Nanobiotechnological Approaches to Enhance Drought Tolerance in Catharanthus roseus Plants Using Salicylic Acid in Bulk and Nanoform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Salicylic Acid Nanoparticles
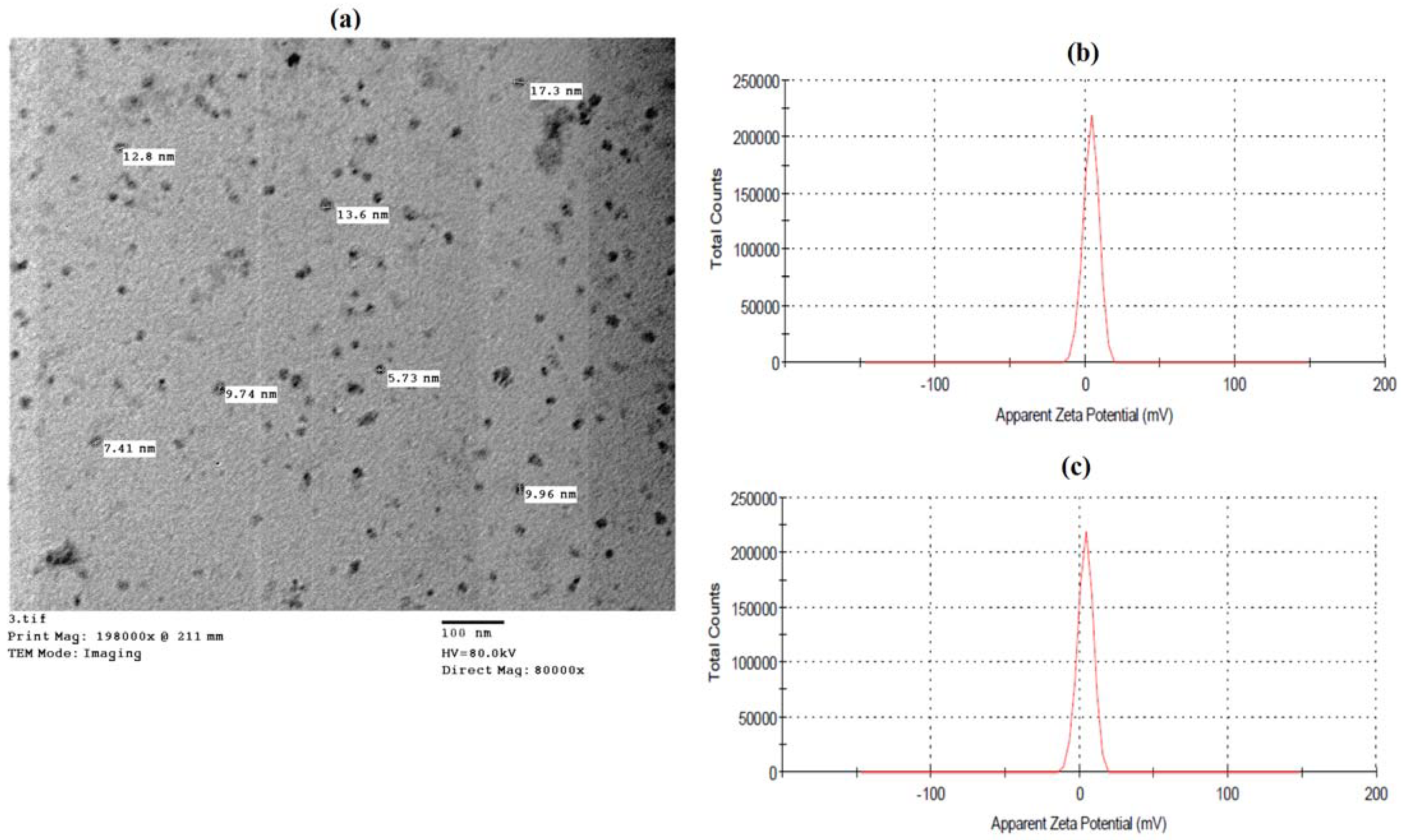
2.1.1. Characterization of Nanomaterials Using Dynamic Light Scattering (DLS)
2.1.2. Transmission Electron Microscopy (TEM)
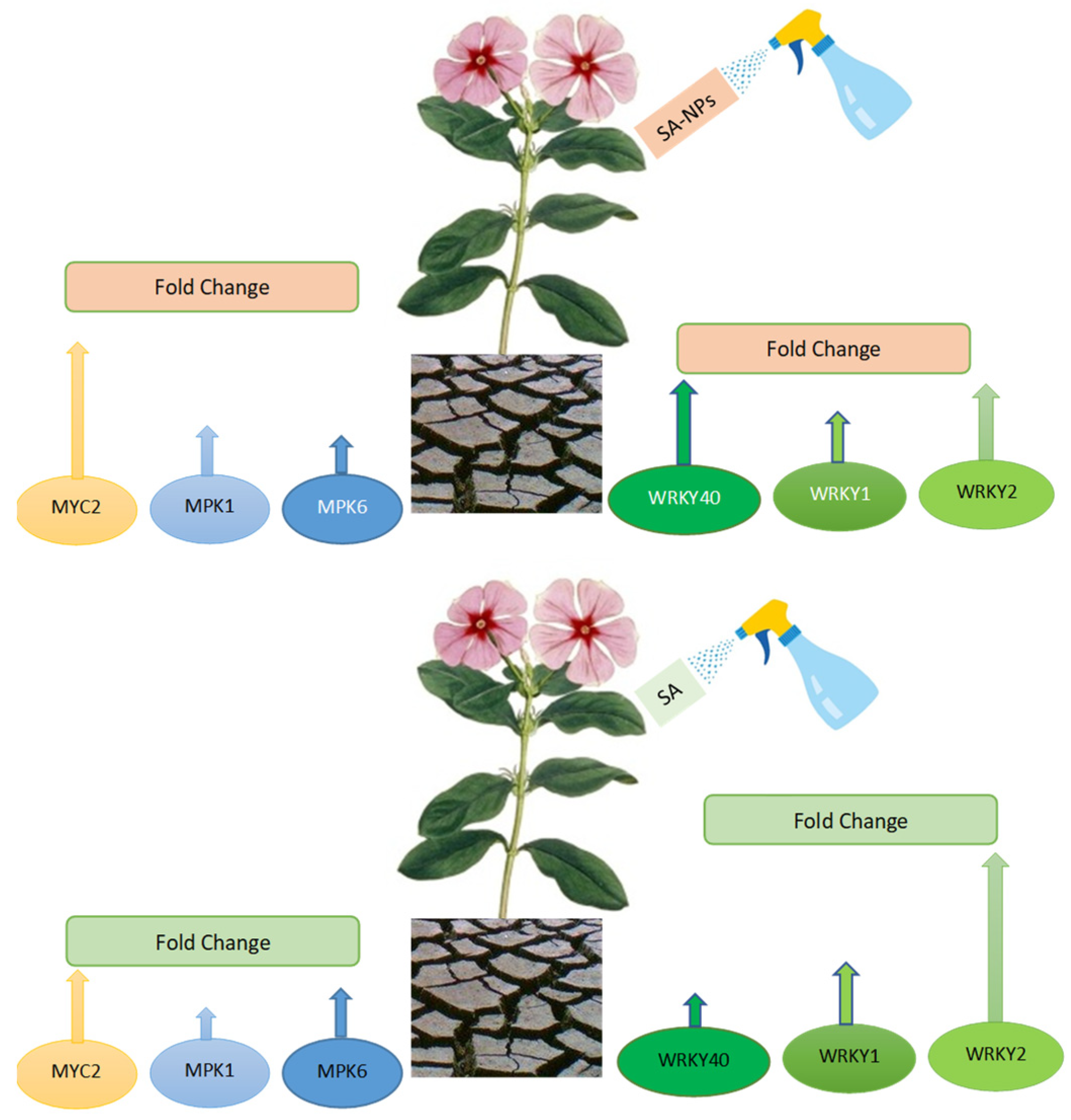
2.2. Plant materials, Drought, and SA Treatments
2.3. Measuring Growth Parameters
2.4. Relative Water Content (RWC) Measurement
2.5. Chlorophyll a, b and Carotenoid Content Measurement
2.6. Total Alkaloid Measurement
2.7. RNA Extraction and Gene Expression Profiling Using Quantitative Real-Time PCR (qRT-PCR)
2.8. Statistical Analysis
3. Results
3.1. Characterization of SA Nanoparticles
3.2. Plant Growth Response
3.3. Chlorophyll a and b and Carotenoid Content
3.4. Relative Water Content and Leaf Area Index
3.5. Alkaloid Content
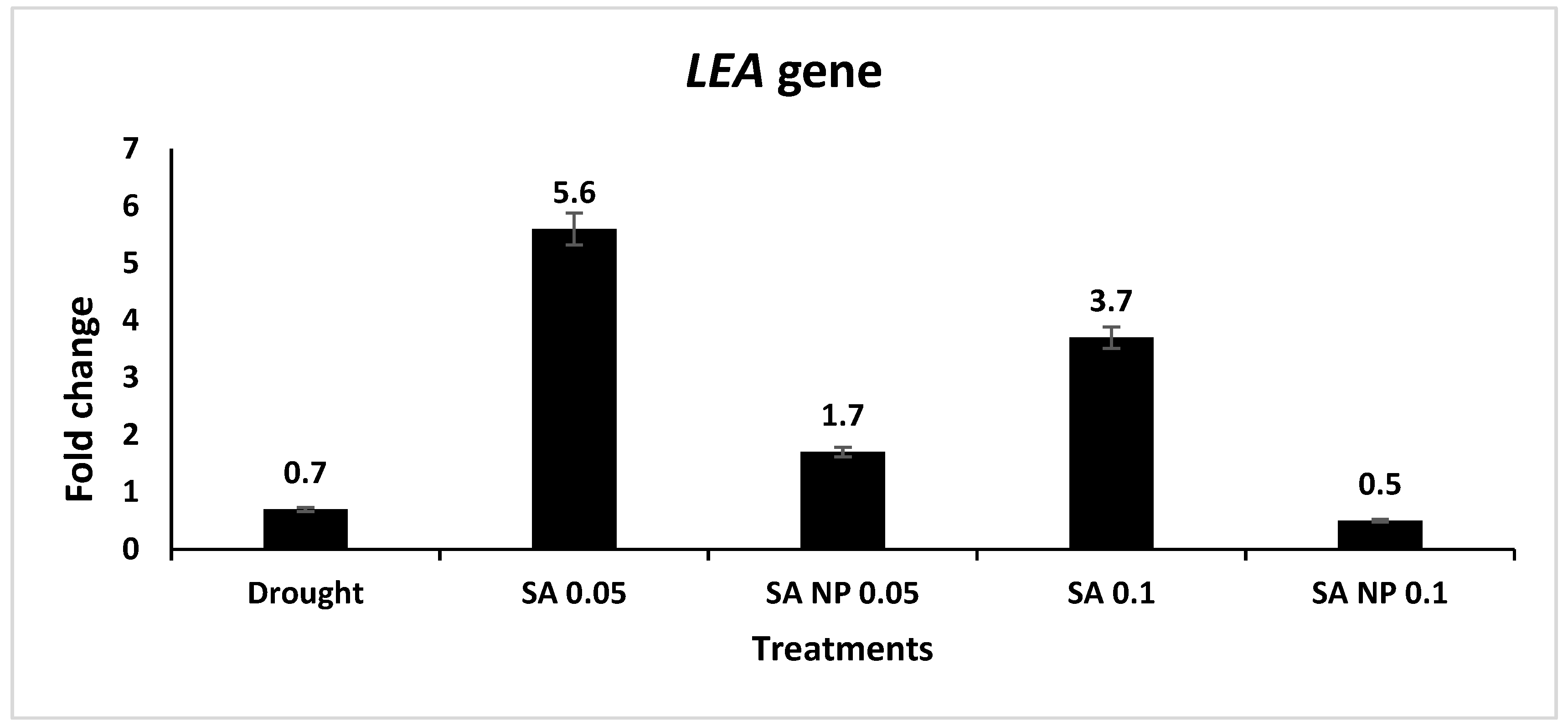
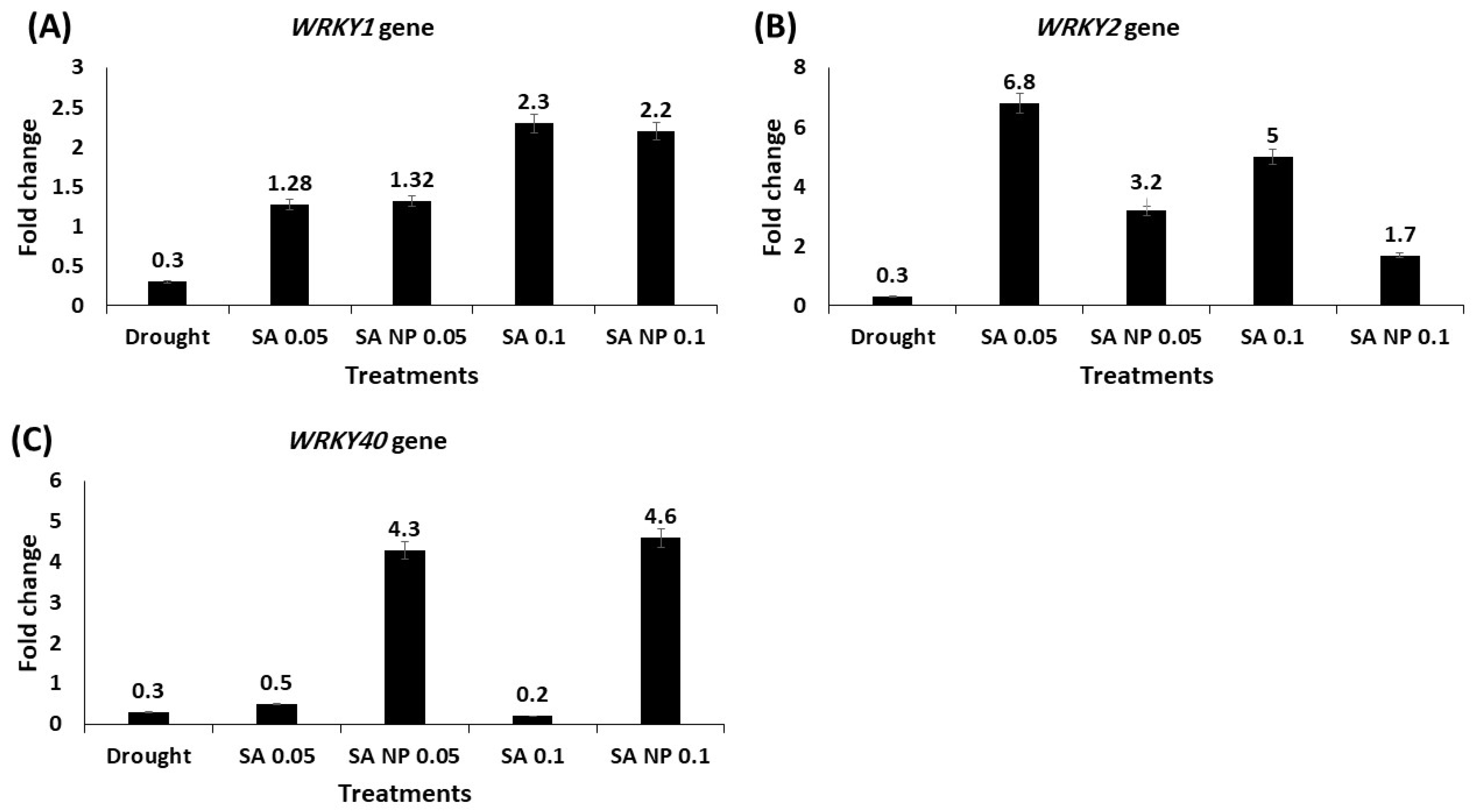
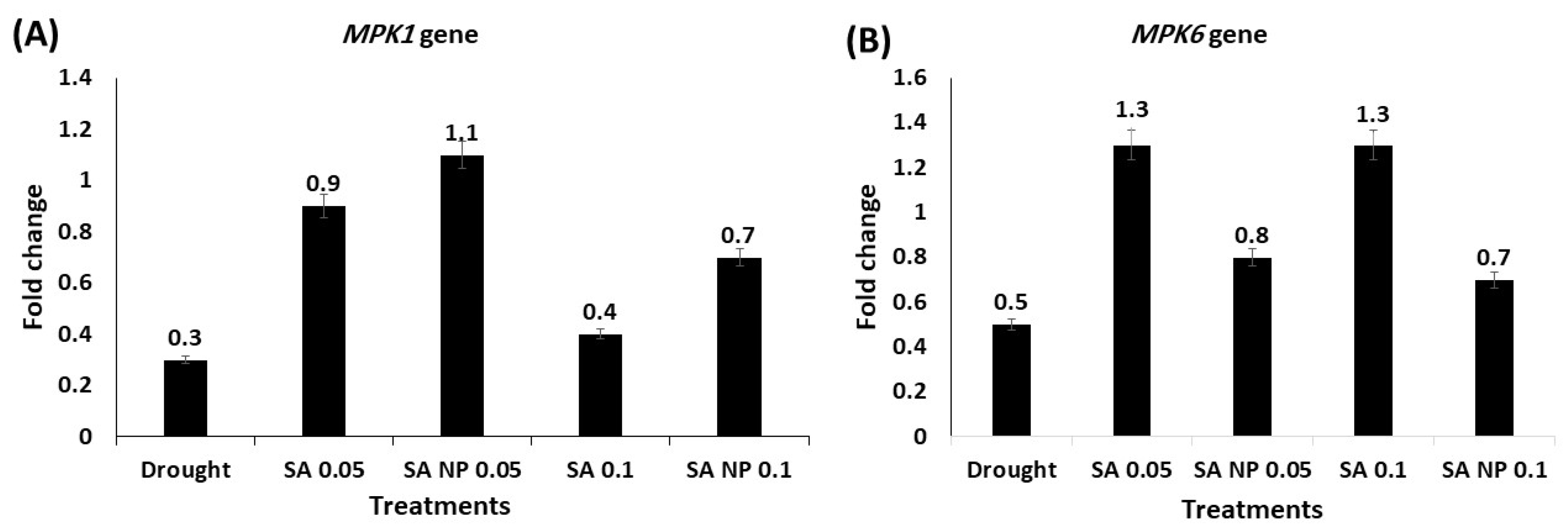
3.6. Effect of Different Treatments on Expression Profiling of Drought Tolerance Associated Genes Using qRT-PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muthu, C.; Ayyanar, M.; Raja, N.; Ignacimuthu, S. Medicinal plants used by traditional healers in Kancheepuram District of Tamil Nadu, India. J. Ethnobiol. Ethnomed. 2006, 2, 43. [Google Scholar] [CrossRef]
- Semenya, S.S.; Potgieter, M.J. Catharanthus roseus (L.) G. Don.: Extraordinary Bapedi medicinal herb for gonorrhoea. J. Med. Plants Res. 2013, 7, 1434–1438. [Google Scholar]
- Barrales-Cureño, H.J.; Reyes, C.R.; García, I.V.; Valdez, L.G.L.; De Jesús, A.G.; Cortés Ruíz, J.A.; Espinoza Perez, J. Alkaloids of Pharmacological Importance in Catharanthus Roseus; Intech Open Ltd.: London, UK, 2019; Volume 1, p. 18. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, S.P.; Lima, A.M.; De Souza, C.R.B. Recent molecular advances on downstream plant responses to abiotic stress. Int. J. Mol. Sci. 2012, 13, 8628–8647. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Xue, L.L.; Zou, C.M. Brassinolide Application Improves the Drought Tolerance in Maize Through Modulation of Enzymatic Antioxidants and Leaf Gas Exchange. J. Agron. Crop Sci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Basra, S.M.A.; Islam, -U.-D. Improving water relations and gas exchange with brassinosteroids in rice under drought stress. J. Agron. Crop Sci. 2009, 195, 262–269. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Rucker, K.S.; Kvien, C.K.; Holbrook, C.C.; Hook, J.E. Identification of peanut genotypes with improved drought avoidance traits. Peanut Sci. 1995, 22, 14–18. [Google Scholar] [CrossRef]
