The Bile Acid Membrane Receptor TGR5 in Cancer: Friend or Foe?
Abstract
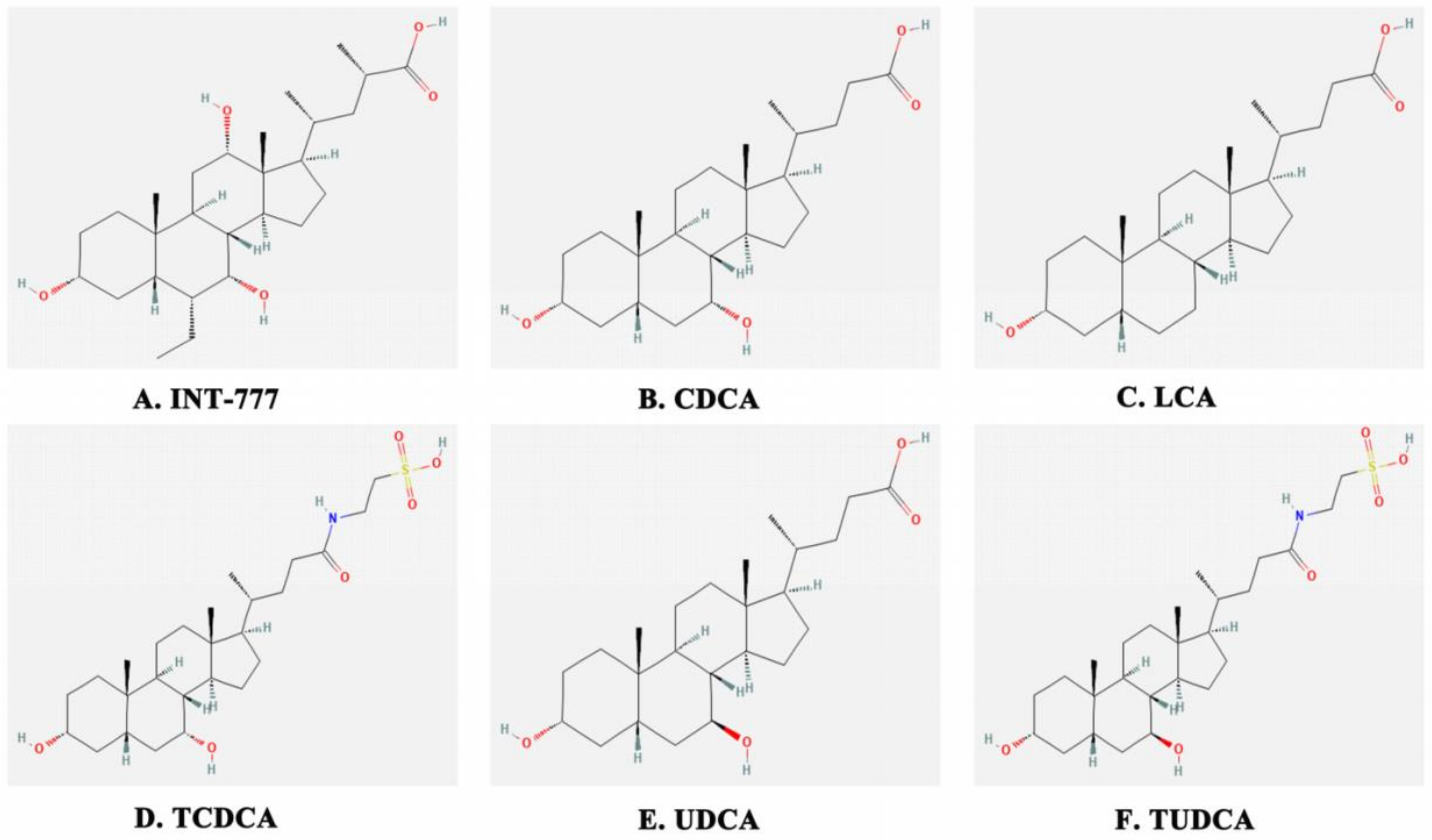
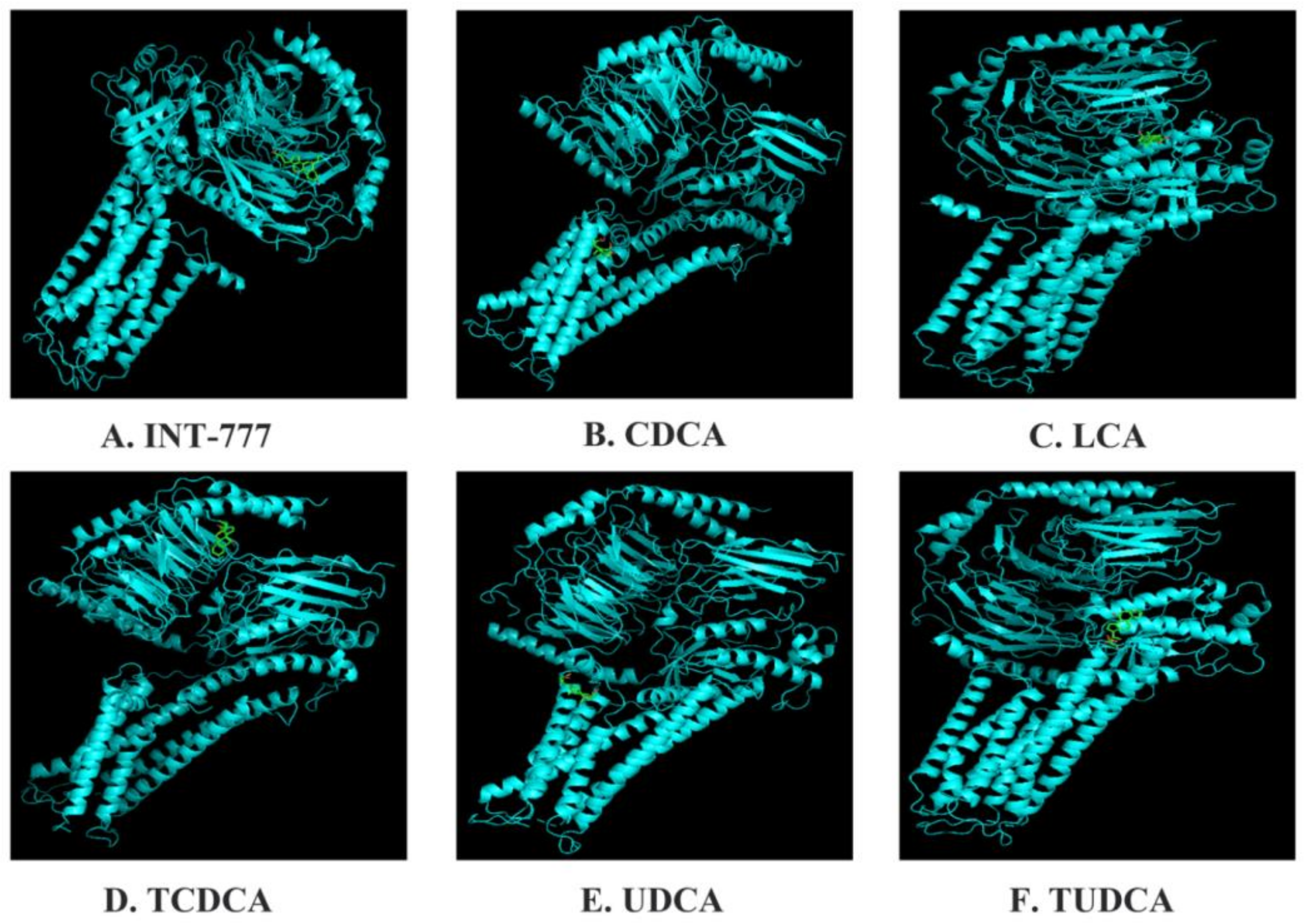
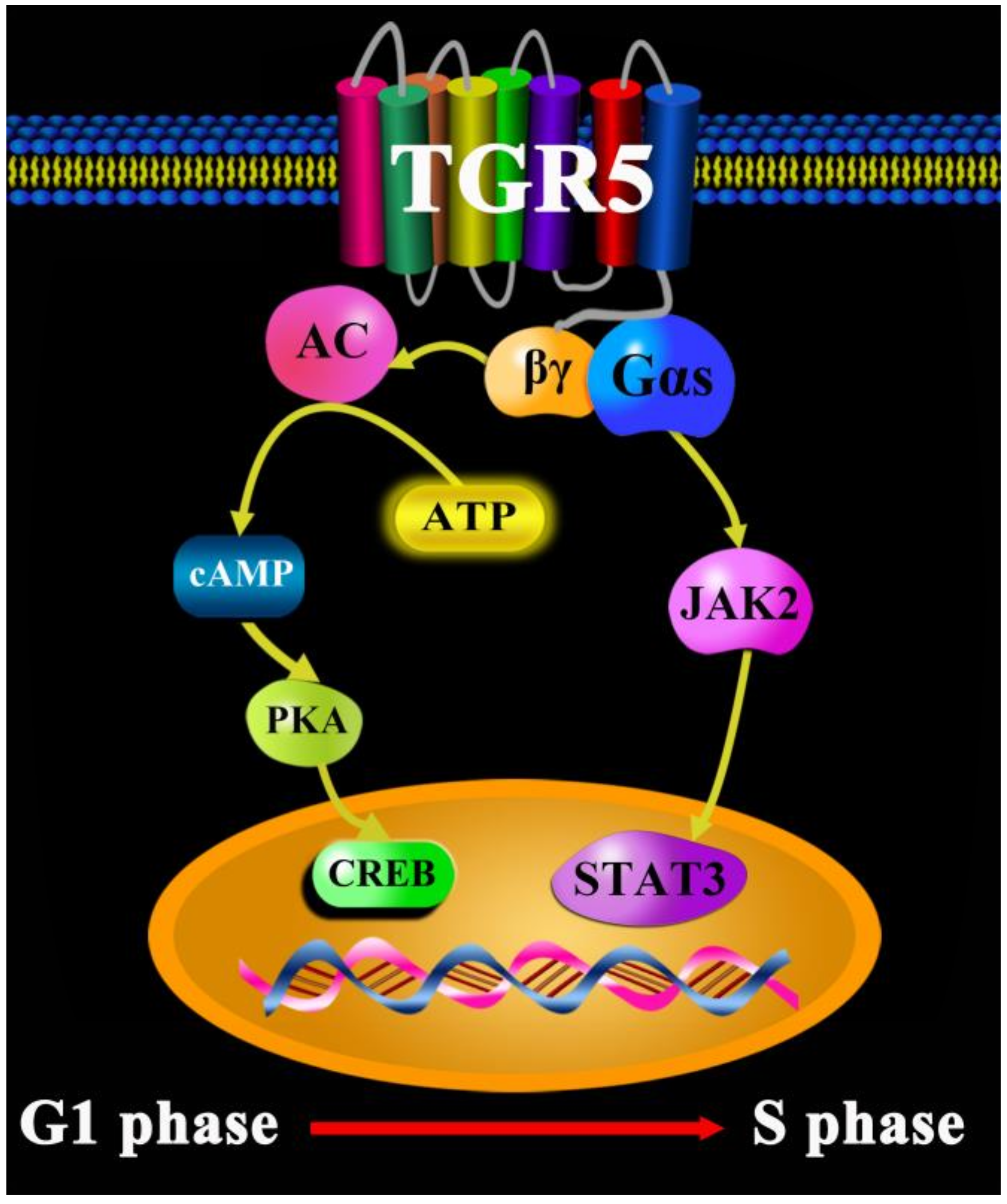
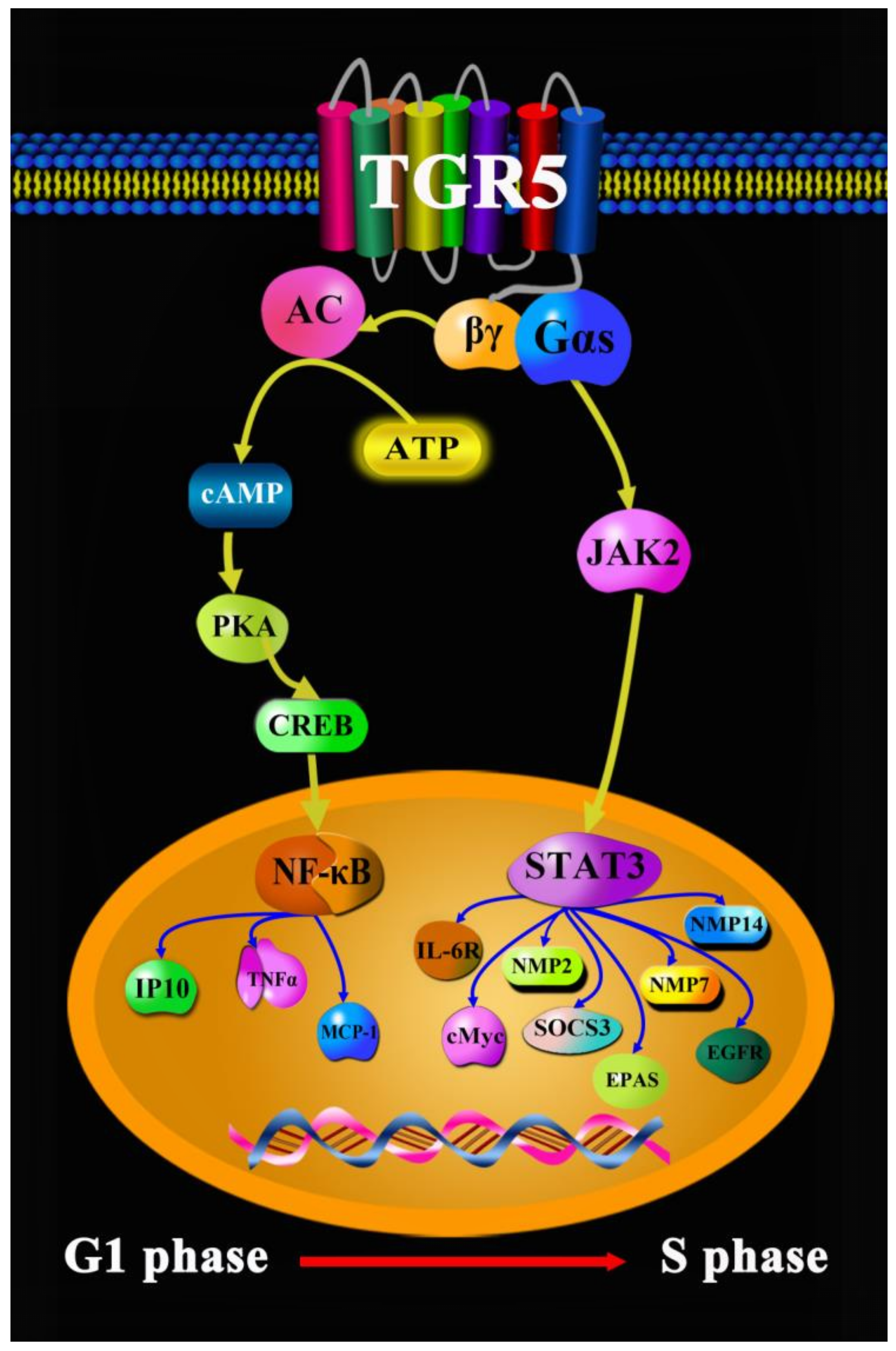
1. Introduction
2. TGR5 in Lung Cancer
3. TGR5 in Liver Cancer
4. TGR5 in Gastric Cancer
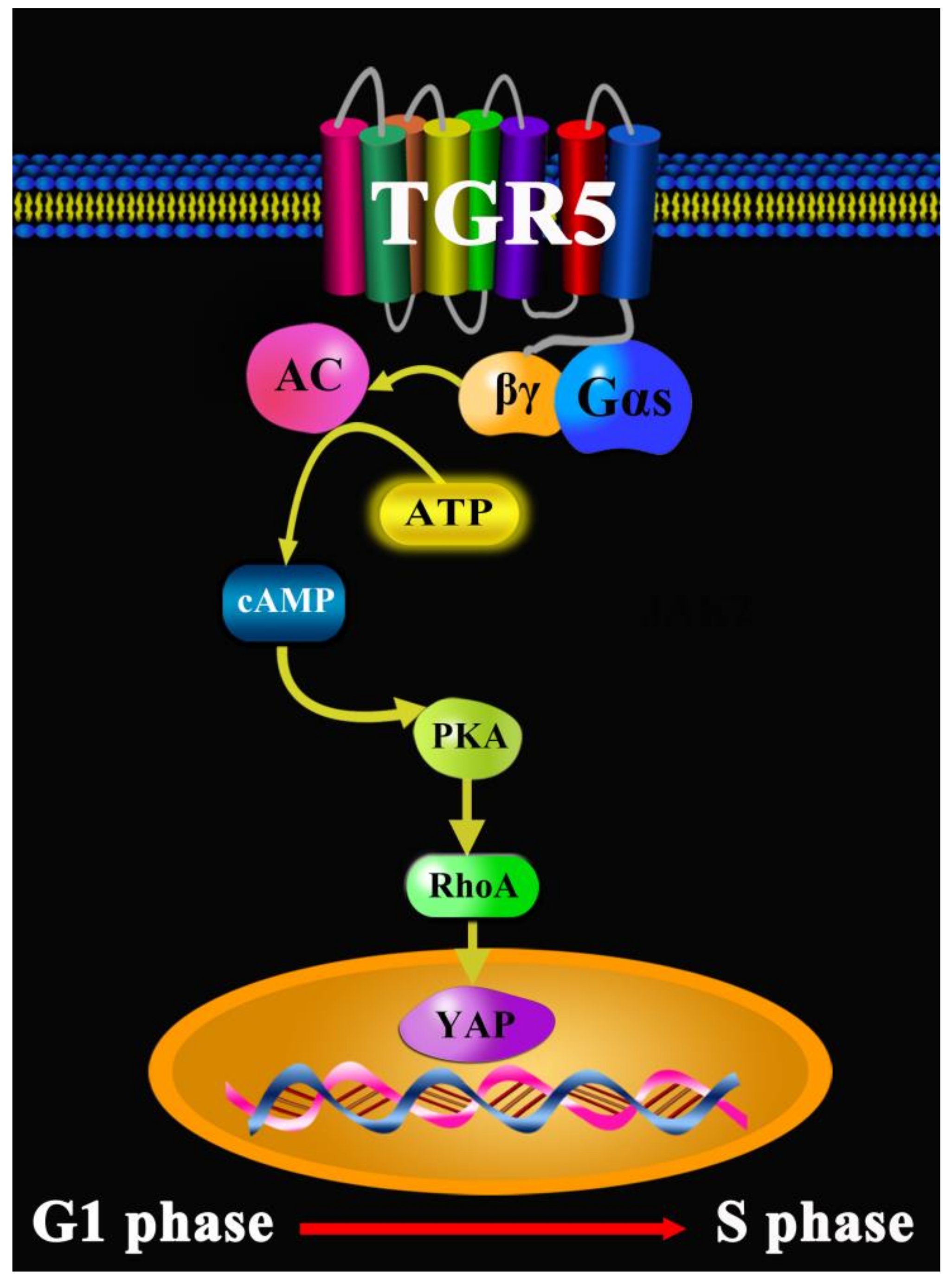
5. TGR5 in Colorectal Cancer
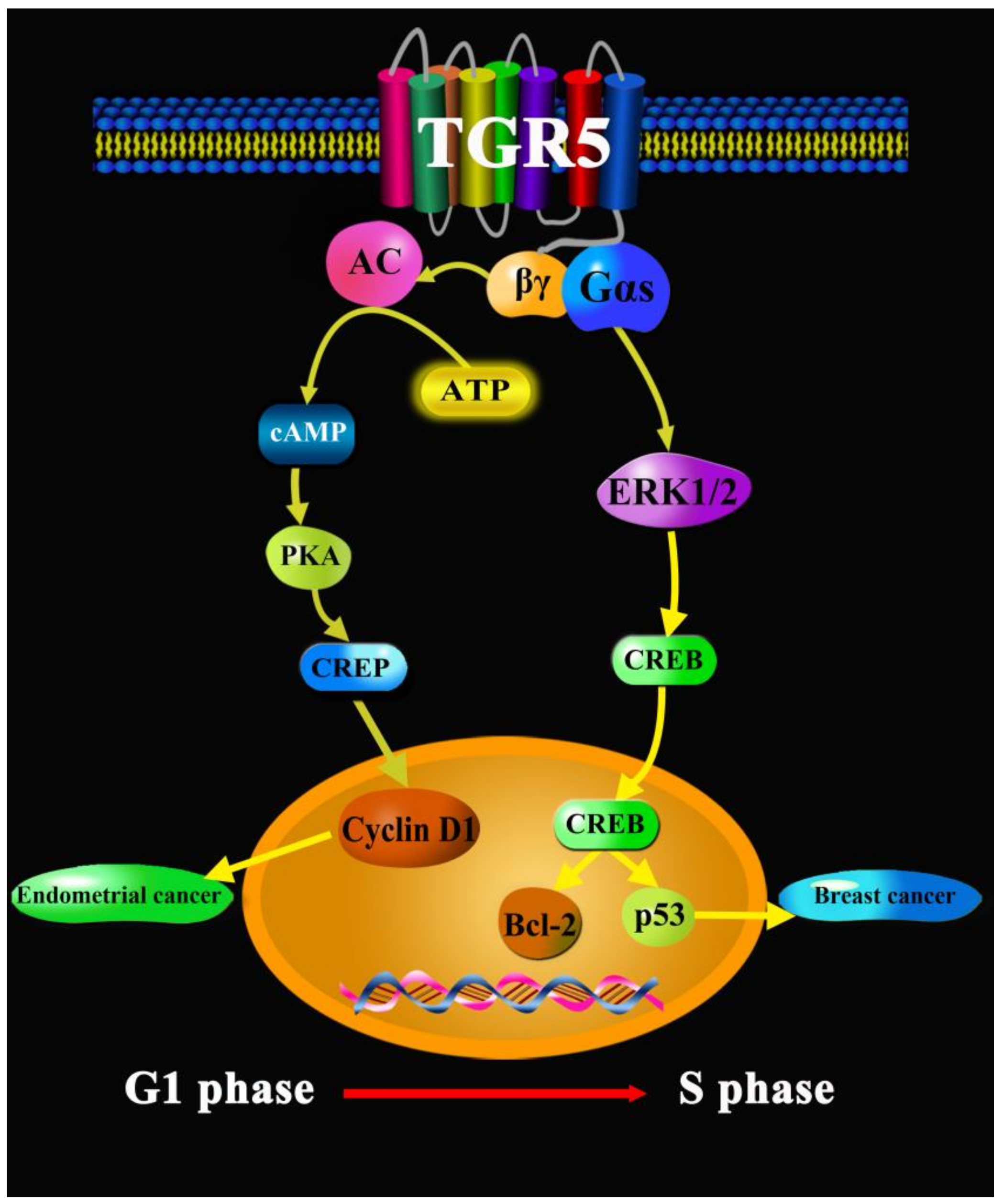
6. TGR5 in Other Cancers
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, L.; Ye, R.D. Role of G protein-coupled receptors in inflammation. Acta Pharmacol. Sin. 2012, 3, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhou, S.; Shi, Y.; Wang, Q.; Wei, S.; Wang, P.; Cheng, F.; Auwerx, J.; Schoonjans, K.; Lu, L. TGR5/Cathepsin E signaling regulates macrophage innate immune activation in liver ischemia and reperfusion injury. Am. J. Transplant. 2021, 4, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.S.; Lou, G.Y.; Meng, Z.P.; Huang, W.D. TGR5: A Novel Target for Weight Maintenance and Glucose Metabolism. J. Diabetes Res. 2011, 2011, 853501. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Chen, W.D.; Wang, Y.D. TGR5, Not Only a Metabolic Regulator. Front. Physiol. 2016, 7, 646. [Google Scholar] [CrossRef] [PubMed]
- Baidya, M.; Chaturvedi, M.; Dwivedi-Agnihotri, H. Allosteric modulation of GPCR-induced β-arrestin trafficking and signaling by a synthetic intrabody. Nat. Commun. 2022, 13, 4634. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, Y.; Li, T.; Feng, X. G-Protein Coupled Receptors (GPCRs): Signaling Pathways, Characterization, and Functions in Insect Physiology and Toxicology. Int. J. Mol. Sci. 2021, 10, 5260. