Abstract
Hydroxymethylthiohydantoin, hydroxymethylthiohydantoin, and hydantoin, containing a pyridine group, were synthesized to study their androgen receptor antagonistic activities. Among them, compounds 6a/6c/7g/19a/19b exhibited excellent androgen receptor antagonistic activity, which was consistent with or even superior to enzalutamide. In addition, compounds 19a and 19b exhibited better antiproliferative activity than enzalutamide in prostate cancer cells. The results show that compound 19a has great potential as a new AR antagonist.
1. Introduction
Prostate cancer, fueled by the androgen axis, is a major public health problem and has a very high leading cause of death among men worldwide [1]. Although androgen deprivation therapy (ADT) has been proved to be effective initially, the tumor will eventually progress and develop into the lethal castration-resistant prostate cancer (CRPC) [2]. Most often, death occurs 2 to 4 years after the onset of the castration-resistant state. However, the over-expression of AR was found in most CRPCs, which is essential for CRPC to adapt to the low levels of androgen. As AR receptor activation plays a crucial role in the progression of CRPC, it has been recognized as an attractive target for the treatment of CRPC [3].
AR antagonists are currently the mainstay of treatments for prostate cancer [4]. Bicalutamide, enzalutamide and apalut salts are marketed as nonsteroidal antiandrogens [5,6,7]. As a first-generation nonsteroidal AR antagonist, bicalutamide diminishes androgenic effects by competitively inhibiting androgen–AR binding. However, due to the occurrence of LBD point mutations and the expression of active AR splice variants, these antiandrogens may become partial AR agonists after a period of treatment (~2 years) [5]. (Figure 1).
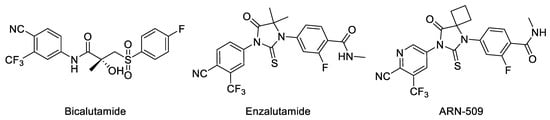
Figure 1.
Structures of known AR antagonists.
Enzalutamide and apalutamide (ARN-509) are second-generation nonsteroidal antiandrogens with high-affinity binding for the AR LBD. In particular, enzalutamide received FDA approval in 2012 for the treatment of patients with metastatic castration-resistant PC who have previously received docetaxel [8,9]. However, a F876L missense mutation in the AR LBD has been shown to confer resistance to enzalutamide and apalutamide (ARN-509) by switching their activity on ARs from antagonist to agonist [10,11]. (Figure 1).
Enzalutamide and apalutamide (ARN-509) are very similar in structure and function to each other. The two agents are potent AR antagonists, which both have a high affinity for the AR. Both of them bind to AR and inhibit androgen-mediated gene transcription in AR-overexpressing prostate cancer cells, but also impair the nuclear localization of AR and DNA binding. However, in murine xenograft models of mCRPC, apalutamide demonstrated greater antitumor activity than enzalutamide. Besides that, apalutamide penetrates less effectively in the BBB (blood–brain barrier) than enzalutamide, suggesting that the chance of developing seizures may be less than with enzalutamide [12,13].
In this work, enzalutamide was used as the lead compound for structural modification. The structural transformation is as follows: (1) the aromatic ring on one side of hydroxymethyl thiohydantoin was transformed to contain both p-CN and m-CF5, and an active derivatized group of enzalutamide was connected to the aromatic ring on the other side, which has been proven to have good properties [14,15,16]; (2) the sulfur atom in the hydroxymethylthiohydantoin was replaced by an oxygen atom, which was shown to have unexpected effects in our previous work [17]; (3) the aromatic ring on the right side of the hydroxymethylthiohydantoin ring was replaced by a pyridine ring, which also contains both p-CN and m-SF5. A total of 30 compounds with CF3 groups were designed and synthesized, and their in vitro activities were tested. (Figure 2).
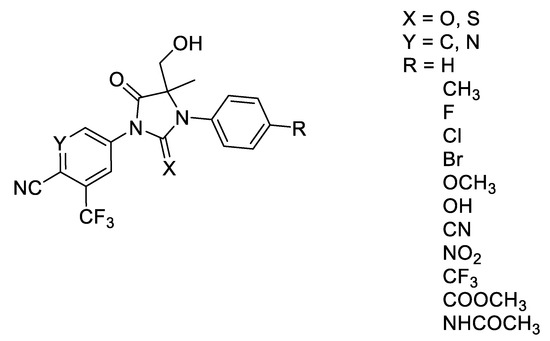
Figure 2.
Design of nonsteroidal androgen receptor antagonists.
2. Results and Discussion
2.1. Chemistry
The design of novel antiandrogen compounds was performed to explore several different chemical modifications around the thiohydanthoin and hydantoin scaffolds as depicted in Scheme 1, Scheme 2 and Scheme 3.
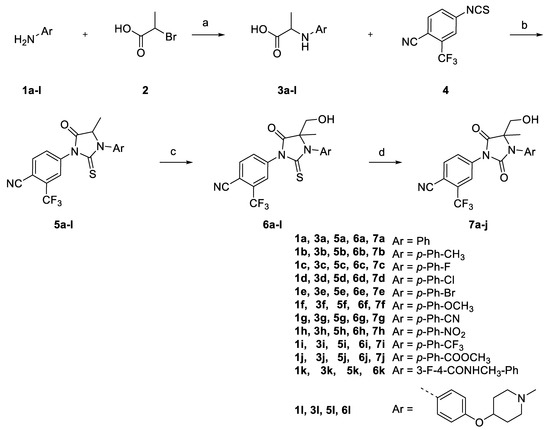
Scheme 1.
Synthesis of antiandrogen derivatives (±)-6a-l, (±)-7a-j. Reagents and conditions: (a) TEA, DCM, rt; (b) TEA, CHCl3, reflux; (c) LiHMDS, HCHO, −78 °C to rt; (d) NaIO4, RuCl3, CCl4, MeCN, Water, rt.
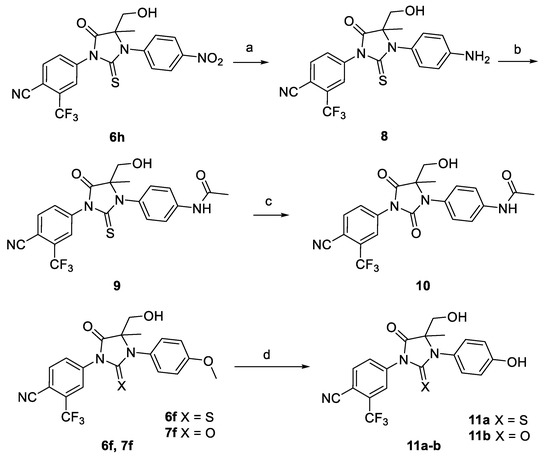
Scheme 2.
Synthesis of antiandrogen derivatives (±)-9, 10, (±)-11a-b. Reagents and conditions: (a) Fe, HCl, EtOH, reflux; (b) Acetyl chloride, THF, −5 °C; (c) NaIO4, RuCl3, CCl4, MeCN, Water, rt; (d) BBr3, DCM, rt.
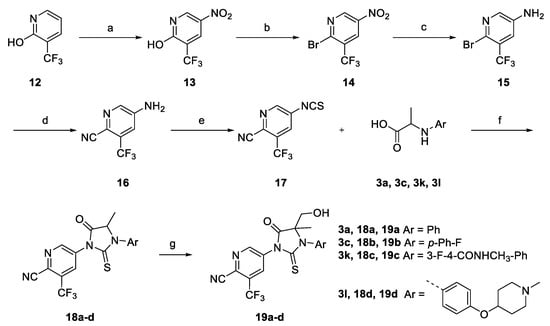
Scheme 3.
Synthesis of antiandrogen derivatives (±)-19a-d. Reagents and conditions: (a) HNO3, H2SO4, rt; (b) POBr3, DMF, 110 °C; (c) Fe, AcOH, EA, reflux; (d) CuCN, NMP, 160 °C; (e) thiophosgene, CCl4/water, rt; (f) TEA, CHCl3, reflux; (g) LiHMDS, HCHO, −78 °C to rt.
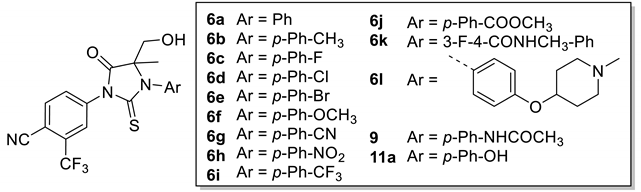
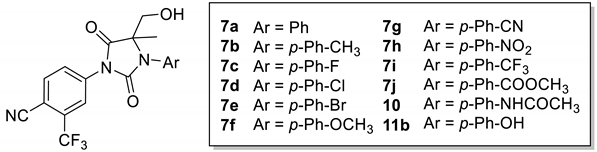
Various compounds (±)-6a-k, (±)-7a-j were synthesized as shown in Scheme 1 by deprotonation–hydroxyalkylation of the carbon of the thiohydanthoin ring and the conversion of thiohydantoin to the corresponding hydantoin. The alkylation of commercially available correponding anilines 1a-k by 2-bromopropionic acid was carried out to obtain compounds 3a-k in yields ranging from 20% to 88%. Immediate cyclization with 4-isothiocyanato-2-(trifluoromethyl)benzonitrile provided phenylthiohydanthoins 5a-k as racemic mixtures in good yield (72–76%). The kithiation of 5a-k with lithium bis(trimethylsilyl)amide at −78 °C was followed by the addition of paraformaldehyde to provide a mixture of diastereoisomeric compounds (±)-6a-k, respectively (in 69–81% yield). Then, various thiohydantoins (±)-6a-k were transformed to the corresponding hydantoins (±)-7a-j in excellent yield (76–80%), via sodium periodate and cat. Ruthenium (III) chloride.
As depicted in Scheme 2, the reduction of 6h with Fe/HCl produced compound 8 (91% yield). The compound 8 was acetylated with acetyl chloride to give the final product (±)-9 in 54% yield. Then, thiohydantoin (±)-9 was transformed to hydantoin (±)-10 in 41% yield, via sodium periodate and catalytic ruthenium trichloride. Compounds (±)-11a,b were obtained in a high yield (40–78%) from the methoxy derivatives ((±)-6f, 6f) by demethylation using boron tribromide (Scheme 2).
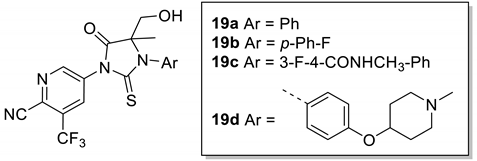
Compounds 19a-c were synthesized according to Scheme 3. The key intermediate 17 was generated from the commercially available compound 1 via nitration, bromination, reduction and cyanation (in 60% yield) [13]. Compound 16 reacted with thiophosgene to form the key intermediate thioisocyanate 17 [17]. Cyclizing 3a, 3c and 3k with 17 followed by TEA afforded the compounds 18a-c. The lithiation of 18a-c with lithium bis(trimethylsilyl)amide at −78 °C was followed by addition of paraformaldehyde to provide a mixture of diastereoisomeric final products (±)-19a-c, respectively (in 46–71% yield).
2.2. Antagonist Affinity of Compounds 6a-l, 7a-j, 9, 10, 11a-b and 19a-d
We examined the AR antagonist activity of the representative compounds 6a-l, 7a-j, 9, 10, 11a-b and 19a-d with a luciferase gene reporter assay in mouse myoblast CV-1 cells.