- Demirevska, K.; Zasheva, D.; Dimitrov, R.; Simova-Stoilova, L.; Stamenova, M.; Feller, U. Drought stress effects on Rubisco in wheat: Changes in the Rubisco large subunit. Acta Physiol. Plant. 2009, 31, 1129–1138. [Google Scholar] [CrossRef]
- Ludlow, M.; Muchow, R.C. A critical evaluation of traits for improving crop yields in water-limited environments. Adv. Agron. 1990, 43, 107–153. [Google Scholar]
- Abaspour Esfaden, M.; Kallaterjari, S.; Fatehi, F. The Effect of Salicylic Acid and L-arginine on Morpho-physiological Properties and Leaf Nutrients of Catharanthus roseus under Drought Stress. J. Hortic. Sci. 2019, 33, 417–432. [Google Scholar] [CrossRef]
- Raskin, I. Role of salicylic acid in plants. Annu. Rev. Plant Biol. 1992, 43, 439–463. [Google Scholar] [CrossRef]
- Janda, T.; Gondor, O.K.; Yordanova, R.; Szalai, G.; Pál, M. Salicylic acid and photosynthesis: Signalling and effects. Acta Physiol. Plant. 2014, 36, 2537–2546. [Google Scholar] [CrossRef]
- Wagner, U.; Edwards, R.; Dixon, D.P.; Mauch, F. Probing the diversity of the Arabidopsis glutathione S-transferase gene family. Plant Mol. Biol. 2002, 49, 515–532. [Google Scholar] [CrossRef]
- El-Garhy, H.A.S.; Abdel-Rahman, F.A.; Shams, A.S.; Osman, G.H.; Moustafa, M.M.A. Comparative analyses of four chemicals used to control black mold disease in tomato and its effects on defense signaling pathways, productivity and quality traits. Plants 2020, 9, 808. [Google Scholar] [CrossRef]
- Maleck, K.; Levine, A.; Eulgem, T.; Morgan, A.; Schmid, J.; Lawton, K.A.; Dangl, J.L.; Dietrich, R.A. The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat. Genet. 2000, 26, 403–410. [Google Scholar] [CrossRef]
- Wang, D.; Amornsiripanitch, N.; Dong, X. A Genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog. 2006, 2, 1042–1050. [Google Scholar] [CrossRef]
- Blanco, F.; Salinas, P.; Cecchini, N.M.; Jordana, X.; van Hummelen, P.; Alvarez, M.E.; Holuigue, L. Early genomic responses to salicylic acid in Arabidopsis. Plant Mol. Biol. 2009, 70, 79–102. [Google Scholar] [CrossRef]
- Misra, N.; Saxena, P. Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Sci. 2009, 177, 181–189. [Google Scholar] [CrossRef]
- Stamou, I.; Antizar-Ladislao, B. The impact of silver and titanium dioxide nanoparticles on the in-vessel composting of municipal solid waste. Waste Manag. 2016, 56, 71–78. [Google Scholar] [CrossRef] [PubMed]
