Abstract
The Eucalyptus genus (Myrtaceae) is characterized by a richness in essential oils (EO) with multiple biological activities. This study reports the chemical composition and the phytotoxic and antimicrobial activities of the EOs from Tunisian E. occidentalis, E. striaticalyx and E. stricklandii. The EOs were analyzed using GC/MS and their phytotoxicities were assessed against the germination and seedling growth of Sinapis arvensis, Trifolium campestre and Lolium rigidum. Antimicrobial activity was investigated against both Gram-negative (Pseudomonas aeruginosa, Escherichia coli and Acinetobacter baumannii) and Gram-positive (Staphylococcus aureus and Listeria monocytogenes) bacteria. The inhibition of biofilm formation and its metabolism was determined at different times. All EOs were rich in oxygenated monoterpenes (36.3–84.8%); the EO of E. occidentalis was rich in sesquiterpenes, both oxygenated and hydrocarbon (40.0% and 15.0%, respectively). Eucalyptol was the main constituent in all samples. The EOs showed phytotoxic activity on seed germination and seedling growth, depending both on chemical composition and weed. The EOs show a remarkable antibacterial potential resulting in a significant inhibition of the formation of bacterial biofilm and its metabolism, depending on the EO and the strain, with activity on the mature biofilm as well. Therefore, these Eucalyptus EOs could have potential applications both in the food and health fields.
1. Introduction
For a few years, the growing interest in natural products has been increased because of the ever-growing problem of antibiotic resistance and the use of synthetic products for pest control that have harmed both human health and the environment. Therefore, the exploration of natural substances from plants and trees appears as an alternative to the microbial resistance issue and a viable way find safe methods for weed control and management [1].
The genus Eucalyptus, which belongs to the Myrtaceae family, includes more than 700 species [2]. It is native to Australia and has become one of the most widely cultivated genera in the world [3]. In Tunisia, 117 Eucalyptus species have been introduced since 1957 [4], and scientific researchers, until today, continue to study their biological and pharmaceutical activities. Eucalyptus has been used in food and traditional medicine. The leaves of some Eucalyptus species have been proposed as a natural additive in the food industry and for health care [5], and their usefulness was also reported in the treatment of diseases and illness [6]. For example, leaves were used for respiratory infections, flu, and neurodegenerative and cardiovascular diseases [7,8].
Many species of this genus were recently reported for their broad spectrum of biological properties. E. globulus Labill., E. citriodora Hook. and E. stricklandii Maiden have been investigated for their antibacterial, cytotoxic, anti-inflammatory and antioxidant activities [9,10,11]. E. occidentalis Endl. has been studied for its insecticidal effects. Such activities can be attributed to the richness of eucalyptus in bioactive molecules, especially essential oils (EOs) that hold good promise as a replacement for antibiotics and herbicides [12]. EOs consist of a complex mixture of terpenes, phenols, ketones and aldehydes that act in synergy to bring the overall biological activities. Several reports investigated the antibacterial activities of Eucalyptus EOs [8] and demonstrated their action potential. Eucalyptus EOs can increase the permeability of the cell membranes of the bacterial strain, alter their microbial enzymes and cause cell death [8].
Eucalyptus EOs have also been investigated for their inhibitory potential on seed germination as well as their allelopathic activity [13]. They can inhibit the germination and growth of competing plants and have a spectrum of inhibitory effects against plants, pathogenic fungi and bacteria [14]. In addition, various bioactive molecules extracted from Eucalyptus species have allelopathic effects against weeds and microorganisms. However, according to our knowledge, no studies on the antibacterial and herbicidal activities of essential oils obtained from Eucalyptus occidentalis Endl., E. striaticalyx Maiden and E. stricklandii W. Fitzg. are known. Furthermore, their capacity to act against Gram-positive and Gram-negative bacteria biofilms, which let them increase their virulence and resistance to conventional antibiotics, is unknown.
We considered the current interest in Eucalyptus species and their multiple potentialities (especially antimicrobial and herbicidal activities). For this reason, our research aimed to study the chemical composition of EOs of three Eucalyptus species (E. occidentalis, E. striaticalyx and E. stricklandii). Furthermore, we evaluated their ability to inhibit biofilm formation and bacterial metabolism against Gram-negative (Pseudomonas aeruginosa, Escherichia coli and Acinetobacter baumannii) and Gram-positive bacteria (Staphylococcus aureus and Listeria monocytogenes). Moreover, this research reports data about the inhibitory effects of the three Eucalyptus EOs on seed germination and seedling growth of Sinapis arvensis L., Trifolium campestre Schreb. and Lolium rigidum Gaudin.
2. Results and Discussion
2.1. Essential Oil Yields
The essential oils were obtained by hydro-distillation of dried leaves in a Clevenger type apparatus, which were separated by the water phase. The extraction yield was calculated on a dry weight basis using the following formula:
where D.M = dry material and VEO = volume of essential oil.
yield = (VEO × 100)/D.M
The average yield of the EOs varied according to the species, resulting in 1.62%, 2.51% and 1.83% (W/DW) for E. occidentalis, E. striaticalyx and E. stricklandii, respectively. The available literature reports yields of 1.1% and 2% respectively for EOs from leaves of Tunisian E. occidentalis and E. stricklandii harvested from Hajeb Layoun Arboreta (Kairouen, central Tunisia) [15]. Bignell and coworkers [16] reported a yield of 1.73% for the EO of E. striaticalyx from Australia.
