Comparison of LC-MS3 and LC-MRM Method for Quantifying Voriconazole and Its Application in Therapeutic Drug Monitoring of Human Plasma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of HPLC Conditions
2.2. Optimization of MS Conditions
2.3. Sample Preparation
2.4. Assay Validation
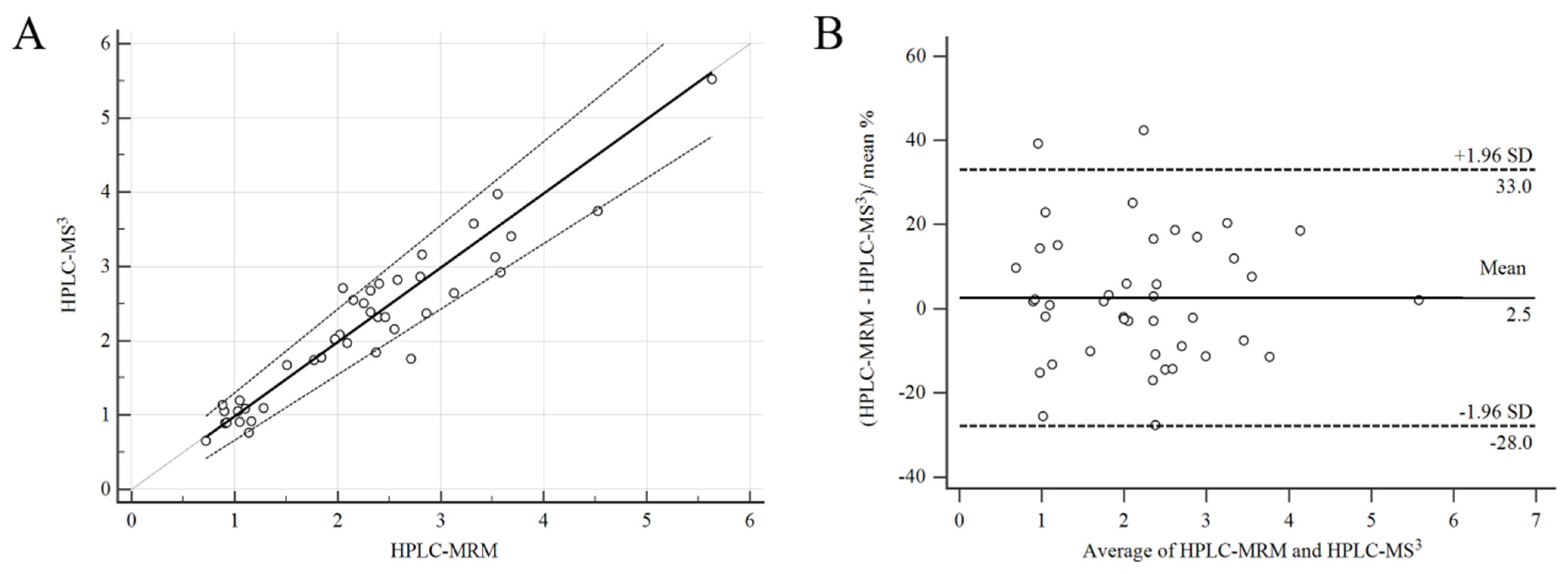
2.5. Comparison of HPLC-MS3 and HPLC-MRM Methods
2.6. The Novelty and Significance of the LC-MS3 Method
2.7. Method Application
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. LC-MS3 Conditions
3.3. Preparation of Calibration Standards and Quality Control Samples
3.4. Assay Validation
3.5. Clinical Application
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Moriyama, B.; Obeng, A.O.; Barbarino, J.; Penzak, S.R.; Henning, S.A.; Scott, S.A.; Agúndez, J.A.G.; Wingard, J.R.; McLeod, H.L.; Klein, T.E.; et al. Clinical pharmacogenetics implementation consortium (CPIC) guidelines for CYP2C19 and voriconazole therapy. Clin. Pharmacol. Ther. 2017, 102, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.C.; Lin, X.B.; Zhang, B.K.; Xiao, Y.W.; Xu, P.; Wang, F.; Xiang, D.X.; Xie, X.B.; Peng, F.H.; Yan, M. Predictors of adverse events and determinants of the voriconazole trough concentration in kidney transplantation recipients. Clin. Transl. Sci. 2020, 14, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Xiang, R.; Liu, F.; Li, Y.; Chen, H.; Yao, P.; Sun, F.; Xia, P. Therapeutic drug monitoring and safety of voriconazole in elderly patients. Int. Immunopharmacol. 2020, 78, 160678. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, X.; Ke, X.; Du, G.; Yang, K.; Zhai, S. Individualized medication of voriconazole: A practice guideline of the division of therapeutic drug monitoring, Chinese pharmacological society. Ther. Drug. Monit. 2018, 40, 12. [Google Scholar] [CrossRef] [PubMed]
- Allegra, S.; Fatiguso, G.; De Francia, S.; Favata, F.; Pirro, E.; Carcieri, C.; De Nicolò, A.; Cusato, J.; Di Perri, G.; D’Avolio, A. Therapeutic drug monitoring of voriconazole for treatment and prophylaxis of invasive fungal infection in children. Br. J. Clin. Pharmacol. 2018, 84, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Han, M.; Han, E.Y.; Lee, K.; Song, J.; Song, S.H. Performance evaluation of enzyme immunoassay for voriconazole therapeutic drug monitoring with automated clinical chemistry analyzers. Pract. Lab. Med. 2017, 8, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Chawla, P.K.; Dherai, A.J.; Ashavaid, T.F. Plasma voriconazole estimation by HPLC. Indian J. Clin. Biochem. 