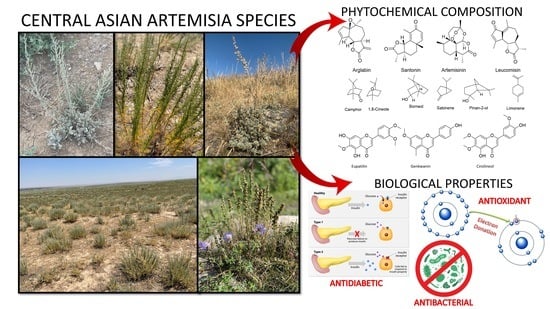
Traditional Use, Phytochemical Profiles and Pharmacological Properties of Artemisia Genus from Central Asia
Abstract
1. Introduction
2. Distribution of Artemisia L. in Central Asia
3. Traditional Use, Phytochemical Profiles and Pharmacological Properties of Artemisia Species from Central Asia
| Chemical Constitutes/Artemisia species | Content, % | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. terraealba | A. frigida | A. glabella | A. rupestris | A. filatovae | A. lercheana | A. sieversiana | A.hyppolyti | A. armeniaca | A. proceriformis | A. dracunculus | A. marschalliana | A. gmelinii | A.kasakorum | A. leucodes | A. serotina | A.aralensis | |
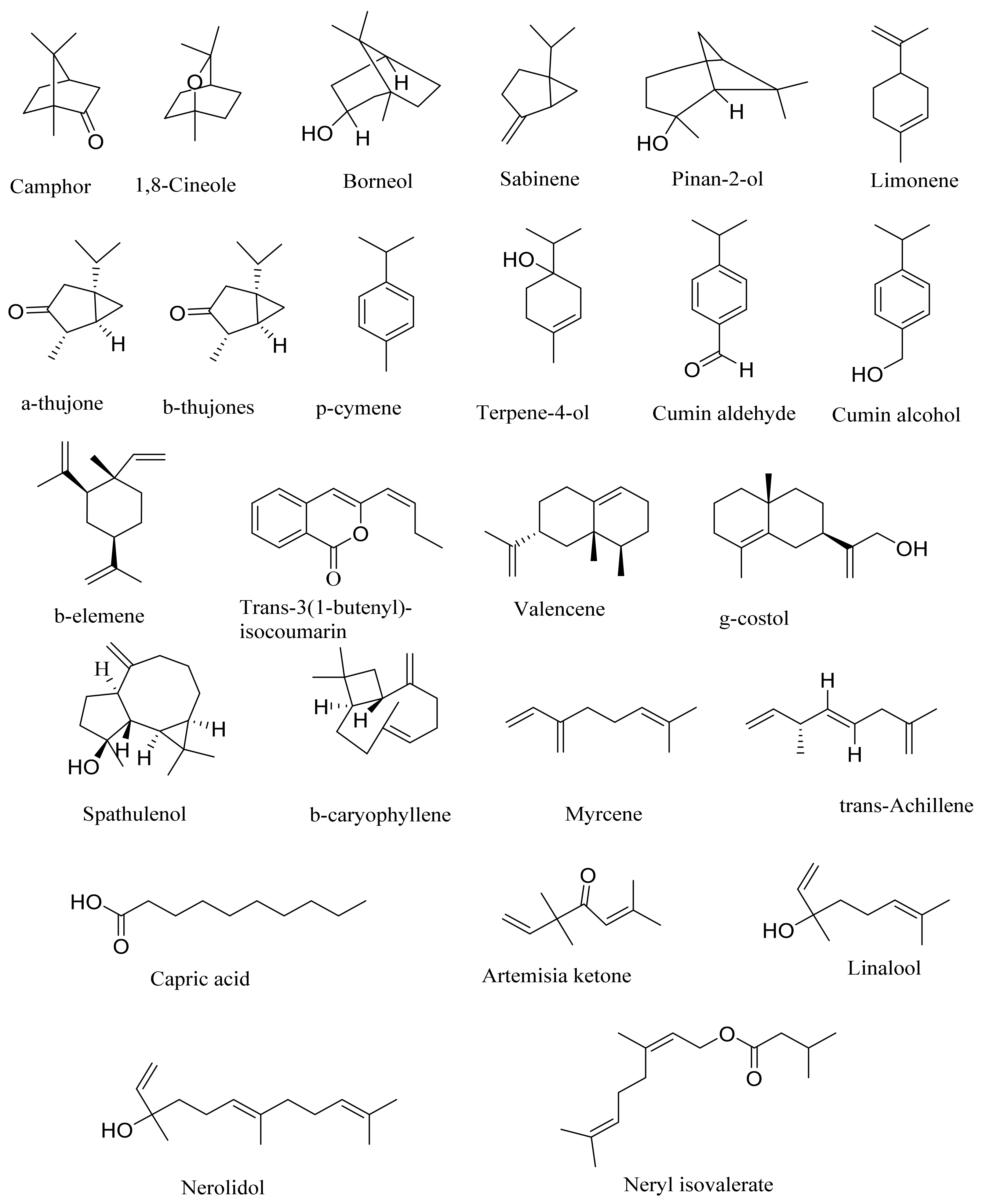
| α-pinene | - * | 1.6 | - | 2.0 | 0.9 | 0.1 | 1.7 | - | 3.4 | - | 4.2 | 4.1 | 0.1 | 0.1 | 5.3 | - | - |
| Camphene | 9.8 | 5 | - | 0.4 | 0.3 | 0.8 | 0.9 | - | - | - | 0.4 | - | - | 0.3 | 9.31 | - | - |
| Sabinene | - | 0.6 | 5 | 0.1 | 3.0 | 0.2 | 0.6 | - | 2.1 | 1.3 | 20.2 | 0.8 | 0.2 | 0.1 | - | - | - |
| Myrcene | - | - | - | 9.5 | 18 | - | 14.2 | - | 6.2 | - | 6.2 | 0.6 | - | - | 0.30 | - | - |
| α-terpenene | - | 1.1 | - | 0.3 | 0.3 | - | 0.1 | - | - | - | 3.1 | 0.2 | 1.0 | 0.3 | - | - | - |
| p-cymene | - | - | - | - | 1.5 | - | 3.4 | - | 3.5 | 0.6 | 1.5 | 2.1 | 3.7 | 3.4 | - | - | - |
| Limonene | - | 0.3 | - | - | - | - | 0.4 | 0.72 | 7.7 | - | 3.3 | 1.0 | - | - | - | - | - |
| 1, 8-cineole | 23.9 | 24.7 | 12 | 0.3 | 4.3 | 4.6 | 9.3 | 23.6 | 1.1 | 3.1 | 0.3 | 13.5 | 28.5 | - | 6.20 | 6.6 | 13.19 |
| Artemisia ketone | - | - | - | - | - | - | - | - | - | - | - | 4.4 | - | - | - | - | - |
| Linalool | - | 0.4 | 8 | 0.2 | 0.3 | 0.1 | 4.2 | 9.87 | - | - | - | 0.7 | 0.1 | 0.2 | - | - | - |
| α-thujone | - | 5.2 | - | 0.1 | - | 24.2 | - | - | - | 66.3 | - | 5.7 | 8.6 | 2.7 | - | 53.9 | - |
| β-thujone | 6.0 | 1.3-2.5 | - | - | - | 45.6 | 0.1 | - | - | 23.4 | - | 1.4 | 1.0 | - | - | - | |
| Camphor | 47.3 | 22.6 | 0.2 | 3.6 | - | 7.5 | - | 1.47 | - | - | - | 9.8 | 11.3 | 3.7 | 39.0 | 2 | - |
| Borneol | - | 3.9 | 5.2 | 0.3 | - | - | - | 6.15 | - | - | - | 3.3 | 9.3 | - | - | ||
| Terpene-4-ol | - | 2.8 | 6.5 | 1.9 | 0.4 | 0.7 | - | 1.42 | 1.4 | 2.0 | - | 2.6 | 3.3 | 2.5 | 0.53 | - | 4.38 |
| Cumin aldehyde | - | - | 9.4 | - | 0.5 | 0.3 | - | - | - | - | - | - | 0.3 | 2.4 | - | - | - |
| β-elemene | - | - | - | 5.4 | 11 | - | 2.0 | - | 6.9 | - | 1.1 | 0.4 | - | - | - | - | - |
| Spathulenol | - | - | 2.4 | 3.8 | 2.0 | 0.2 | 2.1 | 30.4 | - | 3.0 | 3.5 | 0.5 | 1.7 | 3.27 | |||
| Trans-3 (1-butenyl)-isocoumarin | - | - | - | - | - | - | - | - | - | - | 10.3 | - | - | - | - | - | - |
| Capric acid | - | - | - | 5.1 | - | - | - | - | - | - | - | - | - | - | - | - | |
| γ-costol | - | - | - | 3.9 | - | - | 0.4 | - | - | - | - | - | - | - | - | - | - |
| Valencene | - | - | - | 3.7 | 1.42 | - | - | - | - | - | - | - | - | - | - | - | - |
| Cumin alcohol | - | - | 3.7 | - | - | 0.2 | - | - | - | - | - | - | - | - | - | - | - |
| Nerolidol | - | - | - | - | 14 | - | - | - | - | - | - | - | - | - | - | - | - |
| Pinan-2-ol | - | - | - | - | 8.3 | - | - | - | - | - | - | - | - | - | - | - | - |
| Achillene | - | - | - | - | 5 | - | - | - | - | - | - | - | - | - | - | - | - |
| Neryl isovalerate | - | - | - | - | - | - | 3.4 | - | - | - | - | - | - | - | - | - | - |
| Caryophyllene | - | - | - | - | - | - | 3.0 | - | - | - | - | - | - | - | - | - | - |
| Carvone | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 5.7 | - | |
| № | Plants | Structure | Chemical Formula | Compound | Activity |
|---|---|---|---|---|---|
| 1 | Artemisia cina O.C. Berg et C.F. Schmidt | 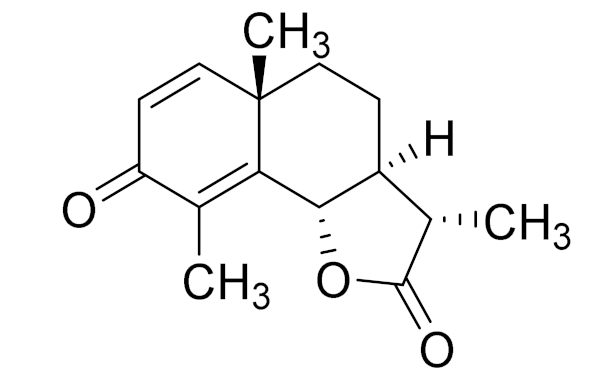 | C15H18O3 | α-Santonin [63] | Anthelmintic [63] and antipyretic activity [104] |
| 2 | Artemisia tschernieviana Besser | 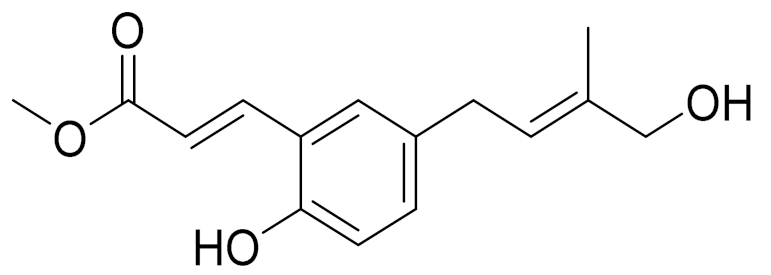 | C15H18O4 | Methyl ester of 3-(5_hydroxyprenyl)-p-coumaric acid [103] | - |
