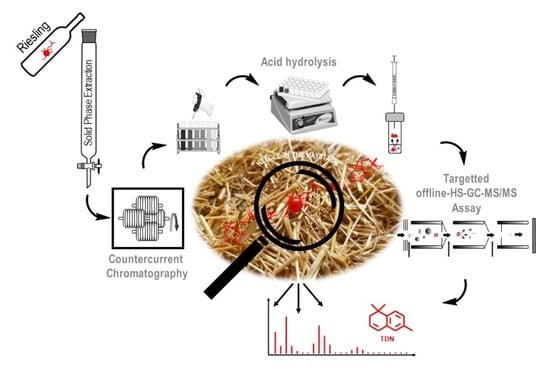
Target-Guided Isolation of Progenitors of 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) from Riesling Wine by High-Performance Countercurrent Chromatography †
Abstract
1. Introduction
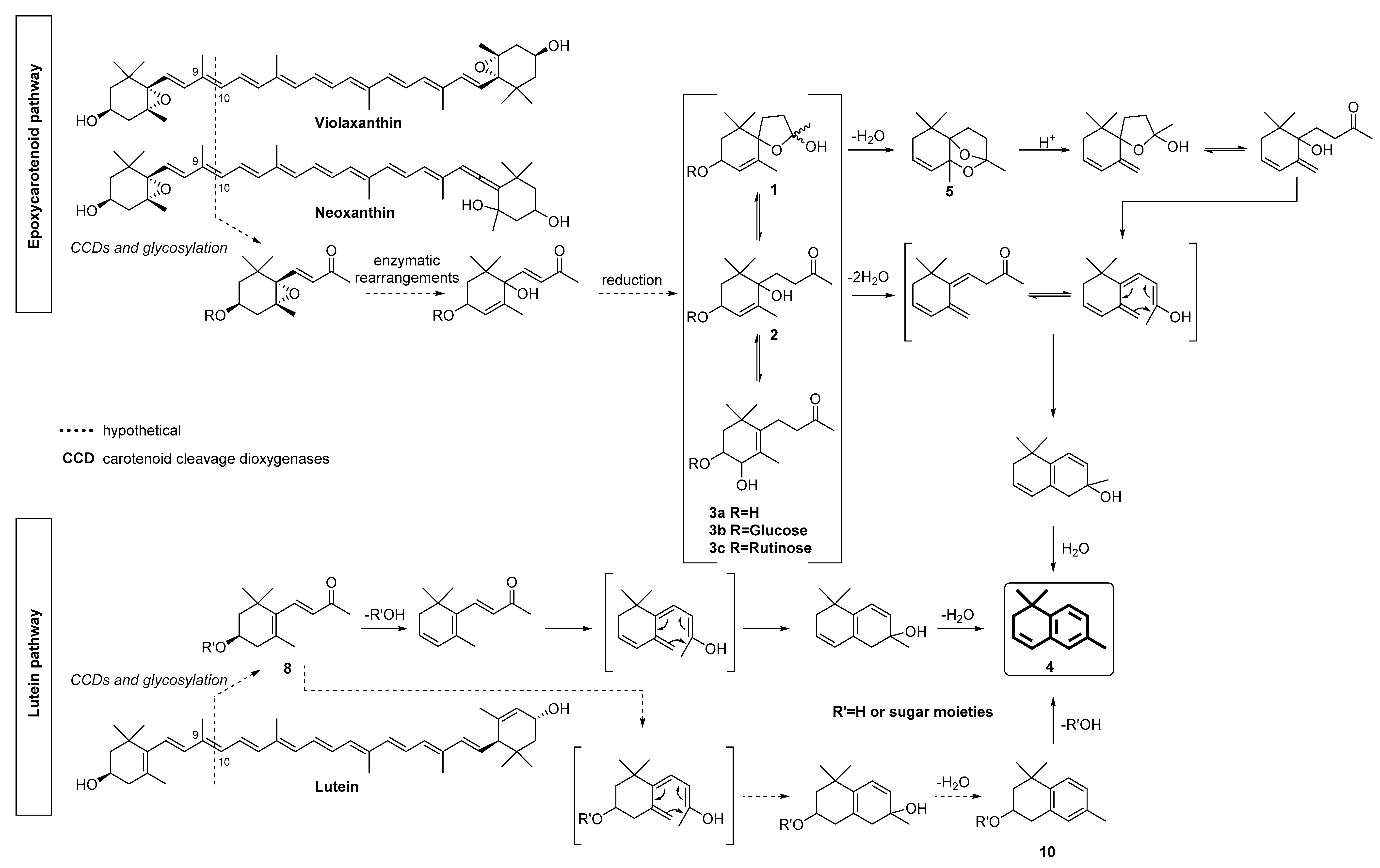
2. Results and Discussion
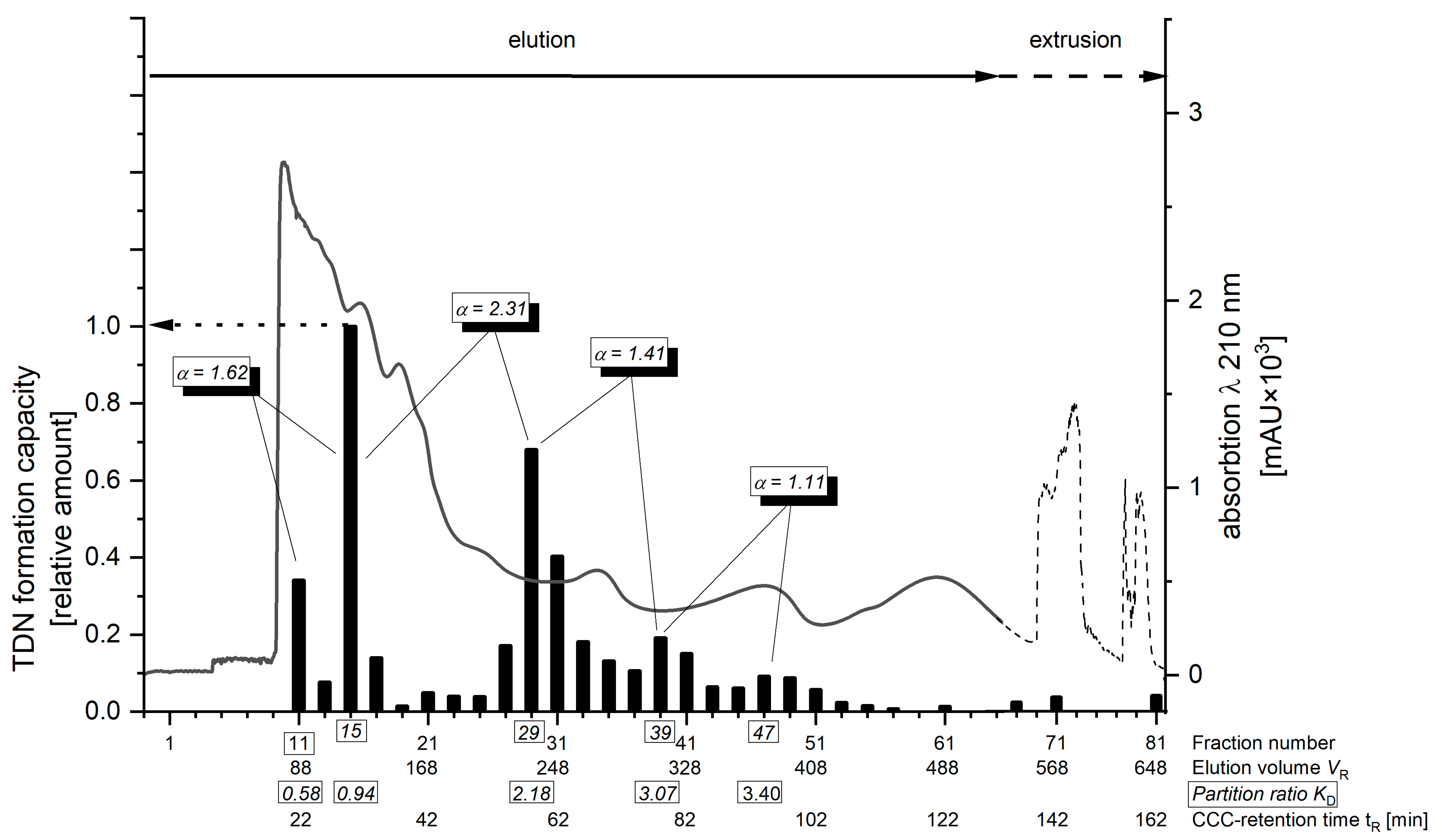
2.1. Target-Guided Isolation of TDN Progenitors by High Performance Countercurrent Chromatography (HPCCC)
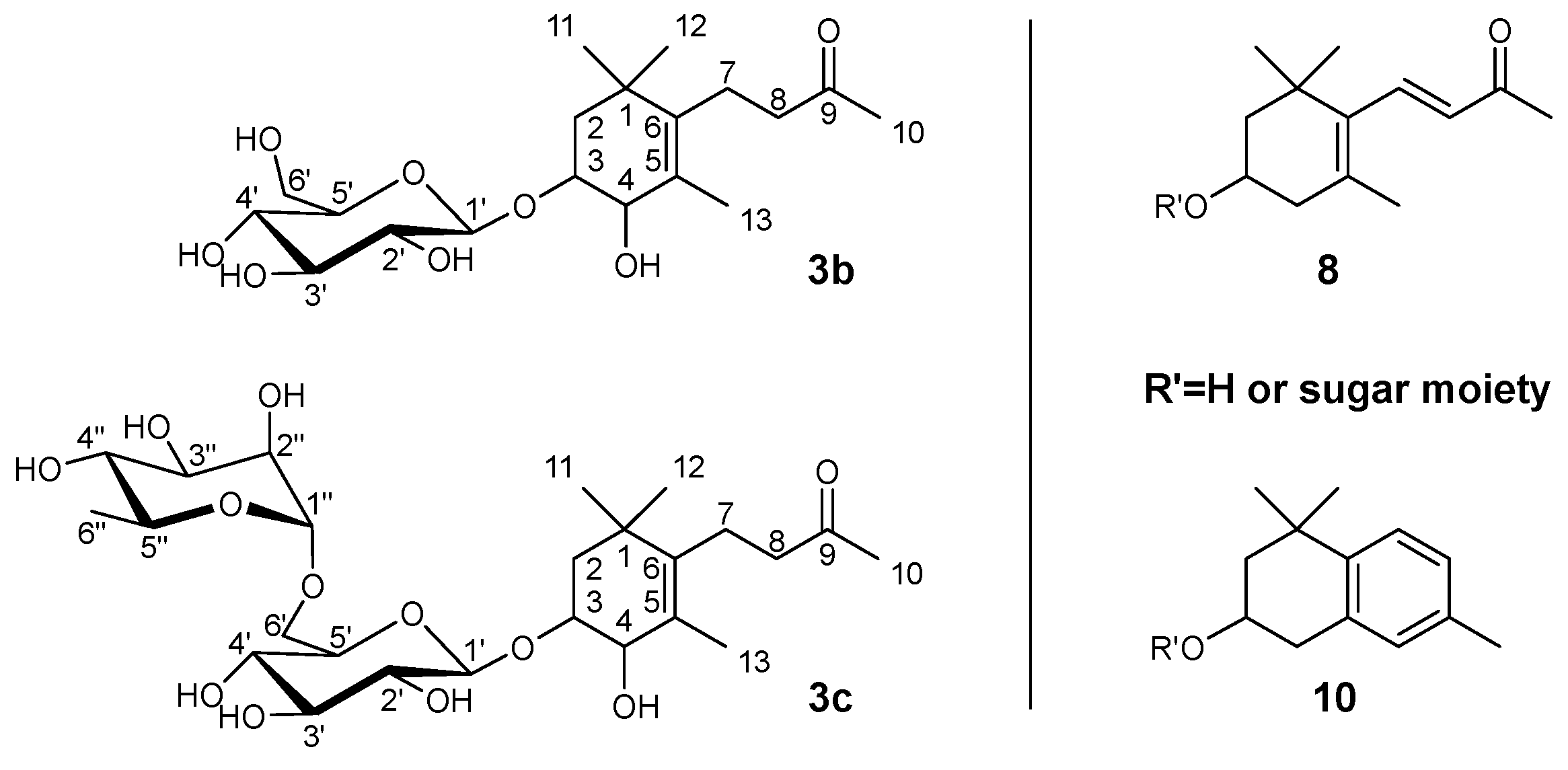
