Synthesis of [B,Al]-EWT-Type Zeolite and Its Catalytic Properties
Abstract
:1. Introduction
2. Result and Discussion
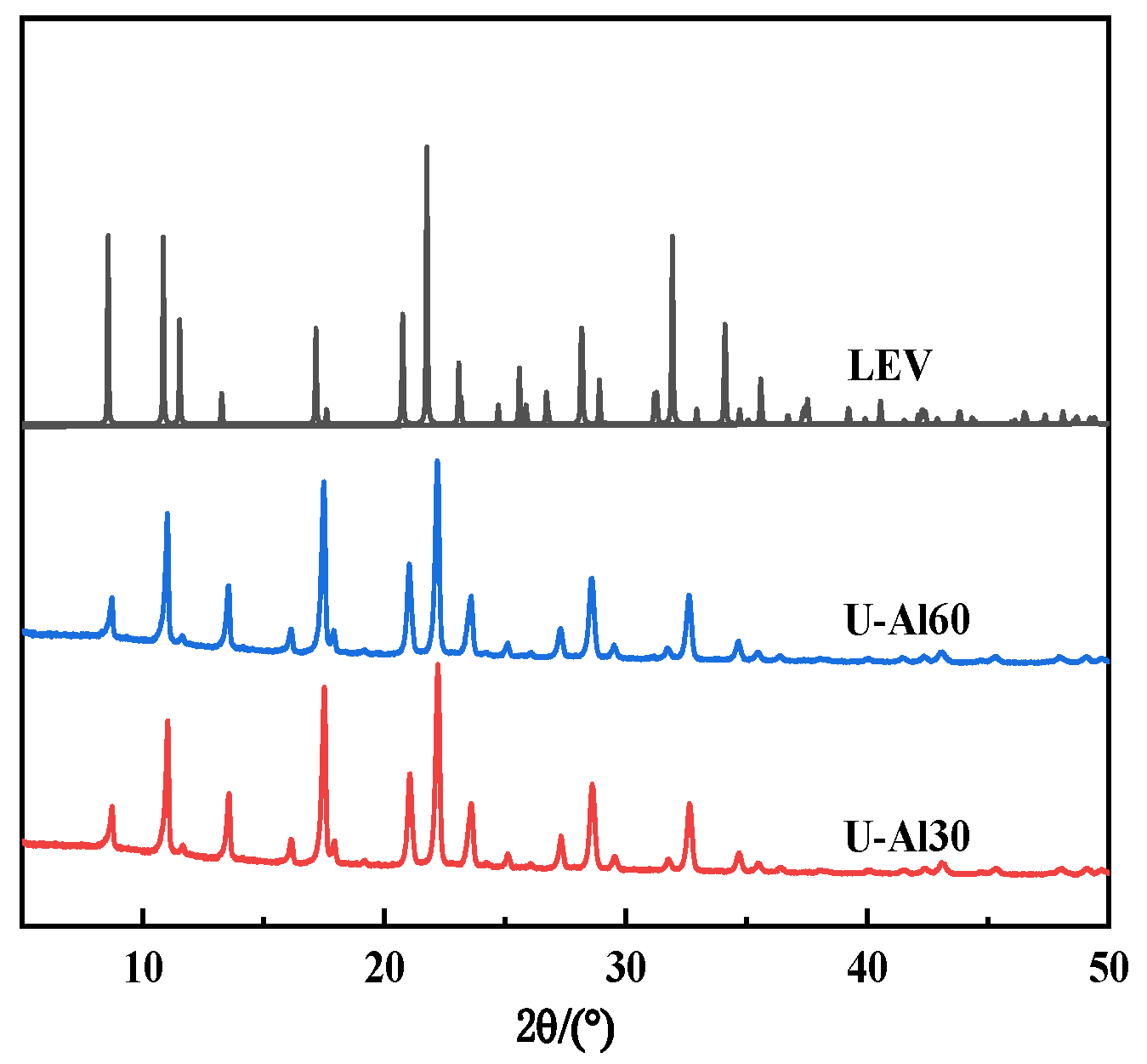
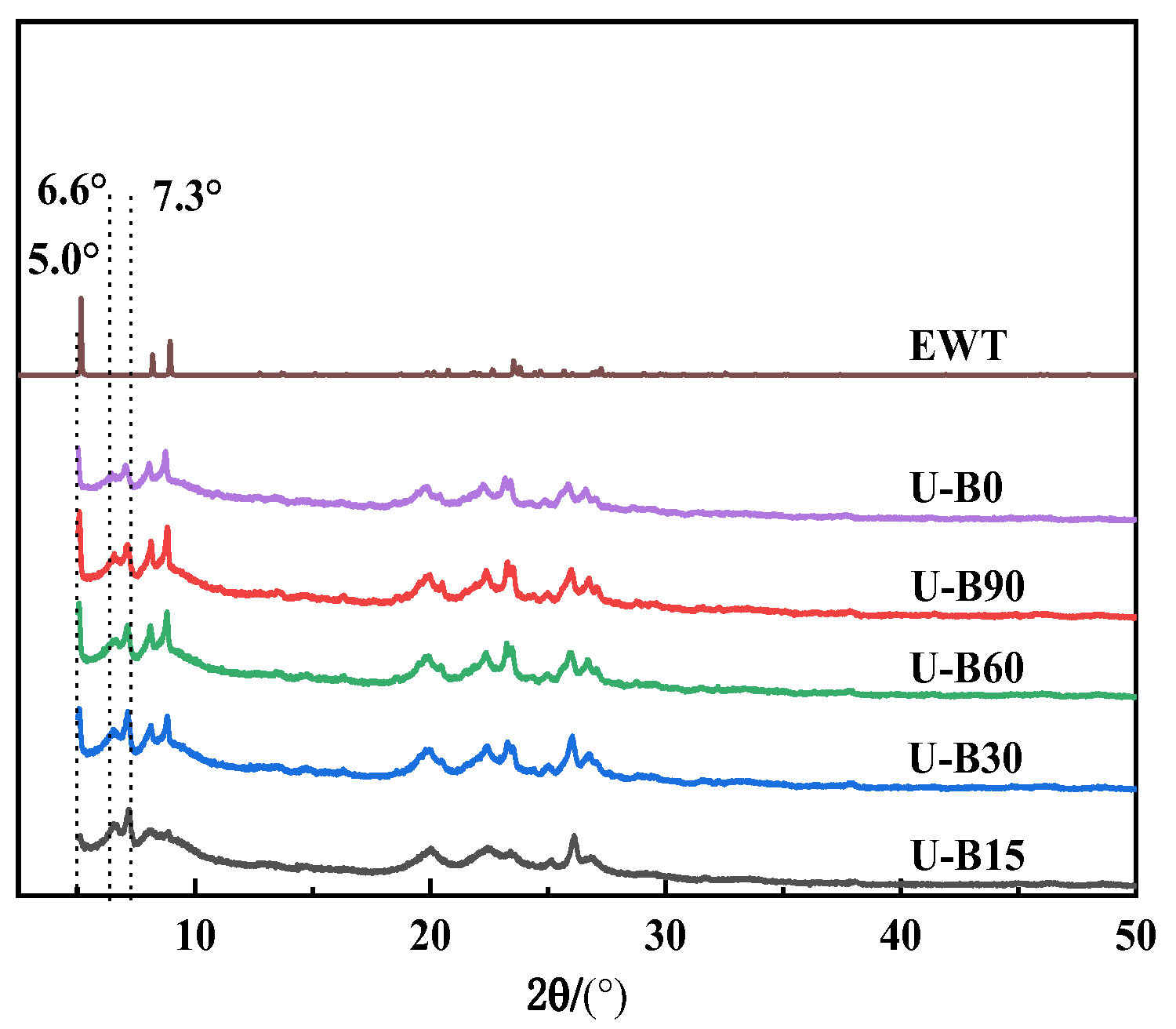
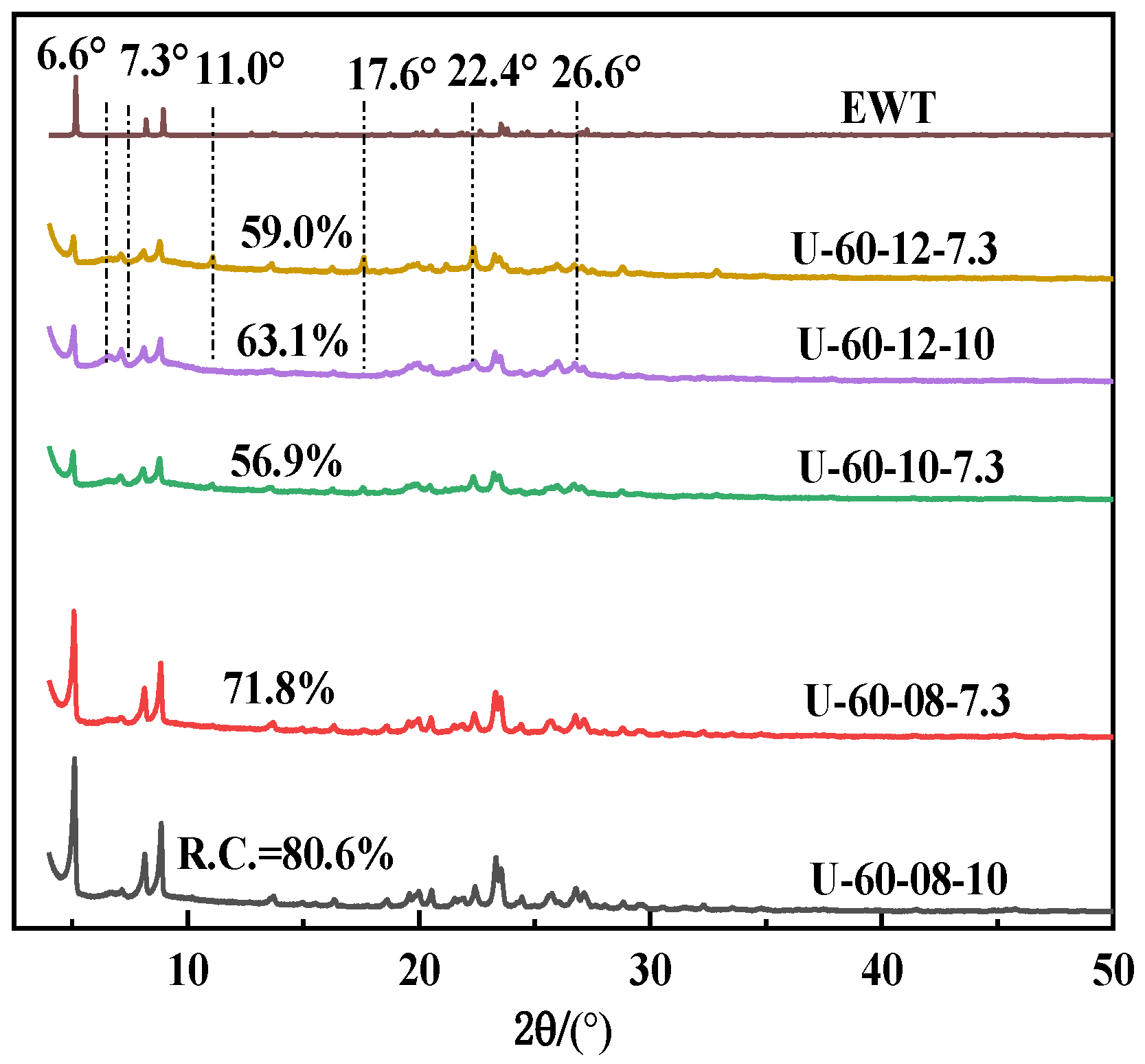
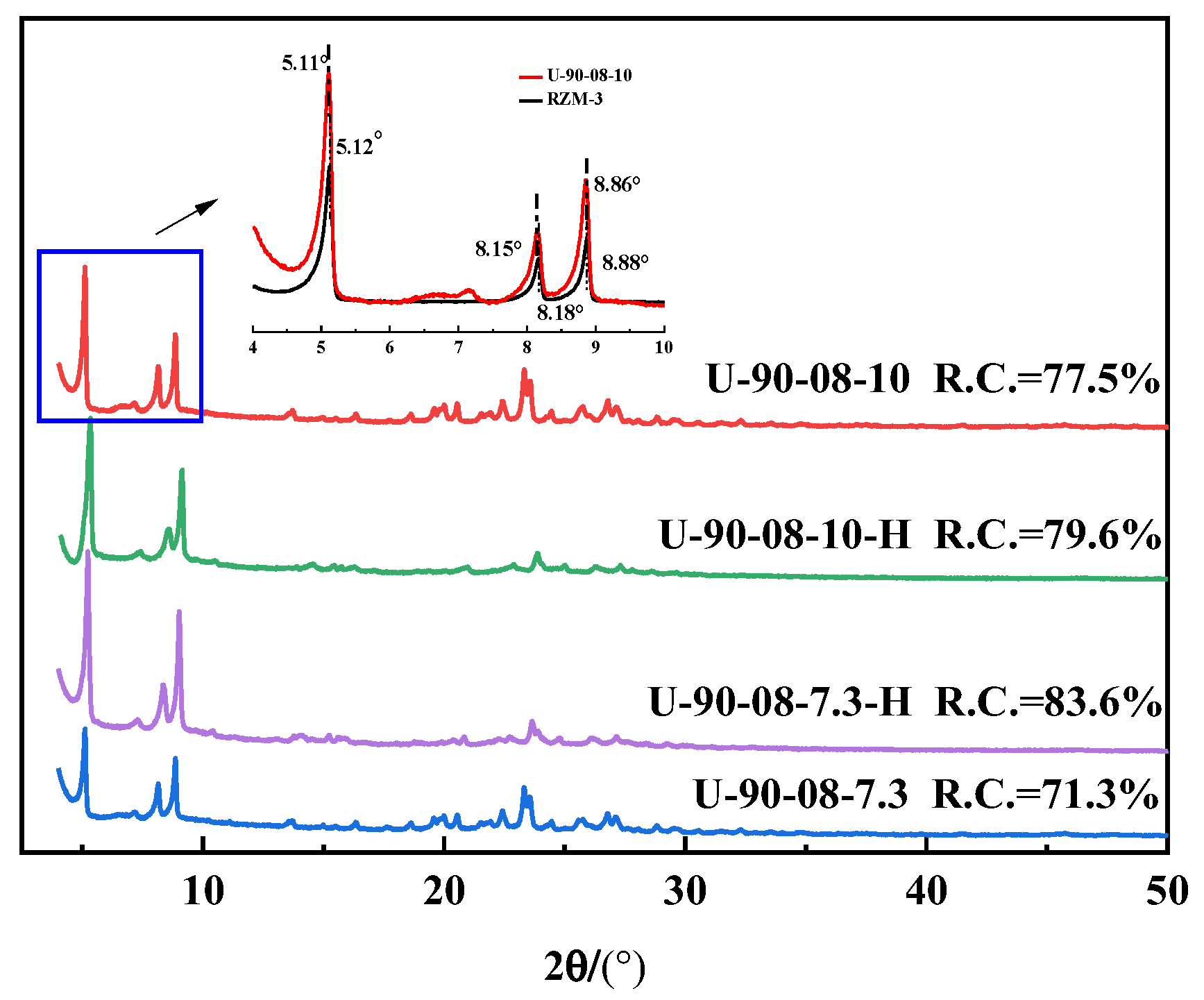
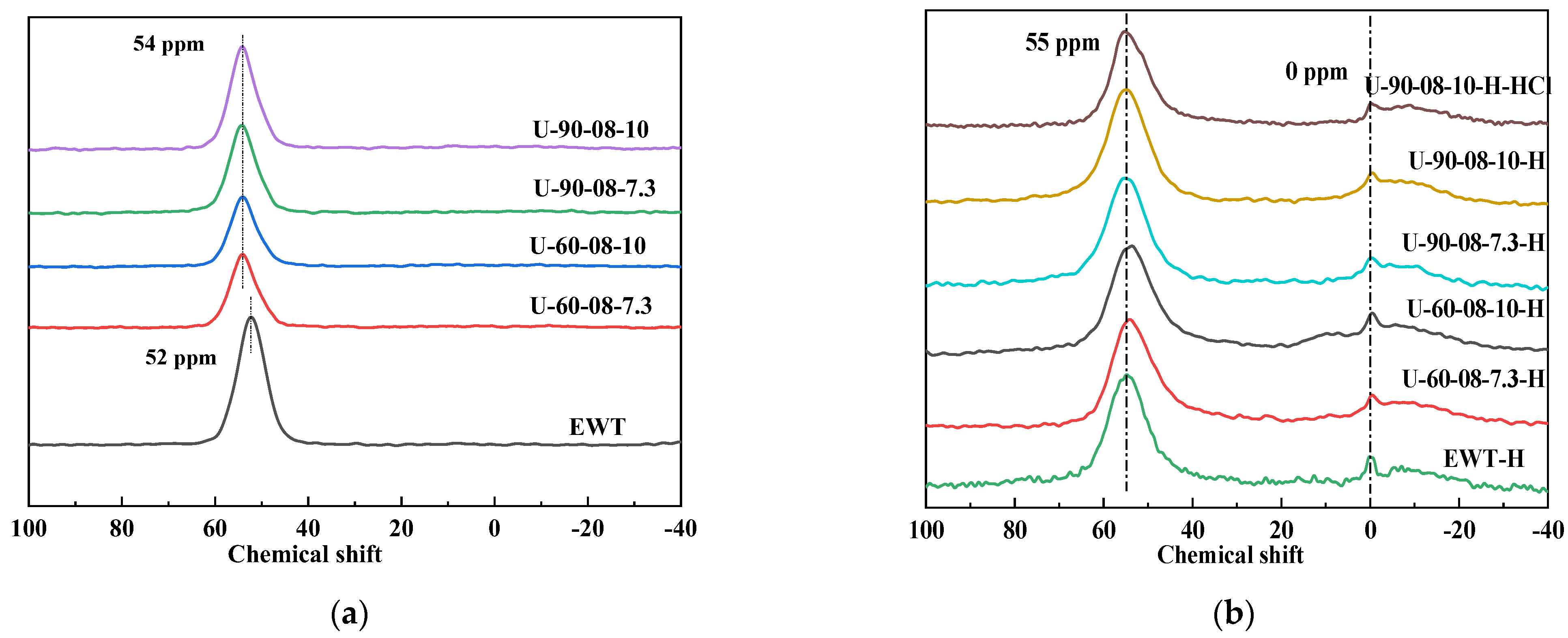
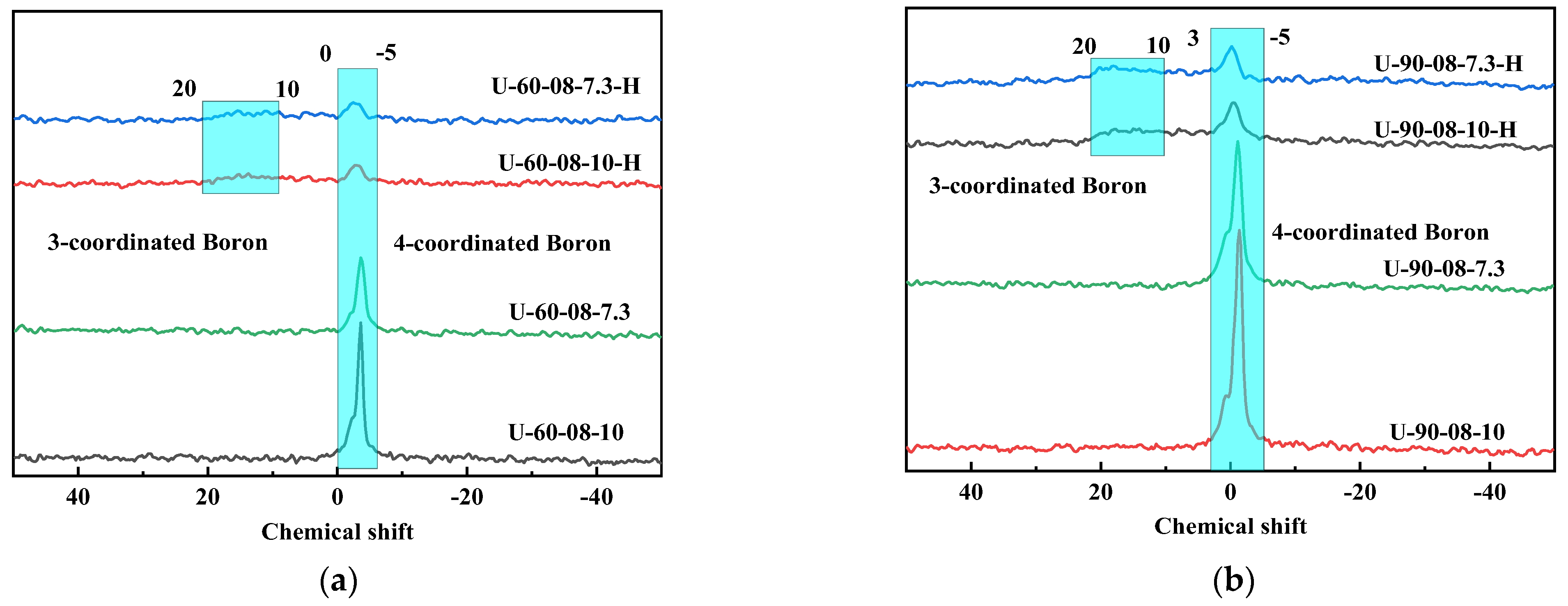
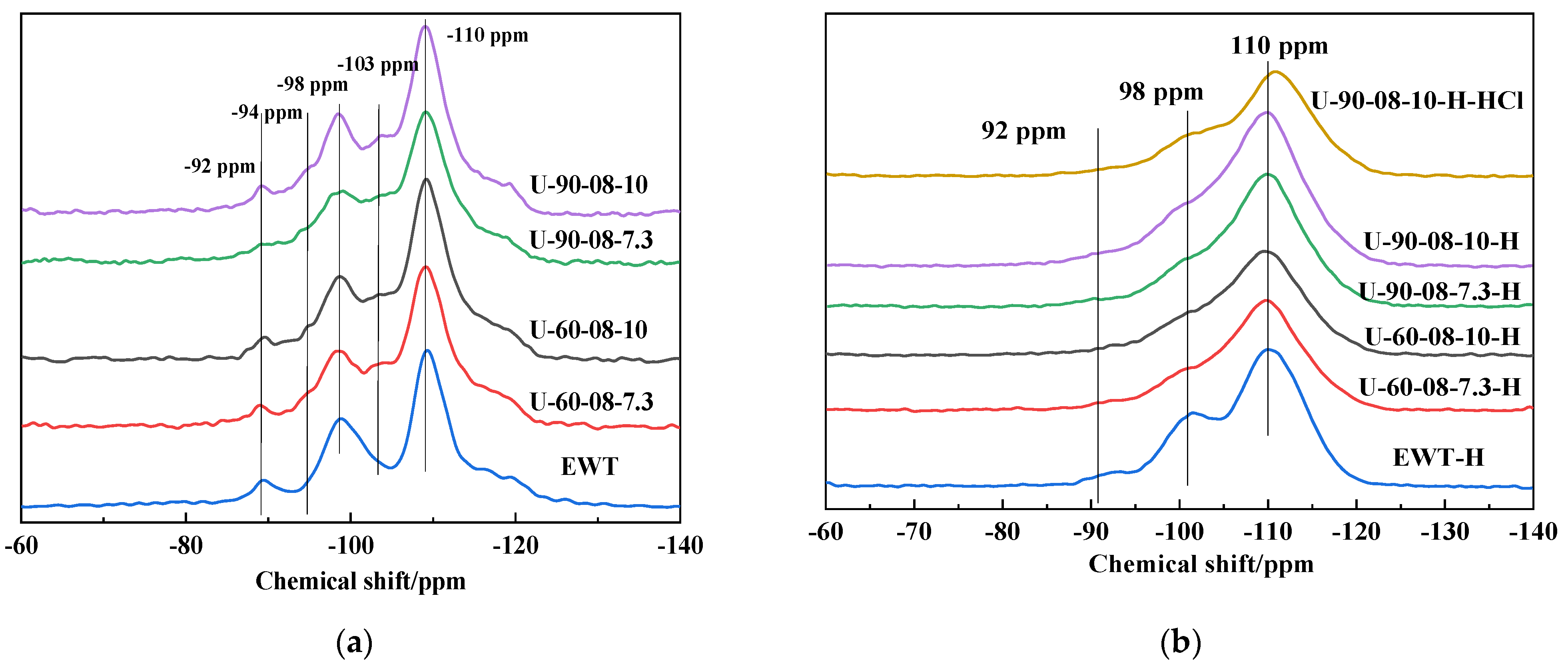
2.1. The Preparation and Characterization of [B,Al]-EWT by Direct Synthesis
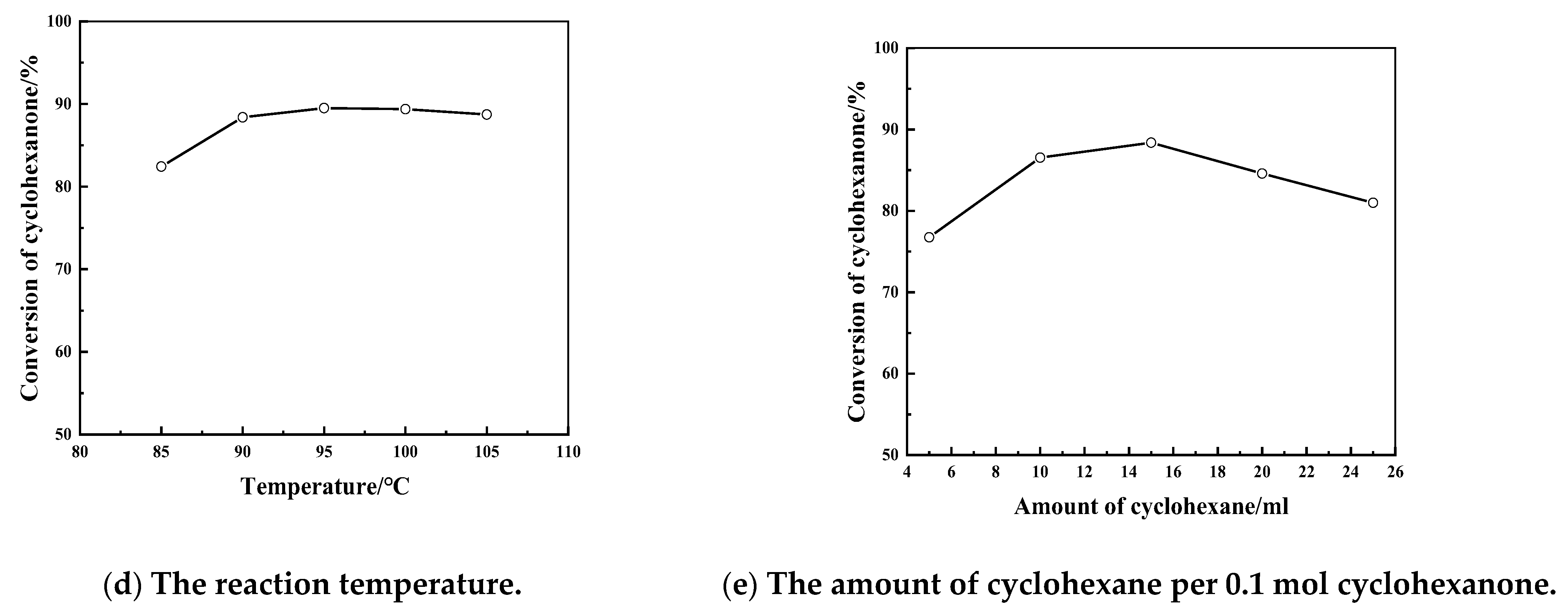
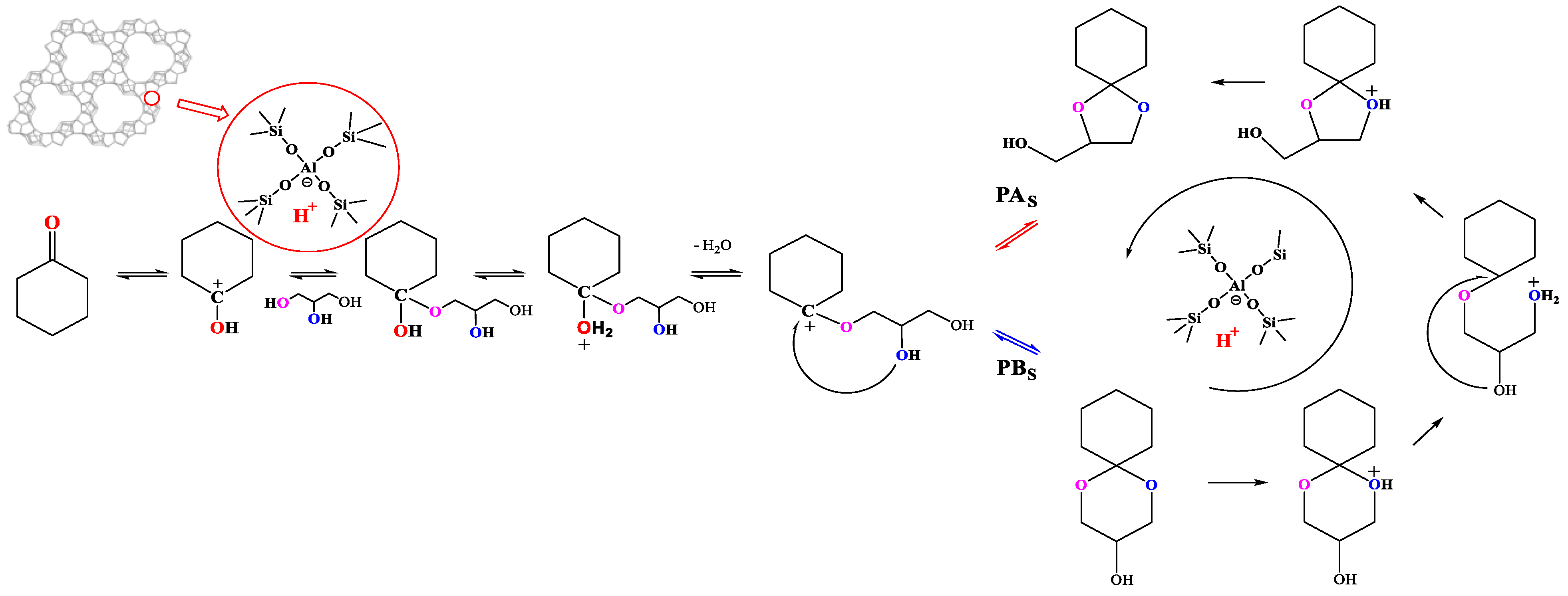
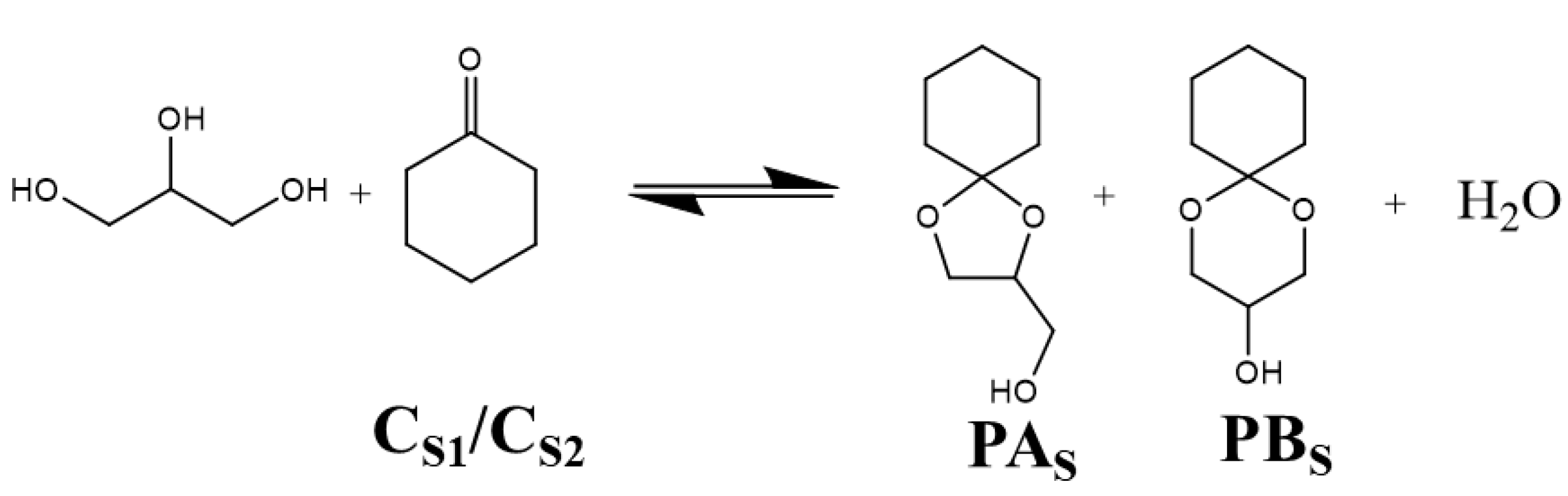
2.2. The Catalytic Property of [Bal]-EWT in the Ketalization Reaction of Glycerol with CycloHexanone
3. Experiment and Methods
3.1. Synthesis of Organic Structure-Directing Agents as Bromide salts
3.2. Anion Exchange from Bromide to Hydroxide
3.3. [B,Al]-EWT Zeolite by Direct Synthesis
3.4. Characterization
3.5. Catalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Burton, A.W.; Stewartsville, N.J. EMM-23 Molecular Sieve Materials, Its Synthesis and Use. U.S. Patent 2012/047910, 1 March 2012. [Google Scholar]
- Willhammar, T.; Burton, A.W.; Yun, Y. EMM-23: A stable high-silica multidimensional zeolite with extra-large trilobe-shaped channels. J. Am. Chem. Soc. 2014, 136, 13570–13573. [Google Scholar] [CrossRef]
- Wu, Q.M.; Meng, X.J.; Lei, C.X.; Feng, S. Method for Synthesizing Macroporous EMM-23 Zeolite Molecular Sieve Using Polyquaternary Ammonium Salt Template. Chinese Patent 106542539, 29 March 2017. [Google Scholar]
- Wang, Y.R.; Zhu, J.C.; Sun, M.Y.; Wang, L.X.; Yang, J.C.; Wu, Q.; Wang, X.G.; Liu, C.L.; Sun, J.L.; Mu, X.H.; et al. The synthesis of RZM-3 zeolite with EWT topology structure using 1,1,6,6-tetramethyl-1,6-diazacyclododecane-1,6-diium dihydroxide as structure-directing agent. Microporous Mesoporous Mater. 2019, 275, 87–94. [Google Scholar] [CrossRef]
