Cold-Active Enzymes and Their Potential Industrial Applications—A Review
Abstract
1. Introduction
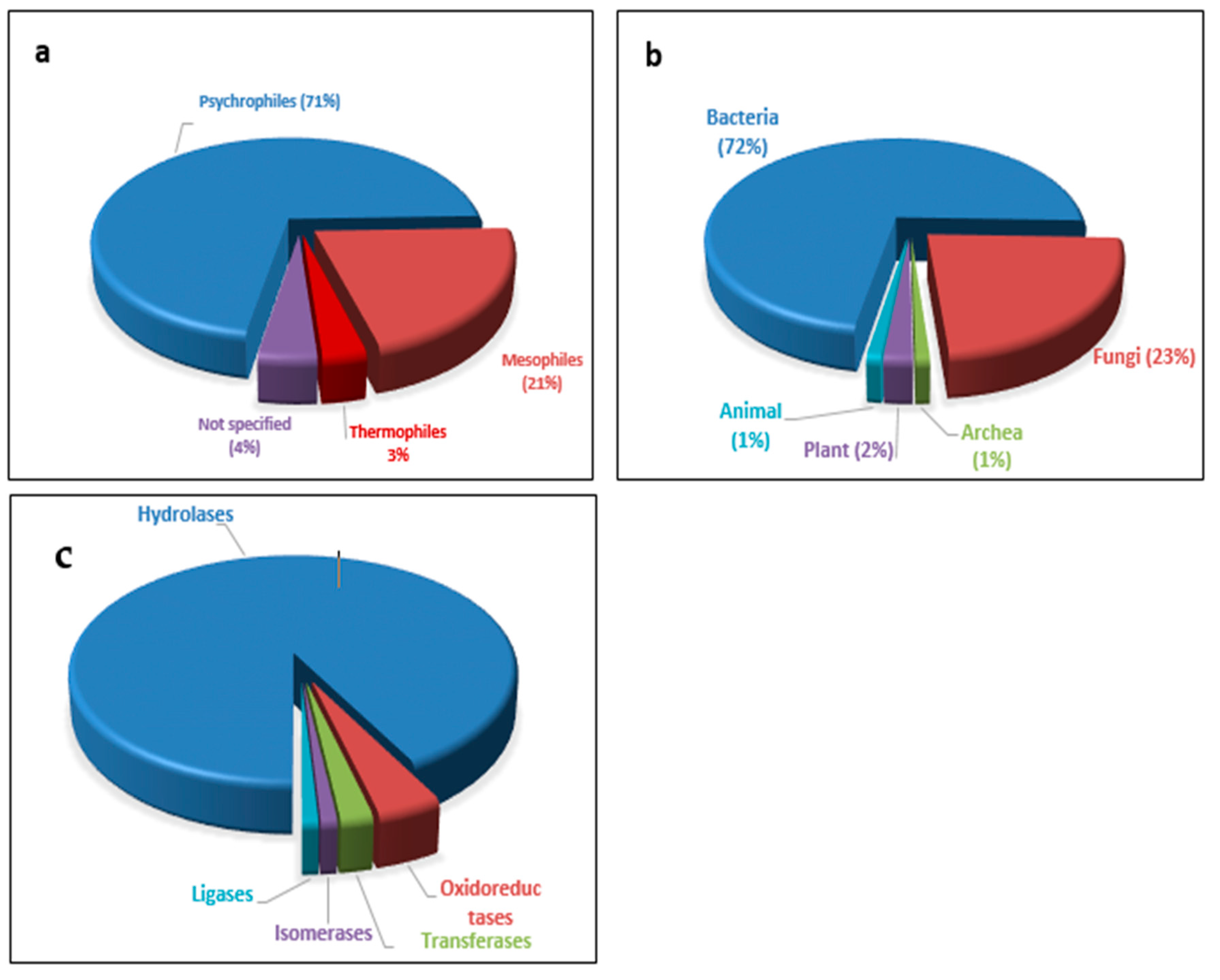
2. Psychrophiles: Habitat and Potentiality
3. Biotechnological Importance of Cold-Active Enzymes
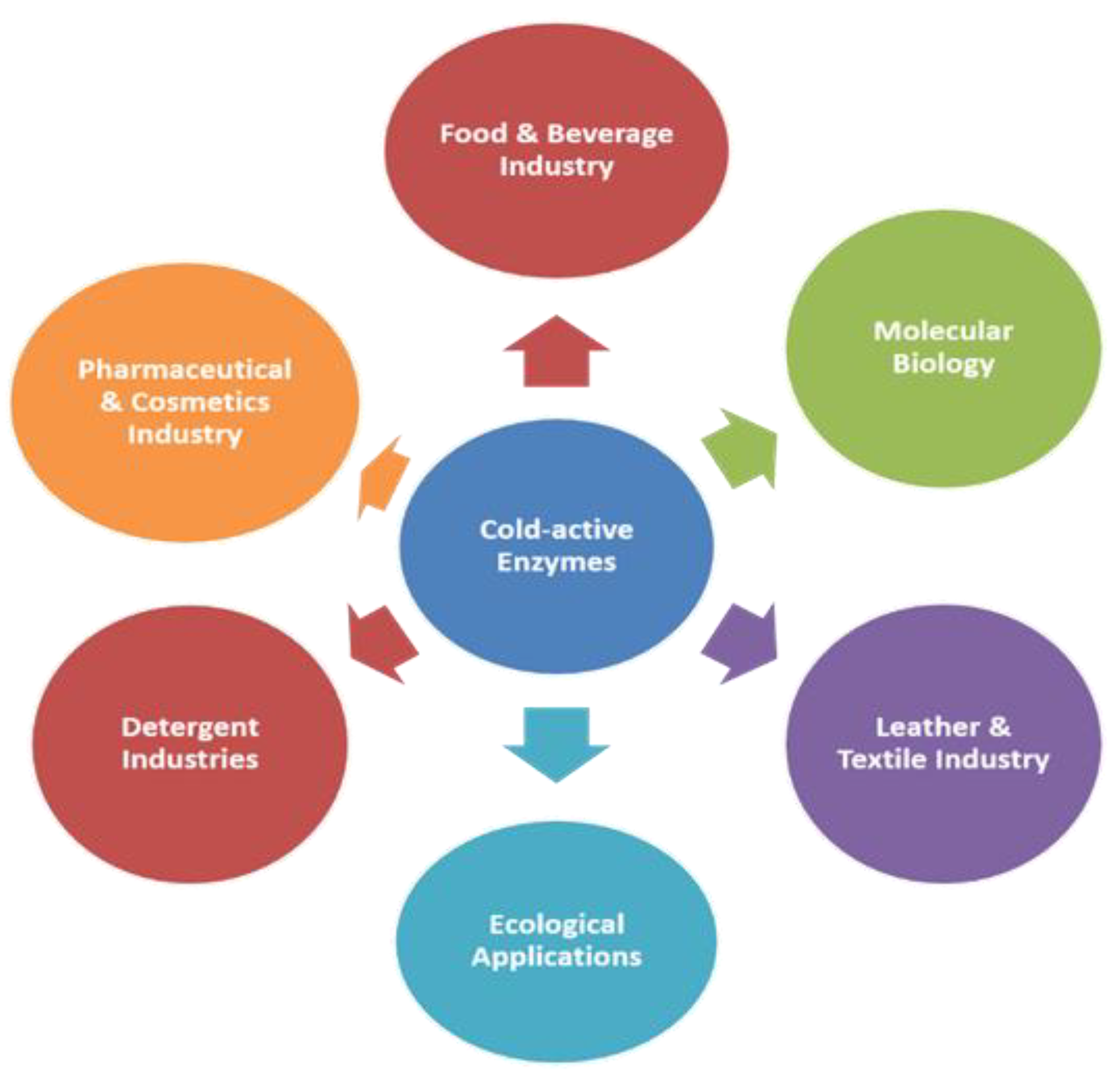
4. Scope of Cold-Active Enzymes in Industries
4.1. Food and Brewing Industry
4.2. Detergent and Cleaning Industry
4.3. Pharmaceutical, Medicine, and Cosmetics
4.4. Molecular Biology
4.5. Bioremediation or Ecological Applications
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- De Mayer, P.; Anderson, D.; Cary, C.; Cowan, D.A. Some like it cold: Understanding the survival strategies of psychrophiles. EMBO Rep. 2014, 15, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.; Margesin, R. Psychrophilic lifestyles: Mechanisms of adaptation and biotechnological tools. Appl. Microbiol. Biotechnol. 2019, 103, 2857–2871. [Google Scholar] [CrossRef] [PubMed]
- Vallesi, A.; Pucciarelli, S.; Buonanno, F.; Fontana, A.; Mangiagalli, M. Bioactive molecules from protists: Perspectives in biotechnology. Eur. J. Protistol. 2020, 75, 125720. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.N.; Verma, P.; Kumar, M.; Pal, K.K.; Dey, R.; Gupta, A.; Padaria, J.C.; Gujar, G.T.; Kumar, S.; Suman, A.; et al. Diversity and phylogenetic profiling of niche-specific bacilli from extreme environments of India. Ann. Microbiol. 2015, 65, 611–629. [Google Scholar] [CrossRef]
- Morita, R.Y. Marine psychrophilic bacteria ocean over. Mar. Biol. Ann. Rev. 1996, 4, 105–121. [Google Scholar]
- Farrell, J.; Rose, A.H. Temperature effects on microorganisms. In Thermobiology; Rose, A.H., Ed.; Academic Press: London, UK, 1967; pp. 147–218. [Google Scholar]
- Bakermans, C. Determining the limits of microbial life at sub-zero temperatures. In Psychrophiles: From Biodiversity to Biotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 21–38. [Google Scholar]
- Rivkina, E.; Abramov, A.; Spirina, E.; Petrovskaya, L.; Shatilovich, A.; Shmakova, L.; Scherbakova, V.; Vishnivetskaya, T. Earth’s perennially frozen environments as a model of cryogenic planet ecosystems. Perm. Periglac. Proc. 2018, 29, 246–256. [Google Scholar] [CrossRef]
- Margesin, R.; Collins, T. Microbial ecology of the cryosphere (glacial and permafrost habitats): Current knowledge. Appl. Microbiol. Biotechnol. 2019, 103, 2537–2549. [Google Scholar] [CrossRef]
- Margesin, R.; Miteva, V. Diversity and ecology of psychrophilic microorganisms. Res. Microbiol. 2011, 162, 346–361. [Google Scholar] [CrossRef]
- Mangiagalli, M.; Lotti, M. Cold-Active β-Galactosidases: Insight into Cold Adaptation Mechanisms and Biotechnological Exploitation. Mar. Drugs 2021, 19, 43. [Google Scholar] [CrossRef]
- Kuddus, M. Cold-active enzymes in food biotechnology: An updated mini review. J. Appl. Biol. Biotechnol. 2018, 6, 58–63. [Google Scholar]
- Wackett, L.P. Microbial industrial enzymes: An annotated selection of World Wide Web sites relevant to the topics in microbial biotechnology. Microb. Biotechnol. 2019, 12, 1090–1091. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.; Ramírez-Sarmiento, C.A.; Zamora, R.A.; Parra, L.P. Discovery, molecular mechanisms, and industrial applications of cold-active enzymes. Front. Microbiol. 2016, 7, 1408. [Google Scholar] [CrossRef]
- Stokes, J.L. General biology and nomenclature of psychrophilic microorganisms. In Recent Progress in Microbiology VIII; University of Toronto Press: Toronto, ON, Canada, 1963; pp. 187–192. [Google Scholar]
- Robinson, C.H. Cold adaptation in the Arctic and Antarctic fungi. New Phytol. 2001, 151, 341–353. [Google Scholar] [CrossRef]
- Troncoso, E.; Barahona, S.; Carrasco, M.; Villarreal, P.; Alcaíno, J.; Cifuentes, V.; Baeza, M. Identification and characterization of yeasts isolated from the South Shetland Islands and the Antarctic Peninsula. Polar Biol. 2017, 40, 649–658. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Tortsen, T. Extremophilic. In Encyclopaedia of Microbiology, 2nd ed.; Lederberg, J., Ed.; Academic Press: London, UK, 2000; pp. 317–337. [Google Scholar]
- Buzzini, P.; Margesin, R. Cold-Adapted Yeasts: Biodiversity, Adaptation Strategies, and Biotechnological Significance; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Bakermans, C.; Skidmore, M.L. Microbial metabolism in ice and brine at −5 degrees C. Environ. Microbiol. 2011, 13, 2269–2278. [Google Scholar] [CrossRef]
- Mykytczuk, N.C.; Foote, S.J.; Omelon, C.R.; Southam, G.; Greer, C.W.; Whyte, L.G. Bacterial growth at -15 degrees C; molecular insights from the permafrost bacterium planococcushalocryophilus Or1. ISME J. 2013, 7, 1211–1226. [Google Scholar] [CrossRef]
