Gradient SERS Substrates with Multiple Resonances for Analyte Screening: Fabrication and SERS Applications
Abstract
:1. Introduction
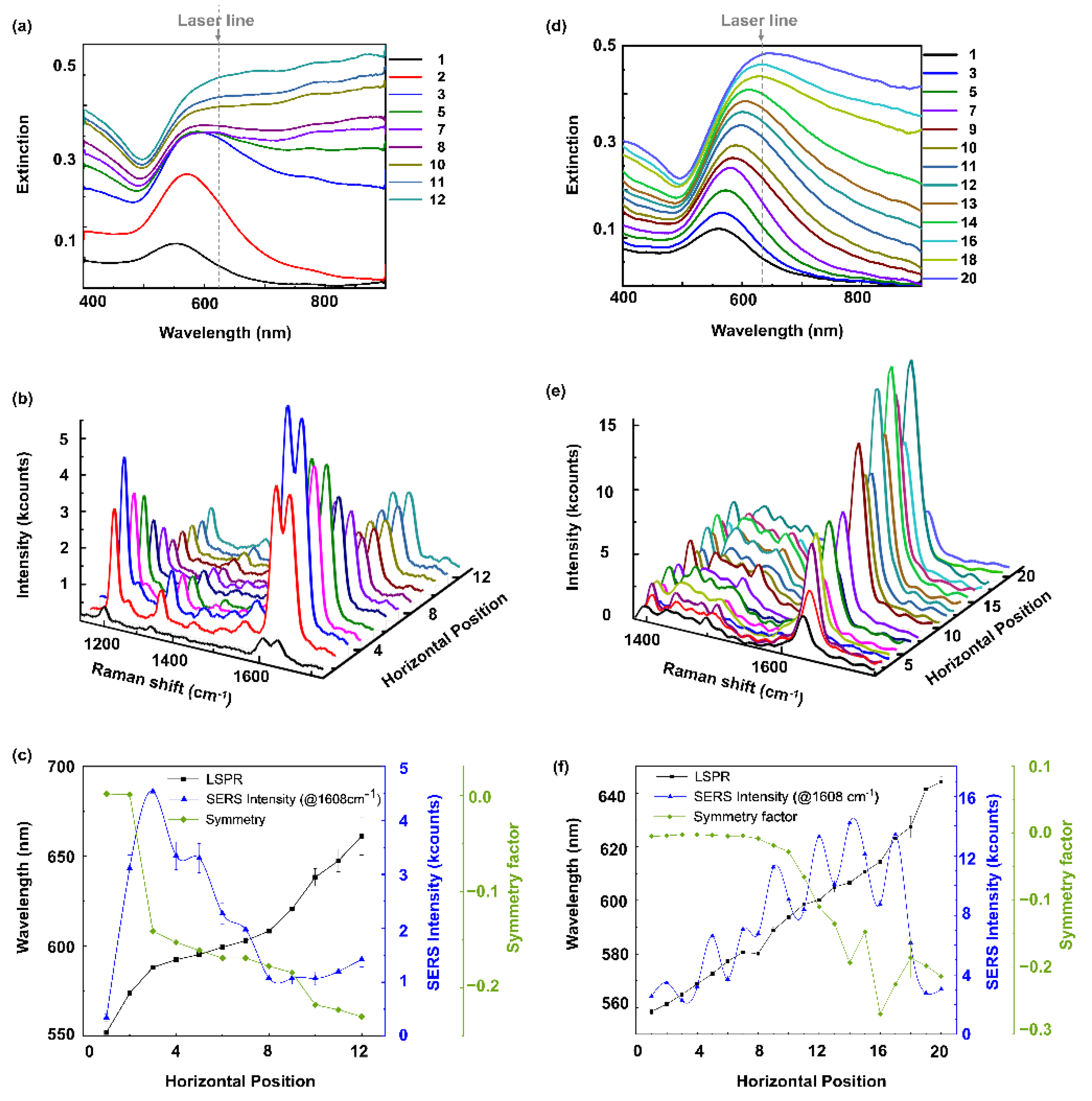
2. Results and Discussion
3. Materials and Methods
3.1. Non-Uniform Metallic Au Particles Substrate Fabrication
3.2. Extinction Spectra
3.3. SERS Experiments
3.4. Scanning Electron Microscopy (SEM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Liu, Q.; Wackenhut, F.; Hauler, O.; Scholz, M.; Zur Oven-Krockhaus, S.; Ritz, R.; Adam, P.-M.; Brecht, M.; Meixner, A.J. Hypericin: Single Molecule Spectroscopy of an Active Natural Drug. J. Phys. Chem. A 2020, 124, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Félidj, N.; Aubard, J.; Lévi, G.; Krenn, J.R.; Salerno, M.; Schider, G.; Lamprecht, B.; Leitner, A.; Aussenegg, F.R. Controlling the optical response of regular arrays of gold particles for surface-enhanced Raman scattering. Phys. Rev. B 2002, 65, 075419. [Google Scholar] [CrossRef]
- Félidj, N.; Aubard, J.; Lévi, G.; Krenn, J.R.; Schider, G.; Leitner, A.; Aussenegg, F.R. Enhanced substrate-induced coupling in two-dimensional gold nanoparticle arrays. Phys. Rev. B 2002, 66, 245407. [Google Scholar] [CrossRef]
- Vosgröne, T.; Meixner, A.J. Surface- and resonance-enhanced micro-Raman spectroscopy of xanthene dyes: From the ensemble to single molecules. Chemphyschem 2005, 6, 154–163. [Google Scholar] [CrossRef]
- Milekhin, A.G.; Rahaman, M.; Rodyakina, E.E.; Latyshev, A.V.; Dzhagan, V.M.; Zahn, D.R.T. Giant gap-plasmon tip-enhanced Raman scattering of MoS2 monolayers on Au nanocluster arrays. Nanoscale 2018, 10, 2755–2763. [Google Scholar] [CrossRef]
- Rahaman, M.; Milekhin, A.G.; Mukherjee, A.; Rodyakina, E.E.; Latyshev, A.V.; Dzhagan, V.M.; Zahn, D.R.T. The role of a plasmonic substrate on the enhancement and spatial resolution of tip-enhanced Raman scattering. Faraday Discuss. 2019, 214, 309–323. [Google Scholar] [CrossRef]
- Rodriguez, R.D.; Sheremet, E.; Deckert-Gaudig, T.; Chaneac, C.; Hietschold, M.; Deckert, V.; Zahn, D.R.T. Surface- and tip-enhanced Raman spectroscopy reveals spin-waves in iron oxide nanoparticles. Nanoscale 2015, 7, 9545–9551. [Google Scholar] [CrossRef] [Green Version]
- Sheremet, E.; Rodriguez, R.D.; Zahn, D.R.T.; Milekhin, A.G.; Rodyakina, E.E.; Latyshev, A.V. Surface-enhanced Raman scattering and gap-mode tip-enhanced Raman scattering investigations of phthalocyanine molecules on gold nanostructured substrates. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2014, 32, 04E110. [Google Scholar] [CrossRef]
