Rapid Detection of Aspergillus flavus and Quantitative Determination of Aflatoxin B1 in Grain Crops Using a Portable Raman Spectrometer Combined with Colloidal Au Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
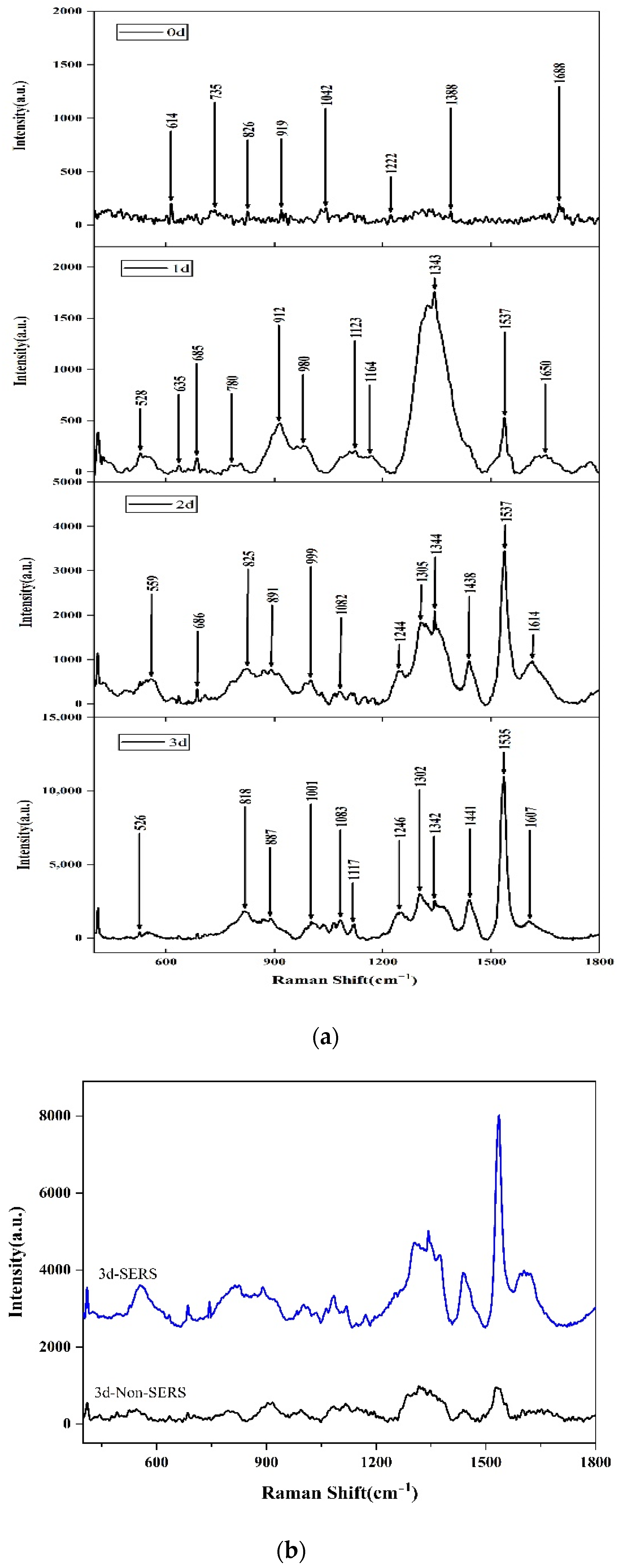
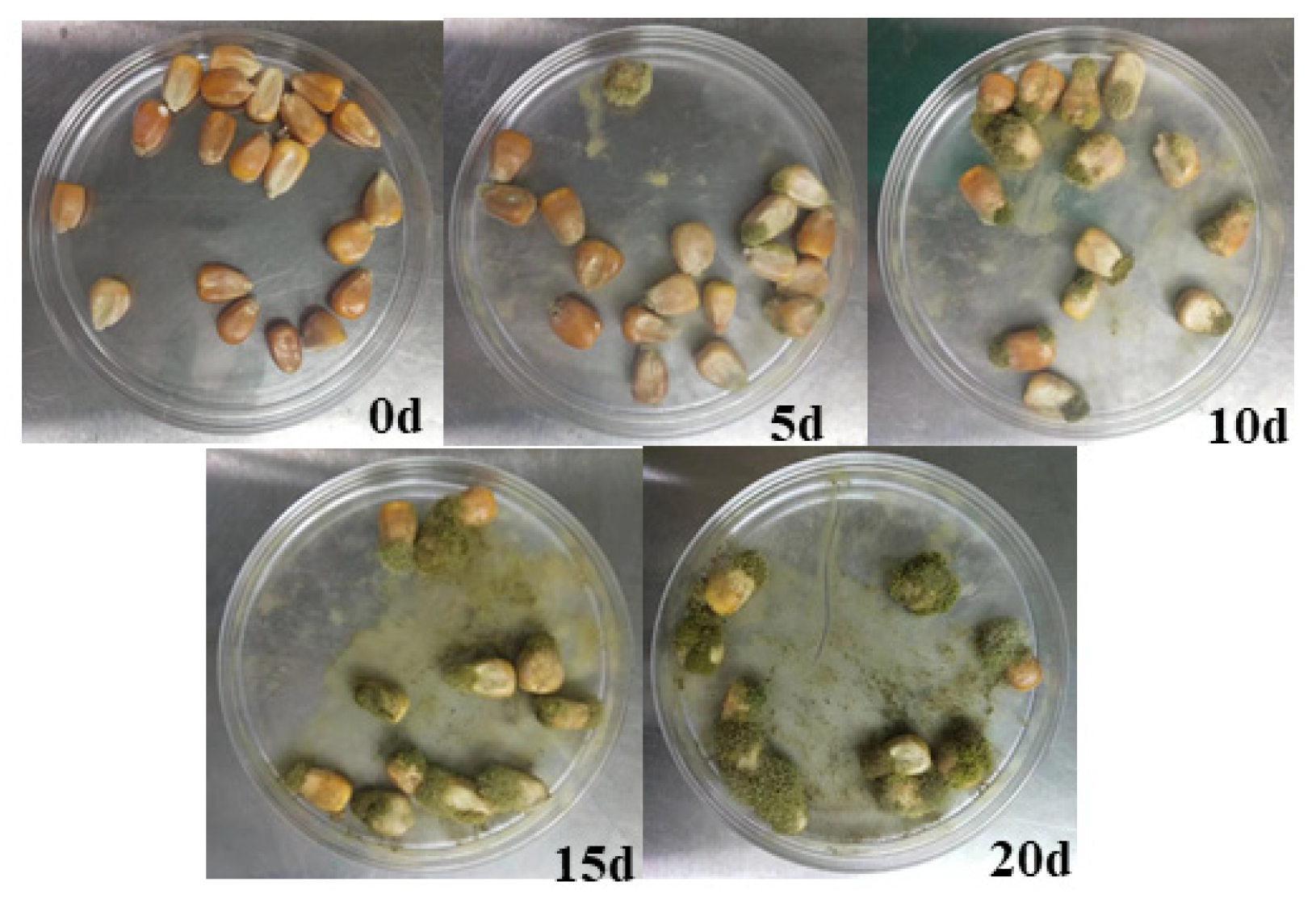
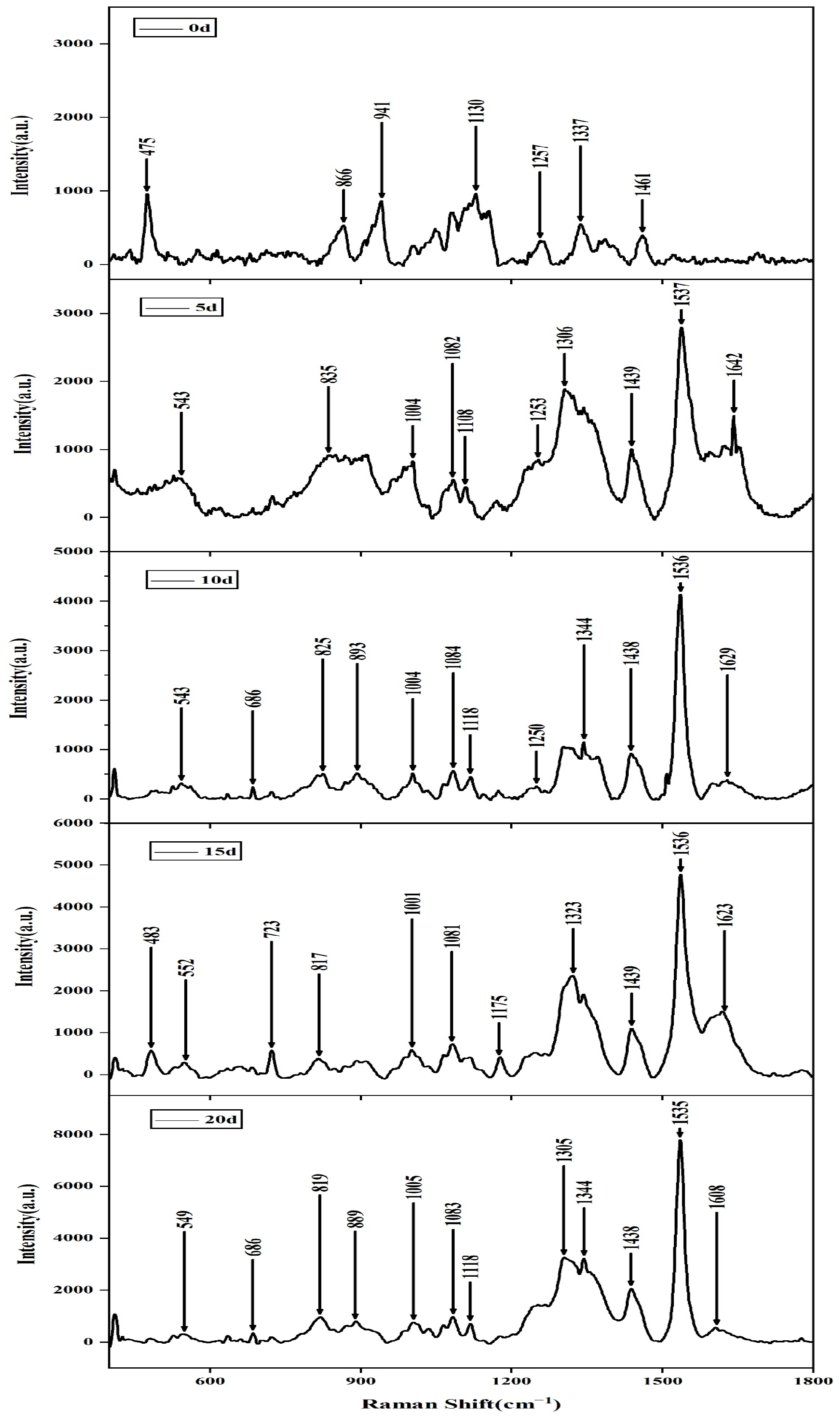
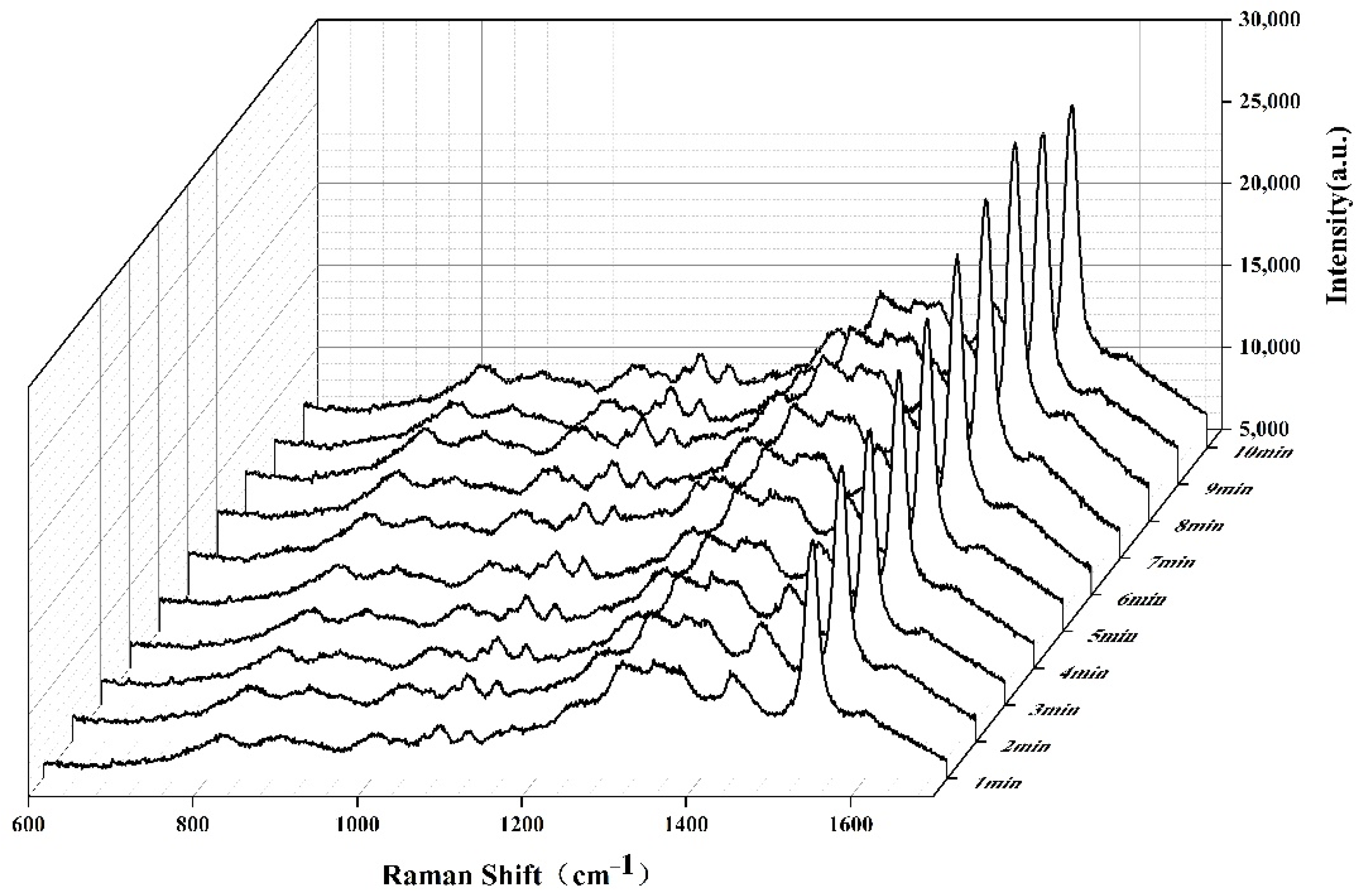
2.1. SERS of Aspergillus flavus on the Medium and on Corn
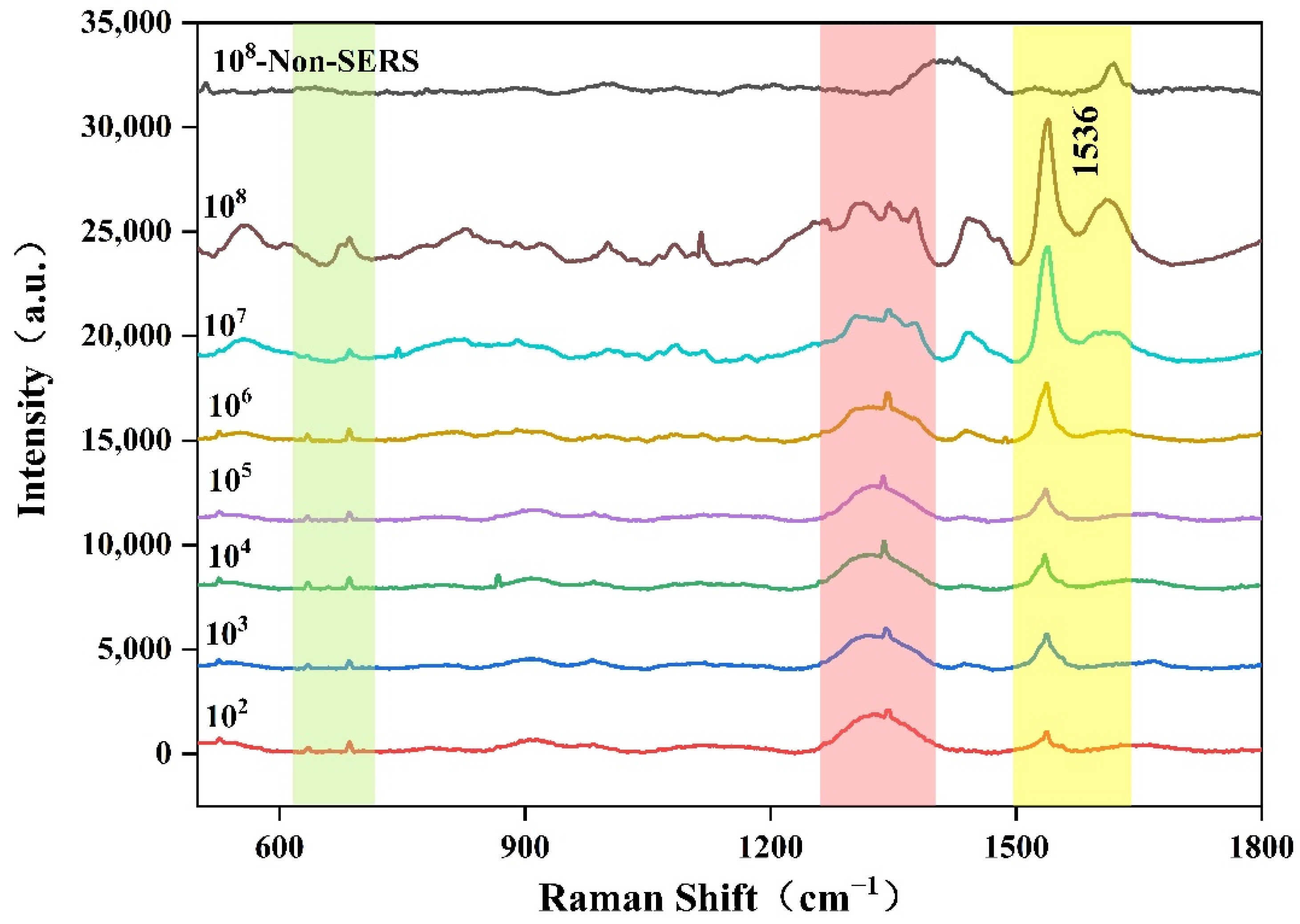
2.2. SERS of Aspergillus flavus with Different Concentrations
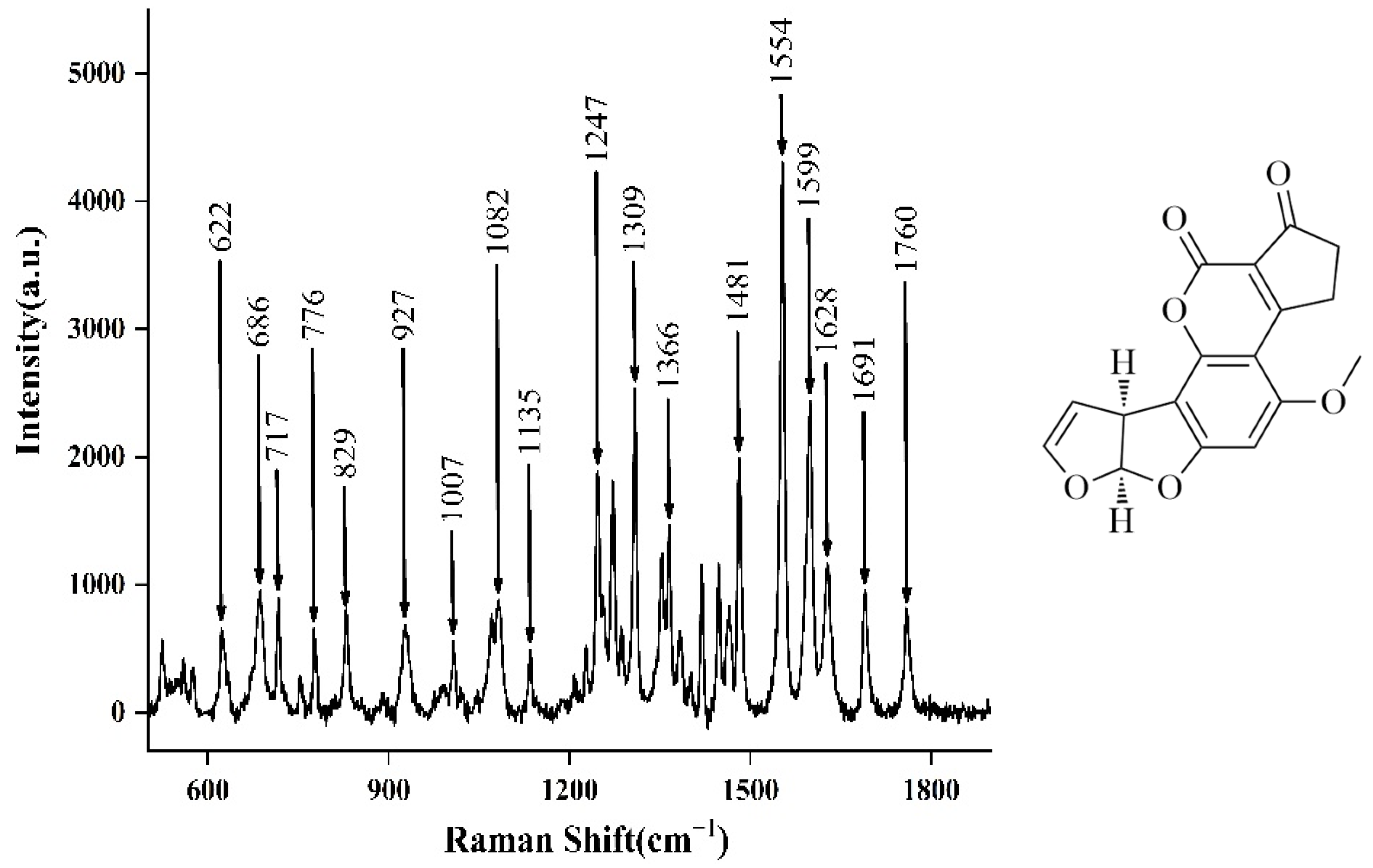
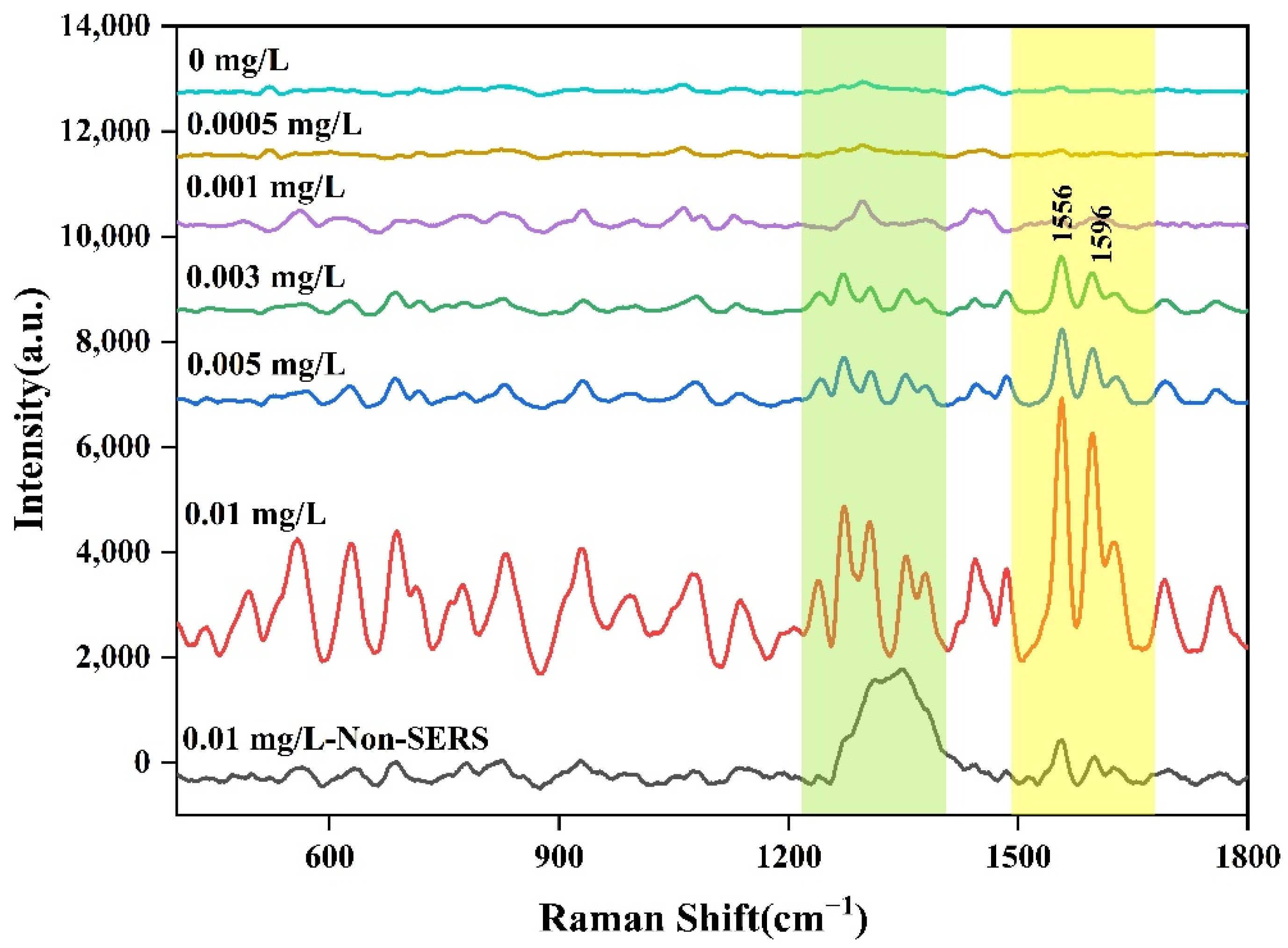
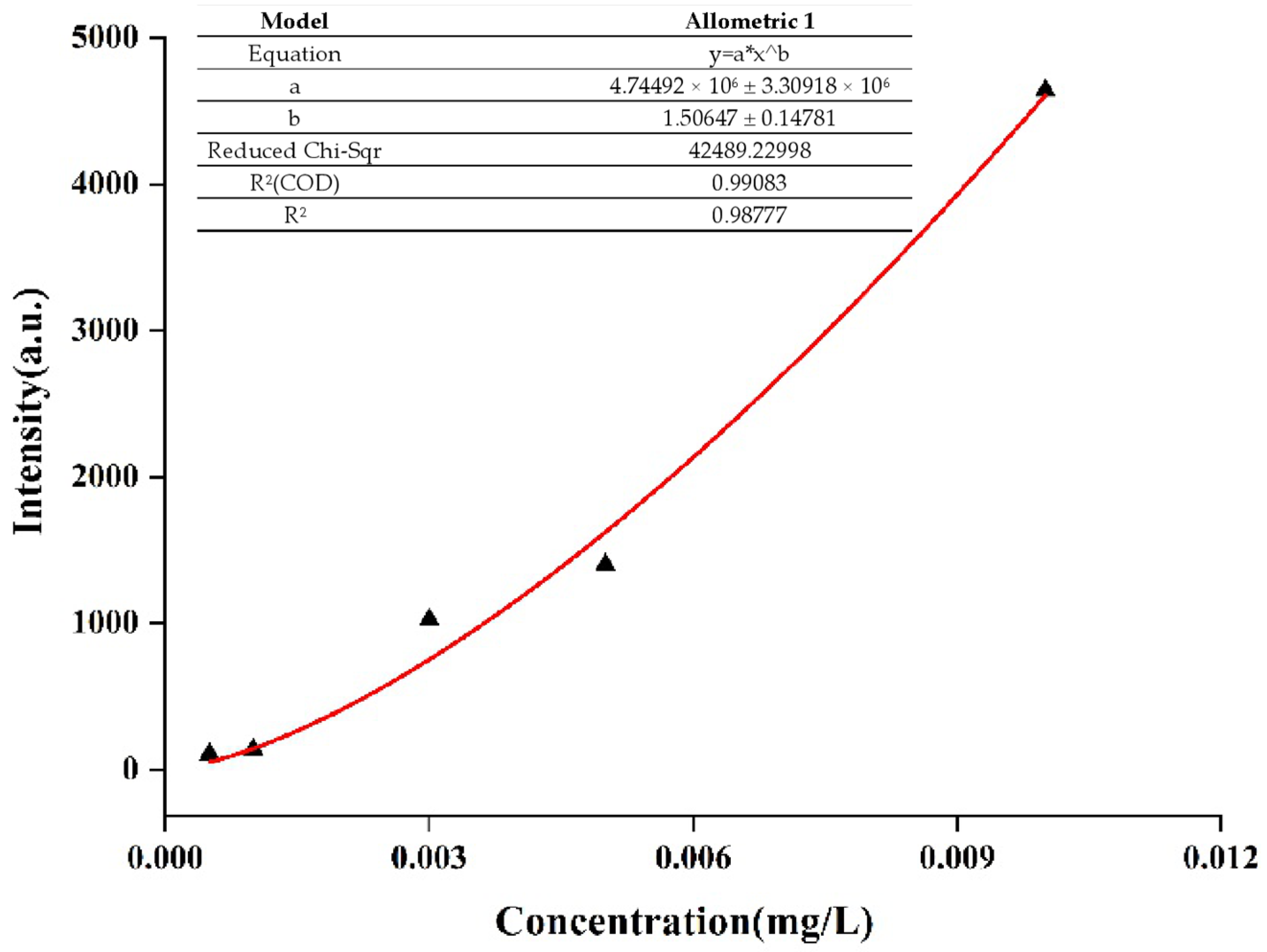
2.3. SERS of Aflatoxin B1
3. Materials and Methods
3.1. Reagents and Materials
3.2. Culture of Aspergillus flavus
3.3. Preparation of Aflatoxin B1 Samples
3.4. SERS Measurement and Spectra Preprocessing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Perrone, G.; Susca, A.; Cozzi, G.; Ehrlich, K.; Varga, J.; Frisvad, J.C.; Meijer, M.; Noonim, P.; Mahakarnchanakul, W.; Samson, R.A. Biodiversity of Aspergillus species in some important agricultural products. Stud. Mycol. 2007, 59, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.W.; Zhang, H.; Liu, R.C.; Liu, C.L. Potential for aflatoxin B1 and B2 production by Aspergillus flavus strains isolated from rice samples. Saudi J. Biol. Sci. 2015, 22, 176–180. [Google Scholar] [CrossRef]
- Kosegarten, C.E.; Ramírez-Corona, N.; Mani-López, E.; Palou, E.; López-Malo, A. Description of Aspergillus flavus growth under the influence of different factors (water activity, incubation temperature, protein and fat concentration, pH, and cinnamon essential oil concentration) by kinetic, probability of growth, and time-to-detection models. Int. J. Food Microbiol. 2017, 240, 115–123. [Google Scholar] [PubMed]
