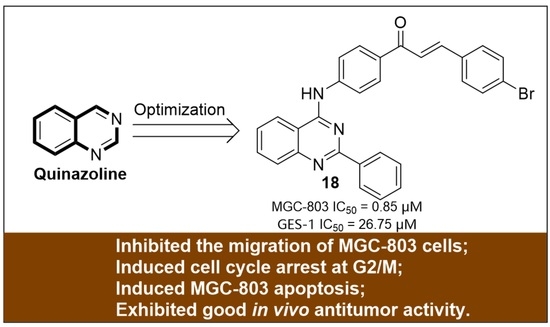
Discovery of Novel Quinazoline Derivatives as Potent Antitumor Agents
Abstract
:1. Introduction
2. Results and Discussion
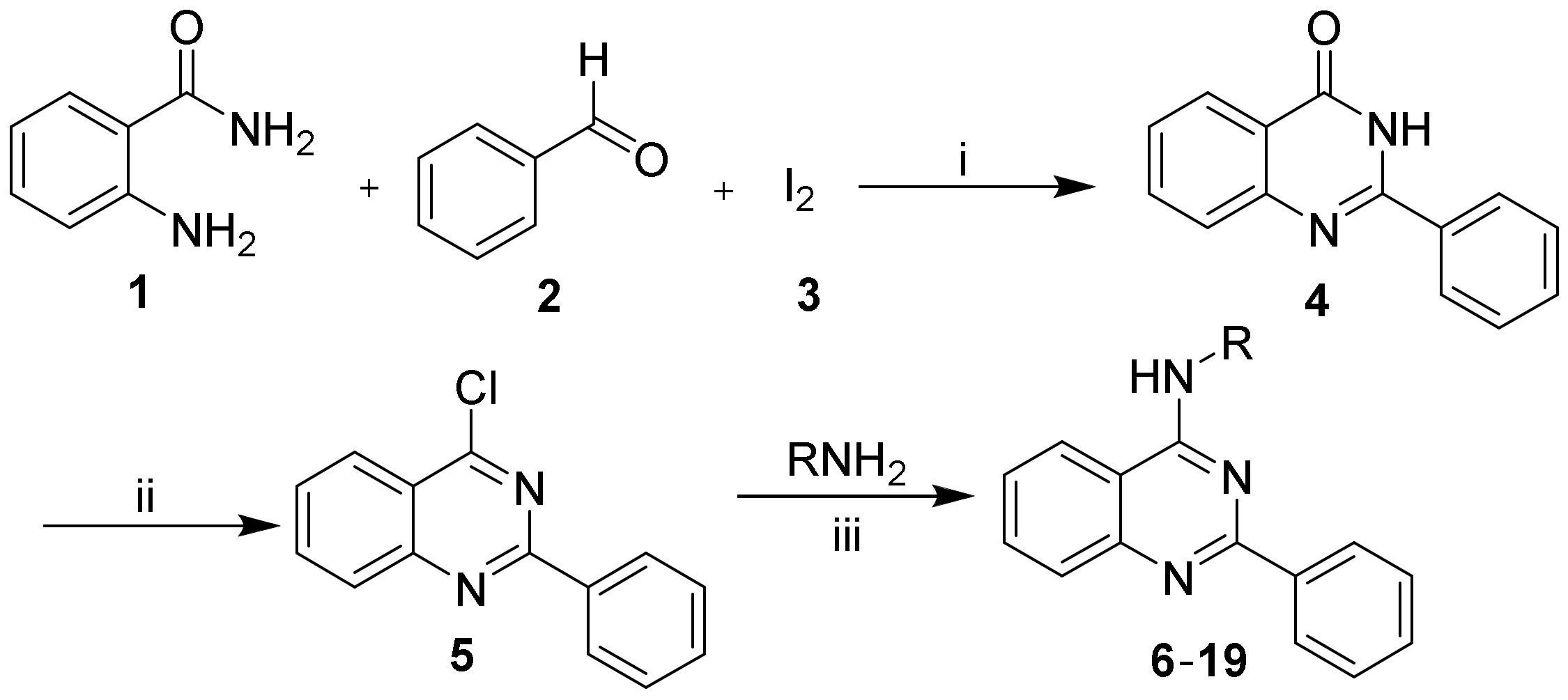
2.1. Chemistry
2.2. Biological Evaluation
2.3. The Effect of Compound 18 on the Migration of MGC-803 Cells
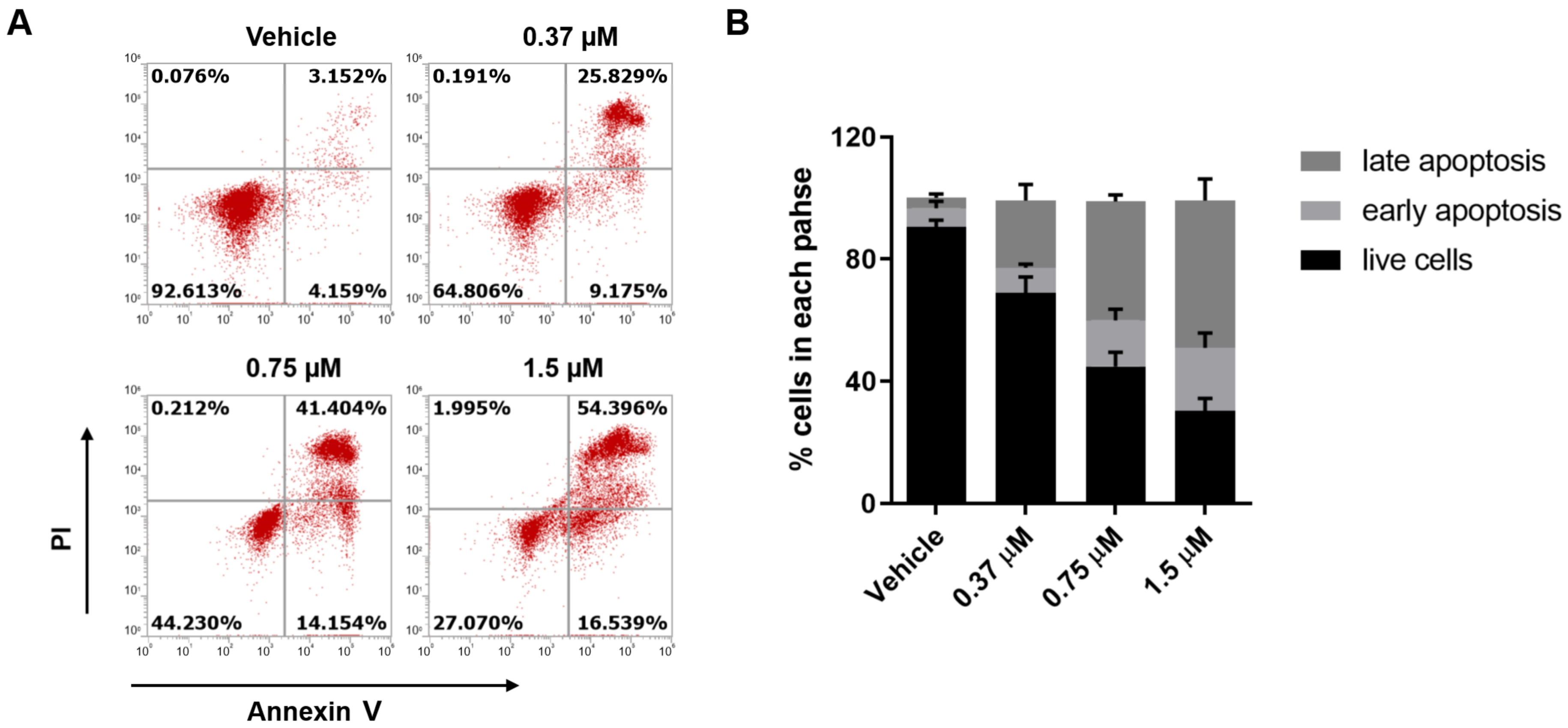
2.4. The Effect of Compound 18 on Apoptosis of MGC-803 Cells
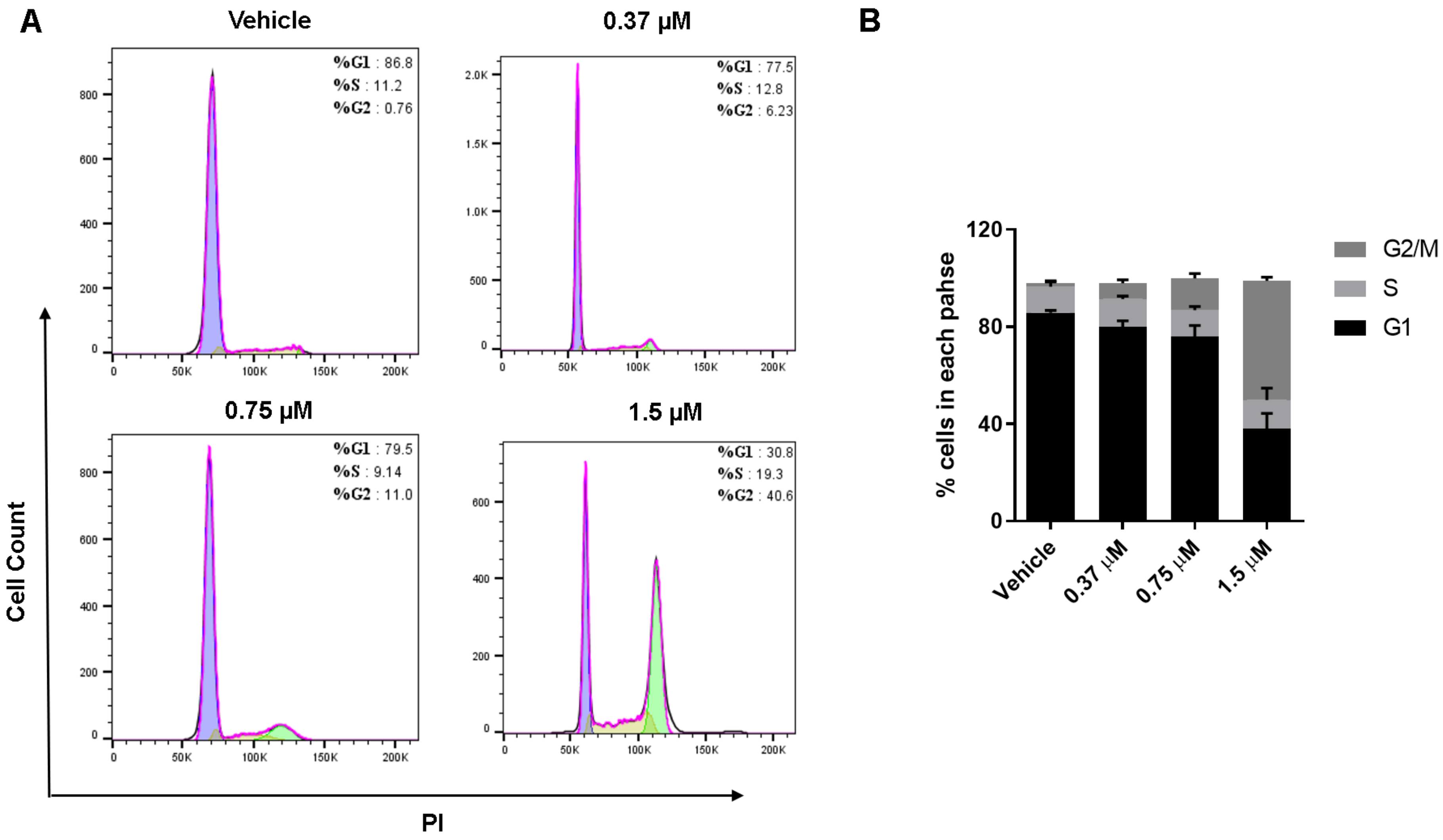
2.5. The Effect of Compound 18 on Cell Cycle Distribution of MGC-803 Cells
2.6. Acute Toxicity Assay
2.7. The Anti-Proliferative Effect of 18 on Gastric Cancer Xenograft Model
3. Experimental Section
3.1. General Information
3.2. Synthesis of Compounds 4 and 5
3.3. Synthesis of Compounds 6–19
3.4. Cell Culture
3.5. MTT Assay
3.6. Migration Assay
3.7. Cell Apoptosis
3.8. Cell Cycle
3.9. Western Blot
3.10. Acute Toxicity Assay
3.11. In Vivo Anti-Tumor Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Macri, G.F.; Greco, A.; Gallo, A.; Fusconi, M.; Marinelli, C.; de Vincentiis, M. Use of electrochemotherapy in a case of neck skin metastasis of oral squamous cell carcinoma: Case report and considerations. Head Neck 2014, 36, E86–E90. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, M.; Kini, S.G. A Review on: The Design and Development of EGFR Tyrosine Kinase Inhibitors in Cancer Therapy. Int. J. Ther. Appl. 2012, 5, 29–37. [Google Scholar]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Ann. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.H.; Chen, Z.S. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist. Updates 2016, 27, 14–29. [Google Scholar] [CrossRef]
