontic therapy. J. Am. Dent. Assoc. 1975, 90, 148–151. [Google Scholar] [CrossRef]
- Nagoba, B.; Deshmukh, S.; Wadher, B.; Mahabaleshwar, L.; Gandhi, R.; Kulkarni, P.; Mane, V.; Deshmukh, J. Treatment of superficial pseudomonal infections with citric acid: An effective and economical approach. J. Hosp. Infect. 1998, 40, 155–157. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef]
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Dizaj, S.M.; Saadat, Y.R.; Khezri, K.; Jafari, S.; Ahmadian, E.; Jahandizi, N.G.; Raeesi, S. Therapeutic benefits of rutin and its nanoformulations. Phytother. Res. 2021, 35, 1719–1738. [Google Scholar] [CrossRef]
- Habtemariam, S.; Lentini, G. The therapeutic potential of rutin for diabetes: An update. Mini Rev. Med. Chem. 2015, 15, 524–528. [Google Scholar] [CrossRef]
- Han, Y. Rutin has therapeutic effect on septic arthritis caused by Candida albicans. Int. Immunopharmacol. 2009, 9, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Nassiri-Asl, M.; Shariati-Rad, S.; Zamansoltani, F. Anticonvulsive effects of intracerebroventricular administration of rutin in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.S.; Jain, D.; Singhal, P.; Awasthi, S.; Singhal, J.; Horne, D. Targeting the mercapturic acid pathway and vicenin-2 for prevention of prostate cancer. Biochim. Biophys. Acta 2017, 1868, 167–175. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, X.; Zhang, W.; Rengarajan, T. Vicenin-2 inhibits Wnt/β-catenin signaling and induces apoptosis in HT-29 human colon cancer cell line. Drug Des. Dev. Ther. 2018, 12, 1303–1310. [Google Scholar] [CrossRef]
- Nagaprashantha, L.D.; Vatsyayan, R.; Singhal, J.; Fast, S.; Roby, R.; Awasthi, S.; Singhal, S.S. Anti-cancer effects of novel flavonoid vicenin-2 as a single agent and in synergistic combination with docetaxel in prostate cancer. Biochem. Pharmacol. 2011, 82, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Li, S.; Zhang, Y.; Zhou, X.; Chen, W. Vicenin-2 is a novel inhibitor of STAT3 signaling pathway in human hepatocellular carcinoma. J. Funct. Foods 2020, 69, 103921. [Google Scholar] [CrossRef]
- Pero, R.W.; Lund, H.; Leanderson, T. Antioxidant metabolism induced by quinic acid. increased urinary excretion of tryptophan and nicotinamide. Phytother. Res. 2008, 23, 335–346. [Google Scholar] [CrossRef]
- Cinkilic, N.; Cetintas, S.K.; Zorlu, T.; Vatan, Ö.; Yilmaz, D.; Cavas, T.; Tunc, S.; Ozkan, L.; Bilaloglu, R. Radioprotection by two phenolic compounds: Chlorogenic and quinic acid, on X-ray induced DNA damage in human blood lymphocytes in vitro. Food Chem. Toxicol. 2013, 53, 359–363. [Google Scholar] [CrossRef]
- Zanello, P.R.; Koishi, A.C.; Júnior, C.D.O.R.; Oliveira, L.A.; Pereira, A.A.; de Almeida, M.V.; dos Santos, C.N.D.; Bordignon, J. Quinic acid derivatives inhibit dengue virus replication in vitro. Virol. J. 2015, 12, 223. [Google Scholar] [CrossRef]
