Facile Preparation and Analytical Utility of ZnO/Date Palm Fiber Nanocomposites in Lead Removal from Environmental Water Samples
Abstract
:1. Introduction
2. Results and Discussion
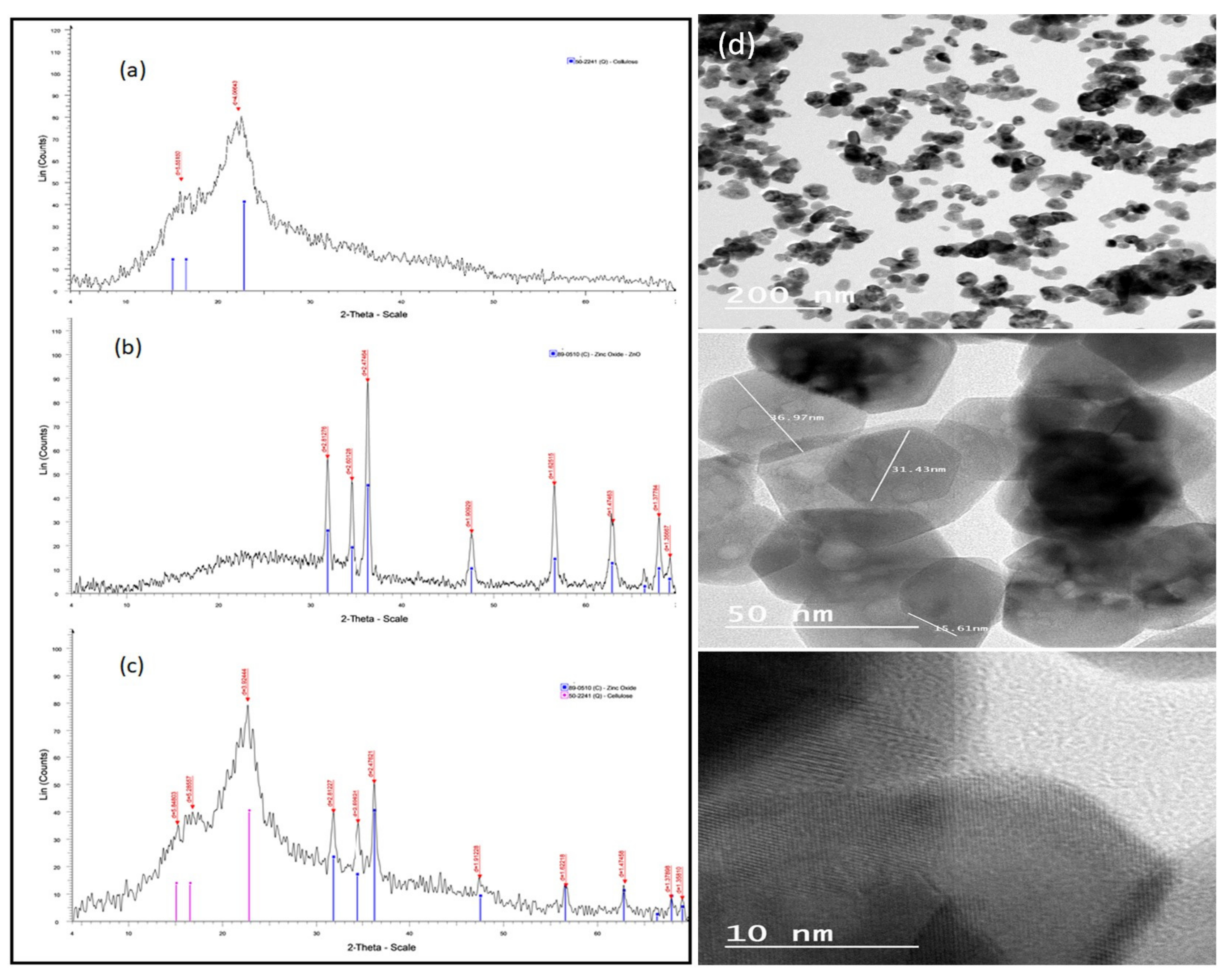
2.1. Characterization of ZnO NPs
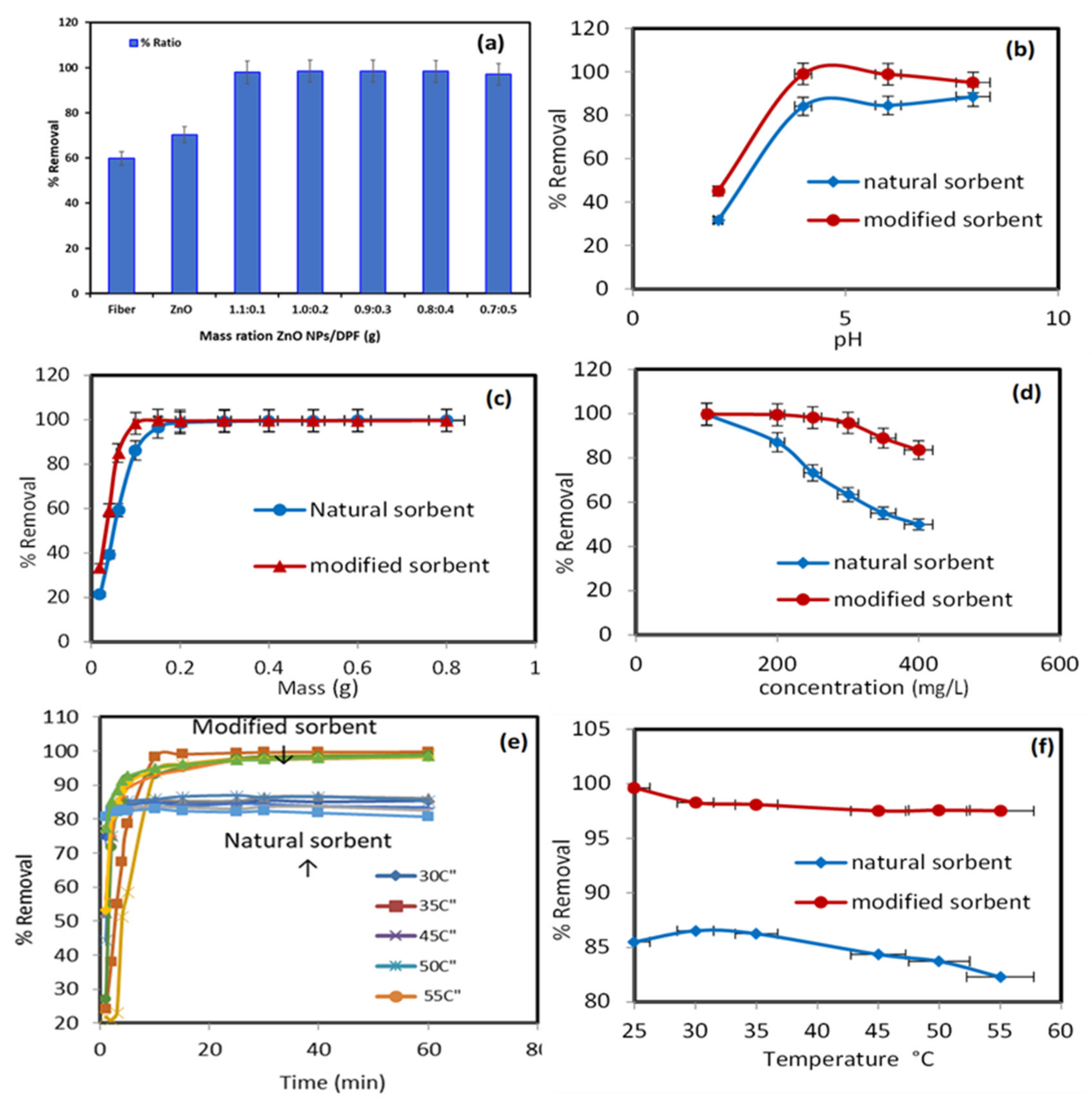
2.2. Sorption Study
2.2.1. Effect of Different Sorbent Ratio
2.2.2. Influence of pH of the Extraction Medium
2.2.3. Impact of Dosage of the Modified ZnO NP/DPF Sorbent
2.2.4. Effect of Lead (II) Concentration
2.2.5. Effect of Contact Time
2.2.6. Effect of Solution Temperature
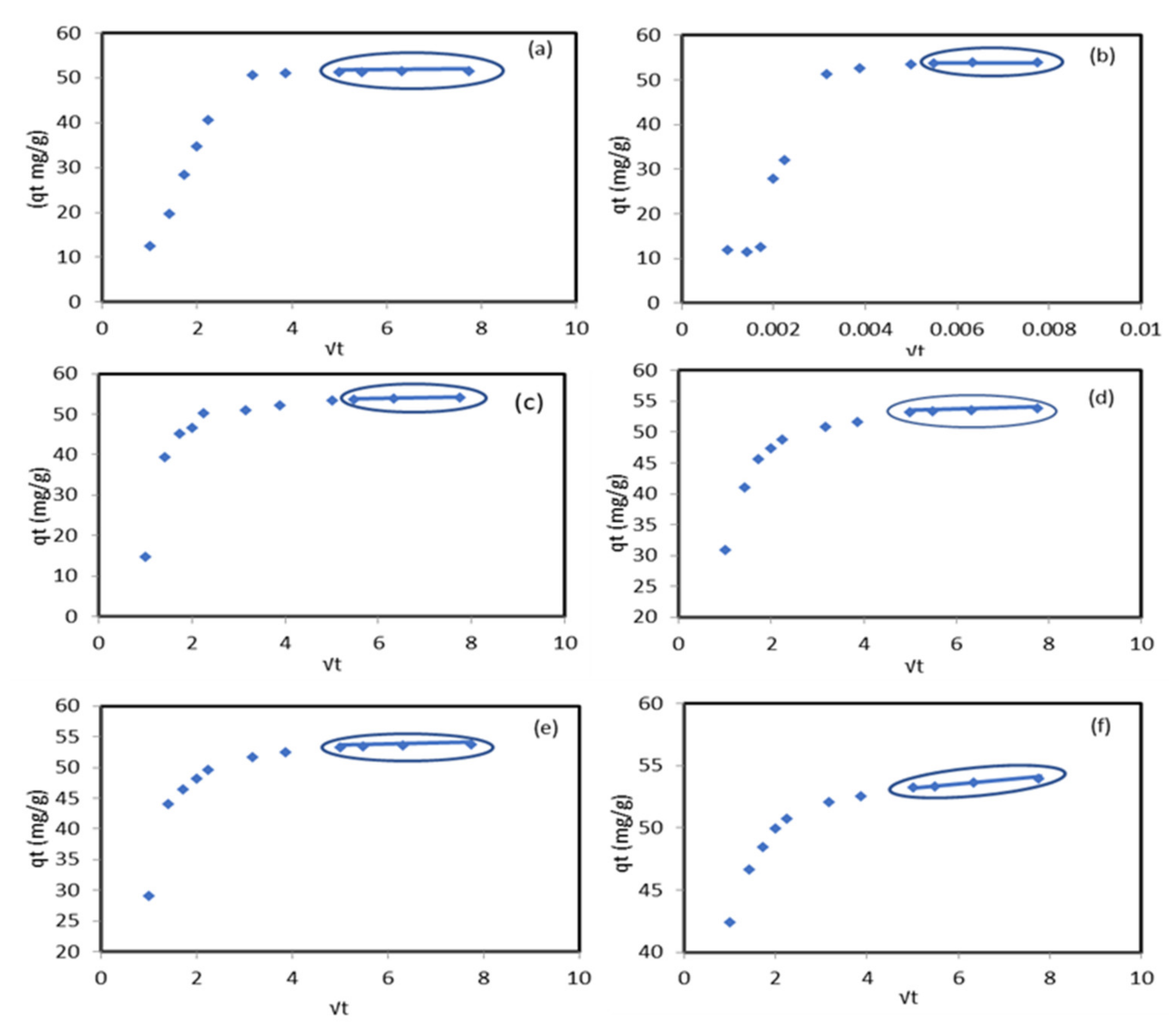
2.3. Kinetic Study of Pb2+ Retention
2.4. Sorption Isotherm Study
2.5. Thermodynamic Study
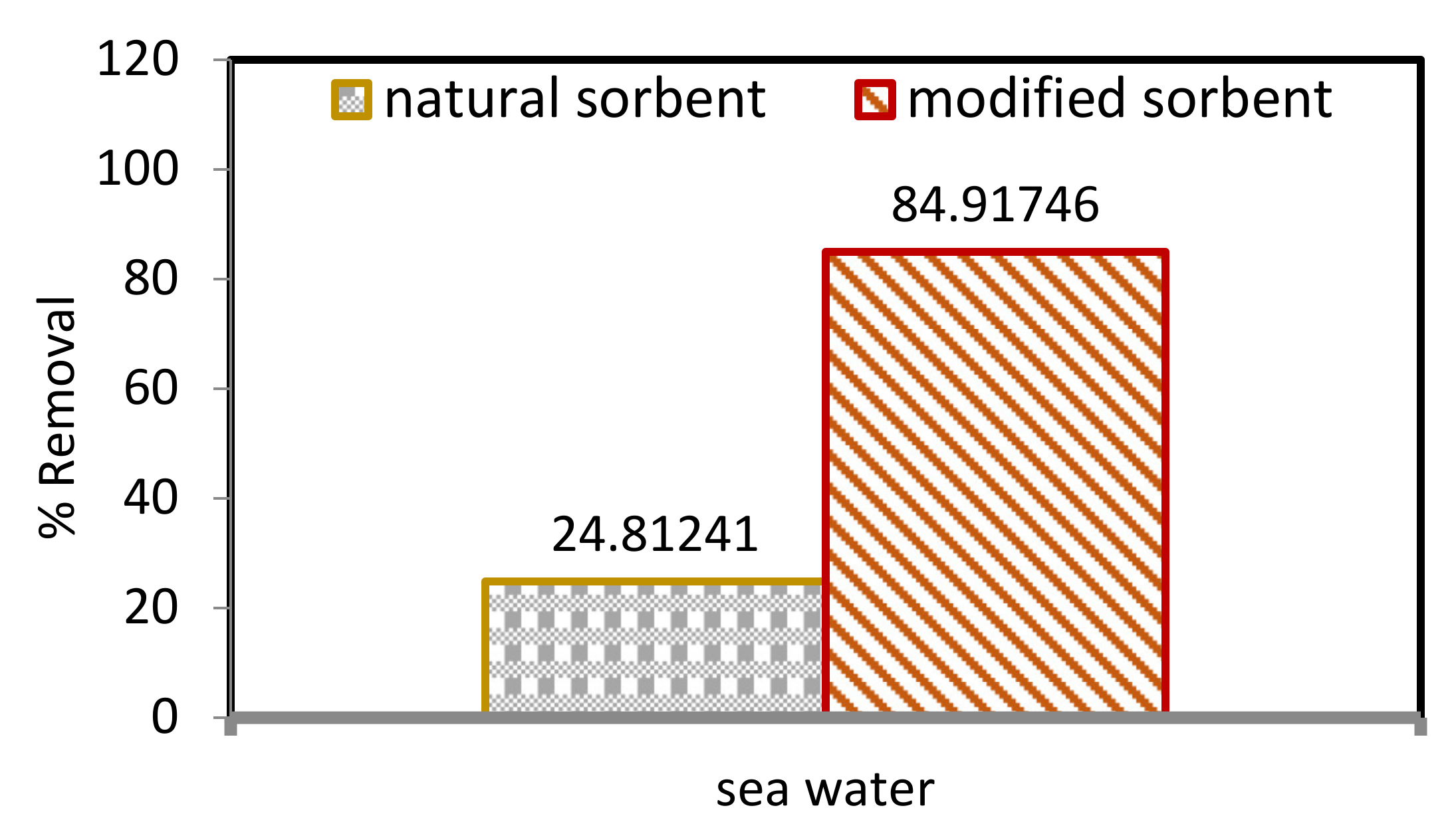
2.6. Environmental Application
3. Materials and Methods
3.1. Synthesized ZnO NPs
3.2. Modified Palm Fiber Preparation
3.3. Characterization of the Solid-Phase Extractor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rezaei, M.; Pourang, N.; Moradi, A.M. Removal of lead from aqueous solutions using three biosorbents of aquatic origin with the emphasis on the affective factors. Sci. Rep. 2022, 12, 751. [Google Scholar] [CrossRef]
- Alhogbi, B.G.; Abdel Salam, M.; Ibrahim, O. Environmental Remediation of Toxic Lead Ions from Aqueous Solution Using Palm Tree Waste Fibers Biosorbent. Desalination Water Treat. 2019, 145, 179–188. [Google Scholar] [CrossRef]
- Alhogbi, B.G. Potential of Coffee Husk Biomass Waste for the Adsorption of Pb (II) Ion from Aqueous Solutions. Sustain. Chem. Pharm. 2017, 6, 21–25. [Google Scholar] [CrossRef]
