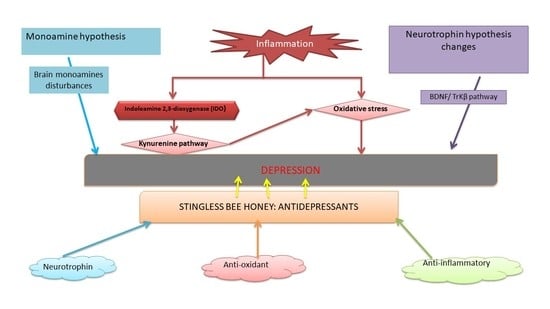
Pathophysiology of Depression: Stingless Bee Honey Promising as an Antidepressant
Abstract
1. Revisiting Depression
2. Pathophysiology of Depression
2.1. Monoamine Hypothesis
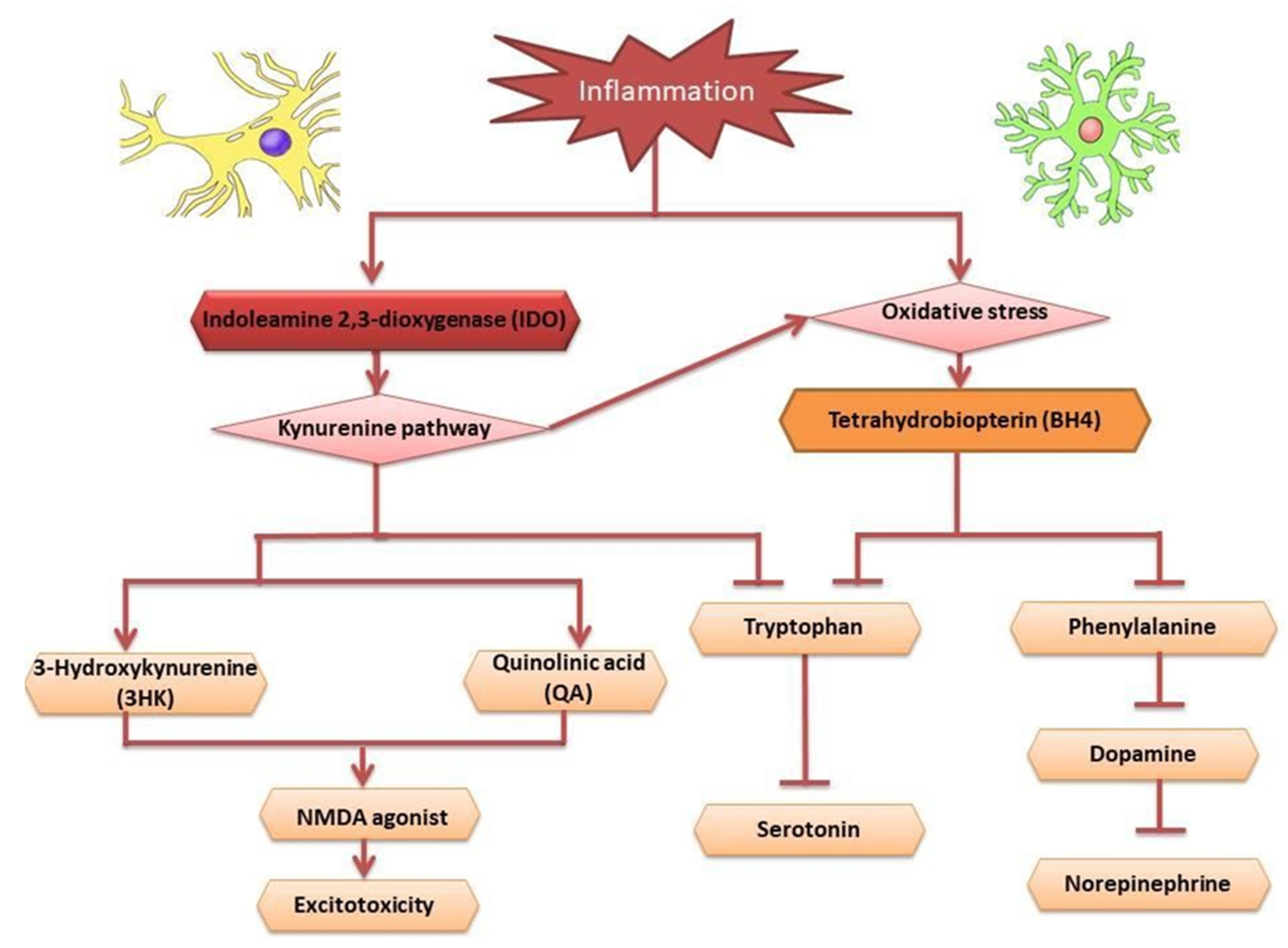
2.2. Inflammation Hypothesis in Depression
2.3. Neurotrophin Hypothesis
3. Stingless Bee Honey (SBH) as an Antidepressant
3.1. Neurotrophic Factors
3.2. Antioxidant
3.3. Anti-Inflammatory
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knol, M.J.; Twisk, J.W.; Beekman, A.T.; Heine, R.J.; Snoek, F.J.; Pouwer, F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia 2006, 49, 837. [Google Scholar] [CrossRef] [PubMed]
- Mauskopf, J.A.; Simon, G.E.; Kalsekar, A.; Nimsch, C.; Dunayevich, E.; Cameron, A. Nonresponse, partial response, and failure to achieve remission: Humanistic and cost burden in major depressive disorder. Depress. Anxiety 2009, 26, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.D.D.; Rodrigues, A.M.; Gregório, M.J.; Branco, J.D.C.; Gouveia, M.J.; Canhão, H.; Dias, S.S. Anxiety and depression in the Portuguese older adults: Prevalence and associated factors. Front. Med. 2017, 4, 196. [Google Scholar] [CrossRef]
- Cohn, D.W.H.; Kinoshita, D.; Palermo-Neto, J. Antidepressants prevent hierarchy destabilization induced by lipopolysaccharide administration in mice: A neurobiological approach to depression. Ann. N. Y. Acad. Sci. 2012, 1262, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.L.; Chen, J.J.; Han, F.; Pan, C.; Zhuang, T.T.; Cai, Y.F.; Lu, Y.P. Novel antidepressant effects of Paeonol alleviate neuronal injury with concomitant alterations in BDNF, Rac1 and RhoA levels in chronic unpredictable mild stress rats. Psychopharmacology 2018, 235, 2177–2191. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Huang, S.; Hao, W. New hypothesis and treatment targets of depression: An integrated view of key findings. Neurosci. Bull. 2015, 31, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Coppen, A. The biochemistry of affective disorders. Br. J. Psychiatry 1967, 113, 1237–1264. [Google Scholar] [CrossRef]
- Schildkraut, J.J. The catecholamine hypothesis of affective disorders: A review of supporting evidence. Am. J. Psychiatry 1965, 122, 509–522. [Google Scholar] [CrossRef]
- Belmaker, R.H.; Agam, G. Major depressive disorder. N. Engl. J. Med. 2008, 358, 55–68. [Google Scholar] [CrossRef]
- Hayley, S.; Poulter, M.O.; Merali, Z.; Anisman, H. The pathogenesis of clinical depression: Stressor-and cytokine-induced alterations of neuroplasticity. Neuroscience 2005, 135, 659–678. [Google Scholar] [CrossRef]
- Hiles, S.A.; Baker, A.L.; de Malmanche, T.; Attia, J. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: Exploring the causes of heterogeneity. Brain Behav. Immun. 2012, 26, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Deyama, S.; Fogaça, M.V. Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants. Eur. J. Neurosci. 2021, 53, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Heninger, G.R.; Nestler, E.J. A molecular and cellular theory of depression. Arch. Gen. Psychiatry 1997, 54, 597–606. [Google Scholar] [CrossRef]
- Kharade, S.M.; Gumate, D.S.; Naikwade, D. A Review: Hypothesis of Depression and Role of Antidepressant Drugs. Depression 2010, 15, 17. [Google Scholar]
- Delgado, P.L. How antidepressants help depression: Mechanisms of action and clinical response. J. Clin. Psychiatry 2004, 65, 25–30. [Google Scholar]
- Taylor, C.; Fricker, A.D.; Devi, L.A.; Gomes, I. Mechanisms of action of antidepressants: From neurotransmitter systems to signaling pathways. Cell. Signal. 2005, 17, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Masand, P.S.; Gupta, S. Long-term side effects of newer-generation antidepressants: SSRIS, venlafaxine, nefazodone, bupropion, and mirtazapine. Ann. Clin. Psychiatry 2002, 14, 175–182. [Google Scholar] [CrossRef]
- Schweitzer, I.; Maguire, K.; Ng, C. Sexual side-effects of contemporary antidepressants. Aust. N. Z. J. Psychiatry 2009, 43, 795–808. [Google Scholar] [CrossRef]
- Mihaljević-Peleš, A.; Šagud, M.; Bajs Janović, M.; Kudlek Mikulić, S.; Jevtović, S. Do we need new therapeutic strategies for depression? Psychiatr. Danub. 2011, 23, 300–301. [Google Scholar] [PubMed]
- Bet, P.M.; Hugtenburg, J.G.; Penninx, B.W.; Hoogendijk, W.J. Side effects of antidepressants during long-term use in a naturalistic setting. Eur. Neuropsychopharmacol. 2013, 23, 1443–1451. [Google Scholar] [CrossRef]
- Richelson, E. Multi-modality: A new approach for the treatment of major depressive disorder. Int. J. Neuropsychopharmacol. 2013, 16, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Guski, L.S.; Freund, N.; Gøtzsche, P.C. Suicidality and aggression during antidepressant treatment: Systematic review and meta-analyses based on clinical study reports. BMJ 2016, 352, i65. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, C.; Fabbri, C.; Porcelli, S.; Drago, A.; Spina, E.; De Ronchi, D.; Serretti, A. Pharmacogenetics of antidepressants. Front. Pharmacol. 2011, 2, 6. [Google Scholar] [CrossRef]
- Ruhé, H.G.; van Rooijen, G.; Spijker, J.; Peeters, F.P.; Schene, A.H. Staging methods for treatment resistant depression. A systematic review. J. Affect. Disord. 2012, 137, 35–45. [Google Scholar] [CrossRef]
