Confined Crystallization of Thin Plasma-Polymerized Nanocomposite Films with Maleic Anhydride and Cellulose Nanocrystals under Hydrolysis
Abstract
1. Introduction
2. Results
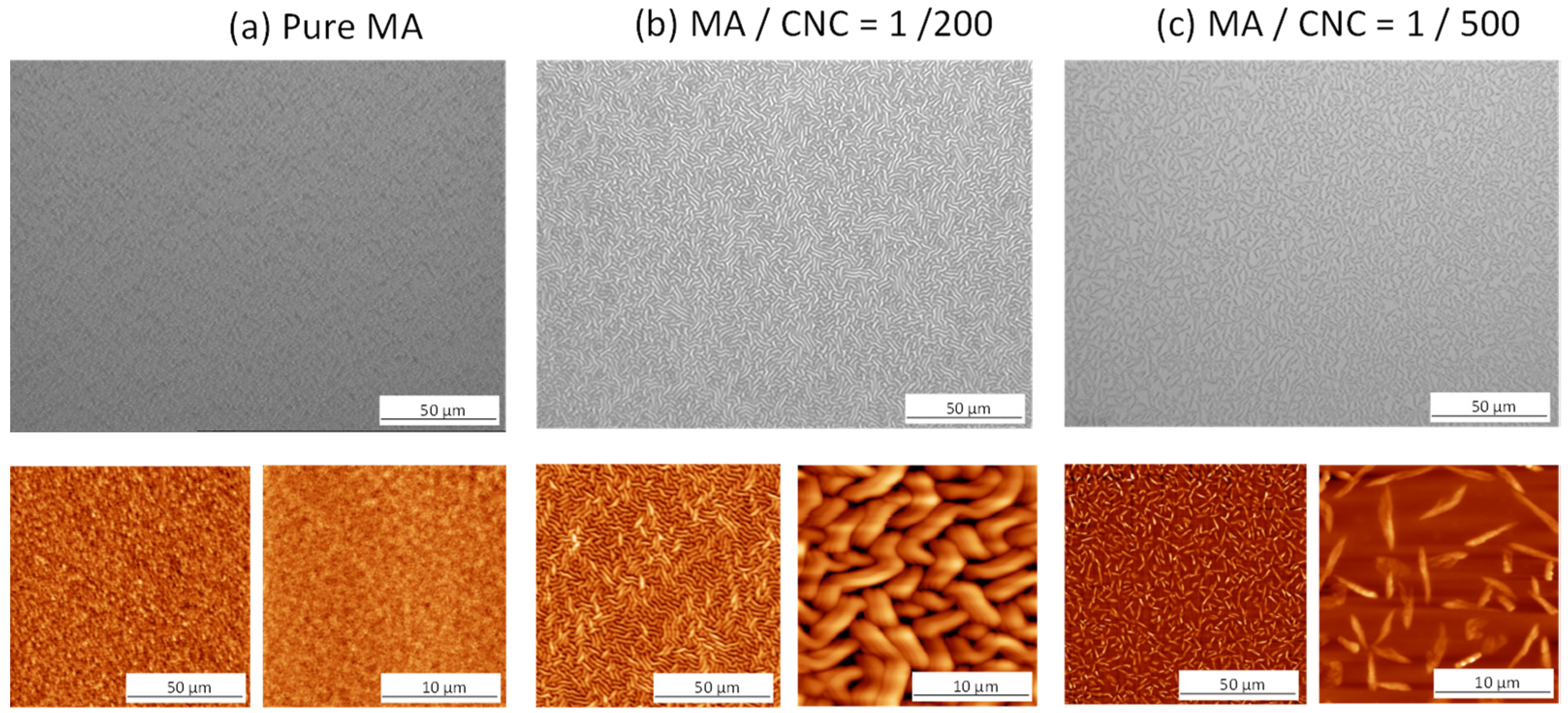
2.1. Morphological Analysis of Plasma-Deposited Coatings
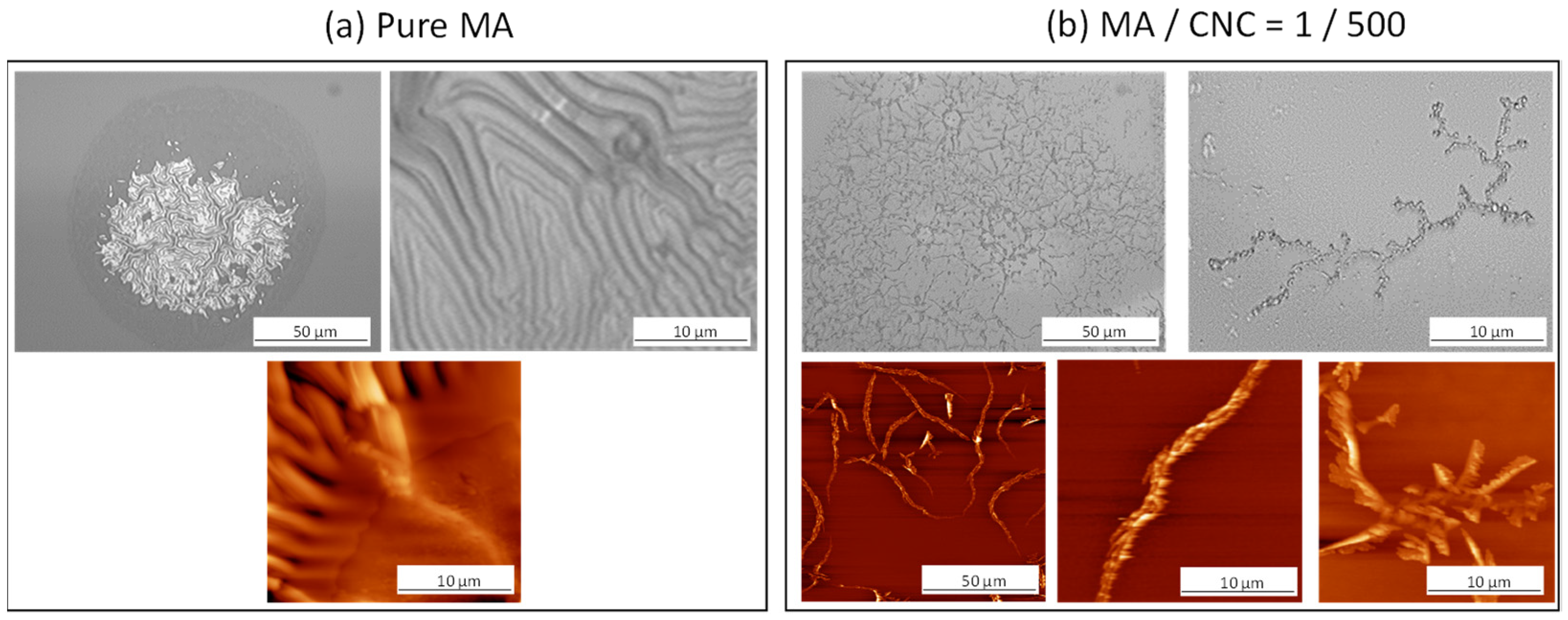
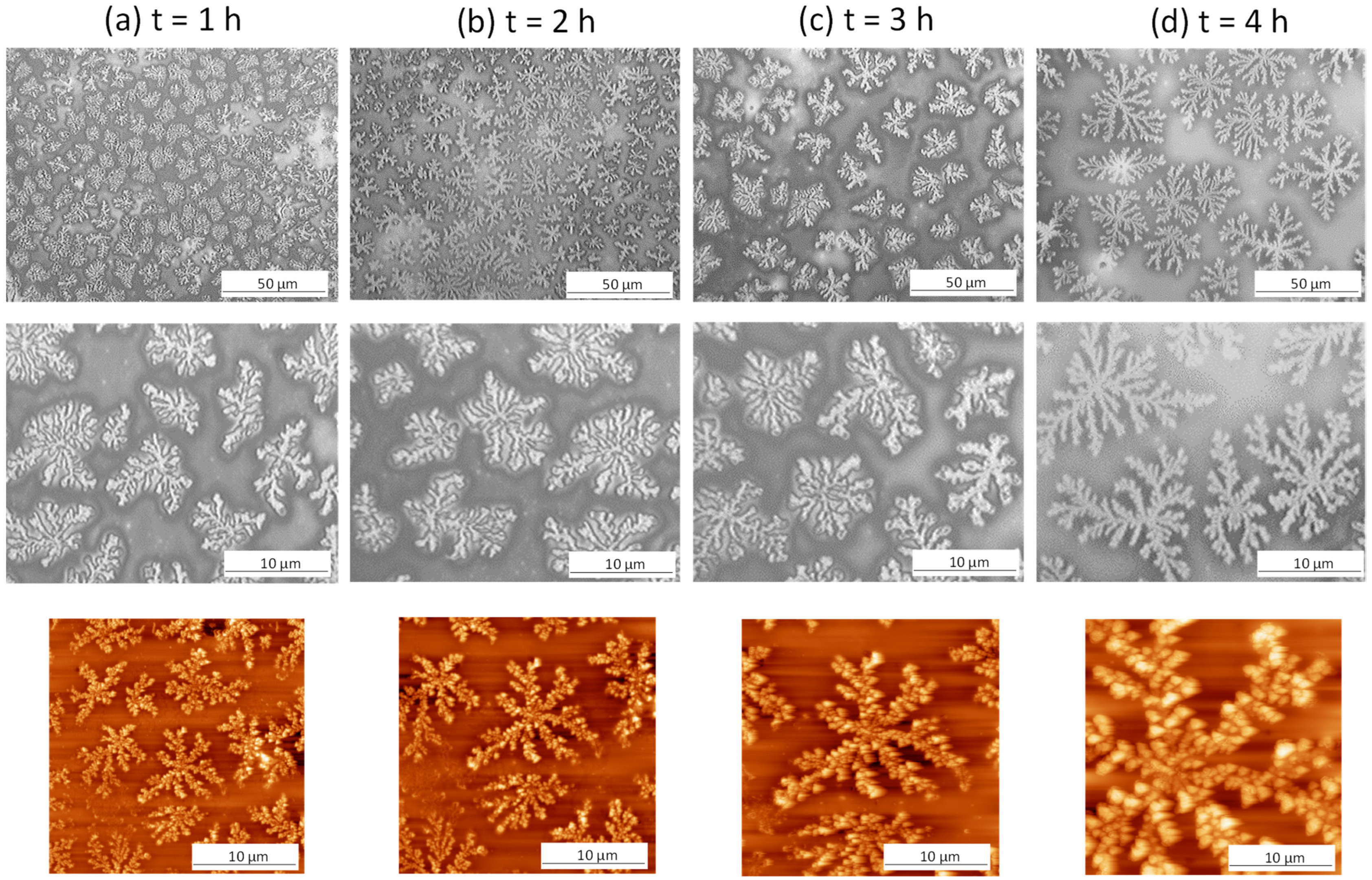
2.2. Morphological Analysis of Hydrolyzed Coatings
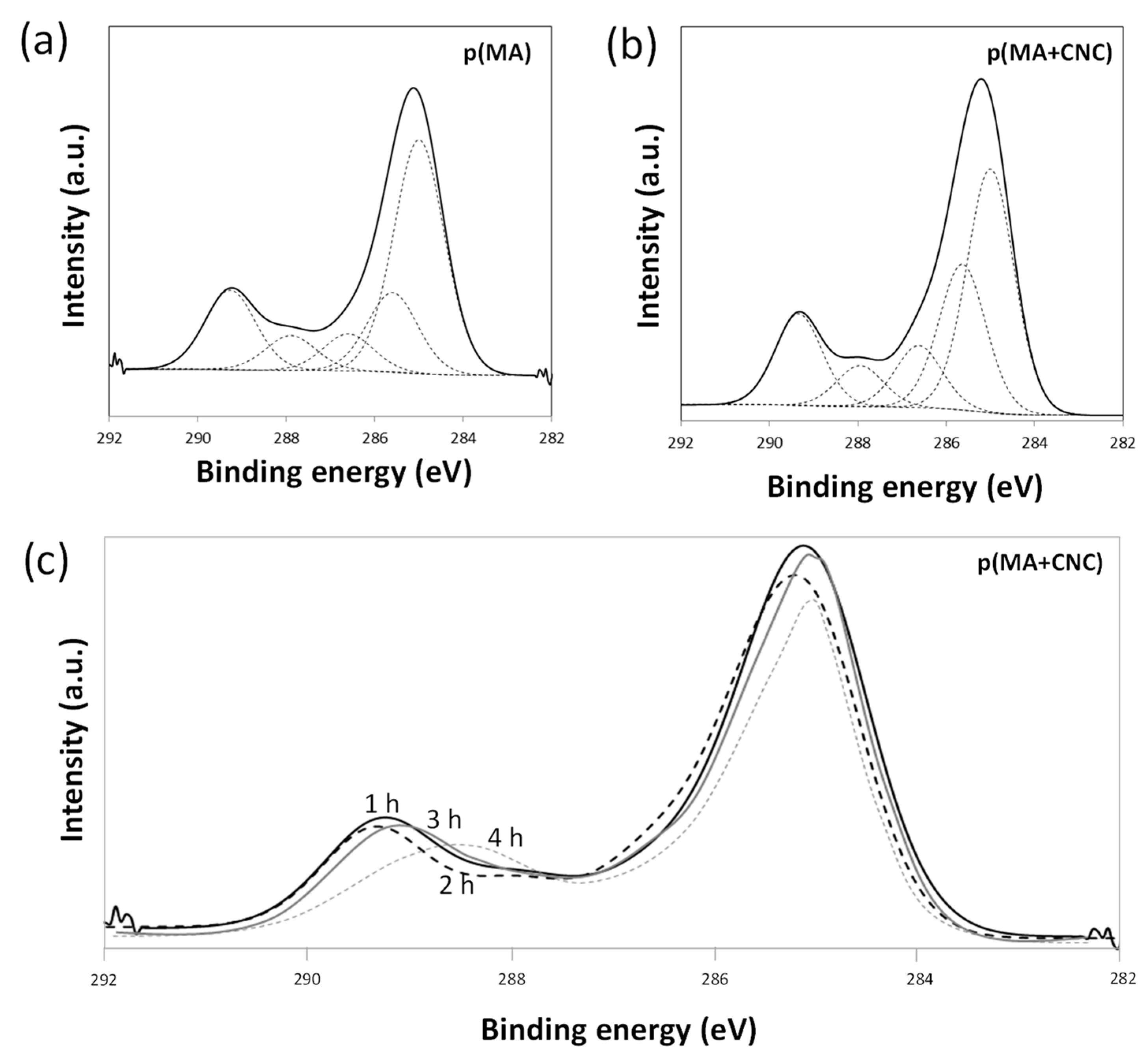
2.3. Chemical Analysis by XPS
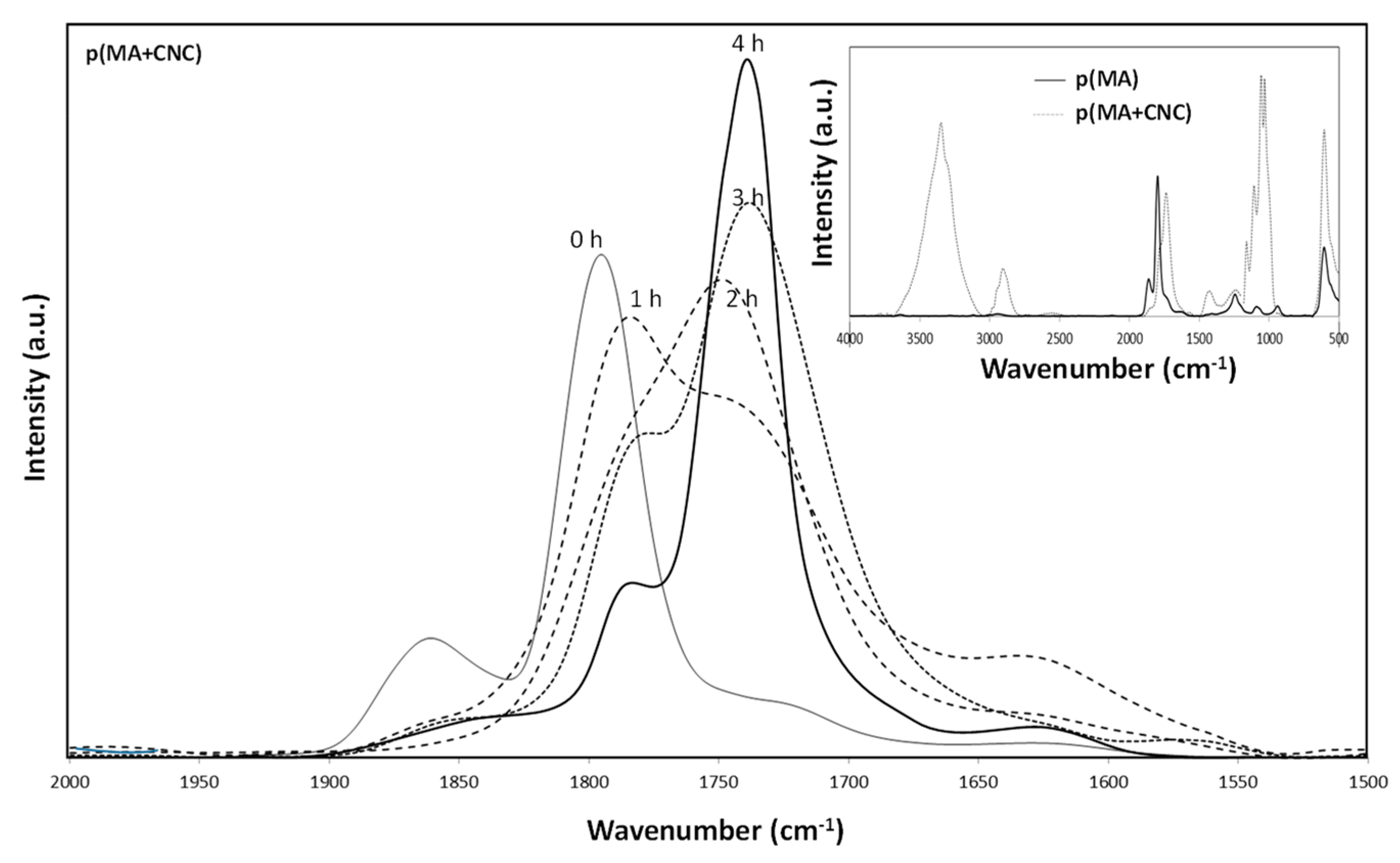
2.4. Chemical Analysis by FTIR Spectroscopy
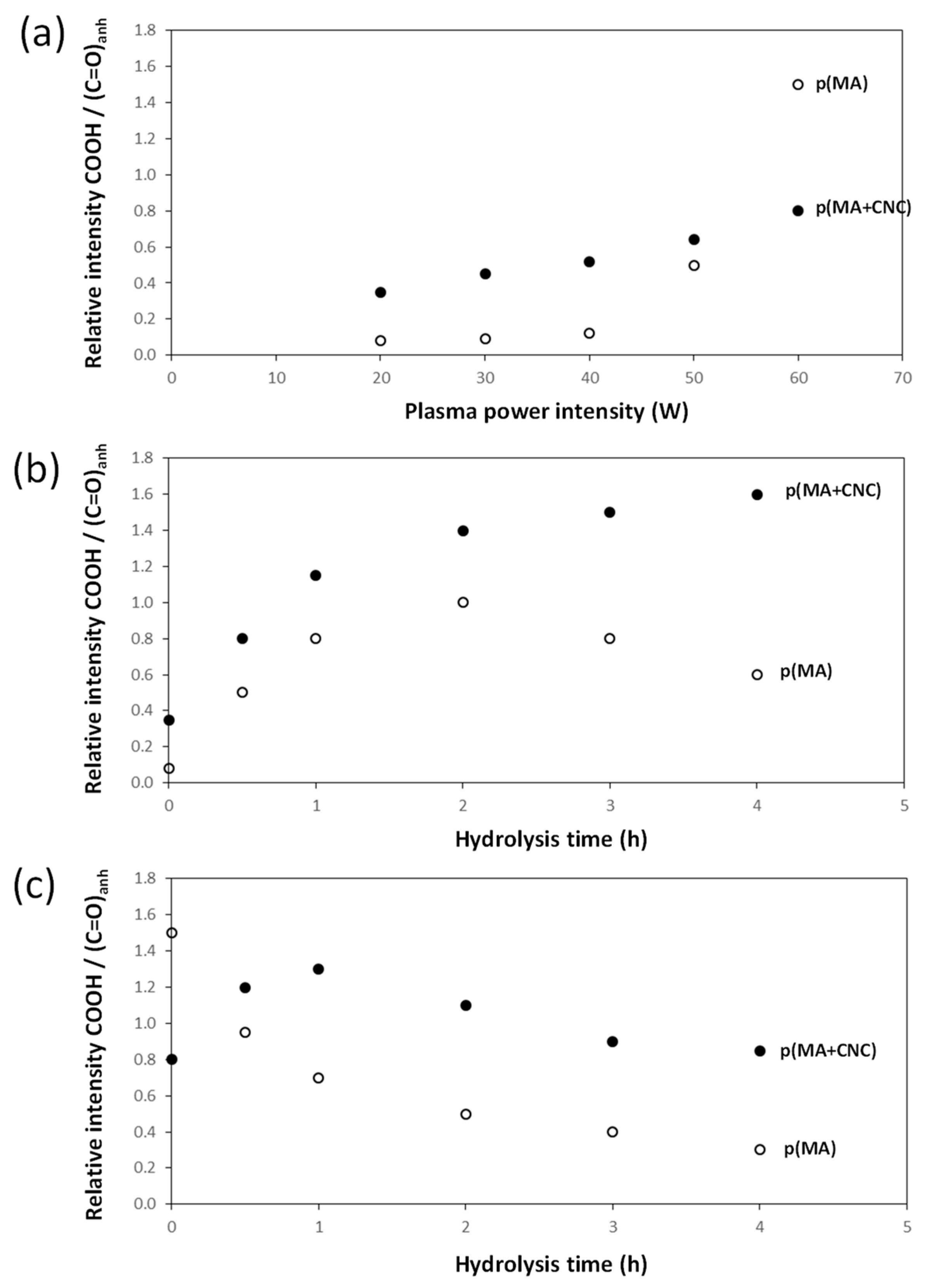