- Staroń, P.; Chwastowski, J. Raphia-Microorganism Composite Biosorbent for Lead Ion Removal from Aqueous Solutions. Materials 2021, 14, 7482. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Hanafiah, M.A.K.M. Removal of Heavy Metal Ions from Wastewater by Chemically Modified Plant Wastes as Adsorbents: A Review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef]
- Gautam, R.K.; Ackmez, M.; Giusy, L.; Chattopadhyaya, M.C. Biomass-Derived Biosorbents for Metal Ions Sequestration: Adsorbent Modification and Activation Methods and Adsorbent Regeneration. J. Environ. Chem. Eng. 2014, 2, 239–259. [Google Scholar] [CrossRef]
- Saka, C.; Şahin, Ö.; Küçük, M.M. Applications on Agricultural and Forest Waste Adsorbents for the Removal of Lead (II) from Contaminated Waters. Int. J. Environ. Sci. Technol. 2012, 9, 379–394. [Google Scholar] [CrossRef]
- Faiad, A.; Alsmari, M.; Ahmed, M.M.Z.; Bouazizi, M.L.; Alzahrani, B.; Alrobei, H. Date Palm Tree Waste Recycling: Treatment and Processing for Potential Engineering Applications. Sustainability. 2022, 14, 1134. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of Nanotechnology in Water and Wastewater Treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef]
- Wally, S.M.; El-Wakil, A.M.; El-Maaty, W.M.A.; Awad, F.S. Efficient removal of Pb(II) and Hg(II) ions from aqueous solution by amine and thiol modified activated carbon. J. Saudi Chem. Soc. 2021, 25, 101296. [Google Scholar] [CrossRef]
- Khajeh, M.; Laurent, S.; Dastafkan, K. Nanoadsorbents: Classification, Preparation, and Applications (with Emphasis on Aqueous Media). Chem. Rev. 2013, 113, 7728–7768. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A. Activated carbon/metal-organic framework composite as a bio-based novel green adsorbent: Preparation and mathematical pollutant removal modeling. J. Mol. Liq. 2019, 277, 310–322. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Oveisi, M.; Taghizadeh, A.; Taghizadeh, M. Synthesis of pearl necklace-like ZIF-8@chitosan/PVA nanofiber with synergistic effect for recycling aqueous dye removal. Carbohydr. Polym. 2020, 227, 115364. [Google Scholar] [CrossRef]
- Kunduru, K.R.; Nazarkovsky, M.; Farah, S.; Pawar, R.P.; Basu, A.; Domb, A.J. Nanotechnology for Water Purification: Applications of Nanotechnology Methods in Wastewater Treatment. Water Purif. 2017, 33–74. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Lu, F.; Didier, A. Nanomaterials for Removal of Toxic Elements from Water. Coord. Chem. Rev. 2018, 356, 147–164. [Google Scholar] [CrossRef]
- Dubey, S.; Banerjee, S.; Upadhyay, S.N.; Sharma, Y.C. Application of Common Nano-Materials for Removal of Selected Metallic Species from Water and Wastewaters: A Critical Review. J. Mol. Liq. 2017, 240, 656–677. [Google Scholar] [CrossRef]
- Gehrke, I.; Geiser, A.; Somborn-Schulz, A. Innovations in Nanotechnology for Water Treatment. Nanotechnol. Sci. Appl. 2015, 8, 1–17. [Google Scholar] [CrossRef]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and Applications. Sci. World J. 2014, 2014, 925494. [Google Scholar] [CrossRef]
- Kim, K.; Choi, M.; Lee, J.K.; Jeong, J.; Kim, Y.; Kim, M.; Paek, S.; Oh, J. Physicochemical Properties of Surface Charge-Modified ZnO Nanoparticles with Different Particle Sizes. Int. J. Nanomed. 2014, 214, 41–56. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Tyagi, I.; Sadegh, H.; Shahryari-Ghoshekand, R.; Makhlouf, A.S.H.; Maazinejad, B. Nanoparticles as Adsorbent; A Positive Approach for Removal of Noxious Metal Ions: A Review. Sci. Technol. Dev. 2015, 34, 195–214. [Google Scholar] [CrossRef]