| 3 | Artemisia sieversiana Ehrh. | 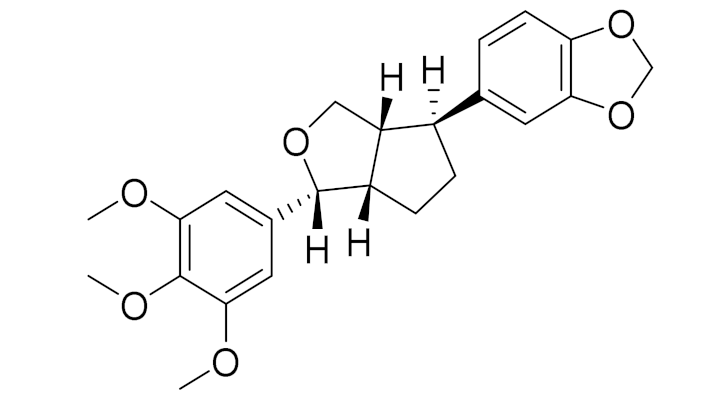 | C22H24O7 | 4-Epiashantin [105] | Reasonable antimicrobial activity toward the aforementioned microorganism strains [105] |
| 4 | Artemisia heptapotamica Poljak. |  | C31H37O13 | Artemisiane E [74] | - |
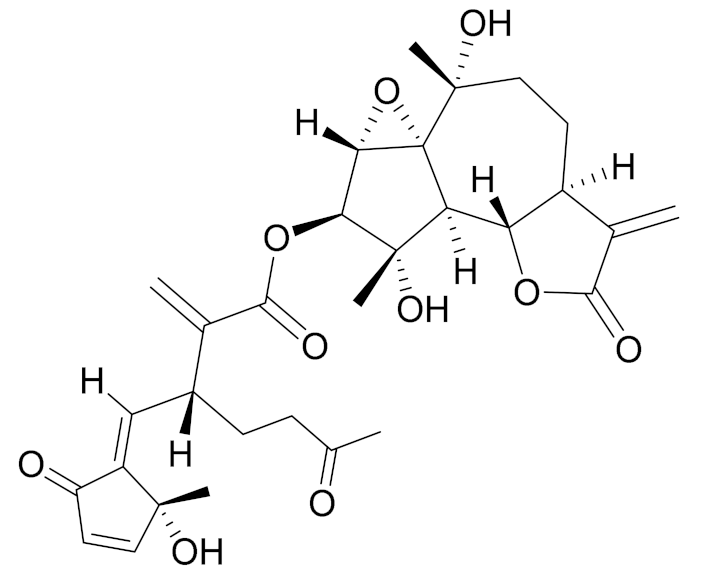 | C30H36O10 | Artemisiane A [74] | - | ||
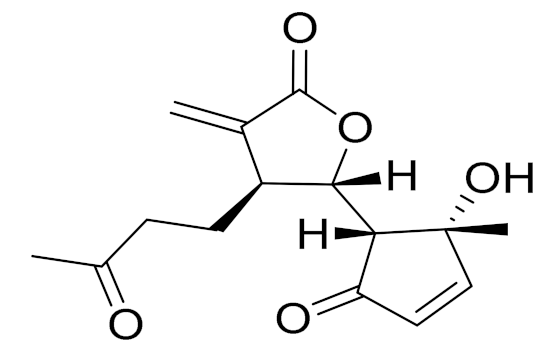 | C15H18O5 | seco-Tanapartholide A [74] | Anti-inflammatory effects [74] | ||
 | C15H18O5 | 5-epi-Secotanapartholide A [74] | Anti-inflammatory effects [74] | ||
 | C16H20O4 | iso-seco-Tanapartholide | Anti-inflammatory effects [74] | ||
 | C16H21O8 | Artemdubolide I. | - | ||
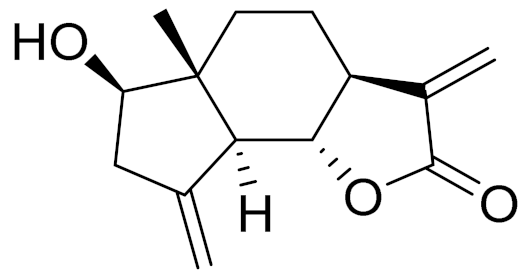 | C14H17O3 | Ajaniaolide B | Anti-inflammatory effects [74] | ||
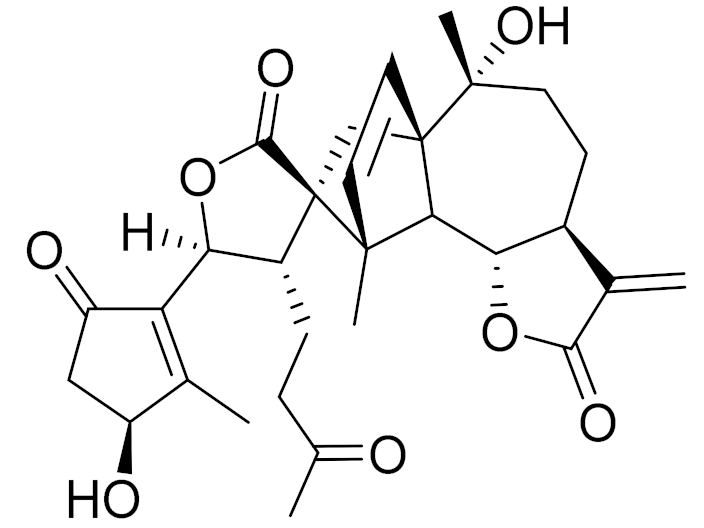 | C30H36O8 | 3β-chloro-4α, 10α-dihydroxy-1α,2α-epoxy-5α, 7αH-guaia-11 (13)-en-12,6α-olide | Anti-inflammatory effects [74] | ||
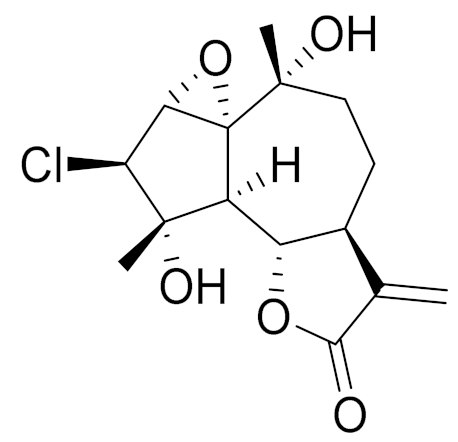 | C15H19ClO5 | 3α-chloro-4β,10α-dihydroxy-1β,2β-epoxy-5α,7α Hguai-11(13)-en-12,6α-olide | Anti-inflammatory effects [74] | ||
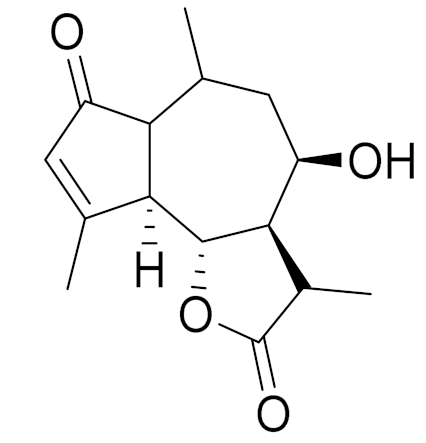 | C15H20O4 | Rupicolin B | Antimicrobial activity [106] | ||
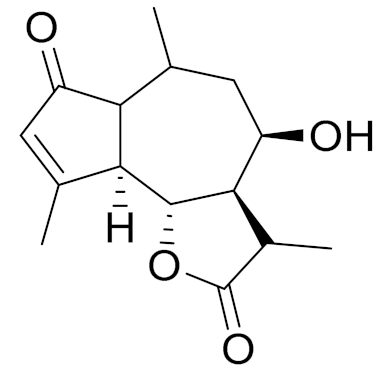 | C15H20O4 | Hydroxyachillin | Anti-inflammatory activity in carrageenan-induced paw edema [107] | ||
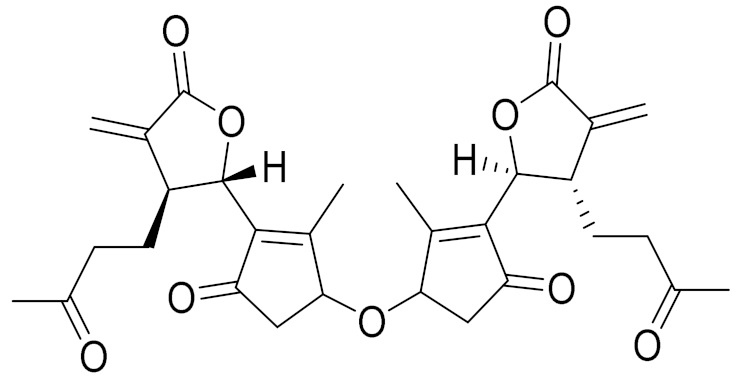 | C30H34O9 | Millifolide A | Tested on the following tumor cell lines: MCF7, HL-60 and PC3; however, it did not exhibit any cytotoxicities [108] | ||
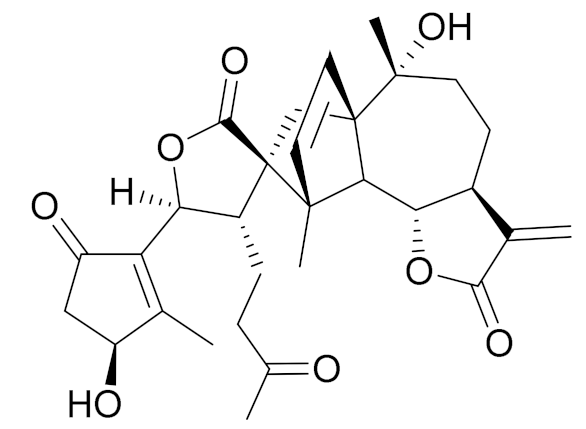 | C30C36O8 | Achillinin C | Antitumor agent [109] | ||
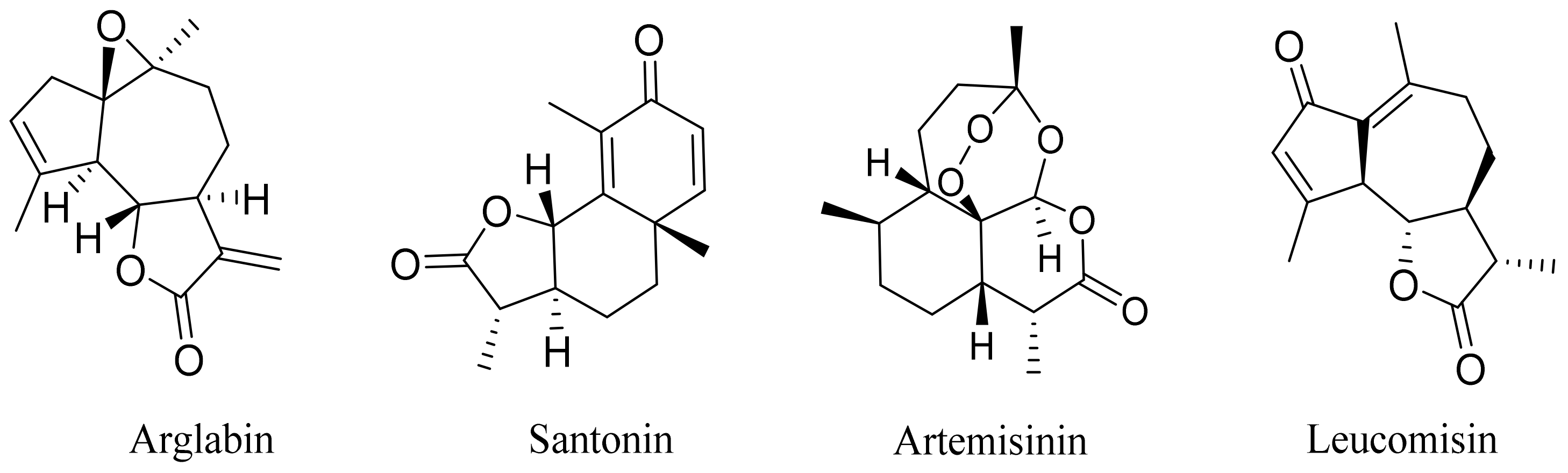
| 5 | Artemisia glabella Kar. & Kir. | 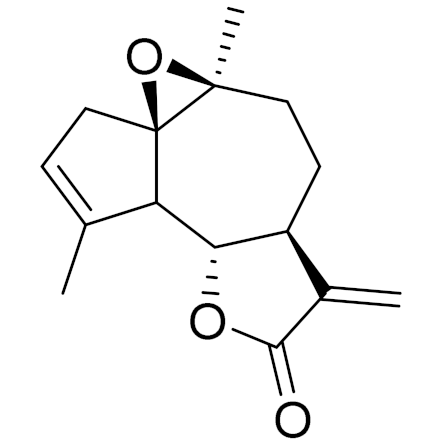 | C15H18O3 | Arglabin [110,111] | Antitumor activity [112] |
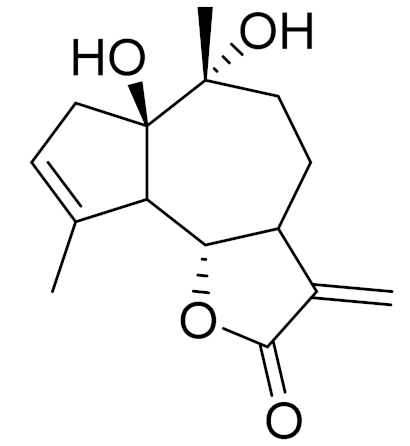 | C15H20O4 | 1β,10α-Dihydro-xyarglabin [113] | - | ||
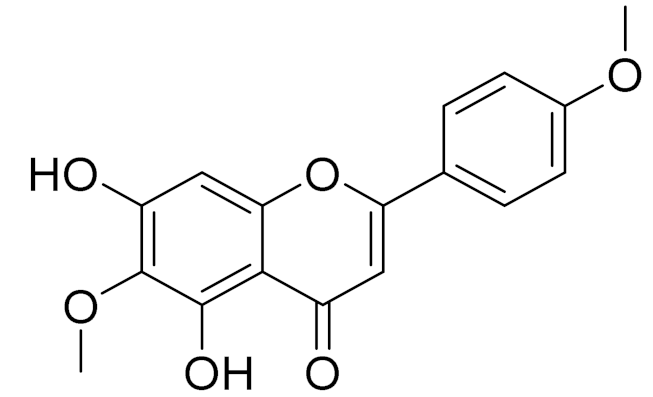 | C17H14O6 | Pectolinarigenin [113] | Anti-inflammatory effects [113] | ||
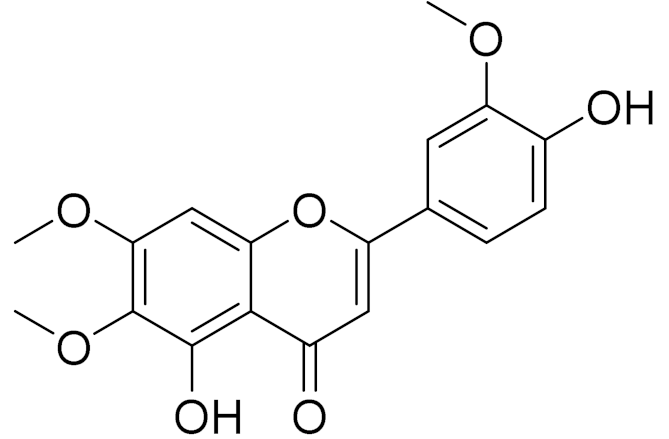 | C18H16O7 | Cirsilineol [114] | Antioxidant, cytostatic, antimicrobial, antifungal, antimalarial and antileishmaniasis activities [114] | ||
 | C15H20O3 | Argolide [113] | Studied for analgesic activity; however it did not show any activities [115] | ||
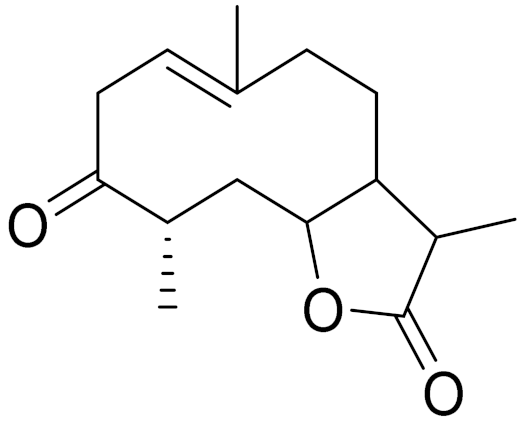 | C15H22O3 | Dihydroargolide | Modulate TCR activation, which is responsible in inflammatory and immune responses [116] | ||
| 6 | Artemisia halophila Krasch. | 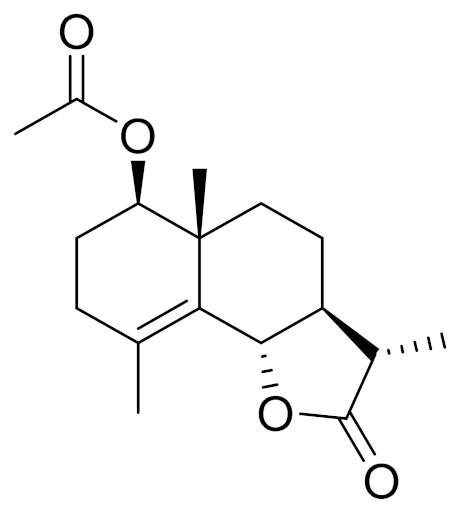 | C17H24O4 | Arhalin [117] | - |
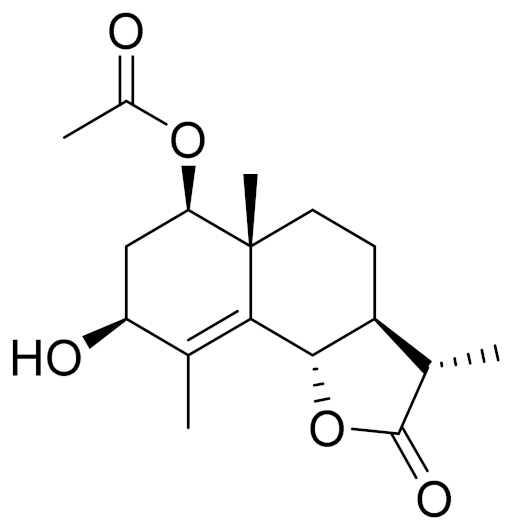 | C17H24O5 | 3-Hydroxyarhalin [117] | Modulate TCR activation [118] | ||
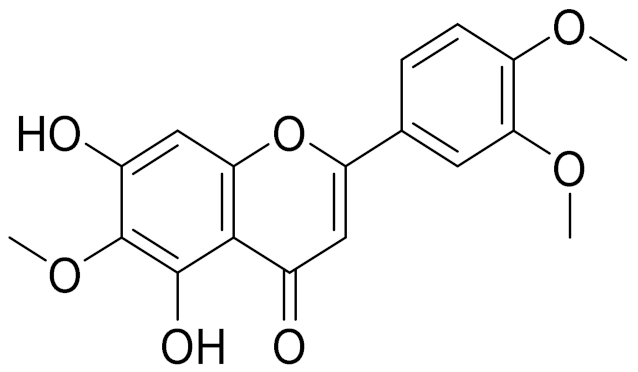 | C18H16O7 | Eupatilin | Anti-inflammatory activity [119] | ||
| 7 | Artemisia semiarida (Krasch. & Lavrenko) Filatova |  | C18H16O8 | 5,7,3-trihydroxy-6,4,5-trimethoxyflavone [120] | Strong inhibitory activity against an FPTase [121] |
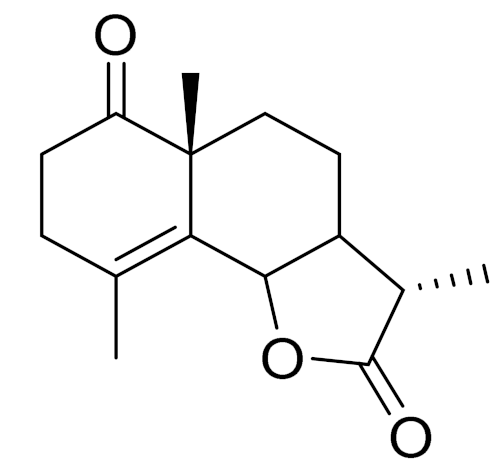 | C15H20O3 | Taurin | Antioxidant [122] | ||
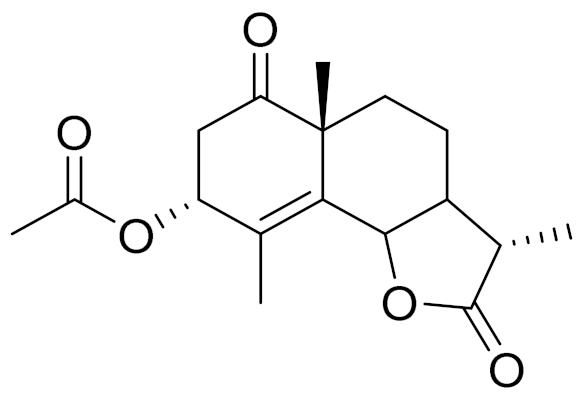 | C17H22O5 | Acetoxytaurin | - | ||
 | C15H20O4 | Hydroxytaurin | Antiprotozol effect against Leishmania dolzovani [122] | ||
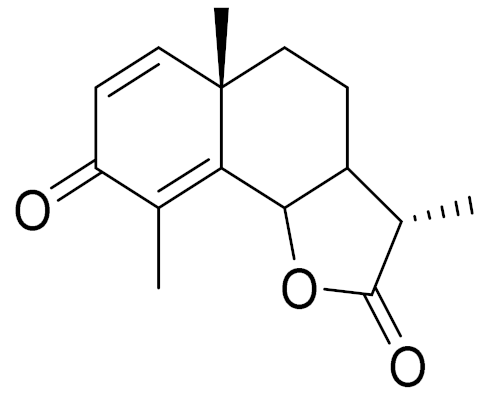 | C15H18O3 | a-Santonin [63] | Anthelmintic [63] and antipyretic activity [104] | ||
| 8 | Artemisia succulenta Ledeb. |  | C15H20O3 | Argolide [123] | Studied for analgesic activity; however, it did not show any activities [112] |
| 9 | Artemisia radicans Kupr. | 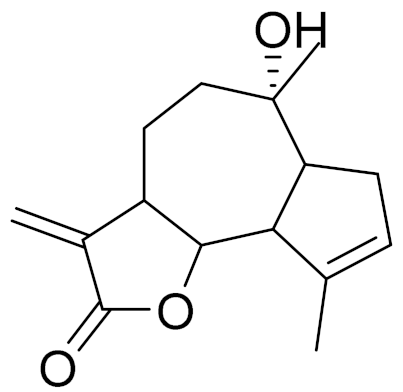 | C15H20O3 | 8-Deoxycumambrin [123] | Aromatase inhibition [124] |
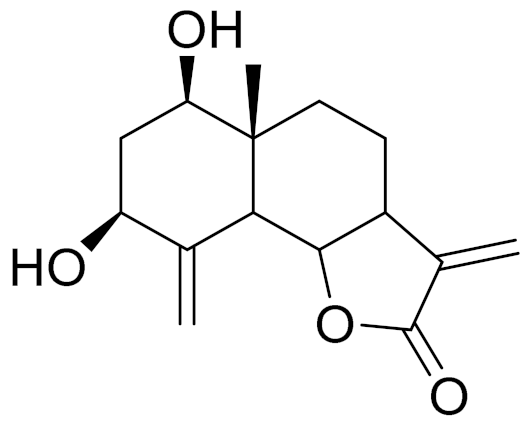 | C15H20O4 | Ridentin B [123] | Studied for action on human adherent cell lines but did not show any activities [125]. | ||
