Gypensapogenin I Ameliorates Isoproterenol (ISO)-Induced Myocardial Damage through Regulating the TLR4/NF-κB/NLRP3 Pathway
Abstract
:1. Introduction
2. Results
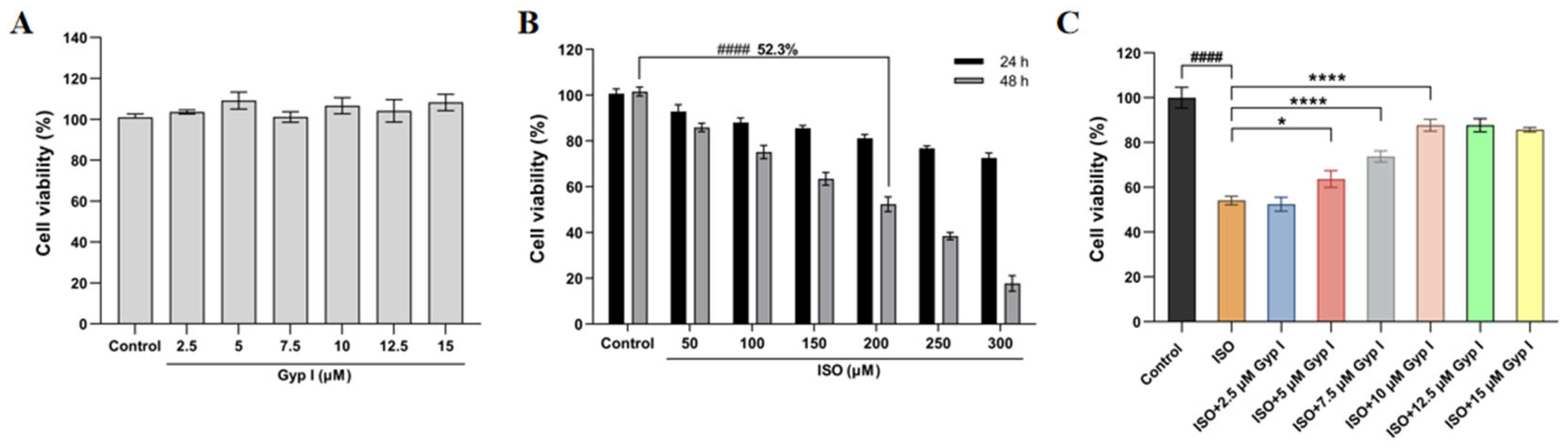
2.1. Gyp I Improves the ISO-Attenuated H9c2 Cell Viability
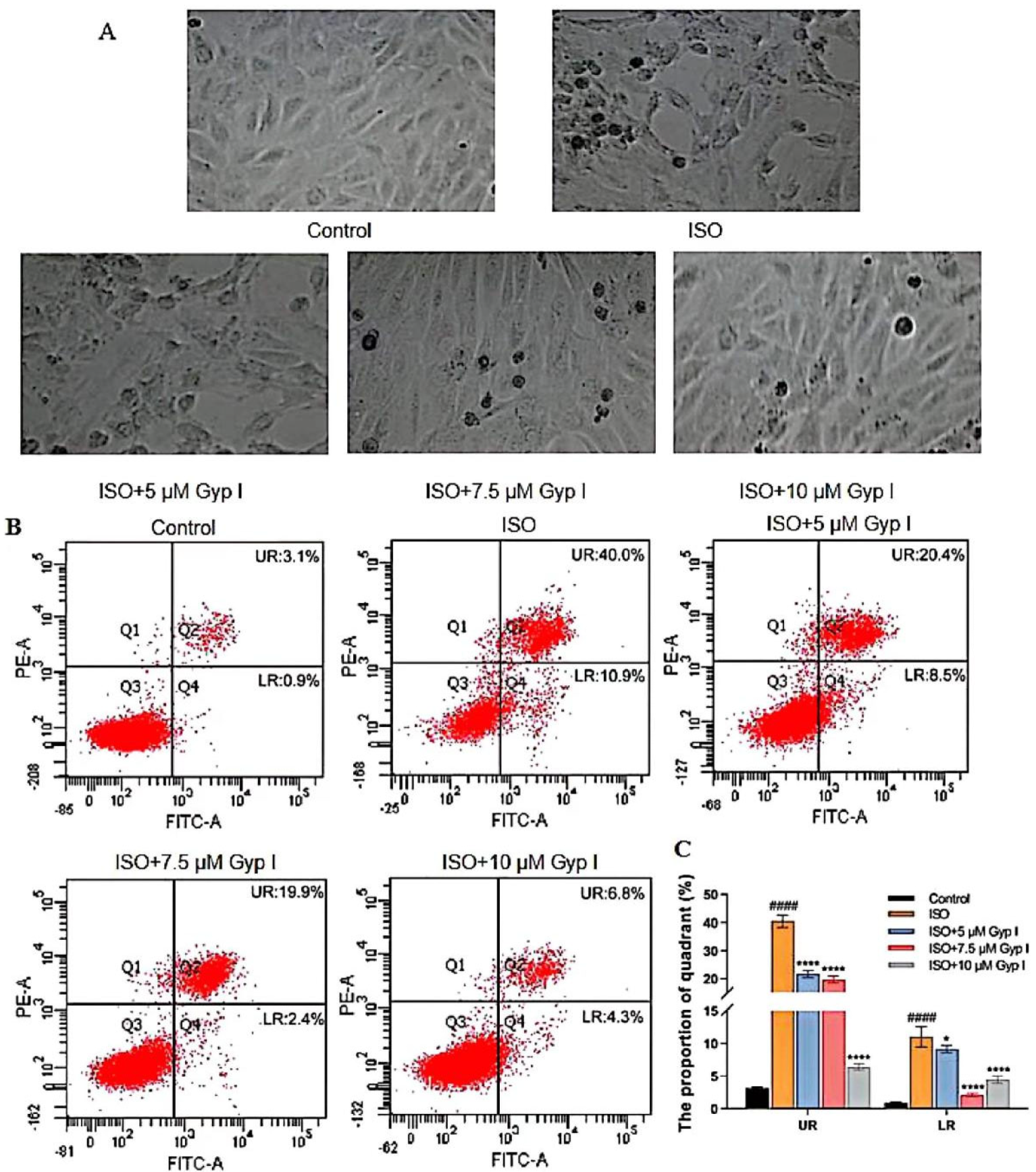
2.2. Gyp I Inhibits ISO-Induced Apoptosis of H9c2 Cardiomyocytes
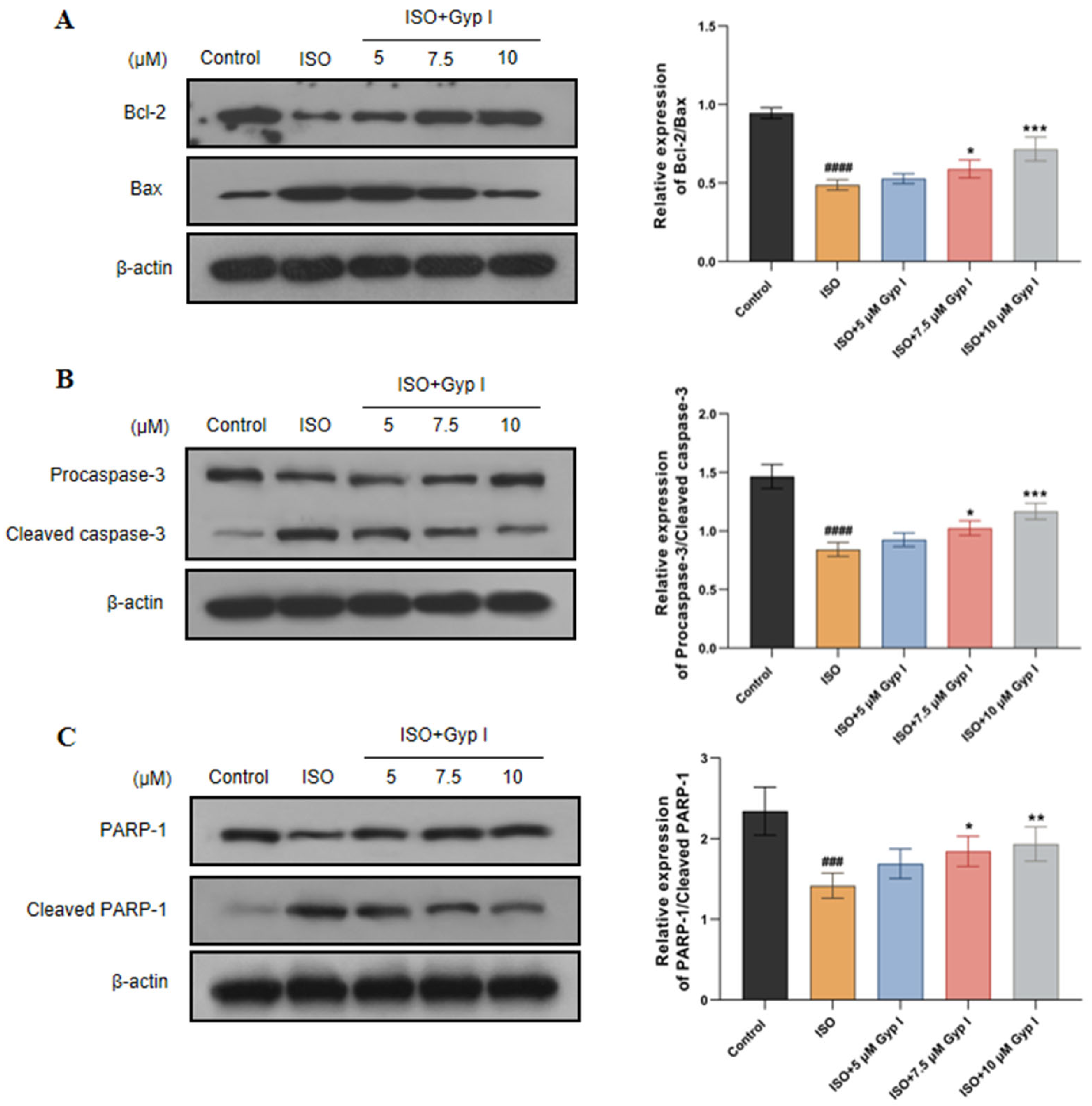
2.3. Gyp I Alters Expression of Apoptosis-Related Proteins in ISO-Treated H9c2 Cells
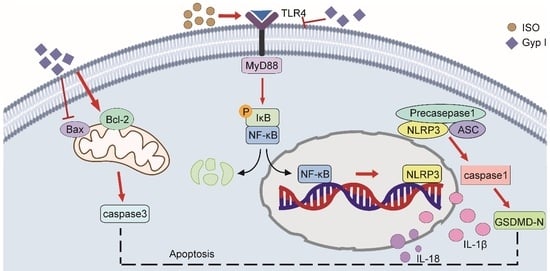
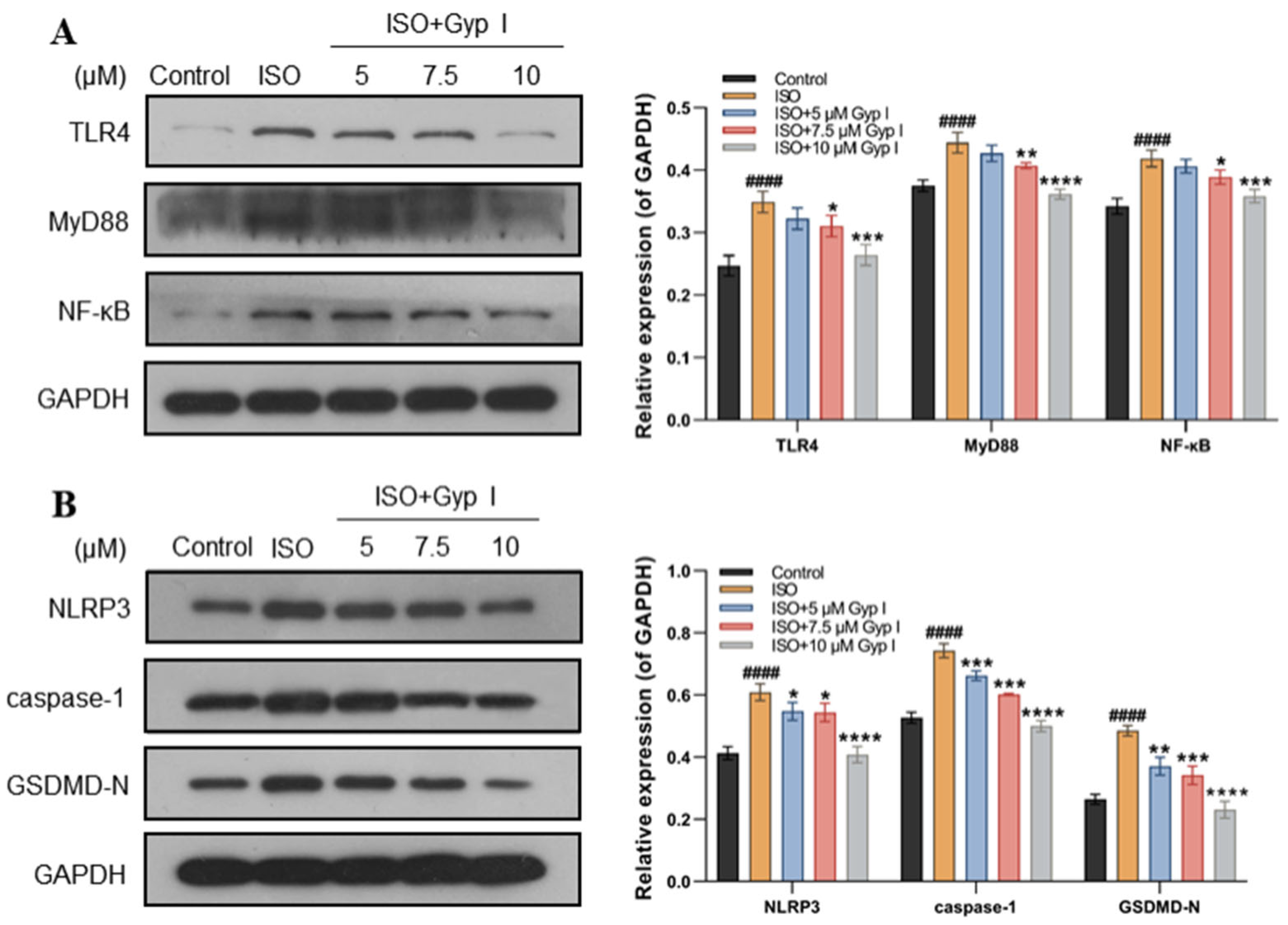
2.4. Gyp I Blocks Activation of the ISO-Induced TLR4/NF-κB/NLRP3 Signaling Pathway
2.5. Effects of Gyp I on Cardiac Morphology and Weight in ISO-Treated Mice
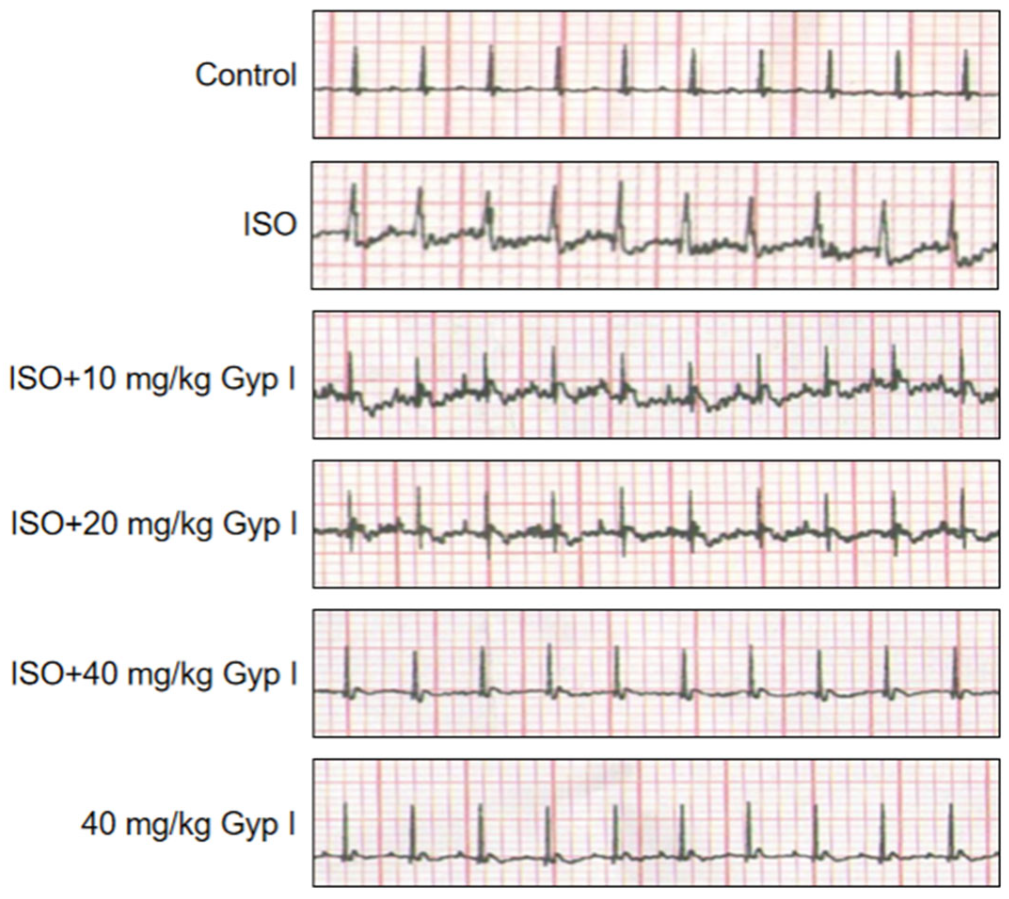
2.6. Electrocardiogram (ECG) Analysis of Various Treatment Group Mice
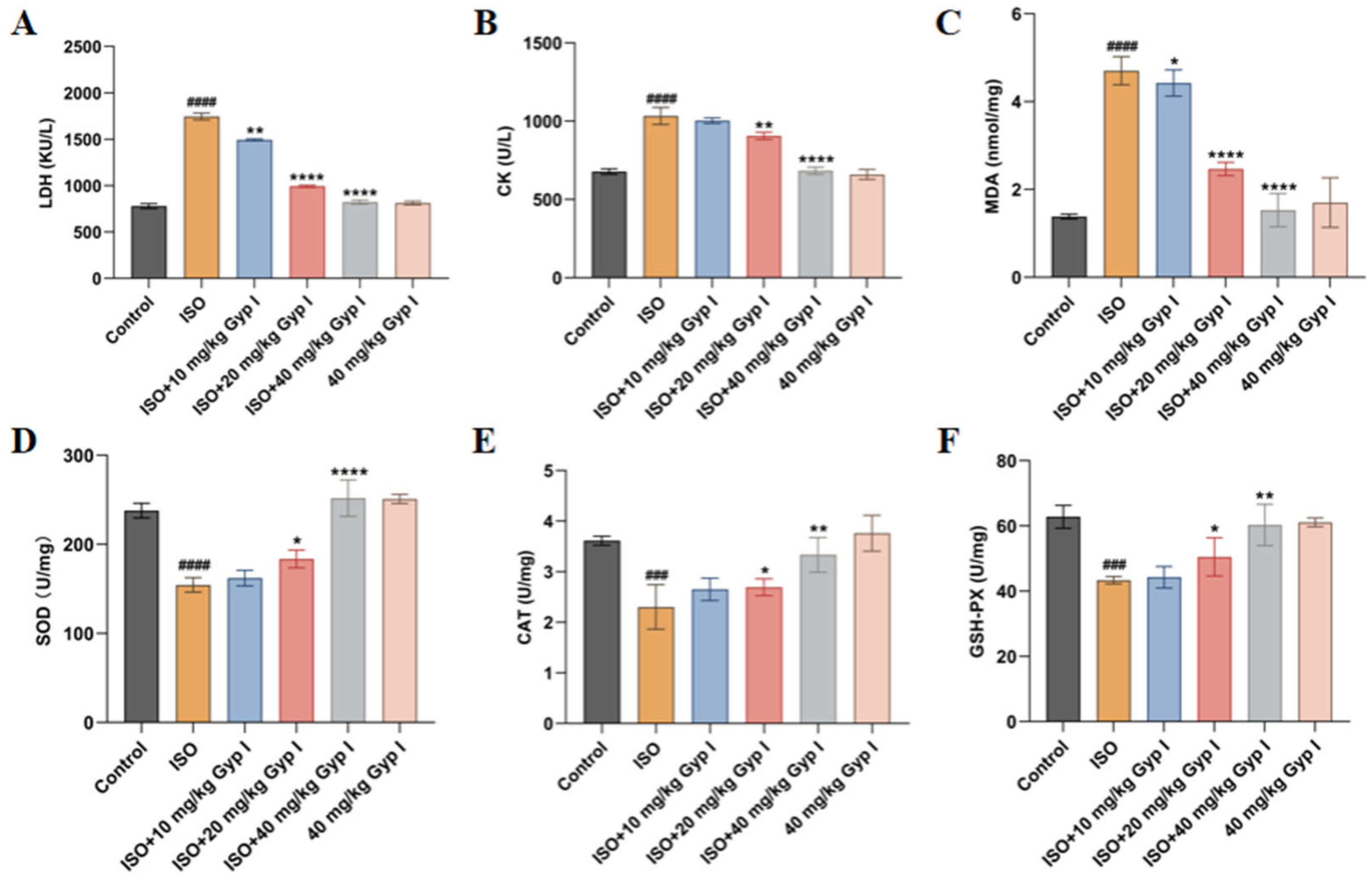
2.7. Effect of Gyp I on ISO-Induced Oxidative Stress in Mice Hearts
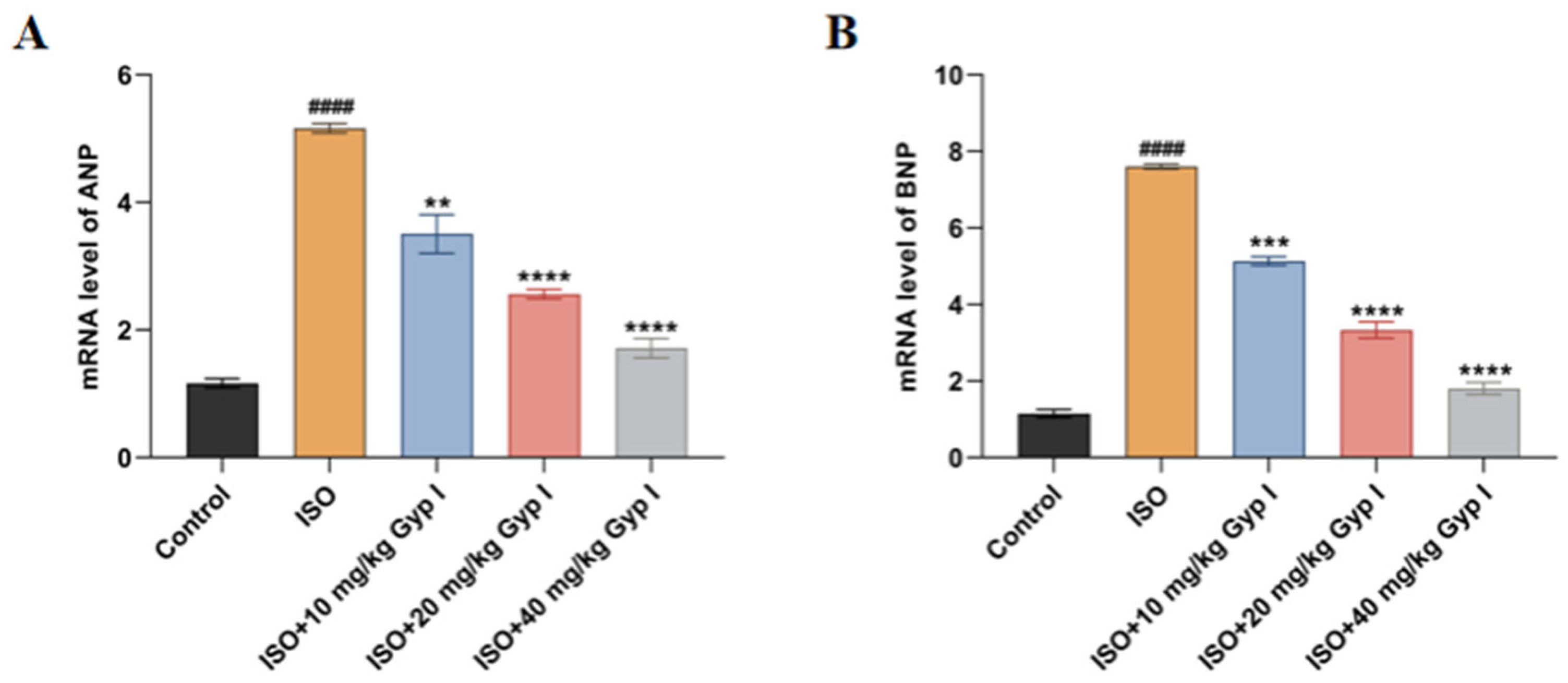
2.8. Effect of Gyp I on the mRNA Expression of ISO-Induced Myocardial Hypertrophy Genes in Mice
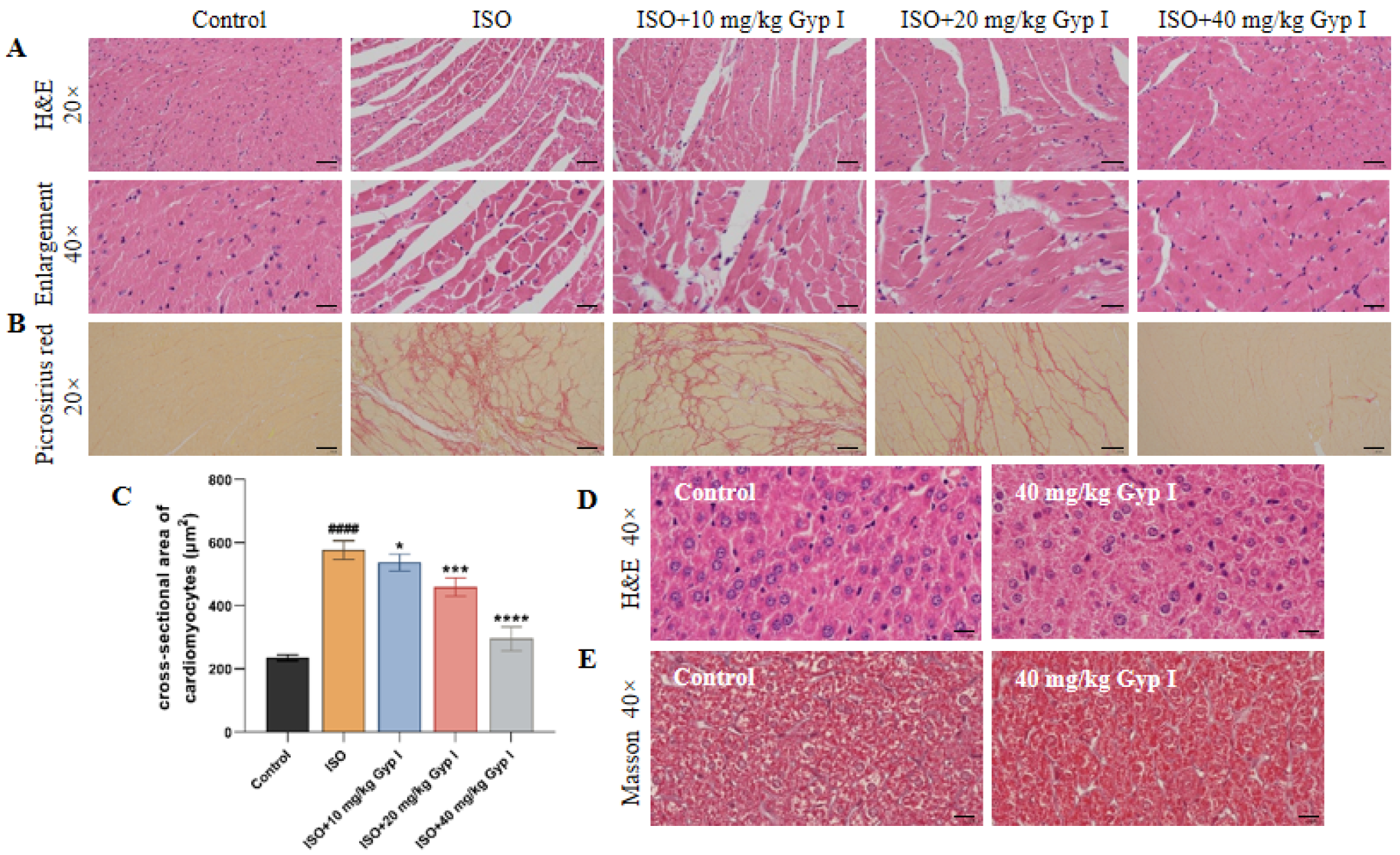
2.9. Gyp I Interferes with ISO-Induced MF in Mice
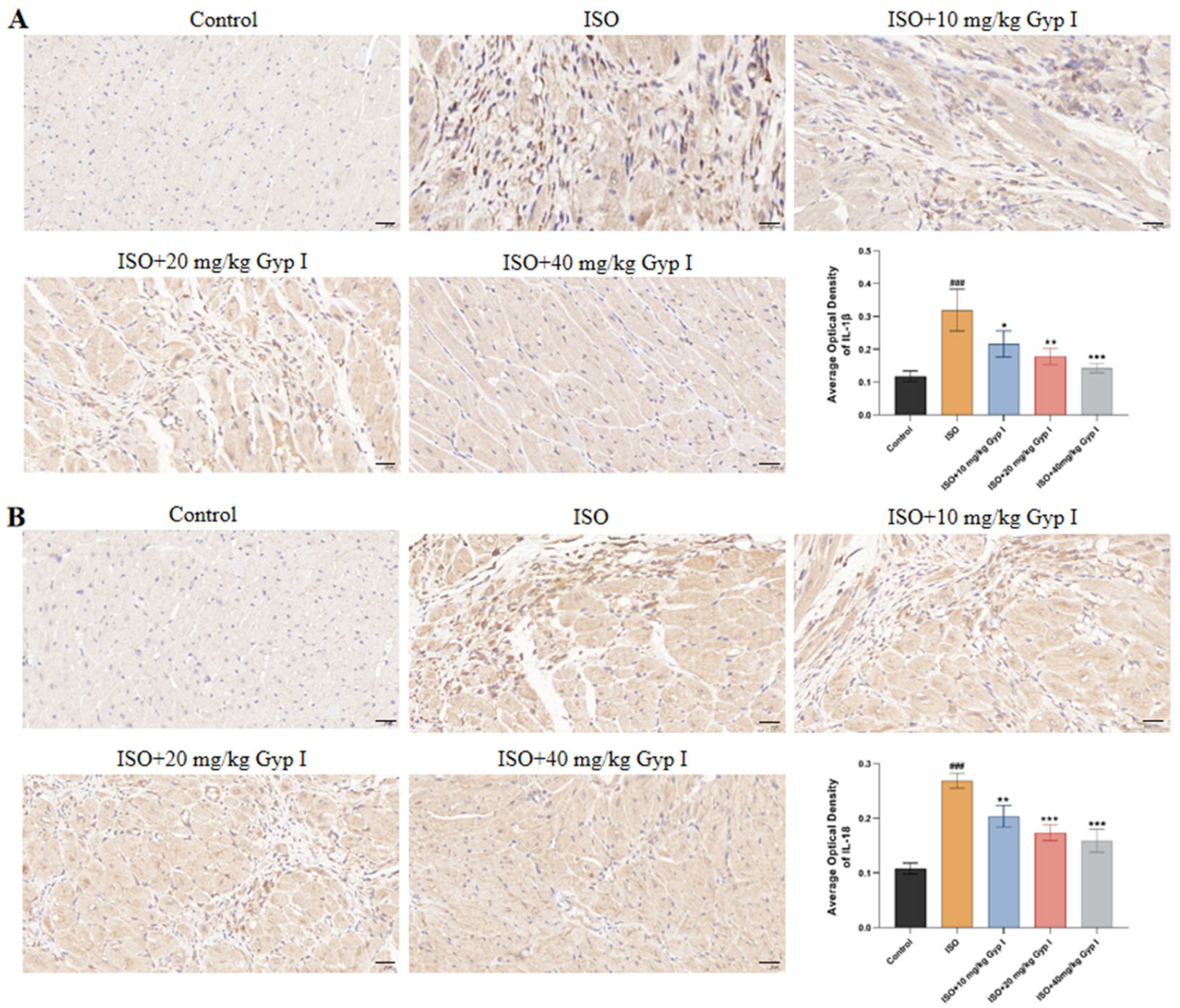
2.10. Effect of Gyp I on IL-1β and IL-18 in the Myocardial Tissue of Mice Induced by ISO
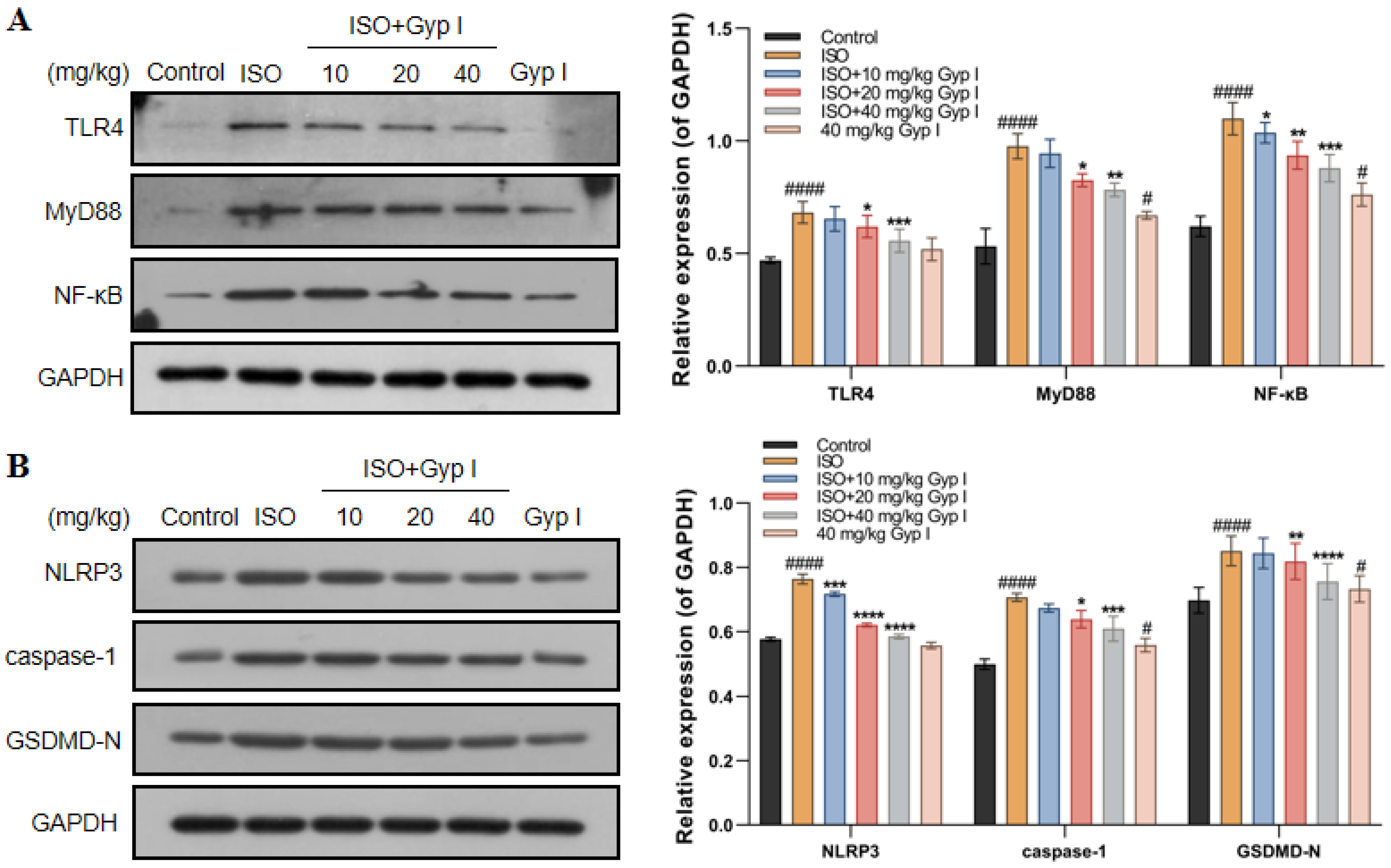
2.11. Gyp I Inhibits the TLR4/NF-κB/NLRP3 Pathway In Vivo
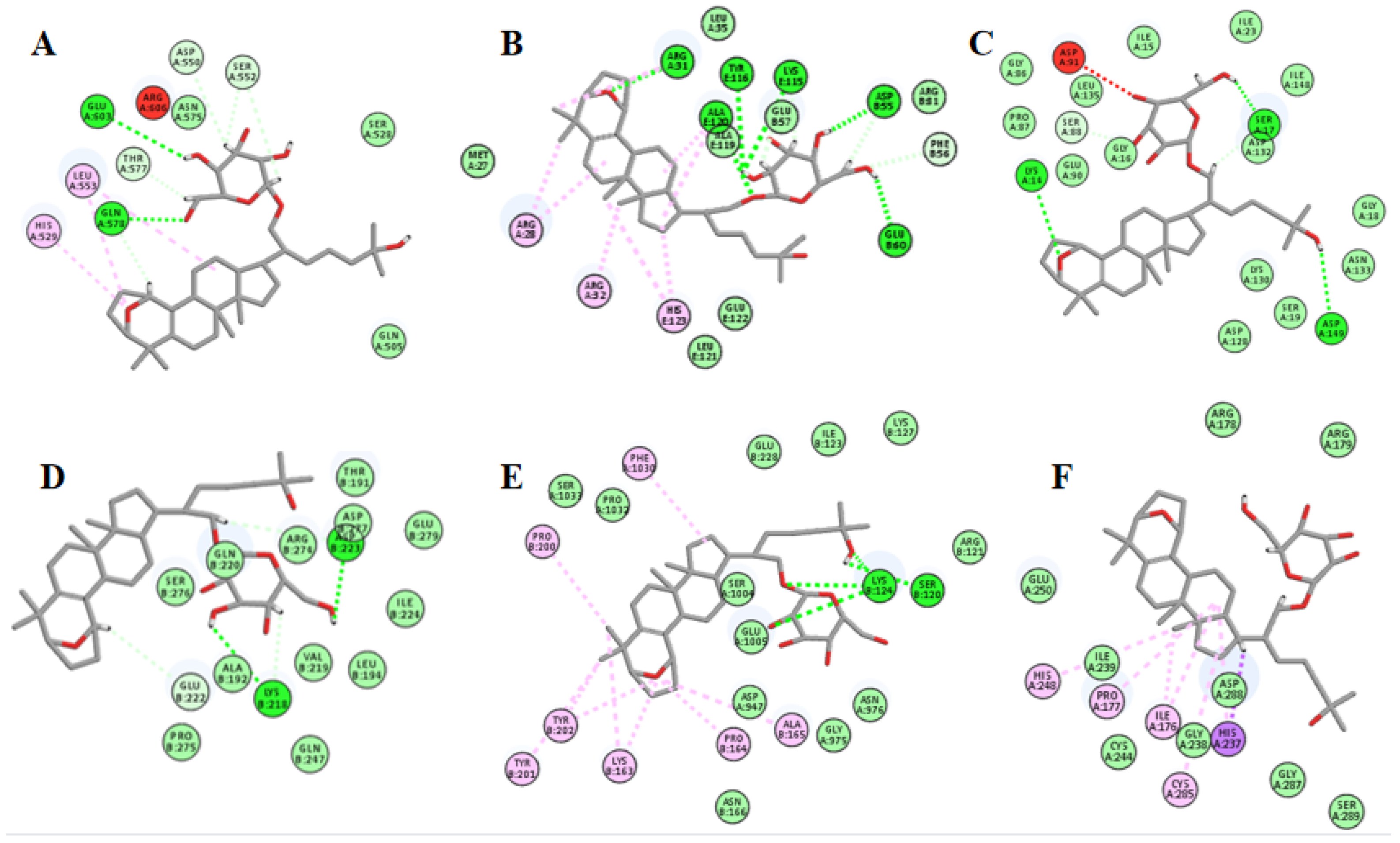
2.12. Molecular Docking Suggests That Gyp I Could Interact with Several Key Proteins of the TLR4/NF-κB/NLRP3 Signaling Pathway
3. Discussion
4. Materials and Methods
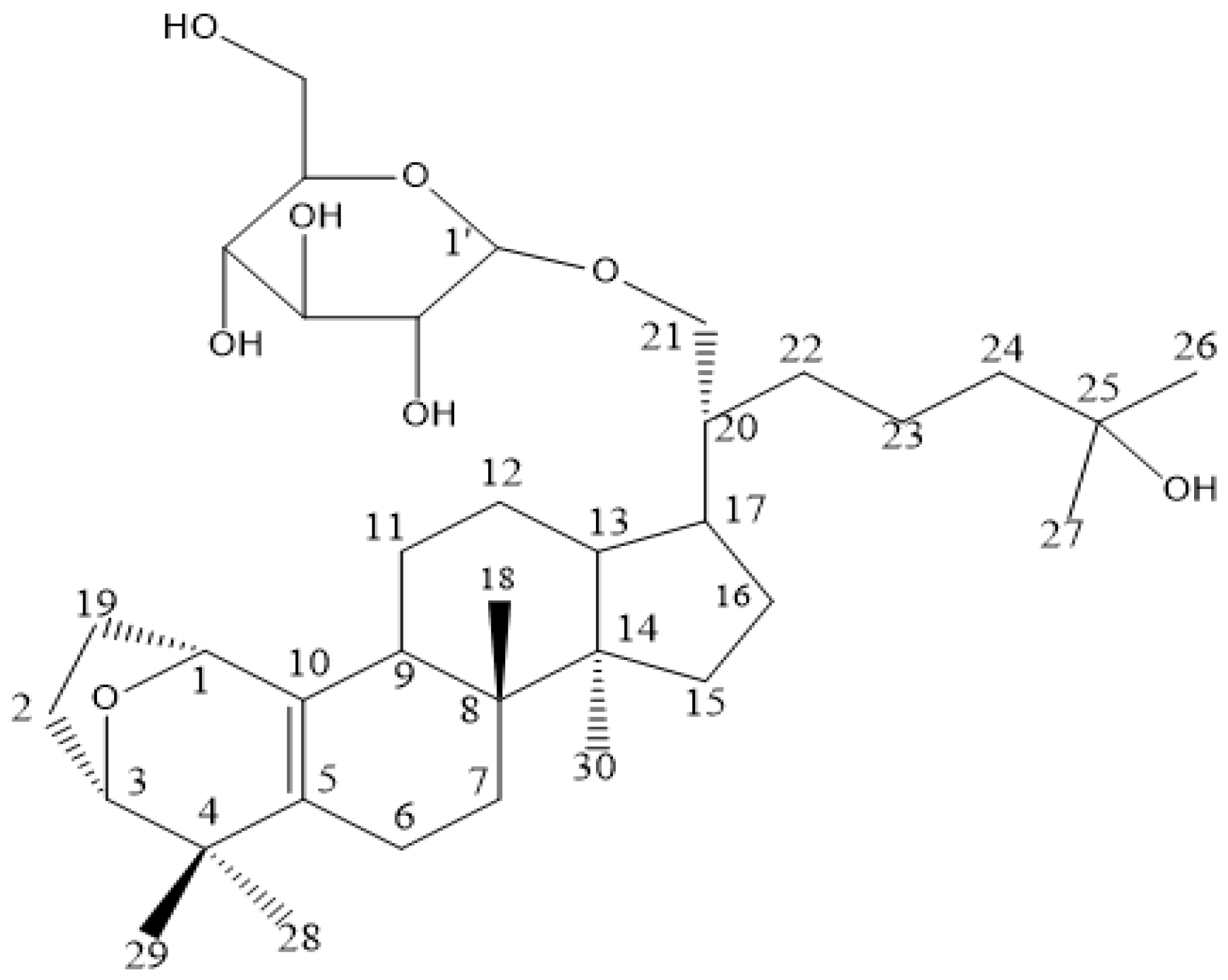
4.1. Sample
4.2. Chemical Reagents and Antibody
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Annexin V/Propidium Iodide (PI) Assay
4.6. Experimental Animals and Groups
4.7. Analysis of HWI and LVWI
4.8. ECG Analysis
4.9. Serum Biomarker Analysis
4.10. Heart Oxidative Stress Markers