- El-Garhy, H.A.S.; Elsisi, A.A.; Mohamed, S.A.; Morsy, O.M.; Osman, G.; Abdel- Rahman, F.A. Transcriptomic changes in green bean pods against grey mould and white rot diseases via field application of chemical elicitor nanoparticles. IET Nanobiotechnol. 2020, 14, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef]
- Javad, S. Nanoagronomy; Springer International Publishing: Cham, Switzerland, 2020; Volume IX, p. 221. [Google Scholar] [CrossRef]
- Nejatzadeh, F. Effect of silver nanoparticles on salt tolerance of Satureja hortensis L. during In Vitro and In Vivo germination tests. Heliyon 2021, 7, e05981. [Google Scholar] [CrossRef]
- Qiao, Z.; Li, C.L.; Zhang, W. WRKY1 regulates stomatal movement in drought-stressed Arabidopsis thaliana. Plant Mol. Biol. 2016, 91, 53–65. [Google Scholar] [CrossRef]
- Chen, J.; Nolan, T.M.; Ye, H.; Zhang, M.; Tong, H.; Xin, P.; Chu, J.; Chu, C.; Li, Z.; Yin, Y. Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef]
- Yamasaki, K.; Kigawa, T.; Inoue, M.; Watanabe, S.; Tateno, M.; Seki, M.; Shinozaki, K.; Yokoyama, S. Structures and evolutionary origins of plant-specific transcription factor DNA-binding domains. Plant Physiol. Biochem. 2008, 46, 394–401. [Google Scholar] [CrossRef]
- Wei, H.; Chen, S.; Niyitanga, S.; Liu, T.; Qi, J.; Zhang, L. Genome-wide identification and expression analysis response to GA3 stresses of WRKY gene family in seed hemp (Cannabis sativa L.). Gene 2022, 822, 146290. [Google Scholar] [CrossRef]
- Xie, T.; Chen, C.; Li, C.; Liu, J.; Liu, C.; He, Y. Genome-wide investigation of WRKY gene family in pineapple: Evolution and expression profiles during development and stress. BMC Genom. 2018, 19, 490. [Google Scholar] [CrossRef] [PubMed]
- An, J.P.; Zhang, X.W.; You, C.X.; Bi, S.Q.; Wang, X.F.; Hao, Y.J. MdWRKY40 promotes wounding-induced anthocyanin biosynthesis in association with MdMYB1 and undergoes MdBT2-mediated degradation. New Phytol. 2019, 224, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Jagodzik, P.; Tajdel-Zielinska, M.; Ciesla, A.; Marczak, M.; Ludwikow, A. Mitogen-activated protein kinase cascades in plant hormone signaling. Front. Plant Sci. 2018, 9, 1387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, C.; Zhang, B.; Yi, J.; Yang, Y.; Kong, C.; Lei, C.; Gong, M. The role of the late embryogenesis-abundant (LEA) protein family in development and the abiotic stress response: A comprehensive expression analysis of potato (Solanum tu-berosum). Genes 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Poku, S.A.; Chukwurah, P.N.; Aung, H.H.; Nakamura, I. Over-expression of a melon Y3SK2-Type LEA gene confers drought and salt tolerance in transgenic tobacco plants. Plants 2020, 9, 1749. [Google Scholar] [CrossRef]