- The effects of plasma deposition conditions on the chemistry and stability were evaluated (Figure 7a) after deposition of p(MA) and p(MA+CNC) films at different plasma power intensities and DC = 2%. In general, the film growth is determined by the formation of a cross-linked polymer network depending on a combination of competitive polymerization and ablation [20]. In particular, the ablation mechanisms become more important and create multiple radical sites, allowing for the formation of a densely cross-linked polymer network at high plasma power. It was also observed for p(MA) films that the formation of ester and carbonyl groups was favored at increased plasma power [33]. The high plasma power consequently results in the creation of films with a more cross-linked polymer network and low penetration rate of water molecules. Otherwise, the structure of the polymer films at low plasma power generally has a more open polymer network with longer polymer chains between the cross-linking points. The differences in the chemistry of deposited films of p(MA) and p(MA+CNC) at plasma power up to 50 W indicate that the p(MA+CNC) films include a higher amount of carboxylic groups owing to the creation of ring-opened anhydride as a result of interface interactions between the maleic anhydride and hydroxyl groups at the surface of CNC. However, the deposition of films with high plasma power of 60 W involves the creation of a higher amount of carboxylic groups relatively to the anhydride carbonyl groups for p(MA), as the relative amount of intact anhydride groups might reduce at a higher plasma power in parallel with the fragmentation of the main polymer chain. Alternatively, the stability of the p(MA+CNC) films is higher at the high plasma power of 60 W, while the anhydride structures in p(MA) films start to fully decompose into ring-opened carboxylic moieties.
