Screening for Astragalus hamosus Triterpenoid Saponins Using HPTLC Methods: Prior Identification of Azukisaponin Isomers
Abstract
:1. Introduction
2. Results and Discussion
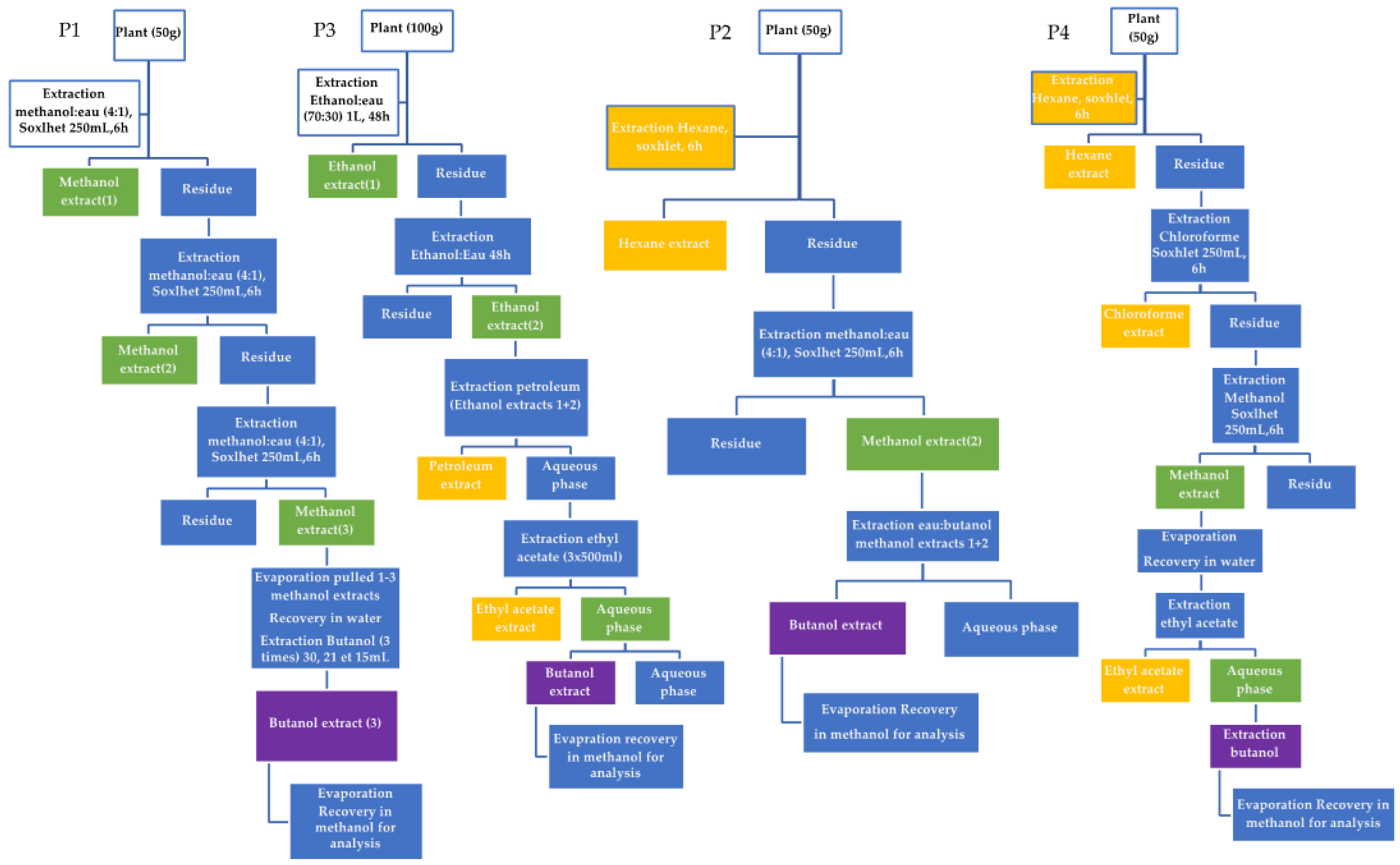
2.1. Extraction of Compounds from Astragalus homosus
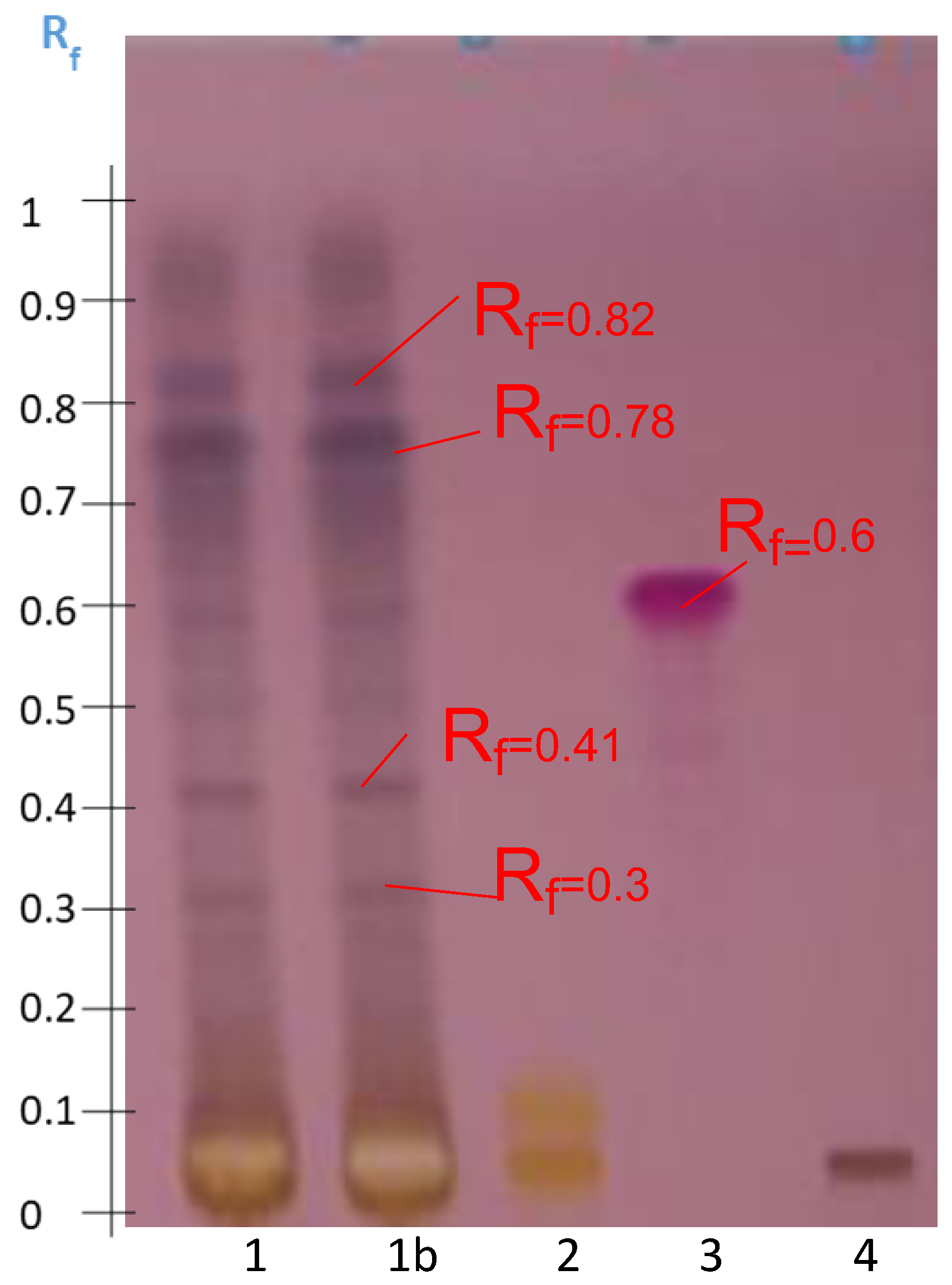
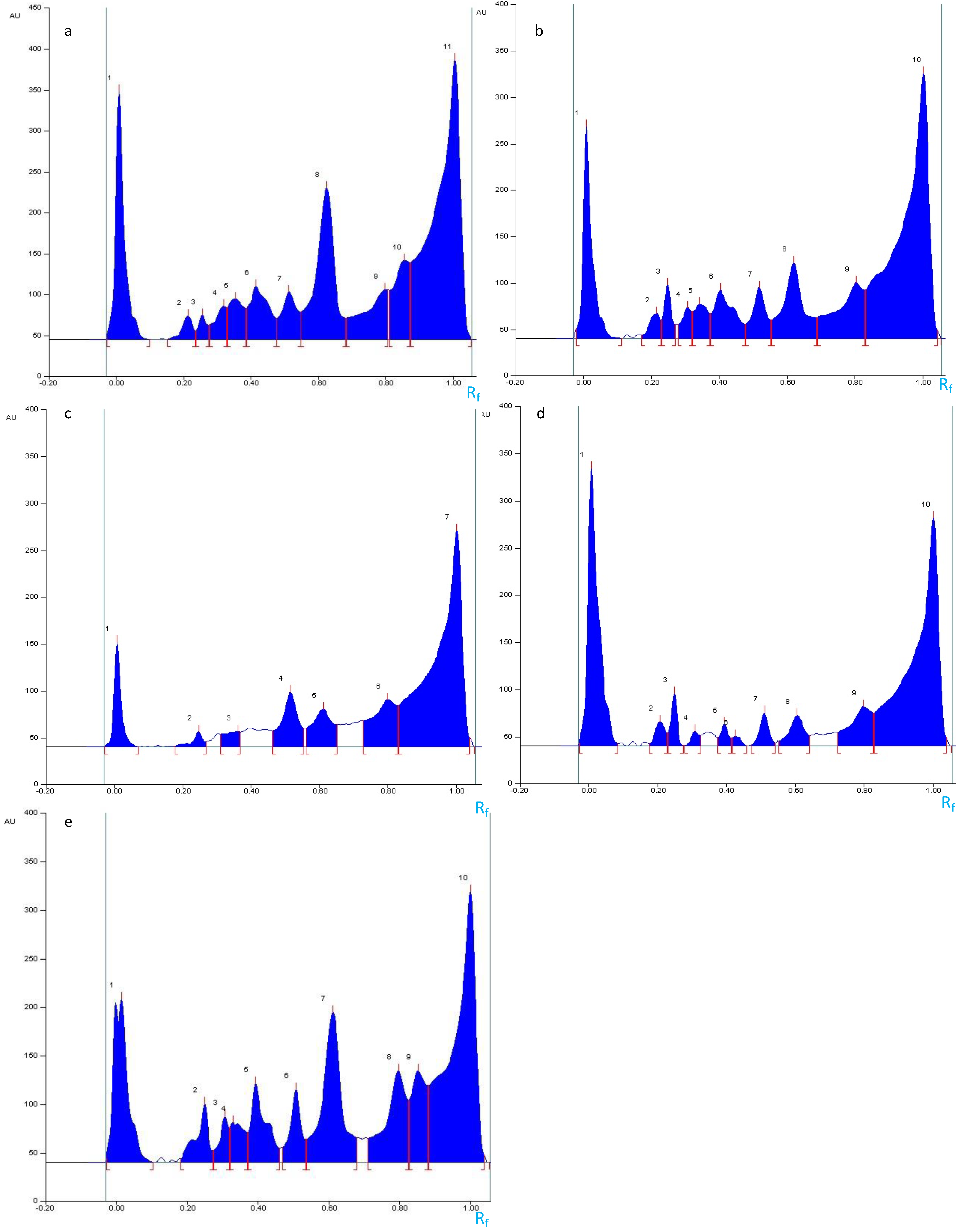
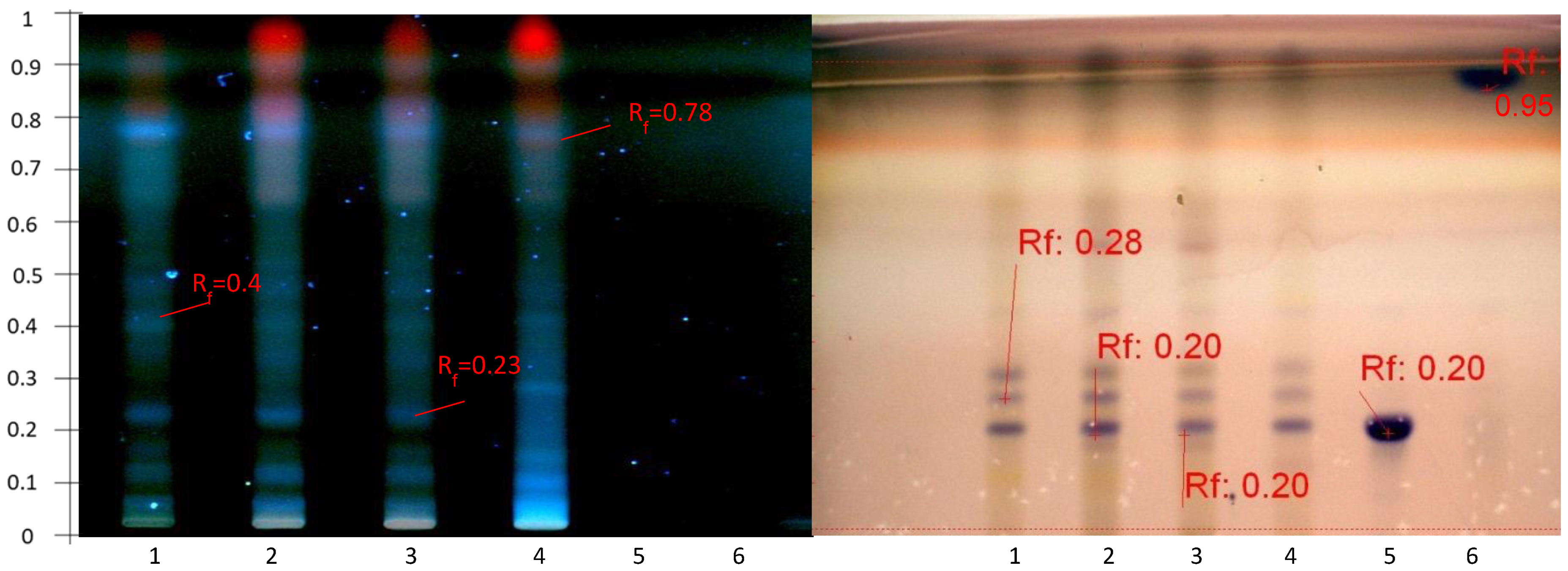
2.2. Preliminary Evaluation of the Abundance of Secondary Metabolites for Screening Triterpenoid Saponins by HPTLC
2.3. Optimization of the Saponin Fraction Purification of Astragalus hamosus
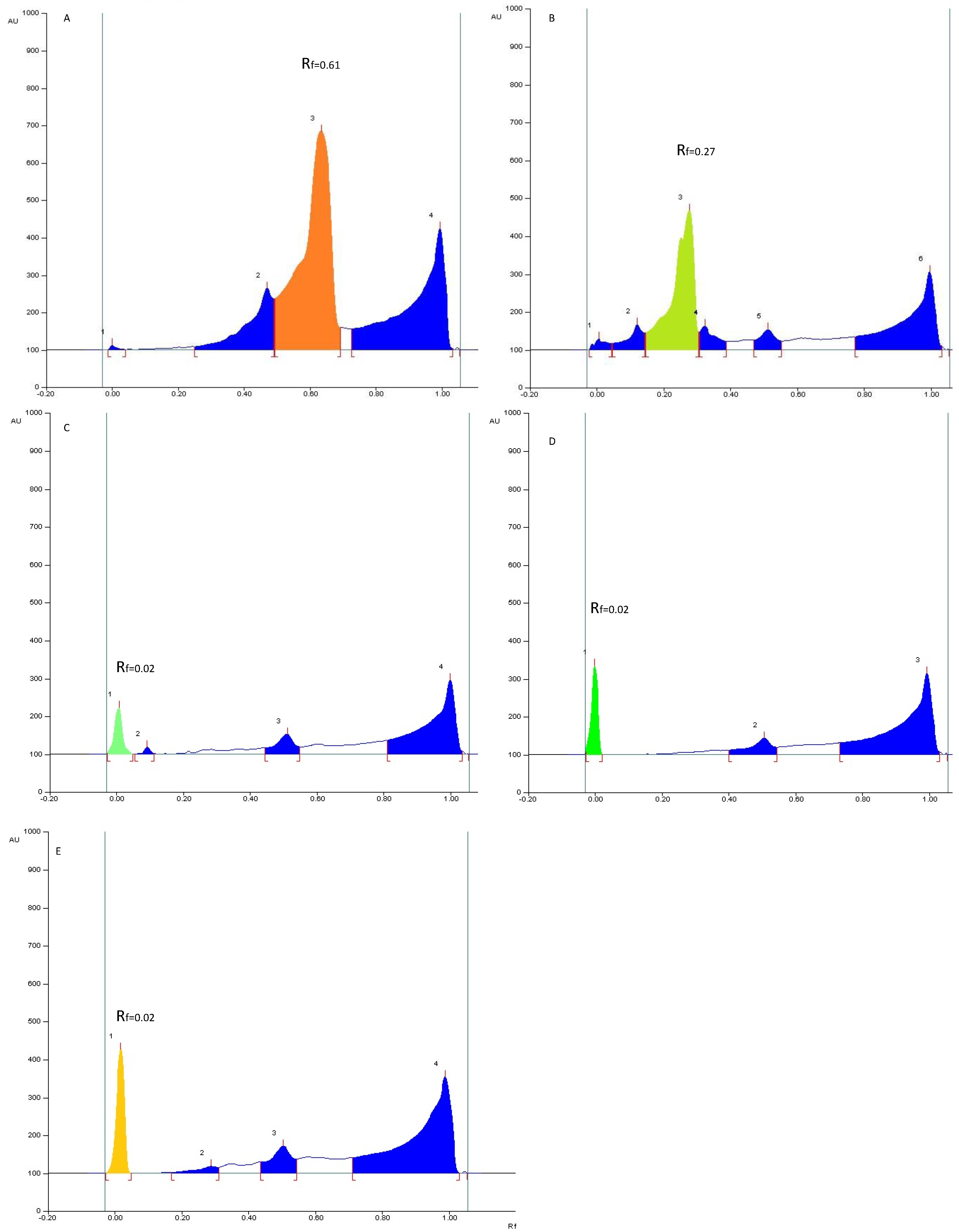
2.4. Optimization of the Elution Systems
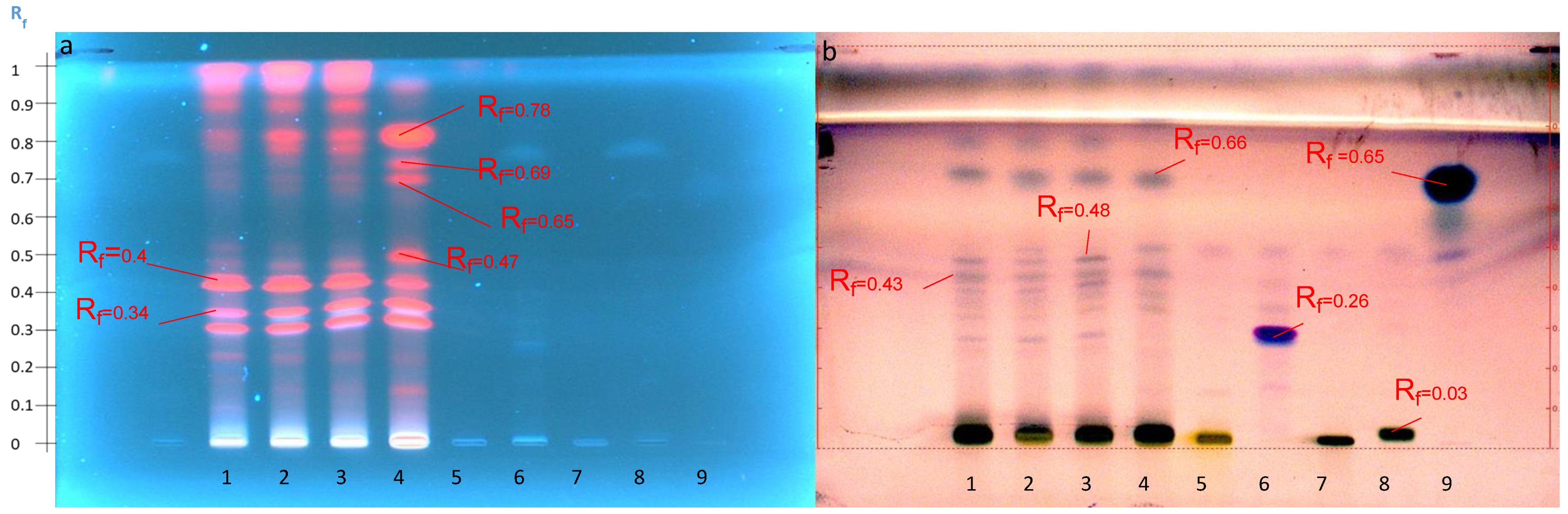
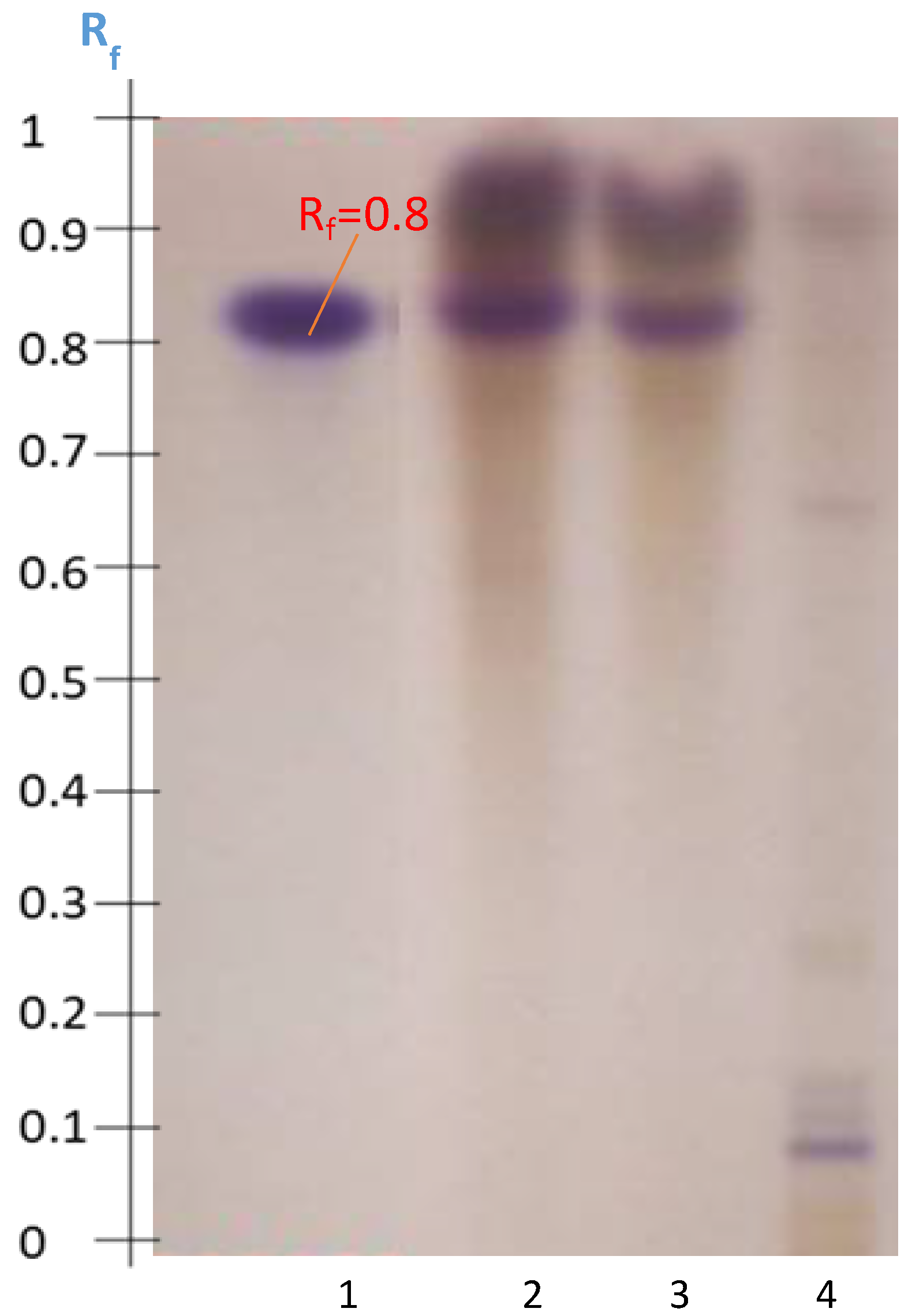
2.4.1. Disclosure of Terpenoids and Preliminary Detection of Soyasaponin Using the Multiple Development System (S2)
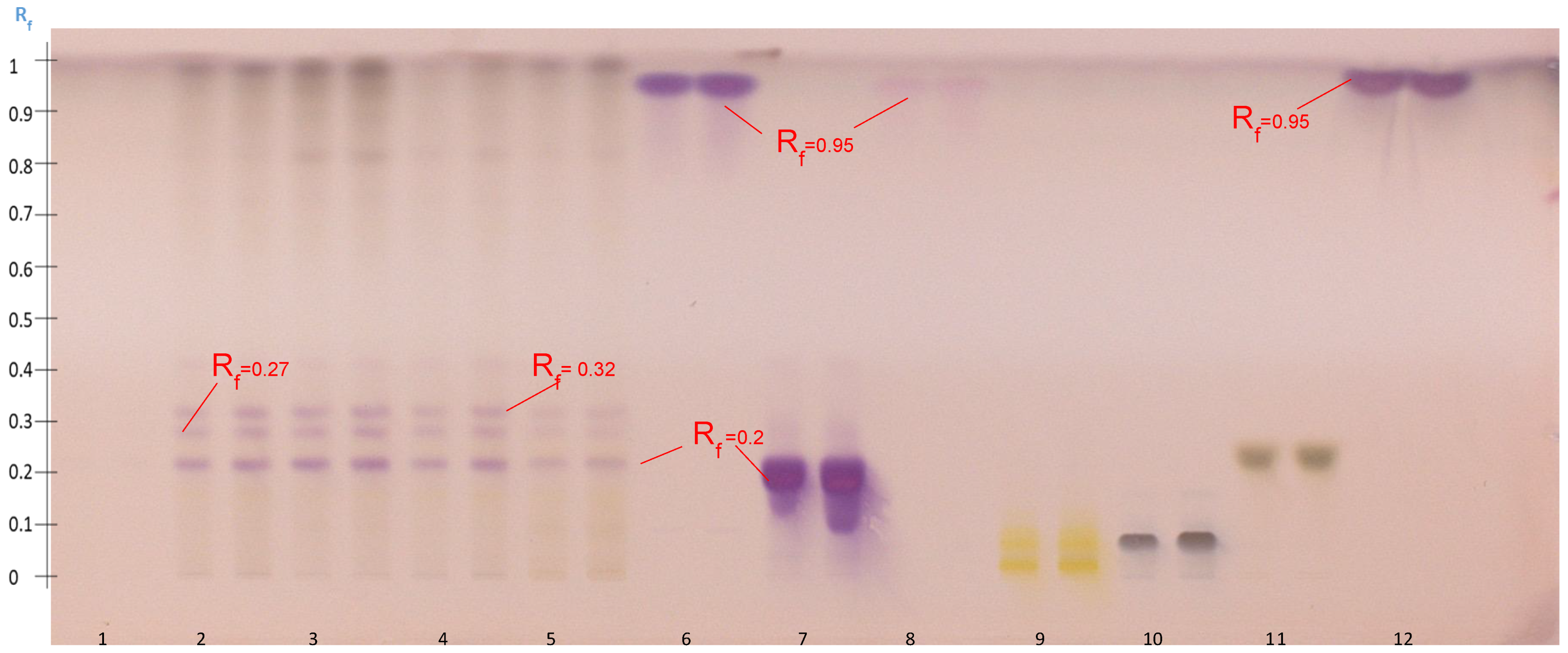
2.4.2. Tentatively Identified Triterpenoid Saponins in the A. hamosus Populations from Bizerte, Siliana and Kairouan
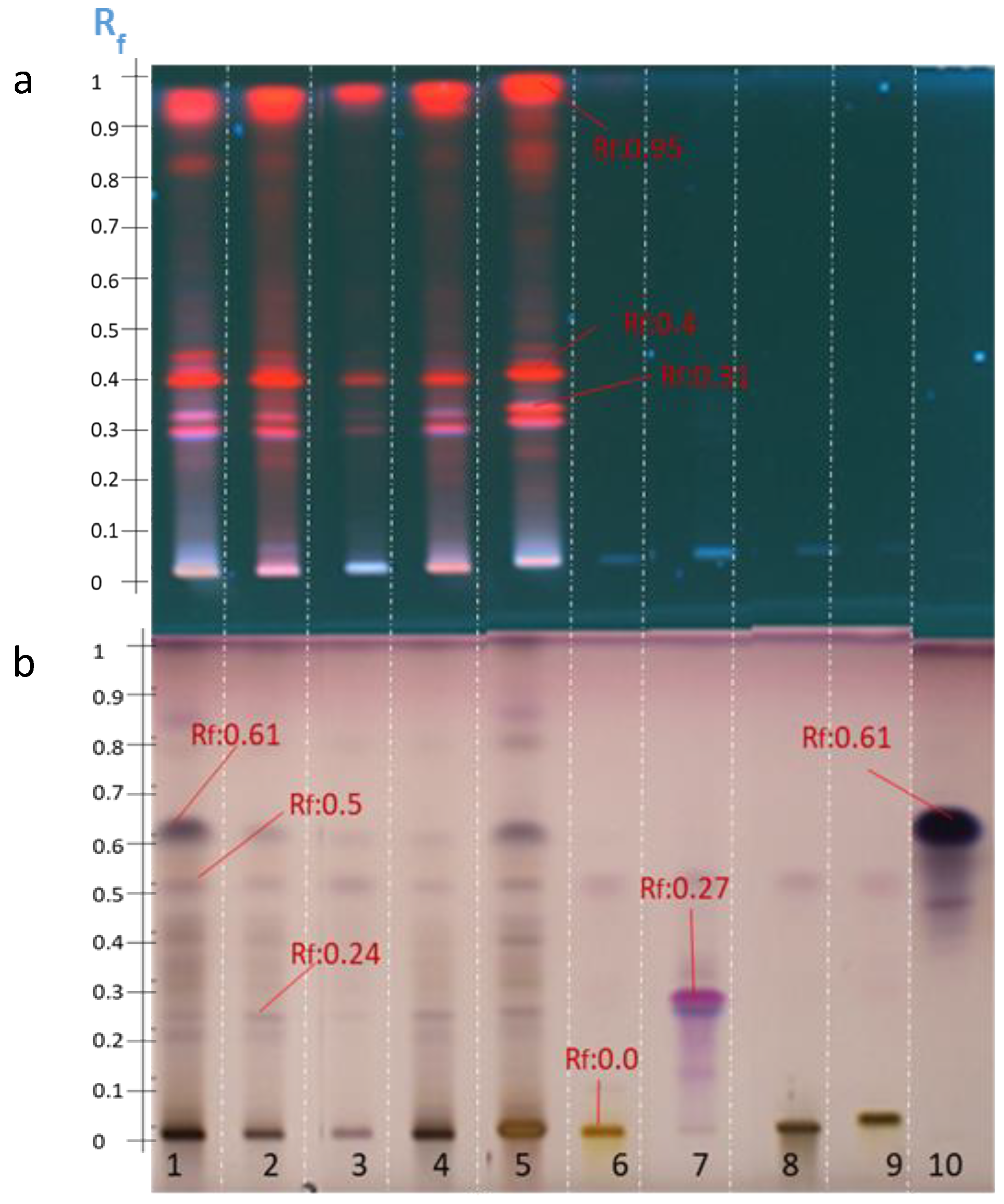
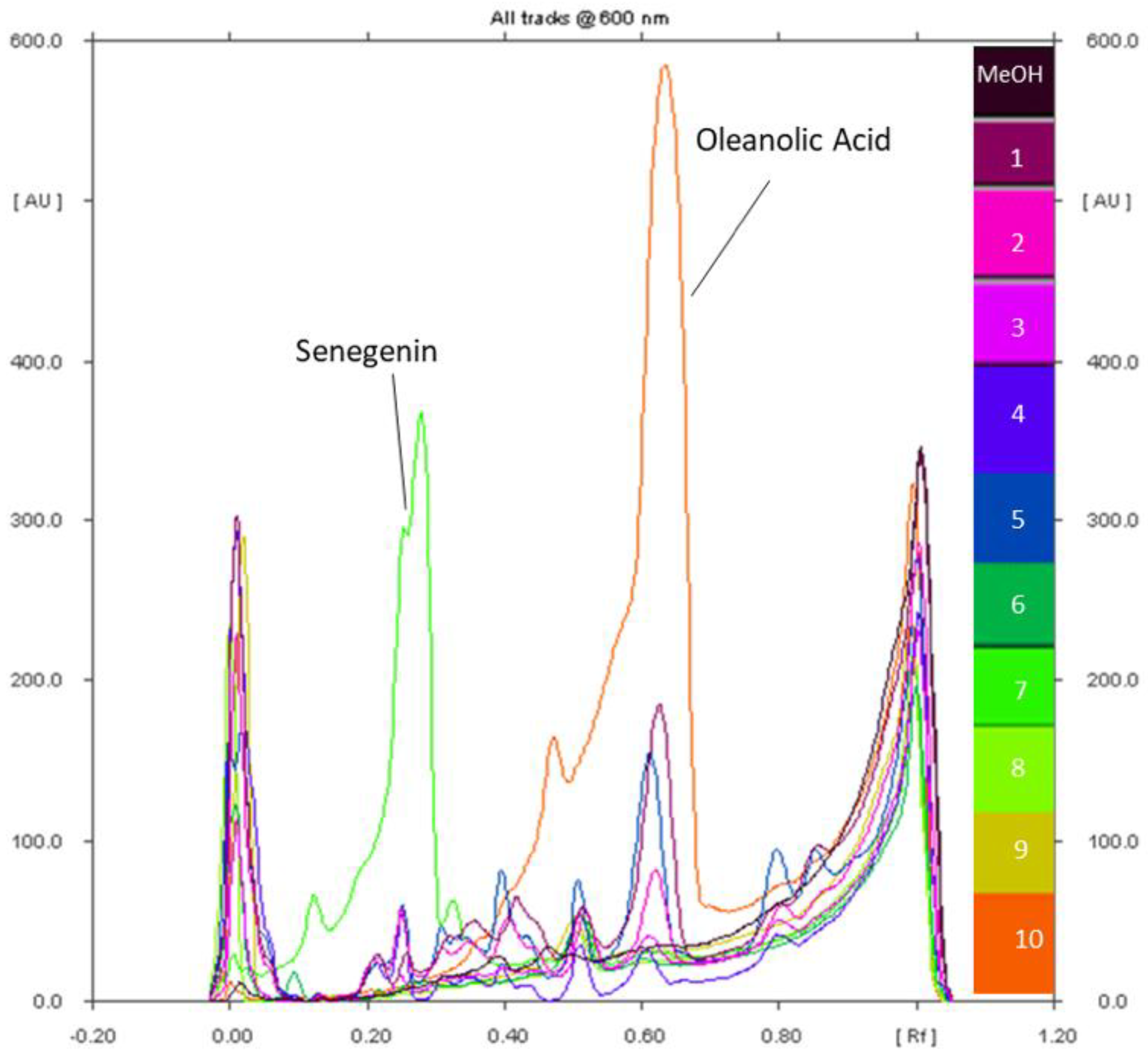
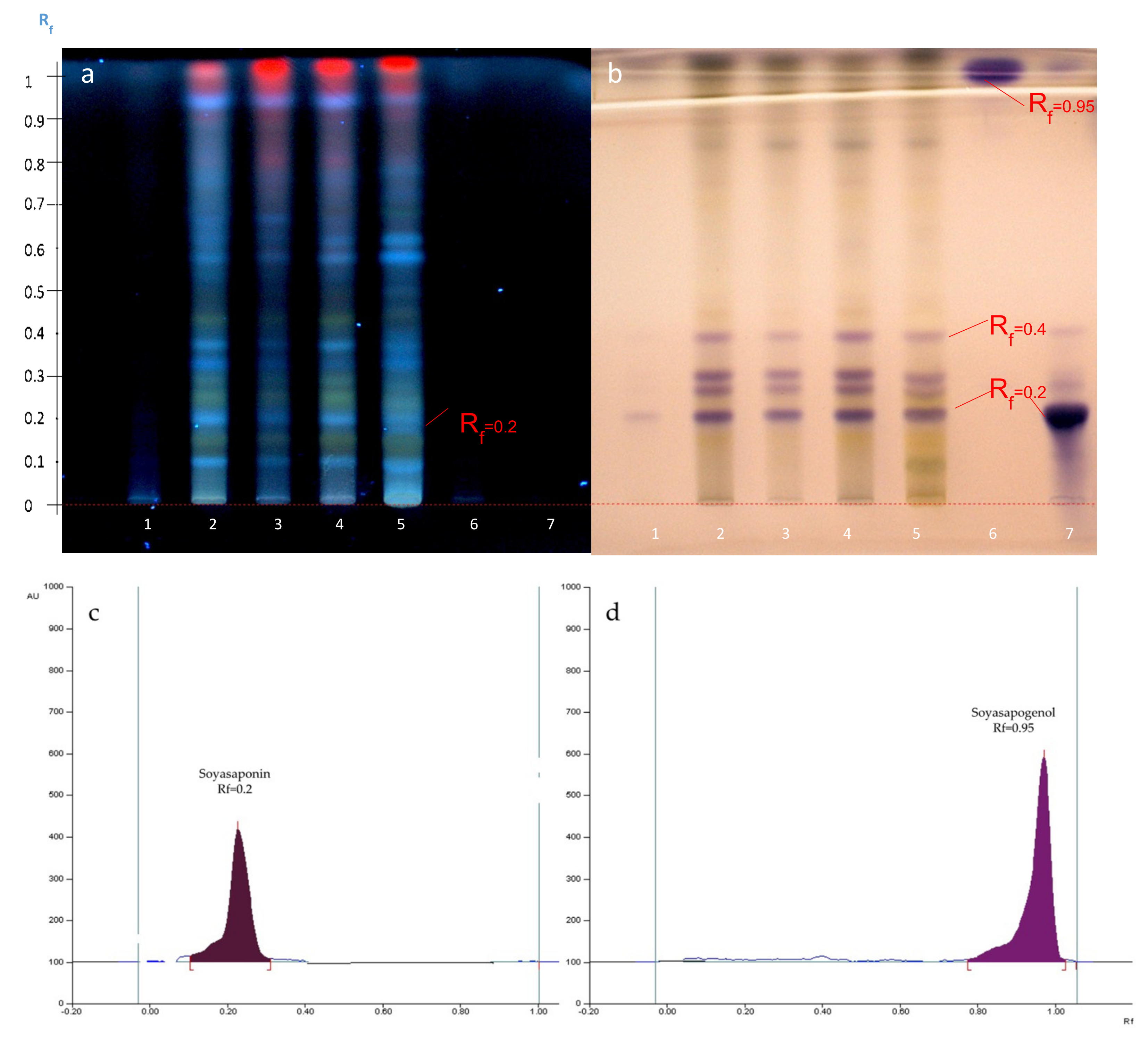
2.5. Hydrolysis of A. hamosus Saponin Extracts
2.6. Quantification of Soyasapogenol
3. Materials and Methods
3.1. Reagent, Chemical and Samples
3.2. Extraction Methods
3.3. High-Performance Thin-Layer Chromatography (HPTLC) Equipment and General Procedure
- −
- SS1: Dichloromethane and MeOH (92: 8) up to 26 mm for methylxanthines;
- −
- SS2: EtOAc, toluene, formic acid, and H2O (8.7: 1.3: 1.7: 0.4) up to 70 mm for phenolic compounds;
- −
- SS3: EtOAc, toluene, formic acid, and H2O (9: 1: 2.5: 1) up to 70 mm for saponins.
3.4. Hydrolysis of Extracts by Acetyl Chloride
3.5. Quantification of Soyasapogenol in Extracts for Densitometry
3.5.1. Quantification of Sayasapogenol by Spectrophotometry
3.5.2. Quantification of Sayasapogenol for Densitometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Al-Snafi, A. Chemical constituents and pharmacological effects of Astragalus hamosus and Astragalus tribuloides grown in iraq. Asian J. Pharm. Sci. Tech. 2015, 5, 321–328. [Google Scholar]
