Uranium Determination in Waters, Wine and Honey by Solid Phase Extraction with New Ion Imprinted Polymer
Abstract
1. Introduction
- -
- -
- -
2. Materials and Methods
2.1. Materials
2.2. Apparatus
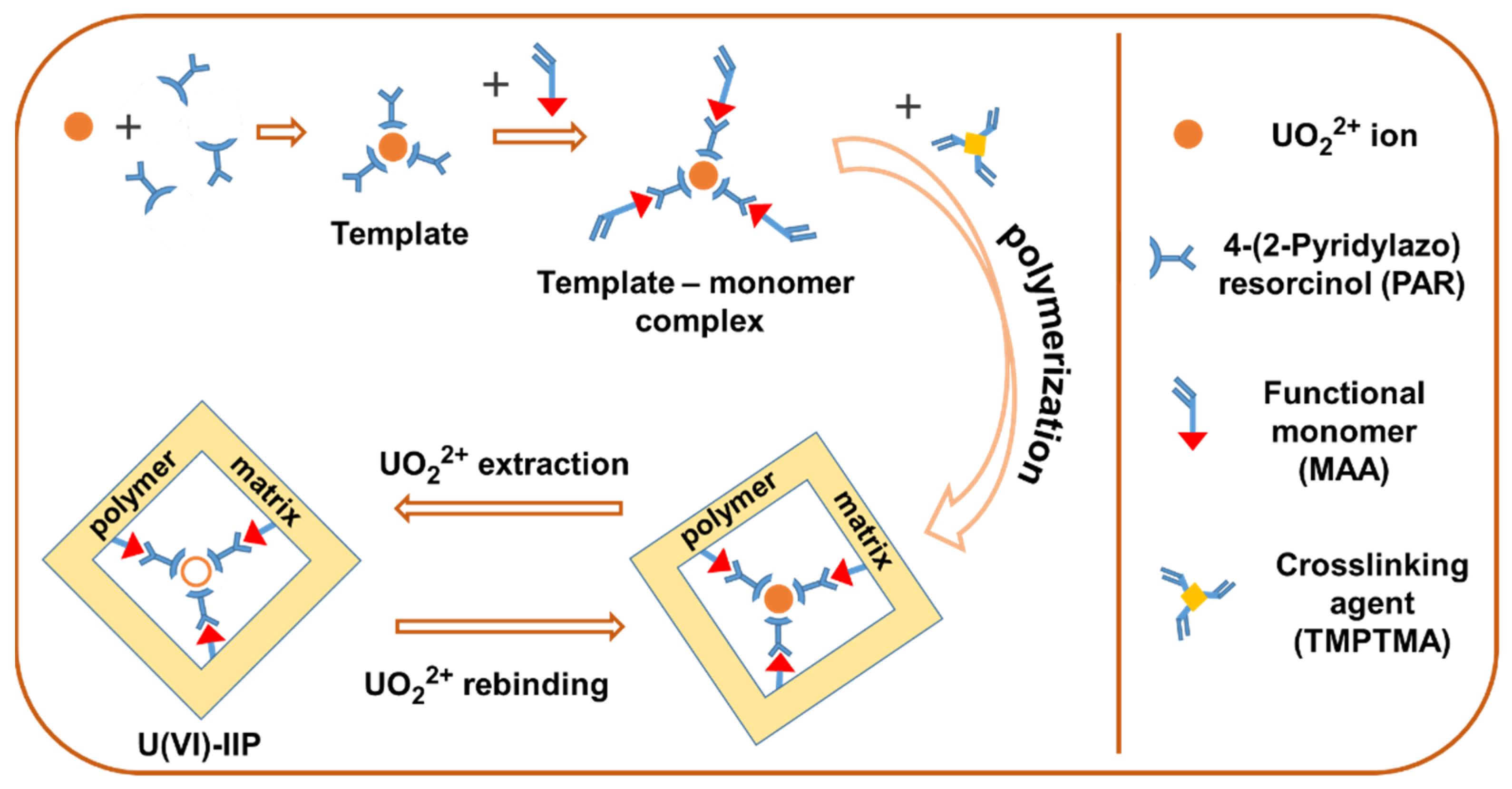
2.3. Synthesis of U(VI)-IIP and NIIP
2.4. Static Adsorption/Desorption Experiments of U
2.5. Isotherm and Kinetic Studies
