A Comparative Study for Nutritional and Phytochemical Profiling of Coffea arabica (C. arabica) from Different Origins and Their Antioxidant Potential and Molecular Docking
Abstract
:1. Introduction
2. Results and Discussion
2.1. Measurement of Phenolic Compounds and Antioxidant Potential of C. arabica
2.2. Pearson’s Correlation Analysis of Phenolic Contents and Antioxidant Activities
2.3. LC-MS/MS Identification of Metabolites from C. arabica
2.3.1. Nutritional Profile of C. arabica
2.3.2. Hormones
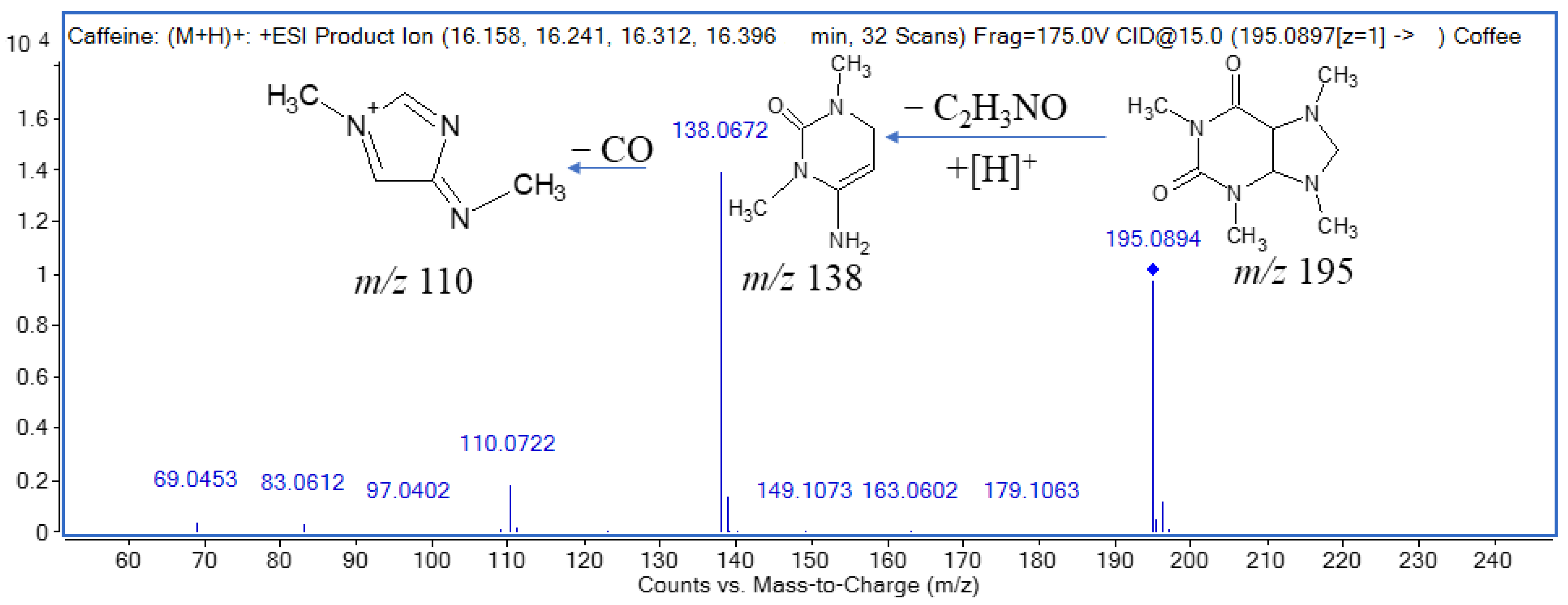
2.3.3. Alkaloids
2.3.4. Triterpenes
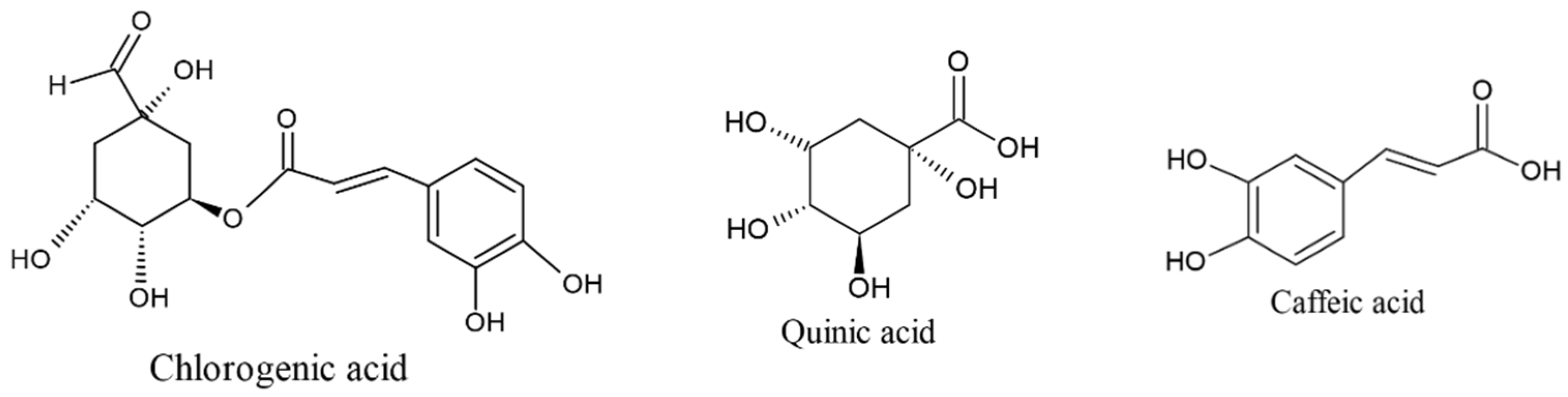
2.3.5. Phenolic Acids
Hydroxybenzoic Acid Derivatives
Hydroxycinnamic Acid Derivatives
2.3.6. Flavonoids
2.3.7. Stilbenes and Lignans
2.3.8. Other Polyphenols
2.4. Distribution of Nutritional and Phytochemical Metabolites
2.5. Heatmap Clustering of Abundant Individual Phenolic Metabolites in Coffee Samples
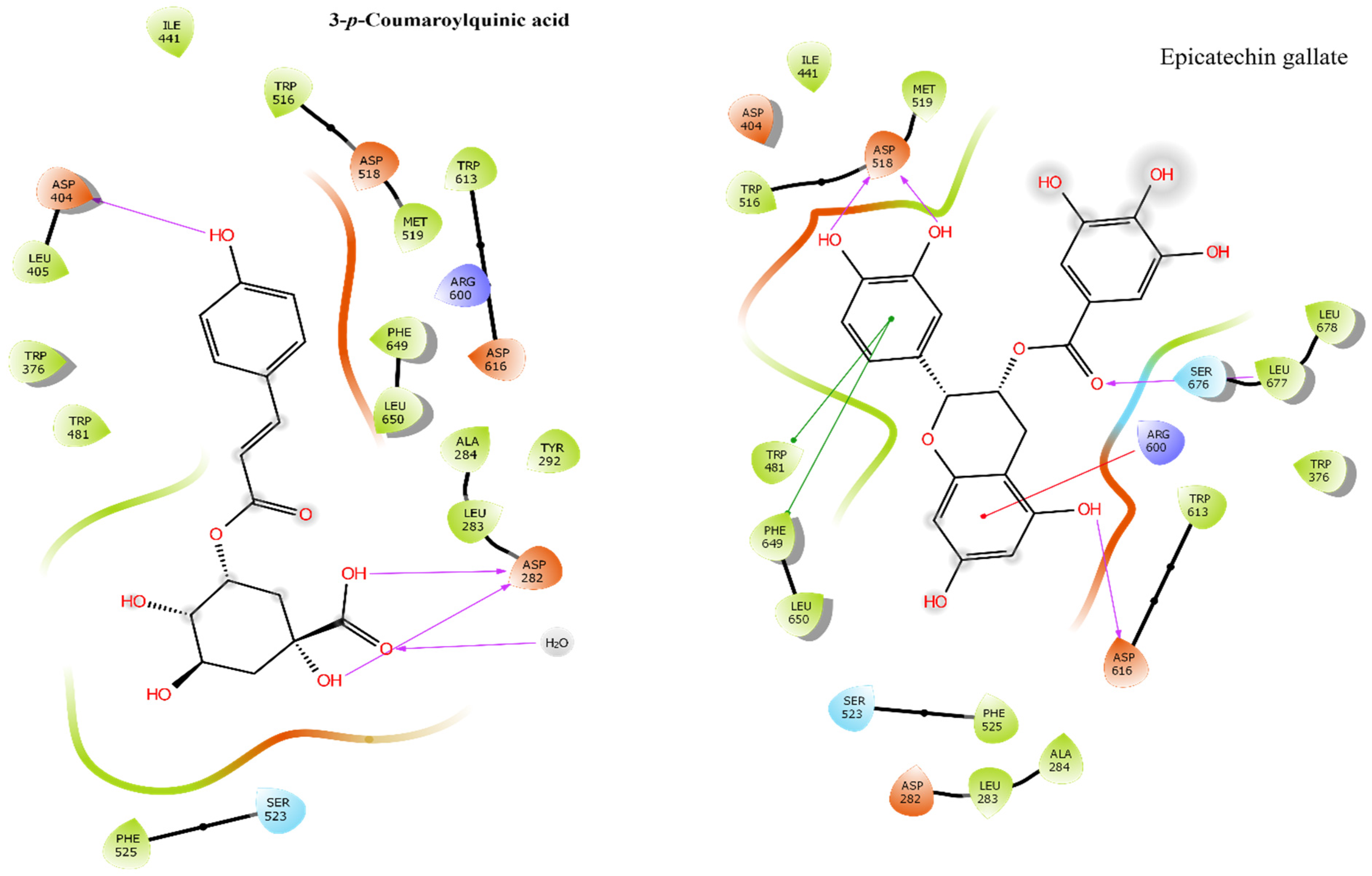
2.6. Molecular Docking
3. Materials and Methods
3.1. Materials
3.2. Extraction and Preparation of C. arabica Samples for Analysis
3.3. Measurement of Total Polyphenols and Total Flavonoids
3.4. Measurement of Antioxidant Potential of C. arabica
3.5. LC-MS/MS Identification and Quantification of Phytochemical Metabolites
3.6. Molecular Docking and Metabolite-Metabolite Networking of Selected Compounds
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Saeed, M.; Naveed, M.; BiBi, J.; Ali Kamboh, A.; Phil, L.; Chao, S. Potential nutraceutical and food additive properties and risks of coffee: A comprehensive overview. Crit. Rev. Food Sci. Nutr. 2019, 59, 3293–3319. [Google Scholar] [CrossRef] [PubMed]
- López-Froilán, R.; Ramírez-Moreno, E.; Podio, N.S.; Pérez-Rodríguez, M.L.; Cámara, M.; Baroni, M.V.; Wunderlin, D.A.; Sánchez-Mata, M.C. In vitro assessment of potential intestinal absorption of some phenolic families and carboxylic acids from commercial instant coffee samples. Food Funct. 2016, 7, 2706–2711. [Google Scholar] [CrossRef] [PubMed]
- Rojas-González, A.; Figueroa-Hernández, C.Y.; González-Rios, O.; Suárez-Quiroz, M.L.; González-Amaro, R.M.; Hernández-Estrada, Z.J.; Rayas-Duarte, P. Coffee chlorogenic acids incorporation for bioactivity enhancement of foods: A review. Molecules 2022, 27, 3400. [Google Scholar] [CrossRef] [PubMed]
- Erskine, E.; Gültekin Subaşı, B.; Vahapoglu, B.; Capanoglu, E. Coffee phenolics and their interaction with other food phenolics: Antagonistic and synergistic effects. ACS Omega 2022, 7, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Liczbiński, P.; Bukowska, B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind. Crops Prod. 2022, 175, 114265. [Google Scholar] [CrossRef]
