The Influence of Yb Doping and Sintering Conditions on the Magnetocaloric and Mechanical Properties of EuS
Abstract
1. Introduction
2. Results
2.1. Sintering of EuS and Yb-Doped EuS
2.2. Magnetizations of EuS and Yb-Doped EuS Bulks
2.3. Specific Heat of EuS and Yb-Doped EuS
2.4. The Influence of Sintering Conditions on the Mechanical Properties of EuS
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Yan, M. Recent progresses in exploring the rare earth based intermetallic compounds for cryogenic magnetic refrigeration. J. Alloys Compd. 2020, 823, 153810. [Google Scholar] [CrossRef]
- Wu, S.; Zhong, X.; Dong, X.; Liu, C.; Huang, J.; Huang, Y.; Yu, H.; Liu, Z.; Huang, Y.; Ramanujan, R. LaFe11.6Si1.4/Pr40Co60 magnetocaloric composites for refrigeration near room temperature. J. Alloys Compd. 2021, 873, 159796. [Google Scholar] [CrossRef]
- Terada, N.; Mamiya, H. High-efficiency magnetic refrigeration using holmium. Nat. Commun. 2021, 12, 1212. [Google Scholar] [CrossRef]
- Tang, X.; Sepehri-Amin, H.; Terada, N.; Martin-Cid, A.; Kurniawan, I.; Kobayashi, S.; Kotani, Y.; Takeya, H.; Lai, J.; Matsushita, Y. Magnetic refrigeration material operating at a full temperature range required for hydrogen liquefaction. Nat. Commun. 2022, 13, 1817. [Google Scholar] [CrossRef]
- Castro, P.B.D.; Terashima, K.; Yamamoto, T.D.; Hou, Z.; Iwasaki, S.; Matsumoto, R.; Adachi, S.; Saito, Y.; Song, P.; Takeya, H. Machine-learning-guided discovery of the gigantic magnetocaloric effect in HoB2 near the hydrogen liquefaction temperature. NPG Asia Mater. 2020, 12, 35. [Google Scholar] [CrossRef]
- Aziz, M. Liquid hydrogen: A review on liquefaction, storage, transportation, and safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- Liu, X.; Hou, Y.; Tang, M.; Wang, L. Atom elimination strategy for MoS2 nanosheets to enhance photocatalytic hydrogen evolution. Chin. Chem. Lett. 2022, in press. [Google Scholar] [CrossRef]