Several researches found that the EO yield depends on the species and is mainly influenced by environmental and genetic factors [17,18]. In addition, the yield can be affected by the extraction conditions and the time of harvest, since temperature and season have an essential effect on the terpene biosynthesis, causing it to be higher in summer than in winter [19,20].
2.2. Chemical Composition
The EOs were analyzed using GC and GC-MS. Table 1 reports the composition of essential oils according to their elution order on a HP-5 MS capillary column. Figure 1 shows the main components of the analyzed essential oils.

Table 1.
Chemical composition (%) of essential oils of E. occidentalis (A), E. striaticalyx (B) and E. stricklandii (C).
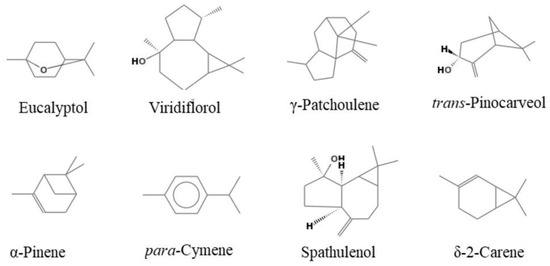
Figure 1.
Main components of the analyzed essential oils.
Sixty-one components were identified, corresponding to 97.1% of the total EOs of E. occidentalis and E. stricklandii and to 96.5% of the total EO of E. striaticalyx.
E. occidentalis EO has significant proportions of oxygenated monoterpenes (41.0%) and oxygenated sesquiterpenes (40.0%). Eucalyptol (40.8%), viridiflorol (29.9%) and γ-patchoulene (5.5%) were the main components identified in this oil. Oxygenated monoterpenes were dominant in E. stricklandii EO, with an amount of 84.8%. Eucalyptol (73.6%), trans-pinocarveol (6%) and α-pinene (4.3%) were the main components.
E. striaticalyx EO was found to be richer in monoterpene hydrocarbons (43.5%) than in oxygenated monoterpenes (36.3%), with eucalyptol (29.6%), p-cymene (19.4%), spathulenol (8.7%), δ-2-carene (8.5%) and α-pinene (7.4%) as the main components. Eucalyptol was the most abundant compound in all analyzed EOs.
The EO compositions of Eucalyptus species from different countries have been investigated in several studies. The EO from Australian E. occidentalis leaves was rich in bicyclogermacrene (28.52%), α-pinene (18.85%), β-caryophyllene (5.44%), viridiflorol (5.13%) and globulol (4.31%) [21]. This composition is different from the Tunisian samples in the current work. However, another study conducted by Elaissi and coworkers [15] on E. occidentalis harvested from Hajeb Layoun Arboreta in Tunisia found that the leaf EOs were rich in oxygenated monoterpenes, with eucalyptol (18.8%), aromadendrene (13.2%), globulol (4.4%), pinene (6.9%) and trans-pinocarveol (5.6%) as the main components. Bande-Borujeni and coworkers (2018) reported that an EO of E. occidentalis leaves from Iran was rich in τ -cadinol (17.2%), 1,8-cineole (15.5%), α-cadinol (14%) and α-pinene (9.21%).
In agreement with our data, Bignell and coworkers [16] reported that EO from the leaves of E. striaticalyx from Australia was rich in 1,8 cineole (77.5%), p-cymene (4.8%) and aromadendrene (2.4%). Our results on the chemical composition of the EO of E. striaticalyx disagree with the composition of EO obtained from leaves harvested at Hajeb Laâyoun arboretum (Tunisia), rich in non-oxygenated monoterpenes with α-terpinene (25.7%), limonene (14.5%), α-pinene (8.5%) and α-thujene (3.6%) as the main components [19].
Our data on the EO from E. stricklandii leaves were similar in composition to those obtained by Elaissi and coworkers [15], but with different percentages. The EO from E. stricklandii leaves from Hajeb Layoun (Tunisia) was rich in oxygenated monoterpenes (34.3%) and monoterpene hydrocarbons (13.0%), with 1,8 cineole (20.4%), α-pinene (11.2%) and trans-pinocarveol (7.5%) as principal constituents.
In our study, Eucalyptus EOs were rich in oxygenated monoterpenes and eucalyptol was the principal constituent. However, we observed a particular variation in the chemical composition among species. This variation can be due to the differences in Eucalyptus species and their geographical origins [22]. The differences can also be attributed to ecological factors, such as climatic conditions, the state of plant material (dry or fresh), the origin of the plant, extraction methods and also the genetic diversity [18,22,23].
2.3. Phytotoxic Activity
The herbicidal activity of the EOs against three very aggressive weeds in Tunisia, Sinapis arvensis and Trifolium campestre (dicots) and Lolium rigidum (monocot), was evaluated (Figure 2). According to the results, remarkable phytotoxic effects were exerted by the tested EOs, resulting in an inhibition of germination and growth of the aerial and root parts of tested weeds, independently from the monocot or dicot class.

Figure 2.
Phytotoxic effects of the studied EOs on S. arvensis (a), L. rigidum (b) and T. campestre (c).
The data relating to germination inhibition effects are shown in Table 2. For all tested EOs, a dose–response effect was highlighted according to the statistical analysis.

Table 2.
Inhibitory effect of the EOs on percentage germination of Lolium rigidum, Sinapis arvensis and Trifolium campestre.