- Hamid, B.; Singh, P.; Rana, R.S.; Sahay, S. Isolation and identification of psychrophilic yeast from the soil of the northern region of India. Inventi Rapid Pharm. Biotechnol. Microbiol. 2012, 1, 1–5. [Google Scholar]
- D’Amico, S.; Claverie, P.; Collins, T.; Georlette, D.; Gratia, E.; Hoyoux, A.; Meuwis, M.A.; Feller, G.; Gerday, C. Molecular basis of cold adaptation. R. Soc. 2002, 357, 917–925. [Google Scholar] [CrossRef]
- Furhan, J. Adaptation, production, and biotechnological potential of cold-adapted proteases from psychrophiles and psychrotrophic: A recent overview. J. Genet. Eng. Biotechnol. 2020, 18, 36. [Google Scholar] [CrossRef]
- Suman, A.; Verma, P.; Yadav, A.N.; Saxena, A.K. Bioprospecting for extracellular hydrolytic enzymes from culturable thermotolerant bacteria isolated from Manikaran thermal springs. Res. J. Biotechnol. 2015, 10, 33–42. [Google Scholar]
- Verma, P.; Yadav, A.N.; Shukla, L.; Saxena, A.K.; Suman, A. Hydrolytic enzymes produced by thermotolerant Bacillus altitudinisIARI-MB-9 and Gulbenkianiamobilis IARI-MB-18 isolated from Manikaran hot springs. Int. J. Adv. Res. 2015, 3, 1241–1250. [Google Scholar]
- Kumar, V.; Yadav, A.N.; Saxena, A. Unraveling rhizospheric diversity and potential of phytase-producing microbes. SM J. Biol. 2016, 2, 1009. [Google Scholar]
- Kumar, V.; Yadav, A.N.; Verema, P. β-Propeller phytases: Diversity, catalytic attributes, current developments, and potential biotechnological applications. Int. J. Biol. Macromol. 2017, 98, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Saxena, A.; Sangwan, P. Production and characterization of a neutral phytase of Penicillium oxalicum EUFR-3 isolated from the Himalayan region. Nusant. Biosci. 2017, 9, 68–76. [Google Scholar] [CrossRef]
- Gerday, C.M.; Aittaleb, M.; Bentahier, J.P.; Chessa, P.; Claverie, T. Collins Cold-adapted enzymes: From fundamentals to biotechnology. Trends Biotechnol. 2000, 18, 103–107. [Google Scholar] [CrossRef]
- Connell, L.; Redman, R.; Craig, S.; Scorzetti, G.; Iszard, M.; Rodriguez, R. Diversity of soil yeasts isolated from South Victoria land, Antarctica. Microb. Ecol. 2008, 56, 448–459. [Google Scholar] [CrossRef]
- Joshi, S.; Satyanarayana, T. Biotechnology of cold-active proteases. Biology 2013, 2, 755–783. [Google Scholar] [CrossRef]
- Margesin, R.; Feller, G. Biotechnological applications of psychrophiles. Environ. Technol. 2010, 31, 835–844. [Google Scholar] [CrossRef]
- Duarte, A.W.F.; Dayo-Owoyemi, I.; Nobre, F.S.; Pagnocca, F.C.; Chaud, L.C.S.; Pessoa, A.; Felipe, M.G.A.; Sette, L.D. Taxonomic assessment and enzyme production by yeasts isolated from marine and terrestrial Antarctic samples. Extremophiles 2013, 17, 1023–1035. [Google Scholar] [CrossRef]
- Sahay, S.; Hamid, B.; Singh, P.; Ranjan, K.; Chauhan, D.; Rana, R.S.; Chaurse, V.K. Evaluation of pectinolytic activities for oenological uses from psychrotrophic yeasts. Lett. Appl. Microbiol. 2013, 57, 115–121. [Google Scholar] [CrossRef]
- Hamid, B.; Rana, R.S.; Chauhan, D.; Singh, P.; Mohiddin, F.A.; Sahay, S.; Abidi, I. Psychrophilic yeasts and their biotechnological applications—A review. Afr. J. Biotechol. 2014, 13, 2188–2197. [Google Scholar]
- Gounot, A.M. Bacterial Life at low temperature: Physiological aspects and biotechnological implications. J. Appl. Bacteriol. 1991, 71, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Munoz, P.A.; Marquez, S.L.; Gonzalez-Nilo, F.D.; Marquez-Miranda, V.; Blamey, J.M. Structure and application of antifreeze proteins from Antarctic bacteria. Microb. Cell Fact. 2017, 16, 138. [Google Scholar] [CrossRef] [PubMed]
- Tribelli, P.M.; Lopez, N.I. Reporting key features in cold-adapted bacteria. Life 2018, 8, 8. [Google Scholar] [CrossRef]
- Kasana, R.C. Proteases from psychrotrophs: An overview. Crit. Rev. Microbiol. 2010, 36, 134–145. [Google Scholar] [CrossRef]
- Staff, B.R. Global Markets for Enzymes in Industrial Applications; BCC Research LLC: Wellesley, MA, USA, 2018. [Google Scholar]
- Chen, J. Food Enzymes: Global Markets; BCC Research: Wellesley, MA, USA, 2018. [Google Scholar]
- Feller, G.; Narinx, E.; Arpigny, J.L.; Aittaleb, M.; Baise, E.; Genicot, S.; Gerday, C. Enzymes from psychrophilic organisms. FEMS Microbiol. Rev. 1996, 18, 189–202. [Google Scholar] [CrossRef]
- Sarmiento, F.; Peralta, R.; Blamey, J.M. Cold, and hot extremozymes: Industrial relevance and current trends. Front. Bioeng. Biotechnol. 2015, 3, 148. [Google Scholar] [CrossRef]
- Bruno, S.; Coppola, D.; di Prisco, G.; Giordano, D.; Verde, C. Enzymes from Marine Polar Regions and Their Biotechnological Applications. Mar. Drugs 2019, 17, 544. [Google Scholar] [CrossRef]
- Mangiagalli, M.; Brocca, S.; Orlando, M.; Lotti, M. The “cold revolution”. Present and future applications of cold-active enzymes and ice-binding proteins. New Biotechnol. 2020, 55, 5–11. [Google Scholar] [CrossRef]