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Pathway Summary for Pathway R-HSA-388396, GPCR Downstream Signalling, Source: Reactome. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/pathway/Reactome:R-HSA-388396 (accessed on 12 August 2022).
- Yu, H.; Lee, H.; Herrmann, A. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Duboc, H.; Taché, Y.; Hofmann, A.F. The bile acid TGR5 membrane receptor: From basic research to clinical application. Dig. Liver Dis. 2014, 4, 302–312. [Google Scholar] [CrossRef]
- Yang, H.; Luo, F.; Wei, Y.; Jiao, Y.; Qian, J.; Chen, S.; Gong, Y.; Tang, L. TGR5 protects against cholestatic liver disease via suppressing the NF-κB pathway and activating the Nrf2/HO-1 pathway. Ann. Transl. Med. 2021, 14, 1158. [Google Scholar] [CrossRef]
- Shotaro, M.; Takashi, S.; Yuki, Y.; Makoto, S.; Ryuichiro, S. Maslinic acid activates mTORC1 and human TGR5 and induces skeletal muscle hypertrophy. Biosci. Biotech. Bioch. 2021, 11, 2311–2321. [Google Scholar]
- Velazquez-Villegas, L.; Perino, A.; Lemos, V. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat. Commun. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.; Zhang, T.; Huang, L.; Araujo, C.; Peng, J.; Travis, Z.; Okada, T.; Ocak, U.; Zhang, G.; Tang, J.; et al. Activation of TGR5 with INT-777 attenuates oxidative stress and neuronal apoptosis via cAMP/PKCε/ALDH2 pathway after subarachnoid hemorrhage in rats. Free Radic. Biol. Med. 2019, 143, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, X.D.; Mao, W.; Li, P.F. Effects of taurochenodeoxycholic acid on Ca2+/CaM signalling mediated by the tgr5 signalling pathway. Die Pharmazie. 2016, 7, 390–393. [Google Scholar]