As shown in Table 1, a series of hydroxymethylthiohydantoin-like SARM compounds exhibited different degrees of AR antagonistic activity. Fortunately, the compounds 6a and 6c are comparable to enzalutamide in both their antagonistic activity and efficacy (IC50 = 46.8, 46.2, 42.82 nM). What’s more, when the aromatic ring contains a methyl or methoxy group such as 6b and 6f, the antagonistic activity is significantly decreased (IC50 = 548.5, 286.2 nM), which also indicates that the donor group on the aromatic ring has a negative effect on the antagonistic activity of the compound. A slight decrease in antagonistic activity was also observed when either chlorine or bromine was attached to the aromatic ring (6d, 6e, IC50 = 79.27, 116.6 nM). When electron-withdrawing groups such as cyano groups, nitro groups, and ester groups are linked to the aromatic ring, it does not show a positive effect on the antagonistic activity of the compound (6j, 6h, 6j, IC50 = 338.4, 79,616, 531.9 nM). However, the trifluoromethyl-group-containing 6i (IC50 = 68.15 nM) retained the same order of magnitude of antagonistic activity as enzalutamide. No increase in antagonistic activity was observed when 6k, containing both fluorine and acyl groups on the aromatic ring, was investigated, nor for 6l, which contains a heterocycloalkoxy group on the aromatic ring (6k, 6l, IC50 = 125.75, 337.35 nM). Compoumd 9 and 11a, containing acyl or hydroxyl groups on the aromatic ring, although inferior to EM, still show a certain antagonistic activity (9, 11a, IC50 = 938.8, 3641 nM).

Table 1.
Bioactivity of compounds 6a-l, 9 and 11a.
As shown in Table 2, after replacing the sulfur atoms on thiohydantoin with oxygen atoms, although no derivatives were found to be more active than the positive compounds, more compounds showed the same magnitude of antagonistic activity as enzalutamide.

Table 2.
Bioactivity of compounds 7a-j, 10 and 11b.
To improve affinity and activity, the methylene group (CH) at the ortho position of the aryl nitrile was replaced with a nitrogen (N) atom, activating the cyano group on the aryl group to form a reversible covalent bond with the endogenous cysteine (Cys784) within the AR ligand-binding pocket. The results in Table 3 show that this strategy was effective. Compound 19a exhibits excellent antagonistic activity (19a, IC50 = 18.4 nM), and the antagonistic activity of compound 19b is almost the same as that of enzalutamide (19b, IC50 = 32.45 nM). However, the antagonistic activity of 19c is inferior to that of 6k (Table 1), which is exactly the opposite of that of 19d, which better than 6l (Table 1).

Table 3.
Bioactivity of compounds 19a-d.
2.3. Anti-Proliferative Activity of Compounds 6a, 6c, 19a and 19b in Prostate Cancer Cell Lines
We performed experiments accordingly to detect the anti-proliferative effect of compounds using the prostate cancer LNCaP cell line. The measurement of proliferative activity was performed after treatment with a concentration range of each compound for 72 h. As can be seen from Table 4, all four compounds exhibited good antiproliferative activity, especially 19b; its inhibitory effect on the proliferation of LNCAP cell line is even better than that of enzalutamide.

Table 4.
Anti-proliferative activity of compounds 6a, 6c, 19a and 19b.
In addition, by using Schrödinger to study the binding mode of compound 19b to AR (PDB ID: 2OZ7), it was found that the binding posture of 19b was very similar to that of enzalutamide. The critical interactions of 19b with the critical residuals are shown in Figure 3a. The cyanyl group of 19b forms an important hydrogen bond with GLY708, Gln711, and TRP741 via a water molecule, similar to enzalutamide and apalutamide (Figure 3b,c). There is a π–π interaction between the pyridine ring and Phe764, which is similar to apalutamide (Figure 3b). Enzalutamide and apalutamide can form a hydrogen bond with ARG779 due to the presence of an acyl group. Obviously, the absence of an acyl group in 19b hinders the formation of this hydrogen bond. However, the extra hydroxyl group in 19b can form a hydrogen bond with ASN705 in the AR, which may be the reason for the high potency of 19b toward AR.
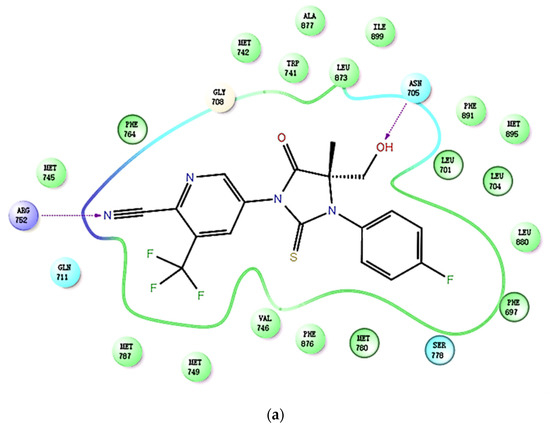
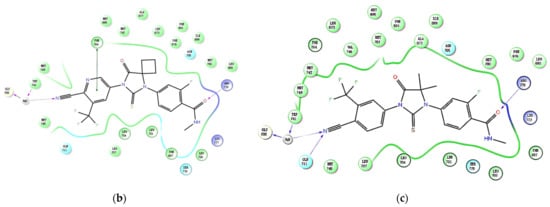
Figure 3.
The predicted binding modes and 2D diagram of (a) compound 19b; (b) apalutamide and (c) enzalutamide in complex with AR (PDB ID: 2OZ7).
3. Conclusions
In this work, a series of hydroxymethylthiohydantoins, hydroxymethylhydantoins and pyridine SARMs were synthesized, and the remarkable AR antagonistic activities of these compounds were revealed by in vitro cell experiments. Among them, compounds 6a, 6c, 6d, 6i, 7a-e, 19a-b, etc., exhibited the same magnitude of antagonistic activity as enzalutamide. In addition, compounds 6a, 6c, 19a and 19b exhibited good anti-proliferative activity. The inhibitory effect of 19b on the proliferation of the LNCAP cell line is even better than that of enzalutamide.
4. Experimental Sections
4.1. Materials and Methods
All reagents are commercially available and were used without further purification. The the solvents used were of analytical grade. Melting points were taken on a Fisher–Johns melting point apparatus, uncorrected and reported in degrees Centigrade. 1H NMR and 13C NMR spectra were scanned on a Bruker DRX-400 (400 MHz) using tetramethylsilane (TMS) as an internal standard and using one or two of the following solvents, DMSO-d6 and CDCl3. Chemical shifts are given in δ, ppm. Splitting patterns were designated as follows: s: singlet; d: doublet; t: triplet; q: quartet; m: multiplet. The mass spectra (MS) were recorded on a Finnigan MAT-95 mass spectrometer. The purity of all tested compounds was established by HPLC to be >95.0%. HPLC analysis was performed at room temperature using an Agilent Eclipse XDBC18 (250 mm × 4.6 mm) and as a mobile phase gradient from 5% MeCN/H2O (1‰ TFA) for 1 min, 5% MeCN/H2O (1‰ TFA) to 95% MeCN/H2O (1‰ TFA) for 9 min and 95% MeCN/H2O (1‰ TFA) for 5 min more, with a flow rate of 1.0 mL/min and plotted at 254 nm (See Supplementary Materials).
4.2. General Synthesis
4.2.1. General Procedure for the Synthesis of 3a-l
In a 100 mL round-bottomed flask, we added amines (1.0 eq), 2-bromopropanoic acid (1.5 eq) and TEA (3.0 eq) in 150 mL DCM to give a colorless suspension. The reaction mixture was held at room temperature with stirring on for 3 days. The mixture was concentrated by rotovap. One-hundred milliliters water was added. An amount of 60 mL 2M HCl(aq) was added to adjusted pH to 5. The aq layer was extracted with EA. The organic was dried Na2SO4, filt and conc to give crude product. Then, 20 mL DCM and 50 mL Et2O was added. The reaction mixture was filtered through a sintered glass funnel with 50 mL Et2O to give 3a-l.
Phenylalanine (3a)
White solid (4.5 g, 51% yield). m.p. 157–159 °C; 1H NMR (400 MHz, DMSO) δ 7.07 (t, J = 7.8 Hz, 2H), 6.61−6.50 (m, 3H), 3.94 (q, J = 7.0 Hz, 0H), 1.37 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 175.85, 147.65, 128.80, 116.28, 112.41, 50.98, 45.49, 18.11, 8.45.
p-tolylalanine (3b)
White solid (2.4 g, 29% yield). m.p. 152–154 °C; 1H NMR (400 MHz, DMSO) δ 6.88 (d, J = 8.2 Hz, 2H), 6.47 (d, J = 8.3 Hz, 2H), 3.90 (q, J = 7.0 Hz, 1H), 2.14 (s, 3H), 1.35 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 175.97, 145.37, 129.24, 124.73, 112.62, 51.27, 20.01, 18.15.
(4-fluorophenyl)alanine (3c)
White solid (2.7 g, 33% yield). m.p. 150–161 °C; 1H NMR (400 MHz, DMSO) δ 6.91 (t, J = 8.8 Hz, 2H), 6.54 (dd, J = 8.9, 4.5 Hz, 2H), 3.90 (q, J = 7.0 Hz, 1H), 1.36 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 175.81, 155.60, 153.31, 144.49, 115.26, 115.04, 113.16, 113.09, 51.40, 18.12.
(4-chlorophenyl)alanine (3d)
White solid (4.2 g, 55% yield). m.p. 143–145 °C; 1H NMR (400 MHz, DMSO) δ 7.09 (d, J = 8.8 Hz, 2H), 6.55 (d, J = 8.8 Hz, 2H), 3.93 (q, J = 7.0 Hz, 1H), 1.36 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 175.52, 146.70, 128.49, 119.48, 113.73, 50.98, 17.99.
(4-bromophenyl)alanine (3e)
White solid (5.2 g, 73% yield). m.p. 138–140 °C; 1H NMR (400 MHz, DMSO) δ 12.53 (s, 1H), 7.29−7.11 (m, 2H), 6.58−6.45 (m, 2H), 3.92 (q, J = 7.0 Hz, 1H), 1.36 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 175.49, 147.10, 131.31, 114.28, 106.83, 50.89, 17.98.
(4-methoxyphenyl)alanine (3f)
White solid (3.8 g, 48% yield). m.p. 150–152 °C; 1H NMR (400 MHz, DMSO) δ 8.89 (s, 1H), 6.71 (d, J = 8.8 Hz, 2H), 6.52 (d, J = 8.8 Hz, 2H), 3.87 (q, J = 6.9 Hz, 1H), 3.62 (s, 3H), 1.34 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 176.15, 151.02, 141.92, 114.49, 113.53, 55.21, 51.71, 18.26.
(4-cyanophenyl)alanine (3g)
White solid (5.0 g, 62% yield). m.p. 144–146 °C; 1H NMR (400 MHz, DMSO) δ 12.73 (s, 1H), 7.47 (d, J = 8.9 Hz, 2H), 6.91 (s, 1H), 6.64 (d, J = 8.8 Hz, 2H), 4.07 (d, J = 6.7 Hz, 1H), 1.40 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 174.79, 151.34, 133.30, 120.39, 112.21, 96.45, 50.40, 17.74.
(4-nitrophenyl)alanine (3h)
Yellow solid (3.3 g, 44% yield). m.p. 148–150 °C; 1H NMR (400 MHz, DMSO) δ 12.83 (s, 1H), 8.01 (d, J = 9.0 Hz, 2H), 7.45 (d, J = 7.5 Hz, 1H), 6.65 (d, J = 9.0 Hz, 2H), 4.17 (p, J = 7.0 Hz, 1H), 1.43 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 174.35, 153.68, 136.25, 126.04, 111.24, 50.61, 17.68.
