Effect of Supplementation with the Combination of Se-Enriched Lentinula edodes Mycelium, Exogenous Enzymes, Acidifiers, Sodium Butyrate and Silicon Dioxide Nanoparticle Feed Additives on Selected Parameters in Calves
Abstract
1. Introduction
2. Results
2.1. ADG of Calves
2.2. Serum Se Concentration
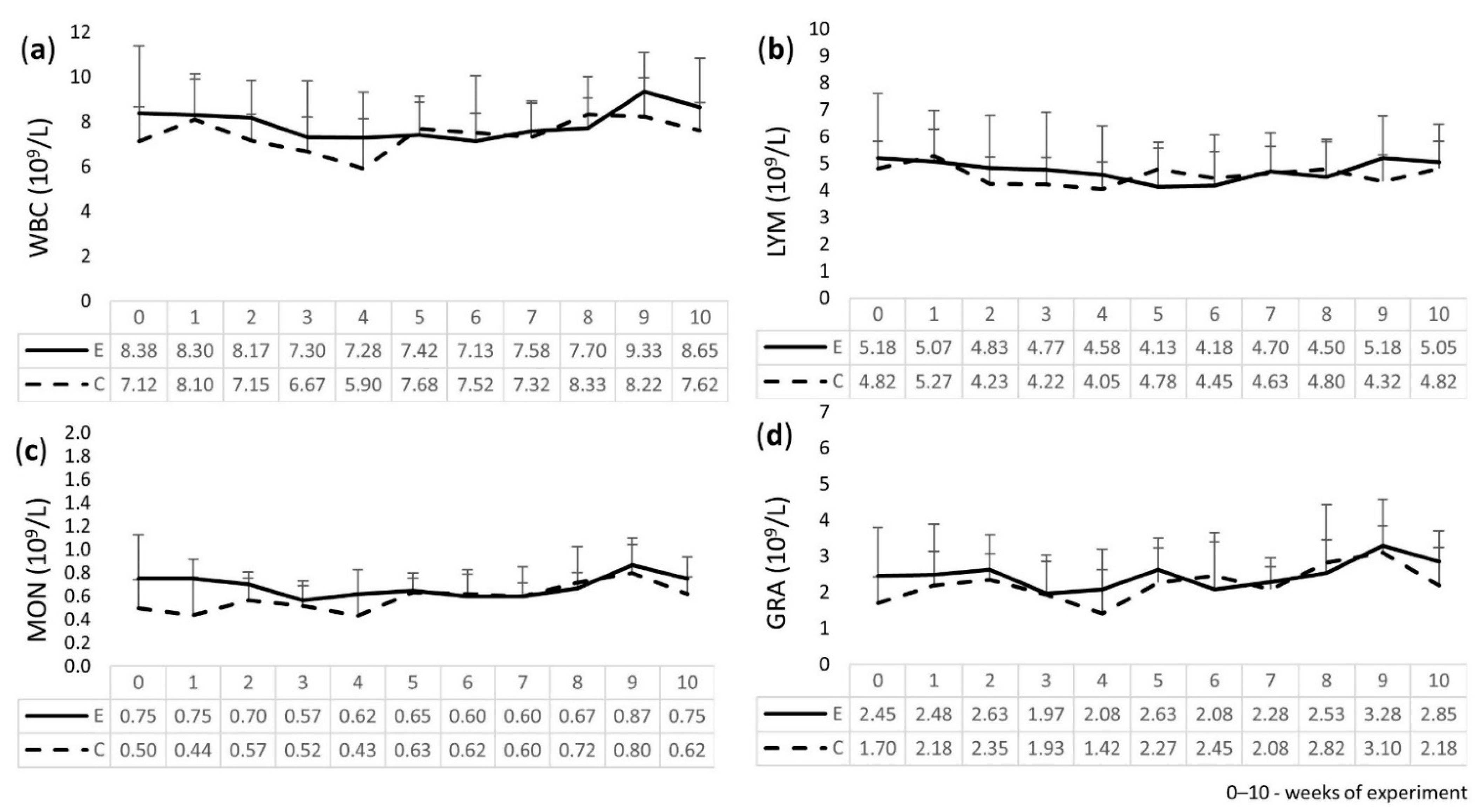
2.3. Leukogram
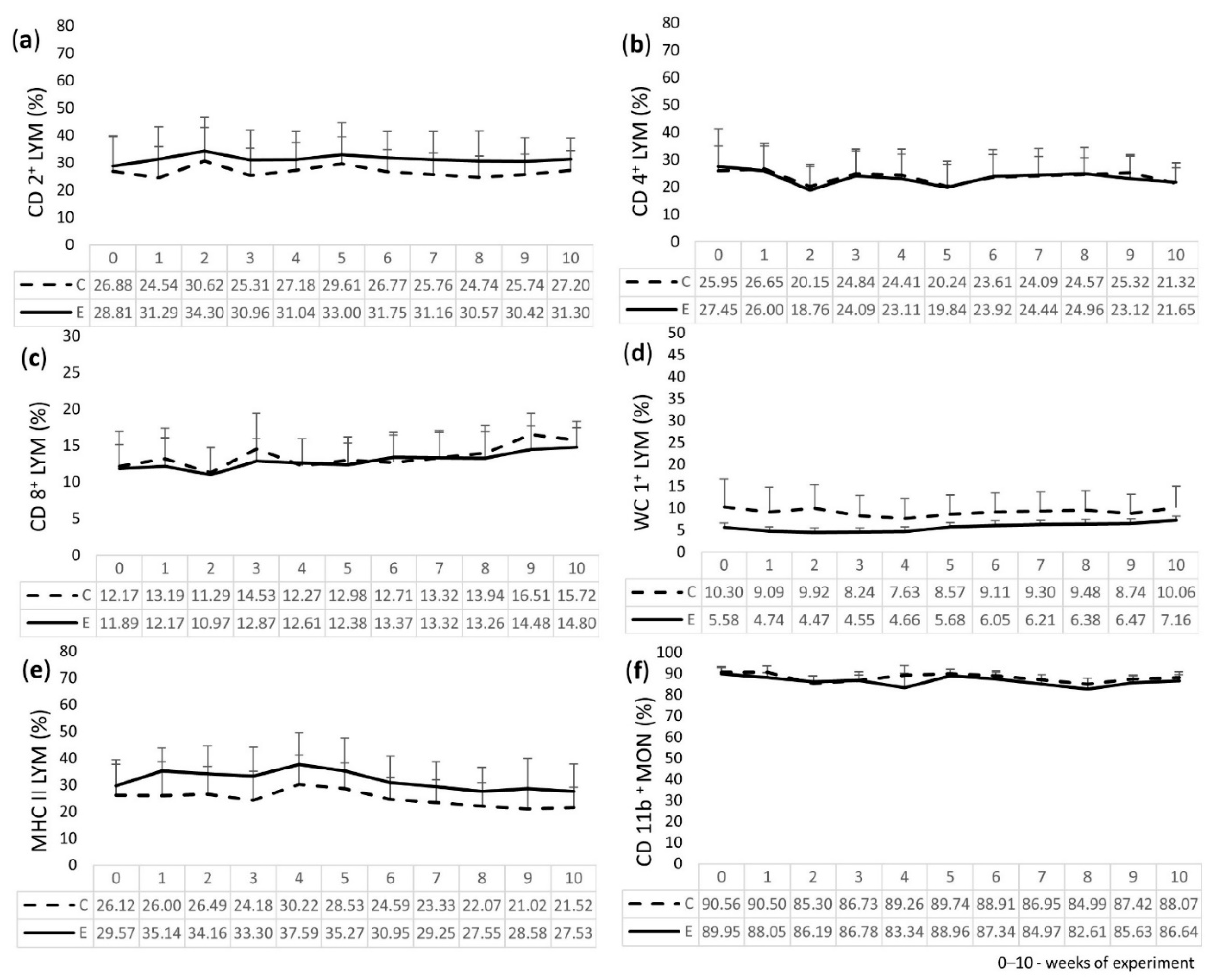
2.4. Immunophenotyping of LEU
2.5. Phagocytic Activity and Oxidative Burst of GRA and MON
2.5.1. Phagocytic Activity of GRA and MON
2.5.2. Oxidative Burst of GRA and MON
2.6. Bovine Ig and Cytokine Concentrations in Calves’ Serum
3. Discussion
4. Materials and Methods
4.1. The Calves and Treatments
4.2. ADG of the Calves
4.3. Serum Se Concentration
4.4. Leukogram
4.5. Immunophenotyping of Leukocytes
4.6. Phagocytic Activity and Oxidative Burst of GRA and MON
4.6.1. Phagocytic Activity of GRA and MON
4.6.2. Oxidative Burst of GRA and MON
4.7. Bovine Ig and Cytokine Concentrations in Calves’ Serum
5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Plattner, B.L.; Hostetter, J.M. Comparative Gamma Delta T Cell Immunology: A Focus on Mycobacterial Disease in Cattle. Vet. Med. Int. 2011, 2011, 214384. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.; Pillai, S. Cellular and Molecular Immunology, 9th ed.; Elsevier: Philadelphia, PA, USA, 2008; pp. 154–196. [Google Scholar]
- Quigley, J.D.; Drewry, J.J.; Murray, L.M.; Ivey, S.J. Body Weight Gain, Feed Efficiency, and Fecal Scores of Dairy Calves in Response to Galactosyl-Lactose or Antibiotics in Milk Replacers. J. Dairy Sci. 1997, 80, 1751–1754. [Google Scholar] [CrossRef]
- Sarker, M.; Ko, S.; Lee, S.; Kim, G.; Choi, J.; Yang, C. Effect of Different Feed Additives on Growth Performance and Blood Profiles of Korean Hanwoo Calves. Asian-Australas. J. Anim. Sci. 2010, 23, 52–60. [Google Scholar] [CrossRef]
- Ribeiro, M.D.; Pereira, J.C.; Queiroz, A.C.D.; Cecon, P.R.; Detmann, E.; Azevêdo, J.A.G. Performance of dairy calves fed milk, milk replacer or post-weaning concentrate with acidifiers. Rev. Bras. Zootec. 2009, 38, 956–963. [Google Scholar] [CrossRef]
- Płaczek, A.; Stępień, P.; Żarczyński, P.; Patorczyk–Pytlik, B. Methods for enrichment of animal diets with selenium. J. Elem. 2019, 24, 1159–1172. [Google Scholar] [CrossRef]