- Umezawa, T.; Sugiyama, N.; Takahashi, F.; Anderson, J.C.; Ishihama, Y.; Peck, S.C.; Shinozaki, K. Genetics and phosphoproteomics reveal a protein phosphorylation network in the abscisic acid signaling pathway in Arabidopsis thaliana. Sci. Signal. 2013, 6, rs8. [Google Scholar] [CrossRef]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef]
- Abdel-Rahman, F.A.; Rashid, I.A.S.; Shoala, T. Nanoactivities of natural nanomaterials rosmarinic acid, glycyrrhizic acid and glycyrrhizic acid ammonium salt against tomato phytopathogenic fungi Alternaria alternata and Penicillium digitatum. J. Plant Prot. Res. 2020, 60, 150–160. [Google Scholar] [CrossRef]
- Amin, B.H.; El-Sharkawy, R.M. Bactericidal Activity of Silver Nanoparticles Produced by Fusarium solani against the Multidrug-Resistant Bacteria. Res. J. Pharm. Biol. Chem. Sci. 2019, 10, 203–211. [Google Scholar]
- Watson, D.J. Comparative physiological studies on the growth of field crops: I. Variation in net assimilation rate and leaf area between species and varieties, and within and between years. Ann. Bot. 1947, 11, 41–76. [Google Scholar] [CrossRef]
- Schonfeld, M.A.; Johnson, R.C.; Carver, B.F.; Mornhinweg, D.W. Water relations in winter wheat as drought resistance indicators. Crop Sci. 1988, 28, 526–531. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Methods of Plant Analysis. In Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; Harborne, J.B., Ed.; Springer: Dordrecht, The Netherlands, 1973; pp. 1–32. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCT Method. Methods 2001, 25, 402e408. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.S.M.S.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 2021, 11, 3195. [Google Scholar] [CrossRef]
- Zafar, Z.; Rasheed, F.; Atif, R.M.; Javed, M.A.; Maqsood, M.; Gailing, O. Foliar application of salicylic acid improves water stress tolerance in Conocarpus erectus L. and Populus deltoides L. saplings: Evidence from morphological, physiological and biochemical changes. Plants 2021, 10, 1242. [Google Scholar] [CrossRef]
- Abbaszadeh, B.; Layeghhaghighi, M.; Azimi, R.; Hadi, N. Improving water use efficiency through drought stress and using salicylic acid for proper production of Rosmarinus officinalis L. Ind. Crops Prod. 2020, 144, 111893. [Google Scholar] [CrossRef]
- Brito, I.P.F.S.; Tropaldi, L.; Carbonari, C.A.; Velini, E.D. Hormetic effects of glyphosate on plants. Pest Manag. Sci. 2018, 74, 1064–1070. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta 2000, 210, 925–931. [Google Scholar] [CrossRef]