- Chiu, C.H.; Chyau, C.C.; Chen, C.C.; Lee, L.Y.; Chen, W.P.; Liu, J.L.; Lin, W.H.; Mong, M.C. Erinacine A-enriched Hericium erinaceus mycelium produces antidepressant-like effects through modulating BDNF/PI3K/Akt/GSK-3β signaling in mice. Int. J. Mol. Sci. 2018, 19, 341. [Google Scholar] [CrossRef]
- Manosso, L.M.; Moretti, M.; Ribeiro, C.M.; Gonçalves, F.M.; Leal, R.B.; Rodrigues, A.L.S. Antidepressant-like effect of zinc is dependent on signaling pathways implicated in BDNF modulation. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 59, 59–67. [Google Scholar]
- Ali, S.S.; Abd El Wahab, M.G.; Ayuob, N.N.; Suliaman, M. The antidepressant-like effect of Ocimum basilicum in an animal model of depression. Biotech. Histochem. 2017, 92, 390–401. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Honey: A novel antioxidant. Molecules 2012, 17, 4400–4423. [Google Scholar] [CrossRef]
- Mijanur Rahman, M.; Gan, S.H.; Khalil, M. Neurological effects of honey: Current and future prospects. Evid.-Based Complement. Altern. Med. 2014, 2014, 958721. [Google Scholar] [CrossRef]
- Münstedt, K.; Voss, B.; Kullmer, U.; Schneider, U.; Hübner, J. Bee pollen and honey for the alleviation of hot flushes and other menopausal symptoms in breast cancer patients. Mol. Clin. Oncol. 2015, 3, 869–874. [Google Scholar] [CrossRef]
- Abd Wahab, M.S.; Othman, N.; Othman, N.H.I.; Jamari, A.A.; Ali, A.A. Exploring the use of and perceptions about honey as complementary and alternative medicine among the general public in the state of Selangor, Malaysia. J. Appl. Pharm. Sci. 2017, 7, 144–150. [Google Scholar]
- Fatima, I.J.; Ab, M.H.; Salwani, I.; Lavaniya, M. Physicochemical characteristics of Malaysian stingless bee honey from trigona species. IIUM Med. J. Malays. 2018, 17, 187–191. [Google Scholar]
- Roowi, S.; Muhamad, S.A.; Sipon, H.; Jaafar, M.; Daud, M.; Hisham, N.; Othman, R. Asid fenolik bebas dalam madu kelulut. Bul. Teknol. MARDI 2012, 2, 145–147. [Google Scholar]
- Biswa, R.; Sarkar, A.; Khewa, S.S. Ethnomedicinal uses of honey of stingless bee by Nepali community of Darjeeling foothills of West Bengal, India. Indian J. Tradit. Knowl. 2017, 16, 648–653. [Google Scholar]
- Özbalci, B.; Boyaci, İ.H.; Topcu, A.; Kadılar, C.; Tamer, U. Rapid analysis of sugars in honey by processing Raman spectrum using chemometric methods and artificial neural networks. Food Chem. 2013, 136, 1444–1452. [Google Scholar] [CrossRef]
- Kek, S.P.; Chin, N.L.; Yusof, Y.A.; Tan, S.W.; Chua, L.S. Total phenolic contents and colour intensity of Malaysian honeys from the Apis spp. and Trigona spp. bees. Agric. Agric. Sci. Procedia 2014, 2, 150–155. [Google Scholar]
- Biluca, F.C.; Della Betta, F.; de Oliveira, G.P.; Pereira, L.M.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. 5-HMF and carbohydrates content in stingless bee honey by CE before and after thermal treatment. Food Chem. 2014, 159, 244–249. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.; Liu, B.; Shi, W.; Shi, J.; Zhang, Z.; Xing, J. Rutin attenuates H2O2-induced oxidation damage and apoptosis in Leydig cells by activating PI3K/Akt signal pathways. Biomed. Pharmacother. 2017, 88, 500–506. [Google Scholar] [CrossRef]
- Kanimozhi, S.; Bhavani, P.; Subramanian, P. Influence of the flavonoid, quercetin on antioxidant status, lipid peroxidation and histopathological changes in hyperammonemic rats. Indian J. Clin. Biochem. 2017, 32, 275–284. [Google Scholar] [CrossRef]
- Nutt, D.J. Relationship of neurotransmitters to the symptoms of major depressive disorder. J. Clin. Psychiatry 2008, 69, 4–7. [Google Scholar]
- Zhang, Z.; Deng, T.; Wu, M.; Zhu, A.; Zhu, G. Botanicals as modulators of depression and mechanisms involved. Chin. Med. 2019, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. The cell biology of synapse formation. J. Cell Biol. 2021, 220, e202103052. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, D.E.; Mattevi, A.; Binda, C.; Li, M.; Hubalek, F. Structure and mechanism of monoamine oxidase. Curr. Med. Chem. 2004, 11, 1983–1993. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.K.; Dhir, A.; Akula, K.K. Potentials of curcumin as an antidepressant. Sci. World J. 2009, 9, 1233–1241. [Google Scholar] [CrossRef]
- Moret, C.; Briley, M. The importance of norepinephrine in depression. Neuropsychiatr. Dis. Treat. 2011, 7 (Suppl 1), 9. [Google Scholar]
- Freis, E.D. Mental depression in hypertensive patients treated for long periods with large doses of reserpine. N. Engl. J. Med. 1954, 251, 1006–1008. [Google Scholar] [CrossRef]
- Pletscher, A. The discovery of antidepressants: A winding path. Experientia 1991, 47, 4–8. [Google Scholar] [CrossRef]
- Radak, Z.; Marton, O.; Nagy, E.; Koltai, E.; Goto, S. The complex role of physical exercise and reactive oxygen species on brain. J. Sport Health Sci. 2013, 2, 87–93. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.B.; Hwang, E.S.; Kim, E.S.; Kim, S.S.; Jeon, T.D.; Song, M.C.; Lee, J.S.; Chung, M.C.; Maeng, S.; et al. Antidepressant-like effects of p-coumaric acid on LPS-induced depressive and inflammatory changes in rats. Exp. Neurobiol. 2018, 27, 189. [Google Scholar] [CrossRef]
- Tunnicliff, G.; Malatynska, E. Central GABAergic systems and depressive illness. Neurochem. Res. 2003, 28, 965–976. [Google Scholar] [CrossRef]
