Quantification of Arbutin in Cosmetics, Drugs and Food Supplements by Hydrophilic-Interaction Chromatography
Abstract
:1. Introduction
2. Results and Discussion
2.1. Spectra of α-Abrutin and β-Arbutin
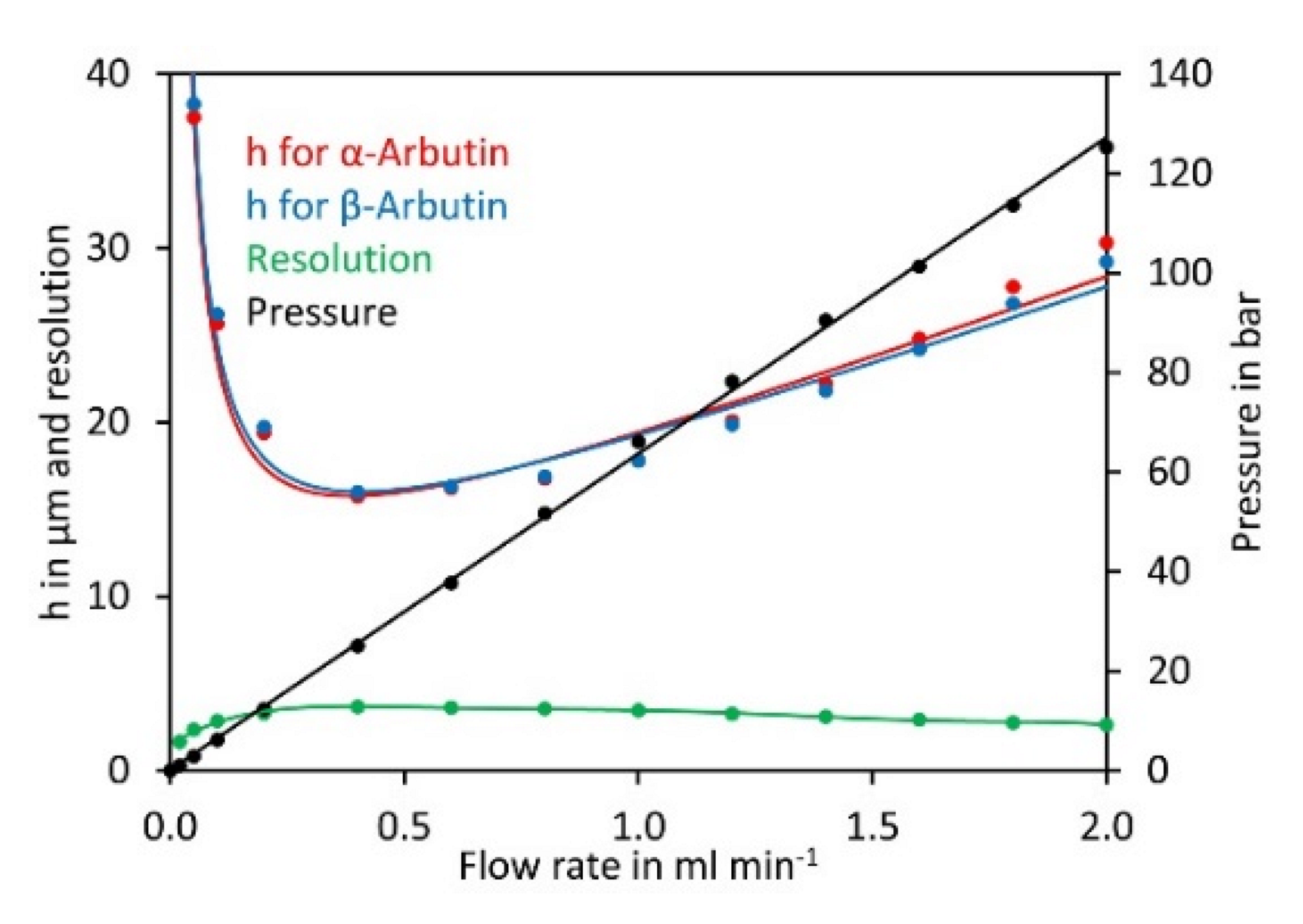
2.2. Optimisation of Chromatographic Conditions
2.3. Sample Preparation
2.4. Validation of the Method
2.5. Assessing the Method under the Principles of Green Chemistry
3. Materials and Methods
3.1. Reagents
3.2. UV Spectroscopy and Measurement of Optical Rotation
3.3. Sample Preparation and HPLC
3.4. HPLC
3.5. Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dadzie, O.E.; Petit, A. Skin bleaching: Highlighting the misuse of cutaneous depigmenting agents. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Lee, S.J.; Chung, M.H.; Park, J.H.; Park, Y.I.; Cho, T.H.; Lee, S.K. Aloesin and arbutin inhibit tyrosinase activity in a synergistic manner via a different action mechanism. Arch. Pharm. Res. 1999, 22, 232–236. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.-S.; Cho, C.; Hong, M.-C.; Shin, M.-K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. 2006, 20, 921–934. [Google Scholar] [CrossRef]
- Thongchai, W.; Liawruangrath, B.; Liawruangrath, S. Arbutin determination in medicinal plants and creams. Int. J. Cosmet. Sci. 2009, 31, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Masse, M.O.; Duvallet, V.; Borremans, M.; Goeyens, L. Identification and quantitative analysis of kojic acid and arbutine in skin-whitening cosmetics. Int. J. Cosmet. Sci. 2001, 23, 219–232. [Google Scholar] [CrossRef]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.-Y.; Park, K.-M.; Choi, K.-W.; Jang, M.K.; Kang, H.Y.; Lee, S.-H.; Park, K.-H.; Cha, J. Inhibitory effects of arbutin-beta-glycosides synthesized from enzymatic transglycosylation for melanogenesis. Biotechnol. Lett. 2008, 30, 743–748. [Google Scholar] [CrossRef]