- Hauler, O.; Jakob, L.A.; Braun, K.; Laible, F.; Fleischer, M.; Meixner, A.J.; Wackenhut, F. Sensitive Interferometric Plasmon Ruler Based on a Single Nanodimer. J. Phys. Chem. C 2021, 125, 6486–6493. [Google Scholar] [CrossRef]
- Macias, G.; Alba, M.; Marsal, L.F.; Mihi, A. Surface roughness boosts the SERS performance of imprinted plasmonic architectures. J. Mater. Chem. C 2016, 4, 3970–3975. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Lei, D.Y.; Chai, Y. Effects of surface roughness of Ag thin films on surface-enhanced Raman spectroscopy of graphene: Spatial nonlocality and physisorption strain. Nanoscale 2014, 6, 1311–1317. [Google Scholar] [CrossRef]
- Muniz-Miranda, M. SERS-active Ag/SiO2 colloids: Photoreduction mechanism of the silver ions and catalytic activity of the colloidal nanoparticles. J. Raman Spectrosc. 2004, 35, 839–842. [Google Scholar] [CrossRef]
- Qian, X.-M.; Nie, S.M. Single-molecule and single-nanoparticle SERS: From fundamental mechanisms to biomedical applications. Chem. Soc. Rev. 2008, 37, 912–920. [Google Scholar] [CrossRef]
- Ye, Z.; Li, C.; Chen, Q.; Xu, Y.; Bell, S.E.J. Self-assembly of colloidal nanoparticles into 2D arrays at water-oil interfaces: Rational construction of stable SERS substrates with accessible enhancing surfaces and tailored plasmonic response. Nanoscale 2021, 13, 5937–5953. [Google Scholar] [CrossRef]
- Aroca, R.; Martin, F. Tuning metal island films for maximum surface-enhanced Raman scattering. J. Raman Spectrosc. 1985, 16, 156–162. [Google Scholar] [CrossRef]
- Jennings, C.A.; Kovacs, G.J.; Aroca, R. Near-infrared surface-enhanced Raman scattering from metal island films. J. Phys. Chem. 1992, 96, 1340–1343. [Google Scholar] [CrossRef]
- Kovacs, G.J.; Loutfy, R.O.; Vincett, P.S.; Jennings, C.; Aroca, R. Distance dependence of SERS enhancement factor from Langmuir-Blodgett monolayers on metal island films: Evidence for the electromagnetic mechanism. Langmuir 1986, 2, 689–694. [Google Scholar] [CrossRef]
- Rodriguez, R.D.; Sheremet, E.; Nesterov, M.; Moras, S.; Rahaman, M.; Weiss, T.; Hietschold, M.; Zahn, D.R. Aluminum and copper nanostructures for surface-enhanced Raman spectroscopy: A one-to-one comparison to silver and gold. Sens. Actuators B Chem. 2018, 262, 922–927. [Google Scholar] [CrossRef]
- Chen, Y.; Si, K.J.; Sikdar, D.; Tang, Y.; Premaratne, M.; Cheng, W. Ultrathin Plasmene Nanosheets as Soft and Surface-Attachable SERS Substrates with High Signal Uniformity. Adv. Opt. Mater. 2015, 3, 919–924. [Google Scholar] [CrossRef]
