Exploring The Relative Astringency of Tea Catechins and Distinct Astringent Sensation of Catechins and Flavonol Glycosides via an In Vitro Assay Composed of Artificial Oil Bodies
Abstract
:1. Introduction
2. Results
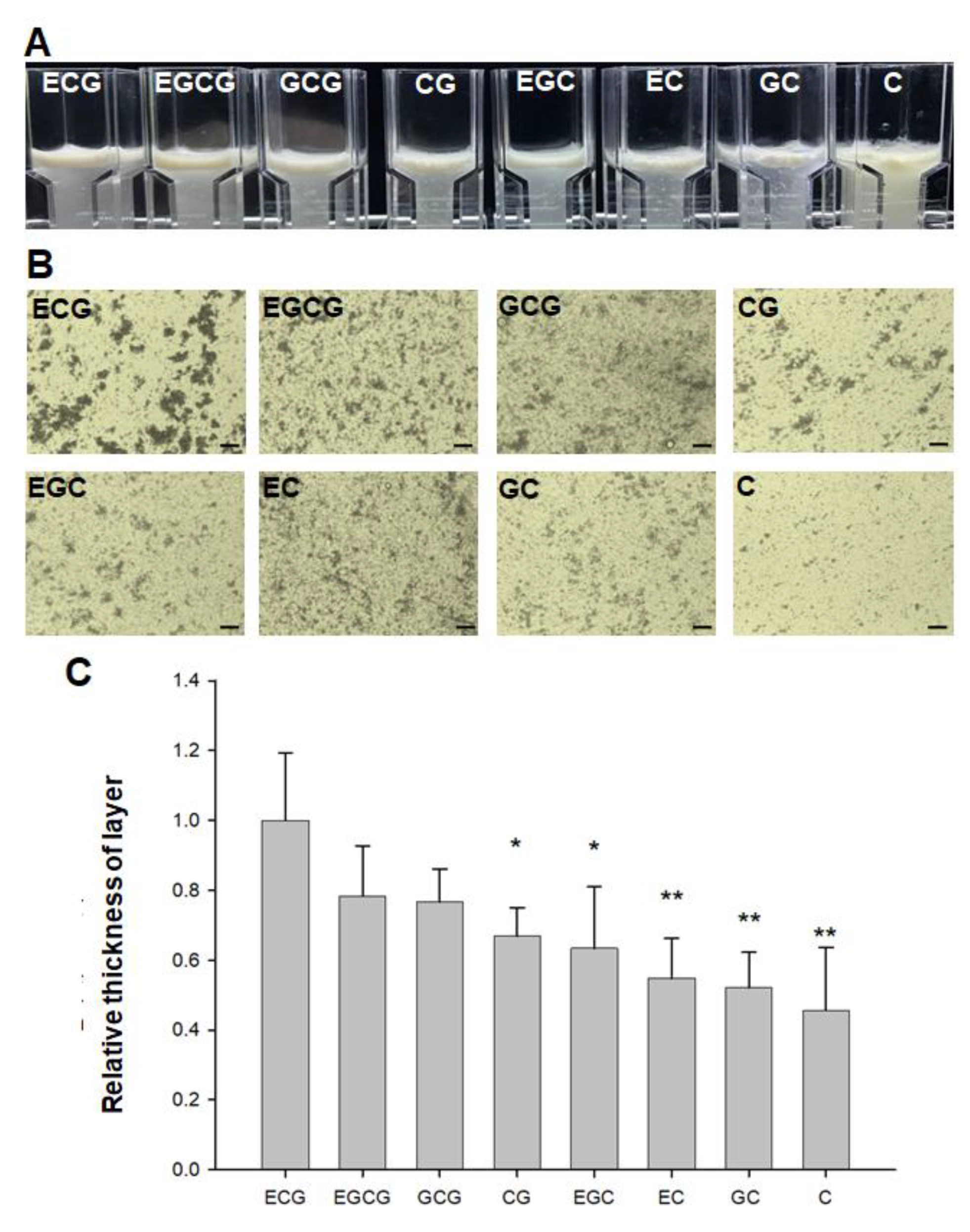
2.1. Detection of Relative Astringency of Eight Catechins in Tea
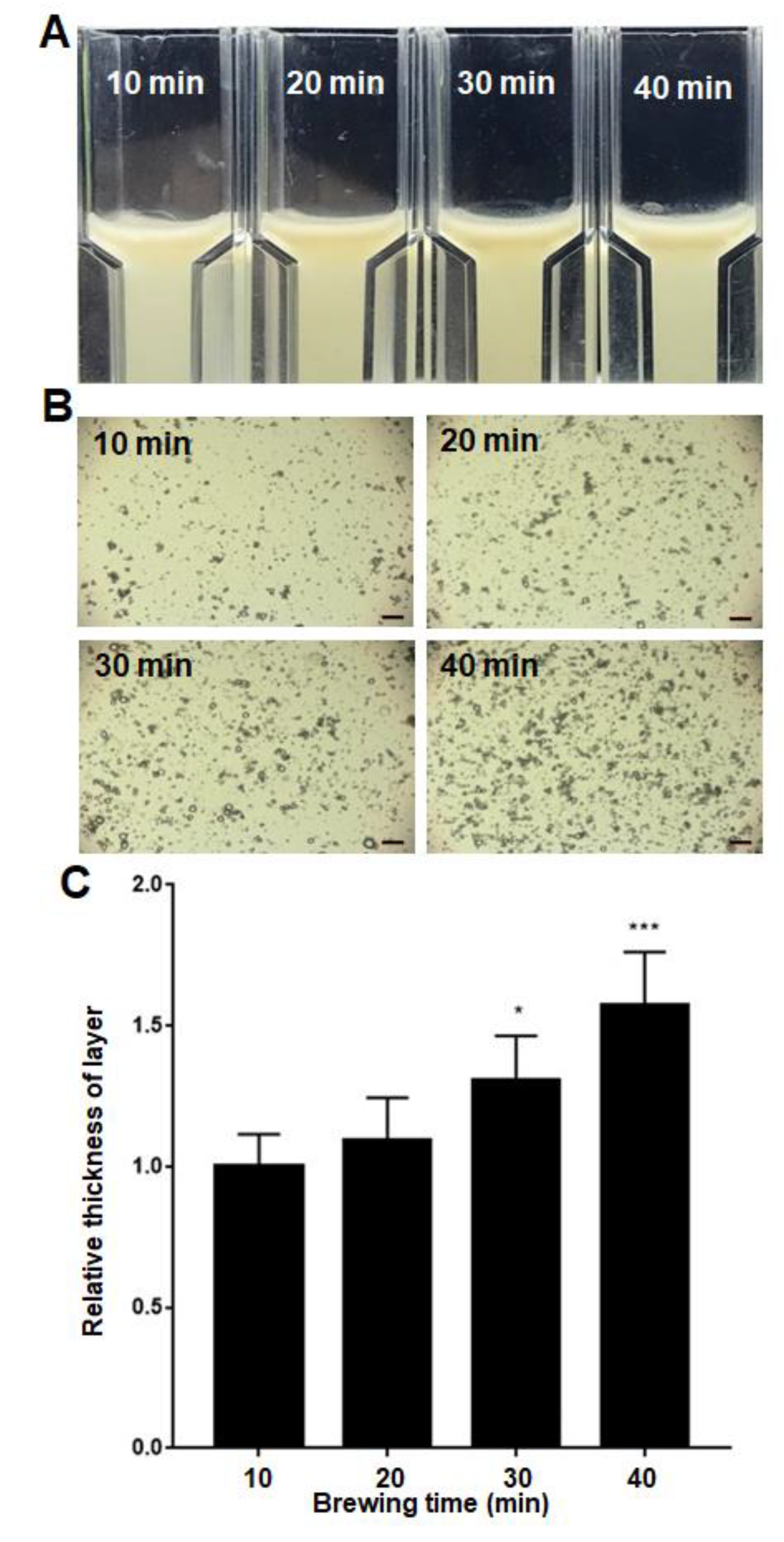
2.2. Relative Astringency of Tea Infusion under Elongated Brewing Time
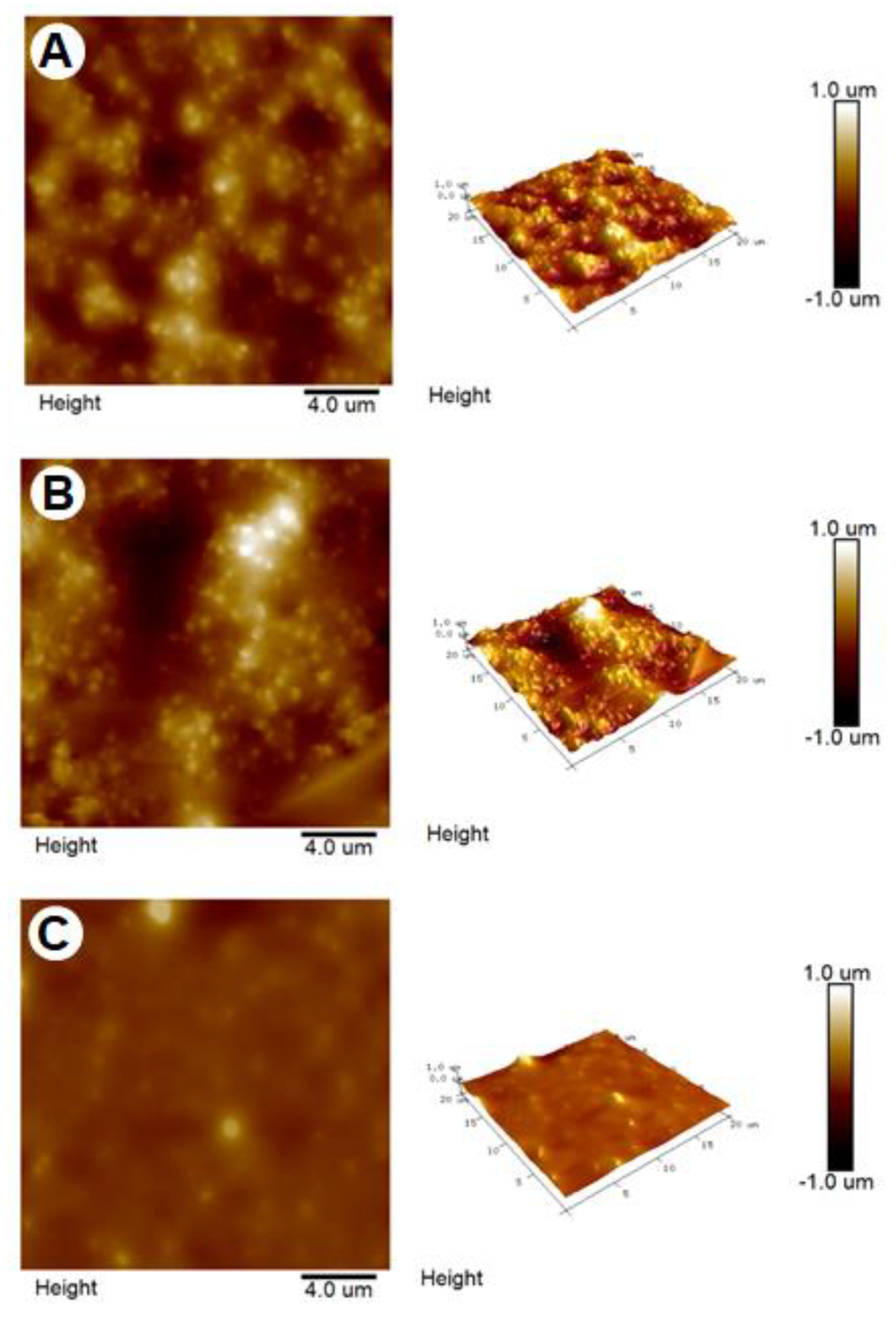
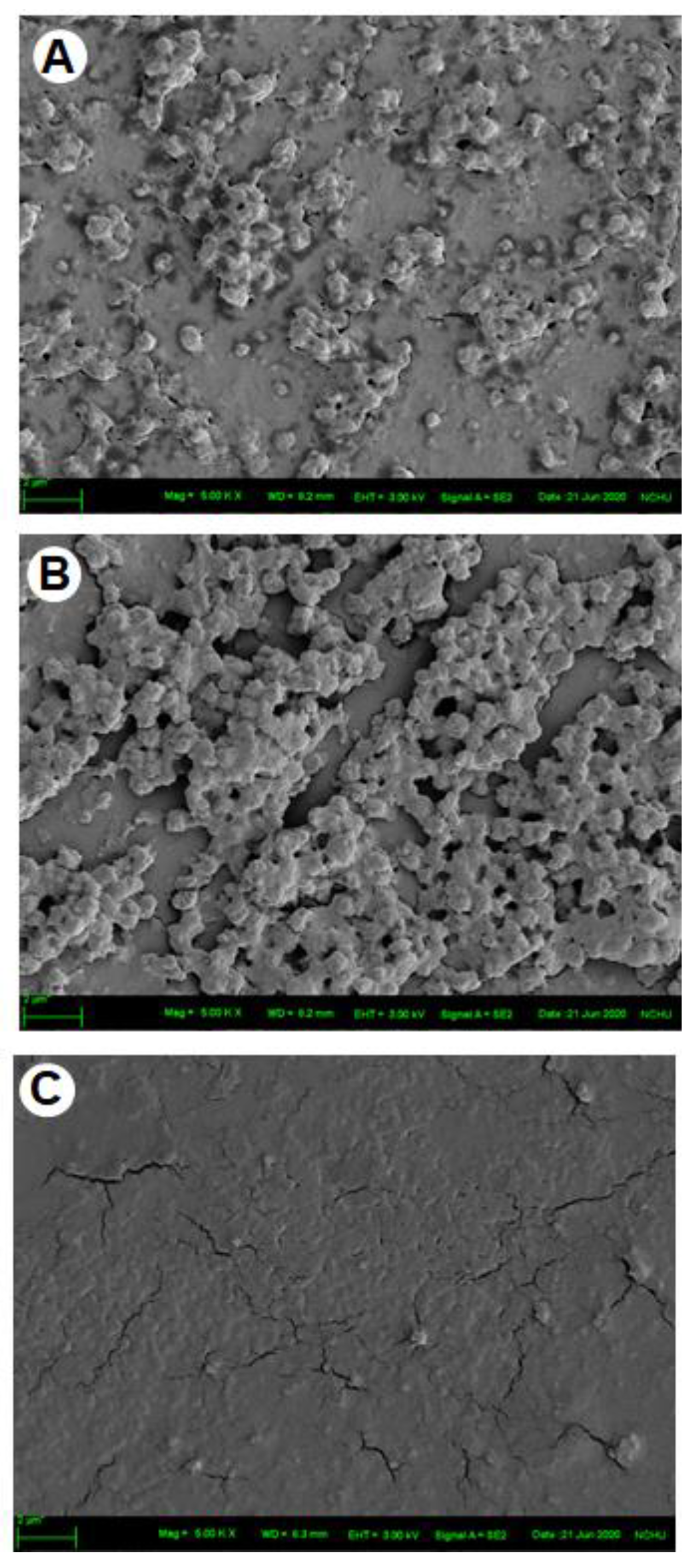
2.3. Surface Roughness of Artificial Oil Bodies in Complex with EGCG or Rutin
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
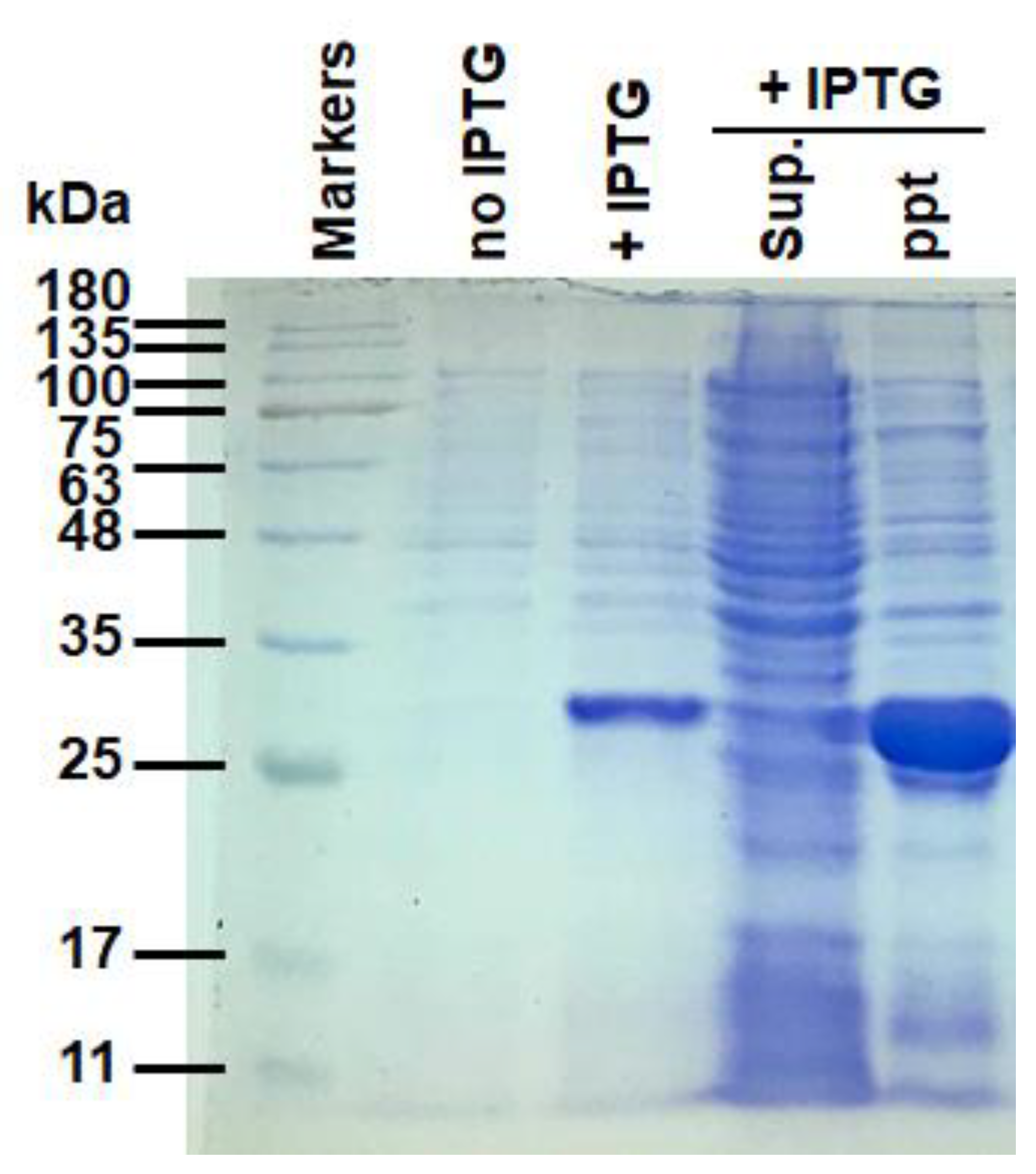
4.2. Generation of Artificial Oil Bodies for the In Vitro Assay of Tea Astringency
4.3. Preparation of Catechin Standards and Tea Infusion
4.4. In Vitro Assay of Relative Astringency
4.5. Light Microscopy of Aggregated Artificial Oil Bodies
4.6. HPLC Analysis of Tea Infusion
4.7. Atomic Force Microscopy Imaging of Artificial Oil Bodies
4.8. Scanning Electron Microscopy Imaging of Artificial Oil Bodies
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Shi, J.; Yang, G.; You, Q.; Sun, S.; Chen, R.; Lin, Z.; Simal-Gandara, J.; Lv, H. Updates on the chemistry, processing characteristics, and utilization of tea flavonoids in last two decades (2001–2021). Crit. Rev. Food Sci. Nutr. 2021, 13, 1–28. [Google Scholar]
- Ng, K.W.; Cao, Z.J.; Chen, H.B.; Zhao, Z.Z.; Zhu, L.; Yi, T. Oolong tea: A critical review of processing methods, chemical composition, health effects, and risk. Crit. Rev. Food Sci. Nutr. 2018, 58, 2957–2980. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y. Elucidation of physiological functions of tea catechins and their practical applications. J. Drug Food Anal. 2012, 20, 296–300. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, T.; Zhao, S.; Zhu, Y.; Feng, C.; Zhan, J.; Li, S.; Ho, C.T.; Gosslau, A. Multifunctional health-promoting effects of oolong tea and its products. Food Sci. Hum. Well. 2022, 11, 512–523. [Google Scholar] [CrossRef]
- Eini, H.; Frishman, V.; Yulzari, R.; Kachko, L.; Lewis, E.C.; Chaimovitz, C.; Douvdevani, A. Caffeine promotes anti-tumor immune response during tumor initiation: Involvement of the adenosine A2A receptor. Biochem. Pharmacol. 2015, 98, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Jhuo, C.F.; Hsu, Y.Y.; Chen, W.Y.; Tzen, J.T.C. Attenuation of tumor development in mammary carcinoma rats by theacrine, an antagonist of adenosine 2A receptor. Molecules 2021, 26, 7455. [Google Scholar] [CrossRef]
- Liao, M.H.; Wang, X.R.; Hsu, W.L.; Tzen, J.T.C. Pu’er tea rich in strictinin and catechins prevents biofilm formation of two cariogenic bacteria, Streptococcus mutans and Streptococcus sobrinus. J. Dent. Sci. 2021, 16, 1331–1334. [Google Scholar] [CrossRef]
- Lin, P.R.; Kuo, P.C.; Li, Y.C.; Jhuo, C.F.; Hsu, W.L.; Tzen, J.T.C. Theacrine and strictinin, two major ingredients for the anti-influenza activity of Yunnan Kucha tea. J. Ethnopharmacol. 2020, 262, 113190. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.H.; Chen, Y.J.; Chang, C.I.; Lin, Y.W.; Chen, C.Y.; Lee, M.R.; Lee, V.S.; Tzen, J.T.C. Teaghrelins, unique acylated flavonoid tetraglycosides in Chin-shin oolong tea, are putative oral agonists of the ghrelin receptor. J. Agric. Food Chem. 2014, 62, 5085–5091. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Q.Q.; Granato, D.; Xu, Y.Q.; Ho, C.T. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar]