- Xu, J.; Li, H.; Xia, Z. Propofol ameliorates hyperglycemia-induced cardiac hypertrophy and dysfunction via heme oxygenase-1/STAT3 signaling pathway in rats. Crit Care Med. 2014, 8, e583-94. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Shi, L.; Duan, G.; Ma, Y.; Li, P. Taurochenodeoxycholic acid increases cAMP content via specially interacting with bile acid receptor TGR5. Molecules 2021, 23, 7066. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, Y.; Nishimaki-Mogami, T.; Yamaguchi, M.; Teraoka, F.; Kaneko, T.; Une, M. Effects of chemical modification of ursodeoxycholic acid on TGR5 activation. Biol. Pharm. Bull. 2011, 34, 1–7. [Google Scholar] [CrossRef][Green Version]
- Naomi, D.; Karina, G.; Luke, C. Tauroursodeoxycholic acid/TGR5 signaling promotes survival and early development of glucose-stressed porcine embryos. Biol Reprod. 2021, 105, 76–86. [Google Scholar]
- Li, B.; Yang, N.; Li, C.; Li, C.; Gao, K.; Xie, X.; Dong, X.; Yang, J.; Yang, Q.; Tong, Z.; et al. INT-777, a bile acid receptor agonist, extenuates pancreatic acinar cells necrosis in a mouse model of acute pancreatitis. Biochem. Biophys. Res. Commun. 2018, 1, 38–44. [Google Scholar] [CrossRef]
- Chen, X.; Yan, L.; Guo, Z.; Chen, Y.; Li, M.; Huang, C.; Chen, Z.; Meng, X. Chenodeoxycholic acid attenuates high-fat diet-induced obesity and hyperglycemia via the G protein-coupled bile acid receptor 1 and proliferator-activated receptor γ pathway. Exp. Ther. Med. 2017, 6, 5305–5312. [Google Scholar] [CrossRef][Green Version]