- Rupprecht, R.; Eser, D.; Zwanzger, P.; Möller, H.J. GABAA receptors as targets for novel anxiolytic drugs. World J. Biol. Psychiatry 2006, 7, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, A.; Turecki, G. Genome wide gene expression studies in mood disorders. Omics A J. Integr. Biol. 2006, 10, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Sanacora, G.; Saricicek, A. GABAergic contributions to the pathophysiology of depression and the mechanism of antidepressant action. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2007, 6, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Schwab, C.; Mcgeer, P.L. Astrocytes are GABAergic cells that modulate microglial activity. Glia 2011, 59, 152–165. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Wichers, M.; Maes, M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. Int. J. Neuropsychopharmacol. 2002, 5, 375–388. [Google Scholar] [CrossRef]
- Karin, M.; Greten, F.R. NF-κB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749. [Google Scholar] [CrossRef]
- Napetschnig, J.; Wu, H. Molecular basis of NF-κB signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef]
- Yu, B.; Chang, J.; Liu, Y.; Li, J.; Kevork, K.; Al-Hezaimi, K.; Graves, D.T.; Park, N.H.; Wang, C.Y. Wnt4 signaling prevents skeletal aging and inflammation by inhibiting nuclear factor-κB. Nat. Med. 2014, 20, 1009. [Google Scholar] [CrossRef]
- FitzGerald, G.A. COX-2 and beyond: Approaches to prostaglandin inhibition in human disease. Nat. Rev. Drug Discov. 2003, 2, 879. [Google Scholar] [CrossRef]
- Munhoz, C.D.; Lepsch, L.B.; Kawamoto, E.M.; Malta, M.B.; de Sá Lima, L.; Avellar, M.C.W.; Sapolsky, R.M.; Scavone, C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-κB in the frontal cortex and hippocampus via glucocorticoid secretion. J. Neurosci. 2006, 26, 3813–3820. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.Q.; Yu, J. Inflammation: A mechanism of depression? Neurosci. Bull. 2014, 30, 515–523. [Google Scholar] [CrossRef]
- Llorens-Martin, M.; Jurado-Arjona, J.; Fuster-Matanzo, A.; Hernandez, F.; Rabano, A.; Avila, J. Peripherally triggered and GSK-3β-driven brain inflammation differentially skew adult hippocampal neurogenesis, behavioral pattern separation and microglial activation in response to ibuprofen. Transl. Psychiatry 2014, 4, e463. [Google Scholar] [CrossRef]
- Norden, D.M.; McCarthy, D.O.; Bicer, S.; Devine, R.D.; Reiser, P.J.; Godbout, J.P.; Wold, L.E. Ibuprofen ameliorates fatigue-and depressive-like behavior in tumor-bearing mice. Life Sci. 2015, 143, 65–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koo, J.W.; Duman, R.S. IL-1β is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl. Acad. Sci. USA 2008, 105, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, G.; Becerril-Villanueva, E.; Arreola, R.; Martínez-Levy, G.; Hernández-Gutiérrez, M.E.; Velasco-Velásquez, M.A.; Alvarez-Herrera, S.; Cruz-Fuentes, C.; Palacios, L.; De La Peña, F.; et al. Inflammatory profiles in depressed adolescents treated with fluoxetine: An 8-week follow-up open study. Mediat. Inflamm. 2018, 2018, 4074051. [Google Scholar] [CrossRef]
- Kasai, T.; Inada, K.; Takakuwa, T.; Yamada, Y.; Inoue, Y.; Shimamura, T.; Taniguchi, S.; Sato, S.; Wakabayashi, G.; Endo, S. Anti-inflammatory cytokine levels in patients with septic shock. Res. Commun. Mol. Pathol. Pharmacol. 1997, 98, 34–42. [Google Scholar]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A Review: Inflammatory Process in Alzheimer’s Disease, Role of Cytokines. Sci. World J. 2012, 2012, 756357. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Leighton, S.P.; Nerurkar, L.; Krishnadas, R.; Johnman, C.; Graham, G.J.; Cavanagh, J. Chemokines in depression in health and in inflammatory illness: A systematic review and meta-analysis. Mol. Psychiatry 2018, 23, 48. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Aghajanian, G.K. Synaptic dysfunction in depression: Potential therapeutic targets. Science 2012, 338, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Miller, A.H. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol. Ther. 2011, 130, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, glutamate, and glia: A trio of trouble in mood disorders. Neuropsychopharmacology 2017, 42, 193. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Dantzer, R.; Kelley, K.W.; McCusker, R.H. Central administration of insulin-like growth factor-I decreases depressive-like behavior and brain cytokine expression in mice. J. Neuroinflamm. 2011, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Dobos, N.; de Vries, E.F.; Kema, I.P.; Patas, K.; Prins, M.; Nijholt, I.M.; Dierckx, R.A.; Korf, J.; den Boer, J.A.; Luiten, P.G.; et al. The role of indoleamine 2, 3-dioxygenase in a mouse model of neuroinflammation-induced depression. J. Alzheimer’s Dis. 2012, 28, 905–915. [Google Scholar] [CrossRef]
- Lawson, M.A.; Parrott, J.M.; McCusker, R.H.; Dantzer, R.; Kelley, K.W.; O’Connor, J.C. Intracerebroventricular administration of lipopolysaccharide induces indoleamine-2, 3-dioxygenase-dependent depression-like behaviors. J. Neuroinflamm. 2013, 10, 875. [Google Scholar] [CrossRef]
- Vancassel, S.; Capuron, L.; Castanon, N. Brain kynurenine and BH4 pathways: Relevance to the pathophysiology and treatment of inflammation-driven depressive symptoms. Front. Neurosci. 2018, 12, 499. [Google Scholar] [CrossRef]
- Hestad, K.A.; Engedal, K.; Whist, J.E.; Farup, P.G. The relationships among tryptophan, kynurenine, indoleamine 2, 3-dioxygenase, depression, and neuropsychological performance. Front. Psychol. 2017, 8, 1561. [Google Scholar] [CrossRef]
