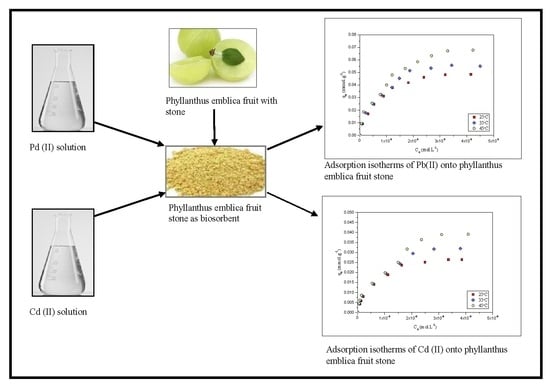
Utilization of Phyllanthus emblica fruit stone as a Potential Biomaterial for Sustainable Remediation of Lead and Cadmium Ions from Aqueous Solutions
Abstract
:1. Introduction
2. Results and Discussion
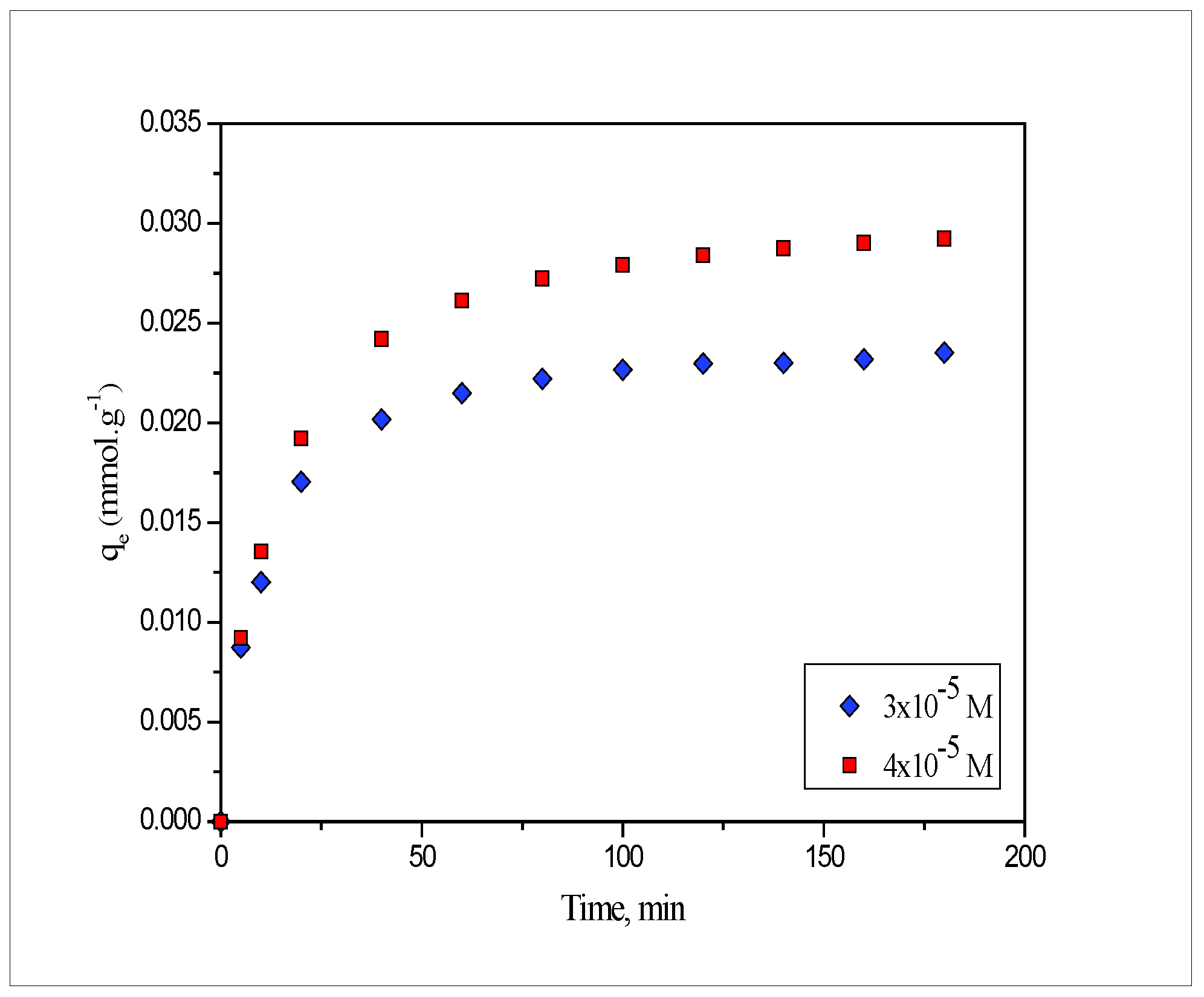
2.1. Effect of Contact Time
2.2. Effect of Initial Metal Ion Concentration
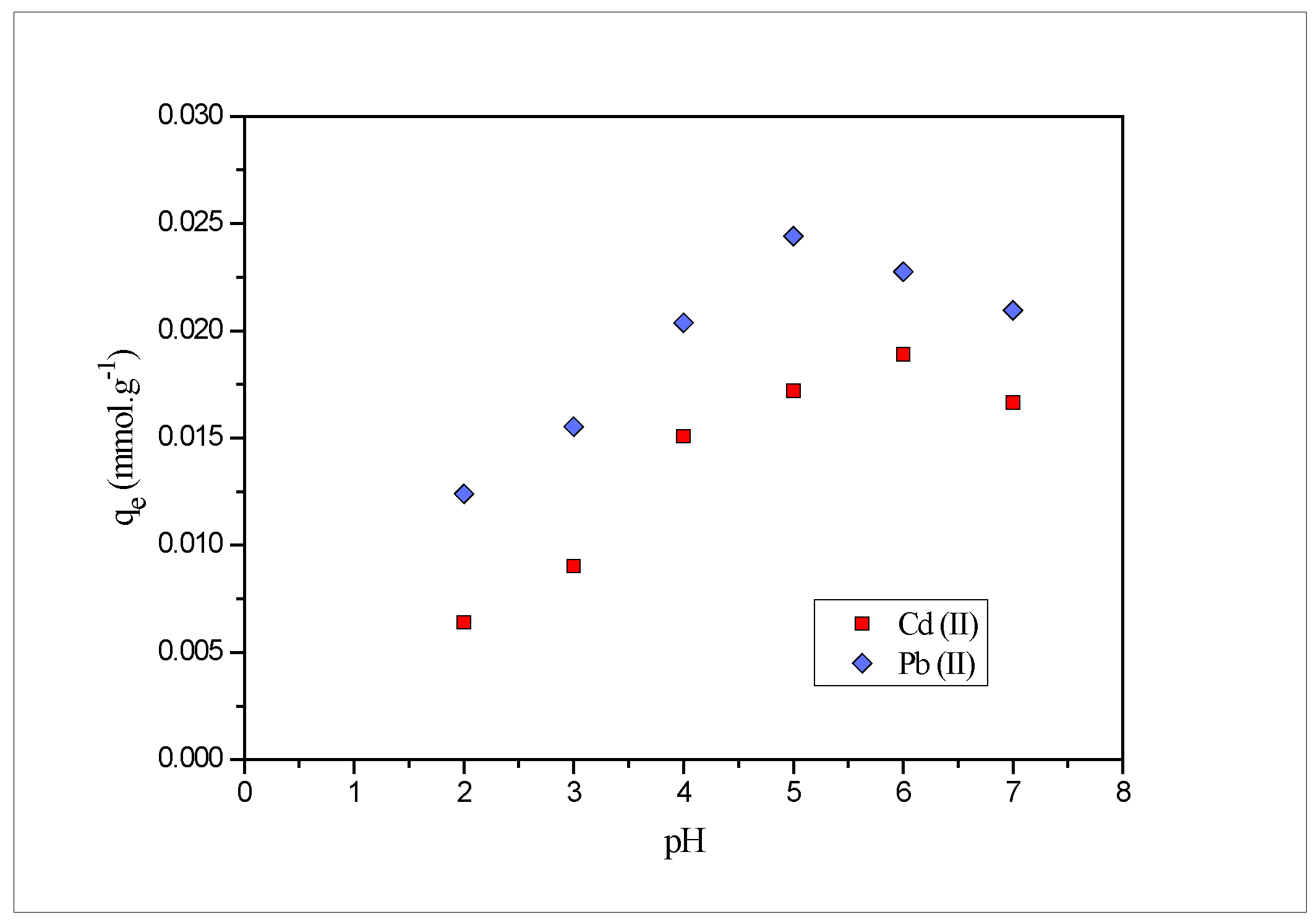
2.3. Effect of pH