- Müller, A.; Bertram, A.; Freude, B. Saisonale und überregionale Unterschiede im Selenversorgungsstatus von Rindern [Differences in the selenium supply of cattle across Europe]. Tierarztl. Prax. Ausg. G Grosstiere-Nutztiere 2014, 42, 131–144. [Google Scholar] [PubMed]
- Kiremidjian-Schumacher, L.; Stotzky, G. Selenium and immune responses. Environ. Res. 1987, 42, 277–303. [Google Scholar] [CrossRef]
- Maplesden, D.C.; Loosli, J.K. Nutritional Muscular Dystrophy in Calves. II. Addition of Selenium and Tocopherol to a Basal, Dystrophogenic Diet Containing Cod-Liver oil. J. Dairy Sci. 1960, 43, 645–653. [Google Scholar] [CrossRef]
- Hefnawy, A.E.G.; Tórtora-Pérez, J.L. The importance of selenium and the effects of its deficiency in animal health. Small Rumin. Res. 2010, 89, 185–192. [Google Scholar] [CrossRef]
- Sordillo, L.M. Selenium-dependent regulation of oxidative stress and immunity in periparturient dairy cattle. Vet. Med. Int. 2013, 2013, 154045. [Google Scholar] [CrossRef]
- Fitak, B.; Grabowski, M.; Suchocki, P. Preparat Przeciwnowotworowy i Sposób Jego Wytwarzania. Polish Patent 176530 (Cl. A61K31/095), 30 June 1999. [Google Scholar]
- Pehrson, B.; Ortman, K.; Madjid, N.; Trafikowska, U. The influence of dietary selenium as selenium yeast or sodium selenite on the concentration of selenium in the milk of suckler cows and on the selenium status of their calves. J. Anim. Sci. 1999, 77, 3371–3376. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Wu, X.; Shen, X.; Zhang, K.; Ren, F.; Huang, K. Effects of selenium form on blood and milk selenium concentrations, milk component and milk fatty acid composition in dairy cows. J. Sci. Food Agric. 2010, 90, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Suchocki, P.; Misiewicz-Krzemińska, I.; Skupińska, K.; Niedźwiecka, K.; Lubelska, K.; Fijałek, Z.; Kasprzycka-Guttman, T. Selenitetriglicerydes affect CYP1A1 and QR activity by involvement of reactive oxygen species and Nrf2 transcription factor. Pharmacol. Rep. 2010, 62, 352–361. [Google Scholar] [CrossRef]