For E. occidentalis, the inhibition of the germination of S. arvensis was total at the dose of 3 µL/mL, whereas, at the same dose, the inhibition was partial for T. campestre (20%) and L. rigidum (33%). These results testify the sensitivity of S. arvensis to E. occidentalis oils and the resistance of the other two seeds.
Similar results were noted for E. stricklandii and E. striaticalyx EOs. In fact, total inhibition of the germination of S. arvensis and T. compestre was recorded at 3µL/mL. However, at the same dose, the germination of L. rigidum was reduced to 6.7% and 10%with E. stricklandii and E. striaticalyx EOs, respectively.
In general, L. rigidum was the most resistant to the action of these EOs, while S. arvensis and T. campestre were the most sensitive. This behavior might be related to the selective resistance in mono and dicot weeds as described in recent papers [1,13]. In addition, E. stricklandii and E. striaticalyx EOs show a remarkable germination-inhibiting potential that exceeds the phytotoxic effect of E. occidentalis, which can be related to the different chemical compositions of the three tested species.
On the other hand, the action of the tested Eucalyptus EOs is accompanied by an inhibitory effect on the growth of the aerial parts when the inhibition of germination is partial (Table 3).

Table 3.
Inhibitory effect of the EOs on shoot growth (cm) of Lolium rigidum, Sinapis arvensis and Trifolium campestre.
According to the statistical analysis, a dose–response effect was recorded. All three EOs show an apparent effect on the growth of the aerial parts of tested weeds, and S. arvensis was always the most sensitive.
In addition, the tested EOs exerted similar effects on the growth of the roots of all tested weeds. This inhibition result was dose dependent, depending both on the tested weed and the EO (Table 4).

Table 4.
Inhibitory effect of the EOs on root growth (cm) of Lolium rigidum, Sinapis arvensis and Trifolium campestre.
This study first describes the phytotoxic effects of these EOs and agrees with the recent literature on EOs and crude extracts of E. erythrocorys F. Muell. that show a phytotoxic effect on the germination and growth of weeds, particularly against the germination and seedling growth of S. arvensis [13,24].
In a study carried out in Australia [25], the phytotoxic effects of 14 Eucalyptus EOs against the germination and growth of Lolium rigidum Gaudin (a monocot weed) were tested, resulting in a significant phytotoxic potential. Moreover, the authors evaluated the phytotoxicity of the EO components, with trans-pinocarveol and α-terpineol as the most active constituents.
In the current study, the EOs of E. occidentalis, E. striaticalyx and E. stricklandii were found to have variable chemical compositions, which can explain their different phytotoxic effects against the tested weeds. Several components are known for their herbicidal activity.
According to a study by De Martino and coworkers [26], 27 monoterpenes were tested for their anti-germinative activity against Lepidium sativum and Raphanus sativus. Some of those monoterpenes were also identified in the tested EOs.
The studied EOs show a specific richness in eucalyptol (1,8-cineole) (29.6–73.6%), an oxygenated monoterpene indeed known for its herbicidal effect. In fact, cineoles have been proposed as lead compounds for herbicides: cinmethylin, a commercial agrochemical, derives from 1,4-cineole (an isomer of eucalyptol), an oxygenated monoterpene found in EOs of various plants [27]. It inhibits germination and the growth of aerial and root parts; it also delays germination, inhibits chlorophyll pigment synthesis and inhibits cellular respiration [28]. Similarly, 1,8-cineole was reported to induce inhibition of germination and growth of weeds, and the application of these compounds induced a decrease in chlorophyll pigments and the mitotic index [29].
p-cymene, a compound representing 19.4% of the EO of E. striaticalyx, was known to possess herbicidal activity and completely inhibited the germination and seedling growth of Amaranthus retroflexus L., Chenopodium album L., and Rumex crispus L. [30]; this can explain the phytotoxic effects of E. striaticalyx EO. Sesquiterpenes are known for their allelopathic properties too, namely β-caryophyllene [31].
The mode of phytotoxic activity of EOs has been described in the literature. Their application generates oxidative stress, a release of malondialdehyde from the peroxidation of fatty acids of membrane phospholipids and an alteration of membrane integrity. The process induces a relative leakage of electrolytes and loss of vital membrane functions [32,33,34]. EOs disturb the synthesis of chlorophyll pigments, inducing an alteration of the energy balance [35]. In addition, Singh and coworkers [33] showed that the activities of antioxidant enzymes, such as superoxide dismutase, guaiacol peroxidase, catalase, ascorbate reductase and glutathione reductase, were significantly elevated, thereby indicating the enhanced generation of reactive oxygen species upon α-pinene exposure. Several monoterpenes affect chlorophyll content in plant seedlings; cell respiration; and the enzymatic activity of proteases, α- and β-amylases, peroxidases, and polyphenol oxidases in a dose-dependent way as a defense mechanism [34].
2.4. Antimicrobial Activity
Table 5 shows the minimal inhibitory concentration of the EOs needed to block the growth of the bacteria used as tester strains. Afterwards, we evaluated the ability of the EOs to affect bacterial adhesion and the mature bacterial biofilm. Then, we investigated the capacity of the EOs to act on the metabolism of the sessile cells, which can lead to an increase in bacterial virulence. These results are shown in Table 6 and Table 7.

Table 5.
MIC (µL/mL) of the EOs necessary to inhibit the growth of A. baumannii, E. coli, L. monocytogenes, P. aeruginosa and S. aureus. Tetracycline was used as positive control.

Table 6.