| 10 | Artemisia filatovae Kuorijanov | 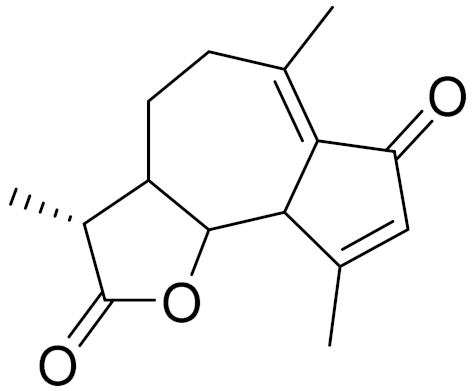 | C15H18O3 | Achillin | Chemosensitizer agent [126] |
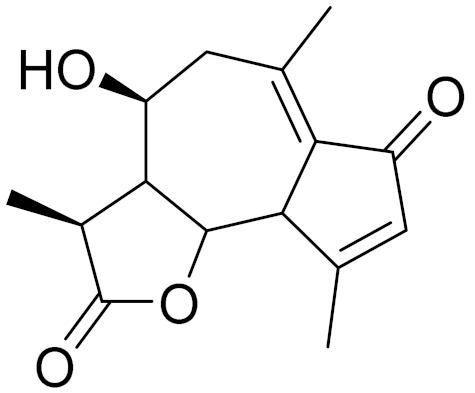 | C15H18O4 | Austricin | Angioprotector and antilipidemic activity [126] | ||
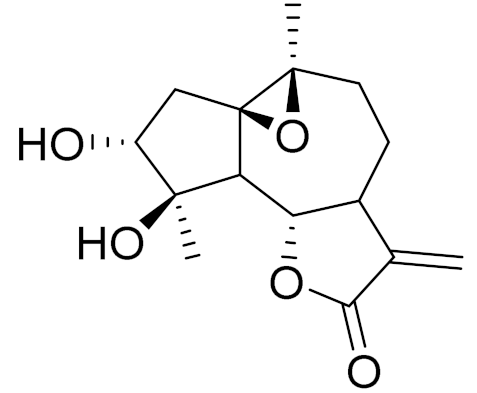 | C15H20O5 | Artefin [127] | Shows neurite outgrowth [128] | ||
| 11 | Artemisia porrecta Krasch. ex Poljakov | 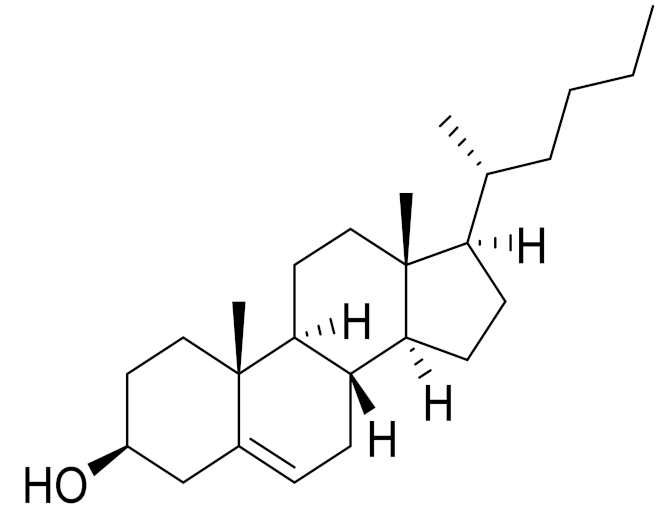 | C25H42O | Nymphayol [129] | Antinociceptive, immunomodulatory and antipyretic activity [130] |
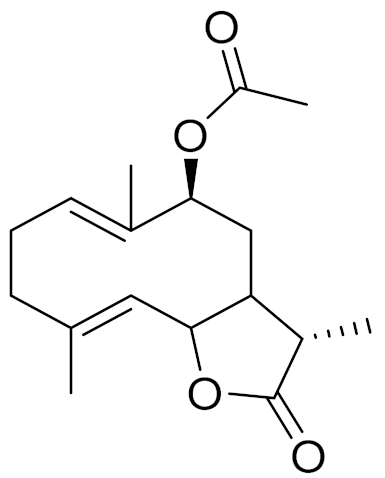 | C17H24O4 | Gerbolide A | - | ||
| 12 | Artemisia albida Willd. ex Ledeb. | 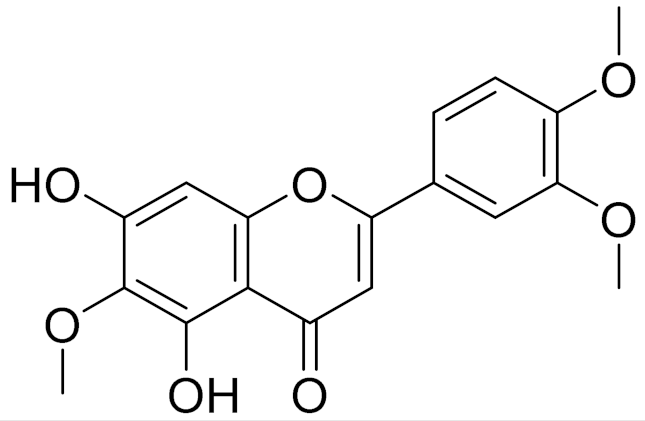 | C18H16O7 | Eupatilin [71] | Anti-inflammatory activity [119] |
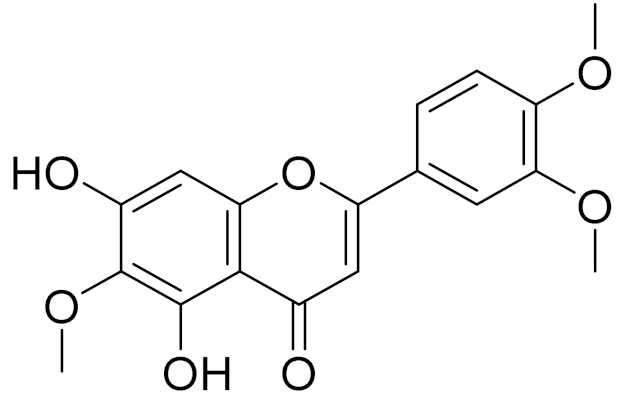 | C18H16O7 | 7-O-Methyl ester of eupatilin | - | ||
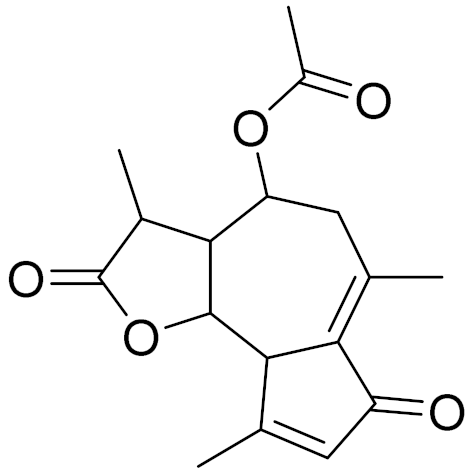 | C17H20O5 | Matricarin | - | ||
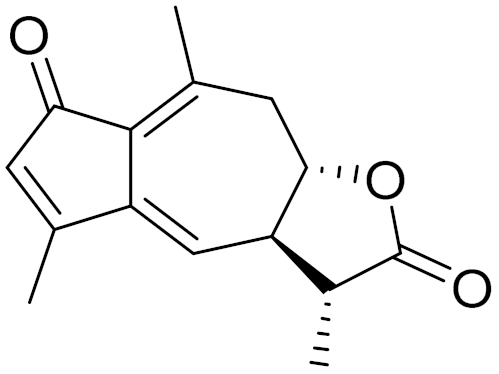 | C15H16O3 | Anhydroaustricin [72] | Low activity against malaria [126] | ||
 | C15H18O4 | Austricin | Angioprotector and antilipidemic activity [126] | ||
| 13 | Artemisia tournefortiana Rchb. | 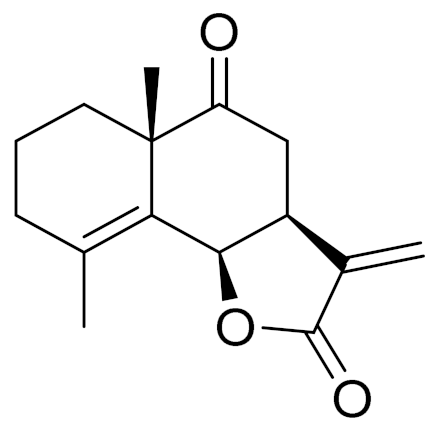 | C15H18O3 | Taurneforin [131] | - |
| 14 | Artemisia pontica L. | 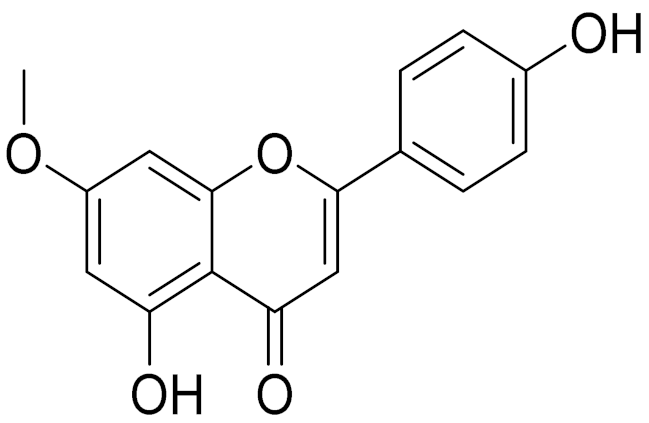 | C16H12O5 | Genkwanin [132] | Anti-inflammatory activity [133] |
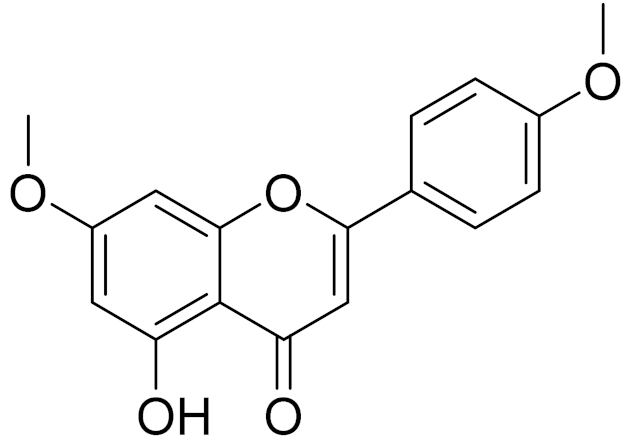 | C17H14O5 | Apigenin 7,4’-dimethyl ether [134] | - | ||
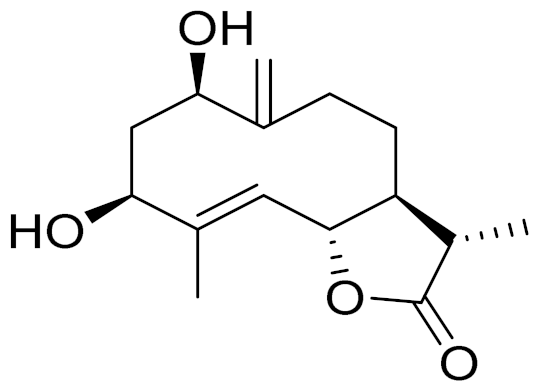 | C15H22O4 | Dihydroridentin [134] | - | ||
| 15 | Artemisia leucodes Schrenk |  | C15H18O4 | 5-β(H)-Austricin [135] | - |
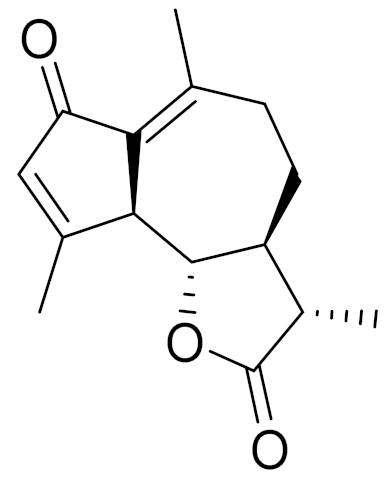 | C15H18O3 | Leucomisin [136] | Antibacterial and phagocytosis-stimulating activity [126] | ||
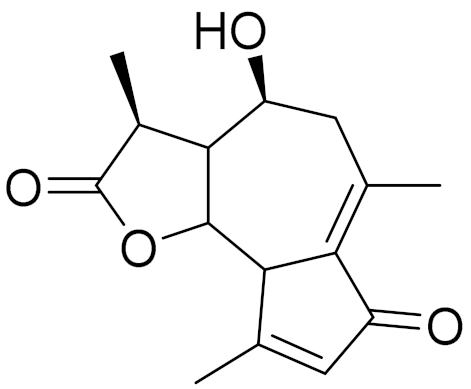 | C15H18O4 | Austricin | Angioprotector and antilipidemic activity [126] | ||
 | C15H16O3 | Grossmizin | Hypolipidemic activity [137] | ||
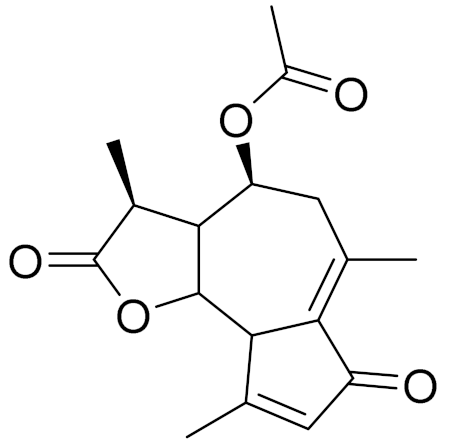 | C17H20O5 | Matricarin | - | ||
| 17 | Artemisia gracilescens Krasch. & Iljin | 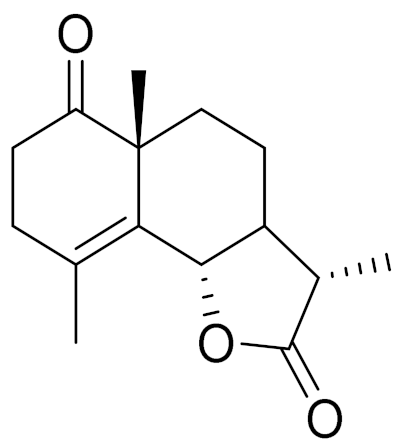 | C15H20O3 | Gracilin [138] | Immunosuppressive activity [139] |
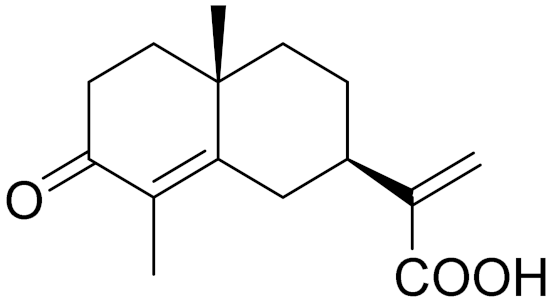 | C15H20O3 | 3-oxocostusic acid [140] | Antibacterial activity [140] | ||
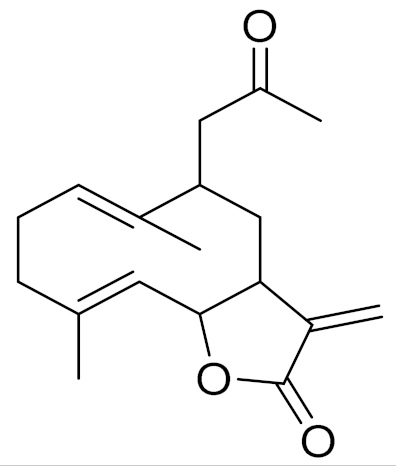 | C18H24O3 | Argracin [141] | TCR activity [141] | ||
| 18 | Artemisia subchrysolepis Filat. | 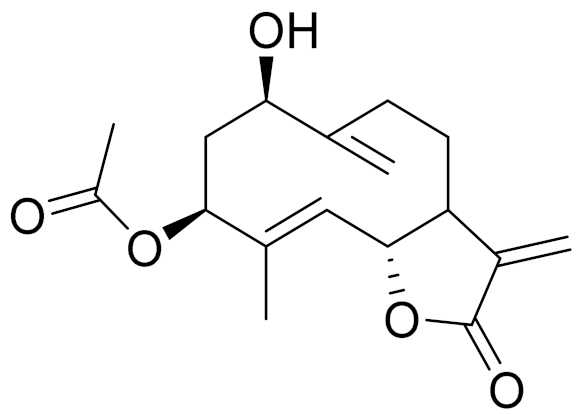 | C17H22O5 | Subchrysin [142] | - |
| 19 | Artemisia altainsis | 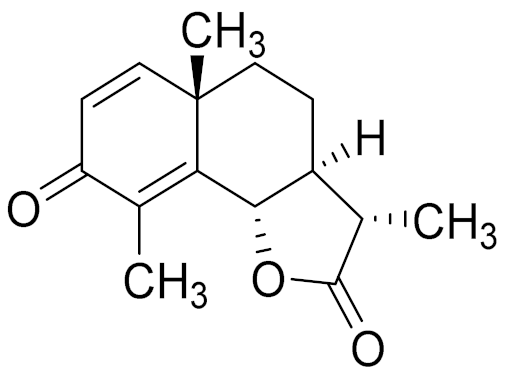 | C15H18O3 | α-Santonin [63] | Anthelmintic [63] and antipyretic activity [104] |
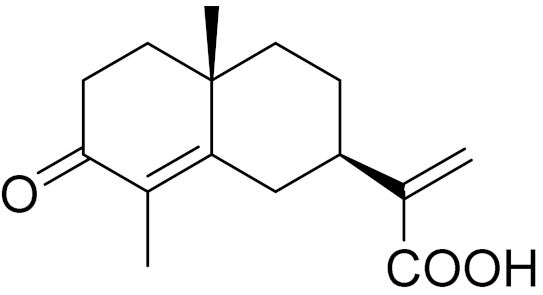 | C15H20O3 | 3-Oxocostus acid [140] | Antibacterial activity [140] | ||
 | C15H22O | α-Cyperone | Antivirulence, antigenotoxic and antibacterial activities [141] | ||
| 20 | Artemesia austriaca Jacq. | 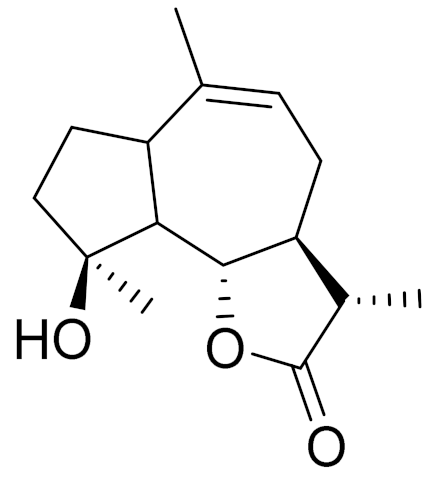 | C15H22O3 | Artaucin [143,144] | - |
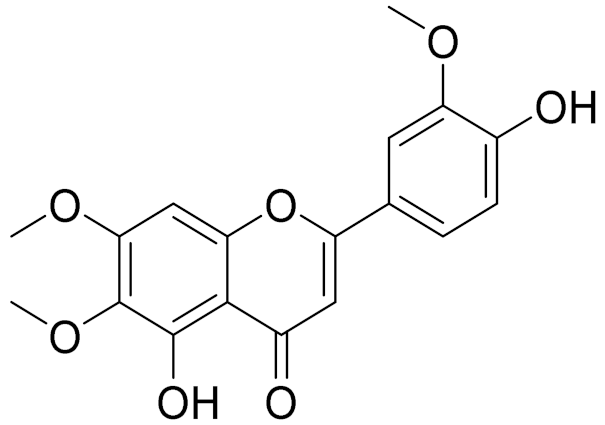 | C18H16O7 | Cirsilineol [112] | Antioxidant, cytostatic, antimicrobial, antifungal, antimalarial and antileishmaniasis activity [112] | ||
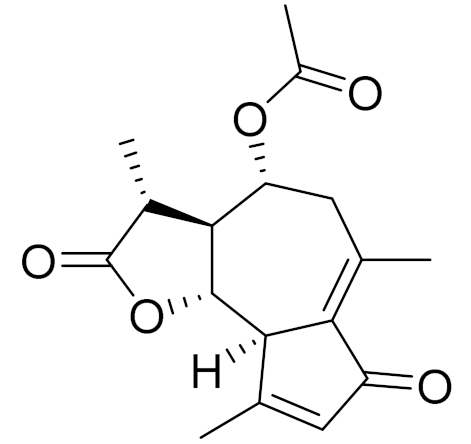 | C17H20O5 | Matricarin | - | ||
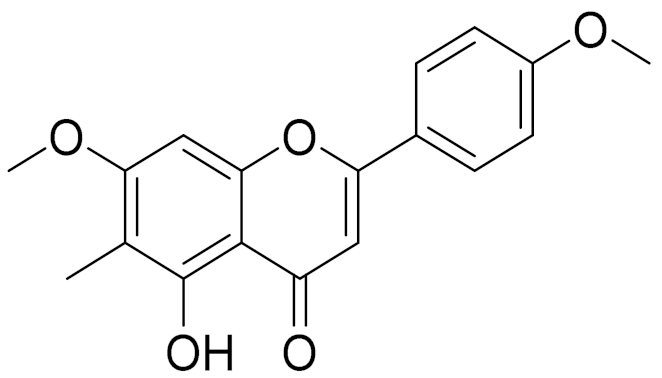 | C18H16O7 | 5-oxy-7,4′-dimethoxy-6-methylflavone [144] | - | ||
 | C15H18O4 | Austricin [143] | Angioprotector and antilipidemic activity [126] | ||
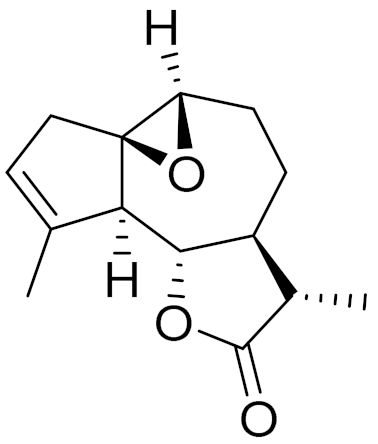 | C14H18O3 | Arborescin [143] | Significant cytotoxic activity in vitro [145] | ||
| 21 | Artemisia latifolia Ledeb. | 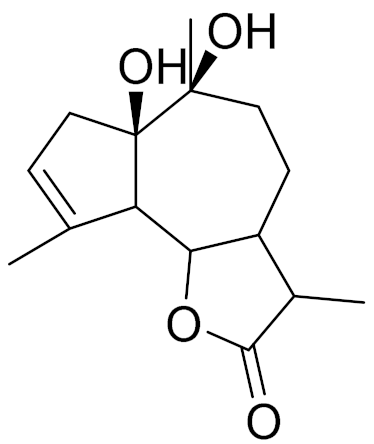 | C15H22O4 | Arlatin [146] | - |
| 22 | Artemisia sublessingiana Krasch. ex Poljakov | 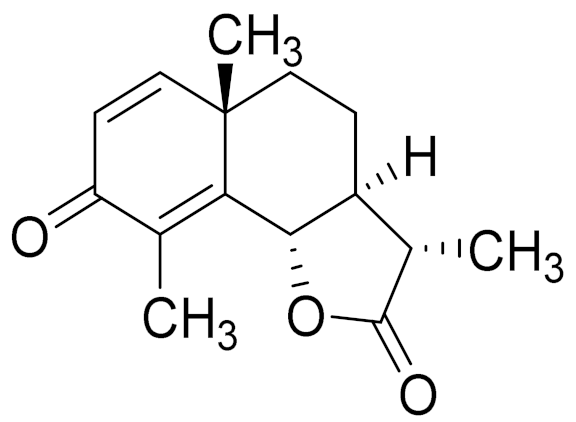 | C15H18O3 | α-Santonin [63] | Anthelmintic [63] and antipyretic activity [104] |
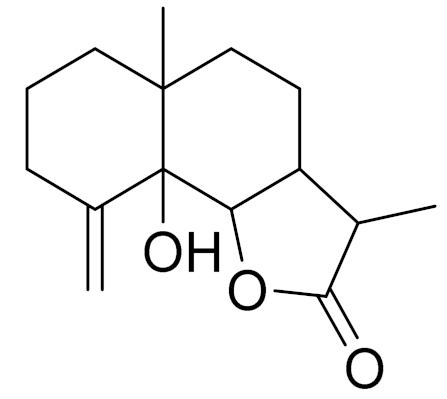 | C15H22O3 | Arsubin [95] | Slightly shows antipyretic actions [96] | ||