- Nakai, V.K.; Rocha, L.O.; Goncalez, E.; Fonseca, H.; Ortega, E.M.M.; Correa, B. Distribution of fungi and aflatoxins in a stored peanut variety. Food Chem. 2008, 106, 285–290. [Google Scholar] [CrossRef]
- Bbosa, G.S.; Kitya, D.; Lubega, A.; Ogwal-Okeng, J.; Anokbonggo, W.W.; Kyegombe, D.B. Review of the Biological and Health Efects of Aflatoxins on Body Organs and Body Systems. In Aflatoxins-Recent Advances and Future Prospects, 1st ed.; Razzaghi-Abyaneh, M., Ed.; InTech: Rijeka, Croatia, 2013; pp. 239–265. [Google Scholar]
- Nguyen, T.B.; Vu, T.B.; Pham, H.M.; Tran, C.S.; Le, H.H.; Vo, N.T. Detection of Aflatoxins B1 in Maize Grains Using Fluorescence Resonance Energy Transfer. Appl. Sci. 2020, 10, 1578–1586. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC Monographs: Lyon, France, 1993; pp. 359–362. [Google Scholar]
- Piermarini, S.; Micheli, L.; Ammida, N.H.S.; Palleschi, G.; Moscone, D. Electrochemical immunosensor array using a 96-well screen-printed microplate for Aflatoxin B1 detection. Biosens. Bioelectron. 2007, 22, 1434–1440. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China, State Administration for Market Regulation. GB 2761-2017[S]; National Food Safety Standard Limit of Mycotoxins in Food. Quality and Standards Publishing & Media Co., Ltd.: Beijing, China, 2017. [Google Scholar]
- Tournas, V.H.; Niazi, N.S. Potentially toxigenic fungi from selected grains and grain products. J. Food Saf. 2018, 38, 12422–12427. [Google Scholar] [CrossRef]
- Aboutalebin, S.; Ahmadikia, K.; Fakhim, H.; Chabavizadeh, J.; Okhovat, A.; Nikaeen, M.; Mirhendi, H. Direct detection and identification of the most common bacteria and fungi causing otitis externa by a stepwise multiplex PCR. Front. Cell. Infect. Microbiol. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Yang, A.F.; Zheng, Q.Y.; Wan, C.; Cao, J.J. Establishment of a real-time PCR method for detection of main fungus in grain. Cereal Feed Ind. 2020, 4, 1–3. [Google Scholar]