- O’connor, J.C.; Lawson, M.A.; Andre, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2, 3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511. [Google Scholar] [CrossRef]
- Raison, C.L.; Dantzer, R.; Kelley, K.W.; Lawson, M.A.; Woolwine, B.J.; Vogt, G.; Spivey, J.R.; Saito, K.; Miller, A.H. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-α: Relationship to CNS immune responses and depression. Mol. Psychiatry 2010, 15, 393. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.Z.; Zulkifli, F.N.; Fernandez, I.; Mariatulqabtiah, A.R.; Sangu, M.; Nor Azfa, J.; Mohamed, M.; Roslan, N. Stingless bee honey improves spatial memory in mice, probably associated with brain-derived neurotrophic factor (BDNF) and inositol 1, 4, 5-triphosphate receptor type 1 (Itpr1) genes. Evid.-Based Complement. Altern. Med. 2019, 2019, 8258307. [Google Scholar] [CrossRef]
- Lu, L.; Ben, X.; Xiao, L.; Peng, M.; Zhang, Y. AMP-activated protein kinase activation in mediating phenylalanine-induced neurotoxicity in experimental models of phenylketonuria. J. Inherit. Metab. Dis. 2018, 41, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Sperner-Unterweger, B.; Fuchs, D.; Gostner, J.M. Mechanisms of inflammation-associated depression: Immune influences on tryptophan and phenylalanine metabolisms. Inflamm.-Assoc. Depress. Evid. Mech. Implic. 2016, 31, 95–115. [Google Scholar]
- Froböse, M.I.; Cools, R. Chemical neuromodulation of cognitive control avoidance. Curr. Opin. Behav. Sci. 2018, 22, 121–127. [Google Scholar] [CrossRef]
- Haroon, E.; Raison, C.L.; Miller, A.H. Psychoneuroimmunology meets neuropsychopharmacology: Translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 2012, 37, 137. [Google Scholar] [CrossRef] [PubMed]
- Neurauter, G.; Schrocksnadel, K.; Scholl-Burgi, S.; Sperner-Unterweger, B.; Schubert, C.; Ledochowski, M.; Fuchs, D. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr. Drug Metab. 2008, 9, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Li, N. A neurotrophic hypothesis of depression: Role of synaptogenesis in the actions of NMDA receptor antagonists. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Begni, V.; Riva, M.A.; Cattaneo, A. Cellular and molecular mechanisms of the brain-derived neurotrophic factor in physiological and pathological conditions. Clin. Sci. 2017, 131, 123–138. [Google Scholar] [CrossRef]
- Galea, R.; Cassar, D. Brain-Derived Neurotrophic Factor—Major Depressive Disorder and Suicide. Open Access Libr. J. 2019, 6, e5106. [Google Scholar] [CrossRef]
- Björkholm, C.; Monteggia, L.M. BDNF–a key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Kim, Y.K. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 2010, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Neurotrophic Factors 2014, 220, 223–250. [Google Scholar]
- Zheleznyakova, G.Y.; Cao, H.; Schiöth, H.B. BDNF DNA methylation changes as a biomarker of psychiatric disorders: Literature review and open access database analysis. Behav. Brain Funct. 2016, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Altar, C.A. Neurotrophins and depression. Trends Pharmacol. Sci. 1999, 20, 59–62. [Google Scholar] [CrossRef]
- Numakawa, T.; Odaka, H.; Adachi, N. Actions of brain-derived neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. Int. J. Mol. Sci. 2018, 19, 3650. [Google Scholar] [CrossRef]
- Zhu, G.; Li, J.; He, L.; Wang, X.; Hong, X. MPTP-induced changes in hippocampal synaptic plasticity and memory are prevented by memantine through the BDNF-TrkB pathway. Br. J. Pharmacol. 2015, 172, 2354–2368. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Song, Z.J.; Wang, X.C.; Zhang, Z.R.; Wu, S.B.; Zhu, G.Q. Curculigoside facilitates fear extinction and prevents depression-like behaviors in a mouse learned helplessness model through increasing hippocampal BDNF. Acta Pharmacol. Sin. 2019, 40, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.B.; Luo, L.; Liu, X.L.; Geng, D.; Liu, Q.; Yi, L.T. 7-Chlorokynurenic acid (7-CTKA) produces rapid antidepressant-like effects: Through regulating hippocampal microRNA expressions involved in TrkB-ERK/Akt signaling pathways in mice exposed to chronic unpredictable mild stress. Psychopharmacology 2015, 232, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; He, B.; Wan, S.; Xu, M.; Yang, H.; Xiao, F.; Bi, K.; Jia, Y. Antidepressant-like effects and cognitive enhancement of Schisandra chinensis in chronic unpredictable mild stress mice and its related mechanism. Sci. Rep. 2017, 7, 6903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, Y.; Bao, T.; Yu, M.; Xu, M.; Guo, Y.; Wang, Y.; Zhang, C.; Zhao, B. Antidepressant-like effects of acupuncture involved the ERK signaling pathway in rats. BMC Complement. Altern. Med. 2016, 16, 380. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Li, C.F.; Chen, S.J.; Liang, W.N.; Wang, M.; Wang, S.S.; Dong, S.Q.; Yi, L.T.; Li, C.D. The antidepressant-like effects of Chaihu Shugan San: Dependent on the hippocampal BDNF-TrkB-ERK/Akt signaling activation in perimenopausal depression-like rats. Biomed. Pharmacother. 2018, 105, 45–52. [Google Scholar] [CrossRef]
- Man, H.Y.; Wang, Q.; Lu, W.Y.; Ju, W.; Ahmadian, G.; Liu, L.; D’Souza, S.; Wong, T.P.; Taghibiglou, C.; Lu, J.; et al. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron 2003, 38, 611–624. [Google Scholar] [CrossRef]