- Yamagata, K. Do coffee polyphenols have a preventive action on metabolic syndrome associated endothelial dysfunctions? An assessment of the current evidence. Antioxidants 2018, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Saimaiti, A.; Zhou, D.D.; Li, J.; Xiong, R.G.; Gan, R.Y.; Huang, S.Y.; Shang, A.; Zhao, C.N.; Li, H.Y.; Li, H.B. Dietary sources, health benefits, and risks of caffeine. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–19. [Google Scholar] [CrossRef]
- Reddy, P.N.; Kolla, H.B.; Konatham, H.R.; Giridhar, P.; Dirisala, V.R. Current understandings of health benefits of coffee on cardiovascular and metabolic syndromes. In Coffee Science; CRC Press: Boca Raton, FL, USA, 2022; pp. 265–271. [Google Scholar]
- Rawangkan, A.; Siriphap, A.; Yosboonruang, A.; Kiddee, A.; Pook-In, G.; Saokaew, S.; Sutheinkul, O.; Duangjai, A. Potential antimicrobial properties of coffee beans and coffee by-products against drug-resistant Vibrio cholerae. Front. Nutr. 2022, 9, 865684. [Google Scholar] [CrossRef] [PubMed]
- Febrianto, N.A.; Sa’diyah, K.; Tejasari, T. Red kidney bean powder substituted milk in cinnamon herbal coffee: Consumer perception, sensory properties and nutrition content. Pelita Perkeb. 2016, 32, 109–119. [Google Scholar] [CrossRef]
- Cottrell, J.J.; Le, H.H.; Artaiz, O.; Iqbal, Y.; Suleria, H.A.; Ali, A.; Celi, P.; Dunshea, F.R. Recent advances in the use of phytochemicals to manage gastrointestinal oxidative stress in poultry and pigs. Anim. Prod. Sci. 2021, 10, 1071. [Google Scholar] [CrossRef]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial role of phytochemicals on oxidative stress and age-related diseases. Biomed. Res. Int. 2019, 2019, 8748253. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Mena, P.; Calani, L.; Cid, C.; Del Rio, D.; Lean, M.E.; Crozier, A. Variations in caffeine and chlorogenic acid contents of coffees: What are we drinking? Food Funct. 2014, 5, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Nowaczewska, M.; Wiciński, M.; Kaźmierczak, W. The ambiguous role of caffeine in migraine headache: From trigger to treatment. Nutrients 2020, 12, 2259. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular docking and structure-based drug design strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, I.D.; Blaney, J.M.; Oatley, S.J.; Langridge, R.; Ferrin, T.E. A geometric approach to macromolecule-ligand interactions. J. Mol. Biol. 1982, 161, 269–288. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The content of polyphenols in coffee beans as roasting, origin and storage effect. Eur. Food Res. Technol. 2020, 246, 33–39. [Google Scholar] [CrossRef]