- Xie, M.G.; Wang, Y.R.; Mu, X.H. Silicon Boron Zeolite, Molecular Sieve Composition and Preparation Method. Chinese Patent 202011145868.2, 23 October 2020. [Google Scholar]
- Breck, D.W. Zeolite Molecular Sieves-Structure, Chemistry and Use; John Wiley&Sons: New York, NY, USA, 1974. [Google Scholar]
- Kerr, G.T. Synthetic Zeolites. Sci. Am. 1989, 261, 100–105. [Google Scholar] [CrossRef]
- Smart, L.; Moore, E. Solid State Chemistry: An Introduction; Chapman and Hall: New York, NY, USA, 1992. [Google Scholar]
- Smith, J.V. Zeolite Chemistry and Catalysis; Rabo, J.A., Ed.; American Chemical Society: Washington, DC, USA, 1976; pp. 1–79. [Google Scholar]
- Van Bekkum, H.; Fanigen, E.M.; Jansen, J.C. (Eds.) Introduction to Zeolite Science and Practice; Elsevier: Amsterdam, The Netherlands, 1991; Volume 58. [Google Scholar]
- Chu, C.T.W.; Chang, C.D. Isomorphous substitution in zeolite frameworks. 1. Acidity of surface hydroxyls in [B]-, [Fe]-, [Ga]-, and [Al]-ZSM-5. J. Phys. Chem. 1985, 89, 1569–1571. [Google Scholar] [CrossRef]
- Zhu, Q.; Kondo, J.N.; Yokoi, T.; Setoyama, T.; Yamaguchi, M.; Takewaki, T.; Domen, K.; Tatsumi, T. The influence of acidities of boron- and aluminium-containing MFI zeolites on co-reaction of methanol and ethene. Phys. Chem. Chem. Phys. 2011, 13, 14598–14605. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, C.; Du, J.; Yue, Y.; Hua, W.; Zhang, C.; Shen, W.; Xu, H. The synthesis of endurable B–Al–ZSM-5 catalysts with tunable acidity for methanol to propylene reaction. Catal. Commun. 2012, 24, 44–47. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Wang, L.; Zhang, H.; Zhang, Y.; Xu, H.; Shen, W.; Tang, Y. Highly stable boron-modified hierarchical nanocrystalline ZSM-5 zeolite for the methanol to propylene reaction. Catal. Sci. Technol. 2014, 4, 2891–2895. [Google Scholar] [CrossRef]