- Ananingsih, V.K.; Sharma, A.; Zhou, W. Green tea catechins during food processing and storage: A review on stability and detection. Food Res. Int. 2013, 50, 469–479. [Google Scholar]
- Rani, A.; Singh, K.; Ahuja, P.S.; Kumar, S. Molecular regulation of catechins biosynthesis in tea [Camellia sinensis (L.) O. Kuntze]. Gene 2012, 495, 205–210. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, L.; Liu, L.; Yang, Q.; Lu, Z.; Nie, Z.; Wang, Y.; Xia, T. Purification and characterization of a novel galloyltransferase involved in catechin galloylation in the tea plant (Camellia sinensis). J. Biol. Chem. 2012, 287, 44406–44417. [Google Scholar] [PubMed] [Green Version]
- Xu, Y.Q.; Zhang, Y.N.; Chen, J.X.; Wang, F.; Du, Q.Z.; Yin, J.F. Quantitative analyses of the bitterness and astringency of catechins from green tea. Food Chem. 2018, 258, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Pradal, C.; Stokes, J.R. Oral tribology: Bridging the gap between physical measurements and sensory experience. Curr. Opin. Food Sci. 2016, 9, 34–41. [Google Scholar]
- Sarkar, A.; Krop, E.M. Marrying oral tribology to sensory perception: A systematic review. Curr. Opin. Food Sci. 2019, 27, 64–73. [Google Scholar] [CrossRef]
- Upadhyay, R.; Brossard, N.; Chen, J. Mechanisms underlying astringency: Introduction to an oral tribology approach. J. Phys. D Appl. Phys. 2016, 49, 104003. [Google Scholar] [CrossRef]