4.11. Real-Time Quantitative RT-PCR
4.12. H&E Straining
4.13. Sirius Red Staining
4.14. Masson Straining
4.15. Immunohistochemistry
4.16. Western Blot Analysis
4.17. Molecular Docking
4.18. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porrello, E.R.; Olson, E.N. A neonatal blueprint for cardiac regeneration. Stem Cell Res. 2014, 13, 556–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souders, C.A.; Bowers, S.L.; Baudino, T.A. Cardiac fibroblast: The renaissance cell. Circ. Res. 2009, 105, 1164–1176. [Google Scholar] [CrossRef]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.L.; Qin, M.; Liu, X.H.; Zhu, Y.Z. The role of hydrogen sulfide on cardiovascular homeostasis: An overview with update on immunomodulation. Front. Pharmacol. 2017, 8, 686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.R.; Nilius, B.; Han, J. Gaseous signaling molecules in cardiovascular function: From mechanisms to clinical translation. Rev. Physiol. Biochem. Pharm. 2018, 174, 81–156. [Google Scholar]
- Krenning, G.; Zeisberg, E.M.; Kalluri, R. The origin of fibroblasts and mechanism of cardiac fibrosis. J. Cell Physiol 2010, 225, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Shinde, A.V.; Frangogiannis, N.G. Fibroblasts in myocardial infarction: A role in inflammation and repair. J. Mol. Cell Cardiol. 2014, 70, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Biernacka, A.; Frangogiannis, N.G. Aging and Cardiac Fibrosis. Aging Dis. 2011, 2, 158–173. [Google Scholar]
- Murtha, L.A.; Schuliga, M.J.; Mabotuwana, N.S.; Hardy, S.A.; Waters, D.W.; Burgess, J.K.; Knight, D.A.; Boyle, A.J. The processes and mechanisms of cardiac and pulmonary fibrosis. Front. Physiol. 2017, 8, 777. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, L.K.; Pang, Y.Z.; Pan, C.S.; Qi, Y.F.; Chen, L.; Wang, X.; Tang, C.S.; Zhang, J. Cardioprotective effects of ghrelin and des-octanoyl ghrelin on myocardial injury induced by isoproterenol in rats. Acta Pharm. Sin. 2006, 27, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Van Linthout, S.; Miteva, K.; Tschope, C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc. Res. 2014, 102, 258–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedayat, M.; Mahmoudi, M.J.; Rose, N.R.; Rezaei, N. Proinflammatory cytokines in heart failure: Double-edged swords. Heart Fail. Rev. 2010, 15, 543–562. [Google Scholar] [CrossRef]
- Razmovski-Naumovski, V.; Huang, T.H.-W.; Tran, V.H.; Li, G.Q.; Duke, C.C.; Roufogalis, B.D. Chemistry and pharmacology of gynostemma pentaphyllum. Phytochem. Rev. 2005, 4, 197–219. [Google Scholar] [CrossRef]
- Xie, Z.; Huang, H.; Zhao, Y.; Shi, H.; Wang, S.; Wang, T.T.; Chen, P.; Yu, L.L. Chemical composition and anti-proliferative and anti-inflammatory effects of the leaf and whole-plant samples of diploid and tetraploid Gynostemma pentaphyllum (Thunb.) Makino. Food Chem. 2012, 132, 125–133. [Google Scholar] [CrossRef]
- Cai, H.; Liang, Q.; Ge, G. Gypenoside attenuates beta amyloid-induced inflammation in N9 microglial cells via SOCS1 signaling. Neural Plast 2016, 2016, 6362707. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Zhang, H.; Zhao, W.; Guo, L.; Li, X.; Li, Y.; Zhang, X.; Sun, Y. Gypenoside protects against myocardial ischemia-reperfusion injury by inhibiting cardiomyocytes apoptosis via inhibition of chop pathway and activation of PI3K/Akt pathway in vivo and in vitro. Cell Physiol. Biochem. 2016, 39, 123–136. [Google Scholar] [CrossRef]
- Gou, S.H.; Huang, H.F.; Chen, X.Y.; Liu, J.; He, M.; Ma, Y.Y.; Zhao, X.N.; Zhang, Y.; Ni, J.M. Lipid-lowering, hepatoprotective, and atheroprotective effects of the mixture Hong-Qu and gypenosides in hyperlipidemia with NAFLD rats. J. Chin. Med. Assoc. 2016, 79, 111–121. [Google Scholar] [CrossRef]
- He, Q.; Li, J.K.; Li, F.; Li, R.G.; Zhan, G.Q.; Li, G.; Du, W.X.; Tan, H.B. Mechanism of action of gypenosides on type 2 diabetes and non-alcoholic fatty liver disease in rats. World J. Gastroenterol. 2015, 21, 2058–2066. [Google Scholar] [CrossRef]