- Cheng, K.C.; Chang, W.T.; Kuo, F.Y. TGR5 activation ameliorates hyperglycemia-induced cardiac hypertrophy in H9c2 cells. Sci. Rep. 2019, 9, 3633. [Google Scholar] [CrossRef]
- Qi, Y.C.; Duan, G.Z.; Mao, W.; Liu, Q.; Zhang, Y.L.; Li, P.F. Taurochenodeoxycholic acid mediates cAMP-PKA-CREB signaling pathway. Chin. J. Nat. Med. 2020, 12, 898–906. [Google Scholar] [CrossRef]
- Shen, Y.; Lu, C.; Song, Z.; Qiao, C.; Wang, J.; Chen, J.; Zhang, C.; Zeng, X.; Ma, Z.; Chen, T.; et al. Ursodeoxycholic acid reduces antitumor immunosuppression by inducing CHIP-mediated TGF-β degradation. Nat. Commun. 2022, 1, 3419. [Google Scholar] [CrossRef] [PubMed]
- Dicks, N.; Gutierrez, K.; Currin, L. Tauroursodeoxycholic acid acts via TGR5 receptor to facilitate DNA damage repair and improve early porcine embryo development. Mol. Reprod. Dev. 2019, 87, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, L.; Zhou, C. Lung cancer in China: Current and prospect. Curr. Opin. Oncol. 2021, 1, 40–46. [Google Scholar] [CrossRef]
- Thomas, A.; Hassan, R. Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol. 2012, 7, e301–e310. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 10066, 299–311. [Google Scholar] [CrossRef]
- Lynch, T.J.; Kass, F.; Kalish, L.A.; Elias, A.D.; Strauss, G.; Shulman, L.N.; Sugarbaker, D.J.; Skarin, A.; Frei, E. Cisplatin, 5-fluorouracil, and etoposide for advanced non-small cell lung cancer. Cancer 1993, 10, 2953–2957. [Google Scholar] [CrossRef]
- Wu, Z.X.; Li, J.; Dong, S.; Lin, L.; Zou, C.; Chen, Z.S. Tepotinib hydrochloride for the treatment of non-small cell lung cancer. Drug Today 2021, 4, 265–275. [Google Scholar] [CrossRef]