- Mihailović, N.; Lazarević, M.; Dželetović, Z.; Vučković, M.; Đurđević, M. Chlorophyllase activity in wheat, Triticum aestivum L. leaves during drought and its dependence on the nitrogen ion form applied. Plant Sci. 1997, 129, 141–146. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.F.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Khazaei, Z.; Esmaielpour, B.; Estaji, A. Ameliorative effects of ascorbic acid on tolerance to drought stress on pepper (Capsicum annuum L.) plants. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2020, 26, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Emam, Y.; Shekoofa, A.; Salehi, F.; Jalali, A.H.; Pessarakli, M. Drought stress effects on two common bean cultivars with contrasting growth habits. Arch. Agron. Soil Sci. 2012, 58, 527–534. [Google Scholar] [CrossRef]
- Baroowa, B. Changes in plant water status, biochemical attributes and seed quality of black gram and green gram genotypes under drought. Int. Lett. Nat. Sci. 2015, 42, 1–12. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.-O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Couchoud, M.; Salon, C.; Girodet, S.; Jeudy, C.; Vernoud, V.; Prudent, M. Pea Efficiency of Post-drought Recovery Relies on the Strategy to Fine-Tune Nitrogen Nutrition. Front. Plant Sci. 2020, 11, 204. [Google Scholar] [CrossRef]
- Abdi, G.; Karami, L. Salicylic acid effects on some physiochemical properties and secondary metabolite accumulation in Mentha piperita L. under water deficit stress. Adv. Hortic. Sci. 2020, 34, 81–91. [Google Scholar] [CrossRef]
- Ababaf, M.; Omidi, H.; Bakhshandeh, A. Changes in antioxidant enzymes activities and alkaloid amount of Catharanthus roseus in response to plant growth regulators under drought condition. Ind. Crops Prod. 2021, 167, 113505. [Google Scholar] [CrossRef]
- Anurag, K.; Anumalik, Y.; Nitika, G.; Swadesh, K.; Nikhil, G.; Santosh, K.; Vinay, Y.; Anuj, P.; Himanshu, G. Metabolites in plants and its classification. World J. Pharm. Pharm. Sci. 2015, 4, 287–305. [Google Scholar]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Mobin, M.; Anjum, N.; Khan, N. Modulation and significance of nitrogen and sulfur metabolism in cadmium-challenged plants. Pl0061nt Growth Regul. 2015, 78, 1–11. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Rahman, M.S.; Narayan, O.P. Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Rep. 2021, 23, 101040. [Google Scholar] [CrossRef]