Percent inhibition of two doses of the EOs on biofilm formation of A. baumannii, E. coli, L. monocytogenes, P. aeruginosa and S. aureus, at 0 and 24 h.

Table 7.
Percent inhibition of two doses of the EOs on biofilm metabolic activity of A. baumannii, E. coli, L. monocytogenes, P. aeruginosa and S. aureus, at 0 and 24 h.
Overall, the EOs were all able to act above all against A. baumanni and S. aureus, inhibiting, ab origine, the formation of biofilm. A. baumanni was very sensitive, and at the higher concentration, the inhibitory capacity of the EOs never dropped below 46.49% (E. stricklandii EO), reaching 86.73% (E. occidentalis EO). S. aureus was also sensitive to all EOs, which inhibited its adhesion capacity by up to 60.46%. This value was reached in the presence of the E. striaticalyx EO, which had a behavior similar to that of A. baumannii. When tested at the highest concentration, the three EOs were also active against E. coli, L. monocytogenes and P. aeruginosa, reaching inhibition rates of 79.48% (EO of E. occidentalis vs. L. monocytogenes). Only the EO of E. stricklandii was ineffective vs. E. coli, showing a poor ability to inhibit adhesion (1.5%).
The inhibitory efficacy on the adhesion capacity had similar results to the activity of the three EOs on the metabolism of bacterial cells: a 67.75% inhibition (E. occidentalis EO vs. L. monocytogenes) was registered and, in any case, was never lower than 17.43%. Exciting results were also provided by the test carried out on mature biofilms after 24 h of growth. In this case, the inhibitory action of the EOs was maintained but with different potency. Even when a decrease by 50% in the inhibitory activity of the EOs was registered, the EO of E. stricklandii kept its inhibitory force practically intact vs. A. baumannii. In other cases, the EOs proved to be much more effective on the mature biofilm than on the initial adhesion capacity of bacterial strains; thus, the EO of E. stricklandii, which vs. L. monocytogenes caused a 36.86% inhibition, proved to be extraordinarily active on the mature biofilm of this strain, resulting in an almost total inhibition (97.67%). The same applies to the EO of E. striaticalyx, which, already, at 10 μL/mL, almost totally inhibited the mature biofilm of L. monocytogenes. The EO of E. stricklandii, also able to inhibit the adhesion of P. aeruginosa, (42.66%), proved to work much more effectively on the mature biofilm of this bacteria, reaching 60.65% inhibition. In the case of S. aureus, all the EOs acted much more powerfully on the mature biofilm, reaching an inhibition up to 83.02% (10 μL/mL of E. stricklanidii EO).
From the metabolic point of view, it seems that the EOs worked mainly on the metabolism of sessile cells. However, the fact that the EOs were utterly incapable of acting on the metabolism of S. aureus may mean that their action could be different, working, for example, on the cellular structure or the genetic material [36].
Considering the chemical composition of the three EOs, it is possible to correlate their effectiveness in preventing or at least limiting the virulence of pathogenic strains to the presence, above all, of the eucalyptol. This compound is an active antimicrobial agent against many Gram-positive and Gram-negative pathogens, including those used in our experiments. It can act on the quorum sensing of bacteria, therefore upstream of the whole process that leads to the formation of the biofilm [37]. According to LaSarre and Federle [38], eucalyptol can act on the quorum sensing mechanism but not on the vital functions of A. baumannii. In the case of S. aureus, the presence of eucalyptol could have determined unwanted apoptosis, and, considering E. coli, the compound could have caused a robust condensation process of the nuclear chromatin present in its bacterial nucleosome [39].
Our results agree with Maczka and coworkers [39] as regards the effects of the EOs on the adhesion of S. aureus, but not about their action on the mature biofilm, where presumably the presence of other components may have somewhat held back the EOs’ effectiveness in acting on the metabolism of its sessile cells.
3. Materials and Methods
3.1. Plant Material
Leaves of Eucalyptus occidentalis, E. striaticalyx and E. stricklandii were collected at Djebbel Mansour arboretum of the governorate of Zaghouen, Tunisia. Six samples harvested from different trees equidistant at at least 20 m were collected for each species. The samples were then stored in a glass greenhouse for drying for 15 days. Mature seeds of annual weeds Sinapis arvensis, Lolium rigidum and Trifolium campestre were harvested from crop fields. The data about arboreta, climatic conditions, and the date of collection are listed in Table 8. Plants were identified by Professor Lamia Hamrouni, and voucher specimens were stored in the herbarium section of the Institut National de la Recherche en Génie Rural, Eaux et Forêts (INRGREF), Tunis.

Table 8.
Species, date and site of harvest of the plant material.
3.2. Isolation and Analysis of the Essential Oils
The EOs were obtained by hydro-distillation of dried leaves in a Clevenger-type apparatus. The EOs were collected and dried over anhydrous sodium sulfate and stored in a brown glass bottle at 4 °C until used. Yield was calculated based on dried weight (w/w%).