- Zheng, X.; Luo, J.; Liu, W.; Ashby, C.R.; Chen, Z.S.; Lin, L. Sotorasib: A treatment for non-small cell lung cancer with the KRAS G12C mutation. Drug Today 2022, 4, 175–185. [Google Scholar] [CrossRef]
- Simone, C.B.; Burri, S.H.; Heinzerling, J.H. Novel radiotherapy approaches for lung cancer: Combining radiation therapy with targeted and immunotherapies. Transl. Lung Cancer Res. 2015, 5, 545–552. [Google Scholar]
- Vansteenkiste, J.F.; Van De Kerkhove, C.; Wauters, E.; Van Mol, P. Capmatinib for the treatment of non-small cell lung cancer. Expert Rev. Anticancer Ther. 2019, 8, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Morias, S.; Loredana, G.; Michala, S.; Eileen, G.; Andrew, P.; Justin, S.; Thanh, G.; Eva, B. Treatment-related adverse effects in lung cancer patients after stereotactic ablative radiation therapy. J. Oncol. 2018, 2018, 6483626. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yuan, Q.; Jiang, N. Dihydroartemisinin regulates immune cell heterogeneity by triggering a cascade reaction of CDK and MAPK phosphorylation. Signal Transduct. Target. Ther. 2022, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Jia, H.; Yu, M.; Yang, Y.; Li, J.; Tian, D.; Zhang, H.; Zou, Z. Salvia miltiorrhiza and Pueraria lobata, two eminent herbs in Xin-Ke-Shu, ameliorate myocardial ischemia partially by modulating the accumulation of free fatty acids in rats. Phytomedicine 2021, 89, 153620. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, X.; Huang, S.; Feng, X.; Zhang, X.; Xiang, D. A monomeric polysaccharide from Polygonatum sibiricum improves cognitive functions in a model of Alzheimer’s disease by reshaping the gut microbiota. Int. J. Biol. Macromol. 