2.6. Interference Studies
2.7. Analytical Application
2.7.1. Surface/Ground Waters
2.7.2. Wine Samples
2.7.3. Honey Samples
3. Results and Discussion
3.1. Synthesis and Characterization of U(VI)-IIP and NIIP
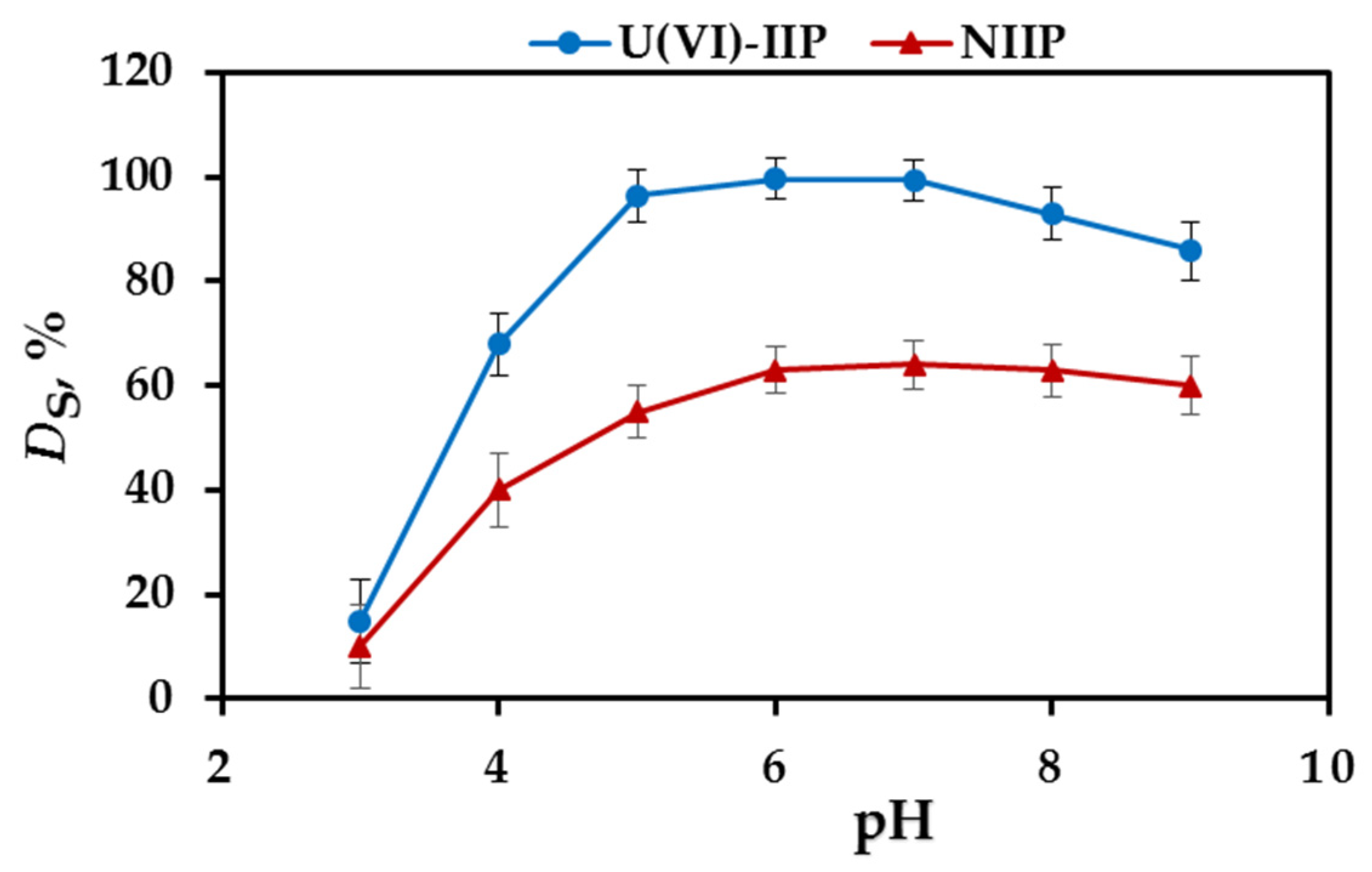
3.2. Effect of pH on SPE Efficiency
3.3. Elution Study
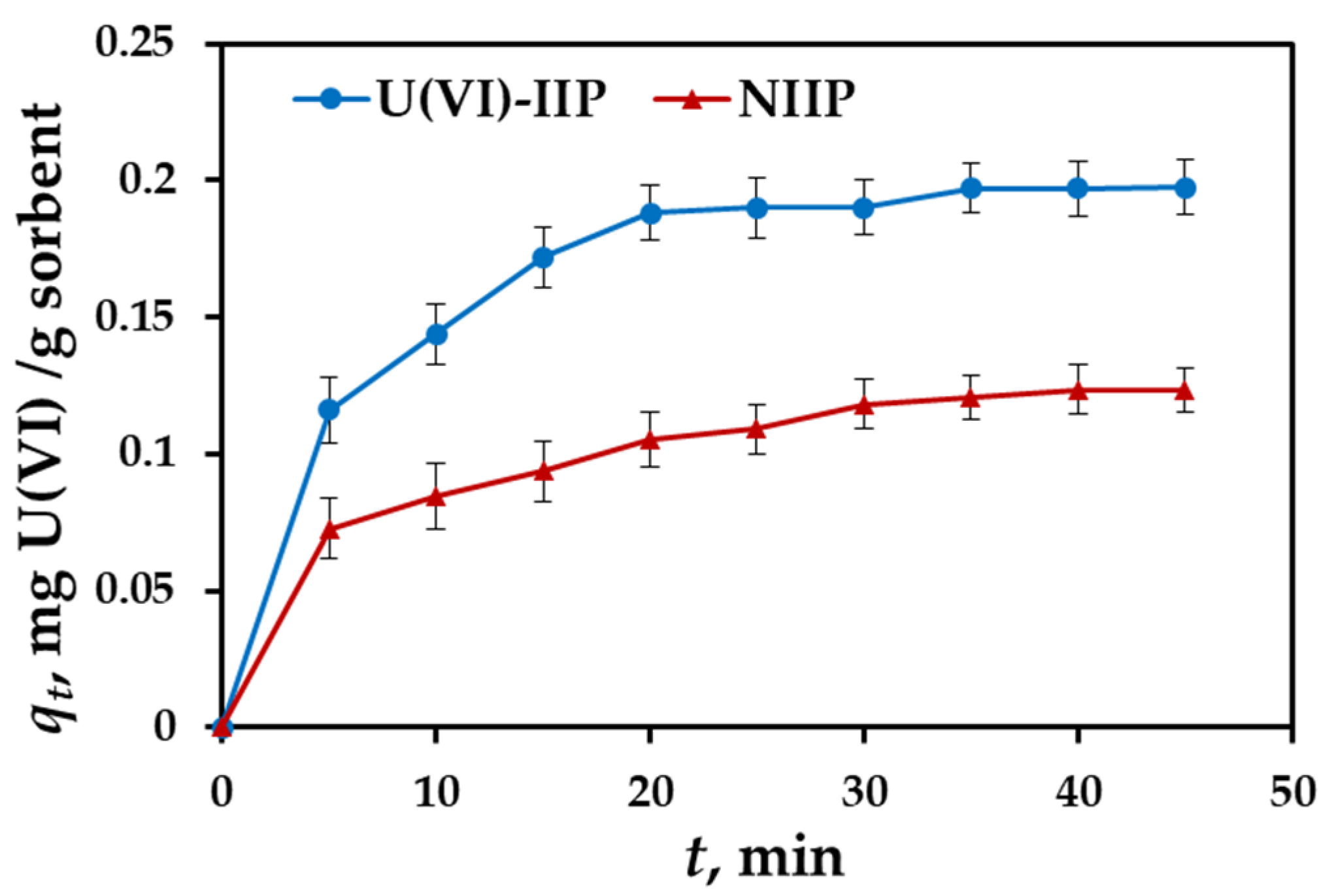
3.4. Effect of Contact Time and Adsorption Kinetics
- pseudo-first-order model:
- Pseudo-second-order model:where: qe, qt—amounts of U(VI) ions retained per mass unit of sorbent at equilibrium and at time t, (mg/g), respectively; k1, k2—rate constants of pseudo-first-order kinetics model (1/min) and pseudo-second-order kinetics model (g/mg·min), respectively.