- Gupta, S.K.; Kataki, S.; Chatterjee, S.; Prasad, R.K.; Datta, S.; Vairale, M.G.; Sharma, S.; Dwivedi, S.K.; Gupta, D.K. Cold adaptation in bacteria with a special focus on cellulase production and its potential application. J. Clean. Prod. 2020, 258, 120351. [Google Scholar] [CrossRef]
- Gurung, N.; Ray, S.; Bose, S.; Rai, V. A broader view: Microbial enzymes and their relevance in industries, medicine, and beyond. Biomed. Res. Int. 2013, 3, 329121. [Google Scholar] [CrossRef] [PubMed]
- Marx, J.C.; Collins, T.; D’Amico, S.; Feller, G.; Gerday, C. Cold-adapted enzymes from marine Antarctic microorganisms. Mar. Biotechnol. 2007, 9, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Cavicchioli, R.; Charlton, T.; Ertan, H.; Mohd Omar, S.; Siddiqui, K.S.; Williams, T.J. Biotechnological uses of enzymes from psychrophiles. Microb. Biotechnol. 2011, 4, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Feller, G. Psychrophilic enzymes: From folding to function and biotechnology. Scientifica 2013, 2013, 512840. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.J.; Hills, M.; Gunton, J.; Nano, F. Heat-labile proteases in molecular biology applications. FEMS Microbiol. Ecol. 2001, 197, 59–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barroca, M.; Santos, G.; Gerday, C.; Collins, T. Biotechnological Aspects of Cold-Active Enzymes. In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Ed.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 461–475. [Google Scholar]
- Cavicchioli, R.; Siddiqui, K.S.; Andrews, D.; Sowers, K.R. Low-temperature extremophiles and their applications. Curr. Opin. Biotechnol. 2002, 13, 253–261. [Google Scholar] [CrossRef]
- Margesin, R.; Feller, G.; Gerday, C.; Russell, N. Cold-Adapted Microorganisms: Adaptation Strategies and Biotechnological Potential. In Encyclopedia of Environmental Microbiology; Bitton, G., Ed.; John Wiley & Sons: New York, NY, USA, 2002; pp. 871–885. [Google Scholar]
- Feller, G.; Gerday, C. Psychrophilic enzymes: Hot topics in cold adaptation. Nat. Rev. Microbiol. 2003, 1, 200–208. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K. Mechanism of bacterial adaptation to low temperature—A review. J. Biosci. 2006, 31, 157–165. [Google Scholar] [CrossRef]
- Berini, F.; Casciello, C.; Marcone, G.L.; Marinelli, F. Metagenomics: Novel enzymes from non-culturable microbes. FEMS Microbiol. Lett. 2017, 364, fnx211. [Google Scholar] [CrossRef]
- Gerlt, J.A. Genomic enzymology: Web tools for leveraging protein family sequence-function space and genome context to discover novel functions. Biochemistry 2017, 56, 4293–4308. [Google Scholar] [CrossRef] [PubMed]
- Ziemert, N.; Alanjary, M.; Weber, T. The evolution of genome mining in microbes-a review. Nat. Prod. Rep. 2016, 33, 988–1005. [Google Scholar] [CrossRef] [PubMed]
- Gerike, U.; Danson, M.J.; Hough, D.W. Cold-active citrate synthase: Mutagenesis of active-site residues. Protein Eng. 2001, 14, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Goomber, S.; Kumar, R.; Singh, R.; Mishra, N.; Kaur, J. Point mutation Gln121-Arg increased temperature optima of Bacilluslipase (1.4 subfamily) by fifteen degrees. Int. J. Biol. Macromol. 2016, 88, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hou, Y.; Xu, Z.; Miao, J.; Li, G. Optimization of cold-active protease production by the psychrophilic bacterium Colwellia sp. NJ341 with response surface methodology. Bioresour. Technol. 2008, 99, 1926–1931. [Google Scholar] [CrossRef]
- Horner, T.W.; Dunn, M.L.; Eggett, D.L.; Ogden, L.V. The beta-galactosidase activity of commercial lactase samples in raw and pasteurized milk at refrigerated temperatures. J. Dairy Sci. 2011, 94, 3242–3249. [Google Scholar] [CrossRef]
- Van de Voorde, I.; Goiris, K.; Syryn, E.; Van den Bussche, C.; Aerts, G. Evaluation of the cold-active Pseudoalteromonas haloplanktis β-galactosidase enzyme for lactose hydrolysis in whey permeate as a primary step of d-tagatose production. Process Biochem. 2014, 49, 2134–2140. [Google Scholar] [CrossRef]
- Hamid, B.; Singh, P.; Mohiddin, F.A.; Sahay, S. Partial characterization of cold-active β-galactosidase activity produced by Cystofilobasidium capitatum SPY11 and Rhodotorula mucilaginosa PT1. J. Endocytobiosis Cell Res. 2013, 24, 23–26. [Google Scholar]
- Mateo, C.; Monti, R.; Pessela, B.C.; Fuentes, M.; Torres, R.; Manuel Guisán, J.; Fernández-Lafuente, R. Immobilization of lactase from Kluyveromyceslactis greatly reduces the inhibition promoted by glucose. Full hydrolysis of lactose in milk. Biotechnol. Prog. 2004, 20, 1259–1262. [Google Scholar] [CrossRef]