- Dong, J.; Qin, Z.; Zhang, W.D.; Cheng, G.; Yehuda, A.G.; Ashby, C.R., Jr.; Chen, Z.S.; Cheng, X.D.; Qin, J.J. Medicinal chemistry strategies to discover P-glycoprotein inhibitors: An update. Drug Resist. Updates 2020, 49, 100681. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S.Q.; Teng, Q.X.; Yang, L.; Lei, Z.N.; Yuan, X.H.; Huo, J.F.; Chen, X.B.; Wang, M.; Yu, B.; et al. Structure-Based Design, Synthesis, and Biological Evaluation of New Triazolo[1,5-a]Pyrimidine Derivatives as Highly Potent and Orally Active ABCB1 Modulators. J. Med. Chem. 2020, 63, 15979–15996. [Google Scholar] [CrossRef] [PubMed]
- Mermer, A.; Keles, T.; Sirin, Y. Recent studies of nitrogen containing heterocyclic compounds as novel antiviral agents: A review. Bioorg. Chem. 2021, 114, 105076. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Kvasnica, M.; Urban, M.; Dickinson, N.J.; Sarek, J. Pentacyclic triterpenoids with nitrogen- and sulfur-containing heterocycles: Synthesis and medicinal significance. Nat. Prod. J. 2015, 32, 1303–1330. [Google Scholar] [CrossRef] [PubMed]
- Pathania, S.; Narang, R.K.; Rawal, R.K. Role of sulphur-heterocycles in medicinal chemistry: An update. Eur. J. Med. Chem. 2019, 180, 486–508. [Google Scholar] [CrossRef] [PubMed]
- Seboletswe, P.; Awolade, P.; Singh, P. Recent Developments on the Synthesis and Biological Activities of Fused Pyrimidinone Derivatives. ChemMedChem 2021, 16, 2050–2067. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Mlala, S.; Oyedeji, O.O.; Aderibigbe, B.A. Pentacyclic Triterpenoids with Nitrogen-Containing Heterocyclic Moiety, Privileged Hybrids in Anticancer Drug Discovery. Molecules 2021, 26, 2401. [Google Scholar] [CrossRef] [PubMed]
- Seth, S. A Comprehensive Review on Recent advances in Synthesis & Pharmacotherapeutic potential of Benzothiazoles. AntiInflamm. Antiallergy Agents Med. Chem. 2015, 14, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef]
- Sak, K. Chemotherapy and dietary phytochemical agents. Chemother. Res. Pract. 2012, 2012, 282570. [Google Scholar] [CrossRef] [Green Version]