- Priyanka, S.R.; Venkatramana, M.; Balakrishna, K.; Murali, H.S.; Batra, H.V. Development and evaluation of a multiplex PCR assay for simultaneous detection of major mycotoxigenic fungi from cereals. J. Food Sci. Tech. 2015, 52, 486–492. [Google Scholar] [CrossRef]
- Sardiñas, N.; Vázquez, C.; Gil-Serna, J.; González-Jaén, T.; Patiño, B. Specific detection and quantification of Aspergillus flavus and Aspergillus parasiticus inwheat flour by SYBR® Green quantitative PCR. Int. J. Food Microbiol. 2011, 145, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Pan, Y.; Wang, Y.; Ahmed, S.; Liu, Z.; Peng, D.; Yuan, Z. Preparation of a broad-spectrum anti-zearalenone and its primary analogues antibody and its application in an indirect competitive enzyme-linked immunosorbent assay. Food Chem. 2018, 247, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.F.; Uriel, N.; Teifoori, F.; Postigo, I.; Suñén, E.; Martínez, J. The major Alternaria alternata allergen, Alt a 1: A reliable and specific marker of fungal contamination in citrus fruits. Int. J. Food Microbiol. 2017, 257, 26–30. [Google Scholar] [CrossRef]
- Henri, A.; Antti, T.; Alexis, N.; Tarja, N. A simple and specific noncompetitive ELISA method for HT-2 toxin detection. Toxins 2017, 9, 145–154. [Google Scholar]
- Jayaratne, W.M.S.C.; Abeyratne, A.H.M.A.K.; Zoysa, H.K.S.; Dissanayake, D.M.R.B.N.; Bamunuarachchige, T.C.; Waisundara, V.Y.; Chang, S. Detection and quantification of Aflatoxin B1 in corn and corn-grown soils in the district of Anuradhapura, Sri Lanka. Heliyon 2020, 6, 5319–5324. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Zheng, P.; Zhang, Z.X.; He, Y.Z. Determination of aflatoxins in high-pigment content samples by matrix solid-phase dispersion and high-performance liquid chromatography. J. Agric. Food Chem. 2006, 54, 4126–4130. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J.; Wang, P.; Wang, Y.; Chen, J. Thin-layer chromatography of mycotoxins and comparison with other chromatographic methods. J. Chromatogr. A 1998, 815, 3–20. [Google Scholar] [CrossRef]