- The effects of hydrolysis on p(MA) and p(MA+CNC) films deposited at a low plasma power of 20 W and duty cycle of 2% (Figure 7b) indicate different stability as a function of hydrolysis time. An increase in the concentration of carboxyl groups for the p(MA+CNC) films suggests ongoing ring opening of the anhydride structure during hydrolysis, whereas a decrease in the concentration of carboxylic groups for the p(MA) film after a certain hydrolysis time is attributed to the progressive dissolution of the polymerized film. Therefore, the p(MA) films were less stable to hydrolysis compared to p(MA+CNC) films, as confirmed by previous optical microscopy showing shrinkage of the film over the surface. In general, the application of higher duty cycles is known to reduce the hydrolysis of pure p(MA) films, while the use of low duty cycles implies a lower cross-linking degree and high ability for water penetration during hydrolysis [17]. However, a DC = 2% was selected in the present work to form the p(MA+CNC) nanocomposite films with distinct nanostructured morphologies, as described before [64]. A preliminary screening of plasma conditions indeed revealed that the morphology of p(MA+CNC) nanocomposite films was not obtained at high DC. The importance of a low DC can be understood as the radical species that are formed during the short ton times, need time for diffusion towards the nucleation sites near CNC during the long toff times, and initiate the growth of crystalline structures.
- The stability during the hydrolysis of p(MA) and p(MA+CNC) films deposited at high plasma power of 60 W and duty cycle of 2% (Figure 7c) is different to films deposited at low plasma power. The power density indeed has a critical influence on the film structure, where the higher power may lead to polymer films with both higher cross-linking density, and probably forms more hydrolyzable polymer fragments from ring-opened anhydride structures (e.g., ester and carboxyl groups) in the main polymer chain. The hydrolysis of the latter film structures is indeed favored and leads to a progressive decrease in carboxyl groups through dissolution of the film. As before, the stability of the p(MA+CNC) film to dissolution is superior to that of p(MA), as there is initially a regular hydrolysis of the film during the first hour of exposure and the concentration of carboxylic groups remains higher in p(MA+CNC) after longer hydrolysis time.
3. Materials and Methods
3.1. Materials
3.2. Plasma Coating and Hydrolysis Treatment
3.3. Sample Characterization
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Iqbal, M.; Dink, D.K.; Abbas, Q.; Imran, M.; Sattar, H.; Al Ahmad, A. Controlled surface wettability by plasma polymer surface modification. Surfaces 2019, 2, 349–371. [Google Scholar] [CrossRef]
- Muir, B.W.; Tarasova, A.; Gengenbach, T.R.; Menzies, D.J.; Meagher, L.; Rovere, F.; Fairbrother, A.; McLean, K.M.; Hartley, P.G. Characterization of low-fouling ethylene glycol containing plasma polymer films. Langmuir 2008, 15, 3828–3835. [Google Scholar] [CrossRef] [PubMed]
- Martinu, L.; Poitras, D. Plasma deposition of optical films and coatings: A review. J. Vac. Sci. Technol. 2000, 18, 2619–2645. [Google Scholar] [CrossRef]
- Ji, M.; Benyahia, L.; Poncin-Epaillard, F. Plasma polymer for enhancing adhesion bonds of metal/elastomer assembly. Plasma Proc. Polym. 2021, 18, e2100035. [Google Scholar] [CrossRef]
- Tiwari, A.; Kumar, R.; Prabaharan, M.; Pandey, R.R.; Kumari, P.; Chaturvedi, A.; Mishra, A.K. Nanofibrous polyaniline thin film prepared by plasma-induced polymerization technique for detection of NO2 gas. Polym. Adv. Technol. 2010, 21, 615–620. [Google Scholar] [CrossRef]
- Bretagnol, F.; Lejeune, M.; Papadopoulou-Bouraoui, A.; Hasiwa, N.; Rauscher, H.; Ceccone, G.; Colpo, P.; Rossi, F. Fouling and non-fouling surfaces produced by plasma polymerization of ethylene oxide monomer. Acta Biomater. 2006, 2, 165–172. [Google Scholar] [CrossRef]
- Hegemann, D.; Hossain, M.M.; Balazs, D.J. Nanostructured plasma coatings to obtain multifunctional textile surfaces. Prog. Org. Coat. 2007, 58, 237–240. [Google Scholar] [CrossRef]
- Shi, F.F. Recent advances in polymer thin films prepared by plasma polymerization: Synthesis, structural characterization, properties and applications. Surf. Coat. Technol. 1996, 82, 1–15. [Google Scholar] [CrossRef]
- Mishra, G.; Easton, C.; Fowler, G.J.; McArthur, S.L. Spontaneously reactive plasma polymer micropatterns. Polymer 2011, 52, 1882–1890. [Google Scholar] [CrossRef]
- Zaitsev, A.; Poncin, F.; Lacoste, A.; Debamot, D. A bottom-up and templateless process for the elaboration of plasma-polymer nanostructures. Plasma Proc. Polym. 2015, 13, 227–235. [Google Scholar] [CrossRef]
- Khakalo, A.; Makela, T.; Johansson, L.S.; Orelma, H.; Tammelin, T. High-throughput tailoring of nanocellulose films: From complex bio-based materials to defined multifunctional architectures. ACS Appl. Biomater. 2020, 3, 7428–7438. [Google Scholar] [CrossRef] [PubMed]
- Wenjie, T.; Qiang, C.; Yuefei, Z.; Yuanjing, G. Investigation of plasma polymerized maleic anhydride film in a middle frequency dielectric barrier discharge. Plasma Sci. Technol. 2008, 10, 176–179. [Google Scholar] [CrossRef]