(4-(trifluoromethyl)phenyl)alanine (3i)
White solid (4.3 g, 83% yield). m.p. 197–199 °C; 1H NMR (400 MHz, DMSO) δ 12.69 (s, 1H), 7.39 (d, J = 8.5 Hz, 2H), 6.66 (d, J = 8.5 Hz, 2H), 6.62 (d, J = 5.0 Hz, 1H), 4.04 (s, 1H), 1.40 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 175.15, 150.84, 129.26, 126.58, 126.18, 126.14, 126.11, 126.07, 123.90, 121.22, 116.30, 115.99, 115.67, 115.35, 111.69, 50.60, 17.85.
(4-(methoxycarbonyl)phenyl)alanine (3j)
White solid (2.6 g, 88% yield). m.p. 85–87 °C; 1H NMR (400 MHz, DMSO) δ 12.69 (s, 1H), 7.38 (d, J = 8.5 Hz, 2H), 6.63 (d, J = 8.5 Hz, 2H), 6.42 (d, J = 5.0 Hz, 1H), 4.04 (q, J = 6.9 Hz, 1H), 3.89 (s, 3H), 1.36 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 175.15, 165.91, 150.84, 129.26, 126.58, 126.18, 126.14, 126.11, 126.07, 123.90, 121.22, 116.30, 115.99, 115.67, 115.35, 111.69, 50.62, 46.21, 17.75.
(3-fluoro-4-(methylcarbamoyl)phenyl)alanine (3k)
White solid (2.7 g, 83% yield). m.p. 217–219 °C; 1H NMR (400 MHz, DMSO) δ 12.69 (s, 1H), 7.64 (s, 1H), 7.48 (t, J = 8.7 Hz, 1H), 6.70 (s, 1H), 6.44 (d, J = 8.5 Hz, 1H), 6.31 (d, J = 14.6 Hz, 1H), 4.04 (s, 1H), 2.74 (d, J = 4.3 Hz, 3H), 1.38 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 174.97, 163.84, 162.37, 159.93, 151.86, 151.74, 131.40, 109.76, 109.62, 108.34, 98.11, 97.83, 50.65, 26.21, 17.79.
(4-((1-methylpiperidin-4-yl)oxy)phenyl)alanine (3l)
White solid (1.6 g, 60% yield). m.p. 104–106 °C; 1H NMR (400 MHz, DMSO) δ 6.75 (d, J = 8.7 Hz, 2H), 6.50 (d, J = 8.7 Hz, 2H), 4.22 (s, 1H), 3.80 (q, J = 6.8 Hz, 1H), 2.98 (s, 2H), 2.73 (s, 2H), 2.50 (s, 3H), 1.94 (s, 2H), 1.75 (s, 2H), 1.32 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 176.62, 147.76, 142.81, 117.83, 113.35, 70.62, 52.07, 50.74, 43.48, 28.55, 18.45.
4.2.2. General Procedure for the Synthesis of 5a-l and 18a-d
In a 100 mL round-bottomed flask, we added 4 or 17 (1.0 eq) and TEA (1.5 eq) in 30 mL CHCl3 to give a yellow solution. The reaction vessel was purged with nitrogen. The reaction was heat to 65 °C with stirring on for 1 hr. The reaction mixture was cooled to 25 °C with stirring on. Then, 3a-l (1.0 eq) was added. The reaction was heated to 65 °C with stirring for 16 h. The mixture was concentrated by rotovap. The crude product was purified by column chromatography to give 5a-l and 18a-d.
4-(4-methyl-5-oxo-3-phenyl-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (5a)
White solid (1.24 g, 50% yield). m.p. 199–201 °C; 1H NMR (400 MHz, CDCl3) δ 7.96 (d, J = 7.8 Hz, 2H), 7.84 (d, J = 8.3 Hz, 1H), 7.53 (t, J = 7.5 Hz, 2H), 7.44 (dd, J = 12.9, 7.3 Hz, 3H), 4.73 (q, J = 7.0 Hz, 1H), 1.55 (d, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 179.81, 171.95, 137.17, 136.19, 135.29, 134.05, 133.72, 133.38, 133.05, 132.32, 129.82, 129.63, 129.06, 127.18, 123.27, 122.70, 120.55, 114.88, 110.19, 61.20, 15.94.
4-(4-methyl-5-oxo-2-thioxo-3-(p-tolyl)imidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (5b)
White solid (750 mg, 88% yield). m.p. 192–194 °C; 1H NMR (400 MHz, CDCl3) δ 7.97 (d, J = 7.5 Hz, 2H), 7.84 (dd, J = 8.3, 1.6 Hz, 1H), 7.33 (d, J = 8.5 Hz, 2H), 7.29 (d, J = 8.5 Hz, 2H), 4.69 (q, J = 7.0 Hz, 1H), 2.42 (s, 3H), 1.55 (d, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 179.87, 172.04, 139.32, 137.17, 135.25, 133.75, 133.52, 133.41, 132.27, 130.47, 127.23, 127.18, 127.13, 127.08, 126.95, 123.25, 120.53, 114.84, 110.20, 61.25, 21.25, 15.93.
4-(3-(4-fluorophenyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (5c)
White solid (1.2 g, 58% yield). m.p. 189–191 °C; 1H NMR (400 MHz, CDCl3) δ 8.04−7.92 (m, 2H), 7.83 (d, J = 8.0 Hz, 1H), 7.41 (dd, J = 8.7, 4.7 Hz, 2H), 7.22 (t, J = 8.4 Hz, 2H), 4.69 (q, J = 7.0 Hz, 1H), 1.57 (d, J = 7.2 Hz, 4H). 13C NMR (101 MHz, CDCl3) δ 180.15, 171.71, 163.57, 161.08, 137.02, 135.29, 133.83, 133.50, 132.19, 132.07, 129.29, 129.20, 127.07, 117.07, 116.84, 114.77, 110.33, 61.21, 15.96.
4-(3-(4-chlorophenyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (5d)
White solid (1.5 g, 66% yield). m.p. 153–155 °C; 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 8.5 Hz, 1H), 7.95 (d, J = 1.6 Hz, 1H), 7.82 (dd, J = 8.3, 2.0 Hz, 1H), 7.50 (d, J = 8.7 Hz, 2H), 7.39 (d, J = 8.7 Hz, 2H), 4.72 (q, J = 7.1 Hz, 1H), 1.56 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 179.84, 171.63, 137.01, 135.31, 134.87, 134.64, 133.81, 132.24, 130.07, 128.47, 127.14, 127.09, 123.22, 120.49, 114.79, 110.32, 60.98, 15.92.
4-(3-(4-bromophenyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (5e)
White solid (1.0 g, 50% yield). m.p. 142–144 °C; 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 8.3 Hz, 1H), 7.95 (d, J = 1.3 Hz, 1H), 7.82 (dd, J = 8.3, 1.9 Hz, 1H), 7.66 (d, J = 8.3 Hz, 2H), 7.33 (d, J = 8.2 Hz, 2H), 4.82−4.65 (m, 1H), 1.56 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 179.74, 171.60, 136.97, 135.31, 135.16, 133.84, 133.51, 133.05, 132.22, 128.71, 127.18, 127.13, 127.09, 127.04, 123.21, 122.87, 120.48, 114.77, 110.36, 60.90, 15.94.
4-(3-(4-methoxyphenyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (5f)
White solid (1.4 g, 67% yield). m.p. 160–162 °C; 1H NMR (400 MHz, CDCl3) δ 7.97 (d, J = 8.2 Hz, 2H), 7.84 (d, J = 9.4 Hz, 1H), 7.32 (d, J = 8.9 Hz, 2H), 7.02 (d, J = 8.9 Hz, 2H), 4.65 (q, J = 7.0 Hz, 1H), 3.86 (s, 3H), 1.55 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 180.14, 172.04, 159.81, 137.18, 135.24, 133.75, 133.42, 132.24, 128.66, 128.53, 127.24, 127.16, 127.10, 127.06, 123.25, 120.52, 115.05, 114.83, 110.19, 61.40, 55.56, 15.95.
4-(3-(4-cyanophenyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (5g)
White solid (1.7 g, 78% yield). m.p. 169–171 °C; 1H NMR (400 MHz, CDCl3) δ 7.99 (d, J = 8.3 Hz, 1H), 7.95 (d, J = 1.6 Hz, 1H), 7.86−7.77 (m, 3H), 7.67 (d, J = 8.7 Hz, 2H), 4.87 (q, J = 7.0 Hz, 1H), 1.58 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 179.54, 171.20, 140.16, 136.87, 135.42, 133.84, 133.53, 132.32, 127.45, 127.22, 127.18, 127.13, 127.08, 123.20, 120.47, 117.81, 114.77, 112.24, 110.46, 60.42, 15.87.
4-(4-methyl-3-(4-nitrophenyl)-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (5h)
White solid (1.6 g, 40% yield). m.p. 193–195 °C; 1H NMR (400 MHz, CDCl3) δ 8.39 (d, J = 9.0 Hz, 2H), 8.01 (d, J = 8.3 Hz, 1H), 7.95 (d, J = 1.4 Hz, 1H), 7.83 (dd, J = 8.3, 1.9 Hz, 1H), 7.75 (d, J = 9.0 Hz, 2H), 4.92 (q, J = 7.0 Hz, 1H), 1.60 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 179.52, 171.09, 146.84, 141.72, 136.79, 135.45, 133.94, 133.60, 132.29, 127.38, 127.22, 127.17, 127.12, 127.08, 126.19, 125.01, 123.17, 120.45, 115.65, 114.72, 110.57, 60.43, 15.92.
4-(4-methyl-5-oxo-2-thioxo-3-(4-(trifluoromethyl)phenyl)imidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (5i)
White solid (2.4 g, 82% yield). m.p. 138–140 °C; 1H NMR (400 MHz, CDCl3) δ 7.99 (d, J = 8.3 Hz, 1H), 7.96 (d, J = 1.6 Hz, 1H), 7.86−7.76 (m, 3H), 7.63 (d, J = 8.3 Hz, 2H), 4.83 (q, J = 7.1 Hz, 1H), 1.58 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 179.70, 171.41, 139.30, 136.92, 135.37, 134.21, 133.88, 133.54, 133.21, 132.27, 130.92, 130.58, 127.34, 127.21, 127.16, 127.11, 127.07, 126.97, 126.94, 126.90, 126.86, 124.86, 123.20, 122.15, 120.48, 114.76, 110.44, 60.73, 15.92.
methyl 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5-methyl-4-oxo-2-thioxoimidazolidin-1-yl)benzoate (5j)
White solid (400 mg, 32% yield). m.p. 83–85 °C; 1H NMR (400 MHz, CDCl3) δ 8.20 (d, J = 8.3 Hz, 2H), 7.99 (d, J = 8.3 Hz, 1H), 7.95 (s, 1H), 7.83 (d, J = 8.0 Hz, 1H), 7.58 (d, J = 8.3 Hz, 2H), 4.84 (d, J = 7.0 Hz, 1H), 3.96 (s, 3H), 1.57 (d, J = 6.9 Hz, 3H). 101 MHz, CDCl3) δ 179.48, 171.53, 165.90, 140.07, 136.95, 135.84, 135.34, 133.52, 132.28, 131.04, 130.27, 127.18, 127.14, 126.72, 123.20, 121.59, 114.77, 112.01, 110.37, 60.70, 52.50, 15.92.
4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5-methyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluoro-N-methylbenzamide (5k)
White solid (350 mg, 36% yield). m.p. 205–207 °C; 1H NMR (400 MHz, DMSO) δ 8.41 (s, 1H), 8.39 (s, 1H), 8.25 (s, 1H), 8.05 (d, J = 8.2 Hz, 1H), 7.77 (t, J = 8.0 Hz, 1H), 7.67 (d, J = 11.4 Hz, 1H), 7.59 (d, J = 8.3 Hz, 1H), 5.28 (q, J = 6.7 Hz, 1H), 2.81 (d, J = 3.8 Hz, 3H), 1.43 (d, J = 6.8 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 179.72, 172.19, 163.36, 159.98, 157.51, 139.37, 139.27, 138.07, 136.29, 133.95, 131.30, 130.98, 130.45, 127.89, 127.84, 123.51, 123.39, 123.24, 122.63, 120.79, 114.97, 114.57, 114.31, 108.67, 60.48, 26.23, 14.66.