2.2. Isolation and Structure Elucidation of 3,4-Dihydroxy-7,8-dihydro-β-ionone 3-0-β-D-glucopyranoside 3b and 3,4-Dihydroxy-7,8-dihydro-β-ionone 3-0-rutinoside 3c from the Polar HPCCC Fractions 11–18
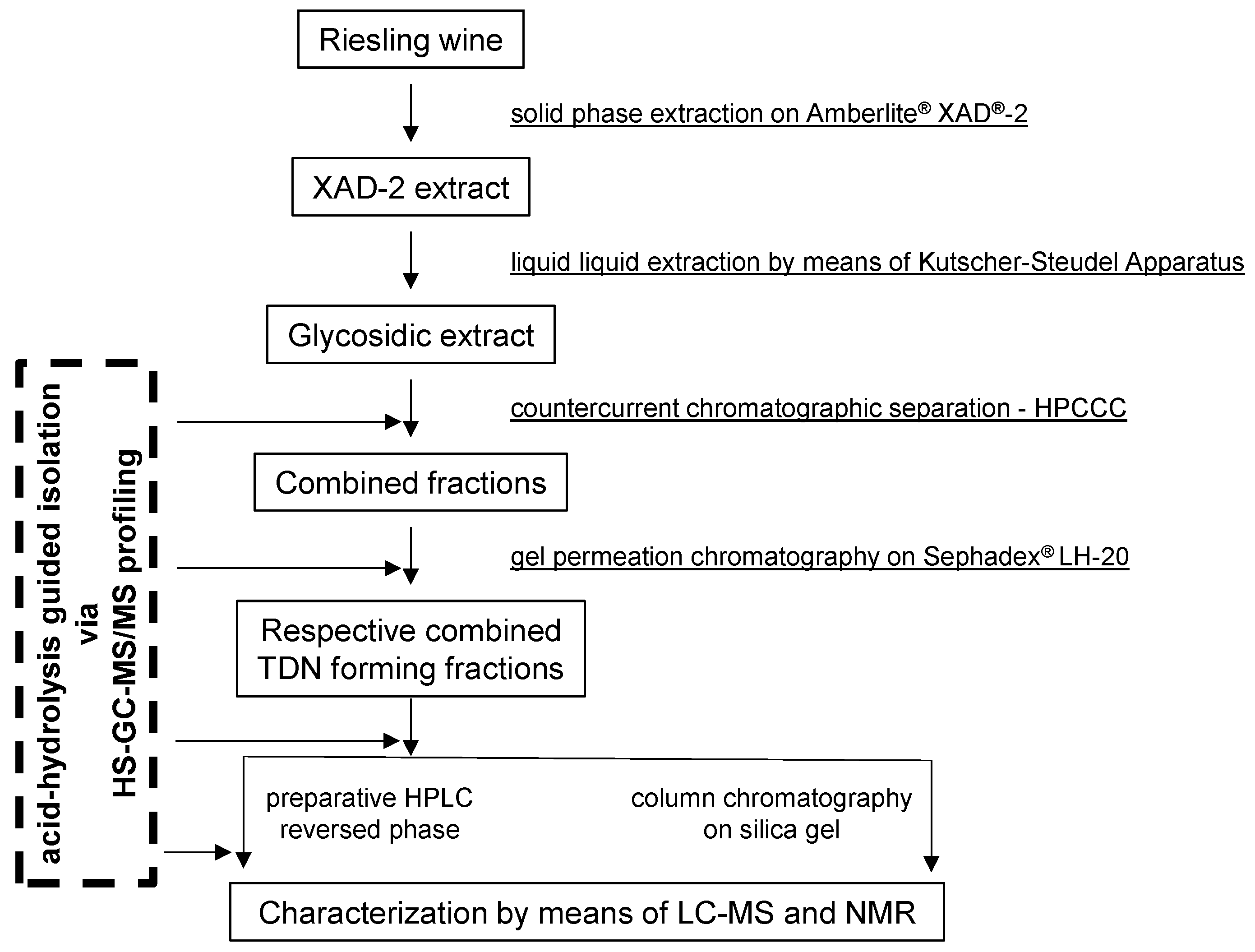
3. Materials and Methods
3.1. Chemicals and General Experimental Conditions
3.2. Isolation of Glycosidically Bound TDN Precursors
3.3. HPCCC Fractionation of the XAD-2 Extract from Riesling Wine
3.4. Gel Permeation Chromatography (GPC)
3.5. Further Purification by Semi-Preparative and Analytical HPLC
3.6. Off-Line Headspace (HS)-GC-MS/MS Screening of TDN-Generating Fractions