- Shagufta; Ahmad, I. An insight into the therapeutic potential of quinazoline derivatives as anticancer agents. MedChemComm 2017, 8, 871–885. [Google Scholar] [CrossRef]
- Bibek Pati, S.B. Quinazolines: An Illustrated Review. J. Adv. Pharm. Educ. Res. 2013, 3, 136–151. [Google Scholar]
- Verhaeghe, P.; Azas, N.; Gasquet, M.; Hutter, S.; Ducros, C.; Laget, M.; Rault, S.; Rathelot, P.; Vanelle, P. Synthesis and antiplasmodial activity of new 4-aryl-2-trichloromethylquinazolines. Bioorg. Med. Chem. Lett. 2008, 18, 396–401. [Google Scholar] [CrossRef]
- Raghavendra, N.M.; Thampi, P.; Gurubasavarajaswamy, P.M.; Sriram, D. Synthesis and antimicrobial activities of some novel substituted 2-imidazolyl-N-(4-oxo-quinazolin-3(4H)-yl)-acetamides. Chem. Pharm. Bull. 2007, 55, 1615–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravanan, G.; Pannerselvam, P.; Prakash, C.R. Synthesis and anti-microbial screening of novel schiff bases of 3-amino-2-methyl quinazolin 4-(3H)-one. J. Adv. Pharm. Technol. Res. 2010, 1, 320–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alagarsamy, V.; Raja Solomon, V.; Sheorey, R.V.; Jayakumar, R. 3-(3-ethylphenyl)-2-substituted hydrazino-3H-quinazolin-4-one derivatives: New class of analgesic and anti-inflammatory agents. Chem. Biol. Drug Des. 2009, 73, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.A.; Adami, M.; Istyastono, E.P.; Zuiderveld, O.P.; van Dam, C.M.; de Kanter, F.J.; Jongejan, A.; Coruzzi, G.; Leurs, R.; de Esch, I.J. Synthesis and QSAR of quinazoline sulfonamides as highly potent human histamine H4 receptor inverse agonists. J. Med. Chem. 2010, 53, 2390–2400. [Google Scholar] [CrossRef]