- Cáceres-Vélez, P.R.; Ali, A.; Fournier-Level, A.; Dunshea, F.R.; Jusuf, P.R. Phytochemical and safety evaluations of finger lime, mountain pepper, and tamarind in zebrafish embryos. Antioxidants 2022, 11, 1280. [Google Scholar] [CrossRef]
- Ali, A.; Bashmil, Y.M.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. LC-MS/MS-QTOF screening and identification of phenolic compounds from australian grown herbs and their antioxidant potential. Antioxidants 2021, 10, 1770. [Google Scholar] [CrossRef]
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive profiling of most widely used spices for their phenolic compounds through LC-ESI-QTOF-MS2 and their antioxidant potential. Antioxidants 2021, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, P.; Liu, Z.; Sharifi-Rad, J.; Suleria, H.A.R. Impact of roasting on the phenolic and volatile compounds in coffee beans. Food Sci. Nutr. 2022, 10, 2408–2425. [Google Scholar] [CrossRef]
- Wanyika, H.N.; Gatebe, E.G.; Gitu, L.M.; Ngumba, E.K.; Maritim, C.W. Determination of caffeine content of tea and instant coffee brands found in the Kenyan market. Afr. J. Food Sci. 2010, 4, 353–358. [Google Scholar]
- Chen, H.-Y.; Lin, Y.-C.; Hsieh, C.-L. Evaluation of antioxidant activity of aqueous extract of some selected nutraceutical herbs. Food Chem. 2007, 104, 1418–1424. [Google Scholar] [CrossRef]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant activity and free radical scavenging capacity between korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Odžaković, B.; Džinić, N.; Kukrić, Z.; Grujić, S. Effect of roasting degree on the antioxidant activity of different Arabica coffee quality classes. Acta Sci. Pol. Technol. Aliment. 2016, 15, 409–417. [Google Scholar] [CrossRef]
- Cho, A.R.; Park, K.W.; Kim, K.M.; Kim, S.Y.; Han, J. Influence of roasting conditions on the antioxidant characteristics of Colombian coffee (Coffea arabica L.) beans. J. Food Biochem. 2014, 38, 271–280. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Kucukaydin, S.; Quradha, M.M.; Ceylan, O.; Ugur, A.; Duru, M.E. Ultrasound-assisted extraction of Syringa Vulgaris Mill., Citrus Sinensis L. and Hypericum Perforatum L.: Phenolic composition, enzyme inhibition and anti-quorum sensing activities. Chem. Afr. 2022, 5, 237–249. [Google Scholar] [CrossRef]
- Jham, G.N.; Fernandes, S.A.; Garcia, C.F.; da Silva, A.A. Comparison of GC and HPLC for the quantification of organic acids in coffee. Phytochem. Anal. 2002, 13, 99–104. [Google Scholar] [CrossRef]