GC and GC-MS were used to examine the composition of the essential oil. GC analyses were performed using a Perkin-Elmer Sigma 115 gas chromatograph equipped with a flame ionization detector (FID) and a non-polar HP-5 MS capillary column of fused silica (30 m × 0.25 mm; 0.25 μm film thickness). The operating conditions were the injector and detector temperatures of 250 °C and 290 °C, respectively. The analysis was conducted on a scheduled basis: 5 min isothermally at 40 °C; subsequently, the temperature was increased by two °C/min until 270 °C, and finally, it was kept in isotherm for 20 min. The analysis was also performed on an HP Innowax column (50 m × 0.20 nm; 0.25 μm film thickness). In both cases, helium was used as a carrier gas (1.0 mL/min). GC-MS analysis was performed using an Agilent 6850 Ser. II Apparatus equipped with a DB-5 fused silica capillary column (30 m × 0.25 mm; 0.25 μm film thickness) and connected to an Agilent Mass Selective Detector (MSD 5973); ionization voltage 70 V; ion multiplier energy 2000 V. The mass spectra were scanned in the range of 40–500 amu, with five scans per second. The chromatographic conditions were as reported above; transfer line temperature, 295 °C. Most of the components were identified by comparing their Kovats indices (Ki) with those in the literature [40,41,42] and by careful analysis of the mass spectra compared to those of pure compounds available in our laboratory or to those present in the NIST 02 and Wiley 257 mass libraries [43]. The Kovats indices were determined with a homologous series of n-alkanes (C10-C35) under the same operating conditions. For some compounds, the identification was confirmed by co-injection with standard compounds.
3.3. Phytotoxic Activity
The seeds of Sinapis arvensis, Lolium rigidum and Trifolium campestre were used in phytotoxic activity assays. Before germination tests, seeds were disinfected with 5% sodium hypochlorite, then rinsed with water. Twenty seeds were put in Petri dishes lined with double-layer filter paper Whatman No.1 and treated with different doses (0, 0.75, 1.50, 2.25 and 3.00 μL/mL) of Eucalyptus EOs in a solution of Tween 20 (0.1%) [13]. The tests were carried out completely randomized, with three replicates for each dose. After 12 days, the germination percentages were calculated, and roots and shoots growth were measured in cm. However, no significant differences (p ≤ 0.05) were found between negative control (pure water) and control (Tween solution), which explains the non-phytotoxic effects of Tween solution.
3.4. Antimicrobial Activity
3.4.1. Microorganisms and Culture Conditions
Acinetobacter baumannii ATCC 19606, Pseudomonas aeruginosa DSM 50071 and Escherichia coli DSM 8579 (Gram-negative bacteria) and Staphylococcus aureus subsp. aureus Rosebach ATCC 25923 and Listeria monocytogenes ATCC 7644 (Gram-positive bacteria) were used as bacterial test strains. They were cultured in Luria broth for 18 h at 37 °C, and centrifuged at 80 rpm (Corning LSE, Pisa, Italy) before the microbial analysis. A. baumannii was cultured at 35 °C under the same conditions.
3.4.2. Minimal Inhibitory Concentration (MIC)
The MIC of each EO was evaluated following the method described by Sarker and coworkers [44], modified as follows. The test was performed using 96 microtiter-plates. The resazurin solution was prepared by dissolving 270 mg homogenously in 40 mL of previously sterilized deionized water. 100 μL of samples, previously resuspended in 10% (v/v) DMSO, was put into the first row of the plate. To all other wells, we added 50 μL of Luria–Bertani broth or normal sterile solution. Then, we performed serial descending concentrations of our samples, and we added to each well 10 μL of resazurin indicator solution. 30 μL of 3.3× strength isosensitized broth and 10 μL of bacterial suspension (5 × 106 cfu/mL) were included in each well. The plates were well covered with parafilm to avoid dehydration, due to the little volume present in each well. As the positive control, wide-spectrum conventional antibiotic tetracycline (previously suspended in DMSO) was added in a column of the plate. Luria–Bertani broth was considered as the negative control. The plates were incubated at 37 °C (or at 35 °C for A. baumannii) for 24 h. The value of the MIC was revealed by the color change from dark purple to colorless. Tetracycline was chosen as a conventional antibiotic with a wide spectrum of action. This compound was used in other similar works [45,46].
3.5. Biofilm Inhibitory Activity
The capacity of the EOs to influence bacterial adhesion was investigated using flat-bottomed 96-well microtiter plates [47]. Before the test, the bacterial cultures were regulated to 0.5 McFarland with fresh culture broth. Then, 10 µL of the bacterial cultures and 10 or 20 µL/mL of the EOs were put in each well, and the wells were filled with different volumes of Luria–Bertani broth to have a final volume of 250 µL/well. The plates were coated with parafilm tape to avoid evaporation and incubated for 48 h at 37 °C (or at 35 °C for A. baumannii). Following the removal of the planktonic cells, sessile cells were delicately washed twice with sterile PBS, which was removed, and the plates were left for 10 min under a laminar flow hood. 200 µL of methanol was incorporated in each well to allow for the fixation of the sessile cells and discarded after 15 min. Each plate was left to allow for the dryness of the samples. The staining of the sessile cells was obtained by adding 200 µL of 2% w/v crystal violet solution/well. After 20 min, the staining solution was discarded; plates were softly washed with sterile PBS and left to dry. The release of the bound dye was allowed by adding 200 µL of glacial acetic acid 20% w/v. The absorbance was measured at λ = 540 nm (Cary Varian, Milano, Italy). The percent value of adhesion was calculated with respect to the control (formed by the cells grown without the presence of the samples, inhibition rate 0%). Triplicate tests were performed, and the average results were taken for reproducibility.