- Zhang, X.S.; Cao, J.Q.; Zhao, C.; Wang, X.D.; Wu, X.J.; Zhao, Y.Q. Novel dammarane-type triterpenes isolated from hydrolyzate of total Gynostemma pentaphyllum saponins. Bioorg. Med. Chem. Lett. 2015, 25, 3095–3099. [Google Scholar] [CrossRef]
- Huang, Z.; Zhuang, X.; Xie, C.; Hu, X.; Dong, X.; Guo, Y.; Li, S.; Liao, X. Exogenous hydrogen sulfide attenuates high glucose-induced cardiotoxicity by inhibiting NLRP3 inflammasome activation by suppressing TLR4/NF-kappaB pathway in H9c2 cells. Cell Physiol. Biochem. 2016, 40, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Dasu, M.R.; Devaraj, S.; Park, S.; Jialal, I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 2010, 33, 861–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.W.; Liao, S.Q.; Zhang, M.J.; Liu, Y.; Li, B.H.; Zhou, Y.; Chen, L.; Gao, C.Y.; Li, J.C.; Zhang, L.L. TLR4-mediated inflammation promotes foam cell formation of vascular smooth muscle cell by upregulating ACAT1 expression. Cell Death Dis. 2014, 5, e1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Du, Q.; Yang, Y.; Wang, J.; Dou, S.; Liu, C.; Duan, J. The protective effect of Luteolin on myocardial ischemia/reperfusion (I/R) injury through TLR4/NF-kappaB/NLRP3 inflammasome pathway. Biomed. Pharm. 2017, 91, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.R.; Zhang, C.; Yang, H.X.; Sun, J.H.; Zhang, Y.; Yao, T.T.; Li, Y.; Ruan, L.; An, R.; Li, A.Y. Modified citrus pectin ameliorates myocardial fibrosis and inflammation via suppressing galectin-3 and TLR4/MyD88/NF-kappaB signaling pathway. Biomed. Pharm. 2020, 126, 110071. [Google Scholar] [CrossRef]