- Kazaz, S.; Miray, R.; Lepiniec, L.; Baud, S. Plant monounsaturated fatty acids: Diversity, biosynthesis, functions and uses. Prog. Lipid Res. 2022, 85, 101138. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Tan, L.; Zhao, J.; Hu, R.; Lu, M. Characterization of fatty acid, amino acid and volatile compound compositions and bioactive components of seven coffee (Coffea robusta) cultivars grown in Hainan province, China. Molecules 2015, 20, 16687–16708. [Google Scholar] [CrossRef] [PubMed]
- Casal, S.; Mendes, E.; Oliveira, M.B.P.P.; Ferreira, M.A. Roast effects on coffee amino acid enantiomers. Food Chem. 2005, 89, 333–340. [Google Scholar] [CrossRef]
- Matemu, A.; Nakamura, S.; Katayama, S. Health benefits of antioxidative peptides derived from legume proteins with a high amino acid score. Antioxidants 2021, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Wang, T.; Luo, Y. A review on plant-based proteins from soybean: Health benefits and soy product development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Martins, S.C.; Araújo, W.L.; Tohge, T.; Fernie, A.R.; DaMatta, F.M. In high-light-acclimated coffee plants the metabolic machinery is adjusted to avoid oxidative stress rather than to benefit from extra light enhancement in photosynthetic yield. PLoS ONE 2014, 9, e94862. [Google Scholar] [CrossRef] [PubMed]
- Kocadağlı, T.; Yılmaz, C.; Gökmen, V. Determination of melatonin and its isomer in foods by liquid chromatography tandem mass spectrometry. Food Chem. 2014, 153, 151–156. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Giridhar, P.; Sankar, K.U.; Ravishankar, G.A. Melatonin and serotonin profiles in beans of Coffea species. J. Pineal Res. 2012, 52, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.S.; Wang, Y.; Weaver, D.F. Phenylindanes in brewed coffee inhibit amyloid-beta and tau aggregation. Front. Neurosci. 2018, 12, 735. [Google Scholar] [CrossRef]
- Corpas, R.; Griñán-Ferré, C.; Palomera-Ávalos, V.; Porquet, D.; García de Frutos, P.; Franciscato Cozzolino, S.M.; Rodríguez-Farré, E.; Pallàs, M.; Sanfeliu, C.; Cardoso, B.R. Melatonin induces mechanisms of brain resilience against neurodegeneration. J. Pineal Res. 2018, 65, e12515. [Google Scholar] [CrossRef]
- Abe, T.; Itoh, T.; Terasaki, M. Total synthesis of fontanesine b and its isomer: Their antiproliferative activity against human colorectal cancer cells. Helv. Chim. Acta 2019, 102, e1900116. [Google Scholar] [CrossRef]