- Muthamil, S.; Balasubramaniam, B.; Balamurugan, K.; Pandian, S.K. Synergistic Effect of Quinic Acid Derived from Syzygium cumini and Undecanoic Acid against Candida spp. Biofilm and Virulence. Front. Microbiol. 2018, 9, 2835. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, X.; Gao, C.-L.; Ruan, W.; Zhao, S.; Kai, G.; Li, F.; Pang, T. Medioresinol as a novel PGC-1α activator prevents pyroptosis of endothelial cells in ischemic stroke through PPARα-GOT1 axis. Pharmacol. Res. 2021, 169, 105640. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Islam, M.S.; Rahman, M.K.; Uddin, M.N.; Akanda, M.R. The pharmacological and biological roles of eriodictyol. Arch. Pharm. Res. 2020, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-Y.; Lee, J.-J.; Kim, Y.; Kim, I.-S.; Han, J.-H.; Lee, S.-G.; Ahn, M.-J.; Jung, S.-H.; Myung, C.-S. Effect of Eriodictyol on Glucose Uptake and Insulin Resistance In Vitro. J. Agric. Food Chem. 2012, 60, 7652–7658. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.F.; Guo, H.J.; Huang, Y.; Wu, C.T.; Zhang, X.F. Eriodictyol, a plant flavonoid, attenuates LPS-induced acute lung injury through its antioxidative and anti-inflammatory activity. Exp. Ther. Med. 2015, 10, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Xuewen, H.; Ping, O.; Zhongwei, Y.; Zhongqiong, Y.; Hualin, F.; Juchun, L.; Changliang, H.; Gang, S.; Zhixiang, Y.; Xu, S.; et al. Eriodictyol protects against Staphylococcus aureus-induced lung cell injury by inhibiting alpha-hemolysin expression. World J. Microbiol. Biotechnol. 2018, 34, 64. [Google Scholar] [CrossRef]
- de Oliveira Ferreira, E.; Fernandes, M.Y.S.D.; de Lima, N.M.R.; Neves, K.R.T.; do Carmo, M.R.S.; Lima, F.A.V.; Fonteles, A.A.; Menezes, A.P.F.; de Andrade, G.M. Neuroinflammatory response to experimental stroke is inhibited by eriodictyol. Behav. Brain Res. 2016, 312, 321–332. [Google Scholar] [CrossRef]
- Kim, M.; Choi, S.-Y.; Lee, P.; Hur, J. Neochlorogenic Acid Inhibits Lipopolysaccharide-Induced Activation and Pro-inflammatory Responses in BV2 Microglial Cells. Neurochem. Res. 2015, 40, 1792–1798. [Google Scholar] [CrossRef]
- Fang, W.; Ma, Y.; Wang, J.; Yang, X.; Gu, Y.; Li, Y. In vitro and in vivo antitumor activity of neochlorogenic acid in human gastric carcinoma cells are complemented with ROS generation, loss of mitochondrial membrane potential and apoptosis induction. J. BUON 2019, 24, 221–226. [Google Scholar]
- Park, S.Y.; Jin, M.L.; Yi, E.H.; Kim, Y.; Park, G. Neochlorogenic acid inhibits against LPS-activated inflammatory responses through up-regulation of Nrf2/HO-1 and involving AMPK pathway. Environ. Toxicol. Pharmacol. 2018, 62, 1–10. [Google Scholar] [CrossRef]
- Gao, X.-H.; Zhang, S.-D.; Wang, L.-T.; Yu, L.; Zhao, X.-L.; Ni, H.-Y.; Wang, Y.-Q.; Wang, J.-D.; Shan, C.-H.; Fu, Y.-J. Anti-inflammatory effects of neochlorogenic acid extract from mulberry leaf (Morus alba L.) against LPS-stimulated inflammatory response through mediating the AMPK/Nrf2 signaling pathway in A549 cells. Molecules 2020, 25, 1385. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bi, C.; Cai, H.; Liu, B.; Zhong, X.; Deng, X.; Wang, T.; Xiang, H.; Niu, X.; Wang, D. The therapeutic effect of chlorogenic acid against Staphylococcus aureus infection through sortase A inhibition. Front. Microbiol. 2015, 6, 1031. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, L.-L.; Xue, N.-N.; Li, C.; Guo, H.-H.; Ren, T.-K.; Zhan, Y.; Li, W.-B.; Zhang, J.; Chen, X.-G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Corradi, I.; De Souza, E.; Sande, D.; Takahashi, J.A. Correlation between phenolic compounds contents, anti-tyrosinase and antioxidant activities of plant extracts. Chem. Eng. Trans. 2018, 64, 109–114. [Google Scholar]
