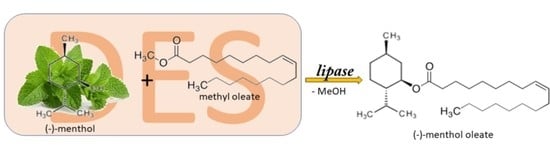
DES-Based Biocatalysis as a Green Alternative for the l-menthyl Ester Production Based on l-menthol Acylation
Abstract
1. Introduction
2. Results and Discussion
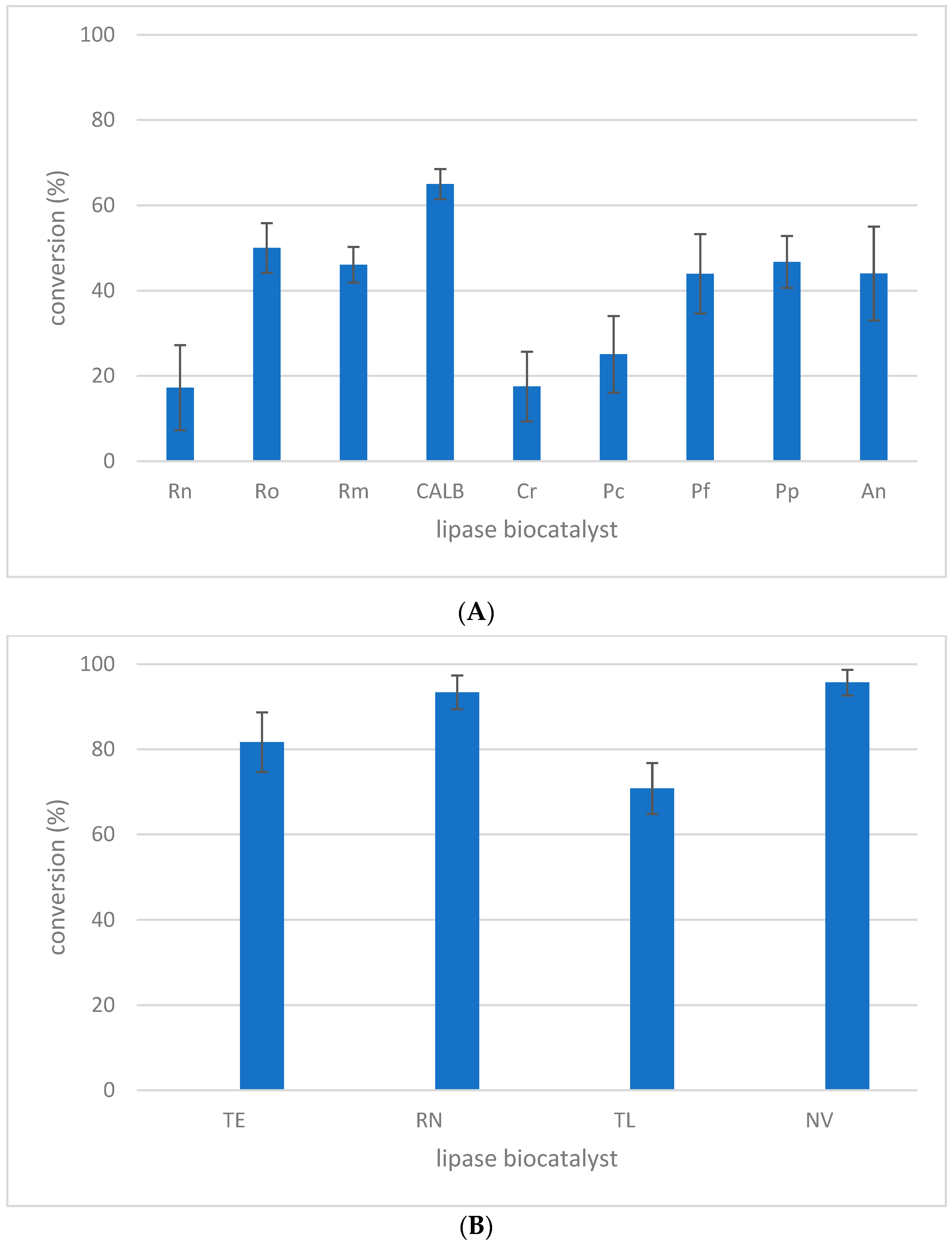
2.1. Lipase Screening for l-menthol Acylation
2.2. Characterization of DES(s) Composition
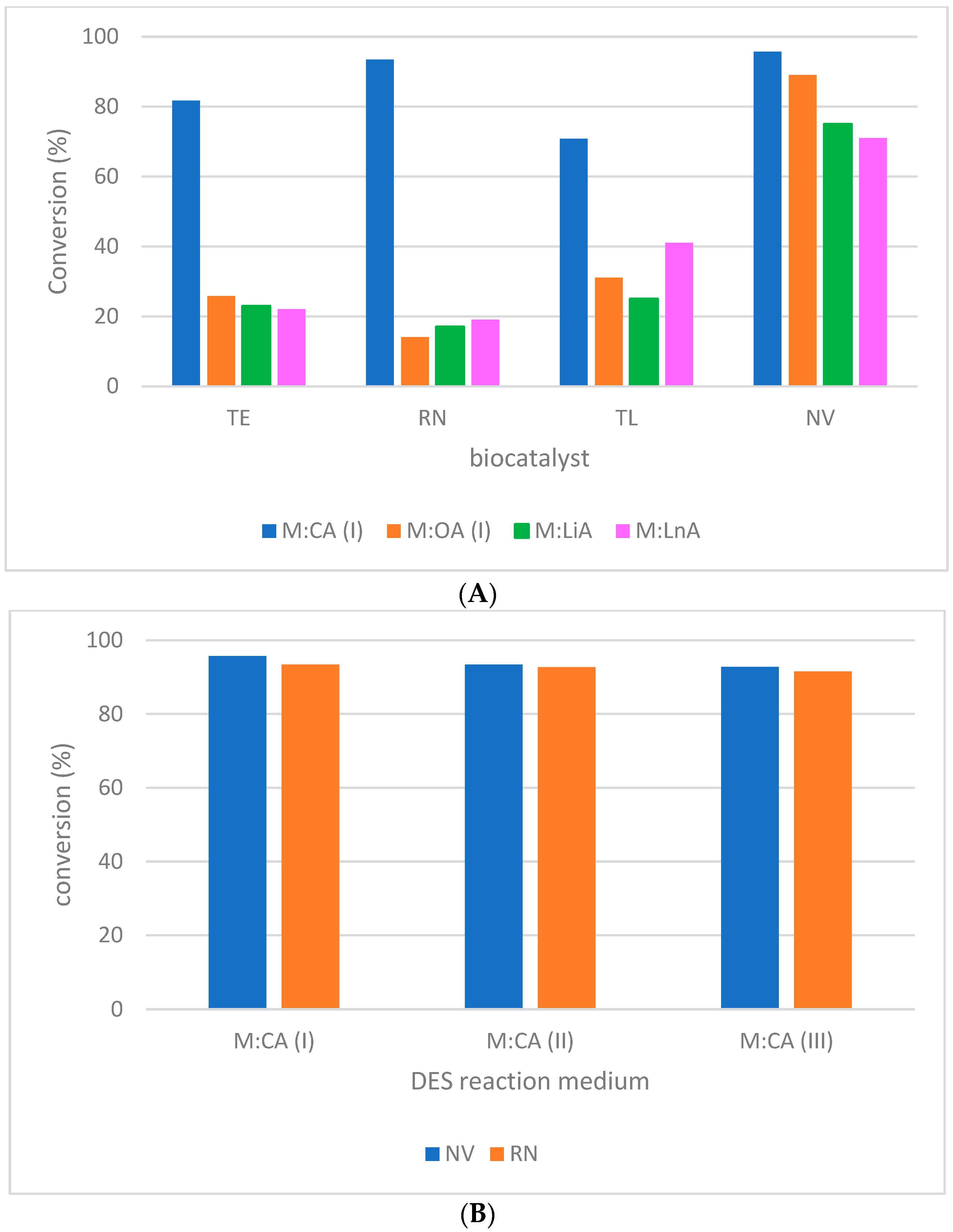
2.3. Influence of DES Composition on the Biocatalytic System
2.4. Testing the Effect of FAME on FME Production
2.5. Stability of the Biocatalyst under Optimal System Conditions
3. Experimental
3.1. Chemicals and Solutions
3.2. DES Preparation
3.3. DES Characterization
3.4. Biocatalytic Approach for l-menthol Acylation
3.5. Evaluation of the Biocatalyst Recyclability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Babali, B.; Tüter, M.; Ustun, G. Enzymatic esterification of (−)-menthol with lauric acid in isooctane by sorbitan monostearate-coated lipase from Candida rugosa. J. Am. Oil Chem. Soc. 2001, 78, 173–175. [Google Scholar] [CrossRef]
- Babali, B.; Aksoy, H.A.; Tüter, M.; Ustun, G. Enzymatic esterification of (−)-menthol with fatty acids in solvent by a commercial lipase from Candida rugosa. J. Am. Oil Chem. Soc. 2001, 78, 53–56. [Google Scholar] [CrossRef]
- Oertling, H.; Reckziegel, A.; Surburg, H.; Bertram, H.-J. Applications of Menthol in Synthetic Chemistry. Chem. Rev. 2007, 107, 2136–2164. [Google Scholar] [CrossRef]
- Craveiro, R.; Meneses, L.; Durazzo, L.; Rocha, A.; Silva, J.M.; Reis, R.L.; Barreiros, S.; Duarte, A.; Paiva, A. Deep Eutectic Solvents for Enzymatic Esterification of Racemic Menthol. ACS Sustain. Chem. Eng. 2019, 7, 19943–19950. [Google Scholar] [CrossRef]