- Cala, O.; Pinaud, N.; Simon, C.; Fouquet, E.; Laguerre, M.; Dufourc, E.J.; Pianet, I. NMR and molecular modeling of wine tannins binding to saliva proteins: Revisiting astringency from molecular and colloidal prospects. FASEB J. 2010, 24, 4281–4290. [Google Scholar]
- Stokes, J.R.; Boehm, M.W.; Baier, S.K. Oral processing, texture and mouthfeel: From rheology to tribology and beyond. Curr. Opin. Colloid Interface Sci. 2013, 18, 349–359. [Google Scholar]
- Linne, B.; Simons, C.T. Quantification of oral roughness perception and comparison with mechanism of astringency perception. Chem. Senses 2017, 42, 525–535. [Google Scholar]
- Bajec, M.R.; Pickering, G.J. Astringency: Mechanisms and perception. Crit. Rev. Food Sci. Nutr. 2008, 48, 858–875. [Google Scholar] [PubMed]
- Laguna, L.; Sarkar, A. Oral tribology: Update on the relevance to study astringency in wines. Tribol.-Mater. Surf. Interfaces 2017, 11, 116–123. [Google Scholar] [CrossRef]
- Schöbel, N.; Radtke, D.; Kyereme, J.; Wollmann, N.; Cichy, A.; Obst, K.; Kallweit, K.; Kletke, O.; Minovi, A.; Dazert, S.; et al. Astringency is a trigeminal sensation that involves the activation of G protein–coupled signaling by phenolic compounds. Chem. Senses 2014, 39, 471–487. [Google Scholar] [PubMed]
- Scharbert, S.; Holzmann, N.; Hofmann, T. Identification of the astringent taste compounds in black tea infusions by combining instrumental analysis and human bioresponse. J. Agric. Food Chem. 2004, 52, 3498–3508. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, I.L.; Noble, A.; Kwiatkowski, M.; Waters, E.J. Taste and mouth-feel properties of different types of tannin-like polyphenolic compounds and anthocyanins in wine. Anal. Chim. Acta 2004, 513, 57–65. [Google Scholar] [CrossRef]