- Yaripour, F.; Shariatinia, Z.; Sahebdelfar, S.; Irandoukht, A. Effect of boron incorporation on the structure, products selectivities and lifetime of H-ZSM-5 nanocatalyst designed for application in methanol-to-olefins (MTO) reaction. Microporous Mesoporous Mater. 2015, 203, 41–53. [Google Scholar] [CrossRef]
- Pang, W.; Li, G.; Li, W.; Zhang, W.; Lin, B. Hetero-atom zeolite(II)-Studies on the crystal structures of B-Si pentasil zeolite molecular sieves. Chem. J. Chin. Univ. 1984, 5, 375–380. [Google Scholar]
- Zhang, Y. The catalytic synthesis of glycerol acetals-investigation on the catalysts and synthesis of glycerol ketals. Master Thesis, Jiangnan University, Wuxi, China, June 2008. [Google Scholar]
- Teketel, S.; Erichsen, M.W.; Bleken, F.L.; Svelle, S.; Lillerud, P.K.; Olsbye, U. Shape selectivity in zeolite catalysis. The Methanol to Hydrocarbons (MTH) reaction. Catalysis 2014, 26, 179–217. [Google Scholar]
- Corma, A.; Corell, C.; Fornés, V.; Kolodziejski, W.; Pérez-Pariente, J. Infrared spectroscopy, thermoprogrammed desorption, and nuclear magnetic resonance study of the acidity, structure, and stability of zeolite MCM-22. Zeolites 1995, 15, 576–582. [Google Scholar] [CrossRef]
- Hwang, S.J.; Chen, C.Y.; Zones, S.I. Boron Sites in Borosilicate Zeolites at Various Stages of Hydration Studied by Solid State NMR Spectroscopy. J. Phys. Chem. B 2004, 108, 18535–18546. [Google Scholar] [CrossRef] [Green Version]