- Meraj, T.A.; Fu, J.; Raza, M.A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional factors regulate plant stress responses through mediating secondary metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Sun, X.; Xi, D.H.; Feng, H.; Du, J.B.; Lei, T.; Liang, H.G.; Lin, H.H. The dual effects of salicylic acid on dehydrin accumulation in water-stressed barley seedlings. Russ. J. Plant Physiol. 2009, 56, 348–354. [Google Scholar] [CrossRef]
- Graether, S.P.; Boddington, K.F. Disorder and function: A review of the dehydrin protein family. Front. Plant Sci. 2014, 5, 576. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Xu, P.; Zhang, Z. Overexpression of a WRKY Transcription Factor TaWRKY2 Enhances Drought Stress Tolerance in Transgenic Wheat. Front. Plant Sci. 2018, 9, 997. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, Z.L.; Zou, X.; Yang, G.; Komatsu, S.; Shen, Q.J. Interactions of two abscisic-acid induced WRKY genes in repressing gibberellin signaling in aleurone cells. Plant J. 2006, 46, 231–242. [Google Scholar] [CrossRef]
- Dong, J.; Chen, C.; Chen, Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol. Biol. 2003, 51, 21–37. [Google Scholar] [CrossRef]
- Wang, C.T.; Ru, J.N.; Liu, Y.W.; Yang, J.F.; Li, M.; Xu, Z.S.; Fu, J.-D. The Maize WRKY Transcription Factor ZmWRKY40 Confers Drought Resistance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2018, 19, 2580. [Google Scholar] [CrossRef]
- He, G.H.; Xu, J.Y.; Wang, Y.X.; Liu, J.M.; Li, P.S.; Chen, M.; Ma, Y.-Z.; Xu, Z.-S. Drought-responsive WRKY transcription factor genes TaWRKY1 and TaWRKY33 from wheat confer drought and/or heat resistance in Arabidopsis. BMC Plant Biol. 2016, 16, 116. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, B.; La, H.; Mamun, M.; Bae, D.W.; Kim, T.H. Characterization of salicylic acid- and abscisic acid-mediated photosynthesis, Ca2+ and H2O2 accumulation in two distinct phases of drought stress intensity in Brassica napus. Environ. Exp. Bot. 2021, 186, 104434. [Google Scholar] [CrossRef]
- Sotoodehnia-Korani, S.; Iranbakhsh, A.; Ebadi, M.; Majd, A.; Oraghi Ardebili, Z. Selenium nanoparticles induced variations in growth, morphology, anatomy, biochemistry, gene expression, and epigenetic DNA methylation in Capsicum annuum, an in vitro study. Environ. Pollut. 2020, 265, 114727. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, J.; Bi, D.; Zhang, Z.; Cheng, F.; Chen, S.; Zhang, Y. MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res. 2008, 18, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon Nanotubes Are Able To Penetrate Plant Seed Coat and Dramatically Affect Seed Germination and Plant Growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef]