4-(4-methyl-3-(4-((1-methylpiperidin-4-yl)oxy)phenyl)-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (5l)
White solid (465 mg, 42% yield). m.p. 121–123 °C; 1H NMR (400 MHz, DMSO) δ 8.38 (d, J = 8.3 Hz, 1H), 8.23 (s, 1H), 8.03 (dd, J = 8.2, 1.0 Hz, 1H), 7.43 (d, J = 8.8 Hz, 2H), 7.08 (d, J = 8.9 Hz, 2H), 5.02 (q, J = 7.0 Hz, 1H), 4.49−4.33 (m, 1H), 2.67−2.58 (m, 2H), 2.22−2.14 (m, 5H), 1.96 (d, J = 10.2 Hz, 2H), 1.71−1.60 (m, 2H), 1.39 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 179.75, 172.60, 156.67, 138.28, 136.13, 133.87, 131.17, 130.85, 129.05, 128.63, 128.51, 127.88, 127.84, 127.79, 127.74, 123.55, 120.82, 115.94, 115.01, 108.40, 72.26, 61.07, 52.37, 45.76, 30.55, 14.78.
5-(4-methyl-5-oxo-3-phenyl-2-thioxoimidazolidin-1-yl)-3-(trifluoromethyl)picolinonitrile (18a)
White solid (330 mg, 45% yield). m.p. 169–171 °C; 1H NMR (400 MHz, DMSO) δ 9.22 (s, 1H), 8.77 (s, 1H), 7.57 (d, J = 8.4 Hz, 4H), 7.45 (s, 1H), 5.22 (d, J = 4.3 Hz, 1H), 1.42 (d, J = 4.1 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 179.14, 172.32, 153.66, 136.41, 135.76, 135.69, 135.65, 135.60, 133.45, 129.26, 128.97, 128.86, 128.63, 128.22, 126.97, 122.89, 120.17, 114.21, 60.99, 14.78.
5-(3-(4-fluorophenyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl)-3-(trifluoromethyl)picolinonitrile (18b)
White solid (500 mg, 65% yield). m.p. 181–183 °C; 1H NMR (400 MHz, CDCl3) δ 9.09 (s, 1H), 8.36 (s, 1H), 7.41 (d, J = 3.4 Hz, 2H), 7.24 (t, J = 8.2 Hz, 2H), 4.74 (d, J = 6.9 Hz, 1H), 1.59 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 179.36, 171.47, 163.66, 161.17, 152.35, 134.13, 132.33, 131.81, 130.70, 130.36, 130.08, 129.26, 129.17, 122.63, 119.90, 117.18, 116.95, 113.78, 61.35, 15.93.
4-(3-(6-cyano-5-(trifluoromethyl)pyridin-3-yl)-5-methyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluoro-N-methylbenzamide (18c)
White solid (460 mg, 58% yield). m.p. 186–188 °C; 1H NMR (400 MHz, DMSO) δ 9.20 (s, 1H), 8.75 (s, 1H), 8.40 (s, 1H), 7.78 (t, J = 8.1 Hz, 1H), 7.67 (d, J = 11.3 Hz, 1H), 7.59 (d, J = 8.3 Hz, 1H), 5.34 (q, J = 6.6 Hz, 1H), 2.80 (d, J = 4.0 Hz, 3H), 1.44 (d, J = 6.8 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 179.22, 171.99, 163.32, 160.01, 157.52, 153.66, 139.12, 139.01, 135.77, 135.73, 133.28, 130.53, 129.31, 129.06, 128.70, 123.57, 123.42, 122.86, 122.64, 122.62, 120.13, 114.53, 114.27, 114.20, 79.12, 60.60, 26.23, 14.69.
5-(4-methyl-3-(4-((1-methylpiperidin-4-yl)oxy)phenyl)-5-oxo-2-thioxoimidazolidin-1-yl)-3-(trifluoromethyl)picolinonitrile (18d)
White solid (600 mg, 70% yield). m.p. 112–114 °C; 1H NMR (400 MHz, CDCl3) δ 9.09 (s, 1H), 8.36 (s, 1H), 7.30 (d, J = 8.4 Hz, 2H), 7.02 (d, J = 8.5 Hz, 2H), 4.69 (q, J = 7.0 Hz, 1H), 4.37 (s, 1H), 2.71 (s, 2H), 2.32 (s, 3H), 2.30 (s, 2H), 2.08−2.00 (m, 2H), 1.88 (dd, J = 14.6, 5.8 Hz, 2H), 1.58 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 179.25, 171.76, 157.96, 152.35, 134.11, 134.07, 132.43, 130.64, 130.30, 129.98, 128.42, 128.16, 122.66, 119.93, 118.42, 116.70, 113.89, 113.77, 72.55, 61.49, 52.61, 46.20, 30.80, 15.95.
4.2.3. General Procedure for the Synthesis of 6a-l and 19a-d
In a 50 mL round-bottomed flask, we added compound 5a-l or 18a-d (1.0 eq) in 10 mL THF to give a colorless solution. The reaction vessel was purged with nitrogen. The reaction mixture was cooled to −78 °C with stirring on. LiHMDS (1.3 eq) was added. The reaction mixture was held at −78 °C with stirring for 10 min. Formaldehyde (3.0 eq) was added. The reaction mixture was warmed up to rt with stirring on for 30 min. Then, 10 mL sat. NH4Cl (aq) was added. The aq layer was extracted with EA. We combined the organic layers and washed them with brine. The organic was dried with Na2SO4, filt and conc to give crude product. The crude product was purified by column chromatography to give 6a-l and 19a-d.
4-(4-(hydroxymethyl)-4-methyl-5-oxo-3-phenyl-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (6a)
White solid (45 mg, 21% yield). m.p. 216–219 °C; 1H NMR (400 MHz, DMSO) δ 8.39 (d, J = 8.2 Hz, 1H), 8.15 (d, J = 1.6 Hz, 1H), 7.99 (dd, J = 8.2, 1.8 Hz, 1H), 7.54 (dq, J = 14.5, 7.2 Hz, 3H), 7.48−7.41 (m, 2H), 5.87 (t, J = 5.1 Hz, 1H), 3.78 (dd, J = 11.7, 4.7 Hz, 1H), 3.40 (dd, J = 11.7, 5.5 Hz, 1H), 1.35 (s, 3H). 13C NMR (101 MHz, DMSO) δ 180.92, 173.96, 137.95, 136.26, 135.22, 133.68, 131.27, 130.95, 129.63, 129.47, 129.22, 127.51, 127.47, 127.42, 127.38, 123.50, 120.78, 114.96, 108.51, 108.49, 71.63, 63.62, 16.78. HRMS(ESI)m/z calcd for C19H14F3N3O2S [M+H]+: 406.0837, Found: 406.0871.
4-(4-(hydroxymethyl)-4-methyl-5-oxo-2-thioxo-3-(p-tolyl)imidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (6b)
White solid (70 mg, 43% yield). m.p. 190–192 °C; 1H NMR (400 MHz, DMSO) δ 8.39 (d, J = 8.2 Hz, 1H), 8.14 (s, 1H), 7.98 (d, J = 8.2 Hz, 1H), 7.36 (d, J = 8.2 Hz, 2H), 7.32 (d, J = 8.3 Hz, 2H), 5.83 (t, J = 5.1 Hz, 1H), 3.77 (dd, J = 11.6, 4.8 Hz, 1H), 3.39 (dd, J = 11.6, 5.6 Hz, 1H), 2.38 (s, 3H), 1.33 (s, 3H). 13C NMR (101 MHz, DMSO) δ 180.95, 174.01, 138.81, 137.99, 136.24, 133.67, 132.55, 131.25, 131.09, 130.92, 129.97, 129.32, 127.46, 127.42, 120.77, 114.96, 108.47, 71.55, 63.58, 20.74, 16.75. HRMS(ESI)m/z calcd for C20H16F3N3O2S [M+H]+: 420.0994, Found: 420.0973.
4-(3-(4-fluorophenyl)-4-(hydroxymethyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (6c)
White solid (90 mg, 42% yield). m.p. 231–233 °C; 1H NMR (400 MHz, DMSO) δ 8.39 (d, J = 8.2 Hz, 1H), 8.14 (d, J = 0.9 Hz, 1H), 7.99 (dd, J = 8.2, 1.3 Hz, 1H), 7.50 (dd, J = 8.8, 5.1 Hz, 2H), 7.42 (t, J = 8.8 Hz, 2H), 5.89 (t, J = 5.2 Hz, 1H), 3.79 (dd, J = 11.7, 4.9 Hz, 1H), 3.40 (dd, J = 11.7, 5.7 Hz, 1H), 1.36 (s, 3H). 13C NMR (101 MHz, DMSO) δ 181.25, 173.90, 163.29, 160.84, 137.88, 136.29, 133.63, 131.96, 131.87, 131.42, 131.39, 131.31, 130.98, 127.41, 127.36, 123.48, 120.76, 116.58, 116.34, 114.94, 108.57, 108.55, 71.62, 63.58, 16.65. HRMS(ESI)m/z calcd for C19H13F4N3O2S [M+H]+: 424.0743, Found: 424.0715.
4-(3-(4-chlorophenyl)-4-(hydroxymethyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (6d)
White solid (73 mg, 34% yield). m.p. 205–207 °C; 1H NMR (400 MHz, DMSO) δ 8.40 (d, J = 8.2 Hz, 1H), 8.15 (s, 1H), 7.99 (d, J = 8.2 Hz, 1H), 7.66 (d, J = 8.5 Hz, 2H), 7.48 (d, J = 8.5 Hz, 2H), 5.90 (t, J = 4.9 Hz, 1H), 3.80 (dd, J = 11.7, 4.6 Hz, 1H), 3.42 (dd, J = 11.7, 5.6 Hz, 1H), 1.37 (s, 3H). 13C NMR (101 MHz, DMSO) δ 181.10, 173.86, 137.83, 136.31, 134.13, 133.95, 133.63, 131.59, 131.31, 130.99, 129.65, 127.41, 127.36, 123.48, 120.76, 114.93, 108.60, 71.71, 63.60, 16.66. HRMS(ESI)m/z calcd for C19H13ClF3N3O2S [M+H]+: 440.0447, Found: 440.0476.
4-(3-(4-bromophenyl)-4-(hydroxymethyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (6e)
White solid (40 mg, 19% yield). m.p. 206–208 °C; 1H NMR (400 MHz, DMSO) δ 8.40 (d, J = 8.3 Hz, 1H), 8.14 (d, J = 1.5 Hz, 1H), 7.98 (dd, J = 8.2, 1.7 Hz, 1H), 7.79 (d, J = 8.6 Hz, 2H), 7.41 (d, J = 8.6 Hz, 2H), 5.89 (t, J = 5.2 Hz, 1H), 3.79 (dd, J = 11.8, 4.7 Hz, 1H), 3.41 (dd, J = 11.8, 5.6 Hz, 1H), 1.36 (s, 3H). 13C NMR (101 MHz, DMSO) δ 181.00, 173.89, 137.79, 136.29, 134.51, 133.64, 132.62, 131.87, 131.33, 131.01, 127.43, 127.38, 123.45, 122.61, 120.72, 114.94, 108.59, 71.69, 63.54, 16.64. HRMS(ESI)m/z calcd for C19H13BrF3N3O2S [M+H]+: 483.9942, Found: 483.9978.