- Qais, A.; Maqtari, A.; Waleed, A.L.; Mahdi, A.A. Cold-active enzymes and their applications in industrial fields—A review. Int. J. Res. Agric. Sci. 2019, 6, 2348–3997. [Google Scholar]
- Pulicherla, K.K.; Mrinmoy Ghosh, P.; Suresh, K.; Sambasiva Rao, K.R.S. Psychrozymes—The next generation industrial enzymes. J. Mar. Sci. Res. Dev. 2011, 1, 1–7. [Google Scholar] [CrossRef]
- Su, H.; Mai, Z.; Yang, J.; Xiao, Y.; Tian, X.; Zhang, S. Cloning, expression, and characterization of a cold-active and organic solvent tolerant lipase from Aeromicrobium sp. SCSIO 25071. J. Microbiol. Biotechnol. 2016, 26, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Mageswari, A.; Subramanian, P.; Chandrasekaran, S.; Karthikeyan, S.; Gothandam, K.M. Systematic functional analysis and application of a cold-active serine protease from a novel Chryseobacterium sp. Food Chem. 2017, 217, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Ramya, L.N.; Pulicherla, K.K. Molecular insights into cold-active polygalacturonase enzyme for its potential application in food processing. J. Food Sci. Technol. 2015, 52, 5484–5496. [Google Scholar] [CrossRef]
- Singh, P.; Hamid, B.; Ahmad Lone, M.; Ranjan, K.; Khan, A.; Chaurse, V. Evaluation of pectinase activity from the psychrophilic fungal strain Truncatella angustata-BPF5 for use in the wine industry. Endocytobiosis Cell Res. 2012, 22, 57–61. [Google Scholar]
- Coker, J.A.; Sheridan, P.P.; Lovel, C.J.; Gutshall, K.R.; Auman, A.J.; Brenchley, J.E. Biochemical characterization of a β-galactosidase with a low-temperature optimum obtained from an Antarctic Arthrobacter isolate. J. Bacteriol. 2003, 185, 5473–5482. [Google Scholar] [CrossRef] [PubMed]
- Kuddus, M.; Saima, R.; Ahmad, I.Z. Cold-active extracellular α-amylase production from novel bacteria Microbacterium foliorum GA2 and Bacillus cereus GA6 isolated from Gangotri glacier, Western Himalaya. J. Genet. Eng. Biotechnol. 2012, 10, 151–159. [Google Scholar] [CrossRef]
- Qin, Y.; Huang, Z.; Liu, Z. A novel cold-active and salt-tolerant alpha-amylase from marine bacterium Zunongwangia profunda: Molecular cloning, heterologous expression, and biochemical characterization. Extremophile 2014, 18, 271–281. [Google Scholar] [CrossRef]
- Wierzbicka-Wos, A.; Cieslinski, H.; Wanarska, M.; Kozlowska-Tylingo, K.; Hildebrandt, P.; Kur, J. A novel cold-active b-D-galactosidase from the Paracoccus sp. 32d-gene cloning, purification, and characterization. Microb. Cell Fact. 2011, 10, 108–120. [Google Scholar] [CrossRef]
- He, Z.; Zhang, L.; Mao, Y.; Gu, J.; Pan, Q.; Zhou, S.; Gao, B.; Wei, D. Cloning of a novel thermostable glucoamylase from thermophilic fungus Rhizomucor pusillus and high-level co-expression with α-amylase in Pichia pastoris. BMC Biotechnol. 2014, 14, 114. [Google Scholar] [CrossRef]
- He, L.; Mao, Y.; Zhang, L.; Wang, H.; Alias, S.A.; Gao, B. Functional expression of a novel α-amylase from Antarctic psychrotolerant fungus for the baking industry and its magnetic immobilization. BMC Biotechnol. 2017, 17, 22. [Google Scholar] [CrossRef]
- Arabaci, N.; Arikan, B. Isolation and characterization of a cold-active, alkaline, detergent stable α-amylase from a novel bacterium Bacillus subtilis N8. Prep. Biochem. Biotechnol. 2018, 48, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Yin, Y.; Zhang, L.; Alias, S.S.; Gao, B.; Wei, D. Development of a novel Aspergillus uracil deficient expression system and its application in expressing a cold-adapted α-amylase gene from Antarctic fungi Geomycespannorum. Process. Biochem. 2015, 50, 1581–1590. [Google Scholar] [CrossRef]
- Dhaulaniya, A.S.; Balan, B.; Kumar, M.; Agrawal, P.K.; Singh, D.K. Cold survival strategies for bacteria, recent advancement, and potential industrial applications. Arch. Microbiol. 2019, 201, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, N.; Tewari, R.; Hoondal, G. Biotechnological aspects of chitinolytic enzymes: A review. Appl. Microbiol. Biotechnol. 2006, 71, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Ramli, A.N.; Mahadi, N.M.; Rabu, A.; Murad, A.M.; Bakar, F.D.; Illias, R.M. Molecular cloning, expression and biochemical characterization of a cold-adapted novel recombinant chitinase from Glaciozyma antarctica PI12. Microb. Cell Fact. 2011, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, M.; Rozas, J.M.; Barahona, S.; Alcaı’no, J.; Cifuentes, V.; Baeza, M. Diversity and extracellular enzymatic activities of yeasts isolated from King George Island, the sub-Antarctic region. BMC Microbiol. 2012, 12, 251. [Google Scholar] [CrossRef]
- Tutino, M.L.; Di Prisco, G.; Marino, G.; Pascale, D. Cold-adapted esterases and lipases: From fundamentals to application. Protein Pept. Lett. 2009, 16, 1172–1180. [Google Scholar]