2022, 213, 404–415. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y.; Hua, H. Complex roles of cAMP-PKA-CREB signaling in cancer. Exp. Hematol. Oncol. 2020, 1, 32. [Google Scholar] [CrossRef]
- Xia, Y.; Zhan, C.; Feng, M.; Leblanc, M.; Ke, E.; Yeddula, N.; Verma, I.M. Targeting CREB pathway suppresses small cell Lung cancer. Mol. Cancer Res. 2018, 5, 825–832. [Google Scholar] [CrossRef]
- Seo, H.S.; Liu, D.D.; Bekele, B.N.; Kim, M.K.; Pisters, K.; Lippman, S.M.; Wistuba, I.I.; Koo, J.S. Cyclic AMP response element-binding protein overexpression: A feature associated with negative prognosis in never smokers with non-small cell lung cancer. Cancer Res. 2008, 15, 6065–6073. [Google Scholar] [CrossRef]
- Liu, X.; Chen, B.; You, W.; Xue, S.; Qin, H.; Jiang, H. The membrane bile acid receptor TGR5 drives cell growth and migration via activation of the JAK2/STAT3 signaling pathway in non-small cell lung cancer. Cancer Lett. 2018, 412, 194–207. [Google Scholar] [CrossRef]
- Li, X.; Ramadori, P.; Pfister, D.; Seehawer, M.; Zender, L.; Heikenwalder, M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer 2021, 9, 541–557. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 1, 65. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Brown, N.J.; Reed, M.W.; Hughes, D.; Woll, P.J. Angiosarcoma. Lancet Oncol. 2010, 10, 983–991. [Google Scholar] [CrossRef]
- World Health Organization. Cancer Today. 2020. Available online: https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 1 December 2020).