- Hao, L.C.; Sapuan, S.M.; Hassan, M.R.; Sheltami, R.M. Natural fiber reinforced vinyl polymer composites. Woodhead Publ. Ser. Compos. Sci. Eng. 2018, 27–70. [Google Scholar] [CrossRef]
- Alharthi, M.N.; Ismail, I.; Bellucci, S.; Salam, M.A. Green synthesis of zinc oxide nanoparticles by Ziziphus jujuba leaves extract: Environmental application, kinetic and thermodynamic studies. J. Phys. Chem. Solids 2021, 158, 110237. [Google Scholar] [CrossRef]
- Mahalakshmi, S.; Hema, N.; Vijaya, P.P. In Vitro Biocompatibility and Antimicrobial activities of Zinc Oxide Nanoparticles (ZnO NPs) Prepared by Chemical and Green Synthetic Route—A Comparative Study. BioNanoScience 2020, 10, 112–121. [Google Scholar] [CrossRef]
- Yang, X.; Cui, X. Adsorption Characteristics of Pb(II) on Alkali Treated Tea Residue. Water Resour. Ind. 2013, 3, 1–10. [Google Scholar] [CrossRef]
- Daneshfozoun, S.; Redza, R.; Vo, D.-V.N.; Ramli, N.M.; Abdullah, M.A.; Abdullah, B. Adsorption Kinetics of Pb(II) Ions from Aqueous Solution Using Modified Magnetic Nano-Composite of OPEFB. Indian J. Sci. Technol. 2017, 10, 1–5. [Google Scholar] [CrossRef]
- Lagergren, S.Y. Zur Theorie Der Sogenannten Adsorption Gelöster Stoffe. Kungliga Svenska Vetenskapsakad. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar] [CrossRef]
- Bouhamed, F.; Elouear, Z.; Bouzid, J.; Ouddane, B. Batch Sorption of Pb (II) Ions from Aqueous Solutions Using Activated Carbon Prepared from Date Stones: Equilibrium, Kinetic, and Thermodynamic Studies. Desalination Water Treat. 2014, 52, 2261–2271. [Google Scholar] [CrossRef]