- Kavitha, M.; Shanthi, C. Alkaline thermostable cold-active lipase from halotolerant Pseudomonas sp. VITCLP4 as detergent additive. Indian J. Biotechnol. 2017, 6, 446–455. [Google Scholar]
- Sahay, S.; Chouhan, D. Study on the potential of cold-active lipases from psychrotrophic fungi for detergent formulation. J. Genet. Biotechnol. 2018, 16, 319–325. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Yan, Q.; Jiang, Z. Gene cloning, functional expression, and characterization of a novel glycogen branching enzyme from Rhizomucor miehei and its application in wheat bread making. Food Chem. 2014, 159, 85–94. [Google Scholar] [CrossRef]
- Huang, H.; Luo, H.; Wang, Y.; Fu, D.; Shao, N.; Yang, P. Novel low-temperature-active phytase from Erwinia carotovora var. carotovota ACCC 10276. J. Microbiol. Biotechnol. 2009, 19, 1085–1091. [Google Scholar] [CrossRef]
- Ranjan, K.; Sahay, S. Identification of phytase-producing yeast and optimization and characterization of extracellular phytase from Candida parapsilosis. Int. J. Sci. Nat. 2013, 4, 583–590. [Google Scholar]
- Yu, P.; Wang, X.T.; Liu, J.W. Purification and characterization of a novel cold-adapted phytase from strain JMUY14 isolated from the Antarctic: Characterization of a novel cold-adapted phytase. J. Basic Microbiol. 2015, 55, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Nagaoka, T.; Miyaji, T.; Tomizuka, N. Cold-active Polygalacturonase from psychrophilic-basidiomycetous yeast Cystofilobasidium capitatum strain PPY-1. Biosci. Biotechnol. Biochem. 2005, 69, 419–421. [Google Scholar] [CrossRef]
- Pan, X.; Tu, T.; Wang, L.; Luo, H.; Ma, R.; Shi, P. A novel low-temperature active pectin methylesterase from Penicillium chrysogenum F46 with high efficiency in fruit firming. Food Chem. 2014, 162, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Conchillo, L.B.; Ruiz, J.; Navascue’s, E.; Marquina, D.; Santos, A. Selection and use of pectinolytic yeasts for improving clarification and phenolic extraction in winemaking. Int. J. Food Microbiol. 2016, 223, 1–8. [Google Scholar] [CrossRef]
- Collins, T.; Gerday, C.; Feller, G. Xylanases, xylanase families, and extremophilic xylanases. FEMS Microbiol. Rev. 2005, 29, 3–23. [Google Scholar] [CrossRef]
- Borges, T.A.; de Souza, A.T.; Squina, F.M.; Riaño-Pacho’n, D.M.; Corrêa dos Santos, R.A.; Machado, E.; Velasco de Castro Oliveira, J.; Dama’sio, A.R.L.; Goldman, G.H. Biochemical characterization of an endoxylanase from Pseudozymabrasiliensis sp. nov. strain GHG001 isolated from the intestinal tract of Chrysomelidae larvae associated to sugarcane roots. Process Biochem. 2014, 49, 77–83. [Google Scholar] [CrossRef]
- Cai, Z.W.; Hui-Hua, G.; Zhi-Wei, Y.; Run-Ying, Z.; Guang-Ya, Z. Characterization of a Novel Psychrophilic and Halophilic β-1, 3-xylanase From Deep-Sea Bacterium, Flammeovirga Pacifica Strain WPAGA1. Int. J. Biol. Macromol. 2018, 118, 2176–2184. [Google Scholar] [CrossRef]
- Miri, S.; Naghdi, M.; Rouissi, T.; Kaur Brar, S.; Martel, R. Recent biotechnological advances in petroleum hydrocarbons degradation under cold climate conditions: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 553–586. [Google Scholar] [CrossRef]
- Margesin, R.; Dieplinger, H.; Hofmann, J.; Sarg, B.; Lindner, H. A cold-active extracellular metalloprotease from Pedobactercryoconitis—production and properties. Res. Microbiol. 2005, 156, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Saba, I.; Qazi, P.H.; Rather, S.A.; Dar, R.A.; Qadri, Q.A.; Ahmad, N.; Johri, S.; Taneja, S.C.; Shawl, S. Purification and characterization of a cold-active alkaline protease from Stenotrophomonas sp., isolated from Kashmir, India. World J. Microbiol. Biotechnol. 2012, 28, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Furhan, J.; Awasthi, P.; Sharma, S. Biochemical characterization and homology modeling of cold-active alkophilic protease from Northwestern Himalayas and its application in detergent industry. Biocatal. Agric. Biotechnol. 2019, 17, 726–735. [Google Scholar] [CrossRef]
- Furhan, J.; Salaria, N.; Jabeen, M.; Qadri, J. Partial purification and characterization of cold-active metalloprotease by Bacillus sp. AP1 from Apharwat peak, Kashmir. Pak. J. Biotechnol. 2019, 16, 47–54. [Google Scholar] [CrossRef]
- Tamaki, H.; Hanada, S.; Kamagata, Y.; Nakamura, K.; Nomura, N.; Nakano, K.; Matsumura, M. Flavobacterium limicola sp. nov., a psychrophilic, organic-polymer-degrading bacterium isolated from freshwater sediments. Int. J. Syst. Evol. Microbiol. 2003, 53, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Salwan, R.; Gulati, A.; Kasana, R.C. Phylogenetic diversity of alkaline protease-producing psychrotrophic bacteria from the glacier and cold environments of Lahaul and Spiti, India. J. Basic Microbiol. 2010, 50, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Białkowska, A.M.; Szulczewska, K.M.; Krysiak, J.; Florczak, T.; Gromek, E.; Kassassir, H.; Kur, J.; Turkiewicz, M. Genetic and biochemical characterization of yeasts isolated from Antarctic soil samples. Polar Biol. 2017, 40, 1787–1803. [Google Scholar] [CrossRef]