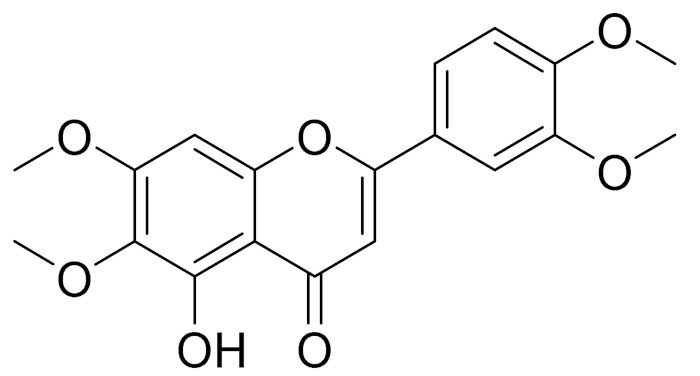 | C19H18O3 | Eupatilin [95,96] | Anti-inflammatory activity [118] | ||
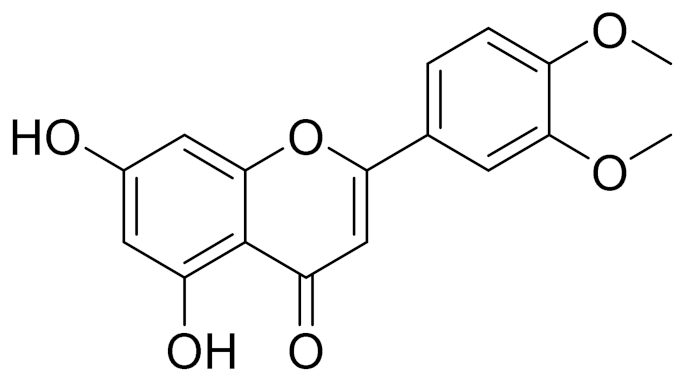 | C17H18O7 | 3′,4′-Dimethoxy-luteolin [95,96] | Potential against the contagious virus SARS-CoV-2 [98] | ||
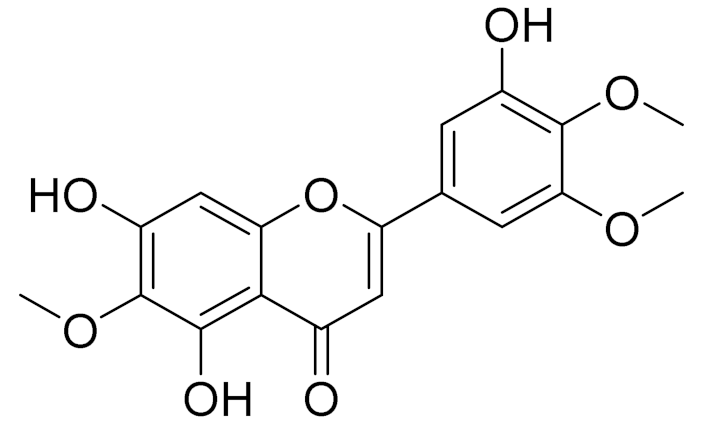 | C18H16O8 | 5, 7, 3′-trihydroxy-6,4′,5′-trimethoxyflavone | - | ||
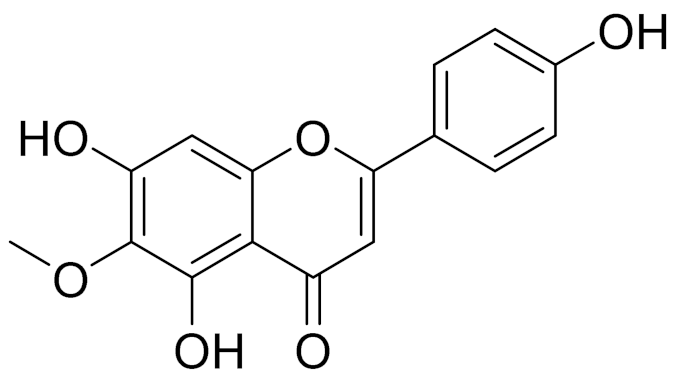 | C16H12O6 | Hispidulin | Anti-tumor effects in a wide array of human cancer cells [147] | ||
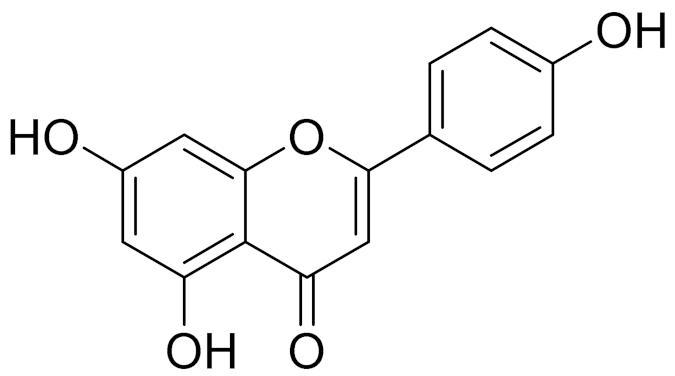 | C15H10O5 | Apigenin | Anti-inflammatory, antibacterial, antiviral and antioxidant agent. [148] | ||
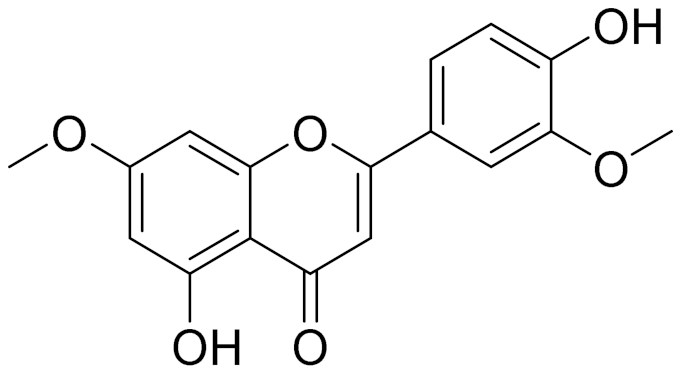 | C17H14O6 | Velutin | Shows improved inhibitory activity against melanin biosynthesis [149] | ||
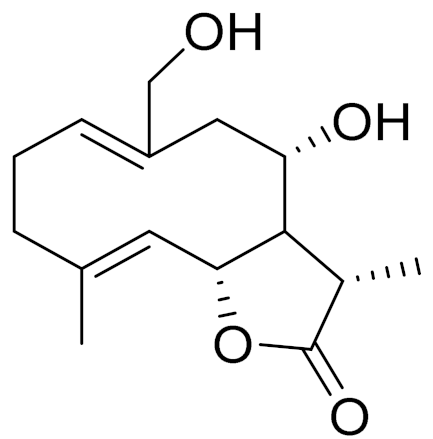 | C15H22O4 | 8α,14-dihydroxy-11,13- dihydromelampolide | - | ||
| 23 | Artemisia nitrosa Weber | 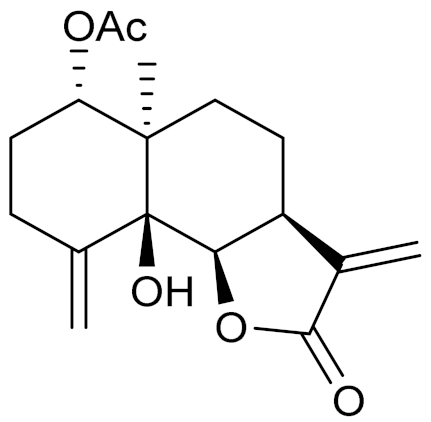 | C17H22O5 | Nitrosin [150] | - |
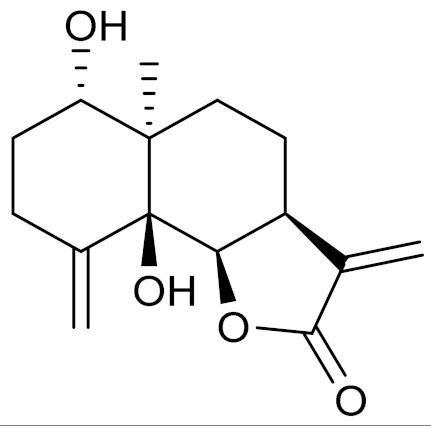 | C15H20O4 | Artemin [151] | Promising candidate for the treatment of neurological disorders [128] | ||
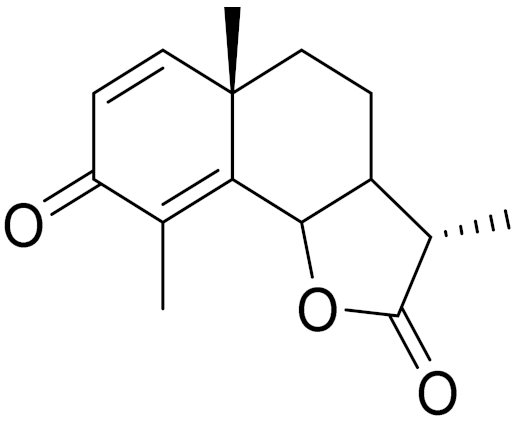 | C15H18O3 | α-Santonin [150,151] | Anthelmintic [63] and antipyretic activity [104] | ||
| 24 | Artemisia pauciflora Weber |  | C15H22O3 | 3-oxo-5,7a,4,6,11b(H)-eudesman-6,12-olide [152] | - |
| 25 | Artemisia transiliensis Poljakov and Artemisia serotina Bunge | 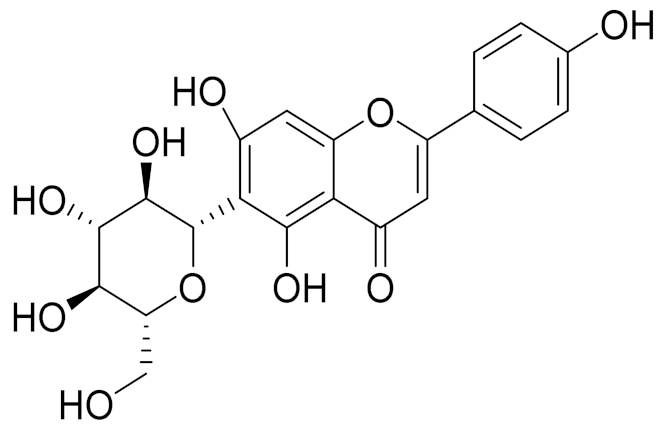 | C21H20O10 | Isovitexin [90] | Antidiabetic agent [153] |
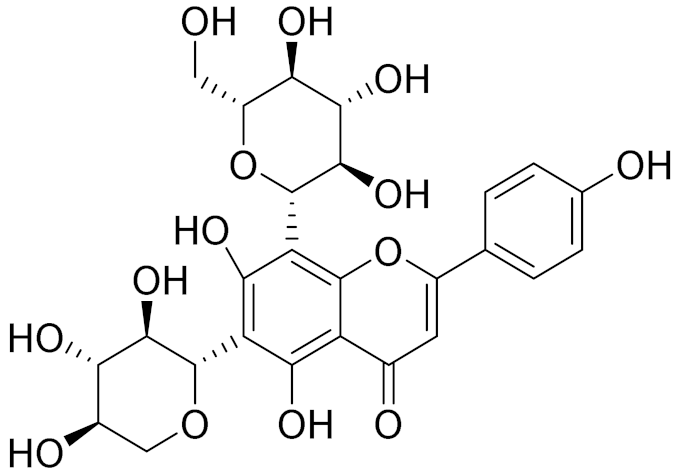 | C26H28O11 | Vicenin 1 | Inhibitory effect on angiotensin-converting enzymes [154] | ||
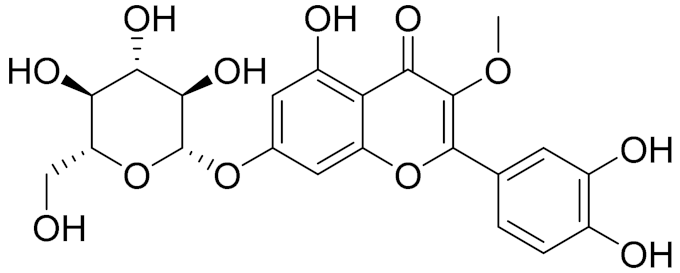 | C22H22O12 | Vransilin | - | ||
 | C27H30O15 | Vicenin 2 | Anti-inflammatory activity [155] | ||
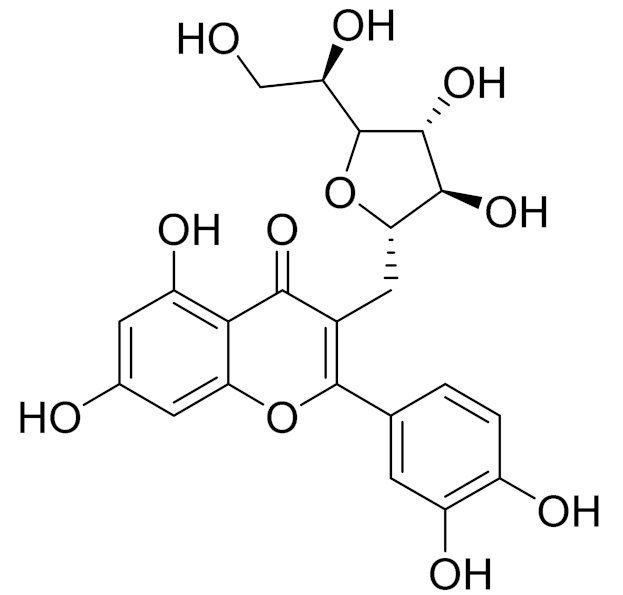 | C22H22O11 | Isoquercitrin | Chemoprotective effects, both in vitro and in vivo, against oxidative stress, cancer, cardiovascular disorders, diabetes and allergic reactions [156] | ||
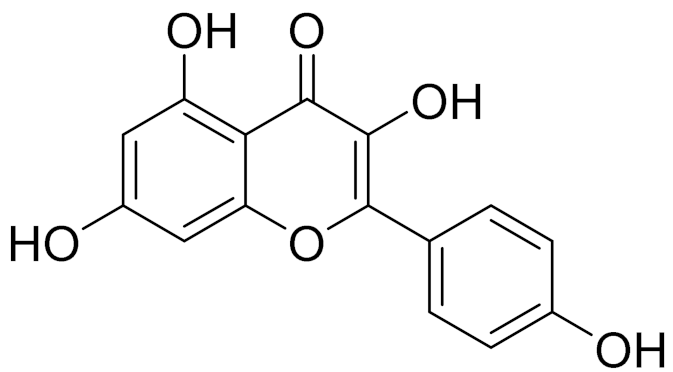 | C16H12O7 | 3-O-Methylquercetin | Possesses antioxidant, antiviral and anticancer properties [157] | ||
 | C15H10O5 | Apigenin | Antioxidant, anti-inflammatory and chemoprevention activity [148] | ||
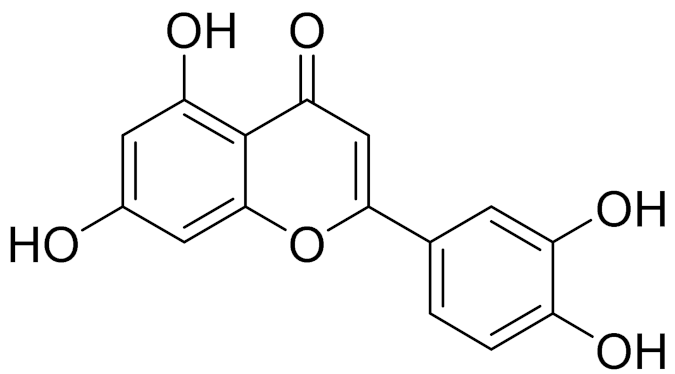 | C15H10O6 | Luteolin | Anticancer, anti-inflammatory, antioxidant, anti-allergic and antimicrobial activity [158,159] | ||
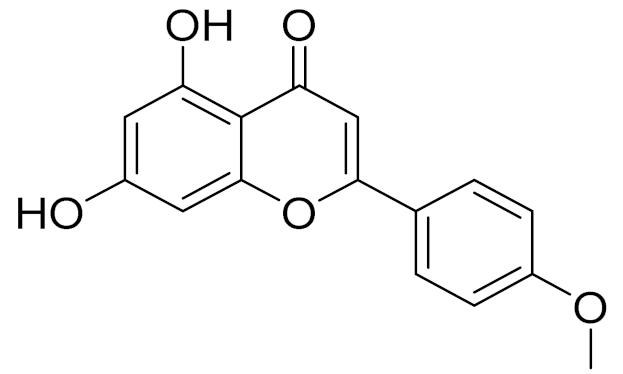 | C16H12O5 | Acacetin | Anticonvulsant [160] | ||
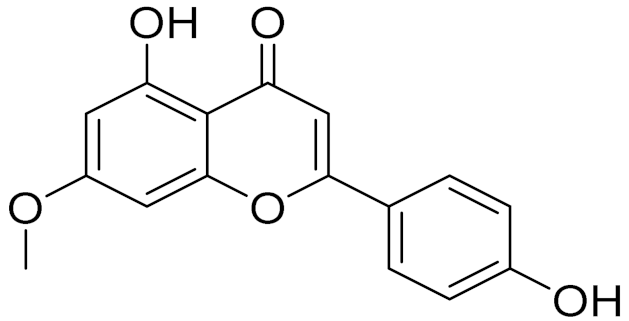 | C16H12O5 | Genkwanin | Anti-inflammatory activity [133] | ||
 | C28H32O14 | Rutin | Antimicrobial, antifungal and anti-allergic agent [161] | ||
 | C15H10O6 | Quercitin | Possesses antioxidant properties and is used in the protection against various diseases such as osteoporosis, lung cancer and cardiovascular disease [162] | ||
| 26 | Artemisia commutata Besser | 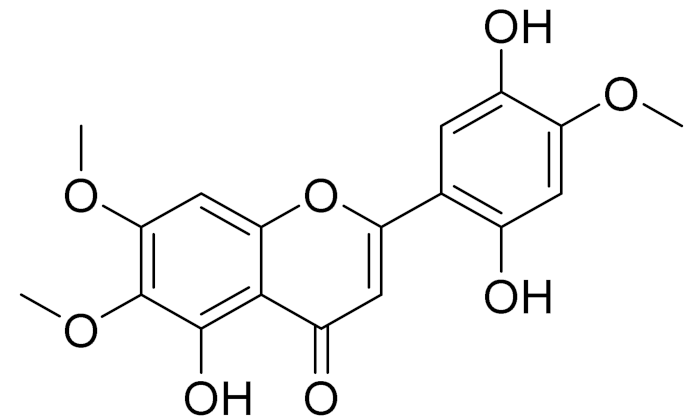 | C18H16O8 | Jusanin [99] | Jusanin showed a high structural similarity degree with X77, the co-crystallized legend of the COVID-19 main protease (PDB ID: 6W 63), Mpro. [97] |
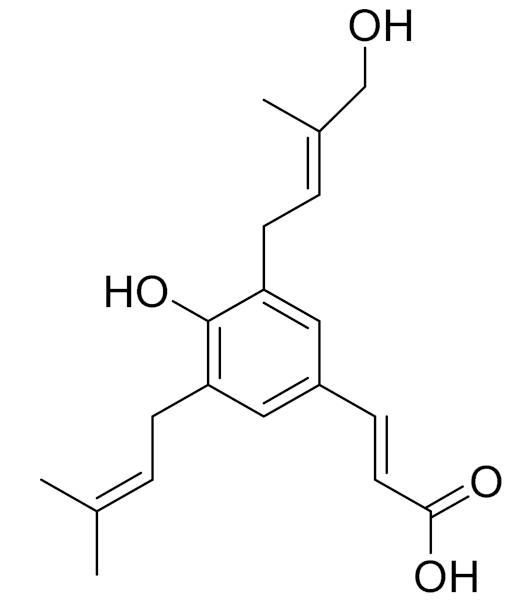 | C19H24O4 | Capillartemisin A | Choleretic activity [163] | ||
 | C15H18O4 | Methyl-3-[S-hydroxyprenyl]-cumarate | - | ||
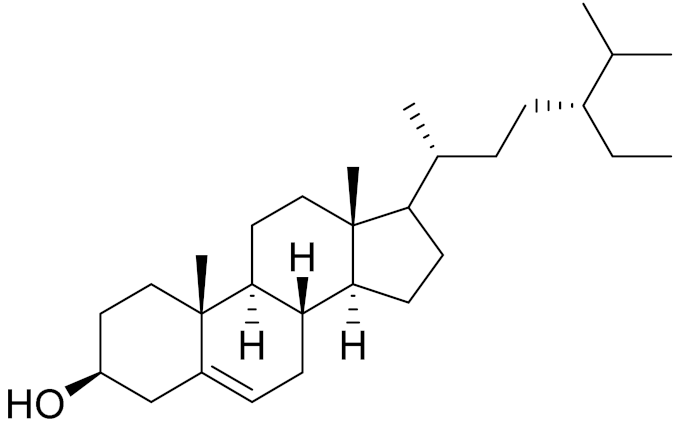 | C29H50O | β-sitosterol | Antifibrotic activity [164] | ||
| 27 | Artemisia glauca Pall. ex Willd | 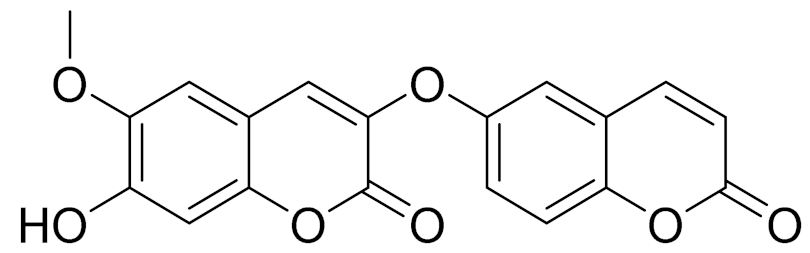 | C19H12O7 | Jusan coumarin [165] | Jusan coumarin demonstrated a high degree of similarity with X77, the co-crystallized ligand of Mpro. [98] |
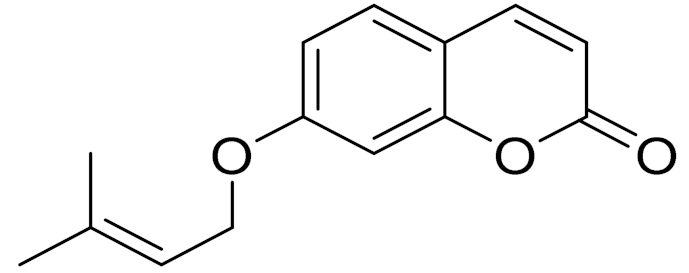 | C14H14O3 | 7-isopentenyloxycoumarin | Antitumor activity [166] | ||
 | C29H50O | β-sitosterol | Antifibrotic activity [164] | ||