- Georgey, H.; Abdel-Gawad, N.; Abbas, S. Synthesis and anticonvulsant activity of some quinazolin-4-(3H)-one derivatives. Molecules 2008, 13, 2557–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, N.B.; Patel, V.N.; Patel, H.R.; Shaikh, F.M.; Patel, J.C. Synthesis and microbial studies of (4-oxo-thiazolidinyl) sulfonamides bearing quinazolin-4(3H)ones. Acta Pol. Pharm. 2010, 67, 267–275. [Google Scholar]
- Zaranappa; Vagdevi, H.M.; Lokesh, M.R.; Gowdarshivannanavar, B.C. Synthesis and antioxidant activity of 3-substituted Schiff bases of quinazoline-2,4-diones. Int. J. ChemTech Res. 2012, 4, 1527–1533. [Google Scholar]
- Ismail, M.A.; Barker, S.; Abou el-Ella, D.A.; Abouzid, K.A.; Toubar, R.A.; Todd, M.H. Design and synthesis of new tetrazolyl- and carboxy-biphenylylmethyl-quinazolin-4-one derivatives as angiotensin II AT1 receptor antagonists. J. Med. Chem. 2006, 49, 1526–1535. [Google Scholar] [CrossRef]
- Wakeling, A.E.; Guy, S.P.; Woodburn, J.R.; Ashton, S.E.; Curry, B.J.; Barker, A.J.; Gibson, K.H. ZD1839 (Iressa): An orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002, 62, 5749–5754. [Google Scholar]
- Moyer, J.D.; Barbacci, E.G.; Iwata, K.K.; Arnold, L.; Boman, B.; Cunningham, A.; DiOrio, C.; Doty, J.; Morin, M.J.; Moyer, M.P.; et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997, 57, 4838–4848. [Google Scholar]
- Wedge, S.R.; Ogilvie, D.J.; Dukes, M.; Kendrew, J.; Chester, R.; Jackson, J.A.; Boffey, S.J.; Valentine, P.J.; Curwen, J.O.; Musgrove, H.L.; et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002, 62, 4645–4655. [Google Scholar] [PubMed]
- Li, D.; Ambrogio, L.; Shimamura, T.; Kubo, S.; Takahashi, M.; Chirieac, L.R.; Padera, R.F.; Shapiro, G.I.; Baum, A.; Himmelsbach, F.; et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008, 27, 4702–4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusnak, D.W.; Lackey, K.; Affleck, K.; Wood, E.R.; Alligood, K.J.; Rhodes, N.; Keith, B.R.; Murray, D.M.; Knight, W.B.; Mullin, R.J.; et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol. Cancer Ther. 2001, 1, 85–94. [Google Scholar] [PubMed]