- Zoghlami, A.; Zouaghi, M. Morphological Variation in Astragalus hamosus L. and Coronilla scorpioides L. Populations of Tunisia. Euphytica 2003, 134, 137–147. [Google Scholar] [CrossRef]
- Hakim, A.; Tajuddin; Ghufran, A.; Nasreen, J. Evaluation of Anti-Inflammatory Activity of the Pods of Iklil-Ul-Malik (Astragalus hamosus Linn.). Indian J. Nat. Prod. Ressour. 2010, 1, 34–37. [Google Scholar]
- Shojaii, A.; Motaghinejad, M.; Norouzi, S.; Motevalian, M. Evaluation of Anti-Inflammatory and Analgesic Activity of the Extract and Fractions of Astragalus hamosus in Animal Models. Iran. J. Pharm. Res. 2015, 14, 263–269. [Google Scholar] [PubMed]
- Bahaeddin, Z.; Yans, A.; Khodagholi, F.; Sahranavard, S. Dietary Supplementation with Allium hirtifolium and/or Astragalus hamosus Improved memory and Reduced Neuro-Inflammation in the Rat Model of Alzheimer’s Disease. Appl. Physiol. Nutr. Metab. 2017, 43, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, M.; Ebrahimi -Barough, S.; Kamian, S.; Azami, M.; Mehri, M.; Abdi, M.; Ai, J. Fabrication and Characterization of a Three-Dimensional Fibrin Gel Model to Evaluate Anti-Proliferative Effects of Astragalus hamosus Plant Extract on Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2022, 2, 731–741. [Google Scholar] [CrossRef]
- Shkondrov, A.; Krasteva, I. Liquid Chromatography–High Resolution Mass Spectrometry Screening of Astragalus hamosus and Astragalus corniculatus. Pharmacia 2021, 68, 135–139. [Google Scholar] [CrossRef]
- Hamedi, A.; Zarshenas, M.M.; Sohrabpour, M. Phytochemical Assessments of Astragalus hamosus Pods (Iklil-Ul-Malik). Trends Pharm. Sci. 2016, 2, 77–84. [Google Scholar]
- Ionkova, I.; Shkondrov, A.; Krasteva, I.; Ionkov, T. Recent Progress in Phytochemistry, Pharmacology and Biotechnology of Astragalus Saponins. Phytochem. Rev. 2014, 13, 343–374. [Google Scholar] [CrossRef]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular Activities, Biosynthesis and Evolution of