| 29 | Artemisia santolinifolia Turcz. ex Bess. | 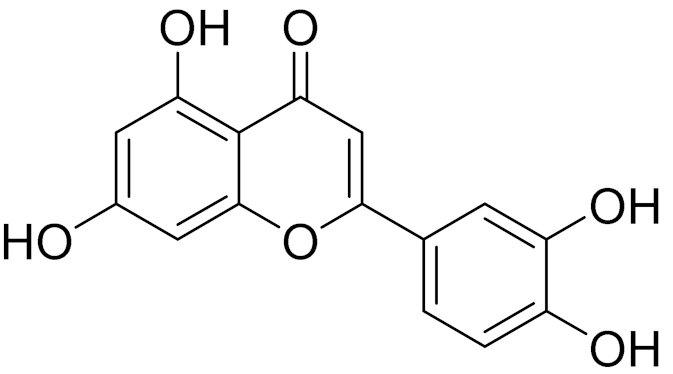 | C15H10O6 | Luteolin | Anticancer, anti-inflammatory, antioxidant, anti-allergic and antimicrobial activity [158,159,160,167] |
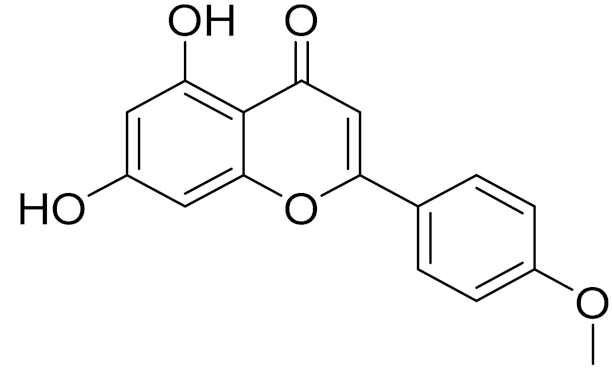 | C16H12O5 | Acacetin | Anticonvulsant [160] | ||
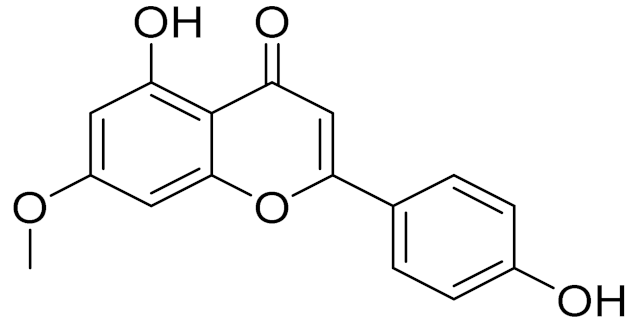 | C16H12O5 | Genkwanin | Anti-inflammatory activity [133] | ||
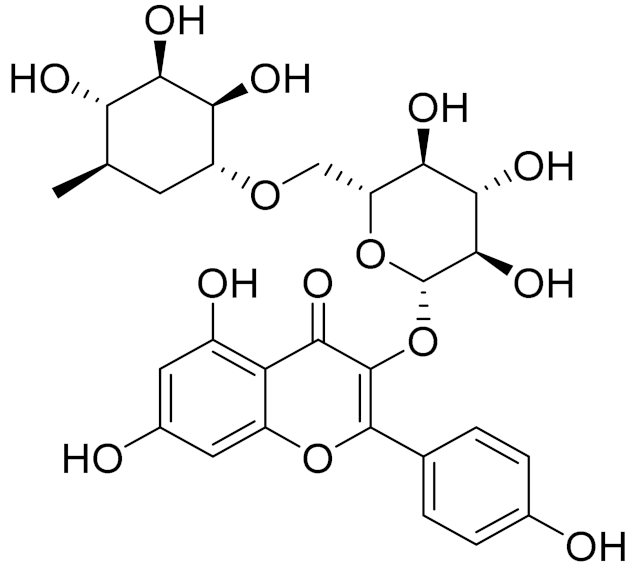 | C28H32O14 | Rutin | Antimicrobial, antifungal and anti-allergic agent [161] | ||
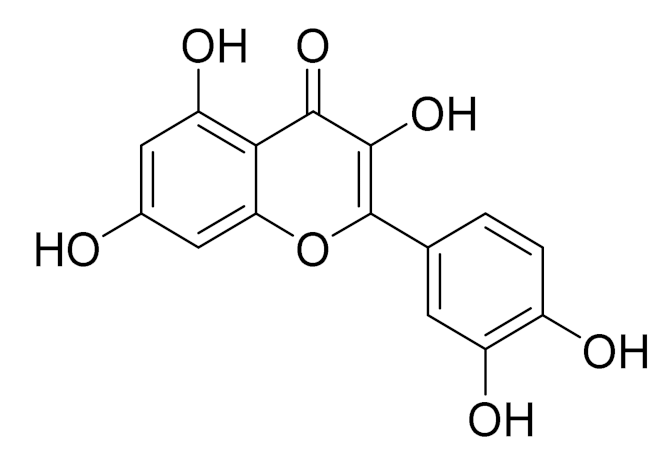 | C15H10O6 | Quercitin | Possesses antioxidant properties and is used in the protection against various diseases such as osteoporosis, lung cancer and cardiovascular disease [162] | ||
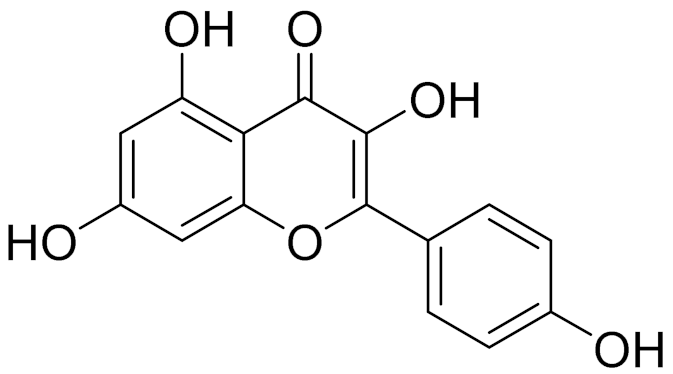 | C15H10O6 | Kaempferol | Antioxidant and antibacterial agent, as well as a plant metabolite [168] | ||
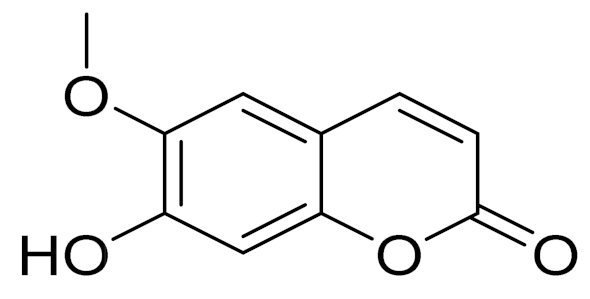 | C10H8O4 | Scopoletin | Potential antineoplastic, antidopaminergic, antioxidant, anti-inflammatory and anticholinesterase effects [169] | ||
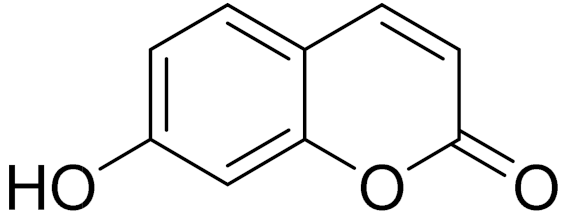 | C9H6O3 | Umbelliferone | Antioxidant properties [170] | ||
| 30 | Artemisia aralensis Krasch. | 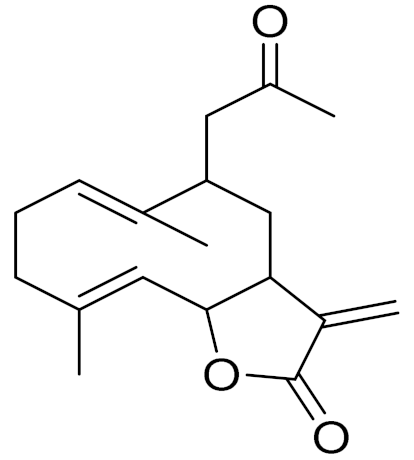 | C18H24O3 | Argracin [91] | TCR activity [139] |
4. Essential Oil Contents of Artemisia Species from Central Asia
5. Chemical Constituents and Bioactivity Ascertainment of Artemisia L. Species from Central Asia
5.1. Materials and Methods
5.1.1. Instruments and Chemicals
5.1.2. Preparation of Extracts for Enzymatic Assays
5.1.3. α-Glucosidase Inhibitory Activity Assay
5.1.4. Assay of PTP1B Inhibitory Activity
5.1.5. BNA Inhibition Assay
5.1.6. Determination of TPC
5.1.7. Determination of TFC
5.1.8. DPPH Radical Scavenging Assay
5.1.9. ABTS Radical Scavenging Activity
5.2. Results and Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dzhumagaliyeva, K.; Sarmurzina, N.; Kayrgaliyeva, G. History of traditional medicine of the Kazakh people. Izv. Samara Sci. Cent. Russ. Acad. Sci. Hist. Sci. 2020, 2, 117–126. [Google Scholar] [CrossRef]
- Shayahemet, K.; Oteyboydak, A.T. Citizen Scientist. Silk Way Youth 2020, 2, 68. [Google Scholar]
- Bakhetbek, T.; Sheypagerlik, B. Treasure of Kazakh’s Traditional Medicine. The Silk Way Youth 2020, 2, 65–67. [Google Scholar]
- Valleás, J. New or rare chromosome counts in Artemisia L. (Asteraceae, Anthemideae) and related genera from Kazakhstan. Bot. J. Linn. Soc. 2001, 137, 399–407. [Google Scholar] [CrossRef]
- Nabiev, R.A.; Galina, G.F. Reflection methods of “traditional medicine” in medical practice in the conditions of Kazakhstan National Public Health formation. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1374–1379. [Google Scholar]
- Xu, X.; Konirhan, B. The Kazakh Herbal Medicine II; China Medical Publishing House: Beijing, China, 2012; pp. 1–489. ISBN 978-7-5067-5523-8. [Google Scholar]
- Xu, X.; Konirhan, B.; Zakaria, B.; Jenis, J. The Kazakh Herbal Medicine I; Ethnic Publishing House: Beijing, China, 2009; pp. 1–450. ISBN 978-7-105-10066-8. [Google Scholar]
- Petrovska, B.B. Historical review of medicinal plants′ usage. Pharmacogn. Rev. 2012, 6, 1. [Google Scholar] [CrossRef]
- Koul, B.; Taak, P.; Kumar, A.; Khatri, T.; Sanyal, I. The Artemisia Genus: A Review on Traditional Uses, Phytochemical Constituents, Pharmacological Properties and Germplasm Conservation. J. Glycom. Lipidom. 2018, 7, 142–149. [Google Scholar] [CrossRef]
- Zaman, W.; Ye, J.; Saqib, S.; Liu, Y.; Shan, Z.; Hao, D.; Chen, Z.; Xiao, P. Predicting potential medicinal plants with phylogenetic topology: Inspiration from the research of traditional Chinese medicine. J. Ethnopharmacol. 2021, 281, 114515. [Google Scholar] [CrossRef]
- Dermene Is a Medicinal Herb. Available online: https://turkystan.kz/article/72807-dermene-d-rilik-sh-pti-t-resi/ (accessed on 22 November 2018).