- Guo, F.C.; Williams, B.A.; Kwakkel, R.P.; Li, H.S.; Li, X.P.; Luo, J.Y.; Li, W.K.; Verstegen, M.W.A. Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on the cecal microbial ecosystem in broiler chickens. Poult. Sci. 2004, 83, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Turlo, J.; Gutkowska, B.; Herold, F.; Dawidowski, M.; Słowiński, T.; Zobel, A. Relationship between selenium accumulation and mycelial cell composition in Lentinula edodes (Berk.) cultures. J. Toxicol. Environ. Health Part A 2010, 73, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, I.; Sitarz, K.; Roslon, M.; Anuszewska, E.; Hoser, G.; Dudkiewicz-Wilczyńska, J.; Iwanowska, M.; Suchocki, P. The influence of an organic selenium(IV) compound on progression of tumor induced using prostate cancer cells and gene expression connected to the oxidative stress response. World J. Pharm. Sci. 2014, 2, 1146–1158. [Google Scholar]
- Drori, A.; Shabat, Y.; Ya’acov, A.B.; Danay, O.; Levanon, D.; Zolotarov, L.; Ilan, Y. Extracts from Lentinula edodes (Shiitake) edible mushrooms enriched with vitamin D exert an anti-inflammatory hepatoprotective effect. J. Med. Food 2016, 19, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, B.; Bederska, D.; Zięba, P. The importance of selenium in the human diet—in the aspect of feeding farm animals. Rocz. Nauk. Zootech. 2018, 45, 135–142. [Google Scholar]
- Murphy, E.A.; Davis, J.M.; Carmichael, M.D. Immune modulating effects of β-glucan. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 656–661. [Google Scholar] [CrossRef]
- Muszyńska, B.; Kała, K.; Włodarczyk, A.; Krakowska, A.; Ostachowicz, B.; Gdula-Argasinska, J.; Suchocki, P. Lentinula edodes as a source of bioelements released into artificial digestive juices and potential anti-inflammatory material. Biol. Trace Elem. Res. 2020, 194, 603–613. [Google Scholar] [CrossRef]
- Friedman, M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, B.; Szacawa, E.; Bederska-Łojewska, D.; Dudek, K.; Pomierny, B.; Włodarczyk, A.; Kała, K.; Lazur, J.; Suchocki, P.; Budziszewska, B.; et al. Preliminary study on Se-enriched Lentinula edodes mycelium as a proposal of new feed additive in selenium deficiency. PLoS ONE 2020, 15, e0233456. [Google Scholar] [CrossRef] [PubMed]
- Szacawa, E.; Dudek, K.; Bednarek, D.; Pieszka, M.; Bederska-Łojewska, D. A pilot study on the effect of a novel feed additive containing exogenous enzymes, acidifiers, sodium butyrate and silicon dioxide nanoparticles on the selected cellular immune indices and body weight gains of calves. J. Vet. Res. 2021, 65, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.C. Essentials of Veterinary Hematology; Lea & Febiger: Philadelphia, PA, USA, 1993. [Google Scholar]