- Mishra, G.; McArthur, S.L. Plasma polymerization of maleic anhydride: Just what are the right deposition conditions? Langmuir 2010, 26, 9645–9658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guinter, E.; Fontana, L.C.; Becker, D. Maleic anhydride film deposition through an active screen plasma system. Bull. Mater. Sci. 2019, 42, 23. [Google Scholar] [CrossRef]
- Ryan, M.E.; Hynes, A.M.; Badyal, J.P.S. Pulsed plasma polymerization of maleic anhydride. Chem. Mater. 1996, 3, 37–42. [Google Scholar] [CrossRef]
- Jebali, S.; Airoudj, A.; Ferreira, I.; Hegemann, D.; Roucoules, V.; Bally-Le Gall, F. Unique combination of spatial and temporal control of maleic anhydride plasma polymerization. Plasma Proc. Polym. 2021, 18, e2000244. [Google Scholar] [CrossRef]
- Schiller, S.; Hu, J.; Jenkins, A.T.A.; Timmons, R.B.; Sanchez-Estrada, F.S.; Knoll, W.; Forch, R. Chemical structure and properties of plasma-polymerized maleic anhydride films. Chem. Mater. 2002, 14, 235–242. [Google Scholar] [CrossRef]
- De Oliveira, J.C.; Brioude, M.; Airoudj, A.; Bally-Le Gall, F.; Roucoules, V. Plasma polymerization in the design of new materials: Looking through the lens of maleic anhydride plasma polymers. Mater. Today Chem. 2022, 23, 100646. [Google Scholar] [CrossRef]
- Homilius, F.; Heilmann, A.; von Borczyskowski, C. Plasma polymer thin films with encapsulated dye molecules and silver nanoparticles. Surf. Coat. Technol. 1995, 74–75, 594–597. [Google Scholar] [CrossRef]
- Saulou-Berion, C.; Despax, B.; Raynaud, P.; Zanna, S.; Marcus, P.; Mercier-Bonin, M. Plasma-engineered polymer thin films with embedded nanosilver for prevention of microbial adhesion. Solid State Phenom. 2009, 151, 95–100. [Google Scholar] [CrossRef]
- Samyn, P.; Airoudj, A.; Laborie, M.P.; Mathew, A.P.; Roucoules, V. Plasma deposition of polymer composite films incorporating nanocellulose whiskers. Eur. J. Phys. J. Appl. Phys. 2011, 56, 24015. [Google Scholar] [CrossRef]
- Kan, K.H.; Cranston, E.D. Mechanical testing of thin film nanocellulose composites using buckling mechanics. TAPPI J. 2013, 12, 9–17. [Google Scholar] [CrossRef]
- Bulbul, E.; Hegemann, D.; Ataka, K.; Lehner, S.; Heberle, J.; Heuberger, M. Confined hydration in nanometer-graded plasma polymer films: Insights from surface-enhanced infrared absorption spectroscopy. Surf. Interfaces 2021, 23, 100922. [Google Scholar] [CrossRef]
- Knobloch, J.; Askew, H.J.; Jarvis, K.; Jobes, R.; Shapter, J.G.; McArthur, S.L.; Köper, I. In situ monitoring of the effect of ionic strength and pH on plasma polymer thin films. Plasma Proc. Polym. 2017, 14, 1700084. [Google Scholar] [CrossRef]
- Cools, P.; Declercq, H.; De Geyter, N.; Morent, R. A stability study of plasma polymerized acrylic acid films. Appl. Surf. Sci. 2017, 432, 214–223. [Google Scholar] [CrossRef]
- Vandenbossche, M.; Hegemann, D. Recent approaches to reduce aging phenomena in oxygen- and nitrogen-containing plasma polymer films: An overview. Curr. Opin. Solid State Mater. Sci. 2018, 22, 26–38. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X. Phase diagrams of instabilities in compressed film-substrate systems. J. Appl. Mech. 2014, 81, 0510041–5100410. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Q.; Knoll, W.; Foerch, R. Effect of aqueous solution on functional plasma polymerized films. Surf. Coat. Technol. 2003, 174–175, 588–590. [Google Scholar] [CrossRef]
- Jarvis, K. Investigating the aqueous swelling and stability of plasma polymerised acrylic acid films. In Neutron Scattering Symposium 2020; Abstract Booklet, 2020; p. 10.
- Pathak, S.C.; Hess, D.W. Dissolution and swelling behaviour of plasma-polymerized polyethylene glycol-like hydrogel films for use as drug delivery reservoirs. ECS Trans. 2008, 6, 1. [Google Scholar] [CrossRef]
- Rosenfeld, J.M.; Murphy, C.B. Hydrolysis study of organic acid anhydrides by differential thermal analysis—II: Maleic anhydride and trimellitic anhydride. Talanta 1967, 14, 91–96. [Google Scholar] [CrossRef]
- Pompe, T.; Renner, L.; Grimmer, M.; Herold, N.; Werner, C. Functional films of maleic anhydride copolymers under physiological conditions. Macromol. Biosci. 2005, 5, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Drews, J.; Launay, H.; Hansen, C.M.; West, K.; Hvilsted, S.; Kingshott, P.; Almdal, K. Hydrolysis stability of thin pulsed plasma polymerised maleic anhydride coatings. Appl. Surf. Sci. 2008, 254, 4720–4725. [Google Scholar] [CrossRef]
- Chu, L.Q.; Foerch, R.; Knoll, W. Pulsed plasma polymerized maleic anhydride films in humid air and in aqueous solutions studied with optical waveguide spectroscopy. Langmuir 2006, 22, 2822–2826. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Josey, B.; Anim-Danso, E.; Maranville, B.; Karapetrova, J.; Jiang, Z.; Zhou, Q.; Dhinojwala, A.; Foster, M.D. In situ nanoscale characterization of water penetration through plasma polymerized coatings. Langmuir 2018, 34, 9634–9644. [Google Scholar] [CrossRef] [PubMed]
- Airoudj, A.; Ploux, L.; Roucoules, V. Effect of plasma duty cycle on silver nanoparticles loading of cotton fabrics for durable antibacterial properties. J. Appl. Polym. Sci. 2015, 132, 41279. [Google Scholar] [CrossRef]