- Karomatov, I.D.; Ruzieva, I.G. Prospects of Application of the Herb Artemisia Cina. Phytother. Electr. Sci. J. Biol. Integ. Med. 2018, 9, 102–109. [Google Scholar]
- Nokerbek, S.; Sakipova, Z.B.; Ulrich, R. Receiving Extract by Different Methods from Stalks of Medicinal Vegetable Raw Materials of Artemisia rupestris. Vestnik KazNMU 2014, 5, 140–141. [Google Scholar]
- Nokerbek, S. Achievements in the Research of Artemisia rupestris L. Pharm. Kazakhstan 2015, 11, 32–35. [Google Scholar]
- Feng, X.; Cao, S.; Qiu, F.; Zhang, B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol. Ther. 2020, 216 Pt 1, 107650. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; van der Kooy, F.; Verpoorte, R. Artemisia afra: A potential flagship for African medicinal plants? S. Afr. J. Bot. 2009, 75, 185–195. [Google Scholar] [CrossRef]
- Nabimeybodi, R.; Zareshahi, R.; Tansaz, M.; Dastjerdi, M.V.; Hajimehdipoor, H. Scientific Evaluation of Medicinal Plants Used for the Treatment of Cervicitis (Qorohe-Rahem) in Iranian Traditional Medicine. Iran. J. Pharm. Res. 2019, 18, 1884–1901. [Google Scholar] [CrossRef] [PubMed]
- Penkala-Gawęcka, D. Mentally ill or chosen by spirits? ‘Shamanic illness’ and the revival of Kazakh traditional medicine in post-Soviet Kazakhstan. Central Asian Surv. 2013, 32, 37–51. [Google Scholar] [CrossRef]
- Watson, L.E.; Bates, P.L.; Evans, T.M.; Unwin, M.M.; Estes, J.R. Molecular phylogeny of Subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera. BMC Evol. Biol. 2002, 2, 17. [Google Scholar] [CrossRef]
- Algieri, F.; Rodriguez-Nogales, A.; Rodriguez-Cabezas, M.E.; Risco, S.; Ocete, M.A.; Galvez, J. Botanical Drugs as an Emerging Strategy in Inflammatory Bowel Disease: A Review. Mediat. Inflamm. 2015, 2015, 179616. [Google Scholar] [CrossRef]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene Lactones from Artemisia Genus: Biological Activities and Methods of Analysis. J. Anal. Methods Chem. 2015, 2015, 1–21. [Google Scholar] [CrossRef]
- European Scientific Cooperative on Phytotherapy. E/S/C/O/P Monographs: The Scientific Foundation for Herbal Medicinal Products; European Scientific Cooperative on Phytotherapy: Exeter, UK, 2003; pp. 1–147. [Google Scholar]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef]
- Krebs, S.; Omer, B.; Omer, T.N.; Fliser, D. Wormwood (Artemisia absinthium) for Poorly Responsive Early-Stage IgA Nephropathy: A Pilot Uncontrolled Trial. Am. J. Kidney Dis. 2010, 56, 1095–1099. [Google Scholar] [CrossRef]
- Wu, C.; Yan, Y.; Wang, Y.; Sun, P.; Qi, R. Antibacterial epoxy composites with addition of natural Artemisia annua waste. e-Polymers 2020, 20, 262–271. [Google Scholar] [CrossRef]
- Orege, J.I.; Adeyemi, S.B.; Tiamiyu, B.B.; Akinyemi, T.O.; Ibrahim, Y.A.; Orege, O.B. Artemisia and Artemisia-based products for COVID-19 management: Current state and future perspective. Adv. Tradit. Med. 2021, 90, 1–12. [Google Scholar] [CrossRef]
- Tanaguzova, B.M.; Sadyrbekov, D.T.; Ivasenko, S.A.; Atazhanova, G.A.; Adekenov, S.M. Unified method for determining essential oils from wild-growing species of Artemisia. Pharm. Kazakhstan 2006, 4, 43–44. [Google Scholar]
- Leonti, M.; Casu, L. Traditional medicines and globalization: Current and future perspectives in ethnopharmacology. Front. Pharmacol. 2013, 4, 92. [Google Scholar] [CrossRef] [PubMed]
- Merrouni, I.A.; Elachouri, M. Anticancer medicinal plants used by Moroccan people: Ethnobotanical, preclinical, phytochemical and clinical evidence. J. Ethnopharmacol. 2021, 266, 113435. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Glenn, A. Medicinal plants used in Northern Peru for reproductive problems and female health. J. Ethnobiol. Ethnomed. 2010, 6, 30. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Al-Shafie’, J.H.; Elgharabah, W.A.; Kherfan, F.A.; Qarariah, K.H.; Khdair, I.S.; Soos, I.M.; Musleh, A.A.; Isa, B.A.; et al. Traditional knowledge of wild edible plants used in Palestine (Northern West Bank): A comparative study. J. Ethnobiol. Ethnomedicine 2008, 4, 13. [Google Scholar] [CrossRef]
- Tan, R.; Zheng, W.; Tang, H. Biologically Active Substances from the Genus Artemisia. Planta Medica 1998, 64, 295–302. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Coronavirus Disease (COVID-19); WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/health-topics/coronavirus (accessed on 4 February 2022).
- Zumla, A.; Chan, J.F.; Azhar, E.I.; Hui, D.S.; Yuen, K.Y. Coronaviruses—Drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016, 15, 327–347. [Google Scholar] [CrossRef]
- Liu, A.L.; Du, G.H. Drug discovery for COVID-19 treatment based on drug targets. Yaoxue Xuebao 2020, 55, 1073–1080. [Google Scholar] [CrossRef]
- Li, S.-Y.; Chen, C.; Zhang, H.-Q.; Guo, H.-Y.; Wang, H.; Wang, L.; Zhang, X.; Hua, S.-N.; Yu, J.; Xiao, P.-G.; et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005, 67, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Zaman, W.; Ye, J.; Ahmad, M.; Saqib, S.; Shinwari, Z.K.; Chen, Z. Phylogenetic exploration of traditional Chinese medicinal plants: A case study on Lamiaceae. Pak. J. Bot. 2022, 54, 1. [Google Scholar] [CrossRef]
- Adjimani, J.P.; Asare, P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol. Rep. 2015, 2, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.J.; Soszynski, A.A.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. Unravelling the antioxidant potential and the phenolic composition of different anatomical organs of the marine halophyte Limonium algarvense. Ind. Crop. Prod. 2015, 77, 315–322. [Google Scholar] [CrossRef]
- Sochor, J.; Ryvolova, M.; Krystofova, O.; Salaš, P.; Hubalek, J.; Adam, V.; Trnkova, L.; Havel, L.; Beklova, M.; Zehnalek, J.; et al. Fully Automated Spectrometric Protocols for Determination of Antioxidant Activity: Advantages and Disadvantages. Molecules 2010, 15, 8618–8640. [Google Scholar] [CrossRef]
- Anthony, K.P.; Saleh, M.A. Free Radical Scavenging and Antioxidant Activities of Silymarin Components. Antioxidants 2013, 2, 398–407. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986. [Google Scholar] [CrossRef]
- Özyürek, M.; Bektaşoğlu, B.; Güçlü, K.; Güngör, N.; Apak, R. A novel hydrogen peroxide scavenging assay of phenolics and flavonoids using cupric reducing antioxidant capacity (CUPRAC) methodology. J. Food Compos. Anal. 2010, 23, 689–698. [Google Scholar] [CrossRef]
- Khatua, S.; Ghosh, S.; Acharya, K. Simplified Methods for Microtiter Based Analysis of In Vitro Antioxidant Activity. Asian J. Pharm. 2017, 11, 327. Available online: https://www.researchgate.net/publication/318275185%0ASimplified (accessed on 5 February 2022).
- Omari, A.; Okemo, P.O.; Machocho, A.; Njagi, P. The role of phytomedicine in the challenges of emerging, re-emerging diseases and pathogens resistance to antibiotics. Int J. Herb Med. 2015, 1, 92–101. Available online: https://www.researchgate.net/publication/279249087 (accessed on 5 February 2022).
- Yang, Z.; Tu, Y.; Baldermann, S.; Dong, F.; Xu, Y.; Watanabe, N. Isolation and identification of compounds from the ethanolic extract of flowers of the tea (Camellia sinensis) plant and their contribution to the antioxidant capacity. LWT 2009, 42, 1439–1443. [Google Scholar] [CrossRef]
- Combs, A.P. Recent Advances in the Discovery of Competitive Protein Tyrosine Phosphatase 1B Inhibitors for the Treatment of Diabetes, Obesity, and Cancer. J. Med. Chem. 2009, 53, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Dodd, G.; Tiganis, T. Protein Tyrosine Phosphatases in Hypothalamic Insulin and Leptin Signaling. Trends Pharmacol. Sci. 2015, 36, 661–674. [Google Scholar] [CrossRef]
- Bertozzi, C.R.; Kiessling, A.L.L. Chemical Glycobiology. Science 2001, 291, 2357–2364. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Yu, M.-M.; Chai, Y.-F.; Shou, S.-T. Sialic Acids in the Immune Response during Sepsis. Front. Immunol. 2017, 8, 1601. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H. Sialidases from Clostridium perfringens and Their Inhibitors. Front. Cell. Infect. Microbiol. 2020, 9, 462. [Google Scholar] [CrossRef]
- Judžentienė, A. Wormwood (Artemisia absinthium L.) Oils. Essent. Oils Food Preserv. Flavor Saf. 2016, 849–856. [Google Scholar] [CrossRef]
- Vallès, J.; Garcia, S.; Hidalgo, O.; Martín, J.; Pellicer, J.; Sanz, M.; Garnatje, T. Biology, Genome Evolution, Biotechnological Issues and Research Including Applied Perspectives in Artemisia (Asteraceae). Adv. Bot. Res. 2011, 60, 349–419. [Google Scholar] [CrossRef]
- Bykov, V.A. Atlas of Medicinal Plants of the USSR; Russian Academy of Science, Zoological Institute: Moscow, USSR, 1962; p. 711. ISBN 9785870190679. [Google Scholar]
- Lazkov, G.; Sultanova, B.A. Checklist of Vascular Plants of Kyrgyzstan; Finnish Museum of Natural History: Helsinki, Finland, 2011; pp. 1–166. ISBN 9789521075889. [Google Scholar]
- Zufarov, K.A. Uzbek Soviet Socialist Republic. Encyclopedia in One Volume; Uzbek Soviet Encyclopedia Publishing House: Uzbek SSR, Tashkent, 1981; p. 260. [Google Scholar]
- Nowak, A.; Nobis, M.; Nowak, S.; Nobis, A.; Wróbel, A.; Świerszcz, S.; Klichowska, E. Illustrated FLORA of TAJIKISTAN and Adjacent Areas; Polish Academy of Sciences, Botanical Garden Center for Biological Diversity Conservation and Polish Botanical Society: Warsaw, Poland, 2020; ISBN 978-83-938900-5-7. [Google Scholar]
- Babayan, A.; Mashkovskii, M.D.; Oboimakova, A.N. From the first Russian State Phamacopoeia to the eleventh edition of the State Pharmacopoeia of the USSR (the 200th anniversary of the first Russian State Pharmacopoeia). Pharm. Chem. J. 1978, 12, 1543–1547. [Google Scholar] [CrossRef]
- Plavlov, H.V. Flora Kazakhstan, Compositae, 9th ed.; Academy of Sciences of the Kazakh SSR, In-t Botany: Almaty, Kazakhstan, 1966. [Google Scholar]
- Adekenov, S. Chemical modification of arglabin and biological activity of its new derivatives. Fitoterapia 2016, 110, 196–205. [Google Scholar] [CrossRef]
- Lone, S.H.; Bhat, K.A.; Khuroo, M.A. Arglabin: From isolation to antitumor evaluation. Chem. Interact. 2015, 240, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Adekenov, S.M. Method and device for production of lyophilized hydrochlo-ride-1,10-epoxy-13-dimethylaminoguai-3(4)-en-6,12-olide hydrochloride lyophilized, antitumor drug «Arglabin». USA Patent 6,242,617,B1, 5 June 2001; Deutschen Patent 697 2504.9-08, 23.10.03.; Europеan Patent 0946565, 15.10.03.; Patent of China ZL 200680055852.4, 26.12.12.
- Sakipova, Z.; Wong, N.S.H.; Bekezhanova, T.; Shukirbekova, A.; Boylan, F. Quantification of santonin in eight species of Artemisia from Kazakhstan by means of HPLC-UV: Method development and validation. PLoS ONE 2017, 12, e0173714. [Google Scholar] [CrossRef] [PubMed]
- Adekenov, S.; Shaimerdenova, Z.; Ermekkyzy, A. Erratum to “Anatomical study and histochemical analysis of Artemisia leucodes Schrenk. Fitoterapia 2021, 155, 105005. [Google Scholar] [CrossRef] [PubMed]
- Ibragimov, T.S.; Kuatbayev, A.T.; Satybaldiyeva, G.K.; Altybayev, Z.M.; Orazbayev, A.Y. Arealogical features of essential oil plants of the natural flora of the foothill semi-desert zone of the Turkestan region according to the seasons. J. Environ. Manag. Tour. 2019, 10, 1601–1608. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Adekenov, S.; Shaimerdenova, Z.; Ermekkyzy, A. Anatomical study and histochemical analysibragimovis of Artemisia leucodes Schrenk. Fitoterapia 2020, 146, 104721. [Google Scholar] [CrossRef]
- Liu, S.-J.; Liao, Z.-X.; Liu, C.; Ji, L.-J.; Sun, H.-F. Two new sesquiterpenes from Artemisia sieversiana. Fitoterapia 2014, 97, 43–49. [Google Scholar] [CrossRef]
- Suleimenov, E.M.; Ozek, T.; Demirci, F.; Baser, K.H.C.; Adekenov, S.M. Component composition of essential oils of Artemisia lercheana and A. sieversiana of the flora of Kazakhstan. Antimicrobial activity of A. sieversiana essential oil. Chem. Nat. Compd. 2009, 45, 120–123. [Google Scholar] [CrossRef]
- Kobaisy, M.; Tellez, M.; Schrader, K. Phytotoxic, Antialgal, and Antifungal Activity of Constituents from Selected Plants of Kazakh-stan. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2006; Volume 927, pp. 142–151. [Google Scholar] [CrossRef]
- Suleimenov, E.M.; Smagulova, F.M.; Morozova, O.V.; Raldugin, V.A.; Bagryanskaya, I.; Gatilov, Y.V.; Yamovoi, V.I.; Adekenov, S.M. Sesquiterpene Lactones and Flavonoids from Artemisia albida. Chem. Nat. Compd. 2005, 41, 689–691. [Google Scholar] [CrossRef]
- Suleimenov, E.M.; Raldugin, V.A.; Adekenov, S.M. Anhydroaustricin from Artemisia albida. Chem. Nat. Compd. 2008, 44, 541–542. [Google Scholar] [CrossRef]
- Suleimenov, E.M.; Tkachev, A.V.; Adekenov, S.M. Essential oil from Kazakhstan Artemisia species. Chem. Nat. Compd. 2010, 46, 135–139. [Google Scholar] [CrossRef]
- Zhamilya, A.; Yuan, J.; Janar, J.; Tang, C.-P.; Ye, Y. Monomeric and dimeric sesquiterpene lactones from Artemisia heptapotamica. Chin. J. Nat. Med. 2019, 17, 785–791. [Google Scholar] [CrossRef]
- Shaimerdenova, Z.R.; Makubayeva, A.I.; Özek, T.; Özek, G.; Süleyman, Y.U.R.; Atazhanova, G.A.; Adekenov, S.M. Chemical composition of essential oils from Artemisia glabella Kar. et Kir. and Artemisia rupestris L. obtained by different methods. Nat. Vol. Essent. Oils. 2018, 5, 1–9. Available online: https://www.researchgate.net/publication/333558803_Chemical_composition_of_essential_oils_from_artemisia_glabella_kar_Et_kir_and_artemisia_rupestris_L_obtained_by_different_extraction_methods (accessed on 24 January 2022).