Triterpenoid Saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; van Staden, J. Biological Activities and Distribution of Plant Saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Osbourn, A.; Goss, R.J.M.; Field, R.A. The Saponins: Polar Isoprenoids with Important and Diverse Biological Activities. Nat. Prod. Rep. 2011, 28, 1261–1268. [Google Scholar] [CrossRef]
- Ligor, M.; Kiełbasa, A.; Ratiu, I.-A.; Buszewski, B. Separation and Quantification of Selected Sapogenins Extracted from Nettle, White Dead-Nettle, Common Soapwort and Washnut. Molecules 2021, 26, 7705. [Google Scholar] [CrossRef] [PubMed]
- Güçlü-Üstündağ, Ö.; Mazza, G. Saponins: Properties, Applications and Processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M.; Haq, A.U.; Byrd, J.A.; Berhow, M.A.; Cartwright, A.L.; Bailey, C.A. Haemolytic and Antimicrobial Activities of Saponin-Rich Extracts from Guar Meal. Food Chem. 2010, 119, 600–605. [Google Scholar] [CrossRef]
- Sun, H.; Chen, L.; Wang, J.; Wang, K.; Zhou, J. Structure–Function Relationship of the Saponins from the Roots of Platycodon grandiflorum for Hemolytic and Adjuvant Activity. Int. Immunopharmacol. 2011, 11, 2047–2056. [Google Scholar] [CrossRef]
- Cheng, T.-C.; Lu, J.-F.; Wang, J.-S.; Lin, L.-J.; Kuo, H.-I.; Chen, B.-H. Antiproliferation Effect and Apoptosis Mechanism of Prostate Cancer Cell PC-3 by Flavonoids and Saponins Prepared from Gynostemma pentaphyllum. J. Agric. Food Chem. 2011, 59, 11319–11329. [Google Scholar] [CrossRef]
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical Study and Medical Application of Saponins as Anti-Cancer Agents. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef]
- Waheed, A.; Barker, J.; Barton, S.J.; Owen, C.P.; Ahmed, S.; Carew, M.A. A Novel Steroidal Saponin Glycoside from Fagonia indica Induces Cell-Selective Apoptosis or Necrosis in Cancer Cells. Eur. J. Pharm. Sci. 2012, 47, 464–473. [Google Scholar] [CrossRef]
- Chan, K.W.; Khong, N.M.H.; Iqbal, S.; Ismail, M. Isolation and Antioxidative Properties of Phenolics-Saponins Rich Fraction from Defatted Rice Bran. J. Cereal Sci. 2013, 57, 480–485. [Google Scholar] [CrossRef]