- Stornetta, R.L.; Zhu, J.J. Ras and Rap signaling in synaptic plasticity and mental disorders. Neuroscientist 2011, 17, 54–78. [Google Scholar] [CrossRef]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Duman, R.S.; Voleti, B. Signaling pathways underlying the pathophysiology and treatment of depression: Novel mechanisms for rapid-acting agents. Trends Neurosci. 2012, 35, 47–56. [Google Scholar] [CrossRef]
- Tomita, H.; Ziegler, M.E.; Kim, H.B.; Evans, S.J.; Choudary, P.V.; Li, J.Z.; Meng, F.; Dai, M.; Neal, C.R.; Myers, R.M.; et al. G protein-linked signaling pathways in bipolar and major depressive disorders. Front. Genet. 2013, 4, 297. [Google Scholar] [CrossRef]
- Plattner, F.; Hayashi, K.; Hernández, A.; Benavides, D.R.; Tassin, T.C.; Tan, C.; Day, J.; Fina, M.W.; Yuen, E.Y.; Yan, Z.; et al. The role of ventral striatal cAMP signaling in stress-induced behaviors. Nat. Neurosci. 2015, 18, 1094. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.C.; Sur, M.; Dobkin, B.H.; O’Brien, C.; Sanger, T.D.; Trojanowski, J.Q.; Rumsey, J.M.; Hicks, R.; Cameron, J.; Chen, D.; et al. Harnessing neuroplasticity for clinical applications. Brain 2011, 134, 1591–1609. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.J.; Boulle, F.; Steinbusch, H.W.; van den Hove, D.L.; Kenis, G.; Lanfumey, L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 2018, 235, 2195–2220. [Google Scholar] [CrossRef] [PubMed]
- Vilar, M.; Mira, H. Regulation of neurogenesis by neurotrophins during adulthood: Expected and unexpected roles. Front. Neurosci. 2016, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Seib, D.R.; Martin-Villalba, A. Neurogenesis in the normal ageing hippocampus: A mini-review. Gerontology 2015, 61, 327–335. [Google Scholar] [CrossRef]
- Eisch, A.J.; Petrik, D. Depression and hippocampal neurogenesis: A road to remission? Science 2012, 338, 72–75. [Google Scholar] [CrossRef]
- Bremner, J.D.; Narayan, M.; Anderson, E.R.; Staib, L.H.; Miller, H.L.; Charney, D.S. Hippocampal volume reduction in major depression. Am. J. Psychiatry 2000, 157, 115–118. [Google Scholar] [CrossRef]
- Cole, J.; Costafreda, S.G.; McGuffin, P.; Fu, C.H. Hippocampal atrophy in first episode depression: A meta-analysis of magnetic resonance imaging studies. J. Affect. Disord. 2011, 134, 483–487. [Google Scholar] [CrossRef]
- Frodl, T.; Schüle, C.; Schmitt, G.; Born, C.; Baghai, T.; Zill, P.; Bottlender, R.; Rupprecht, R.; Bondy, B.; Reiser, M.; et al. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch. Gen. Psychiatry 2007, 64, 410–416. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894. [Google Scholar] [CrossRef] [PubMed]
- Castrén, E.; Rantamäki, T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev. Neurobiol. 2010, 70, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog. Brain Res. 2000, 126, 413–431. [Google Scholar] [PubMed]
- Cook, S.C.; Wellman, C.L. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol. 2004, 60, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, C. Disorders of memory and plasticity in psychiatric disease. Dialogues Clin. Neurosci. 2013, 15, 455. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, D.M.; Wohleb, E.S.; Duman, R.S. Emerging treatment mechanisms for depression: Focus on glutamate and synaptic plasticity. Drug Discov. Today 2016, 21, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Castrén, E.; Hen, R. Neuronal plasticity and antidepressant actions. Trends Neurosci. 2013, 36, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Figurov, A.; Pozzo-Miller, L.D.; Olafsson, P.; Wang, T.; Lu, B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 1996, 381, 706. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, Y.; Hanse, E.; Kafitz, K.W.; Konnerth, A. Postsynaptic induction of BDNF-mediated long-term potentiation. Science 2002, 295, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.I.; Horiike, Y.; Matsuzaki, M.; Miyazaki, T.; Ellis-Davies, G.C.; Kasai, H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science 2008, 319, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Kempton, M.J.; Salvador, Z.; Munafò, M.R.; Geddes, J.R.; Simmons, A.; Frangou, S.; Williams, S.C. Structural neuroimaging studies in major depressive disorder: Meta-analysis and comparison with bipolar disorder. Arch. Gen. Psychiatry 2011, 68, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.R.; Hen, R. The current state of the neurogenic theory of depression and anxiety. Curr. Opin. Neurobiol. 2015, 30, 51–58. [Google Scholar] [CrossRef]
- Arnone, D.; McIntosh, A.M.; Ebmeier, K.P.; Munafò, M.R.; Anderson, I.M. Magnetic resonance imaging studies in unipolar depression: Systematic review and meta-regression analyses. Eur. Neuropsychopharmacol. 2012, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Videbech, P.; Ravnkilde, B. Hippocampal volume and depression: A meta-analysis of MRI studies. Am. J. Psychiatry 2004, 161, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, C.; Duman, R.S. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology 2008, 33, 88. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Diamond, D.M. The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 2002, 3, 453. [Google Scholar] [CrossRef] [PubMed]