- Hygreeva, D.; Pandey, M.; Radhakrishna, K. Potential applications of plant based derivatives as fat replacers, antioxidants and antimicrobials in fresh and processed meat products. Meat Sci. 2014, 98, 47–57. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.-O.; Dommes, J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 2009, 113, 1226–1233. [Google Scholar] [CrossRef]
- Khlebnikov, A.I.; Schepetkin, I.A.; Domina, N.G.; Kirpotina, L.N.; Quinn, M.T. Improved quantitative structure—Activity relationship models to predict antioxidant activity of flavonoids in chemical, enzymatic, and cellular systems. Bioorg. Med. Chem. 2007, 15, 1749–1770. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Horbańczuk, O.K.; Kurek, M.A.; Atanasov, A.G.; Brnčić, M.; Brnčić, S.R. The Effect of Natural Antioxidants on Quality and Shelf Life of Beef and Beef Products. Food Technol. Biotechnol. 2019, 57, 439–447. [Google Scholar] [CrossRef]
- Mukherji, S.; Singh, S. Reaction Mechanism in Organic Chemistry; Macmillan India Press: Madras, India, 1984. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J. Traditional Herbal Medicine Research Methods: Identification, Analysis, Bioassay, and Pharmaceutical and Clinical Studies; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Martins, N.; Barros, L.; Ferreira, I.C. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Mapunya, M.B.; Nikolova, R.V.; Lall, N. Melanogenesis and Antityrosinase Activity of Selected South African Plants. Evid.-Based Complement. Altern. Med. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Zengin, G.; Simirgiotis, M.; Schafberg, M.; Mollica, A.; Vodnar, D.C.; Crişan, G.; Rohn, S. Functional constituents of wild and cultivated Goji (L. barbarum L.) leaves: Phytochemical characterization, biological profile, and computational studies. J. Enzyme Inhib. Med. Chem. 2017, 32, 153–168. [Google Scholar] [CrossRef]
- Kubo, I.; Kinst-Hori, I. Tyrosinase Inhibitors from Cumin. J. Agric. Food Chem. 1998, 46, 5338–5341. [Google Scholar] [CrossRef]
- Younis, M.M.; Ayoub, I.M.; Mostafa, N.M.; El Hassab, M.A.; Eldehna, W.M.; Al-Rashood, S.T.; Eldahshan, O.A. GC/MS Profiling, Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase and Anti-Hyaluronidase Activities of a Stenocarpus sinuatus Leaves Extract. Plants 2022, 11, 918. [Google Scholar] [CrossRef]
- Imen, M.-B.; Chaabane, F.; Nadia, M.; Soumaya, K.-J.; Kamel, G.; Leila, C.-G. Anti-melanogenesis and antigenotoxic activities of eriodictyol in murine melanoma (B16–F10) and primary human keratinocyte cells. Life Sci. 2015, 135, 173–178. [Google Scholar] [CrossRef]
- Khan, H.; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef]
- Chopra, K.; Misra, S.; Kuhad, A. Current perspectives on pharmacotherapy of Alzheimer’s disease. Expert Opin. Pharmacother. 2011, 12, 335–350. [Google Scholar] [CrossRef]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE Inhibitors from Plants and their Contribution to Alzheimer’s Disease Therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef] [PubMed]
- WHO. Diabetes. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 8 August 2022).