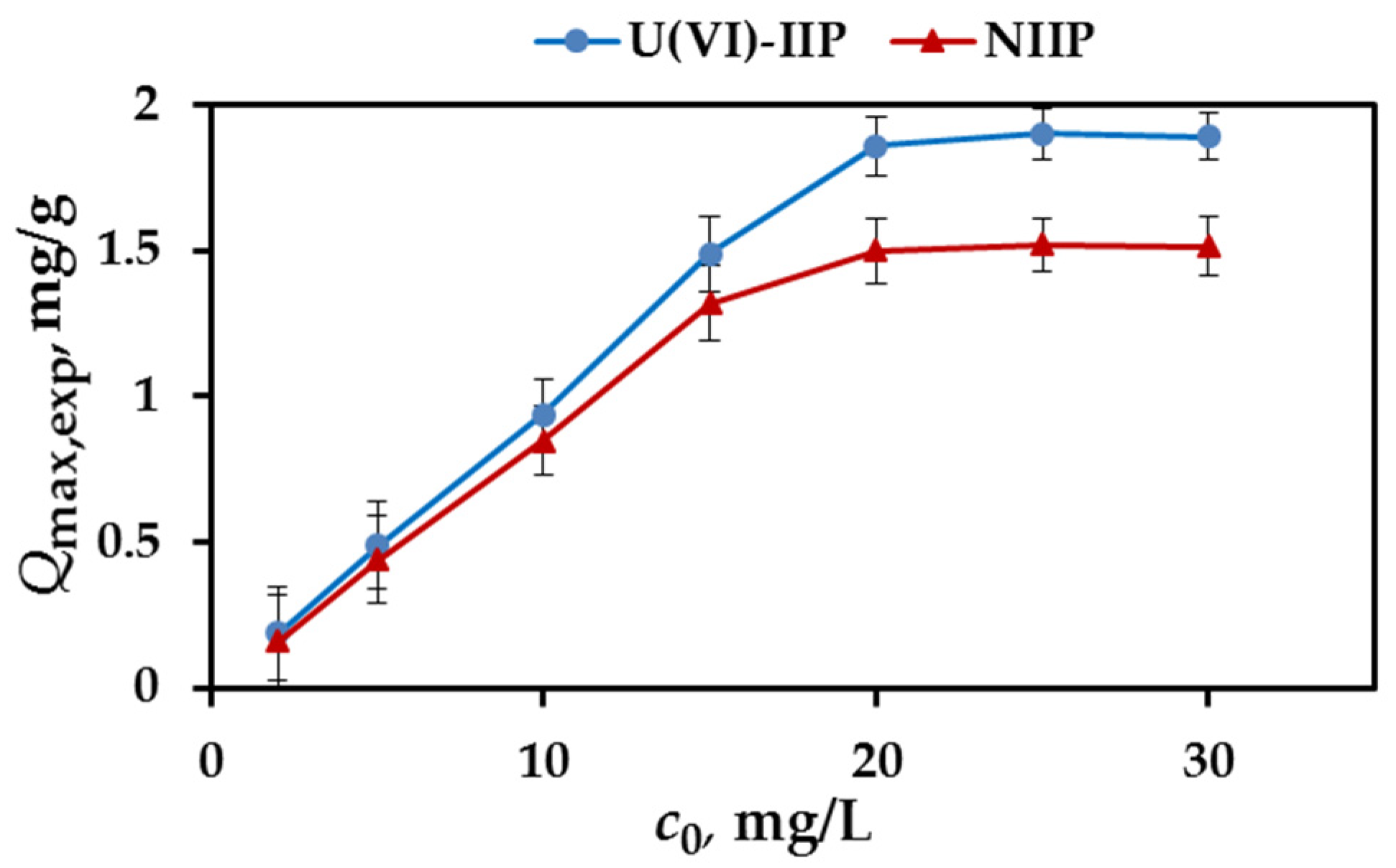
3.5. Effect of Initial U(VI) Concentration and Adsorption Isotherms
3.6. Interference Studies
3.7. Analytical Application
3.8. Analytical Figures of Merit
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cowart, J.B.; Burnett, W.C. The distribution of uranium and thorium decay-series radionuclides in the environment—A review. J. Environ. Qual. 1994, 23, 651–662. [Google Scholar]
- Waseem, A.; Ullah, H.; Rauf, M.K.; Ahmad, I. Distribution of natural uranium in surface and groundwater resources: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2391–2423. [Google Scholar]
- Santana, C.S.; Montalván Olivares, D.M.; Silva, V.H.C.; Luzardo, F.H.M.; Velasco, F.G.; de Jesus, R.M. Assessment of water resources pollution associated with mining activity in a semi-arid region. J. Environ. Manag. 2020, 273, 111148. [Google Scholar]
- Villa-Alfageme, M.; Manjon, G.; Hurtado, S.; Garcia-Tenorio, R. Uranium pollution in an estuary affected by pyrite acid mine drainage and releases of naturally occurring radioactive materials. Mar. Pollut. Bull. 2011, 62, 1521–1529. [Google Scholar]
- Ma, M.; Wang, R.; Xu, L.; Ming Xu, M.; Liu, S. Emerging health risks and underlying toxicological mechanisms of uranium contamination: Lessons from the past two decades. Environ. Int. 2020, 145, 106107. [Google Scholar]
- Rump, A.; Eder, S.; Lamkowski, A.; Hermann, C.; Abend, M.; Port, M. A quantitative comparison of the chemo- and radiotoxicity of uranium at different enrichment grades. Toxicol. Lett. 2019, 313, 159–168. [Google Scholar] [PubMed]
- Zamora, M.L.; Tracy, B.L.; Zielinski, J.M.; Meyerhof, D.P.; Moss, M.A. Chronic ingestion of uranium in drinking water: A study of kidney bioeffects in humans. Toxicol. Sci. 1998, 43, 68–77. [Google Scholar]
- Canu, I.G.; Laurent, O.; Pires, N.; Laurier, D.; Dublineau, I. Health Effects of Naturally Radioactive Water Ingestion: The Need for Enhanced Studies. Environ. Health Perspect. 2011, 119, 1676–1680. [Google Scholar]
- Markich, S.J. Uranium speciation and bioavailability in aquatic systems: An overview. Sci. World J. 2002, 2, 707–729. [Google Scholar]
- Maher, K.; Bargar, J.R.; Brown, G.E. Environmental Speciation of Actinides. Inorg. Chem. 2013, 52, 3510–3532. [Google Scholar]
- Geochemical Atlas of Europe. Part 1: Background Information, Methodology and Maps. Geological Survey of Finland, Espoo, Finland. Available online: http://weppi.gtk.fi/publ/foregsatlas/ (accessed on 31 January 2022).
- Rosenberg, E.; Pinson, G.; Tsosie, R. Uranium Remediation by Ion Exchange and Sorption Methods: A Critical Review. Johns. Matthey Technol. Rev. 2016, 60, 59–77. [Google Scholar]
- Riegel, M.; Schlitt, V. Sorption Dynamics of Uranium onto Anion Exchangers. Water 2017, 9, 268. [Google Scholar]