- Ojha, B.K.; Singh, P.K.; Shrivastava, N. Enzymes in the Animal Feed Industry. In Enzymes in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 93–109. [Google Scholar]
- Hauksson, J.B.; Andresson, O.S.; Asgeirsson, B. Heat-labile bacterial alkaline phosphatase from a marine Vibrio sp. Enzyme. Microb. Technol. 2000, 27, 66–73. [Google Scholar] [CrossRef]
- Georlette, D.; Jonsson, Z.O.; Petegem, F.V.; Chessa, J.P.; Beeumen, J.V.; Hubscher, U.; Gerday, C. A DNA ligase from the psychrophile Pseudoalteromonas haloplanktis gives insight into the adaptation of proteins to low temperatures. Eur. J. Biochem. 2000, 267, 3502–3512. [Google Scholar] [CrossRef]
- Uma, S.; Jadhav, R.S.; Seshu-Kumar, G.; Shivaji, S.; Ray, M.K. An RNA polymerase with transcriptional activity at 0 °C from the Antarctic bacterium. FEBS Lett. 1999, 453, 313–317. [Google Scholar] [CrossRef]
- Siddiqui, K.S. Some like it hot, some like it cold: Temperature-dependent biotechnological applications and improvements in extremophilic enzymes. Biotechnol. Adv. 2015, 33, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, N.R.; Littler, D.; Beddoe, T.; Rossjohn, J.; Illias, R.M.; Mahadi, N.M.; Mackeen, M.M.; Murad, A.M.A.; Abu Bakar, F.D. Crystal structure of fuculosealdolase from the Antarctic psychrophilic yeast Glaciozyma antarctica PI12. Acta Crystallogr. F 2016, 72, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Kobus, S.; Widderich, N.; Hoeppner, A.; Bremer, E.; Smits, S.H.J. Overproduction, crystallization and X-ray diffraction data analysis of ectoine synthase from the cold-adapted marine bacterium Sphingopyxis alaskensis. Acta Crystallogr. F 2015, 71, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Widderich, N.; Kobus, S.; Höppner, A.; Riclea, R.; Seubert, A.; Dickschat, J.S.; Heider, J.; Smits, S.H.; Bremer, E. Biochemistry and crystal structure of ectoinesynthase: A metal-containing member of the cup in the superfamily. PLoS ONE 2016, 11, e0151285. [Google Scholar] [CrossRef]
- Ishibashi, M.; Yamashita, S.; Tokunaga, M. Characterization of Halophilic Alkaline Phosphatase from Halomonas sp. 593, a Moderately Halophilic Bacterium. Biosci. Biotechnol. Biochem. 2005, 69, 1213–1216. [Google Scholar] [CrossRef]
- Wojciechowski, C.L.; Cardia, J.P.; Kantrowitz, E.R. Alkaline phosphatase from the hyperthermophilic bacterium T. maritima requires cobalt for activity. Protein Sci. 2002, 11, 903–911. [Google Scholar] [CrossRef]
- Kumari, U.; Singh, R.; Ray, T.; Rana, S.; Saha, P.; Malhotra, K.; Daniell, H. Validation of leaf enzymes in the detergent and textile industries: Launching of a new platform technology. Plant Biotechnol. J. 2019, 17, 1167–1182. [Google Scholar] [CrossRef]
- Al-Ghanayem, A.A.; Joseph, B. Current perspective in using cold-active enzymes as eco-friendly detergent additive. Appl. Microbiol. Biotechnol. 2020, 104, 2871–2882. [Google Scholar] [CrossRef]
- Gupta, R.; Beg, Q.; Lorenz, P. Bacterial alkaline proteases: Molecular approaches and industrial applications. Appl. Microbiol. Biotechnol. 2002, 59, 15–32. [Google Scholar]
- Chen, K.; Mo, Q.; Liu, H.; Yuan, F.; Chai, H.; Lu, F.; Zhang, H. Identification and characterization of a novel cold-tolerant extracellular protease from Planococcus sp. CGMCC 8088. Extremophiles 2018, 22, 473–484. [Google Scholar] [CrossRef]
- Tariq, A.; Reyaz, A.; Prabakaran, J.J. Purification and characterization of 56 KDa cold-active protease from Serratia marcescens. Afr. J. Microbiol. Res. 2011, 5, 5841–5847. [Google Scholar] [CrossRef]
- Salwan, R.; Kasana, R.C. Purification and characterization of an extracellular low temperature-active and alkaline stable peptidase from psychrotrophic Acinetobacter sp. MN 12 MTCC (10786). Indian J. Microbiol. 2013, 53, 63–69. [Google Scholar] [CrossRef]
- Hao, J.H.; Sun, M. Purification and characterization of a cold alkaline protease from a psychrophilic Pseudomonas aeruginosa HY1215. Appl. Biochem. Biotechnol. 2015, 175, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.M.; Kumar, R.; Panwar, S.; Kumar, A. Microbial alkaline proteases: Optimization of production parameters and their properties. J. Genet. Eng. Biotechnol. 2017, 15, 115–126. [Google Scholar] [CrossRef]
- Nielsen, P.H. Life cycle assessment supports cold-wash enzymes. Int. J. Appl. Sci. 2005, 10, 24–26. [Google Scholar]
- Marshall, R.T.; Goff, H.D.; Hartel, R.W. Ice Cream; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003; pp. 1–11. [Google Scholar]