- Belafriekh, A.; Secundo, F.; Serra, S.; Djeghaba, Z. Enantioselective enzymatic resolution of racemic alcohols by lipases in green organic solvents. Tetrahedron. Asymmetry 2017, 28, 473–478. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nagao, T.; Kawashima, A.; Watanabe, Y.; Shimada, Y. Synthesis of Polyunsaturated Fatty Acid L-Menthyl Esters through Lipase-Catalyzed Esterification in an Organic Solvent-Free System. J. Oleo Sci. 2004, 53, 309–312. [Google Scholar] [CrossRef][Green Version]
- Soel, S.M.; Choi, O.S.; Bang, M.H.; Park, J.H.Y.; Kim, W.K. Influence of conjugated linoleic acid isomers on the metastasis of colon cancer cells in vitro and in vivo. J. Nutr. Biochem. 2007, 18, 650–657. [Google Scholar] [CrossRef]
- Park, Y.; Albright, K.; Storkson, J.; Liu, W.; Pariza, M. Conjugated Linoleic Acid (CLA) Prevents Body Fat Accumulation and Weight Gain in an Animal Model. J. Food Sci. 2007, 72, S612–S617. [Google Scholar] [CrossRef]
- Nagao, K.; Inoue, N.; Wang, Y.-M.; Yanagita, T. Conjugated linoleic acid enhances plasma adiponectin level and alleviates hyperinsulinemia and hypertension in Zucker diabetic fatty (fa/fa) rats. Biochem. Biophys. Res. Commun. 2003, 310, 562–566. [Google Scholar] [CrossRef]
- Ni, Y.; Holtmann, D.; Hollmann, F. How Green is Biocatalysis? To Calculate is To Know. ChemCatChem 2014, 6, 930–943. [Google Scholar] [CrossRef]
- Stamatis, H.; Xenakis, A.; Menge, U.; Kolisis, F.N. Kinetic study of lipase catalyzed esterification reactions in water-in-oil microemulsions. Biotechnol. Bioeng. 1993, 42, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, N.; Goto, M. How Is Enzymatic Selectivity of Menthol Esterification Catalyzed by Surfactant-Coated Lipase Determined in Organic Media? Biotechnol. Prog. 1997, 13, 488–492. [Google Scholar] [CrossRef]