- Rinaldi, A.; Gambuti, A.; Moine, V.; Moio, L. Evaluation of the astringency of commercial tannins by means of the SDS–PAGE-based method. Food Chem. 2010, 122, 951–956. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Brás, N.F.; García-Estévez, I.; Mateus, N.; Rivas-Gonzalo, J.C.; de Freitas, V.; Escribano-Bailón, M.T. Effect of flavonols on wine astringency and their interaction with human saliva. Food Chem. 2016, 209, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Medina, M.B. Determination of the total phenolics in juices and superfruits by a novel chemical method. J. Funct. Foods 2011, 3, 79–87. [Google Scholar]
- Valentova, H.; Skrovankova, S.; Panovska, Z.; Pokorny, J. Determination of astringent taste in model solutions and in beverages. Czech J. Food Sci. 2001, 19, 196–200. [Google Scholar] [CrossRef]
- Han, X.; Jiang, H.; Zhang, D.; Zhang, Y.; Xiong, X.; Jiao, J.; Xu, R.; Yang, M.; Han, L.; Lin, J. A novel quantitative prediction approach for astringency level of herbs based on an electronic tongue. Pharmacogn. Mag. 2017, 13, 492–497. [Google Scholar] [CrossRef]
- Varga, G. Physiology of the salivary glands. Surgery 2012, 30, 578–583. [Google Scholar]
- Obreque-Slier, E.; Pena-Neira, A.; Lopez-Solis, R. Enhancement of both salivary protein−enological tannin interactions and astringency perception by ethanol. J. Agric. Food Chem. 2010, 58, 3729–3735. [Google Scholar] [CrossRef] [PubMed]
- García-Estévez, I.; Ramos-Pineda, A.M.; Escribano-Bailón, M.T. Interactions between wine phenolic compounds and human saliva in astringency perception. Food Funct. 2018, 9, 1294–1309. [Google Scholar] [CrossRef]