- Vasudevan, P.T.; Briggs, M. Production-current state of the art and challenges. J. Ind. Microbiol. Biotechnol. 2008, 35, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Canakci, M.; Sanli, H. Biodiesel production from various feedstocks and their effects on the fuel properties. J. Ind. Microbiol. Biotechnol. 2008, 35, 431–441. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Pina, C.D. From glycerol to valueadded products, Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef]
- Zhou, C.H.; Beltramini, J.N.; Fana, Y.X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef]
- Serafim, H.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Valorization of glycerol into fuel additives over zeolites as catalysts. Chem. Eng. J. 2011, 178, 291–296. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Valorisation of glycerol by condensation with acetone over silica-included heteropolyacids. Appl. Catal. B Environ. 2010, 98, 94–99. [Google Scholar] [CrossRef]
- Güemez, M.B.; Requies, J.; Agirr, I.E.; Arias, P.L.; Barrio, V.L.; Cambra, J.F. Acetalization reaction between glycerol and n-butyraldehyde using an acidic ion exchange resin kinetic modelling. Chem. Eng. J. 2013, 228, 300–307. [Google Scholar] [CrossRef]
- Crotti, C.; Farnetti, E.; Guidolin, N. Alternative intermediates for glycerol valorization: Iridium-catalyzed formation of acetalsand ketals. Green Chem. 2010, 12, 2225–2231. [Google Scholar] [CrossRef]
- Honga, X.; Giverona, O.M.; Kolaha, A.K.; Orjuelab, A.; Peerebooma, L.; Liraa, C.T.; Millera, D.J. Reaction kinetics of glycerol acetal formation via transacetalization with 1,1-diethoxyethane. Chem. Eng. J. 2013, 222, 374–381. [Google Scholar] [CrossRef]
- Talebian-kiakalaieh, A.; AishahN, S.; Amin, N.; Najaafi, S.; Tarighi, S. A Review on the Catalytic Acetalization of Bio-renewable Glycerol to Fuel Additives. Front. Chem. 2018, 6, 573–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayoub, M.; Abdullah, A.Z. Diglycerol synthesis via solvent-free selective glycerol etherification process over lithium-modified clay catalyst. Chem. Eng. J. 2013, 225, 784–789. [Google Scholar] [CrossRef]