- Rossi, C.N.; Takabayashi, C.R.; Ono, M.A.; Saito, G.H.; Itano, E.N.; Kawamura, O.K.; Hirooka, E.Y.; Ono, E.Y.S. Immunoassay based on monoclonal antibody for aflatoxin detection in poultry feed. Food Chem. 2012, 132, 2211–2216. [Google Scholar] [CrossRef]
- Vig, A.; Radoi, A.; Munoz-Berbel, X.; Gyemant, G.; Marty, J.L. Impedimetric aflatoxin M1 immunosensor based on colloidal gold and silver electrodeposition. Sens. Actuators B Chem. 2009, 138, 214–220. [Google Scholar] [CrossRef]
- Yu, L.L.; Zhang, Y.; Hu, C.Y.; Wu, H.; Yang, Y.Y.; Huang, C.S.; Jia, N.Q. Highly sensitive electrochemical impedance spectroscopy immunosensor for the detection of AFB1 in olive oil. Food Chem. 2015, 176, 22–26. [Google Scholar] [CrossRef]
- Chu, X.; Wang, W.; Ni, X.Z.; Zheng, H.T.; Zhao, X.; Zhang, R.; Li, Y.F. Growth Identification of Aspergillus flavus and Aspergillus parasiticus by Visible/Near-Infrared Hyperspectral Imaging. Appl. Sci. 2018, 8, 513–524. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, L.M.; Fang, Y.P.; Zheng, D.W.; Lin, T.F.; Wang, H.Q. Label-free exosomal detection and classification in rapid discriminating different cancer types based on specific Raman phenotypes and multivariate statistical analysis. Molecules 2019, 24, 2947–2961. [Google Scholar] [CrossRef]
- Zheng, D.W.; Liu, X.Y.; Zhang, P.; Su, L.; Wang, L.M.; Wei, X.D.; Wang, H.Q.; Lin, T.F. Rapid identification of mixed enteropathogenic bacteria by means of Au nanoparticles@bacteria using portable Raman spectrometer. J. Nanosci. Nanotechnol. 2018, 18, 6776–6785. [Google Scholar] [CrossRef]