- Draper, E.R.; Mears, L.L.E.; Castilla, A.; King, S.M.; McDonald, T.O.; Akhtar, R.; Adams, D.J. Using the hydrolysis of anhydrides to control gel properties and homogeneity in pH-triggered gelation. RSC Adv. 2015, 5, 95369–95378. [Google Scholar] [CrossRef]
- Habibi, M.; Rahimzadeh, A.; Eslamian, M. On the dewetting of thin films due to crystallization (crystallization dewetting). Eur. Phys. J. E 2016, 39, 30. [Google Scholar] [CrossRef]
- Ramanathan, M.; Darling, S. Mesoscale morphologies in polymer thin films. Prog. Polym. Sci. 2011, 36, 793–812. [Google Scholar] [CrossRef]
- Ferreiro, V.; Douglas, J.F.; Warren, J.A.; Karim, A. Non-equilibrium pattern formation in the crystallization of polymer blend films. Phys. Rev. E 2002, 65, 042802. [Google Scholar] [CrossRef]
- Darko, C.; Botiz, I.; Reiter, G.; Breiby, D.W.; Andreasen, J.W.; Roth, S.V.; Smilgies, D.M.; Metwalli, E.; Papadakis, C.M. Crystallization in diblock copolymer thin films at different degrees of supercoiling. Phys. Rev. E 2009, 79, 041802. [Google Scholar] [CrossRef]
- Jiang, N.; Tang, P.; Zhang, H.; Yang, Y. Study on the thermodynamics of polymer crystallization based on twin-lattice model. J. Phys. Chem. B 2018, 122, 8601–8613. [Google Scholar] [CrossRef] [PubMed]
- Okerberg, B.C.; Berry, B.C.; Garvey, T.R.; Douglas, J.F.; Karim, A.; Soles, C.L. Competition between crystallization and dewetting fronts in thin polymer films. Soft Matter 2009, 5, 562–567. [Google Scholar] [CrossRef]
- Gleadall, A.; Pan, J.; Atkinson, H. A simplified theory of crystallisation induced by polymer chain scissions for biodegradable polyesters. Polym. Degrad. Stab. 2012, 97, 1616–1620. [Google Scholar] [CrossRef]
- Jiang, Q.; Ward, M.D. Crystallization under nanoscale confinement. Chem. Soc. Rev. 2013, 43, 2066–2079. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xie, Q.; Bao, Y.; Shan, G.; Pan, P. Crystalline and spherulitic morphology of polymers crystallized in confined systems. Crystals 2017, 7, 147. [Google Scholar] [CrossRef]
- Carr, J.M.; Langhe, D.S.; Ponting, M.T.; Hiltner, A.; Baer, E. Confined crystallization in polymer nanolayered films: A review. J. Mater. Res. 2012, 27, 1326–1350. [Google Scholar] [CrossRef]
- Samanta, P.; Liu, C.L.; Nandan, B.; Chen, H.L. Crystallization of polymers in confined space. In Crystallization in Multiphase Polymer Systems; Thomas, S., Gowd, E.B., Arif, M.P., Kalarikkal, N., Eds.; Elsevier: New York, NY, USA, 2018; pp. 367–431. [Google Scholar]
- Samyn, P.; Airoudj, A.; Mathew, A.P.; Roucoules, V. Metastable patterning of plasma nanocomposite films by incorporating cellulose nanowhiskers. Langmuir 2012, 28, 1427–1438. [Google Scholar] [CrossRef]
- Airoudj, A.; Schrodj, G.; Vallat, M.F.; Fioux, P.; Roucoules, V. Influence of plasma duty cycle during plasma polymerization in adhesive bonding. Int. J. Adh. Adhes. 2011, 31, 498–506. [Google Scholar] [CrossRef]
- Thiry, D.; Vins, N.; Damman, P.; Rebello, F.J.; Tessier, P.Y.; Moerman, D.; Leclère, P.; Godfroipd, T.; Deprez, S.; Snyders, R. The wrinkling concept applied to plasma-deposited polymer-like thin films: A promising method for the fabrication of flexible electrodes. Plasma Proc. Polym. 2020, 17, 2000119. [Google Scholar] [CrossRef]
- Brioude, M.; Roucoules, V.; Haidara, H.; Vonna, L.; Laborie, M.P. Role of cellulose nanocrystals on the microstructure of maleic anhydride plasma polymer thin films. Appl. Mater. Interfac. 2015, 7, 14079–14088. [Google Scholar] [CrossRef]
- Jing, B.; Zhao, H.; Wang, Y.; Yi, X.; Duan, H. Water-swelling-induced morphological instability of a supported polymethyl methacrylate thin film. Langmuir 2010, 26, 7651–7655. [Google Scholar] [CrossRef] [PubMed]
- Vasiliv, K.; Britcher, L.; Casanal, A.; Griesser, H.J. Solvent-induced porosity in ultrathin amine plasma polymer coatings. J. Phys. Chem. B 2008, 112, 10915–10921. [Google Scholar] [CrossRef] [PubMed]
- Danilaev, M.P.; Bogoslov, E.A.; Plsky, Y.E.; Yanilkin, I.V.; Vakhitov, I.R.; Gumarov, A.I.; Tagirov, R. Internal stresses in plasma deposited polymer film coatings. Inorg. Mater. Appl. Res. 2019, 10, 556–559. [Google Scholar] [CrossRef]
- Yu, Q.S.; Yasuda, H.K. Internal stress in plasma polymer films prepared by cascade arc torch polymerization. J. Appl. Polym. Sci. A 2000, 37, 1577–1587. [Google Scholar] [CrossRef]
- Butler, M.A.; Buss, R.J.; Seager, C.H. Swelling of plasma-polymerized tetrafluoroethylene films. Appl. Phys. Lett. 1991, 59, 2817. [Google Scholar] [CrossRef]
- Gardner, D.J.; Oporto, G.S.; Mills, R.; Samir, M.A.S.A. Adhesion and surface issues in cellulose and nanocellulose. J. Adhes. Sci. Technol. 2008, 22, 545–567. [Google Scholar] [CrossRef]
- Huang, K.Y.; Wee, E.M.; Nagarajan, S. Unique periodic rings composed of fractal-growth dendritic branching in poly(p-dioxanone). Polymers 2022, 14, 805. [Google Scholar] [CrossRef]
- Hernandez-Montero, N.; Meaurio, E.; Elmiloudi, K.; Sarasua, J.R. Novel miscible blends of poly (p-dioxanone) with poly(vinyl phenol). Eur. Polym. J. 2012, 48, 1455–1465. [Google Scholar] [CrossRef]