3.5.1. Activity on Mature Bacterial Biofilm
The overnight bacterial cultures were adjusted to 0.5 McFarland with fresh Luria–Bertani culture broth, and 10 μL were added to flat-bottomed 96-well microtiter plates to have a final volume of 250 μL/well. Then, microplates were fully coated with parafilm tape to avoid evaporation and incubated at 37 °C (35 °C for A. baumannii). After 24 h of bacterial growth, the planktonic cells were removed, and the two concentrations of the EOs, 10 and 20 μL/mL, and Luria-Bertani broth were added to reach a final volume of 250 μL/well. After 24 h of incubation, the sequential steps of the experiment, including the calculation of the percent value of inhibition compared with the untreated bacteria, were performed as previously described.
3.5.2. Effects of EOs on Cell Metabolic Activity within the Biofilm
The effect on the metabolic activity of the bacterial cells of two concentrations (10 and 20 μL/mL) of the EOs, which were added at the beginning of the bacterial growth and after 24 h of incubation, was also investigated through the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric method [46]. After 48 h total of incubation, the bacterial suspension, representing the planktonic cells, was removed; 150 μL of PBS and 30 μL of 0.3% MTT (Sigma, Milano, Italy) were added. The microplates were kept at 37 °C (35 °C for A. baumannii). After two hours, the MTT solution was removed, two washing steps were performed with 200 μL of sterile physiological solution, and 200 μL of dimethyl sulfoxide (DMSO) were added to allow the dissolution of the formazan crystals, measured after two hours at λ = 570 nm (Cary Varian, Milano, Italy).
3.6. Statistical Analysis
Data were subjected to one-way analysis of variance (ANOVA) using the SPSS 18.0 software package. Differences between means were tested through the Student–Newman–Keuls test, and values with p ≤ 0.05 were considered significantly different.
4. Conclusions
The data obtained confirmed the vast literature on the phytotoxic and allelopathic activity of Eucalyptus EOs. These were effective against the Gram-positive and Gram-negative bacteria who are generally more reluctant to undergo the action of conventional antibiotics. It is generally challenging to find a substance capable of decreasing bacterial virulence and limiting all the mechanisms leading to increased bacterial aggression, leading to more difficulty in eradicating the infections they trigger. Therefore, the activity exhibited by these Eucalyptus EOs against the pathogens used in our experiments could be considered of noticeable meaning, both for food and health purposes. In fact, in the last years, the extension of some infections was correlated to the expansion of the presence, in several environments (including foods, workplaces and hospital), of some bacteria, such as P. aeruginosa, S. aureus, E. coli, L. monocytogenes and A. baumannii, which developed robust evolutionary drug resistance due to their careless use, often in situations where their application was to be considered inefficient and inappropriate. Their higher drug resistance lets them form biofilms more quickly, causing a critical problem for food and health. Thus, the interest in natural alternatives to prevent biofilm formation increased the search for natural agents as alternatives to conventional sanitizers to control the biofilm’s development. Furthermore, the EOs were capable of acting not only at the beginning of the biofilm formation process but also on the mature biofilm, when the bacterial cells are more protected in the polymeric niches, becoming less sensitive to the action of the conventional drugs. This fact allows for the hypothesizing of their possible use in both the health and food fields.
Author Contributions
Conceptualization, I.A. and V.D.F.; methodology, I.A., L.H., F.N. and V.D.F.; formal analysis, I.A., L.H. and F.N..; investigation, M.K., F.P., S.K. and F.F.; resources, I.A., V.D.F. and F.N.; data curation, M.K., F.P., S.K. and F.F.; writing—original draft preparation, M.K., F.P., I.A. and F.N.; writing—review and editing, I.A., V.D.F. and F.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the essential oils are available from the authors.
References
- Amri, I.; Khammassi, M.; Gargouri, S.; Hanana, M.; Jamoussi, B.; Hamrouni, L.; Mabrouk, Y. Tunisian pine essential oils: Chemical composition, herbicidal and antifungal properties. J. Essent. Oil-Bear. Plants 2022, 25, 430–443. [Google Scholar] [CrossRef]
- Cheng, S.S.; Huang, C.G.; Chen, Y.J.; Yu, J.J.; Chen, W.J.; Chang, S.T. Chemical compositions and larvicidal activities of leaf essential oils from two Eucalyptus species. Bioresour. Technol. 2009, 100, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, J.; Kong, Q.; Luo, S.; Wang, J.; Feng, S.; Yuan, M.; Chen, T.; Yuan, S.; Ding, C. Chemical composition, antioxidant, antimicrobial, and phytotoxic potential of Eucalyptus grandis × E. urophylla leaves essential oils. Molecules 2021, 26, 1450. [Google Scholar] [CrossRef] [PubMed]