- Cantone, S.; Hanefeld, U.; Basso, A. Biocatalysis in non-conventional media—Ionic liquids, supercritical fluids and the gas phase. Green Chem. 2007, 9, 954–971. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zheng, G.-W.; Zong, M.-H.; Li, N.; Lou, W.-Y. Recent progress on deep eutectic solvents in biocatalysis. Bioresour. Bioprocess. 2017, 4, 1–18. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Florindo, C.; Iff, L.C.; Coelho, M.A.Z.; Marrucho, I. Menthol-based Eutectic Mixtures: Hydrophobic Low Viscosity Solvents. ACS Sustain. Chem. Eng. 2015, 3, 2469–2477. [Google Scholar] [CrossRef]
- Hümmer, M.; Kara, S.; Liese, A.; Huth, I.; Schrader, J.; Holtmann, D. Synthesis of (−)-menthol fatty acid esters in and from (−)-menthol and fatty acids—Novel concept for lipase catalyzed esterification based on eutectic solvents. Mol. Catal. 2018, 458, 67–72. [Google Scholar] [CrossRef]
- Pätzold, M.; Weimer, A.; Liese, A.; Holtmann, D. Optimization of solvent-free enzymatic esterification in eutectic substrate reaction mixture. Biotechnol. Rep. 2019, 22, e00333. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Crespo, E.A.; Pontes, P.V.A.; Silva, L.P.; Bülow, M.; Maximo, G.J.; Batista, E.A.C.; Held, C.; Pinho, S.P.; Coutinho, J.A.P. Tunable Hydrophobic Eutectic Solvents Based on Terpenes and Monocarboxylic Acids. ACS Sustain. Chem. Eng. 2018, 6, 8836–8846. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.; Marrucho, I. Development of hydrophobic deep eutectic solvents for extraction of pesticides from aqueous environments. Fluid Phase Equilib. Ria. 2017, 448, 135–142. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Quest for Green-Solvent Design: From Hydrophilic to Hydrophobic (Deep) Eutectic Solvents. ChemSusChem 2019, 12, 1549–1559. [Google Scholar] [CrossRef]
- Palomo, J.M.; Filice, M.; Romero, O.; Guisan, J.M. Improving Lipase Activity by Immobilization and Post-immobilization. Strategies 2013, 1051, 255–273. [Google Scholar]
- Alnoch, R.C.; Dos Santos, L.A.; De Almeida, J.M.; Krieger, N.; Mateo, C. Recent Trends in Biomaterials for Immobilization of Lipases for Application in Non-Conventional Media. Catalysts 2020, 10, 697. [Google Scholar] [CrossRef]