- Amphlett, J.T.M.; Choi, S.; Parry, S.A.; Moon, E.M.; Sharrad, C.A.; Ogden, M.D. Insights on uranium uptake mechanisms by ion exchange resins with chelating functionalities: Chelation vs. anion exchange. Chem. Eng. J. 2020, 392, 12371. [Google Scholar]
- Madrakian, T.; Mousavi, A. Solid phase extractive preconcentration of uranium (VI)-PVC-CTAB on activated carbon. Can. J. Anal. Sci. Spectrosc. 2008, 53, 232–239. [Google Scholar]
- Kazeraninejad, M.; Shabani, A.M.H.; Dadfarnia, S.; Ahmadi, S.H. Solid phase extraction of trace amounts of uranium (VI) from water samples using 8-hydroxyquinoline immobilized on surfactant coated alumina. J. Anal. Chem. 2011, 66, 373–377. [Google Scholar]
- Sadeghi, S.; Sheikhzadeh, E. Solid phase extraction using silica gel, functionalized with Sulphasalazine for preconcentration of uranium (VI) ions from water samples. Microchim. Acta 2008, 163, 313–320. [Google Scholar]
- Yousefi, S.; Ahmadi, S.J.; Shemirani, F.; Jamali, M.R.; Salavati-Niasary, M. Simultanous extraction and preconcentration of uranium and thorium in aqueous samples by new modified mesoporous silica prior to inductively coupled plasma. Talanta 2009, 80, 212–270. [Google Scholar]
- Ayata, S.; Aydinci, S.; Merdivan, M.; Binzet, G.; Kulcu, N. Sorption of uranium using silica gel with Benzoylthiourea derivatives. J. Radioanal. Nucl. Chem. 2010, 285, 525–529. [Google Scholar]
- Chen, L.; Xu, S.; Li, J. Recent Advances in Molecular Imprinting Technology: Current Status, Challenges and Highlighted Applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar]
- Kusumkar, V.V.; Galamboš, M.; Viglašová, E.; Daňo, M.; Šmelková, J. Ion-Imprinted Polymers: Synthesis, Characterization, and Adsorption of Radionuclides. Materials 2021, 14, 1083. [Google Scholar]
- Gladis, J.M.; Prasada Rao, T. Effect of porogen type on the synthesis of uranium ion imprinted polymer materials for the preconcentration/separation of traces of uranium. Microchim. Acta 2004, 146, 251–258. [Google Scholar] [CrossRef]
- Metilda, P.; Mary Gladis, J.; Prasada Rao, T. Influence of binary/ternary complex of imprint ion on the preconcentration of uranium(VI) using ion imprinted polymer materials. Anal. Chim. Acta 2004, 512, 63–73. [Google Scholar]
- Metilda, P.; Gladis, J.M.; Venkateswaran, G.; Prasada Rao, T. Investigation of the role of chelating ligand in the synthesis of ion-imprinted polymeric resins on the selective enrichment of uranium(VI). Anal. Chim. Acta 2007, 587, 263–271. [Google Scholar]
- Singh, D.K.; Mishra, S. Synthesis and characterization of UO22+ ion imprinted polymer for selective extraction of UO22+. Anal. Chim. Acta 2009, 644, 42–47. [Google Scholar] [PubMed]
- Ahmadi, S.J.; Noori-Kalkhoran, O.; Shirvani-Arani, S. Synthesis and characterization of new ion-imprinted polymer for separation and preconcentration of uranyl (UO22+) ions. J. Hazard. Mater. 2010, 175, 193–197. [Google Scholar]