- Del Re, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Lan, Z.; Xin, Z.; He, C.; Guo, Z.; Xia, X.; Hu, T. Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J. Cell Physiol. 2020, 235, 3207–3221. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Li, W.; Chen, M.; Xu, L.; Lv, Z.; Chen, L.; Li, Y.; He, W. Morroniside alleviates coxsackievirus B3-induced myocardial damage apoptosis via restraining NLRP3 inflammasome activation. RSC Adv. 2019, 9, 1222–1229. [Google Scholar] [CrossRef] [Green Version]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, K.; Liu, W.; She, Y.; Sun, Q.; Shi, J.; Sun, H.; Wang, D.C.; Shao, F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 2016, 535, 111–116. [Google Scholar] [CrossRef] [PubMed]
- El-Sisi, A.E.E.; Sokar, S.S.; Shebl, A.M.; Mohamed, D.Z.; Abu-Risha, S.E. Octreotide and melatonin alleviate inflammasome-induced pyroptosis through inhibition of TLR4-NF-kappaB-NLRP3 pathway in hepatic ischemia/reperfusion injury. Toxicol. Appl. Pharmacol. 2021, 410, 115340. [Google Scholar] [CrossRef]
- Akhter, A.; Gavrilin, M.A.; Frantz, L.; Washington, S.; Ditty, C.; Limoli, D.; Day, C.; Sarkar, A.; Newland, C.; Butchar, J.; et al. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 2009, 5, e1000361. [Google Scholar] [CrossRef] [Green Version]
- Erener, S.; Pétrilli, V.; Kassner, I.; Minotti, R.; Castillo, R.; Santoro, R.; Hassa, P.O.; Tschopp, J.; Hottiger, M.O. Inflammasome-activated caspase 7 cleaves PARP1 to enhance the expression of a subset of NF-κB target genes. Molecular. Cell 2012, 46, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Sagulenko, V.; Vitak, N.; Vajjhala, P.R.; Vince, J.E.; Stacey, K.J. Caspase-1 is an apical caspase leading to caspase-3 cleavage in the AIM2 inflammasome response, independent of caspase-8. J. Mol. Biol. 2018, 430, 238–247. [Google Scholar] [CrossRef]
- Rogers, C.; Erkes, D.A.; Nardone, A.; Aplin, A.E.; Fernandes-Alnemri, T.; Alnemri, E.S. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 2019, 10, 1689. [Google Scholar] [CrossRef]