- Zhang, X.; Li, G.; Wu, D.; Yu, Y.; Hu, N.; Wang, H.; Li, X.; Wu, Y. Emerging strategies for the activity assay and inhibitor screening of alpha-glucosidase. Food Funct. 2020, 11, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Zuiter, A.S. Proanthocyanidin: Chemistry and biology: From phenolic compounds to proanthocyanidins. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Yuan, E.; Liu, B.; Wei, Q.; Yang, J.; Chen, L.; Li, Q. Structure Activity Relationships of Flavonoids as Potent α-Amylase Inhibitors. Nat. Prod. Commun. 2014, 9, 1173–1176. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The inhibitory effects of flavonoids on α-amylase and α-glucosidase. Crit. Rev. Food Sci. Nutr. 2020, 60, 695–708. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cancer. 2021. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 8 August 2022).
- Kuete, V.; Fokou, F.W.; Karaosmanoğlu, O.; Beng, V.P.; Sivas, H. Cytotoxicity of the methanol extracts of Elephantopus mollis, Kalanchoe crenata and 4 other Cameroonian medicinal plants towards human carcinoma cells. BMC Complement. Altern. Med. 2017, 17, 280. [Google Scholar] [CrossRef]
- Ogbole, O.O.; Segun, P.A.; Adeniji, A.J. In vitro cytotoxic activity of medicinal plants from Nigeria ethnomedicine on Rhabdomyosarcoma cancer cell line and HPLC analysis of active extracts. BMC Complement. Altern. Med. 2017, 17, 494. [Google Scholar] [CrossRef]
- Nandi, S.; Vračko, M.; Bagchi, M.C. Anticancer Activity of Selected Phenolic Compounds: QSAR Studies Using Ridge Regression and Neural Networks. Chem. Biol. Drug Des. 2007, 70, 424–436. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Wang, S.; Zhang, Q.; Zhao, J.; Song, L. Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-κB signaling pathway. Oncol. Rep. 2021, 45, 717–727. [Google Scholar] [CrossRef]
- Jiang, Y.; Kusama, K.; Satoh, K.; Takayama, F.; Watanabe, S.; Sakagami, H. Induction of cytotoxicity by chlorogenic acid in human oral tumor cell lines. Phytomedicine 2000, 7, 483–491. [Google Scholar] [CrossRef]
- Li, Y.; But, P.P.; Ooi, V.E. Antiviral activity and mode of action of caffeoylquinic acids from Schefflera heptaphylla (L.) Frodin. Antivir. Res. 2005, 68, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathway of Apoptosis. Sci. Rep. 2016, 6, 24049. [Google Scholar] [CrossRef] [PubMed]
- Metodiewa, D.; Jaiswal, A.K.; Cenas, N.; Dickancaité, E.; Segura-Aguilar, J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic. Biol. Med. 1999, 26, 107–116. [Google Scholar] [CrossRef]