- Qian, X.-P.; Zhang, X.-H.; Sun, L.-N.; Xing, W.-F.; Wang, Y.; Sun, S.-Y.; Ma, M.-Y.; Cheng, Z.-P.; Wu, Z.-D.; Xing, C. Corosolic acid and its structural analogs: A systematic review of their biological activities and underlying mechanism of action. Phytomedicine 2021, 91, 153696. [Google Scholar] [CrossRef]
- Prodea, A.; Mioc, A.; Banciu, C.; Trandafirescu, C.; Milan, A.; Racoviceanu, R.; Ghiulai, R.; Mioc, M.; Soica, C. The role of cyclodextrins in the design and development of triterpene-based therapeutic agents. Int. J. Mol. Sci. 2022, 23, 736. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. Int. Sch. Res. Not. 2014, 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Xiao, J. Protective effects of syringic acid on inflammation, apoptosis and intestinal barrier function in Caco-2 cells following oxygen-glucose deprivation/reoxygenation-induced injury. Exp. Med. 2022, 23, 66. [Google Scholar] [CrossRef] [PubMed]
- Márquez Campos, E.; Jakobs, L.; Simon, M.C. Antidiabetic effects of flavan-3-ols and their microbial metabolites. Nutrients 2020, 12, 1592. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ho, C.T. Polyphenolic chemistry of tea and coffee: A century of progress. J. Agric. Food Chem. 2009, 57, 8109–8114. [Google Scholar] [CrossRef]
- Hamden, K.; Allouche, N.; Damak, M.; Elfeki, A. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytyrosol from olive mill waste in vitro and in rats. Chem. Biol. Interact. 2009, 180, 421–432. [Google Scholar] [CrossRef]
- Wang, L.; Gan, C.; Wang, Z.; Liu, L.; Gao, M.; Li, Q.; Yang, C. Determination and pharmacokinetic study of three diterpenes in rat plasma by UHPLC-ESI-MS/MS after oral administration of Rosmarinus officinalis L. Extract. Molecules 2017, 22, 934. [Google Scholar] [CrossRef]
- Zabot, G.L.; Moraes, M.N.; Rostagno, M.A.; Meireles, M.A.A. Fast analysis of phenolic terpenes by high-performance liquid chromatography using a fused-core column. Anal. Methods 2014, 6, 7457–7468. [Google Scholar] [CrossRef]
- Zhao, L.; Yu, M.; Sun, M.; Xue, X.; Wang, T.; Cao, W.; Sun, L. Rapid determination of major compounds in the ethanol extract of geopropolis from Malaysian stingless bees, Heterotrigona itama, by UHPLC-Q-TOF/MS and NMR. Molecules 2017, 22, 1935. [Google Scholar] [CrossRef] [PubMed]
- Durak, A.; Gawlik-Dziki, U.; Pecio, Ł. Coffee with cinnamon–impact of phytochemicals interactions on antioxidant and anti-inflammatory in vitro activity. Food Chem. 2014, 162, 81–88. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Rai, D.; Sun, D.W.; Tiwari, B.K. Ultrasound-assisted extraction (UAE) of bioactive compounds from coffee silverskin: Impact on phenolic content, antioxidant activity, and morphological characteristics. J. Food Process Eng. 2019, 42, e13191. [Google Scholar] [CrossRef]