- Goldwater, D.S.; Pavlides, C.; Hunter, R.G.; Bloss, E.B.; Hof, P.R.; McEwen, B.S.; Morrison, J.H. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience 2009, 164, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Anwyl, R.; Rowan, M.J. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature 1997, 387, 497. [Google Scholar] [CrossRef] [PubMed]
- Fuenmayor, C.A.; Zuluaga-Domínguez, C.M.; Díaz-Moreno, A.C.; Quicazán, M.C. ‘Miel de Angelita’: Nutritional composition and physicochemical properties of Tetragonisca angustula honey. Interciencia 2012, 37, 142–147. [Google Scholar]
- Manzanares, A.B.; García, Z.H.; Galdón, B.R.; Rodríguez, E.R.; Romero, C.D. Physicochemical characteristics of minor monofloral honeys from Tenerife, Spain. LWT-Food Sci. Technol. 2014, 55, 572–578. [Google Scholar] [CrossRef]
- Ismail, W.W. A review on beekeeping in Malaysia: History, importance and future directions. J. Sustain. Sci. Manag. 2016, 11, 70–80. [Google Scholar]
- Bakar, M.A.; Sanusi, S.B.; Bakar, F.A.; Cong, O.J.; Mian, Z. Physicochemical and antioxidant potential of raw unprocessed honey from Malaysian stingless bees. Pak. J. Nutr. 2017, 16, 888–894. [Google Scholar] [CrossRef]
- Mustafa, M.Z.; Yaacob, N.S.; Sulaiman, S.A. Reinventing the honey industry: Opportunities of the stingless bee. Malays. J. Med. Sci. 2018, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Litwack, G. Chapter 13—Metabolism of Amino Acids. In Human Biochemistry; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- O’Neill, E.; Harkin, A. Targeting the noradrenergic system for anti-inflammatory and neuroprotective effects: Implications for Parkinson’s disease. Neural Regen. Res. 2018, 13, 1332. [Google Scholar] [PubMed]
- Mahar, I.; Bambico, F.R.; Mechawar, N.; Nobrega, J.N. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 2014, 38, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Gotlib, I.H.; Joormann, J. Cognition and depression: Current status and future directions. Annu. Rev. Clin. Psychol. 2010, 6, 285–312. [Google Scholar] [CrossRef] [PubMed]
- Belujon, P.; Grace, A.A. Dopamine system dysregulation in major depressive disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Mallik, S.B.; Mudgal, J.; Nampoothiri, M.; Hall, S.; Anoopkumar-Dukie, S.; Grant, G.; Rao, C.M.; Arora, D. Caffeic acid attenuates lipopolysaccharide-induced sickness behaviour and neuroinflammation in mice. Neurosci. Lett. 2016, 632, 218–223. [Google Scholar] [CrossRef]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Mahmoud, A.M. Stingless bee honey protects against lipopolysaccharide induced-chronic subclinical systemic inflammation and oxidative stress by modulating Nrf2, NF-κB and p38 MAPK. Nutr. Metab. 2019, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Uthurry, C.A.; Hevia, D.; Gomez-Cordoves, C. Role of honey polyphenols in health. J. ApiProduct ApiMedical Sci. 2011, 3, 141–159. [Google Scholar] [CrossRef]
- Khalil, M.I.; Alam, N.; Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. Phenolic acid composition and antioxidant properties of Malaysian honeys. J. Food Sci. 2011, 76, C921–C928. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Facino, R.M. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement. Altern. Med. 2013, 13, 43. [Google Scholar] [CrossRef]
- Esposito, E.; Rotilio, D.; Di Matteo, V.; Di Giulio, C.; Cacchio, M.; Algeri, S. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol. Aging 2002, 23, 719–735. [Google Scholar] [CrossRef]
- Lau, F.C.; Shukitt-Hale, B.; Joseph, J.A. The beneficial effects of fruit polyphenols on brain aging. Neurobiol. Aging 2005, 26, 128–132. [Google Scholar] [CrossRef]
- Schmitt-Schillig, S.; Schaffer, S.; Weber, C.C.; Eckert, G.P.; Muller, W.E. Flavonoids and the aging brain. J. Physiol. Pharmacol. Suppl. 2005, 56, 23–36. [Google Scholar]
- Azman, K.F.; Zakaria, R.; Abdul Aziz, C.B.; Othman, Z. Tualang honey attenuates noise stress-induced memory deficits in aged rats. Oxidative Med. Cell. Longev. 2016, 2016, 1549158. [Google Scholar] [CrossRef]
- Kubera, M.; Obuchowicz, E.; Goehler, L.; Brzeszcz, J.; Maes, M. In animal models, psychosocial stress-induced (neuro) inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 744–759. [Google Scholar] [CrossRef]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.I.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Hritcu, L.; Ionita, R.; Postu, P.A.; Gupta, G.K.; Turkez, H.; Lima, T.C.; Carvalho, C.U.S.; de Sousa, D.P. Antidepressant flavonoids and their relationship with oxidative stress. Oxidative Med. Cell. Longev. 2017, 2017, 5762172. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, M.H.; El-Mahdy, M.A.; Abd-Ellah, M.F.; Helal, G.K.; Khalifa, F.; Hamada, F.M.A. Influence of p-coumaric acid on doxorubicin-induced oxidative stress in rat’s heart. Pharmacol. Res. 2003, 48, 461–465. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Zhu, S.T.; Harris, P.J. Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells. Mol. Nutr. Food Res. 2005, 49, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Corona, G.; Spencer, J.P. Caffeic acid, tyrosol and p-coumaric acid are potent inhibitors of 5-S-cysteinyl-dopamine induced neurotoxicity. Arch. Biochem. Biophys. 2010, 501, 106–111. [Google Scholar] [CrossRef]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Del Fabbro, L.; de Gomes, M.G.; Goes, A.T.R.; Souza, L.C.; Giacomeli, R.; Antunes, M.; Luchese, C.; et al. Neurochemical factors associated with the antidepressant-like effect of flavonoid chrysin in chronically stressed mice. Eur. J. Pharmacol. 2016, 791, 284–296. [Google Scholar] [CrossRef]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Del Fabbro, L.; de Gomes, M.G.; Goes, A.T.R.; Souza, L.C.; Boeira, S.P. Chrysin promotes attenuation of depressive-like behavior and hippocampal dysfunction resulting from olfactory bulbectomy in mice. Chem. Biol. Interact. 2016, 260, 154–162. [Google Scholar] [CrossRef]