- Silva, C.; Gonalves, V.L.C.; Mota, C. Water-tolerant zeolite catalyst for the acetalisation of glycerol. Green Chem. 2009, 11, 38–41. [Google Scholar] [CrossRef]
- Venkatesha, N.J.; Bhat, Y.S.; Jai Prakash, B.S. Dealuminated BEA zeolite for selective synthesis of five-membered cyclic acetal from glycerol under ambient conditions. RSC Adv. 2016, 6, 18824–18833. [Google Scholar] [CrossRef]
- Sonar, S.K.; Shinde, A.S.; Asok, A.; Niphadkar, P.S.; Mayadevi, S.; Joshi, P.N.; Bokade, V.V. Solvent free acetalization of glycerol with formaldehyde over hierarchical zeolite of BEA topology. Environ. Prog. Sustain. Energy 2018, 37, 797–807. [Google Scholar] [CrossRef]
- Díaz-Álvarez, A.E.; Francos, J.; Lastra-Barreira, B. Glycerol and derived solvents: New sustainable reaction media for organic synthesis. Chem. Commun. 2011, 47, 6208–6227. [Google Scholar] [CrossRef]
- Vicente, G.; Melero, J.A.; Morales, G.; Martín, E. Acetalisation of bio-glycerol with acetone to produce solketal over sulfonic mesostructured silicas. Green Chem. 2010, 12, 899–907. [Google Scholar] [CrossRef]
- Laskar, I.B.; Rajkumari, K.; Gupta, R.; Rokhum, L. Acid-functionalized mesoporous polymer-catalyzed acetalization of glycerol to solketal, a potential fuel additive under solvent-free conditions. Energy Fuels. 2018, 32, 12567–12576. [Google Scholar] [CrossRef]
- Rodrigues, R.; Gonçalves, M.; Mandelli, D.; Pescarmona, P.P.; Carvalho, W.A. Solvent-free conversion of glycerol to solketal catalysed by activated carbons functionalised with acid groups. Catal. Sci. Technol. 2014, 4, 2293–2301. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Velty, A. Synthesis of hyacinth, vanilla, and blossom orange fragrances: The benefit of using zeolites and delaminated zeolites as catalysts. Appl. Catal. A General. 2004, 263, 155–161. [Google Scholar] [CrossRef]