- Garcia-Jimenez, A.; Teruel-Puche, J.A.; Berna, J.; Rodriguez-Lopez, J.N.; Tudela, J.; Garcia-Canovas, F. Action of tyrosinase on alpha and beta-arbutin: A kinetic study. PLoS ONE 2017, 12, e0177330. [Google Scholar] [CrossRef]
- European Chemicals Agency. Cosmetic Products Regulation, Annex II—Prohibited Substances. Available online: https://echa.europa.eu/de/cosmetics-prohibited-substances (accessed on 4 July 2022).
- Hu, Z.-M.; Zhou, Q.; Lei, T.-C.; Ding, S.-F.; Xu, S.-Z. Effects of hydroquinone and its glucoside derivatives on melanogenesis and antioxidation: Biosafety as skin whitening agents. J. Dermatol. Sci. 2009, 55, 179–184. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety. Opinion on the Safety of Alpha-Arbutin and Beta-Arbutin in Cosmetic Products. Available online: https://ec.europa.eu/health/system/files/2022-03/sccs_o_264.pdf (accessed on 4 July 2022).
- Chandorkar, N.; Tambe, S.; Amin, P.; Madankar, C. Alpha Arbutin as a Skin Lightening Agent: A Review. Int. J. Pharm. Res. 2021, 13. [Google Scholar] [CrossRef]
- Degen, G.H. Opinion of the Scientific Committee on Consumer Safety (SCCS)—Opinion on the safety of the use of β-arbutin in cosmetic products. Regul. Toxicol. Pharmacol. 2015, 73, 866–867. [Google Scholar] [CrossRef] [PubMed]
- Migas, P.; Krauze-Baranowska, M. The significance of arbutin and its derivatives in therapy and cosmetics. Phytochem. Lett. 2015, 13, 35–40. [Google Scholar] [CrossRef]
- European Medicines Agency. Uvae Ursi Folium. Available online: https://www.ema.europa.eu/en/medicines/herbal/uvae-ursi-folium (accessed on 8 June 2022).