- Yoon, J.H.; Youn, K.; Ho, C.T.; Karwe, M.V.; Jeong, W.S.; Jun, M. p-Coumaric acid and ursolic acid from corni fructus attenuated β-Amyloid25–35-induced toxicity through regulation of the NF-κB signaling pathway in PC12 cells. J. Agric. Food Chem. 2014, 62, 4911–4916. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.; Yousef, A.I.; El-Twab, A.; Sanaa, M.; Abdel Reheim, E.S.; Ashour, M.B. Gallic acid and p-coumaric acid attenuate type 2 diabetes-induced neurodegeneration in rats. Metab. Brain Dis. 2017, 32, 1279–1286. [Google Scholar] [CrossRef]
- Ekinci-Akdemir, F.N.; Gülçin, I.; Gürsul, C.; Alwasel, S.H.; Bayir, Y. Effect of p-coumaric acid against oxidative stress induced by cisplatin in brain tissue of rats. J. Anim. Plant Sci. 2017, 27, 1560–1564. [Google Scholar]
- Sakamula, R.; Thong-Asa, W. Neuroprotective effect of p-coumaric acid in mice with cerebral ischemia reperfusion injuries. Metab. Brain Dis. 2018, 33, 765–773. [Google Scholar] [CrossRef]
- Chhillar, R.; Dhingra, D. Antidepressant-like activity of gallic acid in mice subjected to unpredictable chronic mild stress. Fundam. Clin. Pharmacol. 2013, 27, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Pemminati, S.; Shetty, B.S.; Gopalakrishna, H.N.; Bethi, Y.; Rao, D.; Udaykumar, J.; Rai, A.; Shenoy, A.K. Evaluation of antidepressant activity of gallic acid in mice. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 575–580. [Google Scholar]
- Can, Ö.D.; Turan, N.; Özkay, Ü.D.; Öztürk, Y. Antidepressant-like effect of gallic acid in mice: Dual involvement of serotonergic and catecholaminergic systems. Life Sci. 2017, 190, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Bortolotto, V.C.; Pinheiro, F.C.; Araujo, S.M.; Poetini, M.R.; Bertolazi, B.S.; Mariane Trindade de Paula, M.; Meichtry, L.B.; Polet de Almeida, F.; Shanda de Freitas, C.; Jesse, C.R.; et al. Chrysin reverses the depressive-like behavior induced by hypothyroidism in female mice by regulating hippocampal serotonin and dopamine. Eur. J. Pharmacol. 2018, 822, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Ehrhart, J.; Bai, Y.; Sanberg, P.R.; Bickford, P.; Tan, J.; Shytle, R.D. Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J. Neuroinflamm. 2008, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shahamat, Z.; Abbasi-Maleki, S.; Motamed, S.M. Evaluation of antidepressant-like effects of aqueous and ethanolic extracts of Pimpinella anisum fruit in mice. Avicenna J. Phytomed. 2016, 6, 322. [Google Scholar] [PubMed]
- Li, R.; Wang, X.; Qin, T.; Qu, R.; Ma, S. Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1β production and NLRP3 inflammasome activation in the rat brain. Behav. Brain Res. 2016, 296, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bu, H.; Jiang, Y.; Sun, G.; Jiang, R.; Huang, X.; Duan, H.; Huang, Z.; Wu, Q. The antidepressant effects of apigenin are associated with the promotion of autophagy via the mTOR/AMPK/ULK1 pathway. Mol. Med. Rep. 2019, 20, 2867–2874. [Google Scholar] [CrossRef]
- Balez, R.; Steiner, N.; Engel, M.; Muñoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’Connor, M.; et al. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 2016, 6, 31450. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Garg, V.K.; Buttar, H.S.; Setzer, W.N.; Sethi, G. Apigenin: A natural bioactive flavone-type molecule with promising therapeutic function. J. Funct. Foods 2018, 48, 457–471. [Google Scholar] [CrossRef]
- Nakazawa, T.; Yasuda, T.; Ueda, J.; Ohsawa, K. Antidepressant-like effects of apigenin and 2,4,5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol. Pharm. Bull. 2003, 26, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.T.; Li, J.M.; Li, Y.C.; Pan, Y.; Xu, Q.; Kong, L.D. Antidepressant-like behavioral and neurochemical effects of the citrus-associated chemical apigenin. Life Sci. 2008, 82, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhao, D.; Qu, R.; Fu, Q.; Ma, S. The effects of apigenin on lipopolysaccharide-induced depressive-like behavior in mice. Neurosci. Lett. 2015, 594, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Guo, X.; Li, Y.; Yang, X.; Han, Y. Apigenin reverses depression-like behavior induced by chronic corticosterone treatment in mice. Eur. J. Pharmacol. 2016, 774, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Anusha, C.; Sumathi, T.; Joseph, L.D. Protective role of apigenin on rotenone induced rat model of Parkinson’s disease: Suppression of neuroinflammation and oxidative stress mediated apoptosis. Chem. Biol. Interact. 2017, 269, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.W.; Liu, X.; Yepes, M.; Shepherd, K.R.; Miller, G.W.; Liu, Y.; Wilson, W.D.; Xiao, G.; Blanchi, B.; Sun, Y.E.; et al. A selective TrkB agonist with potent neurotrophic activities by 7, 8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA 2010, 107, 2687–2692. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ogunsuyi, O.B.; Ogunbadejo, M.D.; Adefegha, S.A. Influence of gallic acid on α-amylase and α-glucosidase inhibitory properties of acarbose. J. Food Drug Anal. 2016, 24, 627–634. [Google Scholar] [CrossRef]