4-(4-(hydroxymethyl)-3-(4-methoxyphenyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (6f)
White solid (200 mg, 93% yield). m.p. 183–185 °C; 1H NMR (400 MHz, DMSO) δ 8.39 (d, J = 8.3 Hz, 1H), 8.14 (d, J = 1.6 Hz, 1H), 7.99 (dd, J = 8.2, 1.7 Hz, 1H), 7.37 (d, J = 8.9 Hz, 2H), 7.10 (d, J = 8.9 Hz, 2H), 5.84 (t, J = 5.2 Hz, 1H), 3.83 (s, 3H), 3.78 (dd, J = 11.7, 4.8 Hz, 1H), 3.40 (dd, J = 11.7, 5.7 Hz, 1H), 1.34 (s, 3H). 13C NMR (101 MHz, DMSO) δ 181.17, 174.04, 159.50, 138.02, 136.23, 133.65, 130.73, 127.54, 127.45, 127.40, 123.50, 120.78, 114.96, 114.62, 108.45, 71.52, 63.56, 55.35, 16.71. HRMS(ESI)m/z calcd for C20H16F3N3O3S [M+H]+: 436.0943, Found: 436.0911.
4-(3-(4-cyanophenyl)-4-(hydroxymethyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (6g)
White solid (130 mg, 60% yield). m.p. 234–236 °C; 1H NMR (400 MHz, DMSO) δ 8.40 (d, J = 8.2 Hz, 1H), 8.14 (s, 1H), 8.09 (d, J = 8.5 Hz, 2H), 7.99 (d, J = 8.2 Hz, 1H), 7.67 (d, J = 8.5 Hz, 2H), 5.94 (t, J = 5.3 Hz, 1H), 3.81 (dd, J = 11.9, 4.9 Hz, 1H), 3.42 (dd, J = 11.9, 5.8 Hz, 1H), 1.38 (s, 3H). 13C NMR (101 MHz, DMSO) δ 181.00, 173.70, 139.63, 139.13, 137.69, 136.36, 133.73, 133.63, 131.04, 127.39, 127.34, 124.87, 118.11, 114.92, 112.11, 108.71, 72.03, 63.70, 16.68. HRMS(ESI)m/z calcd for C20H13F3N4O2S [M+H]+: 431.0790, Found: 431.0771.
4-(4-(hydroxymethyl)-4-methyl-3-(4-nitrophenyl)-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (6h)
Yellow solid (330 mg, 39% yield). m.p. 206–208 °C; 1H NMR (400 MHz, DMSO) δ 8.45 (d, J = 8.9 Hz, 2H), 8.42 (d, J = 8.4 Hz, 1H), 8.17 (s, 1H), 8.02 (d, J = 8.3 Hz, 1H), 7.77 (d, J = 9.0 Hz, 2H), 5.98 (t, J = 5.3 Hz, 1H), 3.85 (dd, J = 11.9, 5.0 Hz, 1H), 3.45 (dd, J = 11.9, 5.8 Hz, 1H), 1.41 (s, 3H). 13C NMR (101 MHz, DMSO) δ 181.06, 173.69, 147.57, 141.24, 137.69, 136.38, 133.65, 131.37, 131.06, 127.46, 127.41, 127.37, 127.32, 126.19, 124.85, 123.47, 120.74, 114.91, 108.74, 108.73, 72.13, 63.71, 16.70. HRMS(ESI)m/z calcd for C19H13F3N4O4S [M+H]+: 451.688, Found: 451.0651.
4-(4-(hydroxymethyl)-4-methyl-5-oxo-2-thioxo-3-(4-(trifluoromethyl)phenyl)imidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (6i)
White solid (800 mg, 75% yield). m.p. 178–180 °C; 1H NMR (400 MHz, DMSO) δ 8.41 (d, J = 8.2 Hz, 1H), 8.16 (s, 1H), 7.99 (t, J = 7.8 Hz, 3H), 7.70 (d, J = 8.1 Hz, 2H), 5.94 (t, J = 5.2 Hz, 1H), 3.82 (dd, J = 11.8, 4.9 Hz, 1H), 3.43 (dd, J = 11.8, 5.7 Hz, 1H), 1.39 (s, 3H). 13C NMR (101 MHz, DMSO) δ 181.11, 173.78, 139.09, 137.77, 136.32, 133.65, 131.37, 131.04, 130.86, 130.03, 129.72, 129.40, 129.08, 127.46, 127.42, 127.37, 127.32, 126.71, 126.68, 125.20, 123.47, 123.35, 122.50, 120.75, 119.79, 118.51, 114.91, 108.69, 108.67, 71.93, 63.67, 16.68. HRMS(ESI)m/z calcd for C20H13F6N3O2S [M+Na]+: 493.0530, Found: 496.0558.
methyl 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5-(hydroxymethyl)-5-methyl-4-oxo-2-thioxoimidazolidin-1-yl)benzoate (6j)
White solid (110 mg, 41% yield). m.p. 168–170 °C; 1H NMR (400 MHz, DMSO) δ 8.40 (d, J = 8.3 Hz, 1H), 8.15 (d, J = 8.5 Hz, 3H), 8.00 (dd, J = 8.2, 1.6 Hz, 1H), 7.62 (d, J = 8.5 Hz, 2H), 5.92 (t, J = 5.3 Hz, 1H), 3.90 (s, 3H), 3.81 (dd, J = 11.8, 4.9 Hz, 1H), 3.42 (dd, J = 11.8, 5.7 Hz, 1H), 1.38 (s, 3H). 13C NMR (101 MHz, DMSO) δ 180.90, 173.80, 165.54, 139.58, 137.80, 136.45, 136.32, 133.66, 131.33, 131.00, 130.37, 130.26, 130.22, 127.45, 127.40, 123.48, 121.63, 120.76, 114.92, 108.64, 71.91, 63.69, 52.38, 16.75. HRMS(ESI)m/z calcd for C21H16F3N3O4S [M+H]+: 464.0892, Found: 464.0911.
4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5-(hydroxymethyl)-5-methyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluoro-N-methylbenzamide (6k)
White solid (60 mg, 56% yield). m.p. 123–125 °C; 1H NMR (400 MHz, CDCl3) δ 8.09 (t, J = 8.2 Hz, 1H), 7.92 (d, J = 9.4 Hz, 2H), 7.79 (d, J = 8.2 Hz, 1H), 7.34 (d, J = 8.3 Hz, 1H), 7.29 (d, J = 11.8 Hz, 1H), 6.86 (dd, J = 9.8, 4.6 Hz, 1H), 3.98 (d, J = 11.3 Hz, 1H), 3.69 (s, 1H), 3.53 (d, J = 11.4 Hz, 1H), 2.99 (d, J = 4.1 Hz, 3H), 1.42 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 181.45, 173.70, 163.43, 161.65, 159.16, 139.27, 139.16, 137.28, 135.37, 133.67, 133.34, 132.85, 132.47, 127.26, 127.21, 126.45, 123.22, 122.52, 122.39, 120.50, 118.35, 118.08, 114.86, 110.09, 71.68, 64.19, 27.05, 17.88. HRMS(ESI)m/z calcd for C21H16F4N4O3S [M+H]+: 491.0958, Found: 481.0950.
4-(4-(hydroxymethyl)-4-methyl-3-(4-((1-methylpiperidin-4-yl)oxy)phenyl)-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (6l)
White solid (40 mg, 38% yield). m.p. 117–119 °C; 1H NMR (400 MHz, CDCl3) δ 7.95 (d, J = 7.9 Hz, 2H), 7.85 (d, J = 8.2 Hz, 1H), 7.35 (d, J = 8.8 Hz, 2H), 7.00 (d, J = 8.9 Hz, 2H), 4.42 (s, 1H), 3.96 (d, J = 11.2 Hz, 1H), 3.78 (s, 1H), 3.59 (d, J = 11.2 Hz, 1H), 2.86−2.76 (m, 2H), 2.55 (s, 2H), 2.41 (s, 3H), 2.11 (s, 2H), 1.93 (s, 2H), 1.43 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 181.74, 174.32, 157.98, 137.58, 135.26, 133.56, 133.23, 132.53, 130.84, 127.46, 127.30, 127.25, 123.29, 120.56, 116.54, 114.91, 109.92, 77.28, 71.45, 63.99, 51.88, 45.50, 29.73, 17.85. HRMS(ESI)m/z calcd for C25H25F3N4O3S [M+H]+: 519.1678, Found: 519.1692.
5-(4-(hydroxymethyl)-4-methyl-5-oxo-3-phenyl-2-thioxoimidazolidin-1-yl)-3-(trifluoromethyl)picolinonitrile (19a)
White solid (50 mg, 31% yield). m.p. 203–205 °C; 1H NMR (400 MHz, DMSO) δ 9.18 (d, J = 1.3 Hz, 1H), 8.71 (d, J = 1.4 Hz, 1H), 7.61−7.52 (m, 3H), 7.46 (d, J = 7.4 Hz, 2H), 5.90 (t, J = 5.3 Hz, 1H), 3.82 (dd, J = 11.8, 4.6 Hz, 1H), 3.41 (dd, J = 11.8, 6.0 Hz, 1H), 1.37 (s, 3H). 13C NMR (101 MHz, DMSO) δ 180.36, 173.77, 153.28, 135.28, 135.25, 134.98, 133.12, 129.56, 129.52, 129.38, 128.85, 128.67, 128.34, 114.21, 71.95, 63.60, 16.79. HRMS(ESI) m/z calcd for C18H13F3N4O2S [M+H]+: 407.0790, Found: 407.0789.
5-(3-(4-fluorophenyl)-4-(hydroxymethyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl)-3-(trifluoromethyl)picolinonitrile (19b)
White solid (130 mg, 60% yield). m.p. 181–183 °C; 1H NMR (400 MHz, DMSO) δ 9.21 (s, 1H), 8.73 (s, 1H), 7.53 (s, 2H), 7.46 (s, 2H), 5.96 (s, 1H), 3.87 (d, J = 7.7 Hz, 1H), 3.45 (d, J = 5.5 Hz, 1H), 1.41 (s, 3H). 13C NMR (101 MHz, DMSO) δ 180.68, 173.71, 163.35, 160.90, 153.23, 135.23, 135.19, 133.06, 131.87, 131.77, 131.18, 131.15, 129.03, 128.91, 122.83, 120.11, 116.67, 116.44, 114.18, 71.94, 63.55, 16.65. HRMS(ESI)m/z calcd for C18H12F4N4O2S [M+H]+: 425.0695, Found: 425.0699.
4-(3-(6-cyano-5-(trifluoromethyl)pyridin-3-yl)-5-(hydroxymethyl)-5-methyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluoro-N-methylbenzamide (19c)
White solid (60 mg, 56% yield). m.p. 127–129 °C; 1H NMR (400 MHz, CDCl3) δ 9.01 (s, 1H), 8.32 (s, 1H), 8.12 (t, J = 7.9 Hz, 1H), 7.36 (d, J = 8.0 Hz, 1H), 7.30 (d, J = 11.5 Hz, 1H), 6.87 (s, 1H), 4.16 (s, 1H), 4.02 (d, J = 11.2 Hz, 1H), 3.58 (d, J = 11.2 Hz, 1H), 3.01 (s, 3H), 1.46 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 180.68, 173.43, 163.40, 161.67, 159.17, 152.51, 139.00, 138.88, 134.37, 132.96, 132.57, 130.69, 130.34, 129.93, 126.38, 122.60, 122.46, 119.88, 118.30, 118.03, 113.80, 71.92, 64.21, 27.10, 17.84. HRMS(ESI)m/z calcd for C20H15F4N5O3S [M+H]+: 482.0910, Found: 482.0939.