- Michell, R.M.; Müller, A.J. Confined crystallization of polymeric materials. Prog. Polym. Sci. 2016, 54–55, 183–213. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, W.; Reiter, G. Lamellar crystal orientations biased by crystallization kinetics in polymer thin films. Macromolec 2006, 39, 5159–5164. [Google Scholar] [CrossRef]
- Prud’homme, R.E. Crystallization and morphology of ultrathin films of homopolymers and polymer blends. Prog. Polym. Sci. 2016, 44–45, 214–231. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, E. Polymer crystallization of ultrathin films on solid substrates. Coord. Chem. Rev. 2010, 254, 1011–1037. [Google Scholar] [CrossRef]
- Brener, E.; Müller-Krumbhaar, H.; Temkin, D.; Abel, T. Morphology diagram of possible structures in diffusional growth. Phys. A 1998, 249, 73–81. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Li, L.; Chan, C. The effect of substrate on the annealing of poly(butylene succinate) single crystals. Macromol. Rapid Commun. 2007, 28, 2001–2006. [Google Scholar] [CrossRef]
- Mareau, V.H.; Prud’homme, R.E. In-situ hot stage atomic force microscopy study of poly(#-caprolactone) crystal growth in ultrathin films. Macromolecules 2005, 38, 398–408. [Google Scholar]
- Yu, H.; Qin, Z.; Zhou, Z. Cellulose nanocrystals as green fillers to improve crystallization and hydrophilic property of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Prog. Nat. Sci. 2011, 21, 478–484. [Google Scholar] [CrossRef]
- Jativa, F.; Schütz, C.; Bergström, L.; Zhang, X.; Wicklein, B. Confined self-assembly of cellulose nanocrystals in a shrinking droplet. Soft Matter 2015, 11, 5374–5380. [Google Scholar] [CrossRef]
- Nia, M.H.; Tavakolian, M.; Kiasat, A.R.; van de Ven, T. Hybrid aerogel nanocomposite of dendritic colloidal silica and hairy nanocellulose: An effective dye adsorbent. Langmuir 2020, 36, 11963–11974. [Google Scholar] [CrossRef]
- Zhou, C.; Chu, R.; Wu, R.; Wu, Q. Electrospun polyethylene oxide/cellulose nanocrystal composite nanofibrous mats with homogeneous and heterogeneous microstructures. Biomacromolecules 2011, 12, 2617–2625. [Google Scholar] [CrossRef]
- Briggs, D.; Beamson, G. High Resolution of XPS of Organic Polymers; Wiley: Chichester, UK, 1992. [Google Scholar]
- Greenwood, O.D.; Tasker, S.; Badyal, J.P.S. A comparative study of the silent discharge treatment of saturated and unsaturated hydrocarbon polymers. J. Appl. Polym. Sci. A 1994, 32, 2479–2486. [Google Scholar] [CrossRef]
- Freudenberg, U.; Zschoche, S.; Simon, F.; Janke, A.; Schmidt, K.; Behrens, S.H.; Auweter, H.; Werner, C. Covalent immobilization of cellulose layers onto maleic anhydride copolymer thin films. Biomacromolecules 2005, 6, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Manakhov, A.; Michlicek, M.; Necas, D.; Polcak, J.; Makhneva, Z.; Elias, M.; Zajickova, L. Carboxyl-rich coatings deposited by atmospheric plasma co-polymerization of maleic anhydride and acetylene. Surf. Coat. Technol. 2016, 295, 37–45. [Google Scholar] [CrossRef]
- Sclavons, M.; Carlier, V.; De Roover, B.; Franquinet, P.; Devaux, J.; Legras, R. The anhydride content of some commercial PP-g-MA: FTIR and titration. J. Appl. Polym. Sci. 1996, 62, 1205–1210. [Google Scholar] [CrossRef]
- Yang, C.Q.; Wang, X.; Lu, Y. Infrared spectroscopy studies of cyclic anhydrides as intermediates for ester crosslinking of cotton cellulose by polycarboxylic acids. IV. In situ free radical copolymerization of maleic acid and itaconic acid on cotton. J. Appl. Polym. Sci. 1999, 75, 327–336. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimisation of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Ji, L.; Li, H.; Zhao, F.; Quan, W.; Chen, J.; Zhou, H. Effects of pulse bias duty cycle on fullerenelike nanostructure and mechanical properties of hydrogenated carbon films prepared by plasma enhanced chemical vapor deposition method. J. Appl. Phys. 2009, 105, 106113. [Google Scholar] [CrossRef]


| Sample | %C | %O | O/C |
|---|---|---|---|
| MA monomer | 69.8 | 30.2 | 0.43 |
| p(MA) film p(MA+CNC) film | 74.8 76.0 | 25.2 24.0 | 0.33 0.32 |
| Peak | A | B | C | D | E |
|---|---|---|---|---|---|
| Binding Energy | 285.0 eV | 285.7 eV | 286.6 eV | 287.9 eV | 289.3 eV |
| C-C | C-C(O)=O | C-O | C=O/O-C-O | C(O)=O | |
| p(MA) film p(MA+CNC) film | 61.0 41.7 | 11.0 24.8 | 11.2 10.6 | 5.8 7.1 | 11.0 15.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samyn, P. Confined Crystallization of Thin Plasma-Polymerized Nanocomposite Films with Maleic Anhydride and Cellulose Nanocrystals under Hydrolysis. Molecules 2022, 27, 5683. https://doi.org/10.3390/molecules27175683
Samyn P. Confined Crystallization of Thin Plasma-Polymerized Nanocomposite Films with Maleic Anhydride and Cellulose Nanocrystals under Hydrolysis. Molecules. 2022; 27(17):5683. https://doi.org/10.3390/molecules27175683
Chicago/Turabian StyleSamyn, Pieter. 2022. "Confined Crystallization of Thin Plasma-Polymerized Nanocomposite Films with Maleic Anhydride and Cellulose Nanocrystals under Hydrolysis" Molecules 27, no. 17: 5683. https://doi.org/10.3390/molecules27175683
APA StyleSamyn, P. (2022). Confined Crystallization of Thin Plasma-Polymerized Nanocomposite Films with Maleic Anhydride and Cellulose Nanocrystals under Hydrolysis. Molecules, 27(17), 5683. https://doi.org/10.3390/molecules27175683