- Liu, M.; Li, H.; Liu, S.; Wang, L.; Xie, L.; Zhuang, Z.; Sun, C.; Wang, J.; Tang, M.; Sun, S. Tailoring activation sites of metastable distorted 1T′-phase MoS2 by Ni doping for enhanced hydrogen evolution. Nano Res. 2022, 15, 5946–5952. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, L.; Xie, L.; Sun, C.; Zhao, W.; Liu, X.; Zhuang, Z.; Liu, S.; Zhao, Q. Defect-driven selective oxidation of MoS2 nanosheets with photothermal effect for photo-catalytic hydrogen evolution reaction. Chem. Eng. J. 2022, 439, 135757. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, B.G.; Xu, Z.Y.; Shen, J.; Hu, F.X.; Sun, J.R.; Long, Y. Large reversible magnetocaloric effects in ErFeSi compound under low magnetic field change around liquid hydrogen temperature. Appl. Phys. Lett. 2013, 102, 092401. [Google Scholar] [CrossRef]
- Numazawa, T.; Kamiya, K.; Utaki, T.; Matsumoto, K. Magnetic refrigerator for hydrogen liquefaction. Cryogenics 2014, 62, 185–192. [Google Scholar] [CrossRef]
- Midya, A.; Mandal, P.; Rubi, K.; Chen, R.; Wang, J.S.; Mahendiran, R.; Lorusso, G.; Evangelisti, M. Large adiabatic temperature and magnetic entropy changes in EuTiO3. Phys. Rev. B 2016, 93, 094422. [Google Scholar] [CrossRef]
- Li, D.X.; Yamamura, T.; Nimori, S.; Homma, Y.; Honda, F.; Haga, Y.; Aoki, D. Large reversible magnetocaloric effect in ferromagnetic semiconductor EuS. Solid State Commun. 2014, 193, 6–10. [Google Scholar] [CrossRef]
- Mathimalar, S.; Sasmal, S.; Bhardwaj, A.; Abhaya, S.; Pothala, R.; Chaudhary, S.; Satpati, B.; Raman, K.V. Signature of gate-controlled magnetism and localization effects at Bi2Se3/EuS interface. NPJ Quantum Mater. 2020, 5, 64. [Google Scholar] [CrossRef]