- Dini, I.; Tenore, G.C.; Dini, A. Saponins in Ipomoea Batatas Tubers: Isolation, Characterization, Quantification and Antioxidant Properties. Food Chem. 2009, 113, 411–419. [Google Scholar] [CrossRef]
- Li, J.; Zu, Y.-G.; Fu, Y.-J.; Yang, Y.-C.; Li, S.-M.; Li, Z.-N.; Wink, M. Optimization of Microwave-Assisted Extraction of Triterpene Saponins from Defatted Residue of Yellow Horn (Xanthoceras sorbifolia Bunge.) Kernel and Evaluation of Its Antioxidant Activity. Innov. Food Sci. Emerg. Technol. 2010, 11, 637–643. [Google Scholar] [CrossRef]
- Estrada, A.; Katselis, G.S.; Laarveld, B.; Barl, B. Isolation and Evaluation of Immunological Adjuvant Activities of Saponins from Polygala senega L. Comp. Immunol. Microbiol. Infect. Dis. 2000, 23, 27–43. [Google Scholar] [CrossRef]
- Krasteva, I.; Momekov, G.; Zdraveva, P.; Konstantinov, S.; Nikolov, S. Antiproliferative Effects of a Flavonoid and Saponins from Astragalus hamosus against Human Tumor Cell Lines. Pharmacogn. Mag. 2008, 4, 269–272. [Google Scholar]
- Dineva, I.; Krasteva, I.; Berger, M.; Konstantinov, S. In Vitro Antineoplastic Activity of Some Cytoreductive Drugs versus New Compounds of Plant Origin. Int. J. Curr. Chem. 2010, 1, 281–290. [Google Scholar]
- Verotta, L.; Guerrini, M.; Elsebakhy, N.; Assad, A.; Toaima, S.; Radwan, M.; Luo, Y.-D.; Pezzuto, J. Cycloartane and Oleanane Saponins from Egyptian Astragalus spp. as Modulators of Lymphocyte Proliferation. Planta Med. 2002, 68, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and Quantification of Saponins: A Review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Majinda, R.R.T. Extraction and Isolation of Saponins. In Natural Products Isolation; Sarker, S.D., Nahar, L., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 415–426. [Google Scholar] [CrossRef]
- Oleszek, W.; Bialy, Z. Chromatographic Determination of Plant Saponins—An Update (2002–2005). J. Chromatogr. A 2006, 1112, 78–91. [Google Scholar] [CrossRef]