- Ismail, R.S.M.; Ismail, N.S.M.; Abuserii, S.; Abou El Ella, D.A. Recent advances in 4-aminoquinazoline based scaffold derivatives targeting EGFR kinases as anticancer agents. Future J. Pharm. Sci. 2016, 2, 9–19. [Google Scholar] [CrossRef]
- Wang, C.; Qian, P.-C.; Chen, F.; Cheng, J. Rhodium-catalyzed [4+1] annulation of sulfoxonium ylides: Sequential ortho-C–H functionalization/carbonyl α-amination toward polycyclic quinazolinones. Tetrahedron Lett. 2020, 61, 152441. [Google Scholar] [CrossRef]
- Shi, X.J.; Wang, S.; Li, X.J.; Yuan, X.H.; Cao, L.J.; Yu, B.; Liu, H.M. Discovery of tofacitinib derivatives as orally active antitumor agents based on the scaffold hybridization strategy. Eur. J. Med. Chem. 2020, 203, 112601. [Google Scholar] [CrossRef]





| Compound | R | IC50 (μM) | ||||
|---|---|---|---|---|---|---|
| MGC-803 | MCF-7 | PC-9 | A549 | H1975 | ||
| 6 | 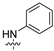 | 6.23 ± 0.12 | 12.62 ± 0.16 | 15.81 ± 0.52 | 10.31 ± 0.98 | 20.08 ± 1.62 |
| 7 | 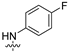 | 4.15 ± 1.16 | 8.75 ± 1.76 | 10.62 ± 2.16 | 4.82 ± 0.99 | 12.58 ± 2.89 |
| 8 | 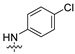 | 3.57 ± 0.89 | 6.96 ± 1.57 | 8.75 ± 1.59 | 6.58 ± 1.31 | 10.63 ± 2.46 |
| 9 |  | 1.89 ± 0.59 | 5.15 ± 1.16 | 4.31 ± 0.96 | 4.75 ± 1.36 | 6.65 ± 1.57 |
| 10 | 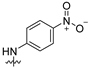 | 8.32 ± 2.06 | 12.15 ± 1.91 | 12.52 ± 2.83 | 15.65 ± 1.89 | 25.75 ± 2.20 |
| 11 |  | 10.96 ± 1.93 | 15.68 ± 1.82 | 20.75 ± 2.16 | 25.37 ± 3.11 | 22.88 ± 2.69 |
| 12 |  | 5.81 ± 2.41 | 10.63 ± 1.67 | 15.32 ± 1.53 | 8.56 ± 1.72 | 19.35 ± 2.38 |
| 13 |  | 4.98 ± 1.26 | 8.36 ± 1.92 | 6.83 ± 1.29 | 5.69 ± 1.68 | >30 |
| 14 |  | 2.12 ± 0.97 | 3.21 ± 2.28 | 5.35 ± 1.82 | 8.72 ± 1.53 | 13.17 ± 1.86 |