3.7. HPLC-ESI-MS/MS-Analysis
3.8. Enzymatic Hydrolysis of TDN-Generating HPCCC Fractions
3.9. Nuclear Magnetic Resonance (NMR) Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Simpson, R.F. 1,1,6-Trimethyl-1,2-dihydronaphthalene: An important contributor to the bottle aged bouquet of wine. Chem. Ind. 1978, 1, 37. [Google Scholar]
- Black, C.; Francis, L.; Henschke, P.; Capone, D.; Anderson, S.; Day, M.; Holt, H.; Pearson, W.; Herderich, M.; Johnson, D. Aged Riesling and the development of TDN. Wine Vitic. J. 2012, 27, 20–26. [Google Scholar]
- Sacks, G.L.; Gates, M.J.; Ferry, F.X.; Lavin, E.H.; Kurtz, A.J.; Acree, T.E. Sensory threshold of 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN) and concentrations in young Riesling and non-Riesling wines. J. Agric. Food Chem. 2012, 60, 2998–3004. [Google Scholar] [CrossRef]
- Ziegler, M.; Gök, R.; Bechtloff, P.; Winterhalter, P.; Schmarr, H.-G.; Fischer, U. Impact of matrix variables and expertise of panelists on sensory thresholds of 1,1,6-trimethyl-1,2-dihydronaphthalene known as petrol off-flavor compound in Riesling wines. Food Qual. Prefer. 2019, 78, 103735. [Google Scholar] [CrossRef]
- Gök, R.; Bechtloff, P.; Ziegler, M.; Schmarr, H.-G.; Fischer, U.; Winterhalter, P. Synthesis of deuterium-labeled 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN) and quantitative determination of TDN and isomeric vitispiranes in Riesling wines by a stable-isotope-dilution assay. J. Agric. Food Chem. 2019, 67, 6414–6422. [Google Scholar] [CrossRef] [PubMed]
- Deutscher Wein: Statistik 2020/2021. Available online: https://www.deutscheweine.de/fileadmin/user_upload/Statistik_2020.pdf (accessed on 22 June 2022).