- Huhman, D.V.; Berhow, M.A.; Sumner, L.W. Quantification of Saponins in Aerial and Subterranean Tissues of Medicago truncatula. J. Agric. Food Chem. 2005, 53, 1914–1920. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H.; Wu, H.; Deng, R.; Sun, M. Deciphering the Metabolic Profile and Pharmacological Mechanisms of Achyranthes bidentata Blume Saponins Using Ultra-Performance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry Coupled with Network Pharmacology-Based Investigation. J. Ethnopharmacol. 2021, 274, 114067. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Yang, W.; Zhang, Z. Characterization of Astragaloside I-IV Based on the Separation of HPTLC from Pleurotus ostreatus Cultivated with Astragalus. J. Food Sci. 2020, 85, 3183–3190. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, Y.; Tao, L.; Zhang, X.; Hao, F.; Zhao, S.; Han, L.; Bai, C. Recent Advances in Separation and Analysis of Saponins in Natural Products. Separation 2022, 9, 163. [Google Scholar] [CrossRef]
- Shawky, E.; Sallam, S.M. Simultaneous Determination of Soyasaponins and Isoflavones in Soy (Glycine max L.) Products by HPTLC-Densitometry-Multiple Detection. J. Chromatogr. Sci. 2017, 55, 1059–1065. [Google Scholar] [CrossRef]
- Gaceb-Terrak, R.; Rahmania, F. Détection et Identification de Saponines Stéroïdes de Type Spirostane Chez Le Palmier Dattier Phœnix Dactylifera L. (Arecaceae). Acta Bot. Gall. 2012, 159, 477–483. [Google Scholar] [CrossRef]
- Mad, T.; Sterk, H.; Mittelbach, M.; Rechberger, G.N. Tandem Mass Spectrometric Analysis of a Complex Triterpene Saponin Mixture of Chenopodium quinoa. J. Am. Soc. Mass Spectrom. 2006, 17, 795–806. [Google Scholar] [CrossRef]
- Le, A.V.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Effect of Solvents and Extraction Methods on Recovery of Bioactive Compounds from Defatted Gac (Momordica cochinchinensis Spreng.) Seeds. Separations 2018, 5, 39. [Google Scholar] [CrossRef]
- da Silva, M.A.; Calabrese, V.; Schmitt, J.; Celebi, D.; Scott, J.L.; Edler, K.J. Alcohol Induced Gelation of TEMPO-Oxidized Cellulose Nanofibril Dispersions. Soft Matter 2018, 14, 9243–9249. [Google Scholar] [CrossRef]