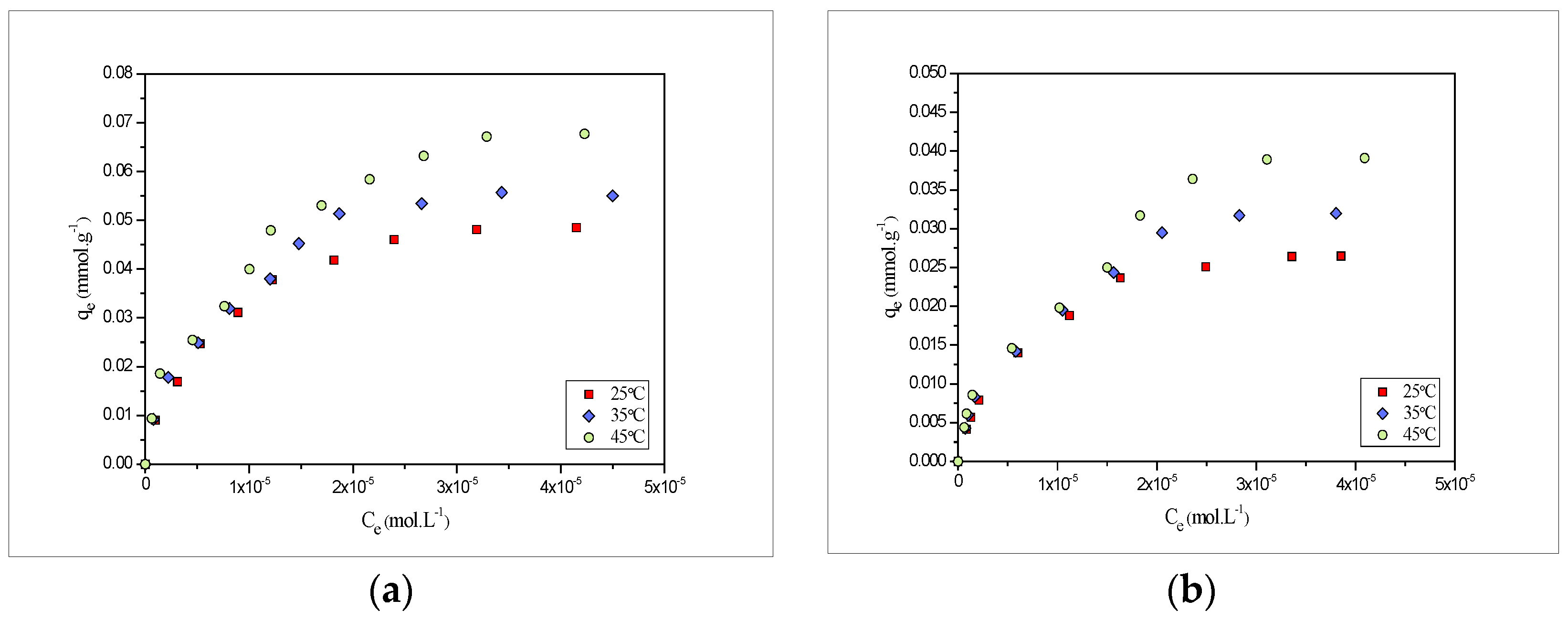
2.4. Adsrption Isotherms
2.5. Characterisation of PEFS and Mechanism
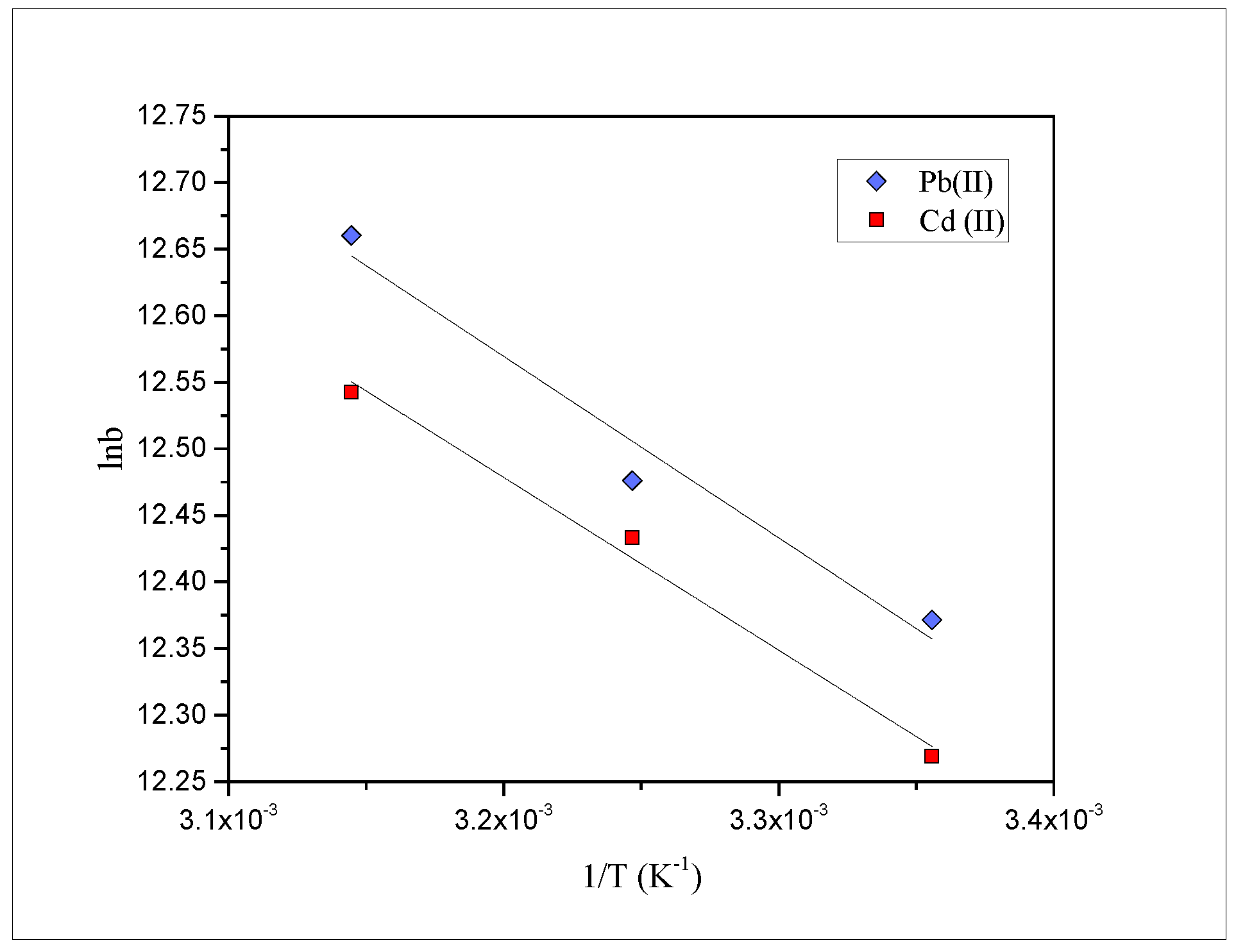
2.6. Effect of Temperature and Thermodynamic Parameters
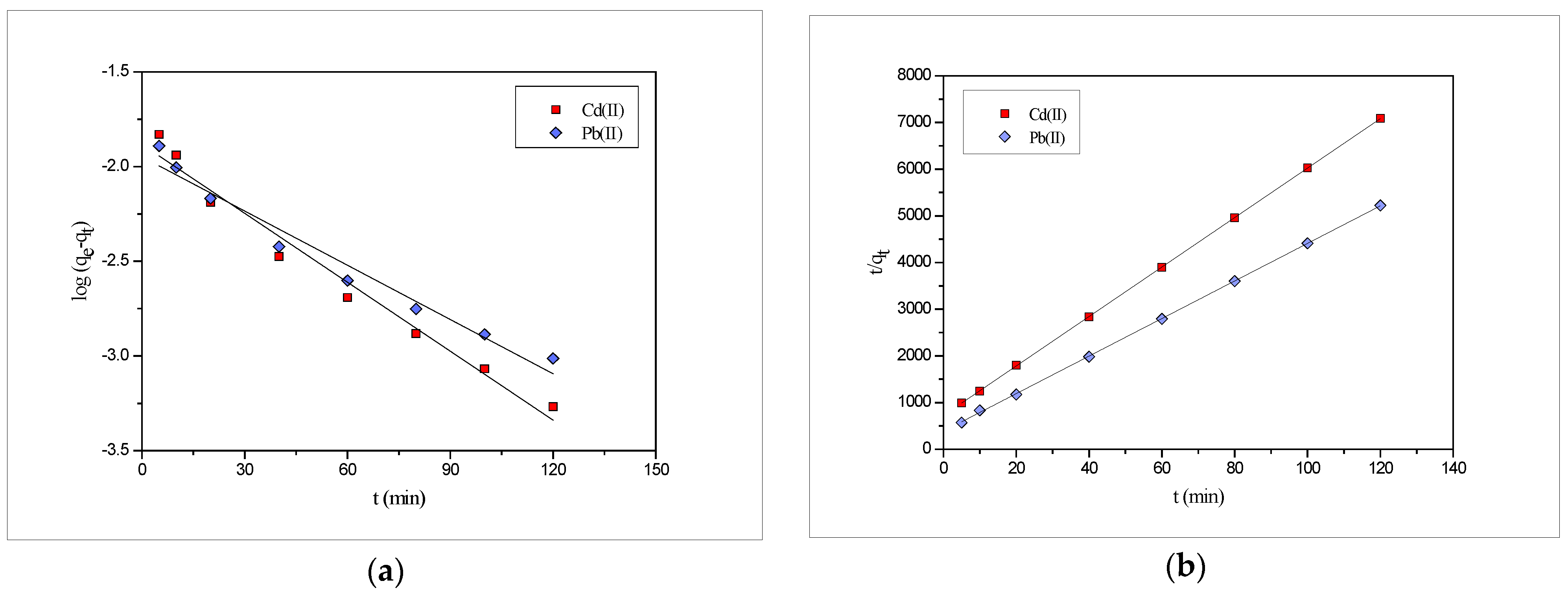
2.7. Biosorption Kinetics
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of PEFS
3.3. Preparation of the Solutions
3.4. Adsorption Studies
3.5. Characterisation of PEFS
4. Conclusions
- The adsorption of Pb(II) and Cd(II) onto PEFS depends on the contact time, initial metal ion concentration, solution pH and temperature. The maximum amount (80%) of Pb(II) and Cd(II) ion was removed in less than 60 min and thereafter, equilibrium was achieved in 120 min.
- The experimental sorption capacity for Pb(II) at pH 5 and Cd(II) at pH 6with contact time 180 min and a biosorbent amount of 0.01 g was found to be 0.048 and 0.026 mmol·g−1, respectively, at 25 °C.
- Comparative results revealed that the removal of Pb(II) is more than that of Cd(II). The biosorption behavior of metal ions on the PEFS is a plausible mechanism with physical adsorption, surface complexation, electrostatic attraction and ion exchange as conventional pathways for explaining the removal of Pb(II) and Cd(II) by PEFS.