- Fang, Y.P.; Lin, T.F.; Zheng, D.W.; Zhu, Y.W.; Wang, L.M.; Fu, Y.Y.; Wang, H.Q.; Wu, X.H.; Zhang, P. Rapid and label-free identification of different cancer types based on surface-enhanced Raman scattering profiles and multivariate statistical analysis. J. Cell. Biochem. 2020, 122, 277–289. [Google Scholar] [CrossRef]
- Cui, X.Y.; Liu, T.; Xu, X.S.; Zhao, Z.Y.; Tian, Y.; Zhao, Y.; Chen, S.; Wang, Z.; Wang, Y.D.; Hu, D.Y.; et al. Label-free detection of multiple genitourinary cancers from urine by surface-enhanced Raman spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 240, 118543–118549. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, M.H.; Huang, S.G.; Yuan, H.C.; Zhao, J.H.; Chen, J. Surface-enhanced Raman spectroscopy method for classification of doxycycline hydrochloride and tylosin in duck meat using gold nanoparticles. Poult. Sci. 2021, 100, 101165–101173. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.X.; Sun, D.W.; Pu, H.B.; Wei, Q.Y. Rapid nondestructive detection of mixed pesticides residues on fruit surface using SERS combined with self-modeling mixture analysis method. Talanta 2020, 217, 120998–121009. [Google Scholar] [CrossRef]
- Audilakshmi, S.; Stenhouse, J.W.; Reddy, T.P. Genetic analysis of grain mold resistance in white seed sorghum genotypes. Euphytica 2005, 145, 95–101. [Google Scholar] [CrossRef]
- Caldwell, J.; Taladriz-Blanco, P.; Rothen-Rutishauser, B.; Petri-Fink, A. Detection of Sub-Micro- and Nanoplastic Particles on Gold Nanoparticle-Based Substrates through Surface-Enhanced Raman Scattering (SERS) Spectroscopy. Nanomaterials 2021, 11, 1149–1166. [Google Scholar] [CrossRef]
- Bharathi, M.S.S.; Byram, C.; Banerjee, D.; Sarma, D.; Barkakaty, B.; Soma, V.R. Gold nanoparticle nanofibres as SERS substrate for detection of methylene blue and a chemical warfare simulant (methyl salicylate). Bull. Mater. Sci. 2021, 44, 103–110. [Google Scholar] [CrossRef]