- Beer, S.M.; Muriqi, A.; Lindner, P.; Winter, M.; Rogalla, D.; Nolan, M.; Ney, A.; Debus, J.; Devi, A. Ferromagnetic europium sulfide thin films: Influence of precursors on magneto-optical properties. Chem. Mater. 2021, 34, 152–164. [Google Scholar] [CrossRef]
- Hua, Q.; Tang, F.; Wang, X.; Li, M.; Gu, X.; Sun, W.; Luan, F.; Tian, C.; Zhuang, X. Electrochemiluminescence sensor based on EuS nanocrystals for ultrasensitive detection of mercury ions in seafood. Sens. Actuators B Chem. 2022, 352, 131075. [Google Scholar] [CrossRef]
- Glaser, P.; Stewart Jr, O.; Atif, R.; Asuigui, D.R.C.; Swanson, J.; Biacchi, A.J.; Hight Walker, A.R.; Morrison, G.; zur Loye, H.C.; Stoll, S.L. Synthesis of mixed-valent lanthanide sulfide nanoparticles. Angew. Chem. 2021, 133, 23318–23325. [Google Scholar] [CrossRef]
- Alenad, A. Novel Routes to Europium Sulfide. Ph.D. Dissertation, University of Manchester, Manchester, UK, 2018. [Google Scholar]
- Gu, S.; He, W.; Zhang, M.; Zhuang, T.; Jin, Y.; ElBidweihy, H.; Mao, Y.; Dickerson, J.H.; Wagner, M.J.; Torre, E.D. Physical justification for negative remanent magnetization in homogeneous nanoparticles. Sci. Rep. 2014, 4, 6267. [Google Scholar] [CrossRef]
- Zhao, F.; Sun, H.L.; Su, G.; Gao, S. Synthesis and size-dependent magnetic properties of monodisperse EuS nanocrystals. Small 2006, 2, 244–248. [Google Scholar] [CrossRef]
- Zhao, F.; Sun, H.-L.; Gao, S.; Su, G. Magnetic properties of EuS nanoparticles synthesized by thermal decomposition of molecular precursors. J. Mater. Chem. 2005, 15, 4209–4214. [Google Scholar] [CrossRef]
- Boncher, W.; Dalafu, H.; Rosa, N.; Stoll, S. Europium chalcogenide magnetic semiconductor nanostructures. Coord. Chem. Rev. 2015, 289, 279–288. [Google Scholar] [CrossRef]