- Kusaczuk, M.; Krętowski, R.; Naumowicz, M.; Stypułkowska, A.; Cechowska-Pasko, M. A Preliminary Study of the Effect of Quercetin on Cytotoxicity, Apoptosis, and Stress Responses in Glioblastoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 1345. [Google Scholar] [CrossRef]
- Uysal, S.; Aktumsek, A. A phytochemical study on Potentilla anatolica: An endemic Turkish plant. Ind. Crop. Prod. 2015, 76, 1001–1007. [Google Scholar] [CrossRef]
- Zengin, G.; Aktumsek, A. Investigation of Antioxidant Potentials of Solvent Extracts from Different Anatomical Parts of Asphodeline anatolica E. Tuzlaci: An Endemic Plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.D.P.; Ruiz-Medina, A.; Zengin, G.; Llorent-Martínez, E.J. Phenolic Characterization, Antioxidant Activity, and Enzyme Inhibitory Properties of Berberis thunbergii DC. Leaves: A Valuable Source of Phenolic Acids. Molecules 2019, 24, 4171. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and Enzyme Inhibitory Potential of Two Potentilla species (P. speciosa L. and P. reptans Willd.) and Their Chemical Composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]

| Samples | Total Phenolic Content (mgGAE/g) | Total Flavonoid Content (mgRE/g) | Total Phenolic Acid Content (mgCE/g) | Total Flavonol Content (mgCAE/g) |
|---|---|---|---|---|
| AV-Aq | 104.42 ± 0.29 a | 10.70 ± 0.63 c | 59.26 ± 0.49 a | 1.63 ± 0.06 b |
| AV-EA | 25.28 ± 0.18 c | 13.81 ± 1.12 b | na | 2.23 ± 0.03 a |
| AV-MEOH | 56.50 ± 0.20 b | 31.38 ± 0.59 a | 32.38 ± 2.02 b | 1.14 ± 0.01 c |
| No. | tR (min) | [M − H]− m/z | m/z (% Base Peak) | Assigned Identification | MeOH | H2O | EA |
|---|---|---|---|---|---|---|---|
| 1 | 1.8 | 191 | MS2 [191]: 173 (46), 127 (14), 111 (100) | Citric acid * | ✓ | ✓ | |
| 2 | 2.5 | 191 | MS2 [191]: 173 (100) | Quinic acid | ✓ | ||
| 3 | 3.7 | 315 | MS2 [315]: 153 (100) MS3 [315→153]: 123 (99), 109 (100), 108 (90) | Dihydroxybenzoic acid-O-hexoside | ✓ | ✓ | |
| 4 | 5.2 | 353 | MS2 [353]: 191 (100), 179 (23), 135 (7) | Neochlorogenic acid * | ✓ | ✓ | |
| 5 | 5.7 | 371 | MS2 [371]: 353 (16), 209 (100), 191 (38) MS3 [371→209]: 191 (100), 173 (5) | Caffeoylglucaric acid | ✓ | ||
| 6 | 8.9 | 353 | MS2 [353]: 191 (100), 179 (5), 173 (5) | Chlorogenic acid * | ✓ | ✓ | ✓ |
| 7 | 10.5 | 387 | MS2 [387]: 369 (8), 207 (100), 163 (84) MS3 [387→207]: 163 (100) | Medioresinol | ✓ | ✓ | ✓ |
| 8 | 11.3 | 533 | MS2 [533]: 371 (100), 353 (10), 209 (24) MS3 [533→371]: 209 (100), 191 (34), 129 (6) MS4 [→→]: | Dicaffeoylglucaric acid | ✓ | ||