- Kinetic data fits well with the pseudo-second order model, while the isotherm data fits well with both adsorption models, i.e., Langmuir and Freundlich.
- The thermodynamic parameter shows that the biosorption process is spontaneous and favorable.
- Keeping in view the results of the removal of Pb(II) and Cd(II), the biosorbent may be tried for wastewater/seawater containing these types of metal ions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Alrumman, S.A.; El-kott, A.F.; Kehsk, M. Water pollution: Source and treatment. Am. J. Environ. Eng. 2016, 6, 88–98. [Google Scholar]
- Gleeson, T.; Wada, Y.; Bierkens, M.F.P.; van Beek, L.P.H. Water balance of global aquifers revealed by groundwater footprint. Nature 2012, 488, 197–200. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Hanafiah, M.A.K.M. Biosorption of copper ions from dilute aqueous solutions on base treatedrubber (Hevea brasiliensis) leaves powder: Kinetics, isotherm, and biosorption mechanisms. J. Environ. Sci. 2008, 20, 1168–1176. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas; Nayak, A.; Agarwal, S.; Chaudhary, M.; Tyagi, I. Removal of Ni (II) ions from water using scrap tire. J. Mol. Liq. 2014, 190, 215–222. [Google Scholar] [CrossRef]
- Song, H.-L.; Liang, L.; Yang, K.-Y. Removal of several metal ions from aqueous solution using powdered stem of Arundo donax L. as a new biosorbent. Chem. Eng. Res. Des. 2014, 92, 1915–1922. [Google Scholar] [CrossRef]
- Wang, N.; Qiu, Y.; Hu, K.; Huang, C.; Xiang, J.; Li, H.; Tang, J.; Wang, J.; Xiao, T. One-step synthesis of cake-like biosorbents from plant biomass for the effective removal and recovery heavy metals: Effect of plant species and roles of xanthation. Chemosphere 2021, 266, 129129. [Google Scholar] [CrossRef]
- Dahaghin, Z.; Mousavi, H.Z.; Sajjadi, S.M. Trace amounts of Cd(II), Cu(II) and Pb(II) ions monitoring using Fe3O4@graphene oxide nanocomposite modified via 2-mercaptobenzothiazole as a novel and efficient nanosorbent. J. Mol. Liq. 2017, 231, 386–395. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Parhi, P.K.; Pandey, S.; Bindhani, B.K.; Thatoi, H.; Panda, C.R. Active and passive biosorption of Pb(II)using live and dead biomass of marine bacterium Bacillus xiamenensis PbRPSD202: Kinetics and isotherm studies. J. Environ. Manag. 2019, 247, 121–134. [Google Scholar] [CrossRef]
- Aksu, Z.; Dönmez, G. A comparative study on the biosorption characteristics of some yeasts for Remazol Blue reactive dye. Chemosphere 2003, 50, 1075–1083. [Google Scholar] [CrossRef]
- Aksu, Z.; Karabayır, G. Comparison of biosorption properties of different kinds of fungi for the removal of Gryfalan Black RL metal-complex dye. Bioresour. Technol. 2008, 99, 7730–7741. [Google Scholar] [CrossRef]
- Tounsadi, H.; Khalidi, A.; Abdennouri, M.; Barka, N. Biosorption potential of Diplotaxis harra and Glebionis coronaria L. biomasses for the removal of Cd(II) and Co(II) from aqueous solutions. J. Environ. Chem. Eng. 2015, 3, 822–830. [Google Scholar] [CrossRef]
- Safa, Y.; Bhatti, H.N. Biosorption of Direct Red-31 and Direct Orange-26 dyes by rice husk: Application of factorial design analysis. Chem. Eng. Res. Des. 2011, 89, 2566–2574. [Google Scholar] [CrossRef]
- Gorgievski, M.; Božić, D.; Stanković, V.; Štrbac, N.; Šerbula, S. Kinetics, equilibrium and mechanism of Cu2+, Ni2+ and Zn2+ ions biosorption using wheat straw. Ecol. Eng. 2013, 58, 113–122. [Google Scholar] [CrossRef]