- Ahmed Ali, A.M.; Yagi, S.; Qahtan, A.A.; Alatar, A.A.; Angeloni, S.; Maggi, F.; Caprioli, G.; Abdel-Salam, E.M.; Sinan, K.I.; Zengin, G. Evaluation of the chemical constituents, antioxidant and enzyme inhibitory activities of six yemeni green coffee beans varieties. Food Biosci. 2022, 46, 101552. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Use of chlorogenic acid against diabetes mellitus and its complications. J. Immunol. Res. 2020, 2020, 9680508. [Google Scholar] [CrossRef]
- Li, Y.J.; He, F.Q.; Zhao, H.H.; Li, Y.; Chen, J. Screening and identification of acetylcholinesterase inhibitors from Terminalia chebula fruits by immobilized enzyme on cellulose filter paper coupled with ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry and molecular docking. J. Chromatogr. A 2022, 1663, 462784. [Google Scholar]
- Solomakou, N.; Loukri, A.; Tsafrakidou, P.; Michaelidou, A.-M.; Mourtzinos, I.; Goula, A.M. Recovery of phenolic compounds from spent coffee grounds through optimized extraction processes. Sustain. Chem. Pharm. 2022, 25, 100592. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Song, S.; Ali, A.; Subbiah, V.; Taheri, Y.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS characterization of phenolic compounds from Pyracantha coccinea M. Roem. And their antioxidant capacity. Cell. Mol. Biol. 2021, 67, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, D.R.A.; Praseptiangga, D.; Van de Walle, D.; Dewettinck, K. Interaction between natural antioxidants derived from cinnamon and cocoa in binary and complex mixtures. Food Chem. 2017, 231, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.F.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Utilization of mango, apple and banana fruit peels as prebiotics and functional ingredients. Agriculture 2021, 11, 584. [Google Scholar] [CrossRef]
- Chou, O.; Ali, A.; Subbiah, V.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS characterisation of phenolics in herbal tea infusion and their antioxidant potential. Fermentation 2021, 7, 73. [Google Scholar] [CrossRef]