- Kavanagh, K.; Dowd, S. Histatins: Antimicrobial peptides with therapeutic potential. J. Pharm. Pharmacol. 2004, 56, 285–289. [Google Scholar] [CrossRef]
- Shih, Y.E.; Lin, Y.C.; Chung, T.Y.; Liu, M.C.; Chen, G.H.; Wu, C.C.; Tzen, J.T.C. In vitro assay to estimate tea astringency via observing flotation of artificial oil bodies sheltered by caleosin fused with histatin 3. J. Agric. Food Chem. 2017, 25, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.H.; Chen, Y.Y.; Wang, G.J. Using a fuzzy analytic hierarchy process to formulate an effectual tea assessment system. Sustainability 2020, 12, 6131. [Google Scholar] [CrossRef]
- Zhu, H.; Ye, Y.; He, H.; Dong, C. Evaluation of green tea sensory quality via process characteristics and image information. Food Bioprod. Process. 2017, 102, 116–122. [Google Scholar] [CrossRef]
- Zou, G.; Xiao, Y.; Wang, M.; Zhang, H. Detection of bitterness and astringency of green tea with different taste by electronic nose and tongue. PLoS ONE 2018, 13, e0206517. [Google Scholar]
- Ye, J.H.; Ye, Y.; Yin, J.F.; Liang, Y.R.; Liu, R.Y.; Tang, P.; Xu, Y.Q. Bitterness and astringency of tea leaves and products: Formation mechanism and reducing strategies. Trends Food Sci. Technol. 2022, 123, 130–143. [Google Scholar] [CrossRef]
- Nikniaz, Z.; Mahdavi, R.; Ghaemmaghami, S.J.; Yagin, N.L.; Nikniaz, L. Effect of different brewing times on antioxidant activity and polyphenol content of loosely packed and bagged black teas (Camellia sinensis L.). Avicenna J. Phytomed. 2016, 6, 313–321. [Google Scholar]