- Guilloteau, P.; Plodari, M.; Romé, V.; Savary, G.; Le Normand, L.; Zabielski, R. Pancreatic enzyme deficiency depends on dietary protein origin in milk-fed calves. J. Dairy Sci. 2011, 94, 1517–1525. [Google Scholar] [CrossRef]
- Pearlin, B.V.; Muthuvel, S.; Govidasamy, P.; Villavan, M.; Alagawany, M.; Farag, M.R.; Dhama, K.; Gopi, M. Role of acidifiers in livestock nutrition and health: A review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Janik, A.; Pieszka, M. Effectiveness of probiotic, acidifier and mannan oligosaccharide use in piglet rearing. Ann. Anim. Sci. Suppl. 2006, 2, 335–340. [Google Scholar] [CrossRef]
- Hill, T.M.; Aldrich, J.M.; Schlotterbeck, R.L.; Bateman, H.G., II. Effects of changing the fat and fatty acid composition of milk replacers fed to neonatal calves. Prof. Anim. Sci. 2007, 23, 135–143. [Google Scholar] [CrossRef]
- Szczurek, P.; Kamyczek, M.; Pierzynowski, S.; Goncharova, K.; Michałowski, P.; Weström, B.; Pryhodko, O.; Grabowski, T.; Pieszka, M. Effects of dietary supplementation with pancreatic-like enzymes of microbial origin (PLEM) and silicon dioxide (SiO2) on the performance of piglets fed creep feed. J. Anim. Sci. 2016, 94, 62–65. [Google Scholar] [CrossRef]
- Khanal, D.; Knight, A.P. Selenium: Its role in livestock health and production. J. Agric. Environ. 2010, 11, 101–106. [Google Scholar] [CrossRef]
- Patorczyk-Pytlik, B.; Kulczycki, G. Content of selenium in arable soils near Wroclaw. J. Elem. 2009, 14, 755–762. [Google Scholar] [CrossRef][Green Version]
- Puls, R. Mineral Levels in Animal Health: Diagnostic Data; Trinity Western University Press; Sherpa International: Clearbrook, MN, Canada, 1988; pp. 168–171. [Google Scholar]
- Żarczyńska, K.; Sobiech, P.; Tobolski, D.; Mee, J.F.; Illek, J. Effect of a single, oral administration of selenitetriglycerides, at two dose rates, on blood selenium status and haematological and biochemical parameters in Holstein-Friesian calves. Ir. Vet. J. 2021, 74, 11. [Google Scholar] [CrossRef]