- de Arriba, S.G.; Naser, B.; Nolte, K.-U. Risk assessment of free hydroquinone derived from Arctostaphylos Uva-ursi folium herbal preparations. Int. J. Toxicol. 2013, 32, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Cela-López, J.M.; Camacho Roldán, C.J.; Gómez-Lizarraga, G.; Martínez, V. A Natural Alternative Treatment for Urinary Tract Infections: Itxasol©, the Importance of the Formulation. Molecules 2021, 26, 4564. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Overview of Skin Whitening Agents: Drugs and Cosmetic Products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Barsoom, B.N.; Abdelsamad, A.M.E.; Adib, N.M. Indirect spectrophotometric determination of arbutin, whitening agent through oxidation by periodate and complexation with ferric chloride. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 64, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, D.; Xie, Z. Rapid and Specific Fluorescence Method for the Quantification of Arbutin in Cosmetics. Anal. Lett. 2022, 55, 318–326. [Google Scholar] [CrossRef]
- Aydar Barutçu, S.; Eskiköy Bayraktepe, D.; Yazan, Z.; Polat, K.; Filik, H. Investigation of electrochemical oxidation mechanism, rapid and low-level determination for whitening cosmetic: Arbutin in aqueous solution by nano sepiolite clay. Chem. Pap. 2021, 75, 3483–3491. [Google Scholar] [CrossRef]
- Glöckl, I.; Blaschke, G.; Veit, M. Validated methods for direct determination of hydroquinone glucuronide and sulfate in human urine after oral intake of bearberry leaf extract by capillary zone electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 2001, 761, 261–266. [Google Scholar] [CrossRef]
- Jin, W.; Wang, W.-Y.; Zhang, Y.-L.; Yang, Y.-J.; Chu, Q.-C.; Ye, J.-N. Determination of phenolic whitening agents in cosmetics by micellar electrokinetic capillary chromatography with amperometric detection. Chin. Chem. Lett. 2013, 24, 636–638. [Google Scholar] [CrossRef]
- Lamien-Meda, A.; Lukas, B.; Schmiderer, C.; Franz, C.; Novak, J. Validation of a quantitative assay of arbutin using gas chromatography in Origanum majorana and Arctostaphylos uva-ursi extracts. Phytochem. Anal. 2009, 20, 416–420. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L.J. Photostability determination of arbutin, a vegetable whitening agent. Il Farm. 2000, 55, 410–413. [Google Scholar] [CrossRef]