- Lim, K.H.; Cho, J.Y.; Kim, B.; Bae, B.S.; Kim, J.H. Red ginseng (Panax ginseng) decreases isoproterenol-induced cardiac injury via antioxidant properties in porcine. J. Med. Food 2014, 17, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Bos, R.; Mougenot, N.; Mediani, O.; Vanhoutte, P.M.; Lechat, P. Potassium canrenoate, an aldosterone receptor antagonist, reduces isoprenaline-induced cardiac fibrosis in the rat. J. Pharmacol. Exp. Ther. 2004, 309, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhao, Q.; Kong, W. Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol. 2018, 68–69, 490–506. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Sakiyama, K.; Shiota, T.; Ito, M. Isoproterenol produces a rapid increase in sialidase activity in rat heart tissue and cardiomyocyte-derived H9c2 cells in culture. FEBS Lett. 2003, 542, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zeng, S.; Zou, J.; Chen, Y.; Yue, Z.; Gao, Y.; Zhang, L.; Cao, W.; Liu, P. Rapamycin attenuated cardiac hypertrophy induced by isoproterenol and maintained energy homeostasis via inhibiting NF-kappaB activation. Mediat. Inflamm. 2014, 2014, 868753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Berlo, J.H.; Maillet, M.; Molkentin, J.D. Signaling effectors underlying pathologic growth and remodeling of the heart. J. Clin. Investig. 2013, 123, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xu, J.; Long, Z.; Wang, C.; Wang, L.; Sun, P.; Li, P.; Wang, T. Hydrogen (H2) inhibits isoproterenol-induced cardiac hypertrophy via antioxidative pathways. Front. Pharmacol. 2016, 7, 392. [Google Scholar] [CrossRef] [Green Version]
- Khatua, T.N.; Padiya, R.; Karnewar, S.; Kuncha, M.; Agawane, S.B.; Kotamraju, S.; Banerjee, S.K. Garlic provides protection to mice heart against isoproterenol-induced oxidative damage: Role of nitric oxide. Nitric Oxide 2012, 27, 9–17. [Google Scholar] [CrossRef]
- Dong, L.; Chen, F.; Xu, M.; Yao, L.; Zhang, Y.; Zhuang, Y. Quercetin attenuates myocardial ischemia-reperfusion injury via downregulation of the HMGB1-TLR4-NF-κB signaling pathway. Am. J. Transl. Res. 2018, 10, 1273–1283. [Google Scholar]