- Deng, Z.Y.J.; Zhang, W.; Wu, G.Y.; Yin, Y.; Ruan, Z.; Li, T.J.; Chu, W.Y.; Kong, X.F.; Zhang, Y.M.; Fan, Y.W.; et al. Dietary supplementation with polysaccharides from Semen cassiae enhances immunoglobulin production and interleukin gene expression in early-weaned piglets. J. Sci. Food Agric. 2007, 87, 1868–1873. [Google Scholar]
- Lee, J.J.; Kang, K.; Park, S.; Cho, J.H.; Oh, S.; Park, D.-J.; Perez-Maldonado, R.; Cho, J.-Y.; Park, I.-H.; Kim, H.B.; et al. Effects of dietary protease on immune responses of weaned pigs. J. Anim. Sci. Technol. 2020, 62, 174–179. [Google Scholar] [CrossRef]
- García, V.E.; Sieling, P.A.; Gong, J.; Barnes, P.F.; Uyemura, K.; Tanaka, Y.; Bloom, B.R.; Morita, C.T.; Modlin, R.L. Single-cell cytokine analysis of gamma delta T cell responses to nonpeptide mycobacterial antigens. J. Immunol. 1997, 159, 1328–1335. [Google Scholar]
- O’Brien, R.L.; Roark, C.L.; Jin, N.; Aydintug, M.K.; French, J.D.; Chain, J.L.; Wands, J.M.; Johnston, M.; Born, W.K. The gamma-delta T-cell receptors: Functional correlations. Immunol. Rev. 2007, 215, 77–88. [Google Scholar] [CrossRef]
- Chanput, W.; Reitsma, M.; Kleinjans, L.; Mes, J.J.; Savelkoul, H.F.; Wichers, H.J. β-Glucans are involved in immune-modulation of THP-1 macrophages. Mol. Nutr. Food Res. 2012, 56, 822–833. [Google Scholar] [CrossRef]
- Wojcicka-Lorenowicz, K.; Kostro, K.; Lisiecka, U.; Gąsiorek, B. Phagocytic activity and oxygen metabolism of peripheral blood granulocytes from rabbits experimentally infected with Trichophyton mentagrophytes. J. Vet. Res. 2018, 62, 43–48. [Google Scholar] [CrossRef]

| Group of Calves | Week 0 | Week 1 | Week 7 | Week 10 |
|---|---|---|---|---|
| experimental | 53.77 ± 5.54 | 90.15 ± 14.85 ** | 131.63 ± 18.02 ** | 92.03 ± 7.14 ** |
| control | 46.80 ± 11.90 | 45.68 ± 7.26 | 48.82 ± 5.12 | 52.00 ± 6.53 |
| Item | Milk Replacer | Calf Starter Feed |
|---|---|---|
| Soybeans (%) | 31 | N/A |
| Wheat flour (%) | 30 | N/A |
| Whey | 28 | N/A |
| Palm oil | 9 | N/A |
| Calcium carbonate | 0.8 | N/A |
| Dextrose | 0.15 | N/A |
| Beet molasses | 0.05 | N/A |
| Crude protein (%) | 21.0 | 18.5 |
| Crude oils and fats (%) | 12.0 | 3.3 |
| Ash (%) | 6.0 | 9.0 |
| Crude fiber (%) | 1.1 | 6.5 |
| Calcium (%) | 0.7 | 1.3 |
| Lysine (%) | 1.4 | - |
| Phosphorus (%) | 0.5 | 0.8 |
| Sodium (%) | 0.3 | 0.23 |