| Accession Number | Gene Name | Forward Primer | Reverse Primer |
|---|---|---|---|
| HQ646368.1 | WRKY1 | ACCACGATGGCTTGGCATGA | TGTACGGAGAGTTCGGCAGC |
| JX241693.1 | WRKY2 | TGCAGGCTCAAAATGGCAGC | CACTGCCATGTGTTGCACGA |
| MG676674.1 | WRKY40 | TCAGGAGGAGGACCAACAATTAC | TGAAATGGCGGCTGCAAGTG |
| KR703580.1 | MPK6 | TCCTCCGTCTCAGCAACAGC | ATGGCTAAGAACGGCCGGAA |
| KR703579.1 | MPKK1 | CTATCTCCTCCGCCGCTACG | GCCGTTACCGTGGCCTAAGA |
| AF283507.2 | MYC2 | ATTTCTTTGTGGCCGCCGTC | AAGCAGGTTGGGCAGGCATA |
| DQ016338.1 | LEA | AAGAGACAGCAGAGGCAGGG | TCAGCAGCACCTTGAGCCAT |
| MG813871.1 (Reference gene) | Actin | GTGCAACGCCTTCTCCGTTC | TGGCTGATGGAGCACAGAGG |
| Soil Water Condition | Treatment | Plant Height (cm) | Shoot Dry Weight (gm) | Root Dry Weight (gm) | Root–Shoot Ratio |
|---|---|---|---|---|---|
| Normal irrigation | Control | 57 c,d ± 0.33 | 4.07 c,d ± 0.18 | 4.14 b ± 0.21 | 1.07 b ± 0.067 |
| SANP (0.05 mM) | 58 b,c ± 0.35 | 3.75 c,d ± 0.49 | 5.3 a ± 0.14 | 1.41 a ± 0.058 | |
| SANP (0.1 mM) | 61 a ± 2.3 | 5.7 a ± 0.66 | 4.3 b± 0.17 | 0.76 d ± 0.01 | |
| SA (0.05 mM) | 56 c,d,e ± 0.18 | 4.63 a,b,c ± 0.43 | 2.94 c ± 0.15 | 0.63 e ± 0.012 | |
| SA (0.1 mM) | 60 a,b ± 2.31 | 5.63 a,b ± 0.35 | 4.42 b ± 0.20 | 0.78 d ± 0.015 | |
| Drought treatment | Control | 48 i ± 0.17 | 1.05 f ± 0.24 | 0.15 d,e,f ± 0.22 | 0.12 g ± 0.009 |
| SANP (0.05 mM) | 51 h ± 0.34 | 2.4 e ± 0.39 | 3.35 c ± 0.11 | 1.16 b ± 0.09 | |
| SANP (0.1 mM) | 55 e,f ± 1.15 | 1.3 e± 0.34 | 1.08 d ± 0.27 | 0.83 c ± 0.012 | |
| SA (0.05 mM) | 53 f,g ± 0.44 | 1.12 f ± 0.10 | 0.60 d,e,f ± 0.18 | 0.13 f,g ± 0.015 | |
| SA (0.1 mM) | 53.5 e,f,g ± 0.88 | 1.4 e ± 0.61 | 0.84 d,e ± 0.31 | 0.60 e,f ± 0.017 |
| Soil Water Condition | Treatment | Chl-a (mg/gm) | Chl-b (mg/gm) | Carotenoids (mg/gm) | Chl-a + Chl-b (mg/gm) |
|---|---|---|---|---|---|
| normal irrigation | Control | 0.95 b ± 0.006 | 0.35 b ± 0.0058 | 0.31 a ± 0.012 | 1.3 b ± 0.02 |
| SANP (0.05 mM) | 0.99 a ± 0.0054 | 0.25 e ± 0.006 | 0.21 d ± 0.01 | 1.24 c ± 0.003 | |
| SANP (0.1 mM) | 0.94 b,c ± 0.012 | 0.23 f ± 0.009 | 0.31 a ± 0.015 | 1.17 de ± 0.014 | |
| SA (0.05 mM) | 0.91 d,e ± 0.015 | 0.22 f ± 0.015 | 0.32 a ± 0.003 | 1.03 g ± 0.012 | |
| SA (0.1 mM) | 0.92 cd ± 0.012 | 0.28 d ± 0.01 | 0.20 d ± 0.017 | 1.12 e,f ± 0.01 | |
| drought stress | Control | 0.75 f ± 0.012 | 0.17 h ± 0.007 | 0.25 c ± 0.009 | 0.92 h ± 0.006 |
| SANP (0.05 mM) | 0.96 b ± 0.015 | 0.16 a ± 0.12 | 0.29 b ± 0.009 | 1.52 a ± 0.044 | |
| SANP (0.1 mM) | 0.75 f ± 0.02 | 0.31 c ± 0.012 | 0.21 d ± 0.006 | 1.06 g ± 0.02 | |