2016, 31, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Leung-Pineda, V. Determination of voriconazole concentrations in serum by GC-MS. J. Clin. Lab. Anal. 2016, 30, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.; Sujishi, K.K.; French, D. Development and validation of a liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay to quantify serum voriconazole. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 986–987, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Peña-Lorenzo, D.; Rebollo, N.; Sánchez-Hernández, J.G.; Zarzuelo-Castañeda, A. Comparison of ultra-performance liquid chromatography and ARK immunoassay for therapeutic drug monitoring of voriconazole. Ann. Clin. Biochem. 2021, 58, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, W.; Li, M.; Zhang, Z.; Liu, S.; Chen, Z. Correlation between enzyme multiplied immunoassay technique and high-performance liquid chromatography in the quantification of voriconazole in a paediatric population. Scand. J. Clin. Lab. Investig. 2021, 81, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Ji, Z.; Cao, H.; Li, Y.; Huang, J. Comparison of LC–MS3 and LC–MRM strategy for quantification of methotrexate in human plasma and its application in therapeutic drug monitoring. J. Pharm. Biomed. Anal. 2021, 205, 114345. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Ji, Z.; Cao, H.; Huang, J.; Zeng, L.; Yin, L. LC–MS3 Strategy for Quantification of Carbamazepine in Human Plasma and Its Application in Therapeutic Drug Monitoring. Molecules 2022, 27, 1224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, R.; Liu, S.; Yin, L.; Xu, T.; Fawcett, J.P.; Gu, J. Liquid chromatography tandem mass spectrometry with triple stage fragmentation for highly selective analysis and pharmacokinetics of alarelin in rat plasma. J. Sep. Sci. 2019, 42, 3033–3040. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Zhang, Z.; Fawcett, J.P.; Sun, D.; Gu, J. Micro-solid phase extraction and LC-MS(3) for the determination of triptorelin in rat plasma and application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2019, 166, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, S.; Vermeersch, P.; Eldere, J.V.; Desmet, K. Fast and simple LC–MS/MS method for quantifying plasma voriconazole. Clin. Chim. Acta 2012, 413, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Kaza, M.; Karaźniewicz-Łada, M.; Kosicka, K.; Siemiątkowska, A.; Rudzki, P.J. Bioanalytical method validation: New FDA guidance vs. EMA guideline. Better or worse? J. Pharm. Biomed. Anal. 2019, 165, 381–385. [Google Scholar] [CrossRef] [PubMed]
- MEA/CHMP/EWP/192217/2009. Guideline on Bioanalytical Method Validation. The European Medicines Agency. 21 July 2011. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 15 July 2022).