- Deng, S.; Zhang, G.; Olayemi-Aluko, O.; Mo, Z.; Mao, J.; Zhang, H.; Liu, X.; Ma, M.; Wang, Q.; Liu, H. Bitter and astringent substances in green tea: Composition, human perception mechanisms, evaluation methods and factors influencing their formation. Food Res. Int. 2022, 157, 111262. [Google Scholar] [CrossRef] [PubMed]
- Ployon, S.; Morzel, M.; Belloir, C.; Bonnotte, A.; Bourillot, E.; Briand, L.; Lesniewska, E.; Lherminier, J.; Aybeke, E.; Canon, F. Mechanisms of astringency: Structural alteration of the oral mucosal pellicle by dietary tannins and protective effect of bPRPs. Food Chem. 2018, 253, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ployon, S.; Belloir, C.; Bonnotte, A.; Lherminier, J.; Canon, F.; Morzel, M. The membrane-associated MUC1 improves adhesion of salivary MUC5B on buccal cells. Application to development of an in vitro cellular model of oral epithelium. Arch. Oral. Biol. 2016, 61, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Zhang, Y.H.; Chen, G.S.; Yin, J.F.; Chen, J.X.; Wang, F.; Xu, Y.Q. Effects of phenolic acids and querce-tin-3-O-rutinoside on the bitterness and astringency of green tea infusion. NPJ Sci. Food 2022, 6, 8. [Google Scholar] [CrossRef]
- Canon, F.; Belloir, C.; Bourillot, E.; Brignot, H.; Briand, L.; Feron, G.; Lesniewska, E.; Nivet, C.; Septier, C.; Schwartz, M.; et al. Perspectives on astringency sensation: An alternative hypothesis on the molecular origin of astringency. J. Agric. Food Chem. 2021, 69, 3822–3826. [Google Scholar] [PubMed]
- Ye, Q.Q.; Chen, G.S.; Pan, W.; Cao, Q.Q.; Zeng, L.; Yin, J.F.; Xu, Y.Q. A predictive model for astringency based on in vitro inter-actions between salivary proteins and (−)-Epigallocatechin gallate. Food Chem. 2021, 340, 127845. [Google Scholar] [CrossRef]