- Mikhail, S.N.; Ekaterina, V.C. Chi-Squared Goodness-of-Fit Tests for Censored Data, 1st ed.; Wiley-ISTE: London, UK, 2017; Volume 3, p. 158. [Google Scholar]
- Alswata, A.A.; Ahmad, M.B.; Al-Hada, N.M.; Kamari, H.M.; Hussein, M.Z.B.; Ibrahim, N.A. Preparation of Zeolite/Zinc Oxide Nanocomposites for Toxic Metals Removal from Water. Results Phys. 2017, 7, 723–731. [Google Scholar] [CrossRef]
- Modwi, A.; Khezami, L.; Taha, K.; Al-Duaij, O.K.; Houas, A. Fast and High Efficiency Adsorption of Pb(II) Ions by Cu/ZnO Composite. Mater. Lett. 2017, 195, 41–44. [Google Scholar] [CrossRef]
- Subramani, S.E.; Thinakaran, N. Isotherm, Kinetic and Thermodynamic Studies on the Adsorption Behaviour of Textile Dyes onto Chitosan. Process Saf. Environ. Prot. 2017, 106, 1–10. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Al-Haidary, A.M.A.; Zanganah, F.H.H.; Al-Azawi, S.R.F.; Khalili, F.I.; Al-Dujaili, A.H. A Study on Using Date Palm Fibers and Leaf Base of Palm as Adsorbents for Pb(II) Ions from Its Aqueous Solution. Water Air Soil Pollut. 2011, 214, 73–82. [Google Scholar] [CrossRef]
- Moyo, M.; Emmanuel, V.; Johannes, S. Biosorption of Lead (II) by Chemically Modified Mangifera Indica Seed Shells: Adsorbent Preparation, Characterization and Performance Assessment. Process Saf. Environ. Prot. 2017, 11, 40–51. [Google Scholar] [CrossRef]
- Yi, Z.; Yao, J.; Zhu, M.; Chen, H.; Wang, F.; Liu, X. Kinetics, Equilibrium, and Thermodynamics Investigation on the Adsorption of lead (II) by Coal-Based Activated Carbon. SpringerPlus 2016, 5, 1160. [Google Scholar] [CrossRef]