- Limam, H.; Ben Jemaa, M.; Tammar, S.; Ksibi, N.; Khammassi, N.; Jallouli, S.; Del Re, G.; Msaada, K. Variation in chemical profile of leaves essential oils from thirteen Tunisian Eucalyptus species and evaluation of their antioxidant and antibacterial properties. Ind. Crops Prod. 2020, 158, 112964. [Google Scholar] [CrossRef]
- Amakura, Y.; Yoshimura, M.; Sugimoto, N.; Yamazaki, T.; Yoshida, T. Marker constituents of the natural antioxidant Eucalyptus leaf extract for the evaluation of food additives. Biosci. Biotechnol. Biochem. 2009, 73, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Sahebkar, A.; Afshani, M.R.; Keshvari, M.; Haghjooyjavanmard, S.; Rafieian Kopaei, M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother. Res. 2014, 28, 193–199. [Google Scholar] [CrossRef]
- Msaada, K.; Salem, N.; Bachrouch, O.; Bousselmi, S.; Tammar, S.; Alfaify, A.; Al Sane, K.; Ben Ammar, W.; Azeiz, S.; Haj Brahim, A.; et al. Chemical composition and antioxidant and antimicrobial activities of wormwood (Artemisia absinthium L.) essential oils and phenolics. J. Chem. 2015, 2015, 804658. [Google Scholar] [CrossRef]
- Harkat-Madouria, L.; Asma, B.; Madania, K.; Bey-Ould Si Saida, Z.; Rigouc, P.; Grenierd, D.; Allaloua, H.; Reminia, H.; Adjaouda, A.; Boulekbache-Makhlouf, L. Chemical composition, antibacterial and antioxidant activities of essential oil of Eucalyptus globulus from Algeria. Ind. Crops Prod. 2015, 78, 148–153. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kohli, R.K.; Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2009, 256, 2166–2174. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Quispe, C.; Llaique, H.; Villalobos, M.; Smeriglio, A.; Trombetta, D.; Ezzat, S.M.; Salem, M.A.; Zayed, A.; et al. Insights into Eucalyptus genus chemical constituents, biological activities and health-promoting effects. Trends Food Sci. Technol. 2019, 91, 609–624. [Google Scholar] [CrossRef]
- Ben Hassine, D.; Kammoun El Euch, S.; Souchard, J.P.; Cazaux, S.; Abderrabba, M.; Bouajila, J. Phytochemical study and pharmaceutical properties of essential oils and organic extracts of two Eucalyptus species: E. stricklandii Maiden and E. brevifolia F. Muell. J. Essent. Oil Res. 2022, 34, 339–351. [Google Scholar] [CrossRef]
- Bande-Borujeni, S.; Zandi-Sohani, N.; Ramezani, L. Chemical composition and bioactivity of essential oil from Eucalyptus occidentalis leaves against two stored product pests. Int. J. Trop. Insect Sci. 2008, 38, 216–223. [Google Scholar] [CrossRef]
- Ben Ghnaya, A.; Hamrouni, L.; Amri, I.; Ahoues, H.; Hanana, M.; Romane, A. Study of allelopathic effects of Eucalyptus erythrocorys L. crude extracts against germination and seedling growth of weeds and wheat. Nat. Prod. Res. 2016, 30, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, S.; Saharkhiz, M.J.; Ramezani, F.; Fotokian, M.H. Use of essential oils as bioherbicides. J. Essent. Oil-Bear. Plants 2008, 11, 319–327. [Google Scholar] [CrossRef]
- Elaissi, A.; Medini, H.; Marzouki, H.; Larbi Khouja, M.; Lynene, F.; Chemli, R.; Harzallah-Skhiri, F. Variation in volatile leaf oils of twelve eucalyptus species harvested from Hajeb Layoun arboreta (Tunisia). Chem. Biodivers. 2010, 27, 705–716. [Google Scholar] [CrossRef]
- Bignell, C.M.; Dunlop, P.J.; Brophy, J.J. Volatile leaf oils of some South-western and Southern Australian species of the genus Eucalyptus (series I). Part XVII: Subgenus Symphyomyrtus (i) Section Bisectaria, Series Calycogonae and (ii) Section Dumaria, Series Dumosae, Series Rigentes and Series Ovulares. Flavour Fragr. J. 1997, 12, 269–275. [Google Scholar] [CrossRef]
- Khammassi, M.; Mighri, H.; Ben Mansour, M.; Amri, I.; Jamoussi, B.; Khaldi, A. Metabolite profiling and potential antioxidant activity of sixteen fennel (Foeniculum vulgare Mill.) populations wild-growing in Tunisia. S. Afr. J. Bot. 2022, 148, 407–414. [Google Scholar] [CrossRef]
- Saoud, I.; Hamrouni, L.; Gargouri, S.; Amri, I.; Hanana, M.; Fezzani, T.; Bouzid, S.; Jamoussi, B. Chemical composition, weed killer and antifungal activities of Tunisian thyme (Thymus capitatus Hoff. et Link.) essential oils. Acta Aliment. 2013, 42, 417–427. [Google Scholar] [CrossRef]
- Elaissi, A.; Chraif, I.; Bannour, F.; Farhat, F.; Ben Salah, M.; Chemli, R.; Khouja, M.L. Contribution to the Qualitative and Quantitative Study of Seven Eucalyptus Species Essential Oil Harvested of Hajeb’s Layoun Arboreta (Tunisia). J. Essent. Oil-Bear. Plants 2007, 10, 15–25. [Google Scholar] [CrossRef]
- Bagheri, H.; Manap, M.Y.B.A.; Solati, Z. Antioxidant activity of Piper nigrum L. essential oil extracted by supercritical CO2 extraction and hydro-distillation. Talanta 2014, 121, 220–228. [Google Scholar] [CrossRef]
- Bignell, C.M.; Dunlop, P.J.; Brophy, J.J.; Jackson, J.F. Volatile leaf oils of some South-western and Southern Australian species of the genus Eucalyptus. Part XI. Subgenus Symphyomyrtus. A—Section Bisectaria. (a) Series Occidentales, (b) unpublished Series Annulatae, (c) Series Micromembranae, (d) Series Obliquae, (e) Series Dundasianae, (f) Series Cooperianae, (g) Series Halophilae, (h) Series Salmonophloiae, and (i) Series Pubescentes. B—Section Dumaria. (a) Series Merrickianae. Flavour Fragr. J. 1996, 11, 107–112. [Google Scholar] [CrossRef]