| 9 | 12.6 | 593 | MS2 [593]: 503 (18), 473 (86), 383 (29), 353 (100), 325 (10) | Vicenin-2 * | ✓ | ✓ | |
| 10 | 13.0 | 337 | MS2 [337]: 191 (100) MS3 [337→191]: 127 (100) | 5-p-Coumaroylquinic acid | ✓ | ✓ | ✓ |
| 11 | 13.8 | 515 | MS2 [515]: 353 (100), 191 (19), 179 (45) MS3 [515→353]: 191 (100), 179 (32), 173 (5), 135 (8) | Dicaffeoylquinic acid isomer | ✓ | ✓ | |
| 12 | 14.5 | 367 | MS2 [367]: 191 (100), 173 (5) | 5-Feruloylquinic acid | ✓ | ✓ | |
| 13 | 15.3 | 463 | MS2 [463]:417 (100), 255 (18), 161 (18) MS3 [463→417]: 255 (100), 161 (46) | Unknown | ✓ | ✓ | ✓ |
| 14 | 19.6 | 609 | MS2 [609]: 301 (100) MS3 [609→301]: 271 (35), 179 (100), 151 (76) | Rutin * | ✓ | ✓ | |
| 15 | 20.7 | 463 | MS2 [463]: 301 (100) MS3 [463→301]: 271 (52), 179 (100), 151 (74) | Quercetin-O-hexoside | ✓ | ✓ | |
| 16 | 22.7 | 515 | MS2 [515]: 353 (100), 191 (13), 179 (20), 173 (30) MS3 [515→353]: 191 (29), 179 (46), 173 (100), 135 (10) | Dicaffeoylquinic acid isomer | ✓ | ✓ | ✓ |
| 17 | 24.1 | 515 | MS2 [515]: 353 (100), 191 (18), 179 (4) MS3 [515→353]: 191 (100), 179 (33), 173 (3), 135 (5) | Dicaffeoylquinic acid isomer | ✓ | ✓ | ✓ |
| 18 | 26.1 | 515 | MS2 [515]: 353 (100), 191 (4), 179 (10), 173 (18) MS3 [515→353]: 191 (22), 179 (40), 173 (100), 135 (6) | Dicaffeoylquinic acid isomer | ✓ | ✓ | ✓ |
| 19 | 27.0 | 549 | MS2 [549]: 387 (100) MS3 [549→387]: 369 (5), 207 (100), 163 (58) MS4 [549→387→207]: 163 (100) | Medioresinol-O-hexoside | ✓ | ✓ | ✓ |
| 20 | 27.9 | 625 | MS2 [625]: 463 (100), 301 (46) MS3 [625→463]: 301 (100) MS4 [625→463→301]: 179 (100), 151 (76) | Quercetin-O-hexoside-O-hexoside | ✓ | ✓ | ✓ |
| 21 | 29.7 | 529 | MS2 [529]: 367 (100), 353 (33), 191 (31) MS3 [529→367]: 191 (100), 173 (4) | Feruloyl-caffeoyl-quinic acid | ✓ | ✓ | ✓ |
| 22 | 30.4 | 515 | MS2 [515]: 353 (100), 179 (12) MS3 [515→353]: 191 (100), 179 (76), 173 (29), 135 (15) | Dicaffeoylquinic acid isomer | ✓ | ✓ | |
| 23 | 32.6 | 287 | MS2 [287]: 269 (3), 151 (100), 135 (5), 125 (4) | Eriodictyol | ✓ | ✓ | |
| 24 | 35.3 | 593 | MS2 [593]: 323 (92), 269 (100) | Unknown | ✓ | ✓ | ✓ |
| 25 | 35.9 | 285 | MS2 [285]: 285 (100), 241 (7) | Luteolin | ✓ | ✓ | ✓ |
| 26 | 36.6 | 345 | MS2 [345]: 330 (100) MS3 [345→330]: 315 (100) MS4 [345→330→315]: 287 (100) | Dimethylated flavonoid | ✓ | ✓ | |
| 27 | 37.1 | 677 | MS2 [677]: 515 (100), 353 (22) MS3 [677→515]: 353 (100), 179 (5), 173 (18) MS4 [677→515→353]: 191 (100), 179 (60) | Tricaffeoylquinic acid | ✓ | ✓ | ✓ |
| 28 | 38.2 | 204 | MS2 [204]: 204 (100), 189 (24) | Unknown | ✓ | ||