- Simpson, R.F.; Miller, G.C. Aroma composition of aged Riesling wine. Vitis 1983, 22, 51–63. [Google Scholar]
- Winterhalter, P.; Sefton, M.A.; Williams, P.J. Volatile C13-norisoprenoid compounds in Riesling wine are generated from multiple precursors. Am. J. Enol. Vitic. 1990, 41, 277–283. [Google Scholar]
- Güldner, A.; Winterhalter, P. Structures of two new ionone glycosides from quince fruit (Cydonia oblonga Mill.). J. Agric. Food Chem. 1991, 39, 2142–2146. [Google Scholar] [CrossRef]
- Winterhalter, P. Bound terpenoids in the juice of the purple passion fruit (Passiflora edulis Sims). J. Agric. Food Chem. 1990, 38, 452–455. [Google Scholar] [CrossRef]
- Humpf, H.-U.; Winterhalter, P.; Schreier, P. 3,4-Dihydroxy-7,8-dihydro-β-ionone-β-D-glucopyranoside: Natural precursor of 2,2,6,8-tetramethyl-7,11-dioxatricyclo [6.2.1.0^1,6] undec-4-ene (Riesling acetal) and 1,1,6-trimethyl-1,2-dihydronaphthalene in red currant (Ribes rubrum L.) leaves. J. Agric. Food Chem. 1991, 39, 1833–1835. [Google Scholar] [CrossRef]
- Stingl, C.; Knapp, H.; Winterhalter, P. 3,4-Dihydroxy-7,8-dihydroβ-ionone-3-O-β-D-glucopyranoside and other glycosidic constituents from apple leaves. Nat. Prod. Lett. 2002, 16, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Roscher, R.; Winterhalter, P. Application of multilayer coil countercurrent chromatography for the study of Vitis vinifera cv. Riesling leaf glycosides. J. Agric. Food Chem. 1993, 41, 1452–1457. [Google Scholar] [CrossRef]
- Winterhalter, P. 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) formation in wine. 1. Studies on the hydrolysis of 2,6,10,10-tetramethyl-1-oxaspiro[4.5]dec-6-ene-2,8-diol rationalizing the origin of TDN and related C13 norisoprenoids in Riesling wine. J. Agric. Food Chem. 1991, 39, 1825–1829. [Google Scholar] [CrossRef]
- Winterhalter, P.; Sefton, M.A.; Williams, P.J. A new C13-norisoprenoid intramolecular acetal in Riesling wine. Chem. Ind. 1990, 14, 463–464. [Google Scholar]
- Daniel, M.A.; Capone, D.L.; Sefton, M.A.; Elsey, G.M. Riesling acetal is a precursor to 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN) in wine. Aust. J. Grape Wine Res. 2009, 15, 93–96. [Google Scholar] [CrossRef]
- Winterhalter, P.; Gök, R. TDN and β-damascenone: Two important carotenoid metabolites in wine. In Carotenoid Cleavage Products; Winterhalter, P., Ebeler, S.E., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2013; Volume 1134, pp. 125–137. ISBN 9780841227781. [Google Scholar]
- Williams, P.J.; Sefton, M.A.; Francis, I.L. Glycosidic precursors of varietal grape and wine flavor. In Flavor Precursors. Thermal and Enzymatic Conversions; Teranishi, R., Takeoka, G.R., Güntert, M., Eds.; ACS Symposium Series 490; American Chemical Society: Washington, DC, USA, 1992; pp. 74–86. ISBN 0-8412-2222-3. [Google Scholar]
- Marais, J. 1,1,6-Trimethyl-l,2-dihydronaphthalene (TDN): A possible degradation product of lutein and beta-carotene. S. Afr. J. Enol. Vitic. 1992, 13, 52–55. [Google Scholar] [CrossRef][Green Version]
- Hixson, J.; Grebneva, Y.; Glameyer, N.; Vollmer, K.; Black, C.; Krstic, M.; Herderich, M. Shedding light on the modulation of key Riesling wine aroma compounds in a changing climate. In Proceedings of the XV Weurmann Flavour Research Symposium; Siegmund, B., Leitner, E., Eds.; Verlag der Technischen Universität Graz: Graz, Austria, 2018; pp. 19–24, ISBN 9783851255935. [Google Scholar]
- Schultz, H.R.; Jones, G.V. Climate induced historic and future changes in viticulture. J. Wine Res. 2010, 21, 137–145. [Google Scholar] [CrossRef]
- Winterhalter, P.; Sefton, M.A.; Williams, P.J. Two-dimensional GC-DCCC analysis of the glycoconjugates of monoterpenes, norisoprenoids, and shikimate-derived metabolites from Riesling wine. J. Agric. Food Chem. 1990, 38, 1041–1048. [Google Scholar] [CrossRef]