- Yong, J.-P.; Aisa, H.A. Synthesis of rupestonic acid amide derivatives and their in vitro activity against type A3 and B flu virus and herpes simplex I and II. Chem. Nat. Compd. 2008, 44, 311–314. [Google Scholar] [CrossRef]
- Yong, J.-P.; Aisa, H.A. Chemical Modification of Rupestonic Acid and Preliminarily In Vitro Antiviral Activity Against Influenza A3and B Viruses. Bull. Korean Chem. Soc. 2011, 32, 1293–1297. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.; Aisa, H.A. Synthesis and anti-influenza activity of aminoalkyl rupestonates. Bioorganic Med. Chem. Lett. 2012, 22, 2321–2325. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Kazakh Folk Medicine I; Xinjiang Science-Technical Publishing House: Urumqi, China, 2009; pp. 26–27. ISBN 978-7-80727-949-5. [Google Scholar]
- Atazhanova, G.A.; Dembitskii, A.D.; Yakovleva, T.D.; Ishmuratova, M.Y.; Mikhailov, V.G.; Adekenov, S.M. Composition of the essential oils of Artemisia radicans and A. frigida. Chem. Nat. Compd. 1999, 35, 427–429. [Google Scholar] [CrossRef]
- Korolyuk, E.A.; Tkachev, A.V. Chemical composition of the essential oil from two wormwood species Artemisia frigida and Artemisia argyrophylla. Russ. J. Bioorganic Chem. 2010, 36, 884–893. [Google Scholar] [CrossRef]
- Ferreira, J.F.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef]
- Aldibek, A.E.; Kudaibergen, A.A.; Dyusebaeva, M.A.; Feng, Y.; Jenis, J. Analysis of amino and fatty acids of the plant Artemisia transiliensis. News Sci. Tech. Soc. “KAHAK” 2019, 3, 61–66. [Google Scholar]
- Kemelbek, M.; Syraiyl, S.; Kudaibergen, A.A.; Mukatay, U.; Ibrahim, M.; Jenis, J. Morphological andphytochemical characteristics of the Artemisia genus from the Ili Alatau region. Vestn. KazNMU 2020, 2, 378–385. [Google Scholar]
- Mukhamatkhanova, R.F.; Bobakulov, K.M.; Sham’ianov, I.D.; Abdullaev, N.D. Terpenoids and other components of Artemisia Serotina and A. Sodiana grown in Uzbekistan. Chem. Plant Raw Mat. 2017, 2, 133–137. [Google Scholar] [CrossRef]
- Goryaev, M.I.; Satdarova, E.I. Analysis of the Essential Oil of Artemisia Serotina. Tr. Inst. Khimicheskikh Nauk. Akad. Nauk. Kazakhskoi SSR. 1959, 4, 37–43. [Google Scholar]
- Nesterova, S.G.; Inelova, Z.A.; Li, T.E.; Korotkov, V.S. Diversity of wormwood (genus Artemisia L.) of the Ile-Balkhash region. Bull. KazNU 2013, 2, 10–13. [Google Scholar]
- Fadeeva, O.V.; Chumbalov, T.K. Flavonoids of Artemisia transiliensis and Artemisia serotina. Fenol’nye Soedin. Ikh Fiziol. Svoistva Vses. Simp. Fenol’nym Soedin. 1973, 2, 134–137. [Google Scholar]
- Bopi, A.K.; Jenis, J.; Dyusebaeva, M.A.; Kudaibergen, A.A.; Feng, Y. Investigation of Chemical Constituents of Artemisia scopae-formis. Vestnik KAZNMU 2019, 4, 320–324. [Google Scholar]
- Bopi, A.K.; Jenis, J.; Dyusebaeva, M.A.; Kudaibergen, A.A.; Feng, Y. Chemical composition of hexane and chloroform extracts from Artemisia scopaeformis. Int. J. Biol. Chem. 2019, 12, 122. [Google Scholar] [CrossRef]
- Adekenov, S.M.; Makubaeva, A.I.; Kokkozov, D.N.; Kanafin, E.N.; Korneev, V.S.; Gatilov, Y.V.; Kishkentaeva, A.S.; Atazhanova, G.A. Chemical Composition of Artemisia aralensis. Chem. Nat. Compd. 2016, 52, 417–420. [Google Scholar] [CrossRef]
- Zhou, Y.; Gilmore, K.; Ramirez, S.; Settels, E.; Gammeltoft, K.A.; Pham, L.V.; Fahnøe, U.; Feng, S.; Offersgaard, A.; Trimpert, J.; et al. In vitro efficacy of artemisinin-based treatments against SARS-CoV-2. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Nair, M.; Huang, Y.; Fidock, D.; Polyak, S.; Wagoner, J.; Towler, M.; Weathers, P. Artemisia annua L. extracts inhibit the in vitro replication of SARS-CoV-2 and two of its variants. J. Ethnopharmacol. 2021, 274, 114016. [Google Scholar] [CrossRef]
- Tarasov, V.A.; Kasymov, S.Z.; Sidyakin, G.P. The structure of the sesquiterpene lactone arsubin. Chem. Nat. Compd. 1971, 7, 722–723. [Google Scholar] [CrossRef]
- Ryakhovskaya, T.V.; Manadilova, A.M.; Sapko, O.A. Flavonoids of Artemisia sublessingiana. Chem. Nat. Compd. 1985, 21, 381–382. [Google Scholar] [CrossRef]
- Jalmakhanbetova, R.I.; Suleimen, Y.M.; Oyama, M.; Elkaeed, E.B.; Eissa, I.H.; Suleimen, R.N.; Metwaly, A.M.; Ishmuratova, M.Y. Isolation and In Silico Anti-COVID-19 Main Protease (Mpro) Activities of Flavonoids and a Sesquiterpene Lactone from Artemisia sublessingiana. J. Chem. 2021, 2021, 5547013. [Google Scholar] [CrossRef]
- Suleimen, Y.M.; Jose, R.A.; Suleimen, R.N.; Arenz, C.; Ishmuratova, M.Y.; Toppet, S.; Dehaen, W.; Alsfouk, B.A.; Elkaeed, E.B.; Eissa, I.H.; et al. Jusanin, a New Flavonoid from Artemisia commutata with an In Silico Inhibitory Potential against the SARS-CoV-2 Main Protease. Molecules 2022, 27, 1636. [Google Scholar] [CrossRef] [PubMed]
- Suleimen, Y.M.; Jose, R.A.; Suleimen, R.N.; Ishmuratova, M.Y.; Toppet, S.; Dehaen, W.; Alsfouk, A.A.; Elkaeed, E.B.; Eissa, I.H.; Metwaly, A.M. Isolation and In Silico SARS-CoV-2 Main Protease Inhibition Potential of Jusan Coumarin, a New Dicoumarin from Artemisia glauca. Molecules 2022, 27, 2281. [Google Scholar] [CrossRef]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D.K. Phytochemistry and pharmacological activity of the genus Artemisia. Arch. Pharmacal Res. 2021, 44, 439–474. [Google Scholar] [CrossRef]
- Atazhanova, G.A.; Dembitskii, A.D.; Yakovleva, T.D.; Mikhailov, V.G.; Adekenov, S.M. About composition of essential oil from Artemisia filatovae. Chem. Nat. Compd. 1999, 35, 529–531. [Google Scholar] [CrossRef]
- Suleimenov, E.M.; Ozek, T.; Demirci, F.; Baser, K.H.C.; Adekenov, S.M. Component composition and antimicrobial activity of essential oil from Artemisia kasakorum. Chem. Nat. Compd. 2008, 44, 263–265. [Google Scholar] [CrossRef]
- Mamatova, A.S.; Korona-Glowniak, I.; Skalicka-Woźniak, K.; Józefczyk, A.; Wojtanowski, K.K.; Baj, T.; Sakipova, Z.B.; Malm, A. Phytochemical composition of wormwood (Artemisia gmelinii) extracts in respect of their antimicrobial activity. BMC Complement. Altern. Med. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Sisengalieva, G.G.; Suleimen, E.M.; Ishmuratova, M.Y.; Iskakova, Z.B.; van Hecke, K. Constituents of Artemisia tschernieviana and Their Biological Activity. Chem. Nat. Compd. 2015, 51, 544–547. [Google Scholar] [CrossRef]
- Mabtín, M.; Morán, A.; Carrón, R.; Montero, M.J.; Roman, L. Antipyretic activity of α- and β-santonin. J. Ethnopharmacol. 1988, 23, 285–290. [Google Scholar] [CrossRef]
- Suleimenov, E.M.; Smagulova, F.M.; Seidakhmetova, R.B.; Aksartov, R.M.; Raldugin, V.A.; Adekenov, S.M. 4-Epiashantin from Artemisia sieversiana. Chem. Nat. Compd. 2007, 43, 232–233. [Google Scholar] [CrossRef]
- Stojanović, G.; Radulović, N.; Hashimoto, T.; Palić, R. In vitro antimicrobial activity of extracts of four Achillea species: The composition of Achillea clavennae L. (Asteraceae) extract. J. Ethnopharmacol. 2005, 101, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.; Bermejo, P.; Valverde, S.; Villar, A. Anti-Inflammatory Activity of Hydroxyachillin, a Sesquiterpene Lactone from Tanacetum microphyllum. Planta Med. 1994, 60, 228–231. [Google Scholar] [CrossRef]
- Li, Y.; Ni, Z.-Y.; Zhu, M.-C.; Zhang, K.; Wu, Y.-B.; Dong, M.; Shi, Q.-W.; Huo, C.-H.; Sauriol, F.; Kiyota, H.; et al. Millifolides A–C. New 1,10-Seco-guaianolides from the Flowers of Achillea millefolium. Z. Nat. B 2012, 67, 438–446. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, M.-C.; Zhang, M.-L.; Wang, Y.-F.; Dong, M.; Shi, Q.-W.; Huo, C.-H.; Sauriol, F.; Kiyota, H.; Gu, Y.-C.; et al. Achillinin B and C, new sesquiterpene dimers isolated from Achillea millefolium. Tetrahedron Lett. 2012, 53, 2601–2603. [Google Scholar] [CrossRef]
- Kagarlitskii, A.D.; Adekenov, S.M. New Sesquiterpene Lactones from Plants of Central Kazakhstan. Izv. Akad. Nauk. Kazakhskoi SSR Seriya Khimicheskaya 1984, 4, 37–40. [Google Scholar]
- Kishkentayeva, A.; Adekenov, S.; Drašar, P. Production Technologies of Pharmacologically Active Sesquiterpene Lactones. Eurasian Chem. J. 2018, 20, 325. [Google Scholar] [CrossRef]
- Yili, A.; Mutalipu, H.; Aisa, A.; Isaev, M.I. Betulinic acid and sterols from Astragalus altaicus. Chem. Nat. Compd. 2009, 45, 592–594. [Google Scholar] [CrossRef]
- Adekenov, S.M.; Turdibekov, K.M.; Aituganoav, K.A.; Lindeman, S.V.; Struchkov, Y.T.; Shaltakov, S.N. 1-β,10-α-Dihydroxyarglabin—A new sesquiterpene lactone from Artemisia glabella. Chem. Nat. Compd. 1993, 6, 825–830. [Google Scholar] [CrossRef]
- Tashenov, Y.; Dzhalmakhanbetova, R.I.; Smagulova, F.M.; Dudkin, R.V.; Gorovoi, P.G.; Suleiman, E.M.; Ross, S.A. Cirsilineol and cubreuva lactone from Artemisia umbrosa and their biological activity. Chem. Nat. Compd. 2013, 49, 97–98. [Google Scholar] [CrossRef]
- Esenbaeva, A.E.; Shul’Ts, E.E.; Gatilov, Y.V.; Shakirov, M.M.; Patrushev, S.S.; Atazhanova, G.A.; Kenesheva, A.B.; Adekenov, S.M. Synthesis of 13-Aryl Derivatives of the Sesquiterpene Lactone Argolide and their Analgesic Activity. Chem. Nat. Compd. 2013, 49, 875–881. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Mitchell, P.T.; Kishkentaeva, A.S.; Shaimerdenova, Z.R.; Atazhanova, G.A.; Adekenov, S.M.; Quinn, M.T. The natural sesquiterpene lactones arglabin, grosheimin, agracin, parthenolide, and estafiatin inhibit T cell receptor (TCR) activation. Phytochemistry 2018, 146, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Adekenov, S.M.; Shaimerdenova, Z.R.; Gatilov, Y.V.; Atazhanova, G.A. Two New Sesquiterpene Lactones from Artemisia halophila. Chem. Nat. Compd. 2017, 53, 284–289. [Google Scholar] [CrossRef]
- Khlebnikov, A.I.; Schepetkin, I.A.; Kishkentaeva, A.S.; Shaimerdenova, Z.R.; Atazhanova, G.A.; Adekenov, S.M.; Kirpotina, L.N.; Quinn, M.T. Inhibition of T Cell Receptor Activation by Semi-Synthetic Sesquiterpene Lactone Derivatives and Molecular Modeling of Their Interaction with Glutathione and Tyrosine Kinase ZAP-70. Molecules 2019, 24, 350. [Google Scholar] [CrossRef]
- Giangaspero, A.; Ponti, C.; Pollastro, F.; Del Favero, G.; Della Loggia, R.; Tubaro, A.; Appendino, G.B.; Sosa, S. Topical Anti-inflammatory Activity of Eupatilin, A Lipophilic Flavonoid from Mountain Wormwood (Artemisia umbelliformis Lam.). J. Agric. Food Chem. 2009, 57, 7726–7730. [Google Scholar] [CrossRef]
- Turdybekov, K.M.; Rakhimova, B.B.; Makhmutova, A.S.; Smailova, Z.R.; Nurkenov, O.A.; Adekenov, S.M. Stereochemistry of Methoxylated Flavonoids from Artemisia semiarida. Chem. Nat. Compd. 2014, 50, 135–136. [Google Scholar] [CrossRef]
- Seo, J.-M.; Kang, H.-M.; Son, K.-H.; Kim, J.H.; Lee, C.W.; Kim, H.M.; Chang, S.-I.; Kwon, B.-M. Antitumor Activity of Flavones Isolated from Artemisia argyi. Planta Medica 2003, 69, 218–222. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Azuma, J.; Mozaffari, M. Role of antioxidant activity of taurine in diabetesThis article is one of a selection of papers from the NATO Advanced Research Workshop on Translational Knowledge for Heart Health (published in part 1 of a 2-part Special Issue). Can. J. Physiol. Pharmacol. 2009, 87, 91–99. [Google Scholar] [CrossRef]
- Adekenov, S.M. Sesquiterpene lactones from endemic species of the family Asteraceae. Chem. Nat. Compd. 2013, 49, 158–162. [Google Scholar] [CrossRef]
- Blanco, J.G.; Gil, R.R.; Bocco, J.L.; Meragelman, T.L.; Genti-Raimondi, S.; Flury, A. Aromatase inhibition by an 11,13-dihydroderivative of a sesquiterpene lactone. J. Pharmacol. Exp. Ther. 2001, 297, 1099–1105. [Google Scholar] [PubMed]
- Hajdú, Z.; Hohmann, J.; Forgo, P.; Máthé, I.; Molnár, J.; Zupkó, I. Antiproliferative Activity of Artemisia asiatica Extract and Its Constituents on Human Tumor Cell Lines. Planta Medica 2014, 80, 1692–1697. [Google Scholar] [CrossRef]
- Plutno, A.B.; Sham, I.D. Modification of the sesquiterpene lactones leukomisin and austricin biological activities of some of their derivatives. Chem. Nat. Compd. 1995, 31, 579–583. [Google Scholar] [CrossRef]
- Buketova, G.K.; Turmukhambetov, A.Z.; Bagryanskaya, I.Y.; Gatilov, Y.V.; Adekenov, S.M. Artefin? A new sesquiterpene lactone from Artemesia filatovii. Chem. Nat. Compd. 1995, 31, 55–57. [Google Scholar] [CrossRef]
- Ilieva, M.; Nielsen, J.; Korshunova, I.; Gotfryd, K.; Bock, E.; Pankratova, S.; Michel, T. Artemin and an Artemin-Derived Peptide, Artefin, Induce Neuronal Survival, and Differentiation Through Ret and NCAM. Front. Mol. Neurosci. 2019, 12, 47. [Google Scholar] [CrossRef]
- Adekenov, S.M.; Kishkentaeva, A.S.; Al’Murzin, N.D.; Esenbaeva, A.E.; Atazhanova, G.A. Nymphayol and Gerbolide A from Artemisia porrecta. Chem. Nat. Compd. 2013, 49, 532. [Google Scholar] [CrossRef]
- Pandurangan, S.-B.; Paul, A.S.; Savarimuthu, I.; Ali, A.A. Antinociceptive, Immunomodulatory and Antipyretic Activity of Nymphayol Isolated from Nymphaea stellata (Willd.) Flowers. Biomol. Ther. 2013, 21, 391–397. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Moradi, M.H. Adaptive fuzzy-sift rule-based registration for 3D cardiac motion estimation. Appl. Intell. 2022, 52, 1615–1629. [Google Scholar] [CrossRef]