- Li, L.; Hirai, S.; Nakamura, E.; Yuan, H. Influences of Eu2O3 characters and sulfurization conditions on the preparation of EuS and its large magnetocaloric effect. J. Alloys Compd. 2016, 687, 413–420. [Google Scholar] [CrossRef]
- Bien, T.N.; Hirai, S.; Vasilyeva, I.G.; Nikolaev, R.; Sekine, C.; Atsunori, K. Study of non-stoichiometric GdSx (0.68≤ x≤ 1.2) processed by reaction sintering. J. Alloys Compd. 2020, 831, 154691. [Google Scholar] [CrossRef]
- Nikolaev, R.; Sulyaeva, V.; Alekseev, A.; Sukhikh, A.; Polyakova, E.; Pomelova, T.; Kuzuya, T.; Hirai, S.; Nhu, B.T. Growth mechanism of helical γ-Dy2S3 single crystals. CrystEngComm 2021, 23, 2196–2201. [Google Scholar] [CrossRef]
- Guo, Q.; Tegus, O.; Ebisu, S. Specific heat in magnetic field and magnetocaloric effects of α-R2S3 (R= Tb, Dy) single crystals. J. Magn. Magn. Mater. 2018, 465, 260–269. [Google Scholar] [CrossRef]
- Hirai, S.; Sumita, E.; Shimakage, K.; Uemura, Y.; Nishimura, T.; Mitomo, M. Synthesis and sintering of cerium (II) monosulfide. J. Am. Ceram. Soc. 2004, 87, 23–28. [Google Scholar] [CrossRef]
- Yuan, H.; Kuzuya, T.; Ohta, M.; Hirai, S. Low-temperature formation of cubic Th3P4-type gadolinium and holmium sesquisulfides. J. MMIJ 2010, 126, 450–455. [Google Scholar] [CrossRef][Green Version]
- Selinsky, R.S.; Han, J.H.; Morales Pérez, E.A.; Guzei, I.A.; Jin, S. Synthesis and magnetic properties of Gd doped EuS nanocrystals with enhanced Curie temperatures. J. Am. Chem. Soc. 2010, 132, 15997–16005. [Google Scholar] [CrossRef]
- Thompson, W.; Holtzberg, F.; McGuire, T.; Petrich, G. Tunneling study of EuS magnetization. AIP Conf. Proc. 1972, 5, 827–836. [Google Scholar]
- Van Houten, S. Magnetic interaction in EuS, EuSe, and EuTe. Phys. Lett. 1962, 2, 215–216. [Google Scholar] [CrossRef]