- Azizi, S.; Shahri, M.M.; Mohamad, R. Green Synthesis of Zinc Oxide Nanoparticles for Enhanced Adsorption of Lead Ions from Aqueous Solutions: Equilibrium, Kinetic and Thermodynamic Studies. Molecules 2017, 22, 831. [Google Scholar] [CrossRef]
- Xie, N.; Wang, H.; You, C. Role of oxygen functional groups in Pb2+ adsorption from aqueous solution on carbonaceous surface: A density functional theory study. J. Hazard. Mater. 2021, 405, 124221. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M. Biosorption of Pb(II) and Cd(II) from aqueous solution using green alga (Ulva lactuca) biomass. J. Hazard. Mater. 2008, 152, 302–308. [Google Scholar] [CrossRef]
- Iddou, A.; Youcef, M.H.; Aziz, A.; Ouali, M.S. Biosorptive removal of lead (II) ions from aqueous solutions using Cystoseira stricta biomass: Study of the surface modification effect. J. Saudi Chem. Soc. 2011, 15, 83–88. [Google Scholar] [CrossRef]
- Yan, C.; Li, G.; Xue, P.; Wei, Q.; Li, Q. Competitive effect of Cu(II) and Zn(II) on the biosorption of lead(II) by Myriophyllum spicatum. J. Hazard. Mater. 2010, 179, 721–728. [Google Scholar] [CrossRef]
- Ayodele, D.T.; Adekola, F.A. Kinetic and thermodynamic studies of the adsorption of lead (II) and zinc (II) Ions onto cockle shell powder. FUW Trends Sci. Technol. J. 2016, 1, 1–10. [Google Scholar]
- Forutan, R.; Ehsandoost, E.; Hadipour, S.; Mobaraki, Z.; Saleki, M.; Mohebbi, G. Kinetic and equilibrium studies on the adsorption of lead by the chitin of pink shrimp (Solenocera melantho). Entomol. Appl. Sci. Lett. 2016, 3, 20–26. [Google Scholar]