- Parejo, I.; Viladomat, F.; Bastida, J.; Codina, C. A single extraction step in the quantitative analysis of arbutin in bearberry (Arctostaphylos uva-ursi) leaves by high-performance liquid chromatography. Phytochem. Anal. 2001, 12, 336–339. [Google Scholar] [CrossRef]
- Lin, C.-H.; Wu, H.-L.; Huang, Y.-L. Microdialysis sampling coupled to on-line high-performance liquid chromatography for determination of arbutin in whitening cosmetics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 829, 149–152. [Google Scholar] [CrossRef]
- Kittipongpatana, N.; Chaiwan, A.; Pusod, U.; Kittipongpatana, O.S. High Performance Liquid Chromatographic Method for Separation and Quantitative Analysis of Arbutin in Plant Tissue Cultures. CMU J. Nat. Sci. 2007, 6, 65–74. [Google Scholar]
- Cheng, P.; Chen, M.; Zhu, Y. Determination of alpha-arbutin, beta-arbutin and niacinamide in cosmetics by high performance liquid chromatography. Chin. J. Chromatogr. 2010, 28, 89–92. [Google Scholar] [CrossRef]
- Huang, S.-C.; Lin, C.-C.; Huang, M.-C.; Wen, K.-C. Simultaneous determination of magnesium ascorbyl phosphate, ascorbyl glucoside, kojic acid, arbutin and hydroquinone in skin whitening cosmetics by HPLC. J. Food Drug Anal. 2004, 12, 1. [Google Scholar] [CrossRef]
- Rychilinska, I.; Nowak, S. Quantitative Determination of Arbutin and Hydroquinone in Different Plant Materials by HPLC. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 109. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Avonto, C.; Avula, B.; Wang, M.; Rua, D.; Khan, I.A. Quantitative Determination of α-Arbutin, β-Arbutin, Kojic Acid, Nicotinamide, Hydroquinone, Resorcinol, 4-Methoxyphenol, 4-Ethoxyphenol, and Ascorbic Acid from Skin Whitening Products by HPLC-UV. J. AOAC Int. 2015, 98, 5–12. [Google Scholar] [CrossRef]
- Keunchkarian, S.; Reta, M.; Romero, L.; Castells, C. Effect of sample solvent on the chromatographic peak shape of analytes eluted under reversed-phase liquid chromatogaphic conditions. J. Chromatogr. A 2006, 1119, 20–28. [Google Scholar] [CrossRef]