- Yu, M.; Moayedpour, S.; Yang, S.; Dardzinski, D.; Wu, C.; Pribiag, V.S.; Marom, N. Dependence of the electronic structure of the EuS/InAs interface on the bonding configuration. Phys. Rev. Mater. 2021, 5, 064606. [Google Scholar] [CrossRef]
- Lin, H.; Luo, Q.; Tong, W.-Y.; Jiang, C.; Huang, R.; Peng, H.; Zhang, L.-C.; Travas-Sejdic, J.; Duan, C.-G. Facile preparation of rare-earth semiconductor nanocrystals and tuning of their dimensionalities. RSC Adv. 2015, 5, 86885–86890. [Google Scholar] [CrossRef]
- Köbler, U.; Hupfeld, D.; Schnelle, W.; Mattenberger, K.; Brückel, T. Fourth-order exchange interactions in GdxEu1-xS. J. Magn. Magn. Mater. 1999, 205, 90–104. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Hirai, S. Fabrication, sintering, heat capacity, magnetic and magnetroresistivity properties of ytterbium sulfides. J. Magn. Magn. Mater. 2019, 476, 289–296. [Google Scholar] [CrossRef]
- Shapira, Y.; Reed, T. In Elastic Constants, Compressibilities and Debye Temperatures of the Eu-Chalcogenides. AIP Conf. Proc. 1972, 5, 837–839. [Google Scholar]
- Lorusso, G.; Sharples, J.W.; Palacios, E.; Roubeau, O.; Brechin, E.K.; Sessoli, R.; Rossin, A.; Tuna, F.; McInnes, E.J.; Collison, D. A dense metal–organic framework for enhanced magnetic refrigeration. Adv. Mater. 2013, 25, 4653–4656. [Google Scholar] [CrossRef]
- Lima, A.; Gschneidner Jr, K.; Pecharsky, V.; Pecharsky, A. Disappearance and reappearance of magnetic ordering upon lanthanide substitution in (Er1−xDyx)Al2. Phys. Rev. B 2003, 68, 134409. [Google Scholar] [CrossRef]
- Yamamoto, T.A.; Nakagawa, T.; Sako, K.; Arakawa, T.; Nitani, H. Magnetocaloric effect of rare earth mono-nitrides, TbN and HoN. J. Alloys Compd. 2004, 376, 17–22. [Google Scholar] [CrossRef]
- Li, L.; Hirai, S.; Yuan, H. Influences of Yb2O3 characters and sulfurization conditions on preparation of Yb2S3. J. Alloys Compd. 2014, 618, 742–749. [Google Scholar] [CrossRef]