| 15 | 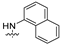 | 18.86 ± 2.82 | >30 | 15.37 ± 1.92 | 12.15 ± 1.59 | 10.39 ± 1.88 |
| 16 | 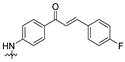 | 2.02 ± 0.38 | 3.96 ± 0.92 | 2.75 ± 0.66 | 1.98 ± 0.26 | 3.67 ± 0.93 |
| 17 | 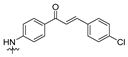 | 1.39 ± 0.26 | 2.37 ± 0.35 | 1.89 ± 0.67 | 1.46 ± 0.16 | 4.75 ± 0.81 |
| 18 | 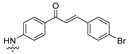 | 0.85 ± 0.14 | 1.61 ± 0.20 | 1.26 ± 0.18 | 1.54 ± 0.38 | 2.12 ± 0.52 |
| 19 | 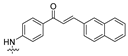 | 1.56 ± 0.92 | 1.96 ± 0.56 | 1.39 ± 0.25 | 2.55 ± 0.49 | 2.61 ± 0.59 |
| 5-Fu | --- | 4.11 ± 0.72 | 4.51 ± 1.23 | 3.68 ± 1.06 | 5.12 ± 1.73 | 2.68 ± 0.86 |
| Compound | IC50 (μM) GES-1 | Compound | IC50 (μM) GES-1 |
|---|---|---|---|
| 6 | >30 | 14 | 19.62 ± 2.58 |
| 7 | 20.82 ± 3.29 | 15 | >30 |
| 8 | >30 | 16 | 18.75 ± 1.56 |
| 9 | 18.57 ± 1.72 | 17 | 23.42 ± 2.83 |
| 10 | >30 | 18 | 26.75 ± 1.85 |
| 11 | 25.41 ± 3.51 | 19 | 19.82 ± 2.21 |
| 12 | 24.28 ± 1.96 | 5-Fu | 3.26 ± 1.07 |
| 13 | 21.31 ± 2.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Z.; Ma, S.; Zhang, L.; Liu, Q.; Zhang, S. Discovery of Novel Quinazoline Derivatives as Potent Antitumor Agents. Molecules 2022, 27, 3906. https://doi.org/10.3390/molecules27123906
Niu Z, Ma S, Zhang L, Liu Q, Zhang S. Discovery of Novel Quinazoline Derivatives as Potent Antitumor Agents. Molecules. 2022; 27(12):3906. https://doi.org/10.3390/molecules27123906
Chicago/Turabian StyleNiu, Zhenxi, Shuli Ma, Lei Zhang, Qibing Liu, and Shengnan Zhang. 2022. "Discovery of Novel Quinazoline Derivatives as Potent Antitumor Agents" Molecules 27, no. 12: 3906. https://doi.org/10.3390/molecules27123906
APA StyleNiu, Z., Ma, S., Zhang, L., Liu, Q., & Zhang, S. (2022). Discovery of Novel Quinazoline Derivatives as Potent Antitumor Agents. Molecules, 27(12), 3906. https://doi.org/10.3390/molecules27123906