- Fasihi, J.; Ammari Alahyari, S.; Shamsipur, M.; Sharghi, H.; Charkhi, A. Adsorption of uranyl ion onto an anthraquinone based ion-imprinted copolymer. React. Funct. Polym. 2011, 71, 803–808. [Google Scholar]
- Pakade, V.E.; Cukrowska, E.M.; Darkwa, J.; Darko, G.; Torto, N.; Chimuka, L. Simple and efficient ion imprinted polymer for recovery of uranium from environmental samples. Water Sci. Technol. 2012, 65, 728–736. [Google Scholar]
- Zhang, H.; Liang, H.; Chen, Q.; Shen, X. Synthesis of a new ionic imprinted polymer for the extraction of uranium from seawater. J. Radioanal. Nucl. Chem. 2013, 298, 1705–1712. [Google Scholar]
- Milja, T.E.; Krupa, V.S.; Prasada Rao, T. Synthesis, characterization and application of uranyl ion imprinted polymers of aniline and 8-hydroxy quinoline functionalized aniline. RSC Adv. 2014, 4, 30718–30724. [Google Scholar]
- Liu, J.; Chen, M.; Cui, H. Synthesis of Ion-imprinted materials with amidoxime groups for enhanced UO22+ adsorption. Inorg. Chim. Acta. 2020, 517, 120196. [Google Scholar]
- Shamsipur, M.; Fasihi, J.; Ashtari, K. Grafting of ion-imprinted polymers on the surface of silica gel particles through covalently surface-bound initiators: A selective sorbent for uranyl ion. Anal. Chem. 2007, 79, 7116–7123. [Google Scholar] [CrossRef] [PubMed]
- Milja, T.E.; Prathish, K.P.; Prasada Rao, T. Synthesis of surface imprinted nanospheres for selective removal of uranium from simulants of Sambhar salt lake and ground water. J. Hazard. Mater. 2011, 188, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Fasihi, J.; Shamsipur, M.; Khanchi, A.; Mahani, M.; Ashtari, K. Imprinted polymer grafted from silica particles for on-line trace enrichment and ICP OES determination of uranyl ion. Microchem. J. 2016, 126, 316–321. [Google Scholar] [CrossRef]
- Hao, X.; Chen, R.; Liu, Q.; Liu, J.; Zhang, H.; Yu, J.; Li, Z.; Wang, J. A novel U(VI)-imprinted graphitic carbon nitride composite for the selective and efficient removal of U(VI) from simulated seawater. Inorg. Chem. Front. 2018, 5, 2218–2226. [Google Scholar] [CrossRef]
- Elsayed, N.H.; Alatawi, R.A.S.; Monier, M. Amidoxime modified chitosan based ion-imprinted polymer for selective removal of uranyl ions. Carbohydr. Polym. 2021, 256, 117509. [Google Scholar] [CrossRef]
- Sadeghi, S.; Aboobakri, E. Magnetic nanoparticles with an imprinted polymer coating for the selective extraction of uranyl ions. Microchim. Acta 2012, 178, 89–97. [Google Scholar] [CrossRef]
- Tavengwa, N.T.; Cukrowska, E.; Chimuka, L. Preparation of magnetic nanocomposite beads and optimizing the conditions for effective removal of U(VI) from aqueous solutions. Toxicol. Environ. Chem. 2014, 96, 998–1011. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, S.; Zhou, Y.; Dong, P.; Hua, D. Synthesis of surface ion-imprinted magnetic microspheres by locating polymerization for rapid and selective separation of uranium(VI). RSC Adv. 2015, 5, 4153–4161. [Google Scholar] [CrossRef]