- Gupta, V.; Jaybhaye, S.; Chandra, N. Biosorption studies of copper, chromium, lead and zinc using fins of Catla catla fish. Int. J. Res. Appl. Sci. Eng. Technol. 2017, 5, 902–909. [Google Scholar] [CrossRef]
- Caccin, M.; Giorgi, M.; Giacobbo, F.; da Ros, M.; Besozzi, L.; Mariani, M. Removal of lead (II) from aqueous solutions by adsorption onto activated carbons prepared from coconut shell. Desalin. Water Treat. 2016, 57, 4557–4575. [Google Scholar] [CrossRef]



| Temperature °C | Pseudo-First-Order | Pseudo-Second-Order | |||
|---|---|---|---|---|---|
| 25 | 51.42 | 1.08 | 54.14 | ||
| R2 | 0.95 | R2 | 0.99 | ||
| 0.20 | 0.11 | ||||
| χ2 | 2358.26 | χ2 | 0.14 | ||
| 30 | 54.02 | 5.83 | 60.99 | ||
| R2 | 0.96 | R2 | 0.98 | ||
| 0.20 | 0.00 | ||||
| χ2 | 398.51 | χ2 | 0.80 | ||
| 35 | 54.03 | 3.99 | 55.18 | ||
| R2 | 0.90 | R2 | 0.99 | ||
| 0.17 | 0.02 | ||||
| χ2 | 627.04 | χ2 | 0.02 | ||
| 45 | 53.80 | 3.81 | 54.41 | ||
| R2 | R2 | 1.0 | |||
| 0.17 | 0.03 | ||||
| χ2 | 656.99 | χ2 | 0.01 | ||
| 50 | 53.80 | 3.50 | 54.34 | ||
| R2 | 0.89 | R2 | 1.0 | ||
| 0.17 | 0.03 | ||||
| χ2 | 722.80 | χ2 | 0.01 | ||
| 55 | 53.98 | 3.09 | 54.20 | ||
| R2 | 0.83 | R2 | 1.0 | ||
| 0.15 | 0.05 | ||||
| χ2 | 836.86 | χ2 | 0.00 | ||
| Temperature °C | C | |
|---|---|---|
| 25 | 0.0266 | 51.221 |
| 30 | 0.1073 | 53.207 |
| 35 | 0.1945 | 52.541 |
| 45 | 0.1864 | 52.349 |
| 50 | 0.1773 | 52.439 |
| 55 | 0.2695 | 51.903 |
| Isotherm Model | Parameter | Value |
|---|---|---|
| Langmuir | 88.76 | |
| 0.76 | ||
| 0.999 | ||
| Freundlich | 43.76 | |
| 0.190 | ||
| 0.889 | ||
| Temkin | 94.79 | |
| 10.37 | ||
| 0.939 | ||
| D–R | 75.89 | |
| 2.69 | ||
| 0.912 |
| Biosorbent | Removal Capacity (mg/g) | Reference |