- American Society of Clinical Oncology. Liver Cancer: Statistics. 2022. Available online: https://www.cancer.net/cancer-types/liver-cancer/statistics (accessed on 1 February 2022).
- Lisa Fayed. Symptoms of Liver Cancer. 2021. Available online: https://www.verywellhealth.com/liver-cancer-symptoms-514170 (accessed on 25 June 2021).
- Chen, H.; Song, Y.P.; Gao, K.; Zhao, L.T.; Ma, L. Efficacy and safety of Jinhua Qinggan granules for coronavirus disease 2019 (COVID-19): A protocol of a systematic review and meta-analysis. Medicine 2020, 24, e20612. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.J.; Liao, J.K.; Wei, L.; Wang, B.Y.; Kai, L.; Tan, D.X. Treatment efficacy of Lianhua Qingwen capsules for eraly-stage COVID-19. Am. J. Transl. Res. 2022, 2, 1332–1338. [Google Scholar]
- Guo, H.; Zheng, J.; Huang, G.; Xiang, Y.; Lang, C.; Li, B.; Huang, D.; Sun, Q.; Luo, Y.; Zhang, Y.; et al. Xuebijing injection in the treatment of COVID-19: A retrospective case-control study. Ann. Palliat. Med. 2020, 5, 3235–3248. [Google Scholar] [CrossRef]
- Cao, P.; Wu, S.L.; Wu, T.T.; Deng, Y.H.; Zhang, Q.L.; Wang, K.P.; Zhang, Y. The important role of polysaccharides from a traditional Chinese medicine-Lung Cleansing and Detoxifying Decoction against the COVID-19 pandemic. Carbohyd. Polym. 2020, 240, 116346. [Google Scholar] [CrossRef]
- Cai, Y.; Zeng, M.; Chen, Y.Z. The pharmacological mechanism of Huashi Baidu Formula for the treatment of COVID-19 by combined network pharmacology and molecular docking. Ann. Palliat. Med. 2021, 4, 3864–3895. [Google Scholar] [CrossRef]
- Li, C.L.; Lin, Y.K.; Chen, H.A.; Huang, C.Y.; Huang, M.T.; Chang, Y.J. Smoking as an independent risk factor for hepatocellular carcinoma due to the α7-nachr modulating the JAK2/STAT3 signaling axis. J. Clin. Med. 2019, 9, 1391. [Google Scholar] [CrossRef]
- Chen, W.D.; Yu, D.; Forman, B.M.; Huang, W.; Wang, Y.D. Deficiency of G-protein-coupled bile acid receptor Gpbar1 (TGR5) enhances chemically induced liver carcinogenesis. Hepatology 2013, 2, 656–666. [Google Scholar] [CrossRef]
- Ma, S.C.; Zhao, Y.; Zhang, T.; Ling, X.L.; Zhao, D. Association between the ERCC1 rs11615 polymorphism and clinical outcomes of oxaliplatin-based chemotherapies in gastrointestinal cancer: A meta-analysis. OncoTargets Ther. 2015, 8, 641–648. [Google Scholar]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Richa, S.N.; Sageena, G. Dietary factors associated with gastric cancer-a review. Transl. Med. Commun. 2022, 7, 7. [Google Scholar] [CrossRef]
- Cai, H.; Ye, F.; Michel, A.; Murphy, G.; Sasazuki, S.; Taylor, P.R.; Qiao, Y.L.; Park, S.K.; Yoo, K.Y.; Jee, S.H.; et al. Helicobacter pylori blood biomarker for gastric cancer risk in East Asia. Int. J. Epidemiol. 2016, 3, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Wang, T.C. Inflammation, atrophy, and gastric cancer. J. Clin. Investig. 2007, 1, 60–69. [Google Scholar] [CrossRef]
- American Society of Clinical Oncology. Stomach Cancer: Symptoms and Signs. 2022. Available online: https://www.cancer.net/cancer-types/stomach-cancer/symptoms-and-signs (accessed on 12 August 2022).