5-(4-(hydroxymethyl)-4-methyl-3-(4-((1-methylpiperidin-4-yl)oxy)phenyl)-5-oxo-2-thioxoimidazolidin-1-yl)-3-(trifluoromethyl)picolinonitrile (19d)
White solid (38 mg, 36% yield). m.p. 119–121 °C; 1H NMR (400 MHz, CDCl3) δ 9.01 (s, 1H), 8.29 (s, 1H), 7.27 (d, J = 8.5 Hz, 2H), 6.94 (d, J = 8.7 Hz, 2H), 4.33 (s, 1H), 4.00 (s, 1H), 3.90 (d, J = 11.0 Hz, 1H), 3.54 (d, J = 11.0 Hz, 1H), 2.70 (s, 2H), 2.37 (s, 2H), 2.29 (s, 3H), 2.00 (s, 2H), 1.83 (s, 2H), 1.38 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 179.95, 173.09, 157.20, 151.55, 133.28, 133.24, 131.82, 129.67, 129.57, 129.23, 128.75, 126.03, 121.67, 118.94, 115.58, 112.83, 76.24, 70.67, 62.93, 51.14, 44.75, 29.09, 16.80. HRMS(ESI)m/z calcd for C24H24F3N5O3S [M+H]+: 520.1630, Found: 520.1649.
4.2.4. General Procedure for the Synthesis of 7a-j and 10
In a 25 mL round-bottomed flask, we added 6a-j and 9 (1.0 eq) in 1 mL CCl4 and 1 mL MeCN to give a colorless solution. The reaction mixture was cooled to 0 °C with stirring on. NaIO4 (4.0 eq) in 2 mL water was added. Ruthenium (III) chloride (0.05 eq) was added. The reaction mixture was held at rt with stirring on for 2 h. Then, 2 mL NaHCO3 (aq) was added. The aq layer was extracted with DCM. We combined the organic layers and washed them with brine. The organic was dried with Na2SO4, filt and conc to give a crude product. The crude product was purified by column chromatography to give 7a-j and 11.
4-(4-(hydroxymethyl)-4-methyl-2,5-dioxo-3-phenylimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (7a)
White solid (40 mg, 48% yield). m.p. 187–189 °C; 1H NMR (400 MHz, DMSO) δ 8.36 (d, J = 8.3 Hz, 1H), 8.20 (s, 1H), 8.08 (d, J = 8.0 Hz, 1H), 7.56−7.43 (m, 5H), 5.72 (t, J = 4.7 Hz, 1H), 3.76 (dd, J = 11.5, 4.7 Hz, 1H), 3.38 (dd, J = 11.5, 5.2 Hz, 1H), 1.32 (s, 3H). 13C NMR (101 MHz, DMSO) δ 173.20, 153.19, 136.59, 136.34, 133.48, 131.73, 131.42, 131.10, 130.78, 129.80, 129.35, 128.63, 123.69, 123.64, 120.82, 115.12, 106.96, 68.41, 63.38, 17.09. HRMS(ESI)m/z calcd for C19H14F3N3O3 [M+H]+: 390.1066, Found: 390.1074.
4-(4-(hydroxymethyl)-4-methyl-2,5-dioxo-3-(p-tolyl)imidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (7b)
White solid (20 mg, 49% yield). m.p. 156–158 °C; 1H NMR (400 MHz, DMSO) δ 8.35 (d, J = 8.4 Hz, 1H), 8.18 (d, J = 1.6 Hz, 1H), 8.06 (dd, J = 8.4, 1.7 Hz, 1H), 7.34 (d, J = 9.0 Hz, 4H), 5.68 (t, J = 5.1 Hz, 1H), 3.73 (dd, J = 11.6, 4.8 Hz, 1H), 3.35 (dd, J = 11.6, 4.8 Hz, 1H), 2.37 (s, 3H), 1.29 (s, 3H). 13C NMR (101 MHz, DMSO) δ 173.25, 153.20, 138.23, 136.64, 136.32, 131.41, 131.09, 130.70, 129.84, 129.77, 129.21, 123.66, 123.61, 123.54, 120.82, 115.12, 106.92, 106.90, 68.29, 63.31, 20.67, 17.05. HRMS(ESI)m/z calcd for C20H16F3N3O3 [M+H]+: 404.1222, Found: 404.1219.
4-(3-(4-fluorophenyl)-4-(hydroxymethyl)-4-methyl-2,5-dioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (7c)
White solid (50 mg, 43% yield). m.p. 208–210 °C; 1H NMR (400 MHz, DMSO) δ 8.36 (d, J = 8.4 Hz, 1H), 8.18 (s, 1H), 8.07 (d, J = 8.4 Hz, 1H), 7.50 (dd, J = 8.8, 5.1 Hz, 2H), 7.38 (t, J = 8.8 Hz, 2H), 5.73 (t, J = 5.2 Hz, 1H), 3.75 (dd, J = 11.7, 5.1 Hz, 1H), 3.36 (dd, J = 11.7, 5.4 Hz, 1H), 1.31 (s, 3H).13C NMR (101 MHz, DMSO) δ 173.13, 162.99, 160.55, 153.28, 136.53, 136.37, 131.70, 131.61, 131.44, 131.11, 129.79, 129.60, 123.67, 123.62, 120.81, 116.40, 116.18, 115.11, 107.00, 68.39, 63.27, 16.91. HRMS(ESI)m/z calcd for C19H13F4N3O3 [M+H]+: 408.0971, Found: 408.0941.
4-(3-(4-chlorophenyl)-4-(hydroxymethyl)-4-methyl-2,5-dioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (7d)
White solid (20 mg, 41% yield). m.p. 152–154 °C; 1H NMR (400 MHz, DMSO) δ 8.36 (d, J = 8.4 Hz, 1H), 8.18 (d, J = 1.4 Hz, 1H), 8.06 (dd, J = 8.4, 1.8 Hz, 1H), 7.61 (d, J = 8.7 Hz, 2H), 7.48 (d, J = 8.6 Hz, 2H), 5.73 (t, J = 5.2 Hz, 1H), 3.76 (dd, J = 11.8, 5.1 Hz, 1H), 3.39 (dd, J = 11.8, 5.4 Hz, 1H), 1.32 (s, 3H). 13C NMR (101 MHz, DMSO) δ 173.07, 153.17, 136.46, 136.37, 136.32, 133.19, 132.47, 131.44, 131.07, 130.79, 129.86, 129.46, 123.72, 123.67, 123.52, 120.80, 118.07, 117.30, 115.54, 115.10, 110.74, 107.06, 68.53, 63.36, 40.11, 39.90, 39.70, 39.49, 39.28, 39.07, 38.86, 16.96. HRMS(ESI)m/z calcd for C19H13ClF3N3O3 [M+H]+: 424.0676, Found: 424.0640.
4-(3-(4-bromophenyl)-4-(hydroxymethyl)-4-methyl-2,5-dioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (7e)
White solid (50 mg, 19% yield). m.p. 102–104 °C; 1H NMR (400 MHz, DMSO) δ 10.71 (s, 1H), 8.29 (s, 1H), 8.18 (dd, J = 8.6, 1.5 Hz, 1H), 8.14 (d, J = 8.7 Hz, 1H), 7.60 (d, J = 8.8 Hz, 2H), 7.31 (d, J = 8.8 Hz, 2H), 4.74 (d, J = 9.2 Hz, 1H), 4.37 (d, J = 9.2 Hz, 1H), 1.64 (s, 3H). 13C NMR (101 MHz, DMSO) δ 173.07, 153.12, 136.46, 136.36, 136.31, 132.94, 132.41, 131.45, 131.35, 131.12, 129.85, 123.77, 123.72, 123.67, 123.62, 123.52, 121.72, 120.79, 115.55, 115.09, 107.08, 68.51, 63.37, 16.97. HRMS(ESI)m/z calcd for C19H13BrF3N3O3 [M+H]+: 468.0171, Found: 468.0135.
4-(4-(hydroxymethyl)-3-(4-methoxyphenyl)-4-methyl-2,5-dioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (7f)
White solid (160 mg, 74% yield). m.p. 128–130 °C; 1H NMR (400 MHz, DMSO) δ 8.35 (d, J = 5.6 Hz, 1H), 8.18 (s, 1H), 8.07 (d, J = 5.8 Hz, 1H), 7.37 (d, J = 5.0 Hz, 2H), 7.06 (d, J = 5.8 Hz, 2H), 5.69 (s, 1H), 3.81 (s, 3H), 3.73 (s, 1H), 3.33 (s, 1H), 1.29 (s, 3H). 13C NMR (101 MHz, DMSO) δ 173.28, 159.26, 153.33, 136.66, 136.32, 130.78, 129.73, 125.59, 123.65, 123.62, 123.57, 123.53, 120.81, 120.74, 120.65, 115.13, 114.54, 113.98, 106.86, 68.21, 63.22, 55.34, 16.95. HRMS(ESI)m/z calcd for C20H16F3N3O4 [M+H]+: 420.1171, Found: 420.1199.
4-(3-(4-cyanophenyl)-4-(hydroxymethyl)-4-methyl-2,5-dioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (7g)
White solid (22 mg, 46% yield). m.p. 169–171 °C; 1H NMR (400 MHz, DMSO) δ 8.43 (d, J = 8.4 Hz, 1H), 8.25 (d, J = 1.3 Hz, 1H), 8.12 (dd, J = 8.4, 1.5 Hz, 1H), 8.07 (d, J = 8.6 Hz, 2H), 7.75 (d, J = 8.6 Hz, 2H), 5.83 (t, J = 5.3 Hz, 1H), 3.85 (dd, J = 11.9, 5.2 Hz, 1H), 3.56 (dd, J = 11.9, 5.5 Hz, 2H), 1.44 (s, 3H). 13C NMR (101 MHz, DMSO) δ 172.88, 153.06, 138.49, 136.43, 136.27, 133.49, 131.48, 131.15, 130.83, 130.03, 129.12, 123.89, 123.84, 123.51, 120.78, 118.27, 115.08, 110.66, 107.29, 68.98, 63.64, 17.11. HRMS(ESI)m/z calcd for C20H13F3N4O3 [M+H]+: 415.1018, Found: 415.1016.
4-(4-(hydroxymethyl)-4-methyl-3-(4-nitrophenyl)-2,5-dioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (7h)
Light-yellow solid (60 mg, 48% yield). m.p. 160–162 °C; 1H NMR (400 MHz, DMSO) δ 8.50−8.30 (m, 3H), 8.20 (d, J = 1.0 Hz, 1H), 8.07 (dd, J = 8.4, 1.4 Hz, 1H), 7.78 (d, J = 8.9 Hz, 2H), 5.80 (t, J = 5.3 Hz, 1H), 3.82 (dd, J = 11.9, 5.3 Hz, 1H), 3.56 (dd, J = 11.9, 5.5 Hz, 1H), 1.42 (s, 3H). 13C NMR (101 MHz, DMSO) δ 172.82, 153.09, 146.28, 140.32, 136.42, 136.22, 131.81, 131.50, 131.18, 130.85, 130.11, 128.80, 124.64, 123.96, 123.92, 123.50, 120.78, 115.05, 107.36, 69.14, 63.70, 30.36, 17.13.HRMS(ESI)m/z calcd for C19H13F3N4O5 [M+Na]+: 457.0736, Found: 457.0722.