- Luo, X.; Liu, W.; Chen, C.; Jiang, G.; Hu, X.; Zhang, H.; Zhong, M. Femtosecond laser micro-nano structured Ag SERS substrates with unique sensitivity, uniformity and stability for food safety evaluation. Opt. Laser Technol. 2021, 139, 106969. [Google Scholar] [CrossRef]
- Wen, P.; Yang, F.; Ge, C.; Li, S.; Xu, Y.; Chen, L. Self-assembled nano-Ag/Au@Au film composite SERS substrates show high uniformity and high enhancement factor for creatinine detection. Nanotechnology 2021, 32, 395502. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, W.; Fu, Q.; Wu, M.; Shayan, K.; Wong, K.M.; Singh, S.; Schober, A.; Schaaf, P.; Lei, Y. Surface-Enhanced Raman Scattering (SERS) Substrate Based on Large-Area Well-Defined Gold Nanoparticle Arrays with High SERS Uniformity and Stability. ChemPlusChem 2014, 79, 1622–1630. [Google Scholar] [CrossRef]
- Schreiber, B.; Gkogkou, D.; Dedelaite, L.; Kerbusch, J.; Hübner, R.; Sheremet, E.; Zahn, D.R.T.; Ramanavicius, A.; Facsko, S.; Rodriguez, R.D. Large-scale self-organized gold nanostructures with bidirectional plasmon resonances for SERS. RSC Adv. 2018, 8, 22569–22576. [Google Scholar] [CrossRef] [Green Version]
- Milekhin, A.G.; Yeryukov, N.A.; Sveshnikova, L.L.; Duda, T.A.; Rodyakina, E.E.; Sheremet, E.S.; Ludemann, M.; Gordan, O.D.; Latyshev, A.V.; Zahn, D.R. Surface enhanced Raman scattering by organic and inorganic semiconductors formed on laterally ordered arrays of Au nanoclusters. Thin Solid Film. 2013, 543, 35–40. [Google Scholar] [CrossRef]
- Baker, B.E.; Kline, N.J.; Treado, P.J.; Natan, M.J. Solution-Based Assembly of Metal Surfaces by Combinatorial Methods. J. Am. Chem. Soc. 1996, 118, 8721–8722. [Google Scholar] [CrossRef]
- Bhat, R.R.; Genzer, J.; Chaney, B.N.; Sugg, H.W.; Liebmann-Vinson, A. Controlling the assembly of nanoparticles using surface grafted molecular and macromolecular gradients. Nanotechnology 2003, 14, 1145–1152. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Tegenfeldt, J.O.; Austin, R.H.; Chou, S.Y. Gradient nanostructures for interfacing microfluidics and nanofluidics. Appl. Phys. Lett. 2002, 81, 3058–3060. [Google Scholar] [CrossRef]
- Gupta, S.; Huda, S.; Kilpatrick, P.K.; Velev, O.D. Characterization and optimization of gold nanoparticle-based silver-enhanced immunoassays. Anal. Chem. 2007, 79, 3810–3820. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Xu, C.; Zhu, D.; Yang, L.; Zhang, J.; Chen, Y.; Ding, K.; Song, F.; Wang, G. Controllable Synthesis of Two-Dimensional Metal Nanoparticle Arrays with Oriented Size and Number Density Gradients. Adv. Mater. 2007, 19, 2979–2983. [Google Scholar] [CrossRef]