- Struvay, C.; Feller, G. Optimization to Low-Temperature Activity in Psychrophilic Enzymes. Int. J. Mol. Sci. 2012, 13, 11643–11665. [Google Scholar] [CrossRef]
- Karan, R.; Capes, M.D.; Das Sarma, S. Function and biotechnology of extremophilic enzymes in low water activity. Aquat. Biol. 2012, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Sondavid, K.N.; Shweta, B.B.; Jun, H.L.; Hak, J.K. Taking advantage of promiscuity of cold-active enzymes. Appl. Sci. 2020, 10, 8128. [Google Scholar]
- Novak, H.R.; Sayer, C.; Panning, J.; Littlechild, J.A. Characterization of an L-Haloacid Dehalogenase from the Marine Psychrophile Psychromonas ingrahamii with Potential Industrial Application. Mar. Biotechnol. 2013, 15, 695–705. [Google Scholar] [CrossRef]
- Huston, A.L. Biotechnological aspects of cold-adapted enzymes. In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Schinner, F., Marx, J.C., Gerday, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 347–363. [Google Scholar]
- Trincone, A. Marine Biocatalysts: Enzymatic Features and Applications. Mar. Drugs 2011, 9, 478–499. [Google Scholar] [CrossRef]
- Pawlak-Szukalska, A.; Wanarska, M.; Popinigis, A.T.; Kur, J. A novel cold-active β-d-galactosidase with transglycosylation activity from the Antarctic Arthrobacter sp. 32cB—Gene cloning, purification, and characterization. Process Biochem. 2014, 49, 2122–2133. [Google Scholar] [CrossRef]
- Fornbacke, M.; Clarsund, M. Cold-adapted proteases as an emerging class of therapeutics. Infect. Dis. Ther. 2013, 2, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Cazarin, C.; Lima, G.; da Silva, J.; Maro’stica, M. Enzymes in meat processing. In Enzymes in Food and Beverage Processing; Chandrasekaran, M., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 337–351. [Google Scholar]
- Balabanova, L.A.; Bakunina, I.Y.; Nedashkovskaya, O.I.; Makarenkova, I.D.; Zaporozhets, T.S.; Besednova, N.N.; Zvyagintseva, T.N.; Rasskazov, V.A. Molecular characterization and therapeutic potential of a marine bacterium Pseudoalteromonas sp. KMM 701 α-galactosidase. Mar. Biotechnol. 2010, 12, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Çelik, A.; Yetis, G. An unusually cold-active nitro reductase for prodrug activations. Bioorg. Med. Chem. 2012, 20, 3540–3550. [Google Scholar] [CrossRef]
- Kobori, H.; Sullivan, C.W.; Shizuya, H. Heat-labile alkaline phosphatase from Antarctic bacteria: Rapid 5’ end-labeling of nucleic acids. Proc. Natl. Acad. Sci. USA 1984, 81, 6691–6695. [Google Scholar] [CrossRef]
- Lanes, O.; Leiros, I.; Smalås, A.O.; Willassen, N.P. Identification, cloning, and expression of uracil-DNA glycosylase from Atlantic cod (Gadusmorhua): Characterization and homology modeling of the cold-active catalytic domain. Extremophiles 2002, 6, 73–86. [Google Scholar] [CrossRef]
- Awazu, N.; Shodai, T.; Takakura, H.; Kitagawa, M.; Mukai, H.; Kato, I. Microorganism-Derived Psychrophilic Endonuclease. U.S. Patent 8,034,597 B2, 11 October 2011. [Google Scholar]
- Muller-Greven, J.C.; Post, M.A.; Kubu, C.J. Recombinant Colwellia psychrerythraea Alkaline Phosphatase and Uses Thereof. U.S. Patent US8486665B2, 16 July 2013. U.S. Patent US8129168B2, 3 June 2012. [Google Scholar]
- Rina, M.; Pozidis, C.; Mavromatis, K.; Tzanodaskalaki, M.; Kokkinidis, M.; Bouriotis, V. Alkaline phosphatase from the Antarctic strain TAB5. Properties and psychrophilic adaptations. Eur. J. Biochem. 2000, 267, 1230–1238. [Google Scholar] [CrossRef]
- Bjerga, G.E.; Lale, R.; Williamson, A.K. Engineering low-temperature expression systems for heterologous production of cold-adapted enzymes. Bioengineered 2016, 7, 33–38. [Google Scholar] [CrossRef]
- Ferrer, M.; Chernikova, T.N.; Timmis, K.N.; Golyshin, P.N. Expression of a temperature-sensitive esterase in a novel chaperone-based Escherichia coli strain. Appl. Environ. Microbiol. 2004, 70, 4499–4504. [Google Scholar] [CrossRef]
- Kim, H.W.; Wi, A.R.; Jeon, B.W.; Lee, J.H.; Shin, S.C.; Park, H.; Jeon, S.J. Cold adaptation of a psychrophilic chaperonin from Psychrobacter sp. and its application for heterologous protein expression. Biotechnol. Lett. 2015, 37, 1887–1893. [Google Scholar] [CrossRef]
- Esteban-Torres, M.; Mancheno, J.M.; de las Rivas, B.; Munoz, R. Characterization of a cold-active esterase from Lactobacillus plantarum suitable for food fermentations. J. Agric. Food Chem. 2014, 62, 5126–5132. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.S.; Deming, J.W. Alkane hydroxylase genes in psychrophile genomes and the potential for cold-active catalysis. BMC Genom. 2014, 15, 1120. [Google Scholar] [CrossRef]
- Kumar, L.; Bharadvaja, N. Chapter 6—Enzymatic bioremediation: A smart tool to fight environmental pollutants. In Smart Bioremediation Technologies; Bhatt, P., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 99–118. [Google Scholar]