| 29 | 39.0 | 327 | MS2 [327]: 291 (36), 229 (68), 211 (47), 171 (100) | Oxo-dihydroxy-octadecenoic acid | ✓ | ✓ | ✓ |
| 30 | 40.4 | 329 | MS2 [329]: 311 (32), 293 (41), 229 (86), 211 (100), 171 (70) | Trihydroxy-octadecenoic acid | ✓ | ✓ | ✓ |
| Total number of metabolites identified = 30 | 25 | 27 | 19 | ||||
| N° | Assigned Identification | mg g−1 DE | ||
|---|---|---|---|---|
| MeOH | H2O | EA | ||
| Phenolic acids | ||||
| 4 | Neochlorogenic acid | 0.88 ± 0.06 b | 3.5 ± 0.2 a | --- |
| 6 | Chlorogenic acid | 6.8 ± 0.4 b | 18 ± 1 a | --- |
| 11 | Dicaffeoylquinic acid | 0.45 ± 0.03 b | 3.6 ± 0.2 a | --- |
| 16 | Dicaffeoylquinic acid | 5.3 ± 0.3 b | 15 ± 1 a | 0.14 ± 0.01 c |
| 17 | Dicaffeoylquinic acid | 44 ± 3 a | 45 ± 3 a | 2.8 ± 0.2 b |
| 18 | Dicaffeoylquinic acid | 19 ± 1 a | 20 ± 1 a | 0.71 ± 0.05 b |
| Total | 76 ± 3b | 105 ± 4a | 3.7 ± 0.2c | |
| Flavonoids | ||||
| 20 | Quercetin-O-Hex-O-Hex | 0.16 ± 0.01 a | 0.13 ± 0.01 b | --- |
| TIPC | 76 ± 3b | 105 ± 4a | 3.7 ± 0.2c | |
| Peak | Compound | MeOH | H2O | EA |
|---|---|---|---|---|
| 1 | Citric acid | 2.72 | 2.19 | 0.00 |
| 2 | Quinic acid | 0.00 | 0.64 | 0.00 |
| 3 | Dihydroxybenzoic acid-O-hexoside | 0.12 | 0.26 | 0.00 |
| 4 | Neochlorogenic acid | 0.63 | 2.52 | 0.00 |
| 5 | Caffeoylglucaric acid | 0.00 | 0.70 | 0.00 |
| 6 | Chlorogenic acid | 6.47 | 7.61 | 0.96 |
| 7 | Medioresinol | 0.85 | 0.70 | 0.47 |
| 8 | Dicaffeoylglucaric acid | 0.00 | 0.85 | 0.00 |
| 9 | Vicenin-2 | 0.38 | 0.97 | 0.00 |
| 10 | 5-p-Coumaroylquinic acid | 0.60 | 0.53 | 0.13 |
| 11 | Dicaffeoylquinic acid isomer | 1.12 | 7.53 | 0.00 |
| 12 | 5-Feruloylquinic acid | 0.00 | 0.98 | 0.40 |
| 13 | Unknown | 1.02 | 0.40 | 3.25 |
| 14 | Rutin | 0.62 | 0.82 | 0.00 |
| 15 | Quercetin-O-hexoside | 0.37 | 0.21 | 0.00 |
| 16 | Dicaffeoylquinic acid isomer | 10.12 | 19.28 | 1.21 |
| 17 | Dicaffeoylquinic acid isomer | 38.86 | 24.49 | 31.16 |
| 18 | Dicaffeoylquinic acid isomer | 26.19 | 23.40 | 7.24 |
| 19 | Medioresinol-O-hexoside | 1.81 | 2.05 | 0.00 |
| 20 | Quercetin-O-hexoside-O-hexoside | 1.31 | 0.52 | 0.17 |
| 21 | Feruloyl-caffeoyl-quinic acid | 1.02 | 0.64 | 6.30 |
| 22 | Dicaffeoylquinic acid isomer | 0.52 | 0.05 | 0.00 |
| 23 | Eriodyctiol | 0.74 | 0.00 | 13.49 |
| 24 | Unknown | 0.11 | 0.14 | 2.44 |
| 25 | Luteolin | 0.07 | 1.29 | 4.42 |
| 26 | Dimethylated flavonoid | 0.40 | 0.00 | 6.28 |
| 27 | Tricaffeoylquinic acid | 1.23 | 0.30 | 0.13 |
| 28 | Unknown | 0.00 | 0.00 | 4.36 |
| 29 | Oxo-dihydroxy-octadecenoic acid | 0.96 | 0.34 | 5.99 |
| 30 | Trihydroxy-octadecenoic acid | 1.78 | 0.60 | 11.61 |