- Filipe-Ribeiro, L.; Milheiro, J.; Matos, C.C.; Cosme, F.; Nunes, F.M. Data on Changes in Red Wine Phenolic Compounds, Headspace Aroma Compounds and Sensory Profile after Treatment of Red Wines with Activated Carbons with Different Physicochemical Characteristics. Data Brief 2017, 12, 188–202. [Google Scholar] [CrossRef]
- Masomian, M.; Rahman, R.; Salleh, A. A Novel Method of Affinity Tag Cleavage in the Purification of a Recombinant Thermostable Lipase from Aneurinibacillus Thermoaerophilus Strain HZ. Catalysts 2018, 8, 479. [Google Scholar] [CrossRef]
- Salleh, A.; Basri, M.; Tan, S.; Rahman, M.; Dzulkefly, K.; Rahman, R.; Razak, C. Synthesis of Fatty Alkanolamides By Using Immobilized Lipases. Malays. J. Anal. Sci. 2001, 7, 281–285. [Google Scholar]
- Munier, R.L.; Drapier, A.M. Nouvelles méthodes de séparation et comportement des alcaloides en chromatographie en milieux tres polaires, neutres ou acides, sur gel de silice non modifié (1ère partie). Chromatographia 1977, 10, 226–232. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Vu-Huynh, K.L.; Nguyen, H.M.; Le, H.T.; Van Le, T.H.; Park, J.H.; Nguyen, M.D. Evaluation of the Saponin Content in Panax vietnamensis Acclimatized to Lam Dong Province by HPLC–UV/CAD. Molecules 2021, 26, 5373. [Google Scholar] [CrossRef] [PubMed]
- Vu-Huynh, K.L.; Nguyen, H.T.; Van Le, T.H.; Ma, C.T.; Lee, G.J.; Kwon, S.W.; Park, J.H.; Nguyen, M.D. Accumulation of Saponins in Underground Parts of Panax vietnamensis at Different Ages Analyzed by HPLC-UV/ELSD. Molecules 2020, 25, 3086. [Google Scholar] [CrossRef]
- Oleszek, W.A. Chromatographic Determination of Plant Saponins. J. Chromatogr. A 2002, 967, 147–162. [Google Scholar] [CrossRef]
- Chua, L.S.; Lau, C.H.; Chew, C.Y.; Dawood, D.a.S. Solvent Fractionation and Acetone Precipitation for Crude Saponins from Eurycoma longifolia Extract. Molecules 2019, 24, E1416. [Google Scholar] [CrossRef]