- Alpert, A.J. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J. Chromatogr. A 1990, 499, 177–196. [Google Scholar] [CrossRef]
- Sugimoto, K.; Nishimura, T.; Kuriki, T. Development of α-Arbutin: Production at Industrial Scale and Application for a Skin-Lightening Cosmetic Ingredient. TIGG 2007, 19, 235–246. [Google Scholar] [CrossRef]
- van Deemter, J.J.; Zuiderweg, F.J.; Klinkenberg, A. Longitudinal diffusion and resistance to mass transfer as causes of nonideality in chromatography. Chem. Eng. Sci. 1995, 50, 3869–3882. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Guideline Q2(R2) on Validation of ANALYTICAL Procedures. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures (accessed on 5 July 2022).
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE-Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Schramm, S.; Boco, M.F.A.C.; Manzer, S.; König, O.; Zhang, T.; Mony, F.T.Z.; Adedeji-Badmus, A.N.; Poppenberger, B.; Rozhon, W. Determination of the [15N]-Nitrate/[14N]-Nitrate Ratio in Plant Feeding Studies by GC–MS. Molecules 2019, 24, 1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]


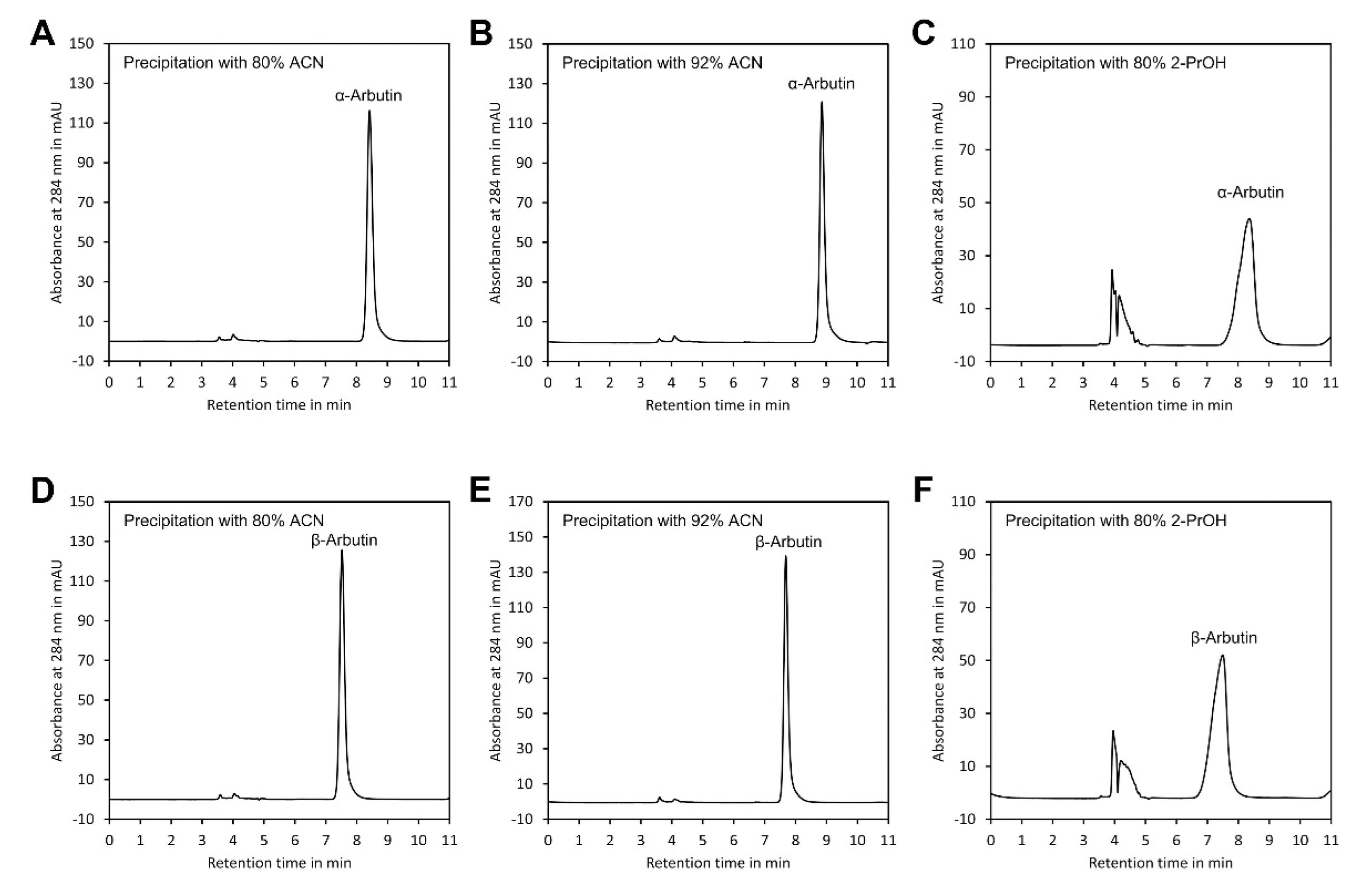


| Solvent, Final Content (v/v) | α-Arbutin Recovery ± SD 2 in % | α-Arbutin W1/2 in Min | β-Arbutin Recovery ± SD in% | β-Arbutin W1/2 in Min |
|---|---|---|---|---|
| ACN/H2O 80/20 | 100.8 ± 2.0 | 0.21 | 103.6 ± 2.5 | 0.19 |
| ACN/H2O 92/8 | 101.4 ± 1.7 | 0.18 | 102.2 ± 0.9 | 0.16 |
| 2-PrOH/H2O 80/20 | 101.3 ± 2.0 | 0.53 | 101.1 ± 2.5 | 0.49 |
| Matrix 2 | Analyte | Unspiked | Spiked | Added | Found | Recovery Rate |
|---|---|---|---|---|---|---|
| Matrix 1 | α-Arbutin | 1.99 ± 0.03% (w/w) | 4.04 ± 0.01% (w/w) | 2% (w/w) | 2.05 ± 0.04% (w/w) | 102.5 ± 1.8% |
| Matrix 2 | α-Arbutin | 2.01 ± 0.03% (w/w) | 4.08 ± 0.02% (w/w) | 2% (w/w) | 2.07 ± 0.03% (w/w) | 103.4 ± 1.7% |
| Matrix 3 | β-Arbutin | 15.18 ± 0.34 g L−1 | 35.62 ± 0.42 g L−1 | 20 g L−1 | 20.44 ± 0.51 g L−1 | 102.2 ± 2.6% |
| Matrix 4 | β-Arbutin | 10.66 ± 0.22 g L−1 | 31.09 ± 0.32 g L−1 | 20 g L−1 | 20.43 ± 0.47 g L−1 | 102.2 ± 2.4% |
| Experiment 1 | Replicates | α-Arbutin 2 Average ± SD (RSD) % (w/w) (%) |
|---|---|---|
| Day 1 | 4 | 1.910 ± 0.029 (1.5) |
| Day 2 | 4 | 1.908 ± 0.025 (1.3) |
| Day 3 | 4 | 1.910 ± 0.010 (0.5) |