| Vitamin A (IU/kg) | 10,000 | 25,000 |
| Vitamin D3 (IU/kg) | 2000 | 5000 |
| Vitamin C (mg/kg) | 100 | - |
| Vitamin E (mg/kg) | 80 | 25.0 |
| Calcium D-pantothenate (mg/kg) | 8.6 | - |
| Niacinamide (mg/kg) | 6.6 | - |
| Vitamin B1 (mg/kg) | 4.3 | - |
| Vitamin B2 (mg/kg) | 4.3 | - |
| Vitamin B6 (mg/kg) | 4.3 | - |
| Vitamin K3 (mg/kg) | 1.0 | - |
| Folic acid (mg/kg) | 0.33 | - |
| Biotin (mg/kg) | 0.07 | - |
| Vitamin B12 (mg/kg) | 0.05 | - |
| Iron (mg/kg) | 80 | - |
| Manganese (mg/kg) | 64 | 0.25 (%) |
| Zinc (mg/kg) | 56 | - |
| Copper (mg/kg) | 8 | - |
| Iodine (mg/kg) | 0.96 | - |
| Selenium (mg/kg) | 0.2 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szacawa, E.; Dudek, K.; Wasiak, M.; Bednarek, D.; Bederska-Łojewska, D.; Muszyńska, B.; Pieszka, M. Effect of Supplementation with the Combination of Se-Enriched Lentinula edodes Mycelium, Exogenous Enzymes, Acidifiers, Sodium Butyrate and Silicon Dioxide Nanoparticle Feed Additives on Selected Parameters in Calves. Molecules 2022, 27, 5163. https://doi.org/10.3390/molecules27165163
Szacawa E, Dudek K, Wasiak M, Bednarek D, Bederska-Łojewska D, Muszyńska B, Pieszka M. Effect of Supplementation with the Combination of Se-Enriched Lentinula edodes Mycelium, Exogenous Enzymes, Acidifiers, Sodium Butyrate and Silicon Dioxide Nanoparticle Feed Additives on Selected Parameters in Calves. Molecules. 2022; 27(16):5163. https://doi.org/10.3390/molecules27165163
Chicago/Turabian StyleSzacawa, Ewelina, Katarzyna Dudek, Magdalena Wasiak, Dariusz Bednarek, Dorota Bederska-Łojewska, Bożena Muszyńska, and Marek Pieszka. 2022. "Effect of Supplementation with the Combination of Se-Enriched Lentinula edodes Mycelium, Exogenous Enzymes, Acidifiers, Sodium Butyrate and Silicon Dioxide Nanoparticle Feed Additives on Selected Parameters in Calves" Molecules 27, no. 16: 5163. https://doi.org/10.3390/molecules27165163
APA StyleSzacawa, E., Dudek, K., Wasiak, M., Bednarek, D., Bederska-Łojewska, D., Muszyńska, B., & Pieszka, M. (2022). Effect of Supplementation with the Combination of Se-Enriched Lentinula edodes Mycelium, Exogenous Enzymes, Acidifiers, Sodium Butyrate and Silicon Dioxide Nanoparticle Feed Additives on Selected Parameters in Calves. Molecules, 27(16), 5163. https://doi.org/10.3390/molecules27165163