4-(4-(hydroxymethyl)-4-methyl-2,5-dioxo-3-(4-(trifluoromethyl)phenyl)imidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (7i)
White solid (190 mg, 66% yield). m.p. 161–163 °C; 1H NMR (400 MHz, DMSO) δ 8.43 (d, J = 8.4 Hz, 1H), 8.25 (d, J = 1.3 Hz, 1H), 8.12 (dd, J = 8.4, 1.5 Hz, 1H), 8.07 (d, J = 8.6 Hz, 2H), 7.75 (d, J = 8.6 Hz, 2H), 5.83 (t, J = 5.3 Hz, 1H), 3.85 (dd, J = 11.9, 5.2 Hz, 1H), 3.56 (dd, J = 11.9, 5.5 Hz, 2H), 1.44 (s, 3H). 13C NMR (101 MHz, DMSO) δ 172.96, 153.18, 137.72, 136.38, 131.48, 131.15, 129.97, 129.51, 128.70, 128.38, 126.49, 126.46, 125.28, 123.83, 123.79, 123.51, 122.58, 120.79, 115.07, 107.21, 68.82, 63.53, 17.09. HRMS(ESI)m/z calcd for C20H13F6N3O3 [M+H]+: 458.0939, Found: 458.0929.
methyl 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5-(hydroxymethyl)-5-methyl-2,4-dioxoimidazolidin-1-yl)benzoate (7j)
White solid (70 mg, 50% yield). m.p. 139–141 °C; 1H NMR (400 MHz, DMSO) δ 8.37 (d, J = 8.4 Hz, 1H), 8.20 (d, J = 1.5 Hz, 1H), 8.08 (dd, J = 13.0, 5.1 Hz, 3H), 7.63 (d, J = 8.6 Hz, 2H), 5.76 (t, J = 5.3 Hz, 1H), 3.89 (s, 3H), 3.79 (dd, J = 11.8, 5.2 Hz, 1H), 3.47 (dd, J = 11.8, 5.4 Hz, 1H), 1.37 (s, 3H). 13C NMR (101 MHz, DMSO) δ 172.99, 165.58, 153.09, 138.35, 136.38, 131.14, 130.22, 129.98, 129.11, 128.67, 123.86, 123.81, 123.51, 120.79, 115.08, 107.18, 68.83, 63.59, 52.29, 17.16. HRMS(ESI)m/z calcd for C21H16F3N3O5 [M+H]+: 448.1120, Found: 448.1154.
N-(4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5-(hydroxymethyl)-5-methyl-2,4-dioxoimidazolidin-1-yl)phenyl)acetamide (10)
White solid (40 mg, 41% yield). m.p. 124–126 °C; 1H NMR (400 MHz, DMSO) δ 10.12 (s, 1H), 8.35 (d, J = 8.4 Hz, 1H), 8.18 (s, 1H), 8.06 (d, J = 8.5 Hz, 1H), 7.69 (d, J = 8.6 Hz, 2H), 7.36 (d, J = 8.6 Hz, 2H), 5.66 (t, J = 5.1 Hz, 1H), 3.73 (dd, J = 11.6, 4.9 Hz, 1H), 3.39−3.34 (m, 1H), 2.07 (s, 3H), 1.30 (s, 3H). 13C NMR (101 MHz, DMSO) δ 173.23, 168.49, 153.26, 139.51, 136.63, 136.32, 131.41, 131.09, 129.78, 127.77, 123.71, 123.65, 123.61, 123.54, 120.82, 119.49, 115.12, 106.90, 68.32, 63.27, 23.96, 17.05. HRMS(ESI)m/z calcd for C21H17F3N4O4 [M+Na]+: 469.1100, Found: 469.1100.
4.2.5. 4-(3-(4-aminophenyl)-4-(hydroxymethyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (8)
In a 10 mL round-bottomed flask, we added 4-(4-(hydroxymethyl)-4-methyl-3-(4-nitrophenyl)-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (6h) (200 mg, 0.444 mmol, 1.0 eq), iron (74.4 mg, 1.332 mmol, 3.0 eq) and HCl (0.222 mL, 0.888 mmol, 2.0 eq) in 5 mL ethanol to give a yellow suspension. The reaction was heated to 90 °C with stirring on for 4 h. The reaction mixture was filtered through celite. The mixture was concentrated by rotovap to give 4-(3-(4-aminophenyl)-4-(hydroxymethyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (8) (170 mg, 91% yield) as a yellow solid. The crude product was used in the next step without further purification.
4.2.6. N-(4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5-(hydroxymethyl)-5-methyl-4-oxo-2-thioxoimidazolidin-1-yl)phenyl)acetamide (9)
In a 25 mL round-bottomed flask, we added 4-(3-(4-aminophenyl)-4-(hydroxymethyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (8) (170 mg, 0.404 mmol, 1.0 eq) in 5 mL THF to give a yellow solution. Acetyl chloride (0.035 mL, 0.485 mmol, 1.2 eq) was added. The reaction mixture was held at 0 °C with stirring on for 1 h. The mixture was concentrated by rotovap. The crude product was purified by column chromatography to give N-(4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5-(hydroxymethyl)-5-methyl-4-oxo-2-thioxoimidazolidin-1-yl)phenyl)acetamide (100 mg, 53.5% yield) as a yellow solid. m.p. 126–128 °C; 1H NMR (400 MHz, DMSO) δ 10.16 (s, 1H), 8.38 (d, J = 8.2 Hz, 1H), 8.14 (d, J = 1.2 Hz, 1H), 7.98 (dd, J = 8.3, 1.4 Hz, 1H), 7.72 (d, J = 8.8 Hz, 2H), 7.35 (d, J = 8.8 Hz, 2H), 5.82 (t, J = 5.1 Hz, 1H), 3.76 (dd, J = 11.6, 4.7 Hz, 1H), 3.39 (dd, J = 11.7, 5.6 Hz, 1H), 2.08 (s, 3H), 1.34 (s, 3H). 13C NMR (101 MHz, DMSO) δ 181.03, 174.00, 168.56, 139.92, 137.98, 136.24, 133.66, 131.26, 130.93, 129.94, 129.56, 127.47, 127.42, 123.49, 119.70, 119.53, 114.97, 108.46, 71.58, 63.56, 23.98, 16.76. HRMS(ESI)m/z calcd for C21H17F3N4O3S [M+Na]+: 485.0871, Found: 485.0886.
4.2.7. General Procedure for the Synthesis of 11a-b
In a 10 mL round-bottomed flask, we added compound 6f or 7f (1.0 eq) in 5 mL DCM to give a colorless solution. BBr3 (5.0 eq) was added. The reaction mixture was held at rt with stirring on for 2 h. Then, 5 mL NaHCO3(aq) was added. The aq layer was extracted with DCM. We combined the organic layers and washed them with brine. The organic was dried with Na2SO4, filt and conc to obtain a crude product. The crude product was purified by column chromatography to give 11a-b.
4-(4-(hydroxymethyl)-3-(4-hydroxyphenyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (11a)
White solid (40 mg, 41% yield). m.p. 203–205 °C; 1H NMR (400 MHz, DMSO) δ 9.84 (s, 1H), 8.38 (d, J = 8.2 Hz, 1H), 8.12 (s, 1H), 7.97 (d, J = 6.5 Hz, 1H), 7.23 (d, J = 8.7 Hz, 2H), 6.89 (d, J = 8.7 Hz, 2H), 5.78 (t, J = 5.1 Hz, 1H), 3.79−3.68 (m, 1H), 3.44−3.34 (m, 1H), 1.32 (s, 3H). 13C NMR (101 MHz, DMSO) δ 181.09, 174.09, 157.94, 138.06, 136.20, 133.65, 131.23, 130.91, 130.62, 127.46, 127.41, 125.96, 123.50, 120.78, 115.88, 114.97, 108.41, 71.45, 63.52, 16.73. HRMS(ESI)m/z calcd for C19H14F3N3O3S [M+H]+: 422.0786, Found: 422.0783.
4-(4-(hydroxymethyl)-3-(4-hydroxyphenyl)-4-methyl-2,5-dioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (11b)
White solid (60 mg, 78% yield). m.p. 110–112 °C; 1H NMR (400 MHz, DMSO) δ 9.77 (s, 1H), 8.34 (d, J = 8.4 Hz, 1H), 8.17 (s, 1H), 8.06 (d, J = 8.5 Hz, 1H), 7.23 (d, J = 8.8 Hz, 2H), 6.86 (d, J = 8.8 Hz, 2H), 5.63 (t, J = 5.1 Hz, 1H), 3.71 (dd, J = 11.5, 4.9 Hz, 1H), 3.34 (d, J = 5.4 Hz, 1H), 1.27 (s, 3H). 13C NMR (101 MHz, DMSO) δ 173.36, 157.63, 153.33, 136.65, 136.29, 131.40, 131.08, 130.80, 129.75, 123.91, 123.65, 123.61, 123.52, 120.80, 115.80, 115.14, 106.81, 68.13, 63.12, 16.95. HRMS(ESI)m/z calcd for C19H14F3N3O4 [M+H]+: 406.1015, Found: 406.1019.
4.2.8. 5-nitro-3-(trifluoromethyl)pyridin-2-ol (13)
In a 500 mL round-bottomed flask, we added added 3-(trifluoromethyl)pyridin-2-ol (12) (9 g, 55 mmol, 1.0 eq) in 40 mL sulfuric acid to give an orange solution. The reaction mixture was cooled to 0 °C with stirring on. HNO3 (19 mL, 414 mmol, 7.5 eq) in 20 mL sulfuric acid was added slowly at 0 °C for 1 h. The reaction mixture was held at room temperature with stirring on for 6 hr. The mixture was poured into 500 mL ice-water with stirring on for 2 h. The reaction mixture was filtered through a Buchner funnel and washed with water to give 5-nitro-3-(trifluoromethyl)pyridin-2-ol (13) (8.6 g, 75% yield) as a white solid. m.p. 160–162 °C; 1H NMR (400 MHz, DMSO) δ 13.48 (s, 1H), 8.95 (d, J = 3.0 Hz, 1H), 8.47 (d, J = 2.6 Hz, 1H). 13C NMR (101 MHz, DMSO) δ 157.98, 142.35, 134.16, 134.11, 128.69, 126.01, 123.32, 120.61, 117.91, 117.31, 116.99, 116.68, 116.37.
4.2.9. 2-bromo-5-nitro-3-(trifluoromethyl)pyridine (14)
In a 100 mL round-bottomed flask, we added 5-nitro-3-(trifluoromethyl)pyridin-2-ol (13) (6 g, 28.8 mmol, 1.0 eq), phosphorus oxybromide (24.80 g, 86 mmol, 3.0 eq) and DMF (0.335 mL, 4.32 mmol, 0.15 eq) to give a yellow suspension. The reaction was heated to 110 °C with stirring on for 3 h. The reaction mixture was added into 200 g ice portion-wise, then, we adjusted the pH to 7. The aq layer was extracted with EA. We combined the organic layers and washed them with water and brine. The organic was dried with Na2SO4, filt and conc to give a crude product. The crude product was purified by column chromatography to give 2-bromo-5-nitro-3-(trifluoromethyl)pyridine (14) (7.61 g, 97% yield) as a light-yellow solid. m.p. 38–40 °C; 1H NMR (400 MHz, CDCl3) δ 9.36 (d, J = 1.2 Hz, 1H), 8.76 (d, J = 1.6 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 147.29, 145.58, 142.82, 131.60, 131.54, 131.49, 131.44, 129.28, 128.93, 128.58, 128.24, 125.24, 122.52, 119.80, 117.08.
4.2.10. 6-bromo-5-(trifluoromethyl)pyridin-3-amine (15)
In a 250 mL round-bottomed flask, we added 2-bromo-5-nitro-3-(trifluoromethyl)pyridine (14) (7.5 g, 27.7 mmol, 1.0 eq), iron (5.41 g, 97 mmol, 3.5 eq), and HOAc (23.77 mL, 415 mmol, 15 eq) in 25 mL ethyl acetate to give a black suspension. The reaction was heated to 65 °C with stirring on for 2 h. sat. Na2CO3 (aq) was added to adjust the pH to 10. The reaction mixture was filtered through a Buchner funnel. The aq layer was extracted with EA. We combined the organic layers and washed them with water and brine. The organic was dried with Na2SO4, filt and conc to give 6-bromo-5-(trifluoromethyl)pyridin-3-amine (15) (6.1 g, 91% yield) as a yellow solid. The product was used in the next step without further purification. 1H NMR (400 MHz, CDCl3) δ 7.97 (s, 1H), 7.26 (s, 1H), 3.81 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 141.94, 138.79, 127.51, 127.19, 125.38, 123.56, 121.82, 121.77, 121.72, 120.85.