- Chand, P.; Bafana, A.; Pakade, Y.B. Xanthate modified apple pomace as an adsorbent for removal of Cd (II), Ni (II) and Pb (II), and its application to real industrial wastewater. Int. Biodeterior. Biodegrad. 2015, 97, 60–66. [Google Scholar] [CrossRef]
- do Nascimento, J.M.; de Oliveira, J.D.; Leite, S.G.F. Chemical characterization of biomass flour of the babassu coconut mesocarp (Orbignya speciosa) during biosorption process of copper ions. Environ. Technol. Innov. 2019, 16, 100440. [Google Scholar] [CrossRef]
- Znad, H.; Awual, M.R.; Martini, S. The Utilization of Algae and Seaweed Biomass for Bioremediation of Heavy Metal-Contaminated Wastewater. Molecules 2022, 27, 1275. [Google Scholar] [CrossRef]
- Guleria, A.; Kumari, G.; Lima, E.C.; Ashish, D.K.; Thakur, V.; Singh, K. Removal of inorganic toxic contaminants from wastewater using sustainable biomass: A review. Sci. Total Environ. 2022, 823, 153689. [Google Scholar] [CrossRef]
- Suhas; Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar] [CrossRef]
- Suhas; Carrott, P.J.M.; Ribeiro Carrott, M.M.L. Lignin—From natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.; Sar, S.K.; Ghosh, P.K. Efficient exclusion of uranyl ion from aqueous medium by a novel magnetic bio adsorbent (Phyllanthus emblica bark). Groundw. Sustain. Dev. 2021, 14, 100625. [Google Scholar] [CrossRef]
- Verma, R.; Kundu, L.M.; Pandey, L.M. Enhanced melanoidin removal by amine-modified Phyllanthus emblica leaf powder. Bioresour. Technol. 2021, 339, 125572. [Google Scholar] [CrossRef] [PubMed]
- Gaire, B.P.; Subedi, L. Phytochemistry, pharmacology and medicinal properties of Phyllanthus emblica Linn. Chin. J. Integr. Med. 2014. Online ahead of print. [Google Scholar] [CrossRef]
- Ahmad, B.; Hafeez, N.; Rauf, A.; Bashir, S.; Linfang, H.; Rehman, M.-u.; Mubarak, M.S.; Uddin, M.S.; Bawazeer, S.; Shariati, M.A.; et al. Phyllanthus emblica: A comprehensive review of its therapeutic benefits. S. Afr. J. Bot. 2021, 138, 278–310. [Google Scholar] [CrossRef]
- Poltanov, E.A.; Shikov, A.N.; Dorman, H.J.D.; Pozharitskaya, O.N.; Makarov, V.G.; Tikhonov, V.P.; Hiltunen, R. Chemical and antioxidant evaluation of Indian gooseberry (emblica officinalis gaertn., syn. Phyllanthus emblica L.) supplements. Phytother. Res. 2009, 23, 1309–1315. [Google Scholar] [CrossRef]
- Pehlivan, E.; Altun, T.; Cetin, S.; Iqbal Bhanger, M. Lead sorption by waste biomass of hazelnut and almond shell. J. Hazard. Mater. 2009, 167, 1203–1208. [Google Scholar] [CrossRef]
- Javanbakht, V.; Zilouei, H.; Karimi, K. Lead biosorption by different morphologies of fungus Mucor indicus. Int. Biodeterior. Biodegrad. 2011, 65, 294–300. [Google Scholar] [CrossRef]
- Hossain, A.; Bhattacharyya, S.R.; Aditya, G. Biosorption of cadmium by waste shell dust of fresh water mussel Lamellidens marginalis: Implications for metal bioremediation. ACS Sustain. Chem. Eng. 2014, 3, 1–8. [Google Scholar] [CrossRef]