|---|---|---|
| ZnO NP/DPF | 51.42 | Present study |
| Green seaweed | 2.25 | [39] |
| Fish scales | 24.26 | [1] |
| Marine brown algae | 64.5 | [40] |
| DPF | 39.50 | [2] |
| Coffee husk biomass waste | 19.07 | [3] |
| Raphia-microorganism composite biosorbent | 94.8 | [4] |
| Aquatic plant | 55.12 | [41] |
| Cockle shell | 24.66 | [42] |
| Chitin of shrimp | 7.00 | [43] |
| Fish fins | 3.00 | [44] |
| Activated carbons (coconut shell) | 32.08 | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhogbi, B.G.; Ibrahim, O.; Abdel Salam, M.; El-Shahawi, M.S.; Aslam, M. Facile Preparation and Analytical Utility of ZnO/Date Palm Fiber Nanocomposites in Lead Removal from Environmental Water Samples. Molecules 2022, 27, 5592. https://doi.org/10.3390/molecules27175592
Alhogbi BG, Ibrahim O, Abdel Salam M, El-Shahawi MS, Aslam M. Facile Preparation and Analytical Utility of ZnO/Date Palm Fiber Nanocomposites in Lead Removal from Environmental Water Samples. Molecules. 2022; 27(17):5592. https://doi.org/10.3390/molecules27175592
Chicago/Turabian StyleAlhogbi, Basma G., Ohowd Ibrahim, Mohamed Abdel Salam, Mohammed S. El-Shahawi, and Mohammed Aslam. 2022. "Facile Preparation and Analytical Utility of ZnO/Date Palm Fiber Nanocomposites in Lead Removal from Environmental Water Samples" Molecules 27, no. 17: 5592. https://doi.org/10.3390/molecules27175592
APA StyleAlhogbi, B. G., Ibrahim, O., Abdel Salam, M., El-Shahawi, M. S., & Aslam, M. (2022). Facile Preparation and Analytical Utility of ZnO/Date Palm Fiber Nanocomposites in Lead Removal from Environmental Water Samples. Molecules, 27(17), 5592. https://doi.org/10.3390/molecules27175592