- Tudorache, M.; Protesescu, L.; Negoi, A.; Parvulescu, V.I. Recyclable biocatalytic composites of lipase-linked magnetic macro-/nano-particles for glycerol carbonate synthesis. Appl. Catal. A. Gen. 2012, 437–438, 90–95. [Google Scholar] [CrossRef]
- Ortega-Zamora, C.; González-Sálamo, J.; Hernández-Sánchez, C.; Hernández-Borges, J. Menthol-Based Deep Eutectic Solvent Dispersive Liquid–Liquid Microextraction: A Simple and Quick Approach for the Analysis of Phthalic Acid Esters from Water and Beverage Samples. ACS Sustain. Chem. Eng. 2020, 8, 8783–8794. [Google Scholar] [CrossRef]
- Alhadid, A.; Mokrushina, L.; Minceva, M. Design of Deep Eutectic Systems: A Simple Approach for Preselecting Eutectic Mixture Constituents. Molecules 2020, 25, 1077. [Google Scholar] [CrossRef]
- Warrag, S.E.E.; Kroon, M.C. Hydrophobic Deep Eutectic Solvents, Deep Eutectic Solvents; WILEY-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2019; pp. 83–93. [Google Scholar]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef]
- Gutierrez-Lazaro, A.; Velasco, D.; Boldrini, D.E.; Yustos, P.; Esteban, J.; Ladero, M. Effect of Operating Variables and Kinetics of the Lipase Catalyzed Transesterification of Ethylene Carbonate and Glycerol. Fermentation 2018, 4, 75. [Google Scholar] [CrossRef]
- Gihaz, S.; Weiser, D.; Dror, A.; Sátorhelyi, P.; Jerabek-Willemsen, M.; Poppe, L.; Fishman, A. Creating an Efficient Methanol-Stable Biocatalyst by Protein and Immobilization Engineering Steps towards Efficient Biosynthesis of Biodiesel. ChemSusChem 2016, 9, 3161–3170. [Google Scholar] [CrossRef]
- Gheorghe, D.; Dragoescu, D.; Teodorescu, M. Volumetric Study for the Binary Nitromethane with Chloroalkane Mixtures at Temperatures in the Range (298.15 to 318.15) K. J. Chem. Eng. Data 2013, 58, 1161–1167. [Google Scholar] [CrossRef]