- Dakova, I.; Karadjova, I.; Georgieva, V.; Georgiev, G. Ion-imprinted polymethacrylic microbeads as new sorbent for preconcentration and speciation of mercury. Talanta 2009, 78, 523–529. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, J.; Jiang, Y.; Liu, Z.; Meng, M.; Ni, L.; Qin, C.; Peng, J. Selective Ce(III) ion-imprinted polymer grafted on Fe3O4. nanoparticles supported by SBA-15 mesopores microreactor via surface-initiated RAFT polymerization. Microporous Mesoporous Mater. 2016, 234, 176–185. [Google Scholar] [CrossRef]
- Ruiz, R.; Rosés, M.; Ràfols, C.; Bosch, E. Critical validation of a new simpler approach to estimate aqueous pKa of drugs sparingly soluble in water. Anal. Chim. Acta 2005, 550, 210–221. [Google Scholar] [CrossRef]
- Krestou, A.; Panias, D. Uranium (VI) speciation diagrams in the UO22+/CO32−/H2O system at 25 °C. Eur. J. Miner. Process. Environ. Prot. 2004, 4, 113–129. [Google Scholar]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Lăcrămioara, N.; Bulgariu, L. Optimization of process parameters for heavy metals biosorption onto mustard waste biomass. Open Chem. 2016, 14, 175–187. [Google Scholar]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Intra-particle diffusion processes during acid dye adsorption onto chitosan. Biores. Technol. 2007, 98, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; de Oliveira, F.M.; Tarley, M.; Del Pilar, C.R.T.; Sotomayo, T. Study on the cross-linked molecularly imprinted poly(methacrylic acid) and poly(acrylic acid) towards selective adsorption of diuron. React. Funct. Polym. 2016, 100, 26–36. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 56, 2–10. [Google Scholar] [CrossRef]
- Tavengwa, N.T.; Cukrowska, E.; Chimuka, L. Synthesis of bulk ion-imprinted polymers (IIPs) embedded with oleic acid coated Fe3O4 for selective extraction of hexavalent uranium. Water SA 2014, 40, 623–630. [Google Scholar] [CrossRef][Green Version]
- Tavengwa, N.T.; Cukrowska, E.; Chimuka, L. Sequestration of U(VI) from aqueous solutions using precipitate ion imprinted polymers endowed with oleic acid functionalized magnetite. J. Radioanal. Nucl. Chem. 2015, 304, 933–943. [Google Scholar] [CrossRef]

| Model | Parameters | U(VI)-IIP | NIIP |
|---|---|---|---|
| Pseudo-first-order model | qe,exp (mg/g) | 0.19 | 0.13 |
| qe,calc (mg/g) | 4.20 | 11.00 | |
| k1 (1/min) | 0.151 | 0.072 | |
| R2 | 0.945 | 0.974 | |
| Pseudo-second-order model | qe,calc (mg/g) | 0.21 | 0.14 |
| k2 (g/mg.min) | 1.078 | 1.108 | |
| R2 | 0.998 | 0.996 | |
| Intra-particle diffusion model Region 1 | kdiff (mg/g min1/2) | 0.033 | 0.014 |
| C (mg/g) | 0.042 | 0.040 | |
| R2 | 0.996 | 0.990 | |
| Intra-particle diffusion model Region 2 | kdiff (mg/g min1/2) | 0.005 | 0.008 |
| C (mg/g) | 0.164 | 0.072 | |