- Okada, M.; Morioka, S.; Kanazawa, H.; Yamawaki, H. Canstatin inhibits isoproterenol-induced apoptosis through preserving mitochondrial morphology in differentiated H9c2 cardiomyoblasts. Apoptosis 2016, 21, 887–895. [Google Scholar] [CrossRef]
- Wang, M.; Qian, L.; Li, J.; Ming, H.; Fang, L.; Li, Y.; Zhang, M.; Xu, Y.; Ban, Y.; Zhang, W. GHSR deficiency exacerbates cardiac fibrosis: Role in macrophage inflammasome activation and myofibroblast differentiation. Cardiovasc. Res. 2020, 116, 2091–2102. [Google Scholar] [CrossRef]
- Yu, S.Y.; Tang, L.; Zhao, G.J.; Zhou, S.H. Statin protects the heart against ischemia-reperfusion injury via inhibition of the NLRP3 inflammasome. Int. J. Cardiol. 2017, 229, 23–24. [Google Scholar] [CrossRef]
- Feng, H.; Xie, B.; Zhang, Z.Q.; Yan, J.; Cheng, M.Y.; Zhou, Y.F. MiR-135a protects against myocardial injury by targeting TLR4. Chem. Pharm. Bull. 2021, 69, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Aguirre, S.A.; Evering, W.E.; Hirakawa, B.P.; May, J.R.; Palacio, K.; Wang, J.; Zhang, Y.; Stevens, G.J. miR-208a as a biomarker of isoproterenol-induced cardiac injury in Sod2+/- and C57BL/6J wild-type mice. Toxicol. Pathol. 2014, 42, 1117–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, H.; Nagai, Y.; Matsunaga, T.; Okamoto, N.; Watanabe, Y.; Tsuneyama, K.; Hayashi, H.; Fujii, I.; Ikutani, M.; Hirai, Y.; et al. Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. J. Leukoc. Biol. 2014, 96, 1087–1100. [Google Scholar] [CrossRef] [PubMed]


| Primer Name | Primer Sequence (5′-3′) | Fragment Length (bp) | |
|---|---|---|---|
| ANP | forward | CTTCTTCCTCGTCTTGGCCTTT | 114 |
| reverse | TCCAGGTGGTCTAGCAGGTTCT | 114 | |
| BNP | forward | GCTGCTGGAGCTGATAAGAGAA | 194 |
| reverse | CGATCCGGTCTATCTTGTGCC | 194 | |
| GAPDH | forward | CCTCGTCCCGTAGACAAAATG | 133 |
| reverse | TGAGGTCAATGAAGGGGTCGT | 133 | |