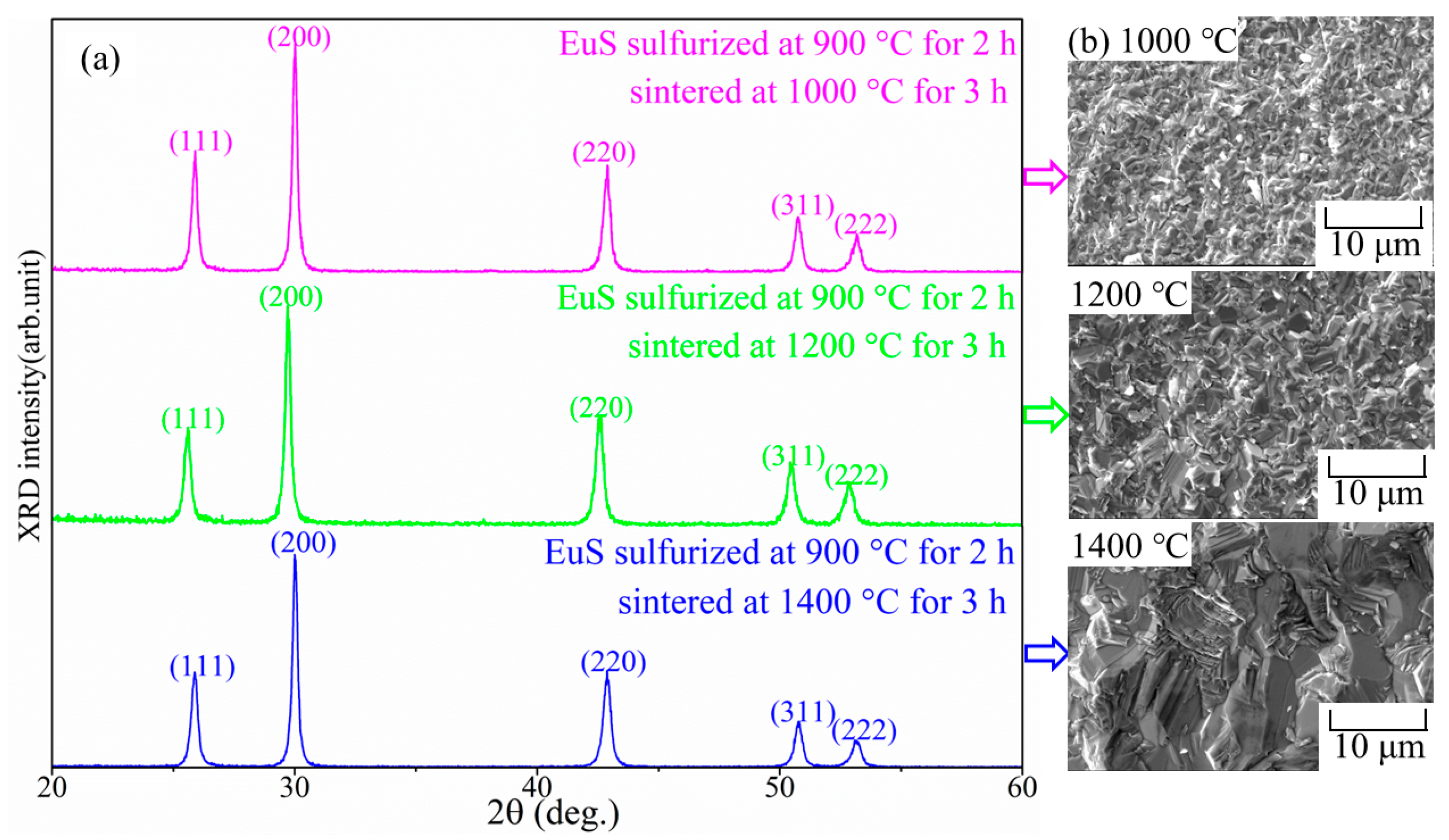


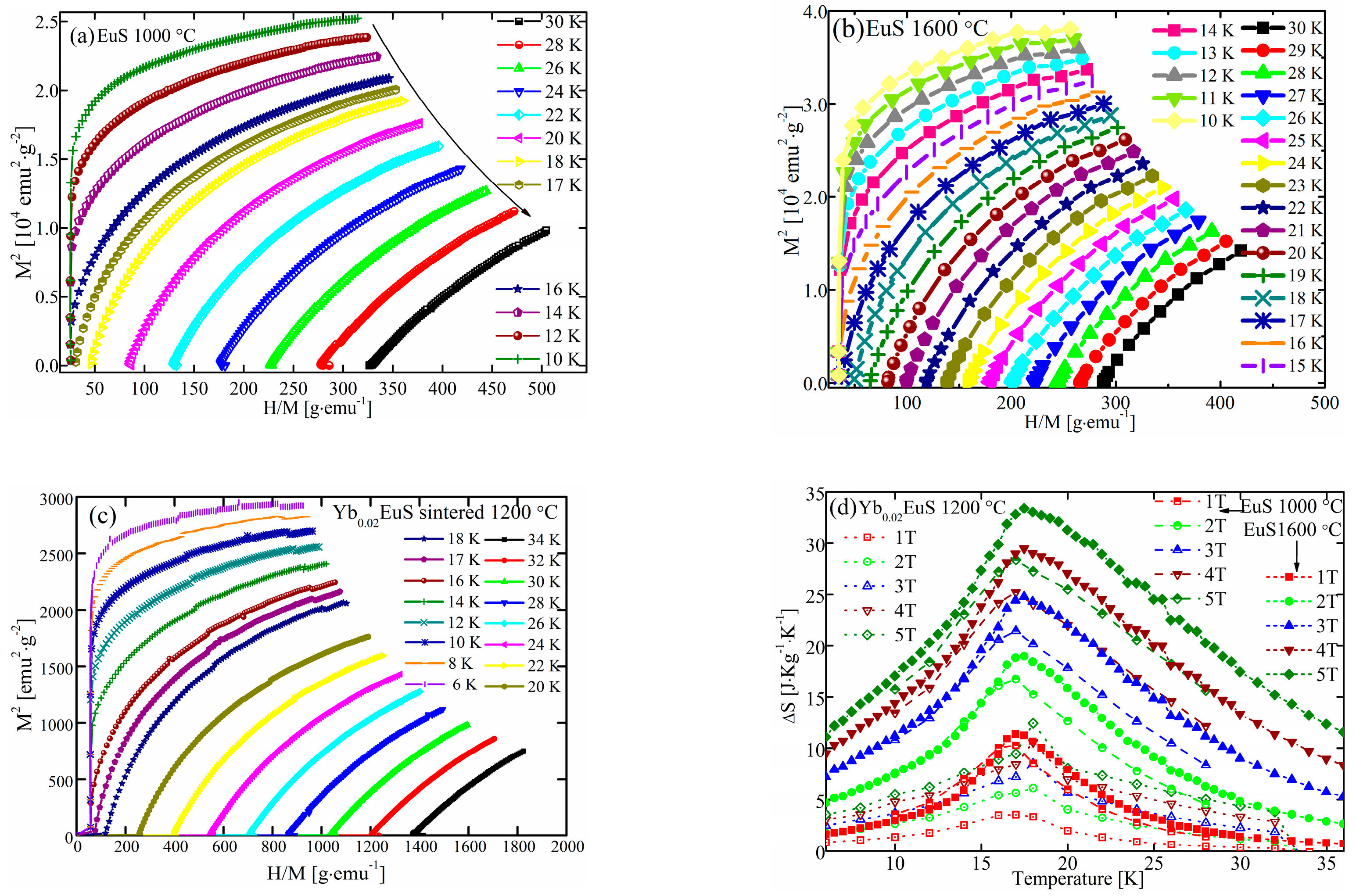


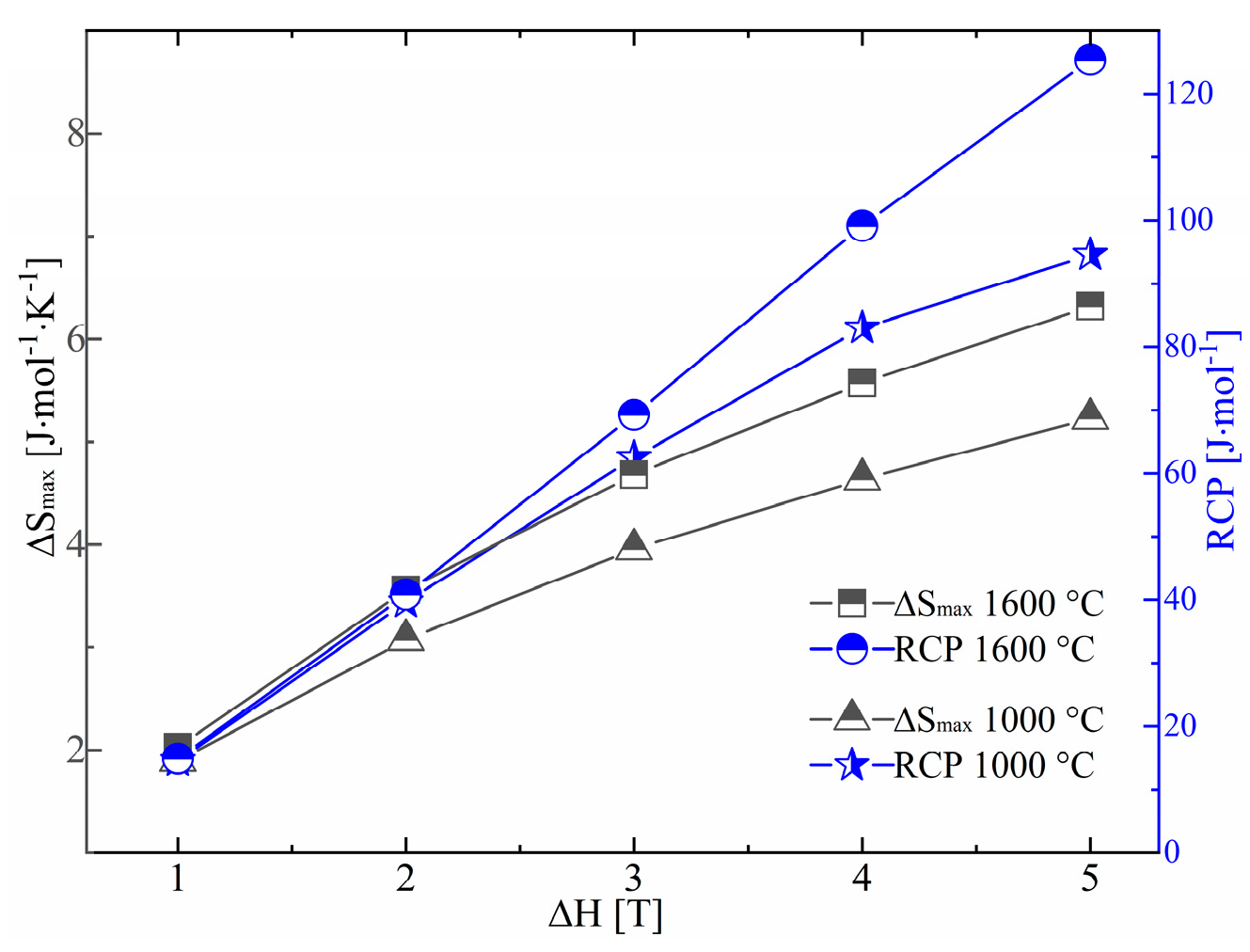
| Sample | Sulf. Condition | Lattice Constant |
|---|---|---|
| EuS | 1000 °C | 5.959182 |
| EuS | 1200 °C | 5.969099 |
| EuS | 1400 °C | 5.979182 |
| Yb0.02EuS | 1000 °C | 5.971716 |
| Yb0.02EuS | 1200 °C | 5.962835 |
| Yb0.02EuS | 1400 °C | 5.971736 |
| Sintering Temperature | M(2 K) [emu/g] | Tc [K] | C | θp [K] | μeff (μB) |
|---|---|---|---|---|---|
| 1000 °C | 0.0500 | 18.0 | 8.13 | 18.8 | 8.06 |
| 1400 °C | 0.0681 | 18.0 | 8.54 | 19.6 | 8.26 |
| 1600 °C | 0.0001 | 18.2 | 6.48 | 17.0 | 7.20 |
| Single-crystal EuS (100) plane [13] | 0.0430 | 19.0 | 7.58 | 17.2 | 7.79 |
| Single-crystal EuS (110) plane [13] | 0.0500 | 19.0 | 16.9 | 7.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Chen, Y.; He, J.; Zhou, A. The Influence of Yb Doping and Sintering Conditions on the Magnetocaloric and Mechanical Properties of EuS. Molecules 2022, 27, 5660. https://doi.org/10.3390/molecules27175660
Li L, Chen Y, He J, Zhou A. The Influence of Yb Doping and Sintering Conditions on the Magnetocaloric and Mechanical Properties of EuS. Molecules. 2022; 27(17):5660. https://doi.org/10.3390/molecules27175660
Chicago/Turabian StyleLi, Liang, Yuqi Chen, Junbao He, and Aiguo Zhou. 2022. "The Influence of Yb Doping and Sintering Conditions on the Magnetocaloric and Mechanical Properties of EuS" Molecules 27, no. 17: 5660. https://doi.org/10.3390/molecules27175660
APA StyleLi, L., Chen, Y., He, J., & Zhou, A. (2022). The Influence of Yb Doping and Sintering Conditions on the Magnetocaloric and Mechanical Properties of EuS. Molecules, 27(17), 5660. https://doi.org/10.3390/molecules27175660