- Margesin, R.S.; Gander, G.; Zacke, A.M.; Gounot, A.M.; Schinner, F. Hydrocarbon degradation and enzyme activities of cold-adapted bacteria and yeasts. Extremophiles 2003, 7, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, L.; Vazquez, S.; Lobalbo, A.; Mac Cormack, W. Psychrotolerant hydrocarbon-degrading Rhodococcus strains isolated from polluted Antarctic soils. Antarct. Sci. 2005, 17, 47–56. [Google Scholar] [CrossRef]
- Aislabie, J.; Saul, D.J.; Foght, J.M. Bioremediation of hydrocarbon-contaminated polar soils. Extremophiles 2006, 10, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yang, B.; Shen, J.; Du, N. Biodegradation of crude oil by an Arctic psychrotrophic bacterium Pseudoalteromomas sp. P29. Curr. Microbiol. 2009, 59, 341–345. [Google Scholar] [CrossRef]
| Cold-Active Enzyme | Source of Isolation | Potential Applications | References |
|---|---|---|---|
| Lactases/β-galactosidase | Paracoccus sp., Cystofilobasidium capitatum SPY11, Rhodotorula sp. | Dairy industry (lactose hydrolysis) | [11,45,66,77] |
| α-Amylases | Geomycespannorum, Bacillus subtilis N8, Geomyces pannorum | Food, baking, and detergent industries | [78,79,80] |
| Cellulases | Pyrococcus sp. | Food and textile industry, ethanol fermentation | [81,82] |
| Chitinases | Metschnikowia sp., Glaciozyma antarctica, Mrakia psychrophila, Sporobolomyces salmonicolor | Meat tenderization, degradation of chitin rich wastes, control of phytopathogens | [83,84,85] |
| Lipases | Pseudoalteromonas haloplanktis TAC125, Penicillium canesense, Pseudomonas sp. VITCLP4 | Animal feed, detergent, and textile industries | [86,87,88] |
| Glycogen branching enzyme | Rhizomucor miehei | Wheat bread manufacturing and baking industry | [89] |
| Phytases | Erwinia carotovora, Candida carpophila, Cryptococcus laurentii, Yarrowia lipolytica | Food and feed industry | [90,91,92] |
| Pectinases (polygalacturonase and pectin-methylesterase) | Cystofilobasidium capitatum PPY-1, Rhodotorula mucilaginosa PT1, Cystofilobasidium capitatum SPY11, Leucosporidium drummii, Sporobolomyces salmonicolor, Penicillium chrysogenum F46 | Food and fruit industries, pectin degradation, juice extraction | [35,93,94,95] |
| Xylanases | Pseudoalteromonas haloplanktis TAH3A, Flavobacterium sp. MSY-2, Rhodococcus sp., Pseudomonas sp., Flammeovirga pacifica WPAGA1, Cryptococcus adeliensis | Baking industry, xylan hydrolysis, osmoprotectants, biofuel production | [96,97,98,99] |
| Metalloproteases | Pedobacter cryoconitis | Bioremediation of wastewater at a lower temperature | [100] |
| Alkaline protease | Stenotrophomonas sp., Bacillus subtilis WLCP1 | Detergent and textile industries | [101,102,103] |
| Proteases | Flavobacterium limicola, Acinetobacter sp., Geomyces pannorum, Naganishia albida | Textile and leather industries, organic polymer mineralization in freshwater sediments, food and feed industry | [104,105,106,107] |
| Serine proteases | Pseudoalteromonas sp. | Low-temperature food processing, leather industry | [63] |
| Alkaline phosphates | Vibrio sp. | Molecular biology | [108] |
| DNA ligase | Pseudoalteromonas haloplanktis | Molecular biology and recombinant DNA technology | [109] |
| RNA polymerase | Pseudomonas syringae | Molecular biology | [110] |
| Fuculose aldolase | Glaciozyma antarctica PI12 | Pharmaceutical industry | [111,112] |
| Ectoine synthase | Sphingopyxis alaskensis | Cosmetics, biomedical industry | [113,114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamid, B.; Bashir, Z.; Yatoo, A.M.; Mohiddin, F.; Majeed, N.; Bansal, M.; Poczai, P.; Almalki, W.H.; Sayyed, R.Z.; Shati, A.A.; et al. Cold-Active Enzymes and Their Potential Industrial Applications—A Review. Molecules 2022, 27, 5885. https://doi.org/10.3390/molecules27185885
Hamid B, Bashir Z, Yatoo AM, Mohiddin F, Majeed N, Bansal M, Poczai P, Almalki WH, Sayyed RZ, Shati AA, et al. Cold-Active Enzymes and Their Potential Industrial Applications—A Review. Molecules. 2022; 27(18):5885. https://doi.org/10.3390/molecules27185885
Chicago/Turabian StyleHamid, Burhan, Zaffar Bashir, Ali Mohd Yatoo, Fayaz Mohiddin, Neesa Majeed, Monika Bansal, Peter Poczai, Waleed Hassan Almalki, R. Z. Sayyed, Ali A. Shati, and et al. 2022. "Cold-Active Enzymes and Their Potential Industrial Applications—A Review" Molecules 27, no. 18: 5885. https://doi.org/10.3390/molecules27185885
APA StyleHamid, B., Bashir, Z., Yatoo, A. M., Mohiddin, F., Majeed, N., Bansal, M., Poczai, P., Almalki, W. H., Sayyed, R. Z., Shati, A. A., & Alfaifi, M. Y. (2022). Cold-Active Enzymes and Their Potential Industrial Applications—A Review. Molecules, 27(18), 5885. https://doi.org/10.3390/molecules27185885