- Nadeem, M.; Mahmood, A.; Shahid, S.A.; Shah, S.S.; Khalid, A.M.; McKay, G. Sorption of lead from aqueous solution by chemically modified carbon adsorbents. J. Hazard. Mater. 2006, 138, 604–613. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, Q.; Ji, X.; Wang, F.; Gao, X.; Zhao, M. High and fast adsorption of Cd(II) and Pb(II) ions from aqueous solutions by a waste biomass based hydrogel. Sci. Rep. 2020, 10, 3285. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraja, G.; Krishnaiah, N.; Subbaiah, M.V.; Krishnaiah, A. Biosorption of Pb(II) from aqueous solution by Solanum melongena leaf powder as a low-cost biosorbent prepared from agricultural waste. Colloids Surf. B Biointerfaces 2014, 114, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Fifi, U.; Winiarski, T.; Emmanuel, E. Assessing the mobility of lead, copper and cadmium in a calcareous soil of Port-au-Prince, Haiti. Int. J. Environ. Res. Public Health 2013, 10, 5830–5843. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Suhas; Gupta, V.K.; Singh, L.P.; Chaudhary, M.; Kushwaha, S. A novel approach to develop activated carbon by an ingenious hydrothermal treatment methodology using Phyllanthus emblica fruit stone. J. Clean. Prod. 2021, 288, 125643. [Google Scholar] [CrossRef]
- Rambabu, K.; Thanigaivelan, A.; Bharath, G.; Sivarajasekar, N.; Banat, F.; Show, P.L. Biosorption potential of Phoenix dactylifera coir wastes for toxic hexavalent chromium sequestration. Chemosphere 2021, 268, 128809. [Google Scholar] [CrossRef]
- Cholico-González, D.; Ortiz Lara, N.; Fernández Macedo, A.M.; Chavez Salas, J. Adsorption Behavior of Pb(II), Cd(II), and Zn(II) onto Agave Bagasse, Characterization, and Mechanism. ACS Omega 2020, 5, 3302–3314. [Google Scholar] [CrossRef]
- Fabre, E.; Lopes, C.B.; Vale, C.; Pereira, E.; Silva, C.M. Valuation of banana peels as an effective biosorbent for mercury removal under low environmental concentrations. Sci. Total Environ. 2020, 709, 135883. [Google Scholar] [CrossRef]
- Huang, D.; Li, B.; Ou, J.; Xue, W.; Li, J.; Li, Z.; Li, T.; Chen, S.; Deng, R.; Guo, X. Megamerger of biosorbents and catalytic technologies for the removal of heavy metals from wastewater: Preparation, final disposal, mechanism and influencing factors. J. Environ. Manag. 2020, 261, 109879. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Yang, Z.; Han, W.; Yuan, L.; Zhang, L.; Huang, X. Efficient removal of Pb(II) and Cd(II) from aqueous solutions by mango seed biosorbent. Chem. Eng. J. Adv. 2022, 11, 100295. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J.; Dai, G.; Wu, J.; Yan, H. Adsorption characteristics of Pb(II) from aqueous solution onto a natural biosorbent, fallen Cinnamomum camphora leaves. Desalination 2010, 262, 174–182. [Google Scholar] [CrossRef]
- Mwandira, W.; Nakashima, K.; Kawasaki, S.; Arabelo, A.; Banda, K.; Nyambe, I.; Chirwa, M.; Ito, M.; Sato, T.; Igarashi, T.; et al. Biosorption of Pb(II) and Zn(II) from aqueous solution by Oceanobacillus profundus isolated from an abandoned mine. Sci. Rep. 2020, 10, 21189. [Google Scholar] [CrossRef]
- Taşar, Ş.; Kaya, F.; Özer, A. Biosorption of lead(II) ions from aqueous solution by peanut shells: Equilibrium, thermodynamic and kinetic studies. J. Environ. Chem. Eng. 2014, 2, 1018–1026. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Kungliga Sven. Vetensk. Handlingar. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ho, Y.S.; Chiang, C.C. Sorption Studies of Acid Dye by Mixed Sorbents. Adsorption 2001, 7, 139–147. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]

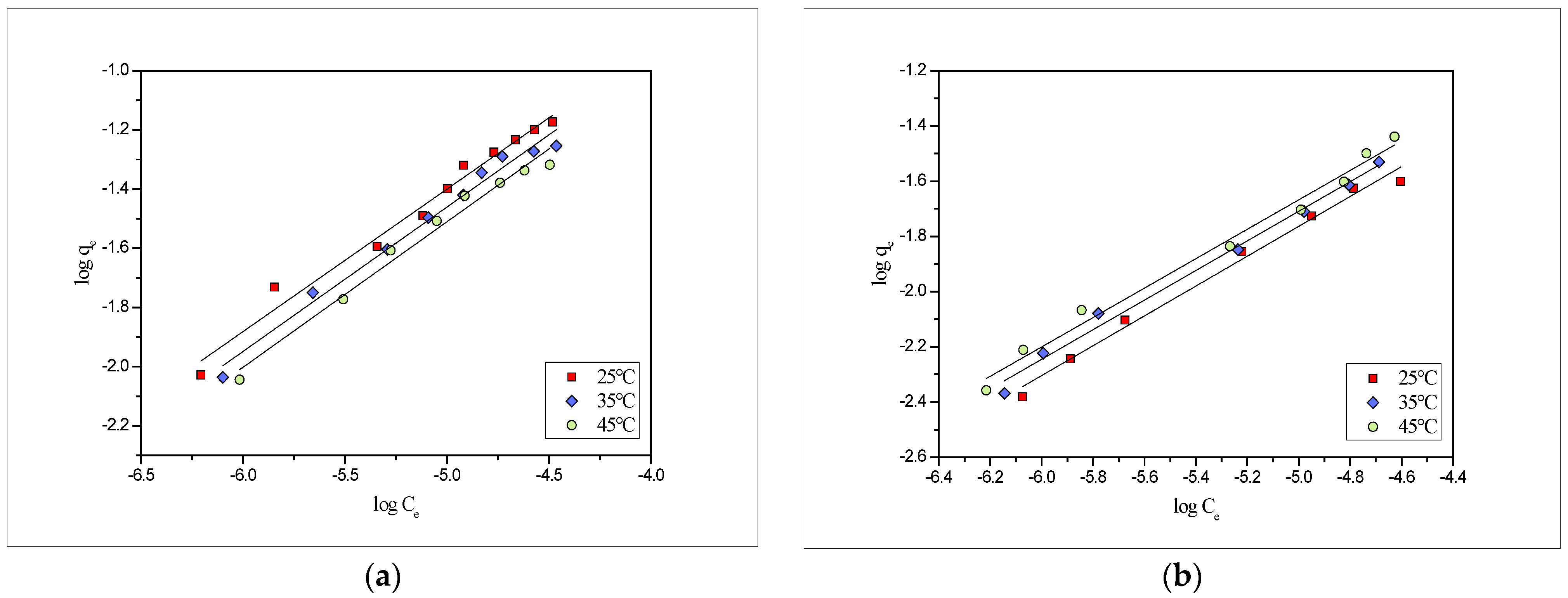
| Metals | Temp (°C) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| qmax (mmol·g−1) | b(L·mol−1) | R2 | Kf (mmol·g−1) | n | R2 | ||