- Meyer, H.J.; Wilke, H. Treatment strategies in gastric cancer. Dtsch. Arztebl. Int. 2011, 41, 698–705. [Google Scholar] [CrossRef]
- Guo, C.; Qi, H.; Yu, Y.; Zhang, Q.; Su, J.; Yu, D.; Huang, W.; Chen, W.D.; Wang, Y.D. The G-protein-coupled bile acid receptor Gpbar1 (TGR5) inhibits gastric inflammation through antagonizing NF-κB signaling pathway. Front. Pharmacol. 2015, 6, 287. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, L.; Chen, X.; Lu, Q.; Yang, Y.; Liu, J.; Ma, X. SIRT1 counteracted the activation of STAT3 and NF-κB to repress the gastric cancer growth. Int. J. Clin. Exp. Med. 2014, 12, 5050–5058. [Google Scholar]
- Bosch-Presegue, L.; Vaquero, A. The dual role of sirtuins in cancer. Gene Cancer 2011, 2, 648–662. [Google Scholar] [CrossRef]
- Yuan, H.; Su, L.; Chen, W.Y. The emerging and diverse roles of sirtuins in cancer: A clinical perspective. Onco Targets Ther. 2013, 6, 1399–1416. [Google Scholar]
- Kume, S.; Haneda, M.; Kanasaki, K.; Sugimoto, T.; Araki, S.; Isshiki, K.; Isono, M.; Uzu, T.; Guarente, L.; Kashiwagi, A.; et al. SIRT1 inhibits transforming growth factor beta-induced apoptosis in glomerular mesangial cells via Smad7 deacetylation. J. Biol. Chem. 2007, 282, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Su, J.; Li, Z.; Xiao, R.; Wen, J.; Li, Y.; Zhang, M.; Zhang, X.; Yu, D.; Huang, W.; et al. The G-protein-coupled bile acid receptor Gpbar1 (TGR5) suppresses gastric cancer cell proliferation and migration through antagonizing STAT3 signaling pathway. Oncotarget 2015, 33, 34402–34413. [Google Scholar] [CrossRef] [PubMed]
- American Society of Clinical Oncology. Colorectal Cancer. 2022. Available online: https://www.cancer.net/cancer-types/colorectal-cancer/statistics (accessed on 1 May 2022).
- Rogler, G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014, 2, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 10, 101174. [Google Scholar] [CrossRef] [PubMed]
- McQuade, R.M.; Stojanovska, V.; Bornstein, J.C.; Nurgali, K. Colorectal cancer chemotherapy: The evolution of treatment and new approaches. Curr. Med. Chem. 2017, 15, 1537–1557. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Zhang, C. Ursodeoxycholic acid suppresses the malignant progression of colorectal cancer through TGR5-YAP axis. Cell Death Discov. 2021, 7, 207. [Google Scholar] [CrossRef]
- Ward, J.B.J.; Lajczak, N.K.; Kelly, O.B.; O’Dwyer, A.M.; Giddam, A.K.; Ni Gabhann, J.; Franco, P.; Tambuwala, M.M.; Jeferies, C.A.; Keely, S.; et al. Ursodeoxycholic acid and lithocholic acid exert anti-infammatory actions in the colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G550–G558. [Google Scholar] [CrossRef]
- Pols, T.W.; Noriega, L.G.; Nomura, M.; Auwerx, J.; Schoonjans, K. The bile acid membrane receptor TGR5: A valuable metabolic target. Dig. Dis. 2011, 1, 37–44. [Google Scholar] [CrossRef]
- Pols, T.W.; Noriega, L.G.; Nomura, M.; Auwerx, J.; Schoonjans, K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J. Hepatol. 2011, 6, 1263–1272. [Google Scholar] [CrossRef]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 3, 167–177. [Google Scholar] [CrossRef]
- Casaburi, I.; Avena, P.; Lanzino, M.; Sisci, D.; Giordano, F.; Maris, P.; Catalano, S.; Morelli, C.; Andò, S. Chenodeoxycholic acid through a TGR5-dependent CREB signaling activation enhances cyclin D1 expression and promotes human endometrial cancer cell proliferation. Cell Cycle 2012, 14, 2699–2710. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.H.; Bard, J.M.; Carbonnelle, D.; Chaillou, C.; Huvelin, J.M.; Bobin-Dubigeon, C.; Nazih, H. Lithocholic bile acid inhibits lipogenesis and induces apoptosis in breast cancer cells. Cell. Oncol. 2018, 1, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kovács, P.; Csonka, T.; Kovács, T.; Sári, Z.; Ujlaki, G.; Sipos, A.; Karányi, Z.; Szeőcs, D.; Hegedűs, C.; Uray, K.; et al. Lithocholic Acid, a Metabolite of the Microbiome, Increases Oxidative Stress in Breast Cancer. Cancers 2019, 9, 1255. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Y.; He, S.J.; Ma, J.J.; Hu, H.; Gong, Y.P.; Wang, Y.L.; Hu, B.J.; Xie, J.Z.; Tu, W.Z.; Huang, Q.; et al. High expression of TGR5 predicts a poor prognosis in patients with pancreatic cancer. Int. J. Clin. Exp. Pathol. 2018, 7, 3567–3574. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Duan, G.; Wei, D.; Zhao, C.; Ma, Y. The Bile Acid Membrane Receptor TGR5 in Cancer: Friend or Foe? Molecules 2022, 27, 5292. https://doi.org/10.3390/molecules27165292
Qi Y, Duan G, Wei D, Zhao C, Ma Y. The Bile Acid Membrane Receptor TGR5 in Cancer: Friend or Foe? Molecules. 2022; 27(16):5292. https://doi.org/10.3390/molecules27165292
Chicago/Turabian StyleQi, Youchao, Guozhen Duan, Dengbang Wei, Chengzhou Zhao, and Yonggui Ma. 2022. "The Bile Acid Membrane Receptor TGR5 in Cancer: Friend or Foe?" Molecules 27, no. 16: 5292. https://doi.org/10.3390/molecules27165292
APA StyleQi, Y., Duan, G., Wei, D., Zhao, C., & Ma, Y. (2022). The Bile Acid Membrane Receptor TGR5 in Cancer: Friend or Foe? Molecules, 27(16), 5292. https://doi.org/10.3390/molecules27165292