- Liu, T.H.; Chyan, C.L.; Li, F.Y.; Chen, Y.J.; Tzen, J.T.C. Engineering lysine-rich caleosins as carrier proteins to render biotin as a hapten on artificial oil bodies for antibody production. Biotechnol. Prog. 2011, 27, 1760–1767. [Google Scholar]
- Rasband, W.S. ImageJ, 1997–2018; U.S. National Institutes of Health: Bethesda, MD, USA, 2018. Available online: https://imagej.nih.gov/ij/ (accessed on 15 March 2020).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-T.; Tzen, J.T.C. Exploring The Relative Astringency of Tea Catechins and Distinct Astringent Sensation of Catechins and Flavonol Glycosides via an In Vitro Assay Composed of Artificial Oil Bodies. Molecules 2022, 27, 5679. https://doi.org/10.3390/molecules27175679
Liu C-T, Tzen JTC. Exploring The Relative Astringency of Tea Catechins and Distinct Astringent Sensation of Catechins and Flavonol Glycosides via an In Vitro Assay Composed of Artificial Oil Bodies. Molecules. 2022; 27(17):5679. https://doi.org/10.3390/molecules27175679
Chicago/Turabian StyleLiu, Chao-Tzu, and Jason T.C. Tzen. 2022. "Exploring The Relative Astringency of Tea Catechins and Distinct Astringent Sensation of Catechins and Flavonol Glycosides via an In Vitro Assay Composed of Artificial Oil Bodies" Molecules 27, no. 17: 5679. https://doi.org/10.3390/molecules27175679
APA StyleLiu, C.-T., & Tzen, J. T. C. (2022). Exploring The Relative Astringency of Tea Catechins and Distinct Astringent Sensation of Catechins and Flavonol Glycosides via an In Vitro Assay Composed of Artificial Oil Bodies. Molecules, 27(17), 5679. https://doi.org/10.3390/molecules27175679