- He, L.; Chen, X.; Mu, Y.; Song, F.; Han, M. Two-dimensional gradient Ag nanoparticle assemblies: Multiscale fabrication and SERS applications. Nanotechnology 2010, 21, 495601. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; He, X.; Tang, C.; Du, K.; He, Z. Gradient nanoporous gold: A novel surface-enhanced Raman scattering substrate. RSC Adv. 2017, 7, 15747–15753. [Google Scholar] [CrossRef] [Green Version]
- Huwiler, C.; Kunzler, T.P.; Textor, M.; Vörös, A.J.; Spencer, N.D. Functionalizable Nanomorphology Gradients via Colloidal Self-Assembly. Langmuir 2007, 23, 5929–5935. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Cai, Y.; Newby, B.-M.Z. Fabricating tunable nanoparticle density gradients with the contact printing based approach. Appl. Surf. Sci. 2006, 253, 2393–2398. [Google Scholar] [CrossRef]
- Bhat, R.R.; Fischer, A.D.A.; Genzer, J. Fabricating Planar Nanoparticle Assemblies with Number Density Gradients. Langmuir 2002, 18, 5640–5643. [Google Scholar] [CrossRef]
- Oates, T.W.H.; Noda, S. Thickness-gradient dependent Raman enhancement in silver island films. Appl. Phys. Lett. 2009, 94, 053106. [Google Scholar] [CrossRef]
- Pashley, D.W.; Stowell, M.J.; Jacobs, M.H.; Law, T.J. The growth and structure of gold and silver deposits formed by evaporation inside an electron microscope. Philos. Mag. 1964, 10, 127–158. [Google Scholar] [CrossRef]
- Farooq, S.; de Araujo, R.E. Engineering a Localized Surface Plasmon Resonance Platform for Molecular Biosensing. Open J. Appl. Sci. 2018, 8, 126–139. [Google Scholar] [CrossRef] [Green Version]
- Göeken, K.L.; Subramaniam, V.; Gill, R. Enhancing spectral shifts of plasmon-coupled noble metal nanoparticles for sensing applications. Phys. Chem. Chem. Phys. 2015, 17, 422–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starowicz, Z.; Wojnarowska-Nowak, R.; Ozga, P.; Sheregii, E.M. The tuning of the plasmon resonance of the metal nanoparticles in terms of the SERS effect. Colloid Polym. Sci. 2018, 296, 1029–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabor, C.; van Haute, D.; El-Sayed, M.A. Effect of orientation on plasmonic coupling between gold nanorods. ACS Nano 2009, 3, 3670–3678. [Google Scholar] [CrossRef]
- Khaywah, M.Y.; Jradi, S.; Louarn, G.; Lacroute, Y.; Toufaily, J.; Hamieh, T.; Adam, P.-M. Ultrastable, Uniform, Reproducible, and Highly Sensitive Bimetallic Nanoparticles as Reliable Large Scale SERS Substrates. J. Phys. Chem. C 2015, 119, 26091–26100. [Google Scholar] [CrossRef]