4.2.11. 5-amino-3-(trifluoromethyl)picolinonitrile (16)
In a 250 mL round-bottomed flask, we added 6-bromo-5-(trifluoromethyl)pyridin-3-amine (15) (6 g, 24.90 mmol, 1.0 eq) and copper(I) cyanide (2.56 g, 28.6 mmol, 1.2 eq) in 60 mL NMP to give a black suspension. The reaction vessel was purged with nitrogen. The reaction mixture was heated to 160 °C with stirring on for 2 h. Then, 200 mL 25% EDA (aq) was added. The aq layer was extracted with EA. We combined the organic layers and washed them with 25% EDA (aq) and brine. The organic was dried with Na2SO4, filt and conc to give a crude product (7g). The crude product was purified by column chromatography to give 5-amino-3-(trifluoromethyl)picolinonitrile (16) (4.2 g, 90% yield) as a yellow solid. M.p. 148–150 °C; 1H NMR (400 MHz, DMSO) δ 8.17 (d, J = 1.7 Hz, 1H), 7.27 (d, J = 1.9 Hz, 1H), 6.97 (s, 2H). 13C NMR (101 MHz, DMSO) δ 147.96, 139.37, 130.34, 130.02, 129.70, 129.38, 126.28, 123.56, 120.84, 118.12, 116.01, 115.07, 115.02, 114.97, 114.93, 112.92.
4.2.12. 5-isothiocyanato-3-(trifluoromethyl)picolinonitrile (17)
In a 100 mL round-bottomed flask, we added 5-amino-3-(trifluoromethyl)picolinonitrile (16) (1.0 g, 5.34 mmol, 1.0 eq) in 20 mL DCM and 10 mL water to give an orange solution. Thiophosgene (0.451 mL, 5.88 mmol, 1.1 eq) was added. The reaction mixture was held at rt with stirring on for 16 h. The aq layer was extracted with DCM. We combined the organic layers and washed them with water and brine. The organic was dried with Na2SO4, filt and conc to give a crude product. The crude product was purified by column chromatography to give 5-isothiocyanato-3-(trifluoromethyl)picolinonitrile (17) (930 mg, 76% yield) as a yellow oil. The product was used in the next step without further purification. m.p. 30–32 °C; 1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 2.4 Hz, 1H), 8.72 (d, J = 2.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 113.61, 121.04, 127.41, 130.38, 131.44, 133.55, 144.75, 150.30.
4.3. Bioassays
4.3.1. Cell Preparation
C2C12 cells were provided and certified by the Cell Bank at Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences and confirmed as being negative for mycoplasma contamination. C2C12 were cultured in phenol red-free HG–DMEM medium (Life Technologies, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum, 50 IU/mL penicillin, 50 μg /mL streptomycin and 1% sodium pyruvate. Cells were maintained at 37 °C in a 5% CO2 incubator and seeded onto 10 cm cell culture dishes before transfection. After overnight culture, the cells were transiently co-transfected with AR-expressing plasmid (pSVAR0) and luciferase reporter gene vector (MMTV-Luc) using FuGENE® HD Transfection Reagent (Promega, Madison, WI, USA), while cells reached 80–90% confluence. After 18 h, the transfected cells were distributed to 384-well plates at a density of 15,000 cells per well and incubated for a further 6 h at 37 °C before compound treatment.
4.3.2. Antagonist Assay
Enzalutamide was used as a positive control. The antagonist activity of testing compounds was measured by Steadylite plus the Reporter Gene Assay System (PerkinElmer, Boston, MA, USA) according to the manufacturer’s instructions. In brief, after incubation for 6 h as mentioned above, 5 μL testing of compounds diluted in culture medium with eight different working concentrations (384 pM to 30 μM; enzalutamide (128 pM–10 μM)) was added to the cell well followed by 5 μL DHT (final concentration as EC80). After 24 h incubation, Steadylite reagent (50 μL, equal volume) was introduced, gently shaken for 2 min and kept at room temperature for 15 min before luminescence 384 measurement on an EnSpire multilabel plate reader (PerkinElmer).
4.3.3. Cytotoxicity
CellTiter-Glo® 2.0 Assay (Promega) was applied to assess cytotoxicity. In brief, cells were seeded onto 384-well plates at a density of 1500 cells per well and incubated for 24 h. Ten microliters of testing compounds diluted in culture medium was added and reacted for 24 h. CellTiter-Glo reagent was then introduced and the luminescence 384 was measured as above.
4.3.4. Anti-Proliferative Activity Assay
LNCaP cells were cultured in RPMI-1640 medium (Life Technologies, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum, 1% sodium pyruvate and 1% L-Glutamine. The cells were seeded into 96-well plates at a density of 8 × 104 cells per well. After 24 h incubation, the culture medium was removed and the cells were cultivated in the medium supplemented with 2% (v/v) fetal bovine serum for 2 days and then treated with different concentrations of test compounds for 3 days. Cell viability was measured with the CCK-8 kit (Dojindo).
4.4. Molecular Docking
All docking procedures were completed with programs as implemented in Schrodinger Suite.
The crystal structure of the androgen receptor (PDB: 2OZ7) was prepared in Protein Preparation Wizard with all water molecules deleted and bond orders assigned. The 2D structures of 19b, enzalutamide and apalutamide subjected to LigPrep and possible tautomeric states at pH 7.0 ± 2.0 were generated using Epik. Induced-fit docking was performed using the program Induced Fit and briefly consisted of several steps: first, the initial docking was performed using Glide, and then the sampling and minimization of sidechain residues within 5 Å of the docked ligand was achieved using Prime, followed by re-docking using Glide.
In the initial docking, the receptor van der Waals radii scaling was set at 0.50 and the ligand van der Waals radii was scaled at 0.5 to soften the potentials. Finally, the binding energy for each output pose was estimated as the IFDScore.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules27185867/s1, 1H, 13C, NMR and HRMS spectra of compounds.
Author Contributions
Conceptualization, W.L. and J.J.; methodology, J.J.; validation, M.-W.W., W.L. and J.J.; formal analysis, L.M.; investigation, L.M., Y.Z. and D.Y.; data curation, L.M. and J.J.; writing—original draft preparation, L.M.; writing—review and editing, L.M., J.J. and W.L.; visualization, J.J.; supervision, W.L.; project administration, W.L.; funding acquisition, W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the “Personalized Medicines-Molecular Signature-based Drug Discovery and Development Strategic Priority Research Program” of Chinese Academy of Sciences grants (XDA12020347 to M.-W.W.; XDA12040308 to D.Y.) and the Thousand Talents Program in China (to M.-W.W.). We are grateful for the financial support for Shanghai Science and Technology Council (16DZ2280100).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Yang Feng for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Heemers, H.V.; Verhoeven, G.; Swinnen, J.V. Androgen activation of the sterolregulatory element-binding protein pathway: Current insights. Mol. Endocrinol. 2006, 20, 2265–2277. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.J.; Mawji, N.R.; Wang, J.; Wang, G.; Haile, S.; Myung, J.K.; Watt, K.; Tam, T.; Yang, Y.C.; Bañuelos, C.A.; et al. Regression of cas-trate-recurrent prostate cancer by a small-molecule in hibitor of the amino-terminus domain of the androgen receptor. Cancer Cell 2010, 17, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Mellado, B.; Codony, J.; Ribal, M.J.; Visa, L.; Gascón, P. Molecular biology of androgen-independent prostate cancer; the role of the androgen receptor pathway. Clin. Transl. Oncol. 2009, 11, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Centenera, M.M.; Harris, J.M.; Tilley, W.D.; Butler, L.M. The contribution of different androgen receptor domains to receptor dimerization and signaling. Mol. Endocrinol. 2008, 22, 2373–2382. [Google Scholar] [CrossRef] [PubMed]
- Carver, B.S. Strategies for targeting the androgen receptor axis in prostate cancer. Drug Discov. Today 2014, 19, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Beer, T.M.; Higano, C.S.; Anand, A.; Taplin, M.E.; Efstathiou, E.; Rathkopf, D.; Shelkey, J.; Yu, E.Y.; Alumkal, J.; et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1-2 study. Lancet 2010, 375, 1437–1446. [Google Scholar] [CrossRef]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a Second-Generation Antiandrogen for Treatment of Advanced Prostate Cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Clegg, N.J.; Wongvipat, J.; Joseph, J.D.; Tran, C.; Ouk, S.; Dilhas, A.; Chen, Y.; Grillot, K.; Bischoff, E.D.; Cal, L.; et al. ARN-509: A Novel Antiandrogen for Prostate Cancer Treatment. Cancer Res. 2012, 72, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Shore, N.D.; Chowdhury, S.; Villers, A.; Klotz, L.; Siemens, D.R.; Phung, D.; van Os, S.; Hasabou, N.; Wang, F.; Bhattacharya, S.; et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): A randomised, double-blind, phase 2 study. Lancet Oncol. 2016, 17, 153–163. [Google Scholar] [CrossRef]
- Korpal, M.; Korn, J.M.; Gao, X.L.; Rakiec, D.P.; Ruddy, D.A.; Doshi, S.; Yuan, J.; Kovats, S.G.; Kim, S.; Cooke, V.G.; et al. An F876L Mutation in Androgen Receptor Confers Genetic and Phenotypic Resistance to MDV3100 (Enzalutamide). Cancer Discov. 2013, 3, 1030–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, J.D.; Lu, N.; Qian, J.; Sensintaffar, J.; Shao, G.; Brigham, D.; Moon, M.; Maneval, E.C.; Chen, I.; Darimont, B.; et al. A Clinically Relevant Androgen Receptor Mutation Confers Resistance to Second-Generation Antiandrogens Enzalutamide and ARN-509. Cancer Discov. 2013, 3, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Rathkopf, D.E.; Antonarakis, E.S.; Shore, N.D.; Tutrone, R.; Alumkal, J.J.; Ryan, C.J.; Saleh, M.N.; Hauke, R.J.; Maneval, E.C.; Scher, H.I. ARN-509 in men with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2013, 31, 48. [Google Scholar] [CrossRef]
- Pang, X.; Wang, Y.; Chen, Y. Design, synthesis, and biological evaluation of deuterated apalutamide with improved pharmacokinetic profiles. Bioorg. Med. Chem. Lett. 2017, 27, 2803–2806. [Google Scholar] [CrossRef] [PubMed]
- Savoie, P.R.; Welch, J.T. Preparation and utility of organic pentafluorosulfanylcontaining compounds. Chem. Rev. 2015, 115, 1130–1190. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.X.; Linton, A.; Kephart, S.; Ornelas, M.; Pairish, M.; Gonzalez, J.; Greasley, S.; Nagata, A.; Burke, B.J.; Edwards, M.; et al. Discovery of Aryloxy Tetramethylcyclobutanes as Novel Androgen Receptor Antagonists. J. Med. Chem. 2011, 54, 7693–7704. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.E.; Ouk, S.; Yoo, D.; Sawyers, C.L.; Chen, C.; Tran, C.; Wongvipat, J. Structure-Activity Relationship for Thiohydantoin Androgen Receptor Antagonists for Castration-Resistant Prostate Cancer (CRPC). J. Med. Chem. 2010, 53, 2779–2796. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.Y.; Huang, S.L.; Wang, L.; Leng, Y.; Lu, W. Design and synthesis of Atglistatin derivatives as adipose triglyceride lipase inhibitors. Chem. Biol. Drug Des. 2017, 90, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).