- Talzhanov, N.A.; Mukanov, R.M.; Raldugin, V.A.; Shakirov, M.M.; Atazhanova, G.A.; Adekenov, S.M. Dihydroridentin from Artemisia pontica and its Stereochemistry. Chem. Nat. Compd. 2005, 41, 423–425. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, F.; Fang, L.; Cai, R.; Zong, C.; Qi, Y. Genkwanin Inhibits Proinflammatory Mediators Mainly through the Regulation of miR-101/MKP-1/MAPK Pathway in LPS-Activated Macrophages. PLoS ONE 2014, 9, e96741. [Google Scholar] [CrossRef]
- Talzhanov, N.A.; Sadyrbekov, D.T.; Smagulova, F.M.; Mukanov, R.M.; Raldugin, V.A.; Shakirov, M.M.; Tkachev, A.V.; Atazhanova, G.A.; Tuleuov, B.I.; Adekenov, S.M. Components of Artemisia pontica. Chem. Nat. Compd. 2005, 41, 178–181. [Google Scholar] [CrossRef]
- Talzhanov, N.A.; Yamovoi, V.I.; Kulyyasov, A.T.; Turdybekov, K.M.; Adekenov, S.M. 5 (H)-Austricin, a New Guaianolide from Artemisia leucodes. Chem. Nat. Compd. 2004, 40, 129–133. [Google Scholar] [CrossRef]
- Arystan, L.I.; Sariev, A.K.; Akhmetova, S.B.; Adekenov, S.M. Experimental evaluation of the antibacterial and phagocytosis-stimulating properties of leucomisine. Eksperimental’naia I Klin. Farmakol. 2009, 72, 35–37. [Google Scholar]
- Ratkin, A.K. Hypolipidemic action of Grossmizin in hyperlipidemia induced by triton WR 1339. Education 2015, 2, 121–123. [Google Scholar]
- Abbasov, M.E.; Alvariño, R.; Chaheine, C.M.; Alonso, E.; Sánchez, J.A.; Conner, M.L.; Alfonso, A.; Jaspars, M.; Botana, L.M.; Romo, D. Simplified immunosuppressive and neuroprotective agents based on gracilin A. Nat. Chem. 2019, 11, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Turmukhambetov, A.Z.; Adekenov, S.M.; Turdybekov, K.M.; Gatilov, Y.V. Gracilin—A new sesquiterpene lactone from Artemisia gracilescens. Chem. Nat. Compd. 1991, 27, 292–295. [Google Scholar] [CrossRef]
- Khanina, M.A.; Kulyyasov, A.T.; Bagryanskaya, I.; Gatilov, Y.V.; Adekenov, S.M.; Raldugin, V.A. 3-Oxocostusic acid from Artemisia altaiensis. Chem. Nat. Compd. 1998, 34, 145–147. [Google Scholar] [CrossRef]
- Adekenov, S.M.; Turmukhambetov, A.Z.; Buketova, G.K. Structure of argracin—A new sesquiterpene lactone from Artemesia gracilescens. Chem. Nat. Compd. 1992, 28, 387–388. [Google Scholar] [CrossRef]
- Turdybekov, K.M.; Edil’Baeva, T.T.; Raldugin, V.A.; Shakirov, M.M.; Kulyyasov, A.T.; Adekenov, S.M. Structure of subchrysin. Chem. Nat. Compd. 1998, 34, 141–144. [Google Scholar] [CrossRef]
- Adekenov, S.M.; Aituganov, K.A.; Golovtsov, N.I. Artausin? A new sesquiterpene lactone from Artemesia austriaca. Chem. Nat. Compd. 1987, 23, 127–128. [Google Scholar] [CrossRef]
- Adekenov, S. Chemical study of Artemisia austriaca Jacq. Int. J. Biol. Chem. 2021, 14, 156–163. [Google Scholar] [CrossRef]
- Dylenova, E.P.; Randalova, T.E.; Tykheev, Z.A.; Zhigzhitzhapova, S.V.; Radnaeva, L.D. Artemisia jacutica Drob. as the source of terpenoids. IOP Conf. Series Earth Environ. Sci. 2019, 320, 012054. [Google Scholar] [CrossRef]
- Adekenov, S.M.; Kadirberlina, G.M.; Kagarlitskii, A.D.; Mukhametzhanov, M.N.; Fomichev, A.A.; Golovtsov, N.I. Arlatin—A new sesquiterpene lactone from Artemisia latifolia. Chem. Nat. Compd. 1984, 20, 755–757. [Google Scholar] [CrossRef]
- Gao, H.; Gao, M.-Q.; Peng, J.-J.; Han, M.; Liu, K.-L.; Han, Y. Hispidulin mediates apoptosis in human renal cell carcinoma by inducing ceramide accumulation. Acta Pharmacol. Sin. 2017, 38, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- Cadoná, F.C.; Machado, A.K.; Bodenstein, D.; Rossoni, C.; Favarin, F.R.; Ourique, A.F. Natural product–based nanomedicine: Polymeric nanoparticles as delivery cargoes of food bioactives and nutraceuticals for anticancer purposes. In Advances and Avenues in the Development of Novel Carriers for Bioactives and Biological Agents; Academic Press: Cambridge, MA, USA, 2020; pp. 37–67. [Google Scholar] [CrossRef]
- Jung, S.-H.; Kim, J.; Eum, J.; Choe, J.W.; Kim, H.H.; Kee, Y.; Lee, K. Velutin, an Aglycone Extracted from Korean Mistletoe, with Improved Inhibitory Activity against Melanin Biosynthesis. Molecules 2019, 24, 2549. [Google Scholar] [CrossRef] [PubMed]
- Adekenov, S.M.; Kharasov, R.M.; Kuprinov, A.N.; Turmukhambetov, A.Z. Nitrosin—A new sesquiterpene lactone from Artemisia nitrosa. Chem. Nat. Compd. 1986, 22, 608–609. [Google Scholar] [CrossRef]
- Adekenov, S.M.; Turmukhambetov, A.Z.; Turdybekov, K.M. Structure of Nitrosin and Biogenesis of Sesquiterpene Lactones of Artemisia Nitrosa. Izv. Akad. Nauk. Resp. Kazakhstan Seriya Khimicheskaya 1992, 1, 79–86. [Google Scholar]
- Adekenov, S.M. Sesquiterpene lactones from plants of the family Asteraceae in the Kazakhstan flora and their biological activity. Chem. Nat. Compd. 1995, 31, 21–25. [Google Scholar] [CrossRef]
- Kamakura, R.; Son, M.J.; de Beer, D.; Joubert, E.; Miura, Y.; Yagasaki, K. Antidiabetic effect of green rooibos (Aspalathus linearis) extract in cultured cells and type 2 diabetic model KK-Ay mice. Cytotechnology 2014, 67, 699–710. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Luo, J.-G.; Han, C.; Xu, J.-F.; Kong, L.-Y. Bioassay-guided preparative separation of angiotensin-converting enzyme inhibitory C-flavone glycosides from Desmodium styracifolium by recycling complexation high-speed counter-current chromatography. J. Pharm. Biomed. Anal. 2015, 102, 276–281. [Google Scholar] [CrossRef]
- Wu, H.; Guo, J.; Chen, S.; Liu, X.; Zhou, Y.; Zhang, X.; Xu, X. Recent developments in qualitative and quantitative analysis of phytochemical constituents and their metabolites using liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2012, 72, 267–291. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Schwingel, L.C.; Schwingel, G.O.; Storch, N.; Barreto, F.; Bassani, V.L. 3-O-Methylquercetin from organic Nicotiana tabacum L. trichomes: Influence of the variety, cultivation and extraction parameters. Ind. Crop. Prod. 2014, 55, 56–62. [Google Scholar] [CrossRef]
- Shukla, R.; Pandey, V.; Vadnere, G.P.; Lodhi, S. Role of Flavonoids in Management of Inflammatory Disorders. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Academic Press: Cambridge, MA, USA, 2019; pp. 293–322. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Zhang, Z.; Chen, M.; Zhang, D.; Tian, C.; Liu, M.; Jiang, G. The Antibacterial Activity and Mechanism of Action of Luteolin Against Trueperella pyogenes. Infect. Drug Resist. 2020, 13, 1697–1711. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5280442, Acacetin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acacetin (accessed on 27 June 2022).
- Al-Dhabi, N.A.; Arasu, M.V.; Park, C.H.; Park, S.U. An up-to-date review of rutin and its biological and pharmacological activities. EXCLI J. 2015, 14, 59–63. [Google Scholar] [CrossRef] [PubMed]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Kitagawa, I.; Fukuda, Y.; Yoshihara, M.; Yamahara, J.; Yoshikawa, M. Capillartemisin A and B, two new choleretic principles from Artemisiae Capillaris Herba. Chem. Pharm. Bull. 1983, 31, 352–355. [Google Scholar] [CrossRef]
- Kim, K.-S.; Yang, H.J.; Lee, J.-Y.; Na, Y.-C.; Kwon, S.-Y.; Kim, Y.-C.; Lee, J.-H.; Jang, H.-J. Effects of β-sitosterol derived from Artemisia capillaris on the activated human hepatic stellate cells and dimethylnitrosamine-induced mouse liver fibrosis. BMC Complement. Altern. Med. 2014, 14, 363. [Google Scholar] [CrossRef]
- Sokolova, A.S.; Yarovaya, O.; Shernyukov, A.; Pokrovsky, A.; Pokrovsky, A.; Lavrinenko, V.A.; Zarubaev, V.V.; Tretiak, T.S.; Anfimov, P.M.; Kiselev, O.I.; et al. New quaternary ammonium camphor derivatives and their antiviral activity, genotoxic effects and cytotoxicity. Bioorganic Med. Chem. 2013, 21, 6690–6698. [Google Scholar] [CrossRef]
- Baba, M.; Jin, Y.; Mizuno, A.; Suzuki, H.; Okada, Y.; Takasuka, N.; Tokuda, H.; Nishino, H.; Okuyama, T. Studies on Cancer Chemoprevention by Traditional Folk Medicines XXIV. Inhibitory Effect of a Coumarin Derivative, 7-Isopentenyloxycoumarin, against Tumor-Promotion. Biol. Pharm. Bull. 2002, 25, 244–246. [Google Scholar] [CrossRef]
- Suleimen, E.M.; Dzhalmakhanbetova, R.I.; Ishmuratova, M.Y. Flavonoids from Artemisia santolinifolia. Chem. Nat. Compd. 2014, 50, 918–919. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem Compound Summary for CID 5280863, Kaempferol. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Kaempferol (accessed on 29 February 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5280460, Scopoletin. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Scopoletin (accessed on 29 February 2022).
- Mazimba, O. Umbelliferone: Sources, chemistry and bioactivities review. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 223–232. [Google Scholar] [CrossRef]
- Jenis, J.; Baiseitova, A.; Yoon, S.H.; Park, C.; Kim, J.Y.; Li, Z.P.; Lee, K.W.; Park, K.H. Competitive α-glucosidase inhibitors, dihydrobenzoxanthones, from the barks of Artocarpus elasticus. J. Enzym. Inhib. Med. Chem. 2019, 34, 1623–1632. [Google Scholar] [CrossRef]
- Li, Z.P.; Song, Y.H.; Uddin, Z.; Wang, Y.; Park, K.H. Inhibition of protein tyrosine phosphatase 1B (PTP1B) and α-glucosidase by xanthones from Cratoxylum cochinchinense, and their kinetic characterization. Bioorganic Med. Chem. 2018, 26, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Kim, J.Y.; Li, Z.P.; Jenis, J.; Ban, Y.J.; Baiseitova, A.; Park, K.H. Effectiveness of Prenyl Group on Flavonoids from Epimedium koreanum Nakai on Bacterial Neuraminidase Inhibition. Molecules 2019, 24, 317. [Google Scholar] [CrossRef] [PubMed]
- Baiseitova, A.; Jenis, J.; Kim, J.Y.; Li, Z.P.; Park, K.H. Phytochemical analysis of aerial part of Ikonnikovia kaufmanniana and their protection of DNA damage. Nat. Prod. Res. 2019, 35, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]



| Countries | Number of Present Species | Number of Endemic Species |
|---|---|---|
| China | 186 | 82 |
| EX-USSR | 180 | 45 |
| Russian Federation | 80 | - |
| Kyrgyzstan | 55 | 5 |
| Uzbekistan | 47 | 19 |
| Tajikistan | 48 | 1 |
| Turkmenistan | 33 | 1 |
| Kazakhstan | 81 | 19 |
| No | Species | α-Glucosidase, Inhibition (%), 50 μg/ml | PTP1B, Inhibition (%), 50 μg/ml | BNA, Inhibition (%), 20 μg/ml |
|---|---|---|---|---|
| 1 | A. albida | 55.8 | 85.5 | 95.5 |
| 2 | A. terrae-alba | 25.5 | 65.2 | 96.5 |
| 3 | A. serotina | 25.0 | 65.0 | 92.1 |
| 4 | A. marschalliana | 43.1 | 75.1 | 88.1 |
| 5 | A. schrenkiana | 59.3 | 76.2 | 87.2 |
| 6 | A. rutifolia | 39.3 | 71.2 | 85.6 |
| 7 | A. nitrosa | 47.1 | 76.1 | 95.6 |
| 8 | A. scopaeformis | 83.1 | 95.6 | 99.8 |
| 9 | A. transiliensis | 64.0 | 92.3 | 85.6 |
| 10 | A. scoparia | 28.7 | 66.5 | 89.8 |
| 11 | A. albicerata | 67.8 | 77.8 | 95.2 |
| 12 | Deoxynojirimycin * | 100.0 | - | - |
| 13 | Ursolic acid * | - | 100.0 | - |
| 14 | Quercetin * | - | - | 100.0 |
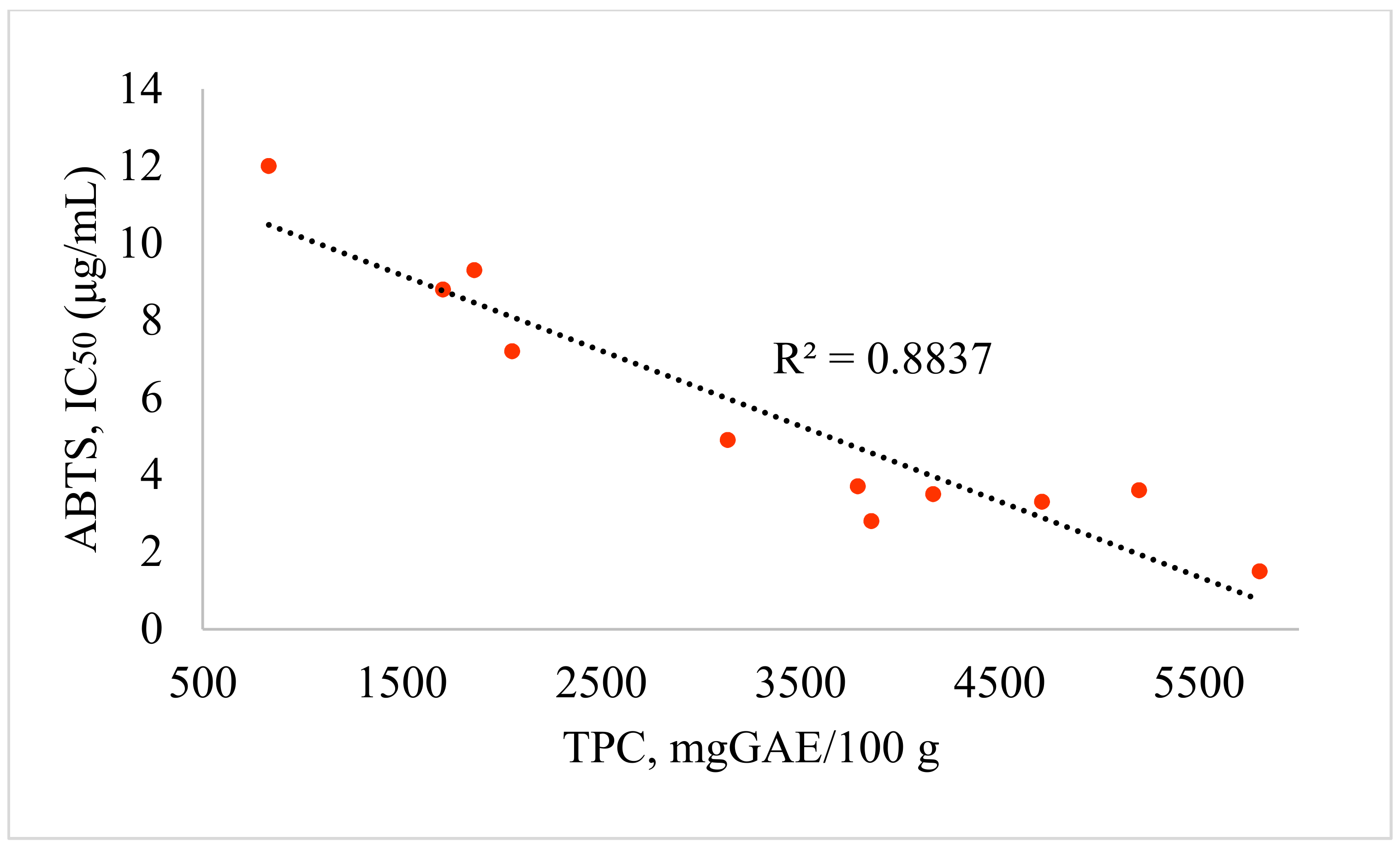
| No | Species | TPC mgGAE/100 g | TFC mgQE/100 g | DPPH, IC50 (μg/mL) | ABTS, IC50 (μg/mL) |
|---|---|---|---|---|---|
| 1 | A. albida | 832 | 698 | 29.9 | 12.0 |
| 2 | A. terrae-alba | 1706 | 709 | 14.2 | 8.8 |
| 3 | A. serotina | 1864 | 748 | 13.9 | 9.3 |
| 4 | A. marschalliana | 2053 | 1419 | 18.0 | 7.2 |
| 5 | A. schrenkiana | 5199 | 2080 | 11.7 | 3.6 |
| 6 | A. rutifolia | 3787 | 2024 | 10.7 | 3.7 |
| 7 | A. nitrosa | 3856 | 1892 | 11.3 | 2.8 |
| 8 | A. scopaeformis | 5804 | 2745 | 8.4 | 1.5 |
| 9 | A. transiliensis | 4166 | 2975 | 9.4 | 3.5 |
| 10 | A. scoparia | 4711 | 1951 | 8.5 | 3.3 |
| 11 | A. albicerata | 3135 | 1290 | 13.4 | 4.9 |
| 12 | Trolox * | - | - | 36.5 | 19.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurlybekova, A.; Kudaibergen, A.; Kazymbetova, A.; Amangeldi, M.; Baiseitova, A.; Ospanov, M.; Aisa, H.A.; Ye, Y.; Ibrahim, M.A.; Jenis, J. Traditional Use, Phytochemical Profiles and Pharmacological Properties of Artemisia Genus from Central Asia. Molecules 2022, 27, 5128. https://doi.org/10.3390/molecules27165128
Nurlybekova A, Kudaibergen A, Kazymbetova A, Amangeldi M, Baiseitova A, Ospanov M, Aisa HA, Ye Y, Ibrahim MA, Jenis J. Traditional Use, Phytochemical Profiles and Pharmacological Properties of Artemisia Genus from Central Asia. Molecules. 2022; 27(16):5128. https://doi.org/10.3390/molecules27165128
Chicago/Turabian StyleNurlybekova, Aliya, Aidana Kudaibergen, Aizhan Kazymbetova, Magzhan Amangeldi, Aizhamal Baiseitova, Meirambek Ospanov, Haji Akber Aisa, Yang Ye, Mohamed Ali Ibrahim, and Janar Jenis. 2022. "Traditional Use, Phytochemical Profiles and Pharmacological Properties of Artemisia Genus from Central Asia" Molecules 27, no. 16: 5128. https://doi.org/10.3390/molecules27165128
APA StyleNurlybekova, A., Kudaibergen, A., Kazymbetova, A., Amangeldi, M., Baiseitova, A., Ospanov, M., Aisa, H. A., Ye, Y., Ibrahim, M. A., & Jenis, J. (2022). Traditional Use, Phytochemical Profiles and Pharmacological Properties of Artemisia Genus from Central Asia. Molecules, 27(16), 5128. https://doi.org/10.3390/molecules27165128