- Dragoescu, D.; Sirbu, F.; Almásy, L. Study of the thermophysical properties for aqueous alkanediols binary mixtures. J. Mol. Liq. 2021, 335, 116150. [Google Scholar] [CrossRef]
- Mach, H.; Middaugh, C.; Lewis, R.V. Statistical determination of the average values of the extinction coefficients of tryptophan and tyrosine in native proteins. Anal. Biochem. 1992, 200, 74–80. [Google Scholar] [CrossRef]


| Molar Ratio (%) of Acceptor vs. Donor | |
|---|---|
| M:CA (I) 1 | 73:27 |
| M:CA (II) 1 | 65:35 |
| M:CA (III) 1 | 50:50 |
| M:OA (I) 2 | 83:17 |
| M:OA (II) 2 | 62:38 |
| M:OA (III) 2 | 50:50 |
| M:LiA 3 | 83:17 |
| M:LnA 4 | 83:17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ion, S.; Olănescu, F.; Teodorescu, F.; Tincu, R.; Gheorghe, D.; Pârvulescu, V.I.; Tudorache, M. DES-Based Biocatalysis as a Green Alternative for the l-menthyl Ester Production Based on l-menthol Acylation. Molecules 2022, 27, 5273. https://doi.org/10.3390/molecules27165273
Ion S, Olănescu F, Teodorescu F, Tincu R, Gheorghe D, Pârvulescu VI, Tudorache M. DES-Based Biocatalysis as a Green Alternative for the l-menthyl Ester Production Based on l-menthol Acylation. Molecules. 2022; 27(16):5273. https://doi.org/10.3390/molecules27165273
Chicago/Turabian StyleIon, Sabina, Florentina Olănescu, Florina Teodorescu, Robert Tincu, Daniela Gheorghe, Vasile I. Pârvulescu, and Mădălina Tudorache. 2022. "DES-Based Biocatalysis as a Green Alternative for the l-menthyl Ester Production Based on l-menthol Acylation" Molecules 27, no. 16: 5273. https://doi.org/10.3390/molecules27165273
APA StyleIon, S., Olănescu, F., Teodorescu, F., Tincu, R., Gheorghe, D., Pârvulescu, V. I., & Tudorache, M. (2022). DES-Based Biocatalysis as a Green Alternative for the l-menthyl Ester Production Based on l-menthol Acylation. Molecules, 27(16), 5273. https://doi.org/10.3390/molecules27165273