- Dai, F.; Horrer, A.; Adam, P.-M.; Fleischer, M. Accessing the Hotspots of Cavity Plasmon Modes in Vertical Metal–Insulator–Metal Structures for Surface Enhanced Raman Scattering. Adv. Opt. Mater. 2020, 8, 1901734. [Google Scholar] [CrossRef]
- Commission Internationale de L’Eclairag. Colorimetry, 3rd ed.; CIE, Central Bureau: Vienna, Austria, 2004; ISBN 3901906339. [Google Scholar]
- Guergouri, M.; Bensegueni, R.; Bencharif, L. Synthesis, photophysical properties and electrochemical polymerization of a new blue fluorescent compound based on 3,4-ethylenedioxythiophene moiety. Mater. Sci. Pol. 2020, 38, 151–158. [Google Scholar] [CrossRef]
- Selvam, S.; Sarkar, I. Bile salt induced solubilization of methylene blue: Study on methylene blue fluorescence properties and molecular mechanics calculation. J. Pharm. Anal. 2017, 7, 71–75. [Google Scholar] [CrossRef]
- Kim, A.; Ou, F.S.; Ohlberg, D.A.A.; Hu, M.; Williams, R.S.; Li, Z. Study of molecular trapping inside gold nanofinger arrays on surface-enhanced Raman substrates. J. Am. Chem. Soc. 2011, 133, 8234–8239. [Google Scholar] [CrossRef]
- Altun, A.O.; Youn, S.K.; Yazdani, N.; Bond, T.; Park, H.G. Metal-dielectric-CNT nanowires for femtomolar chemical detection by surface enhanced Raman spectroscopy. Adv. Mater. 2013, 25, 4431–4436. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, A.; Liu, Q.; Wackenhut, F.; Dai, F.; Fleischer, M.; Adam, P.-M.; Meixner, A.J.; Brecht, M. Gradient SERS Substrates with Multiple Resonances for Analyte Screening: Fabrication and SERS Applications. Molecules 2022, 27, 5097. https://doi.org/10.3390/molecules27165097
Mukherjee A, Liu Q, Wackenhut F, Dai F, Fleischer M, Adam P-M, Meixner AJ, Brecht M. Gradient SERS Substrates with Multiple Resonances for Analyte Screening: Fabrication and SERS Applications. Molecules. 2022; 27(16):5097. https://doi.org/10.3390/molecules27165097
Chicago/Turabian StyleMukherjee, Ashutosh, Quan Liu, Frank Wackenhut, Fang Dai, Monika Fleischer, Pierre-Michel Adam, Alfred J. Meixner, and Marc Brecht. 2022. "Gradient SERS Substrates with Multiple Resonances for Analyte Screening: Fabrication and SERS Applications" Molecules 27, no. 16: 5097. https://doi.org/10.3390/molecules27165097
APA StyleMukherjee, A., Liu, Q., Wackenhut, F., Dai, F., Fleischer, M., Adam, P.-M., Meixner, A. J., & Brecht, M. (2022). Gradient SERS Substrates with Multiple Resonances for Analyte Screening: Fabrication and SERS Applications. Molecules, 27(16), 5097. https://doi.org/10.3390/molecules27165097