- Zavyalova, E.; Ambartsumyan, O.; Zhdanov, G.; Gribanyov, D.; Gushchin, V.; Tkachuk, A.; Rudakova, E.; Nikiforova, M.; Kuznetsova, N.; Popova, L.; et al. SERS-Based Aptasensor for Rapid Quantitative Detection of SARS-CoV-2. Nanomaterials 2021, 11, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Kozhina, E.P.; Bedin, S.A.; Nechaeva, N.L.; Podoynitsyn, S.N.; Tarakanov, V.P.; Andreev, S.N.; Grigoriev, Y.V.; Naumov, A.V. Ag-Nanowire Bundles with Gap Hot Spots Synthesized in Track-Etched Membranes as Effective SERS-Substrates. Appl. Sci. 2021, 11, 1375–1387. [Google Scholar] [CrossRef]
- Zhu, C.H.; Meng, G.W.; Zheng, P.; Huang, Q.; Li, Z.B.; Hu, X.Y.; Wang, X.J.; Huang, Z.L.; Li, F.D.; Wu, N.Q. A Hierarchically Ordered Array of Silver-Nanorod Bundles for Surface-Enhanced Raman Scattering Detection of Phenolic Pollutants. Adv. Mater. 2016, 28, 4871–4876. [Google Scholar] [CrossRef] [PubMed]
- Tommasini, M.; Zanchi, C.; Lucotti, A.; Bombelli, A.; Villa, N.S.; Casazza, M.; Ciusani, E.; Grazia, U.; Santoro, M.; Fazio, E.; et al. Laser-Synthesized SERS Substrates as Sensors toward Therapeutic Drug Monitoring. Nanomaterials 2019, 9, 677–691. [Google Scholar] [CrossRef]
- Halas, N.J.; Moskovits, M. Surface-enhanced Raman spectroscopy: Substrates and materials for research and applications. MRS Bull. 2013, 38, 607–611. [Google Scholar] [CrossRef]
- Alsammarraie, F.K.; Lin, M.S.; Mustapha, A.; Lin, H.T.; Chen, X.; Chen, Y.H.; Wang, H.; Huang, M.Z. Rapid determination of thiabendazole in juice by SERS coupled with novel gold nanosubstrates. Food Chem. 2018, 259, 219–225. [Google Scholar] [CrossRef]
- Wang, K.Q.; Sun, D.W.; Pu, H.B.; Wei, Q.Y. Shell thickness-dependent Au@Ag nanoparticles aggregates for high-performance SERS applications. Talanta 2019, 195, 506–515. [Google Scholar] [CrossRef]