- Poly, S.S.; Jamil, M.; Touchy, A.S.; Yasumura, S.; Shimizu, K.I. Acetalization of glycerol with ketones and aldehydes catalyzed by high silica Hβ zeolite. Mol. Catal. 2019, 479, 110608–110613. [Google Scholar] [CrossRef]



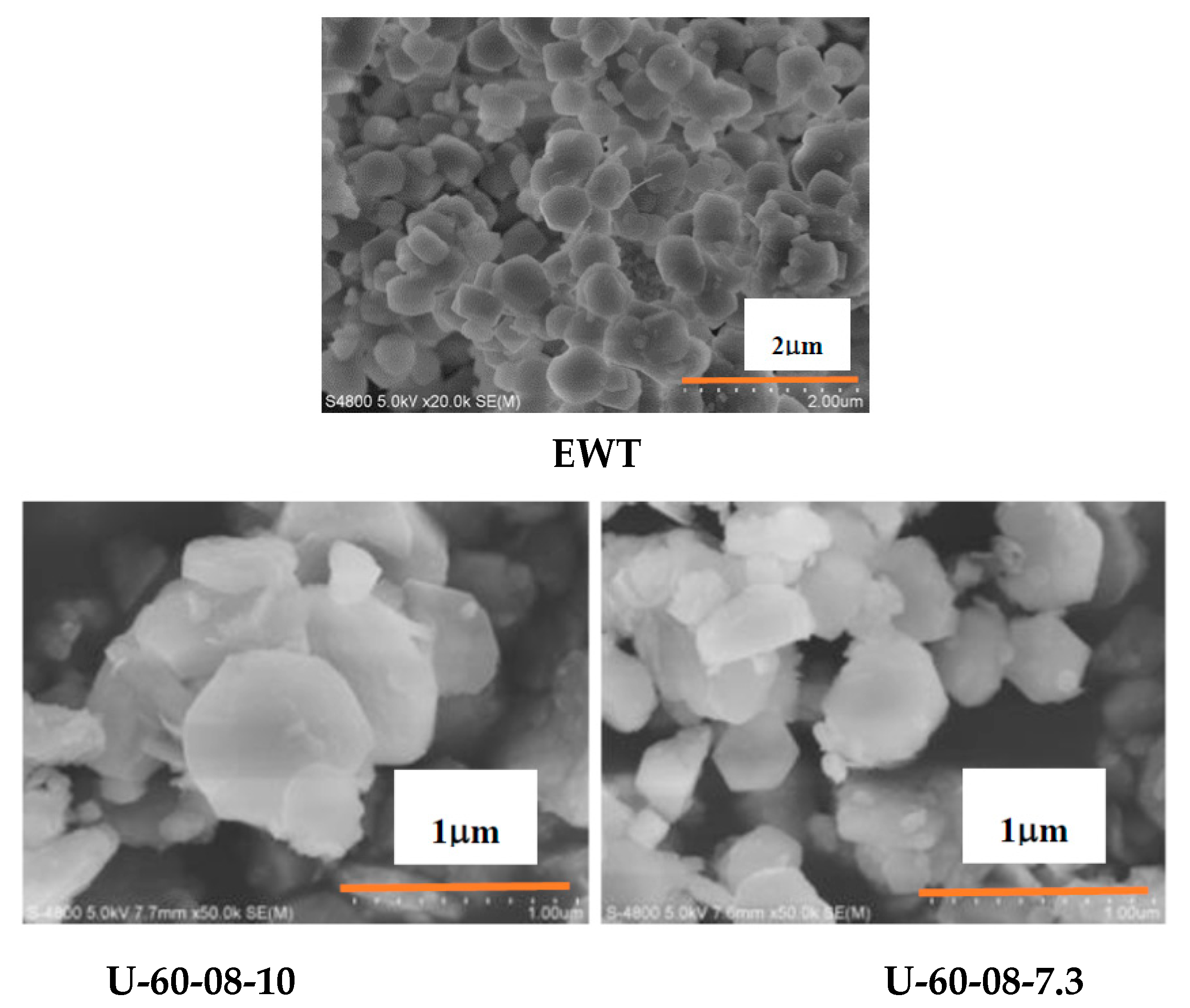
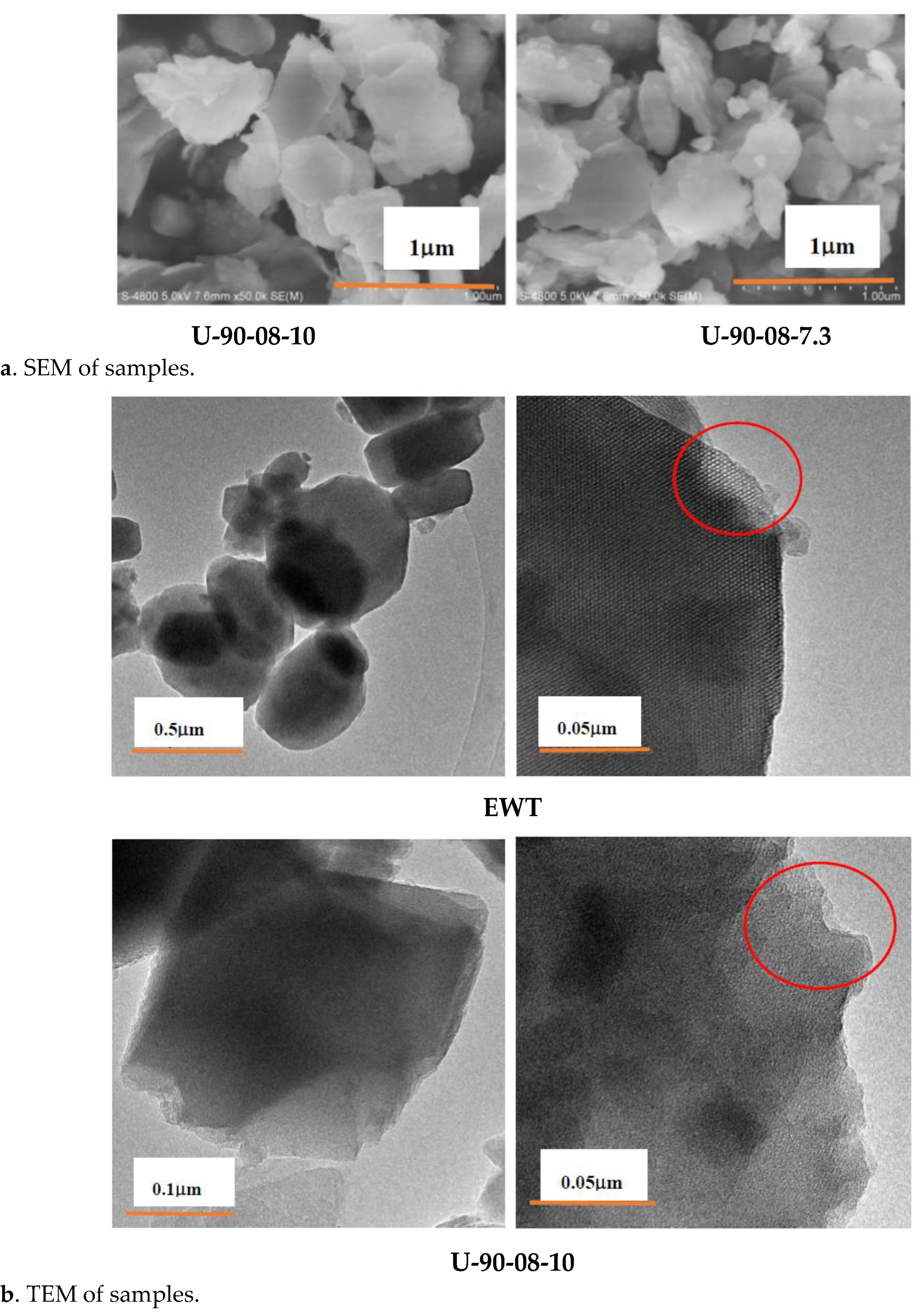
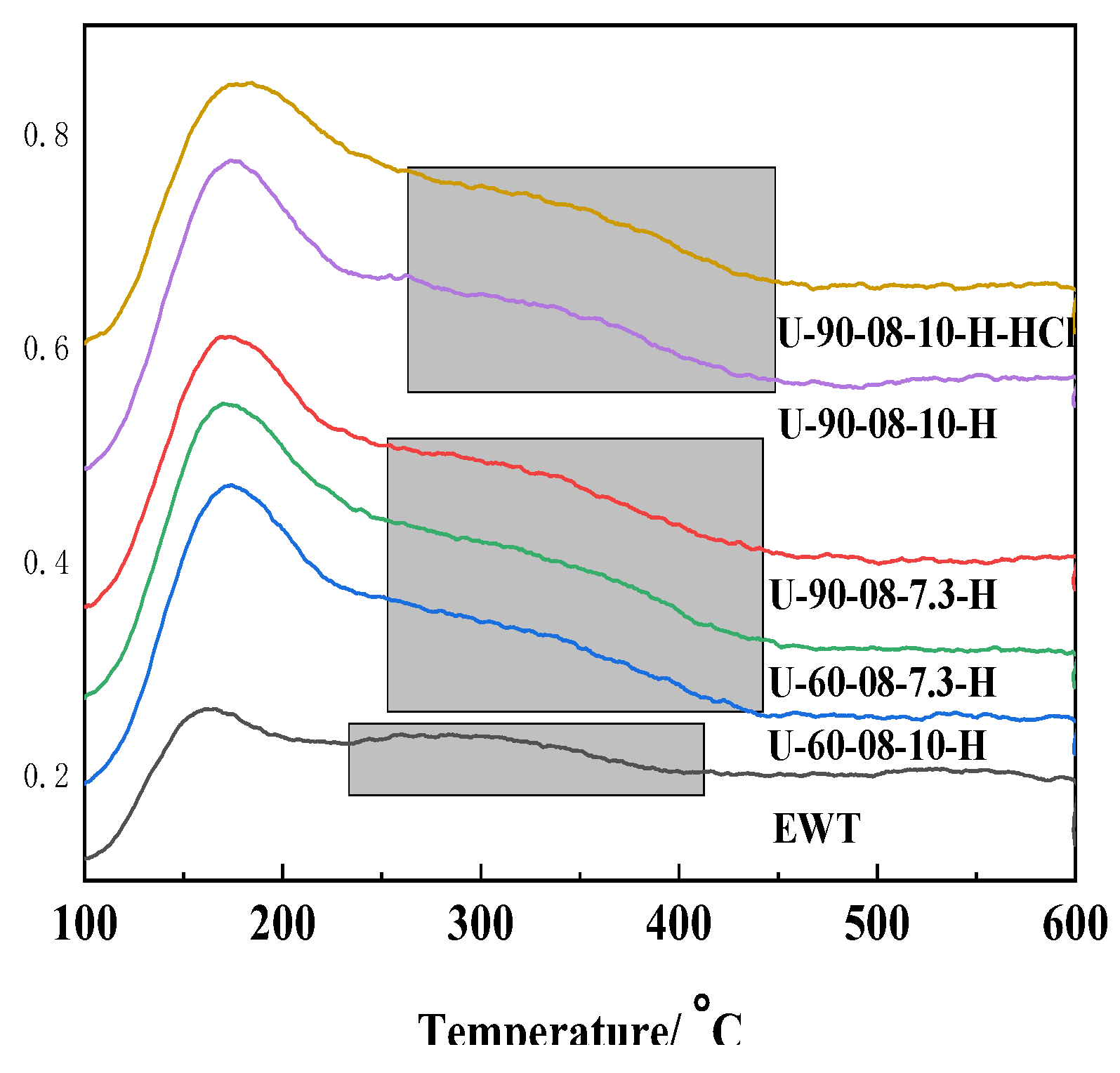
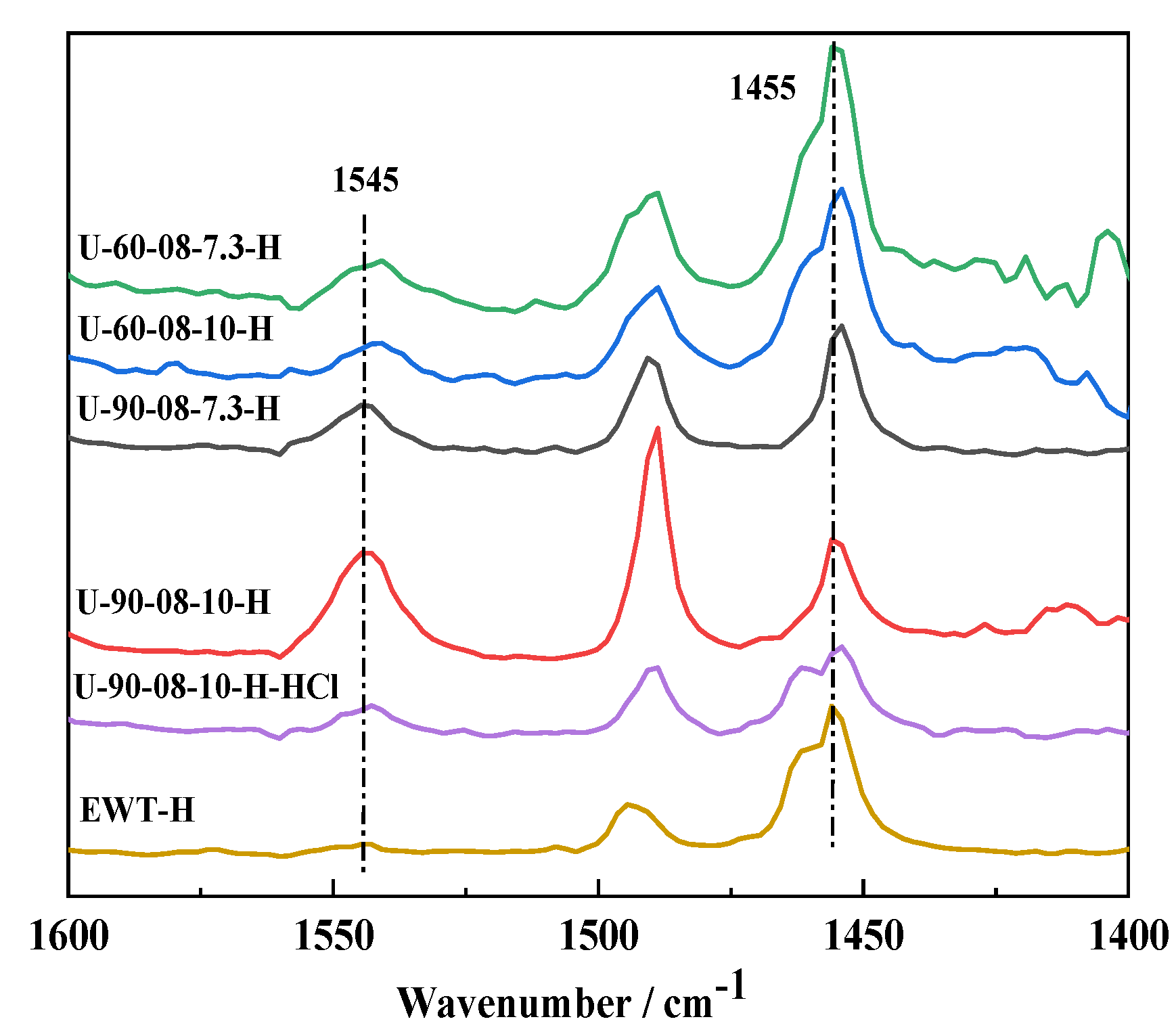


| Samples | Ratio of Feed | Product | Relative Crystallinity a/% | |||||
|---|---|---|---|---|---|---|---|---|
| n(SiO2)/ n(B2O3) | n(SiO2)/ n(Al2O3) | n(NaOH)/ n(SiO2) | n(SDA)/ n(SiO2) | n(H2O)/ n(SiO2) | ||||
| I | U-Al30 | 30 | 30 | 0.10 | 0.15 | 10 | LEV | / |
| U-Al60 | 60 | 30 | 0.10 | 0.15 | 10 | LEV | / | |
| II | U-B15 | 15 | 60 | 0.10 | 0.15 | 10 | EWT + MCM-22 | / |
| U-B30 | 30 | 60 | 0.10 | 0.15 | 10 | EWT + MCM-22 | / | |
| U-B60 | 60 | 60 | 0.10 | 0.15 | 10 | EWT + MCM-22 | / | |
| U-B90 | 90 | 60 | 0.10 | 0.15 | 10 | EWT + MCM-22 | / | |
| U-B∞ | ∞ | 60 | 0.10 | 0.15 | 10 | EWT + MCM-22 | / | |
| III | U-30-08-10 | 30 | 60 | 0.08 | 0.15 | 10.0 | EWT + MCM-22 | / |
| U-30-08-7.3 | 30 | 60 | 0.08 | 0.15 | 7.3 | EWT + MCM-22 | / | |
| U-30-10-7.3 | 30 | 60 | 0.10 | 0.15 | 7.3 | EWT + MCM-22 + MEL | / | |
| U-30-12-10 | 30 | 60 | 0.12 | 0.15 | 10.0 | EWT + MCM-22 + MEL | / | |
| U-30-12-7.3 | 30 | 60 | 0.12 | 0.15 | 7.3 | EWT + MCM-22 + MEL | / | |
| U-60-10-7.3 | 60 | 60 | 0.10 | 0.15 | 7.3 | EWT + MCM-22 | / | |
| U-60-12-10 | 60 | 60 | 0.12 | 0.15 | 10.0 | EWT + MCM-22 | / | |
| U-60-12-7.3 | 60 | 60 | 0.12 | 0.15 | 7.3 | EWT + MCM-22 | / | |
| U-60-08-10 | 60 | 60 | 0.08 | 0.15 | 10 | EWT | 80.6 | |
| U-60-08-7.3 | 60 | 60 | 0.08 | 0.15 | 7.3 | EWT | 71.8 | |