| SA (0.05 mM) | 0.94 b,c ± 0.009 | 0.21 g ± 0.012 | 0.31 a ± 0.012 | 1.15 e,f ± 0.017 | |
| SA (0.1 mM) | 0.90 d,e ± 0.026 | 0.30 c ± 0.009 | 0.28 b ± 0.006 | 1.2 d ± 0.007 |
| Soil Water Condition | Treatments | Relative Water Content % (RWC) | Leaf Area Index |
|---|---|---|---|
| normal irrigation | Control | 88.83 b,c ± 0.68 | 3.4 g,h ± 0.25 |
| SA-NPs (0.05 mM) | 87.06 d,e ± 0.06 | 7.03 a ± 0.22 | |
| SA-NPs (0.1 mM) | 91.10 a ± 0.33 | 4.5 d,e ± 0.21 | |
| SA (0.05 mM) | 86.82 e,f ± 0.09 | 4.06 e,f ± 0.30 | |
| SA (0.1 mM) | 88.00 b,c,d ± 0.18 | 5.67 b ± 0.18 | |
| drought stress | Control | 66 i ± 0.18 | 2.07 i ± 0.19 |
| SA-NPs (0.05 mM) | 86.10 e,f ± 0.83 | 5.03 c ± 0.26 | |
| SA-NPs (0.1 mM) | 89.17 b ± 0.44 | 3.77 f,g ± 0.15 | |
| SA (0.05 mM) | 71.66 h ± 0.74 | 2.97 h ± 0.12 | |
| SA (0.1 mM) | 85.33 f ± 0.33 | 4.87 c,d ± 0.24 |
| Soil Water Condition | Treatment | Total Alkaloid Percentage |
|---|---|---|
| Normal irrigation | Control | 0.72 e ± 0.008 |
| SANP (0.05 mM) | 0.75 d ± 0.012 | |
| SANP (0.1 mM) | 0.77 c ± 0.006 | |
| SA (0.05 mM) | 0.65 g ± 0.012 | |
| SA (0.1 mM) | 0.70 f ± 0.013 | |
| Drought stress | Control | 0.77 c ± 0.006 |
| SANP (0.05 mM) | 0.83 a ± 0.007 | |
| SANP (0.1 mM) | 0.80 b ± 0.007 | |
| SA (0.05 mM) | 0.76 c ± 0.009 | |
| SA (0.1 mM) | 0.82 a ± 0.0006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salem, D.; El-Garhy, H.A.S.; Ismail, I.A.; Dessoky, E.S.; Samra, B.N.; Shoala, T. Nanobiotechnological Approaches to Enhance Drought Tolerance in Catharanthus roseus Plants Using Salicylic Acid in Bulk and Nanoform. Molecules 2022, 27, 5112. https://doi.org/10.3390/molecules27165112
Salem D, El-Garhy HAS, Ismail IA, Dessoky ES, Samra BN, Shoala T. Nanobiotechnological Approaches to Enhance Drought Tolerance in Catharanthus roseus Plants Using Salicylic Acid in Bulk and Nanoform. Molecules. 2022; 27(16):5112. https://doi.org/10.3390/molecules27165112
Chicago/Turabian StyleSalem, Dina, Hoda A. S. El-Garhy, Ismail A. Ismail, Eldessoky S. Dessoky, Bassem N. Samra, and Tahsin Shoala. 2022. "Nanobiotechnological Approaches to Enhance Drought Tolerance in Catharanthus roseus Plants Using Salicylic Acid in Bulk and Nanoform" Molecules 27, no. 16: 5112. https://doi.org/10.3390/molecules27165112
APA StyleSalem, D., El-Garhy, H. A. S., Ismail, I. A., Dessoky, E. S., Samra, B. N., & Shoala, T. (2022). Nanobiotechnological Approaches to Enhance Drought Tolerance in Catharanthus roseus Plants Using Salicylic Acid in Bulk and Nanoform. Molecules, 27(16), 5112. https://doi.org/10.3390/molecules27165112