| Day 4 | 4 | 1.902 ± 0.018 (0.9) |
| Inter-day | 16 | 1.907 ± 0.020 (1.0) |
| Experiment 1 | Replicates | α-Arbutin 2 Average ± SD (RSD) % (w/w) (%) |
|---|---|---|
| Day 1 | 3 3 | 1.879 ± 0.043 (2.3) |
| Day 2 | 4 | 1.909 ± 0.038 (2.0) |
| Day 3 | 4 | 1.898 ± 0.009 (0.5) |
| Day 4 | 4 | 1.884 ± 0.014 (0.7) |
| Inter-day | 15 | 1.893 ± 0.028 (1.5) |
| Experiment 1 | Replicates | β-Arbutin 2 Average ± SD (RSD) mg per Pill (%) |
|---|---|---|
| Day 1 | 4 | 156.2 ± 1.7 (1.1) |
| Day 2 | 4 | 157.2 ± 1.8 (1.2) |
| Day 3 | 4 | 163.5 ± 1.1 (0.7) |
| Day 4 | 4 | 161.1 ± 2.9 (1.8) |
| Inter-day | 16 | 159.5 ± 3.6 (2.2) |
| Experiment 1 | Replicates | β-Arbutin 2 Average ± SD (RSD) mg per Pill (%) |
|---|---|---|
| Day 1 | 4 | 103.1 ± 0.6 (0.6) |
| Day 2 | 4 | 104.2 ± 1.1 (1.1) |
| Day 3 | 4 | 104.6 ± 0.2 (0.2) |
| Day 4 | 4 | 103.1 ± 1.0 (1.0) |
| Inter-day | 16 | 103.8 ± 1.0 (1.0) |
| Experiment 1 | α-Arbutin Equation (r) 2 | β-Arbutin Equation (r) |
|---|---|---|
| Day 1 | y = 14.452x − 17.940 (0.9999) | y = 14.669x − 34.583 (0.9999) |
| Day 2 | y = 14.429x − 26.208 (0.9999) | y = 14.570x − 29.548 (0.9999) |
| Day 3 | y = 14.353x − 21.773 (0.9999) | y = 14.579x − 38.618 (0.9998) |
| Day 4 | y = 14.254x − 4.355 (0.9997) | y = 14.483x − 7.216 (0.9998) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Repert, S.; Matthes, S.; Rozhon, W. Quantification of Arbutin in Cosmetics, Drugs and Food Supplements by Hydrophilic-Interaction Chromatography. Molecules 2022, 27, 5673. https://doi.org/10.3390/molecules27175673
Repert S, Matthes S, Rozhon W. Quantification of Arbutin in Cosmetics, Drugs and Food Supplements by Hydrophilic-Interaction Chromatography. Molecules. 2022; 27(17):5673. https://doi.org/10.3390/molecules27175673
Chicago/Turabian StyleRepert, Sarah, Sandra Matthes, and Wilfried Rozhon. 2022. "Quantification of Arbutin in Cosmetics, Drugs and Food Supplements by Hydrophilic-Interaction Chromatography" Molecules 27, no. 17: 5673. https://doi.org/10.3390/molecules27175673
APA StyleRepert, S., Matthes, S., & Rozhon, W. (2022). Quantification of Arbutin in Cosmetics, Drugs and Food Supplements by Hydrophilic-Interaction Chromatography. Molecules, 27(17), 5673. https://doi.org/10.3390/molecules27175673