- Wurzelmann, M.; Romeika, J.; Sun, D. Therapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury. Neural Regen. Res. 2017, 12, 7. [Google Scholar]
- Rahvar, M.; Owji, A.A.; Mashayekhi, F.J. Effect of quercetin on the brain-derived neurotrophic factor gene expression in the rat brain. Bratisl. Lek. Listy 2018, 119, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Caviedes, A.; Lafourcade, C.; Soto, C.; Wyneken, U. BDNF/NF-κB Signaling in the Neurobiology of Depression. Curr. Pharm. Des. 2017, 23, 3154–3163. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Borsato, D.M.; Prudente, A.S.; Doell-Boscardin, P.M.; Borsato, A.V.; Luz, C.F.; Maia, B.H.; Cabrini, D.A.; Otuki, M.F.; Miguel, M.D.; Farago, P.V.; et al. Topical anti-inflammatory activity of a monofloral honey of Mimosa scabrella provided by Melipona marginata during winter in Southern Brazil. J. Med. Food 2014, 17, 817–825. [Google Scholar] [CrossRef]
- Kaster, M.P.; Gadotti, V.M.; Calixto, J.B.; Santos, A.R.; Rodrigues, A.L.S. Depressive-like behavior induced by tumor necrosis factor-α in mice. Neuropharmacology 2012, 62, 419–426. [Google Scholar] [CrossRef]
- Tavares, R.G.; Schmidt, A.P.; Abud, J.; Tasca, C.I.; Souza, D.O. In vivo quinolinic acid increases synaptosomal glutamate release in rats: Reversal by guanosine. Neurochem. Res. 2005, 30, 439–444. [Google Scholar] [CrossRef]
- McNally, L.; Bhagwagar, Z.; Hannestad, J. Inflammation, glutamate, and glia in depression: A literature review. CNS Spectr. 2008, 13, 501–510. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Lawson, M.A.; Kelley, K.W. Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology 2011, 36, 426–436. [Google Scholar] [CrossRef]
- Yu, S.; Holsboer, F.; Almeida, O.F. Neuronal actions of glucocorticoids: Focus on depression. J. Steroid Biochem. Mol. Biol. 2008, 108, 300–309. [Google Scholar] [CrossRef]
- Zappella, M.; Biamonte, F.; Balzamino, B.O.; Manieri, R.; Cortes, M.; Santucci, D.; Di Stasio, E.; Rizzuto, M.; Micera, A. Relaxation Response in Stressed Volunteers: Psychometric Tests and Neurotrophin Changes in Biological Fluids. Front. Psychiatry 2021, 12, 790. [Google Scholar] [CrossRef]
- Sadighparvar, S.; Darband, S.G.; Yousefi, B.; Kaviani, M.; Ghaderi-Pakdel, F.; Mihanfar, A.; Babaei, G.; Mobaraki, K.; Majidinia, M. Combination of quercetin and exercise training attenuates depression in rats with 1,2-dimethylhydrazine-induced colorectal cancer: Possible involvement of inflammation and BDNF signalling. Exp. Physiol. 2020, 105, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Yao, W.; Hashimoto, K. Brain-derived Neurotrophic Factor (BDNF)-TrkB Signaling in Inflammation-related Depression and Potential Therapeutic Targets. Curr. Neuropharmacol. 2016, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Felger, J.C.; Miller, A.H. Inflammation and treatment resistance in major depression: A perfect storm. Psychiatr. Times 2013, 30, 17. [Google Scholar]
- Strawbridge, R.; Young, A.H.; Cleare, A.J. Inflammation as a marker of clinical response to treatment: A focus on treatment-resistant depression. In Inflammation and Immunity in Depression; Academic Press: Cambridge, MA, USA, 2018; pp. 473–487. [Google Scholar]
- Strawbridge, R.; Hodsoll, J.; Powell, T.R.; Hotopf, M.; Hatch, S.L.; Breen, G.; Cleare, A.J. Inflammatory profiles of severe treatment-resistant depression. J. Affect. Disord. 2019, 246, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.I.; Strawbridge, R.; Stokes, P.R.; Young, A.H. Anti-inflammatory treatments for mood disorders: Systematic review and meta-analysis. J. Psychopharmacol. 2017, 31, 1137–1148. [Google Scholar] [CrossRef]

| Type | Compounds | Therapeutic Effects | ||
|---|---|---|---|---|
| Phenolic acids | p-Coumaric acid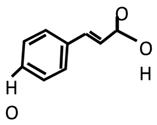 | Anti-inflammatory | Antidepressant | Antioxidant |
| [49,170,171] | [49] | [171,172,173] | ||
Gallic acid | [171] | [174,175,176] | [171,174] | |
Caffeic acid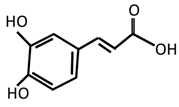 | [149] | |||
| Flavonoids | Chrysin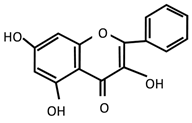 | [168,169] | [177] | [168,169,177] |
Apigenin | [178,179,180,181,182,183] | [180,181,184,185,186,187] | [180,183,188] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, F.H.; Samhani, I.; Mustafa, M.Z.; Shafin, N. Pathophysiology of Depression: Stingless Bee Honey Promising as an Antidepressant. Molecules 2022, 27, 5091. https://doi.org/10.3390/molecules27165091
Zakaria FH, Samhani I, Mustafa MZ, Shafin N. Pathophysiology of Depression: Stingless Bee Honey Promising as an Antidepressant. Molecules. 2022; 27(16):5091. https://doi.org/10.3390/molecules27165091
Chicago/Turabian StyleZakaria, Fatin Haniza, Ismail Samhani, Mohd Zulkifli Mustafa, and Nazlahshaniza Shafin. 2022. "Pathophysiology of Depression: Stingless Bee Honey Promising as an Antidepressant" Molecules 27, no. 16: 5091. https://doi.org/10.3390/molecules27165091
APA StyleZakaria, F. H., Samhani, I., Mustafa, M. Z., & Shafin, N. (2022). Pathophysiology of Depression: Stingless Bee Honey Promising as an Antidepressant. Molecules, 27(16), 5091. https://doi.org/10.3390/molecules27165091