| IV | U-90-08-10 | 90 | 60 | 0.08 | 0.15 | 10 | EWT | 77.5 |
| U-90-08-7.3 | 90 | 60 | 0.08 | 0.15 | 7.3 | EWT | 71.3 | |
| EWT b | ∞ | 90 | 0.10 | 0.15 | 10 | EWT | 100 | |
| Samples | Composition of Zeolites a | Surface Area and Pore Volume b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| w(Na2O)/% | n(SiO2)/ n(Al2O3) | n(SiO2)/ n(B2O3) | n(Al)/ n(B) | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | |||||
| Total | Micro | Meso | Total | Micro | Meso | |||||
| U-60-08-10 | 0.65 | 42.4 | 150.7 | 3.6 | 536 | 501 | 35 | 0.31 | 0.23 | 0.08 |
| U-60-08-7.3 | 0.65 | 43.7 | 132.7 | 3.0 | 546 | 490 | 56 | 0.37 | 0.23 | 0.14 |
| U-90-08-10 | 0.95 | 41.4 | 180.0 | 4.4 | 522 | 481 | 42 | 0.32 | 0.22 | 0.10 |
| U-90-08-7.3 | 0.90 | 45.4 | 184.7 | 4.1 | 516 | 463 | 53 | 0.35 | 0.21 | 0.14 |
| U-90-08-10-HCl | / | 65.5 | 492.3 | 7.5 | 519 | 470 | 49 | 0.35 | 0.22 | 0.13 |
| EWT | 0.62 | 92.0 | / | / | 578 | 556 | 22 | 0.26 | 0.23 | 0.03 |
| Sample | Acidity by NH3-TPD a (μmol g−1) | Acidity by Py-IR b-200 °C (μmol g−1) | Acidity by Py-IR b-350 °C (μmol g−1) | The Fraction of Al (IV) Species c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Weak (100~290 °C) | Strong (290~600 °C) | Bronsted/(μmol·g−1) | Lewis/(μmol·g−1) | B/L | Bronsted/(μmol·g−1) | Lewis/(μmol·g−1) | B/L | ||
| U-60-08-10 | 762.1 | 421.0 | 341.1 | 119.5 | 71.7 | 1.7 | 44.3 | 144.9 | 0.3 | 6.07 |
| U-60-08-7.3 | 703.3 | 420.1 | 283.2 | 107.1 | 115.3 | 0.9 | 32.0 | 111.9 | 0.3 | 5.22 |
| U-90-08-10 | 847.3 | 437.5 | 409.8 | 100.7 | 134.9 | 0.7 | 88.7 | 159.0 | 0.6 | 6.73 |
| U-90-08-7.3 | 711.6 | 357.2 | 354.4 | 99.4 | 180.5 | 0.6 | 54.0 | 174.5 | 0.3 | 5.04 |
| U-90-08-10-HCl | 755.8 | 417.0 | 338.8 | 87.4 | 41.0 | 2.1 | 43.4 | 59.5 | 0.7 | 7.02 |
| EWT-H | 626.3 | 264.7 | 361.6 | 31.6 | 50.8 | 0.6 | 10.7 | 68.0 | 0.2 | 4.88 |