| Entry | Protein | PDB ID | LibDock Score | Interaction |
|---|---|---|---|---|
| 1 | TLR4 | 3FXI | 115.271 | H bonds: GLN578, GLU603 Interacting residues: ASP550, HIS529, SER552, LEU553, THR577 |
| 2 | MyD88 | 3MOP | 129.773 | H bonds: ARE31, ASP55, GLU57, ALU60, LYS115, TYR116, ALA119, ALA120 Interacting residues: ARG28, ARG31, ARG32, PHE56, ALA120, HIS123 |
| 3 | I-κBα | 4KBA | 113.576 | H bonds: LYS14, SER17, ASP132, ASP149 Interacting residues: SER88 |
| 4 | NF-κB | 1MY5 | 121.299 | H bonds: LYS218, ASP223, ARG274 Interacting residues: GLU222 |
| 5 | NLRP3 | 6NPY | 110.726 | H bonds: SER120, LYS124 Interacting residues: LYS163, PRO164, ALA165, PRO200, TYR201, TYR202, PHE1030 |
| 6 | caspase-1 | 1RWX | 116.82 | Interacting residues: ILE176, PRO177, HIS237, HIS248, CYS285 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Tan, H.; Gao, T.; Han, L.; Teng, X.; Wang, F.; Zhang, X. Gypensapogenin I Ameliorates Isoproterenol (ISO)-Induced Myocardial Damage through Regulating the TLR4/NF-κB/NLRP3 Pathway. Molecules 2022, 27, 5298. https://doi.org/10.3390/molecules27165298
Li M, Tan H, Gao T, Han L, Teng X, Wang F, Zhang X. Gypensapogenin I Ameliorates Isoproterenol (ISO)-Induced Myocardial Damage through Regulating the TLR4/NF-κB/NLRP3 Pathway. Molecules. 2022; 27(16):5298. https://doi.org/10.3390/molecules27165298
Chicago/Turabian StyleLi, Mengyuan, Hongyan Tan, Ting Gao, Linlin Han, Xinhang Teng, Fang Wang, and Xiaoshu Zhang. 2022. "Gypensapogenin I Ameliorates Isoproterenol (ISO)-Induced Myocardial Damage through Regulating the TLR4/NF-κB/NLRP3 Pathway" Molecules 27, no. 16: 5298. https://doi.org/10.3390/molecules27165298
APA StyleLi, M., Tan, H., Gao, T., Han, L., Teng, X., Wang, F., & Zhang, X. (2022). Gypensapogenin I Ameliorates Isoproterenol (ISO)-Induced Myocardial Damage through Regulating the TLR4/NF-κB/NLRP3 Pathway. Molecules, 27(16), 5298. https://doi.org/10.3390/molecules27165298