- Hu, B.X.; Sun, D.W.; Pu, H.B.; Wei, Q.Y. Recent advances in detecting and regulating ethylene concentrations for shelf-life extension and maturity control of fruit: A review. Trends Food Sci. Technol. 2019, 91, 66–82. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhai, W.L.; Han, C.R.; Wang, H.; Zhao, Z.L.; Wang, M. Determination of aflatoxin B1 in peanut by QuEChERS-surface-enhanced Raman spectroscopy. J. Food Saf. Qual. 2022, 13, 1502–1508. [Google Scholar]
- Yang, X.Q.; Yu, H.C.; Yin, Y.; Yuan, Y.X.; Wu, H.; Li, X. Detection of Aflatoxin B1 and Zearalenone in maize by Raman Spectroscopy. J. Nucl. Agric. Sci. 2021, 35, 159–166. [Google Scholar]
- Egging, V.; Nguyen, J.; Kurouski, D. Detection and Identification of Fungal Infections in Intact Wheat and Sorghum Grain Using a Hand-Held Raman Spectrometer. Anal. Chem. 2018, 90, 8616–8621. [Google Scholar] [CrossRef] [PubMed]
- Shetty, G.; Kendall, C.; Shepherd, N.; Stone, N.; Barr, H. Raman spectroscopy: Elucidation of biochemical changes in carcinogenesis of oesophagus. Br. J. Cancer 2006, 94, 1460–1464. [Google Scholar] [CrossRef]
- Bittel, M.; Assaf, A.; Cordella, C.B.Y.; Durand, M.J.; Thouand, G. Potential of Raman Spectroscopy to Monitor Arsenic Toxicity on Bacteria: Insights toward Multiparametric Bioassays. Environ. Sci. Technol. 2015, 49, 12324–12332. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.D.; Zheng, D.W.; Zhang, P.; Lin, T.F.; Wang, H.Q.; Zhu, Y.W. Surface enhanced Raman scattering investigation of bovine albumin with different size of Au nanoparticles. J. Appl. Biomater. Func. 2018, 16, 157–162. [Google Scholar]
- Shi, Y.Y.; Liu, W.F.; Chen, C.P. Two-step centrifugation method for subpicomolar surface-enhanced Raman scattering detection. Anal. Chem. 2016, 88, 5009–5015. [Google Scholar] [CrossRef] [PubMed]
- Futamata, M.; Ishikura, M.; Iida, C.; Handa, S. The critical importance of gap modes in surface enhanced Raman scattering. Faraday Discuss. 2015, 178, 203–220. [Google Scholar] [CrossRef]
- Wu, X.; Gao, S.; Wang, J.S.; Wang, H.; Huang, Y.W.; Zhao, Y. The surface-enhanced Raman spectra of aflatoxins: Spectral analysis, density functional theory calculation. Detect Differ. Anal. 2012, 137, 4226–4234. [Google Scholar] [CrossRef]