- Jiménez, G.G.; Durán, A.G.; Macías, F.A.; Simonet, A.M. Structure, Bioactivity and Analytical Methods for the Determination of Yucca Saponins. Molecules 2021, 26, 5251. [Google Scholar] [CrossRef]
- Andriamasinoro, N.A. Purification et Caractérisation Des Métabolites Secondaires Extraits de Plantes de La Famille Des Asparagaceae et Caprifoliaceae, et Évaluation de Leurs Activités Biologiques. Ph.D. Thesis, Université Bourgogne Franche-Comté, Besançon, France, 2018. [Google Scholar]
- Gupta, P.; Patil, D.; Patil, A. Qualitative HPTLC Phytochemical Profiling of Careya Arborea Roxb. Bark, Leaves and Seeds. 3 Biotech 2019, 9, 311. [Google Scholar] [CrossRef]
- Podolak, I.; Hubicka, U.; Żuromska-Witek, B.; Janeczko, Z.; Krzek, J. Quantification of Saponins in Different Plant Parts of Lysimachia L. Species by Validated HPTLC-Densitometric Method. JPC-J. Planar Chromatogr.-Mod. TLC 2013, 26, 248–253. [Google Scholar] [CrossRef]
- Khakimov, B.; Tseng, L.; Godejohann, M.; Bak, S.; Engelsen, S. Screening for Triterpenoid Saponins in Plants Using Hyphenated Analytical Platforms. Molecules 2016, 21, 1614. [Google Scholar] [CrossRef]
- Ma, X.Q.; Shi, Q.; Duan, J.A.; Dong, T.T.X.; Tsim, K.W.K. Chemical Analysis of Radix Astragali (Huangqi) in China: A Comparison with Its Adulterants and Seasonal Variations. J. Agric. Food Chem. 2002, 50, 4861–4866. [Google Scholar] [CrossRef]
- Kambouche, N.; Merah, B.; Derdour, A.; Bellahouel, S.; Younos, C.; Soulimani, R. Activité antihyperglycémiante d’un stérol β-sitoglucoside isolé de la plante Anabasis articulata (Forssk) Moq. Phytothérapie 2011, 9, 2–6. [Google Scholar] [CrossRef]
- Maamria, L.; Long, C.; Haba, H.; Lavaud, C.; Cannac, A.; Benkhaled, M. Cycloartane Glycosides from Astragalus gombo. Phytochem. Lett. 2015, 11, 286–291. [Google Scholar] [CrossRef]
- Pistelli, L.; Giachi, I.; Lepori, E.; Bertoli, A. Further Saponins and Flavonoids from Astragalus verrucosus Moris. Pharm. Biol. 2003, 41, 568–572. [Google Scholar] [CrossRef]