| R2 | 0.790 | 0.844 |
| Polymer Gel | Qmax,exp mg/g | Langmuir Isotherm Model | Freundlich Isotherm Model | |||||
|---|---|---|---|---|---|---|---|---|
| Qmax,teor, mg/g | b, L/mg | R2 | RL | kF | n | R2 | ||
| U(VI)-IIP | 1.89 | 1.91 | 1,80 | 0.9986 | 0.02–0.22 | 20.25 | 2.35 | 0.9128 |
| NIIP | 1.35 | 1.37 | 4.85 | 0.9997 | 0.01–0.10 | 1.83 | 3. 42 | 0.8507 |
| Interferent | Recovery, % [Mean ± SD] at Concentration: | |||
|---|---|---|---|---|
| 10 mg/L | 50 mg/L | 100 mg/L | 200 mg/L | |
| HCO3− | 98 ± 2 | 95 ± 3 | 90 ± 3 | 85 ± 4 |
| SO42− | >99 | >99 | 91 ± 3 | 93 ± 3 |
| Cl− | >99 | >99 | >99 | 98 ± 2 |
| Na+ | >99 | >99 | 98 ± 2 | 96 ± 3 |
| K+ | >99 | >99 | 97 ± 2 | 97 ± 2 |
| Ca2+ | >99 | >99 | 97 ± 3 | 95 ± 3 |
| Mg2+ | >99 | >99 | 96 ± 3 | 95 ± 2 |
| tartrate | >99 | >99 | 93 ± 3 | 92 ± 4 |
| Humic substances, 2 mg/L | 98 ± 2 | |||
| Mineral Water Sample | HCO3−, mg/L | CO32−, mg/L | SO42−, mg/L | Cl−, mg/L | Recovery, % [Mean ± SD] |
|---|---|---|---|---|---|
| Gorna Bania | 17 | 22 | 22 | 9 | 95 ± 2 |
| Bankia | 62 | 12 | 51 | 10 | 94 ± 2 |
| Devin | 89 | 21 | 28 | 11 | 91 ± 3 |
| Bachkovo | 92 | 18 | 31 | 7 | 90 ± 2 |
| Hisar | 120 | 15 | 21 | 9 | 88 ± 3 |
| Water Sample | Recovery, % Mean | RSD, % |
|---|---|---|
| River Iskar | 95 | 4 |
| River Maritsa | 92 | 5 |
| Lake Ogosta | 91 | 5 |
| Black sea water (Burgas gulf) | 93 | 2 |
| Tap water Sofia | 94 | 3 |
| Wine Sample | Recovery, % Mean ± SD | ||
|---|---|---|---|
| 10 mL Sample | 20 mL Sample | 30 mL Sample | |
| Red (merlot) | 97 ± 3 | 92 ± 3 | 80 ± 5 |
| Rose | 98 ± 2 | 94 ± 3 | 82 ± 5 |
| White (Muskat) | 98 ± 2 | 93 ± 3 | 81 ± 5 |
| Honey Sample (5% Aqueous Solution) | Recovery, %, Mean ± SD | ||
|---|---|---|---|
| 10 mL Sample | 20 mL Sample | 30 mL Sample | |
| Honey (lime) | >99 | 96 ± 2 | 84 ± 4 |
| Honey (rapeseed) | >99 | 97 ± 2 | 86 ± 4 |
| Honey (sunflower) | >99 | 96 ± 2 | 85 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiev, V.; Dakova, I.; Karadjova, I. Uranium Determination in Waters, Wine and Honey by Solid Phase Extraction with New Ion Imprinted Polymer. Molecules 2022, 27, 5516. https://doi.org/10.3390/molecules27175516
Georgiev V, Dakova I, Karadjova I. Uranium Determination in Waters, Wine and Honey by Solid Phase Extraction with New Ion Imprinted Polymer. Molecules. 2022; 27(17):5516. https://doi.org/10.3390/molecules27175516
Chicago/Turabian StyleGeorgiev, Valentin, Ivanka Dakova, and Irina Karadjova. 2022. "Uranium Determination in Waters, Wine and Honey by Solid Phase Extraction with New Ion Imprinted Polymer" Molecules 27, no. 17: 5516. https://doi.org/10.3390/molecules27175516
APA StyleGeorgiev, V., Dakova, I., & Karadjova, I. (2022). Uranium Determination in Waters, Wine and Honey by Solid Phase Extraction with New Ion Imprinted Polymer. Molecules, 27(17), 5516. https://doi.org/10.3390/molecules27175516