- Gao, S.M.; Wang, H.Y.; Lin, Y.X.; Li, R.H. Surface-enhanced Raman spectra of aflatoxin B1 adsorbed on silver clusters. Acta Phys. -Chim. Sin. 2012, 28, 2044–2050. [Google Scholar]
- Liu, M.J.; Ren, X.F.; Zheng, D.W.; Lin, T.F.; Zhang, P.; Wang, H.Q. Determination of aflatoxin B1 in corn or corn products by post-column photochemical derivatization. China Feed 2022, 9, 92–95. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, M.; Zhang, Y.; Zhao, H.; Lu, W.; Lin, T.; Zhang, P.; Zheng, D. Rapid Detection of Aspergillus flavus and Quantitative Determination of Aflatoxin B1 in Grain Crops Using a Portable Raman Spectrometer Combined with Colloidal Au Nanoparticles. Molecules 2022, 27, 5280. https://doi.org/10.3390/molecules27165280
Wang H, Liu M, Zhang Y, Zhao H, Lu W, Lin T, Zhang P, Zheng D. Rapid Detection of Aspergillus flavus and Quantitative Determination of Aflatoxin B1 in Grain Crops Using a Portable Raman Spectrometer Combined with Colloidal Au Nanoparticles. Molecules. 2022; 27(16):5280. https://doi.org/10.3390/molecules27165280
Chicago/Turabian StyleWang, Huiqin, Mengjia Liu, Yumiao Zhang, Huimin Zhao, Wenjing Lu, Taifeng Lin, Ping Zhang, and Dawei Zheng. 2022. "Rapid Detection of Aspergillus flavus and Quantitative Determination of Aflatoxin B1 in Grain Crops Using a Portable Raman Spectrometer Combined with Colloidal Au Nanoparticles" Molecules 27, no. 16: 5280. https://doi.org/10.3390/molecules27165280
APA StyleWang, H., Liu, M., Zhang, Y., Zhao, H., Lu, W., Lin, T., Zhang, P., & Zheng, D. (2022). Rapid Detection of Aspergillus flavus and Quantitative Determination of Aflatoxin B1 in Grain Crops Using a Portable Raman Spectrometer Combined with Colloidal Au Nanoparticles. Molecules, 27(16), 5280. https://doi.org/10.3390/molecules27165280