| Samples | DPPH (mgTE/g) | ABTS (mgTE/g) | FRAP (mgTE/g) | CUPRAC (mgTE/g) | Metal Chelating (mgEDTAE/g) | Phosphomolybdenum (mmolTE/g) |
|---|---|---|---|---|---|---|
| AV-Aq | 370.26 ± 0.89 a | 669.46 ± 10.48 a | 565.77 ± 3.57 a | 875.90 ± 17.72 a | 21.39 ± 0.84 a | 3.39 ± 0.12 a |
| AV-EA | 33.46 ± 1.71 c | 85.11 ± 1.92 c | 87.33 ± 3.66 c | 177.60 ± 0.69 c | 18.87 ± 1.63 a,b | 2.91 ± 0.17 b,c |
| AV-MEOH | 192.59 ± 0.58 b | 307.93 ± 4.82 b | 243.60 ± 0.68 b | 462.67 ± 15.18 b | 12.01 ± 0.89 c | 2.69 ± 0.17 c |
| Samples | AChE Inhibition (mgGALAE/g) | BChE Inhibition (mgGALAE/g) | Tyrosinase Inhibition (mgKAE/g) | Amylase Inhibition (mmolACAE/g) | Glucosidase Inhibition (mmolACAE/g) |
|---|---|---|---|---|---|
| AV-Aq | Na c | Na c | 53.26 ± 1.61 c | 0.09 ± 0.01 a | 0.31 ± 0.02 c |
| AV-EA | 5.48 ± 0.09 a | 6.83 ± 0.72 b | 122.14 ± 2.36 b | 0.68 ± 0.04 b,c | 1.71 ± 0.01 a |
| AV-MEOH | 3.28 ± 0.10 b | 2.93 ± 0.13 b | 126.99 ± 1.71 a | 0.63 ± 0.03 c | 0.49 ± 0.09 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suroowan, S.; Llorent-Martínez, E.J.; Zengin, G.; Dall’Acqua, S.; Sut, S.; Buskaran, K.; Fakurazi, S.; Mahomoodally, M.F. Phytochemical Characterization, Anti-Oxidant, Anti-Enzymatic and Cytotoxic Effects of Artemisia verlotiorum Lamotte Extracts: A New Source of Bioactive Agents. Molecules 2022, 27, 5886. https://doi.org/10.3390/molecules27185886
Suroowan S, Llorent-Martínez EJ, Zengin G, Dall’Acqua S, Sut S, Buskaran K, Fakurazi S, Mahomoodally MF. Phytochemical Characterization, Anti-Oxidant, Anti-Enzymatic and Cytotoxic Effects of Artemisia verlotiorum Lamotte Extracts: A New Source of Bioactive Agents. Molecules. 2022; 27(18):5886. https://doi.org/10.3390/molecules27185886
Chicago/Turabian StyleSuroowan, Shanoo, Eulogio Jose Llorent-Martínez, Gokhan Zengin, Stefano Dall’Acqua, Stefania Sut, Kalaivani Buskaran, Sharida Fakurazi, and Mohamad Fawzi Mahomoodally. 2022. "Phytochemical Characterization, Anti-Oxidant, Anti-Enzymatic and Cytotoxic Effects of Artemisia verlotiorum Lamotte Extracts: A New Source of Bioactive Agents" Molecules 27, no. 18: 5886. https://doi.org/10.3390/molecules27185886
APA StyleSuroowan, S., Llorent-Martínez, E. J., Zengin, G., Dall’Acqua, S., Sut, S., Buskaran, K., Fakurazi, S., & Mahomoodally, M. F. (2022). Phytochemical Characterization, Anti-Oxidant, Anti-Enzymatic and Cytotoxic Effects of Artemisia verlotiorum Lamotte Extracts: A New Source of Bioactive Agents. Molecules, 27(18), 5886. https://doi.org/10.3390/molecules27185886