- Diäaz-Maroto, M.C.; Perez-Coello, M.S.; Esteban, J.; Sanz, J.S. Comparison of the volatile composition of wild fennel samples (Foeniculum vulgare Mill.) from central Spain. J. Agric. Food Chem. 2006, 54, 6814–6818. [Google Scholar] [CrossRef] [PubMed]
- Telci, I.; Toncer, O.G.; Sahbaz, N. Yield, essential oil content and composition of Coriandrum sativum varieties (var. vulgare Alef and var. microcarpum DC.) grown in two different locations. J. Essent. Oil Res. 2006, 18, 189–193. [Google Scholar] [CrossRef]
- Amri, I.; Mancini, E.; De Martino, L.; Marandino, A.; Lamia, H.; Mohsen, H.; Bassem, J.; Scognamiglio, M.R.; Reverchon, E.; De Feo, V. Chemical composition and biological activities of the essential oils from three Melaleuca species grown in Tunisia. Int. J. Mol. Sci. 2012, 13, 16580–16591. [Google Scholar] [CrossRef]
- Li, A.; Wu, H.; Feng, Y.; Deng, S.; Hou, A.; Che, F.; Liu, Y.; Geng, Q.; Ni, H.; Wei, Y. A strategy of rapidly screening out herbicidal chemicals from Eucalyptus essential oils. Pest. Manag. Sci. 2020, 76, 917–927. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; Mancini, E.; Rolim de Almeida, L.F.; De Feo, V. The antigerminative activity of twenty-seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Cavers, P.B.; Bernards, M.A. Isolation and structural characterization of a water-soluble germination inhibitor from scotch thistle (Onopordum acanthium) cypselas. J. Chem. Ecol. 2003, 29, 2425–2438. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathic effect of two volatile monoterpenes against bill goat weed (Ageratum conyzoides L.). Crop. Prot. 2002, 21, 347–350. [Google Scholar] [CrossRef]
- Romagni, J.G.; Allen, S.N.; Dayan, F.E. Allelopathic effects of volatile cineoles on two weedy plant species. J. Chem. Ecol. 2000, 26, 303–313. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef]
- Araniti, F.; Sanchéz Moreiras, A.M.; Graña, E.; Reigosa, M.G.; Abenavoli, M. R: Terpenoid trans-caryophyllene inhibits weed germination and induces plant water status alteration and oxidative damage in adult Arabidopsis. Plant Biol. 2017, 19, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Abrahim, D.; Francischini, A.C.; Pergo, E.M.; Kelmer-Bracht, A.M.; Ishii-Iwamoto, E.L. Effects of α-pinene on the mitochondrial respiration of maize seedlings. Plant Physiol. Biochem. 2003, 41, 985–991. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Arora, K.; Kohli, R.K. a-Pinene inhibits growth and induces oxidative stress in roots. Ann. Bot. 2006, 98, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Chowhan, N.; Singh, H.P.; Kohl, R.K. Phytotoxic effects of β-pinene on early growth and associated biochemical changes in rice. Acta Physiol. Plant. 2011, 33, 2369–2376. [Google Scholar] [CrossRef]
- Podesta, F.E.; Plaxton, W.C. Regulation of cytosolic carbon metabolism in germinating Ricinus communis cotyledons: I. Developmental profiles for the activity, concentration, and molecular structure of the pyrophosphate-and ATP-dependent phosphofructokinase, phosphoenolpyruvate carboxylase and pyruvate kinase. Planta 1994, 194, 374–380. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R. Quorum sensing and phytochemicals. Int. J. Mol. Sci. 2013, 14, 12607–12619. [Google Scholar] [CrossRef]
- LaSarre, B.; Federle, M.J. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. Rev. 2013, 77, 73–111. [Google Scholar] [CrossRef]
- Mączka, W.; Duda-Madej, A.; Górny, A.; Grabarczyk, M.; Wińska, K. Can eucalyptol replace antibiotics? Molecules 2021, 26, 4933. [Google Scholar] [CrossRef]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavour and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Co.: Carol Stream, IL, USA, 2007. [Google Scholar]
- McLafferty, F.W. Wiley Registry of Mass Spectral Data, with NIST Spectral Data CD Rom, 7th ed.; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Polito, F.; Amato, G.; Caputo, L.; Francolino, R.; d’Acierno, A.; Fratianni, F.; Candido, V.; Coppola, R.; De Feo, V. Chemical composition of essential oils and bulbs and aerial parts of two cultivars of Allium sativum and their biofilm activity agaianst food and nosocomial pathogens. Antibiotics 2022, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; d’Acierno, A.; Ombra, M.N.; Amato, G.; De Feo, V.; Ayala-Zaval, J.F.; Coppola, R.; Nazzaro, F. Fatty acid composition and in vitro anti-inflammatory activity of five cold-pressed Prunus seed oils, and their anti-biofilm effect against pathogenic bacteria. Front. Nutr. 2021, 8, 775751. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Ombra, M.N.; d’Acierno, A.; Caputo, L.; Amato, G.; De Feo, V.; Coppola, R.; Nazzaro, F. Polyphenols content and in vitro α-glycosidase activity of different italian monofloral honeys, and their effect on selected pathogenic and probiotic bacteria. Microorganisms 2021, 9, 1694. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).