- Versini, G.; Rapp, A.; Marais, J.; Mattivi, F.; Spraul, M. A new 1,1,6-trimethyl-l,2-dihydronaphthalene (TDN) precursor isolated from Riesling grape products: Partial structure elucidation and possible reaction mechanism. Vitis 1996, 35, 15–21. [Google Scholar]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef]
- Stahl, E. Dünnschicht-Chromatographie–Ein Laboratoriumshandbuch, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1967. [Google Scholar]
- Achenbach, H.; Waibel, R.; Raffelsberger, B.; Addae-Mensah, I. Iridoid and other constituents of Canthium subcordatum. Phytochemistry 1981, 20, 1591–1595. [Google Scholar] [CrossRef]
- Schievano, E.; D’Ambrosio, M.; Mazzaretto, I.; Ferrarini, R.; Magno, F.; Mammi, S.; Favaro, G. Identification of wine aroma precursors in Moscato Giallo grape juice: A nuclear magnetic resonance and liquid chromatography–mass spectrometry tandem study. Talanta 2013, 116, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Nyemba, A.M.; Ngando Mpondo, T.; Kimbu, S.F.; Connolly, J.D. Stilbene glycosides from Guibourtia tessmannii. Phytochemistry 1995, 39, 895–898. [Google Scholar] [CrossRef]
- Gök, R. Einfluss von Hefen auf die glykosidisch gebundenen Aromavorstufen und Untersuchungen zur Vermeidung der TDN-Fehlnote in Riesling. Ph.D. Thesis, Technische Universität Braunschweig, Braunschweig, Germany, 2015. [Google Scholar]
- Gunata, Z.; Bayonove, C.L.; Baumes, R.L.; Cordonnier, R.E. The aroma of grapes I. Extraction and determination of free and glycosidically bound fractions of some grape aroma components. J. Chromatogr. A 1985, 331, 83–90. [Google Scholar] [CrossRef]
- Winterhalter, P.; Schreier, P. Free and bound C13 norisoprenoids in quince (Cydonia oblonga, Mill.) fruit. J. Agric. Food Chem. 1988, 36, 1251–1256. [Google Scholar] [CrossRef]
- Berthod, A.; Friesen, J.B.; Inui, T.; Pauli, G.F. Elution-extrusion countercurrent chromatography: Theory and concepts in metabolic analysis. Anal. Chem. 2007, 79, 3371–3382. [Google Scholar] [CrossRef]
- McIlvaine, T.C. A buffer solution for colorimetric comparison. J. Biol. Chem. 1921, 49, 183–186. [Google Scholar] [CrossRef]
- Mulisch, M.; Welsch, U. Romeis–Mikroskopische Technik, 19th ed.; Springer Spektrum: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-642-55189-5. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gök, R.; Selhorst, P.; Kiene, M.; Noske, T.; Ziegler, M.; Fischer, U.; Winterhalter, P. Target-Guided Isolation of Progenitors of 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) from Riesling Wine by High-Performance Countercurrent Chromatography. Molecules 2022, 27, 5378. https://doi.org/10.3390/molecules27175378
Gök R, Selhorst P, Kiene M, Noske T, Ziegler M, Fischer U, Winterhalter P. Target-Guided Isolation of Progenitors of 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) from Riesling Wine by High-Performance Countercurrent Chromatography. Molecules. 2022; 27(17):5378. https://doi.org/10.3390/molecules27175378
Chicago/Turabian StyleGök, Recep, Pia Selhorst, Mats Kiene, Theresa Noske, Michael Ziegler, Ulrich Fischer, and Peter Winterhalter. 2022. "Target-Guided Isolation of Progenitors of 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) from Riesling Wine by High-Performance Countercurrent Chromatography" Molecules 27, no. 17: 5378. https://doi.org/10.3390/molecules27175378
APA StyleGök, R., Selhorst, P., Kiene, M., Noske, T., Ziegler, M., Fischer, U., & Winterhalter, P. (2022). Target-Guided Isolation of Progenitors of 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) from Riesling Wine by High-Performance Countercurrent Chromatography. Molecules, 27(17), 5378. https://doi.org/10.3390/molecules27175378