| Pb(II) | 25 | 0.048 | 2.36 × 105 | 0.980 | 8.90 | 2.03 | 0.980 |
| 35 | 0.052 | 2.62 × 105 | 0.984 | 9.54 | 2.05 | 0.984 | |
| 45 | 0.057 | 3.15 × 105 | 0.974 | 10.20 | 2.08 | 0.981 | |
| Cd(II) | 25 | 0.027 | 2.13 × 105 | 0.996 | 8.71 | 1.85 | 0.990 |
| 35 | 0.028 | 2.51 × 105 | 0.992 | 9.37 | 1.87 | 0.991 | |
| 45 | 0.031 | 2.80 × 105 | 0.987 | 9.92 | 1.88 | 0.988 | |
| Elemental Analysis of PEFS | |||||||
|---|---|---|---|---|---|---|---|
| C% | N% | S% | H% | ||||
| 46.5 | 0.07 | 0.14 | 6.2 | ||||
| Inorganic Amount in 1 kg | |||||||
| K (mg) | Na (mg) | Mg (mg) | Ca (mg) | Fe (mg) | P (mg) | Cu (mg) | Mn (mg) |
| 432.2 | 253 | 101.3 | 860.14 | 26.7 | 267.99 | 5.4 | 0.81 |
| FTIR Analysis of PEFS | |||||||
| O–H stretching | C–H stretching | C=O stretching (carboxyl, aldehyde, ketone and ester) | carboxylate anion stretching | stretching due to Methoxy group | stretching due to ether and epoxide | C–O stretching of alcohol | -OH bending |
| 3448 | 3000–2800 cm−1 | 1740–1700 cm−1 | 1637 cm−1 | 1453 and 1423 cm−1 | 1254 cm−1 | 1044 cm−1 | 615 cm−1 |
| XRD-analysis of PEFS | |||||||
| 2θ = 16° and 22° Corresponding to cellulose | |||||||
| N2 adsorption isotherms | |||||||
| Low surface area and non-porous | |||||||
| SEM analysis | |||||||
| Compact surface structure | |||||||
| Metals | Temperature (°C) | −ΔG° (kJ·mol−1) | ΔS° (J·mol−1·K−1) | ΔH° (kJ·mol−1) |
|---|---|---|---|---|
| Pb(II) | 25 | 30.7 | ||
| 35 | 30.9 | 141 | 11.3 | |
| 45 | 31.4 | |||
| Cd(II) | 25 | 30.4 | ||
| 35 | 30.8 | 138 | 10.8 | |
| 45 | 31.1 |
| Metal | Co (mol·L−1) | qe (exp) (mmol·g−1) | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|---|---|
| qe (mmol·g−1) | K1 (min−1) | R2 | qe (mmol·g−1) | K2 (g·mmol−1·min−1) | R2 | |||
| Pb(II) | 3 × 10−5 | 0.024 | 0.013 | 0.019 | 0.743 | 0.025 | 4.19 | 0.998 |
| Cd(II) | 3 × 10−5 | 0.018 | 0.011 | 0.022 | 0.969 | 0.019 | 3.88 | 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushwaha, S.; Suhas; Chaudhary, M.; Tyagi, I.; Bhutiani, R.; Goscianska, J.; Ahmed, J.; Manila; Chaudhary, S. Utilization of Phyllanthus emblica fruit stone as a Potential Biomaterial for Sustainable Remediation of Lead and Cadmium Ions from Aqueous Solutions. Molecules 2022, 27, 3355. https://doi.org/10.3390/molecules27103355
Kushwaha S, Suhas, Chaudhary M, Tyagi I, Bhutiani R, Goscianska J, Ahmed J, Manila, Chaudhary S. Utilization of Phyllanthus emblica fruit stone as a Potential Biomaterial for Sustainable Remediation of Lead and Cadmium Ions from Aqueous Solutions. Molecules. 2022; 27(10):3355. https://doi.org/10.3390/molecules27103355
Chicago/Turabian StyleKushwaha, Sarita, Suhas, Monika Chaudhary, Inderjeet Tyagi, Rakesh Bhutiani, Joanna Goscianska, Jahangeer Ahmed, Manila, and Shubham Chaudhary. 2022. "Utilization of Phyllanthus emblica fruit stone as a Potential Biomaterial for Sustainable Remediation of Lead and Cadmium Ions from Aqueous Solutions" Molecules 27, no. 10: 3355. https://doi.org/10.3390/molecules27103355
APA StyleKushwaha, S., Suhas, Chaudhary, M., Tyagi, I., Bhutiani, R., Goscianska, J., Ahmed, J., Manila, & Chaudhary, S. (2022). Utilization of Phyllanthus emblica fruit stone as a Potential Biomaterial for Sustainable Remediation of Lead and Cadmium Ions from Aqueous Solutions. Molecules, 27(10), 3355. https://doi.org/10.3390/molecules27103355