| Samples | HZSM-5 | HBeta | EWT | U-60-08-10 | U-60-08-7.3 | U-90-08-10 | U-90-08-7.3 | U-90-08-10-HCl |
|---|---|---|---|---|---|---|---|---|
| n(SiO2)/n(Al2O3) | 49 | 20 | 92 | 42 | 44 | 41 | 45 | 65.5 |
| acidity amount a/mmol NH3·g−1 | 0.93 | 1.38 | 0.66 | 0.76 | 0.70 | 0.85 | 0.66 | 0.76 |
| acidity amount with medium and strong strength a/mmol NH3·g−1 | 0.50 | 0.46 | 0.36 | 0.34 | 0.28 | 0.41 | 0.27 | 0.34 |
| Brönsted acidity (350 °C) b/μmol g−1 | 87 | 62 | 11 | 44 | 32 | 89 | 54 | 43 |
| B/L (350 °C) | 2.8 | 2.5 | 0.2 | 0.3 | 0.3 | 0.6 | 0.3 | 0.7 |
| cyclohexanone conversion c/% | 66.86 | 86.11 | 66.35 | 81.80 | 81.29 | 86.21 | 84.54 | 88.17 |
| cyclohexanone conversion *105/mol acid site with medium and strong strength | 6.69 | 8.39 | 9.22 | 12.04 | 14.84 | 9.97 | 15.24 | 12.82 |
| Selectivity of PA/% | 96.56 | 98.12 | 94.91 | 97.51 | 99.85 | 97.63 | 97.78 | 98.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Bai, Y.; Chen, P.; Chen, Q.; Wang, Y.; Shu, X. Synthesis of [B,Al]-EWT-Type Zeolite and Its Catalytic Properties. Molecules 2022, 27, 5625. https://doi.org/10.3390/molecules27175625
Wang Y, Bai Y, Chen P, Chen Q, Wang Y, Shu X. Synthesis of [B,Al]-EWT-Type Zeolite and Its Catalytic Properties. Molecules. 2022; 27(17):5625. https://doi.org/10.3390/molecules27175625
Chicago/Turabian StyleWang, Youju, Yongyue Bai, Pohua Chen, Qiang Chen, Yongrui Wang, and Xingtian Shu. 2022. "Synthesis of [B,Al]-EWT-Type Zeolite and Its Catalytic Properties" Molecules 27, no. 17: 5625. https://doi.org/10.3390/molecules27175625
APA StyleWang, Y., Bai, Y., Chen, P., Chen, Q., Wang, Y., & Shu, X. (2022). Synthesis of [B,Al]-EWT-Type Zeolite and Its Catalytic Properties. Molecules, 27(17), 5625. https://doi.org/10.3390/molecules27175625