- Peng, D.; Zahid, H.F.; Ajlouni, S.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS profiling of australian mango peel by-product polyphenols and their potential antioxidant activities. Processes 2019, 7, 764. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from Northeast Portugal: Individual cap and stipe activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Bashmil, Y.M.; Ali, A.; BK, A.; Dunshea, F.R.; Suleria, H.A.R. Screening and characterization of phenolic compounds from Australian grown bananas and their antioxidant capacity. Antioxidants 2021, 10, 1521. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Anil, D.A.; Aydin, B.O.; Demir, Y.; Turkmenoglu, B. Design, synthesis, biological evaluation and molecular docking studies of novel 1H-1,2,3-triazole derivatives as potent inhibitors of carbonic anhydrase, acetylcholinesterase and aldose reductase. J. Mol. Struct. 2022, 1257, 132613. [Google Scholar] [CrossRef]



| Variables | TPC mg GAE/g | TFC mg QE/g | DPPH mg AAE/g | ABTS mg AAE/g | OH-RSA mg AAE/g |
|---|---|---|---|---|---|
| Australian Coffee | 10.97 ± 0.74 c | 1.13 ± 0.05 b | 3.11 ± 0.06 b | 10.96 ± 0.40 a | 19.66 ± 0.31 c |
| Colombian Coffee | 17.74 ± 0.32 a | 1.36 ± 0.05 a | 3.61 ± 0.06 a | 17.17 ± 0.15 a | 23.62 ± 0.47 a |
| Ethiopian Coffee | 14.30 ± 0.27 b | 1.13 ± 0.16 b | 3.12 ± 0.09 b | 11.54 ± 0.09 b | 21.96 ± 0.74 b |
| Peruvian Coffee | 10.24 ± 0.73 c | 1.01 ± 0.13 bc | 1.55 ± 0.03 c | 6.17 ± 0.28 c | 17.99 ± 0.82 d |
| Variables | TPC | TFC | DPPH | ABTS |
|---|---|---|---|---|
| TFC | 0.92 * | |||
| DPPH | 0.76 | 0.84 | ||
| ABTS | 0.92 * | 0.99 ** | 0.92 * | |
| •OH-RSA | 0.97 ** | 0.90 | 0.87 | 0.94 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Zahid, H.F.; Cottrell, J.J.; Dunshea, F.R. A Comparative Study for Nutritional and Phytochemical Profiling of Coffea arabica (C. arabica) from Different Origins and Their Antioxidant Potential and Molecular Docking. Molecules 2022, 27, 5126. https://doi.org/10.3390/molecules27165126
Ali A, Zahid HF, Cottrell JJ, Dunshea FR. A Comparative Study for Nutritional and Phytochemical Profiling of Coffea arabica (C. arabica) from Different Origins and Their Antioxidant Potential and Molecular Docking. Molecules. 2022; 27(16):5126. https://doi.org/10.3390/molecules27165126
Chicago/Turabian StyleAli, Akhtar, Hafza Fasiha Zahid, Jeremy J. Cottrell, and Frank R. Dunshea. 2022. "A Comparative Study for Nutritional and Phytochemical Profiling of Coffea arabica (C. arabica) from Different Origins and Their Antioxidant Potential and Molecular Docking" Molecules 27, no. 16: 5126. https://doi.org/10.3390/molecules27165126
APA StyleAli, A., Zahid, H. F., Cottrell, J. J., & Dunshea, F. R. (2022). A Comparative Study for Nutritional and Phytochemical Profiling of Coffea arabica (C. arabica) from Different Origins and Their Antioxidant Potential and Molecular Docking. Molecules, 27(16), 5126. https://doi.org/10.3390/molecules27165126