- Giacinti, G.; Raynaud, C.; Capblancq, S.; Simon, V. Evaluation and Prevention of the Negative Matrix Effect of Terpenoids on Pesticides in Apples Quantification by Gas Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2017, 1483, 8–19. [Google Scholar] [CrossRef]
- Kaltbach, P.; Ballert, S.; Kabrodt, K.; Schellenberg, I. New HPTLC Methods for Analysis of Major Bioactive Compounds in Mate (Ilex paraguariensis) Tea. J. Food Compos. Anal. 2020, 92, 103568. [Google Scholar] [CrossRef]
- Ncube, B.; Ngunge, V.N.P.; Finnie, J.F.; Van Staden, J. A Comparative Study of the Antimicrobial and Phytochemical Properties between Outdoor Grown and Micropropagated Tulbaghia violacea Harv. Plants. J. Ethnopharmacol. 2011, 134, 775–780. [Google Scholar] [CrossRef]

| Extraction Protocol | Population of A. hamosus | Extraction Yield (DW/DW%) |
|---|---|---|
| M1 | Zaghouan Siliana Bizerte Kairouan | 11.5 ± 0.7 7.2 9.3 9.4 |
| P1 | Zaghouan Siliana Bizerte Kairouan | 6.3 8.1 8.0 6.3 |
| P2 | Zaghouan | 4.5 |
| P3 | Zaghouan | 4.8 |
| P4 | Zaghouan | 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nafti, K.; Giacinti, G.; Marghali, S.; Raynaud, C.D. Screening for Astragalus hamosus Triterpenoid Saponins Using HPTLC Methods: Prior Identification of Azukisaponin Isomers. Molecules 2022, 27, 5376. https://doi.org/10.3390/molecules27175376
Nafti K, Giacinti G, Marghali S, Raynaud CD. Screening for Astragalus hamosus Triterpenoid Saponins Using HPTLC Methods: Prior Identification of Azukisaponin Isomers. Molecules. 2022; 27(17):5376. https://doi.org/10.3390/molecules27175376
Chicago/Turabian StyleNafti, Khouloud, Geraldine Giacinti, Sonia Marghali, and Christine Delgado Raynaud. 2022. "Screening for Astragalus hamosus Triterpenoid Saponins Using HPTLC Methods: Prior Identification of Azukisaponin Isomers" Molecules 27, no. 17: 5376. https://doi.org/10.3390/molecules27175376
APA StyleNafti, K., Giacinti, G., Marghali, S., & Raynaud, C. D. (2022). Screening for Astragalus hamosus Triterpenoid Saponins Using HPTLC Methods: Prior Identification of Azukisaponin Isomers. Molecules, 27(17), 5376. https://doi.org/10.3390/molecules27175376