| Parameters | MS3 | |
|---|---|---|
| Voriconazole | IS | |
| MS3 transitions | 350.3→224.3→197.3 | 240.3→196.3→181.3 |
| Declustering potential (V) | 80 | 80 |
| Entrance potential (V) | 10 | 10 |
| Collision energy (eV) | 23 | 20 |
| Excitation energy (AF2) (V) | 0.1 | 0.1 |
| Scan rate (Da/s) | 10,000 | 10,000 |
| LIT fill time (ms) | 80 | 80 |
| Excitation time (ms) | 25 | 25 |
| Turboheater temperature (°) | 450 | 450 |
| Ionspray voltage (V) | 5500 | 5500 |
| Curtain gas (N2, psi) | 30 | 30 |
| Nebulizer gas (N2, psi) | 50 | 50 |
| Heater gas (N2, psi) | 50 | 50 |
| Drug | QC (μg/mL) | Intra-Day Precision (RSD%) | Inter-Day Precision (RSD%) | Accuracy (RE%) |
|---|---|---|---|---|
| 0.5 | 8.72 | 0.52 | 2.63 | |
| Voriconazole | 2.5 | 6.64 | 2.37 | 4.96 |
| 10 | 3.66 | 3.68 | 5.45 |
| Drug | QC | Matrix Effects (%) | Recovery (%) |
|---|---|---|---|
| Voriconazole | 0.5 2.5 10 | 99.6 ± 4.5 100.4 ± 5.6 107.2 ± 2.8 | 107.2 ± 7.7 103.6 ± 5.7 96.1 ± 6.0 |
| Low QC | Middle QC | High QC | |
|---|---|---|---|
| Long-term (−80 °C) | 98.5 ± 12.1 | 101.7 ± 5.8 | 98.2 ± 5.1 |
| Three Freeze-thaw | 100.7 ± 4.2 | 108.0 ± 1.7 | 95.1 ± 5.6 |
| Under autosampler conditions (8 °C) | 95.3 ± 6.7 | 101.9 ± 9.0 | 101.9 ± 3.2 |
| Short-terms (4 h, RT) | 97.2 ± 5.3 | 102.1 ± 4.0 | 102.9 ± 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, W.; Yin, L.; Zhang, G.; Zhai, T.; Huang, J. Comparison of LC-MS3 and LC-MRM Method for Quantifying Voriconazole and Its Application in Therapeutic Drug Monitoring of Human Plasma. Molecules 2022, 27, 5609. https://doi.org/10.3390/molecules27175609
Ren W, Yin L, Zhang G, Zhai T, Huang J. Comparison of LC-MS3 and LC-MRM Method for Quantifying Voriconazole and Its Application in Therapeutic Drug Monitoring of Human Plasma. Molecules. 2022; 27(17):5609. https://doi.org/10.3390/molecules27175609
Chicago/Turabian StyleRen, Wenbo, Lei Yin, Gaixia Zhang, Taiyu Zhai, and Jing Huang. 2022. "Comparison of LC-MS3 and LC-MRM Method for Quantifying Voriconazole and Its Application in Therapeutic Drug Monitoring of Human Plasma" Molecules 27, no. 17: 5609. https://doi.org/10.3390/molecules27175609
APA StyleRen, W., Yin, L., Zhang, G., Zhai, T., & Huang, J. (2022). Comparison of LC-MS3 and LC-MRM Method for Quantifying Voriconazole and Its Application in Therapeutic Drug Monitoring of Human Plasma. Molecules, 27(17), 5609. https://doi.org/10.3390/molecules27175609







