Facile Solvent-Free Synthesis of Metal Thiophosphates and Their Examination as Hydrogen Evolution Electrocatalysts
Abstract
1. Introduction
2. Results and Discussion
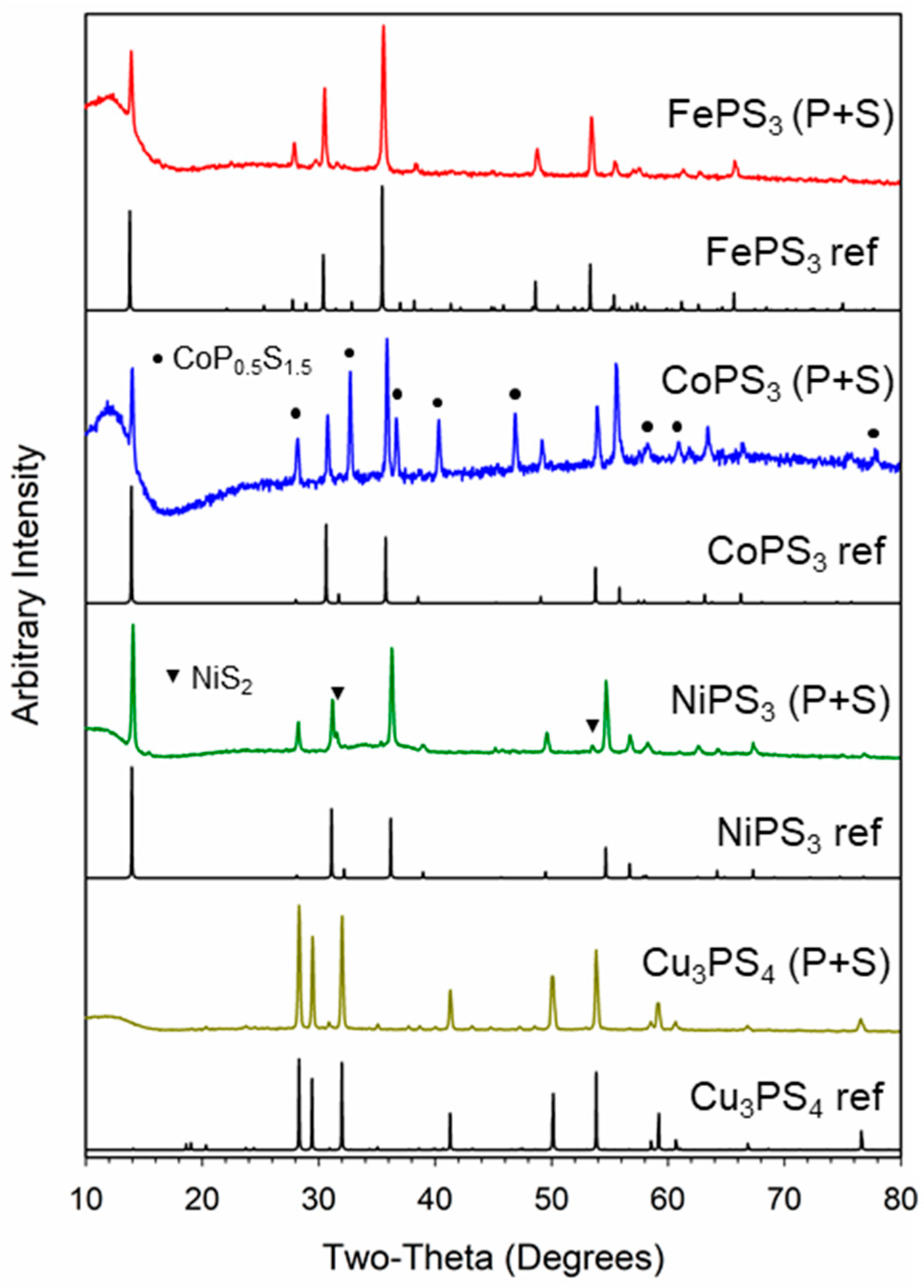
2.1. M-P-S Synthesis from Elemental Phosphorus and Sulfur
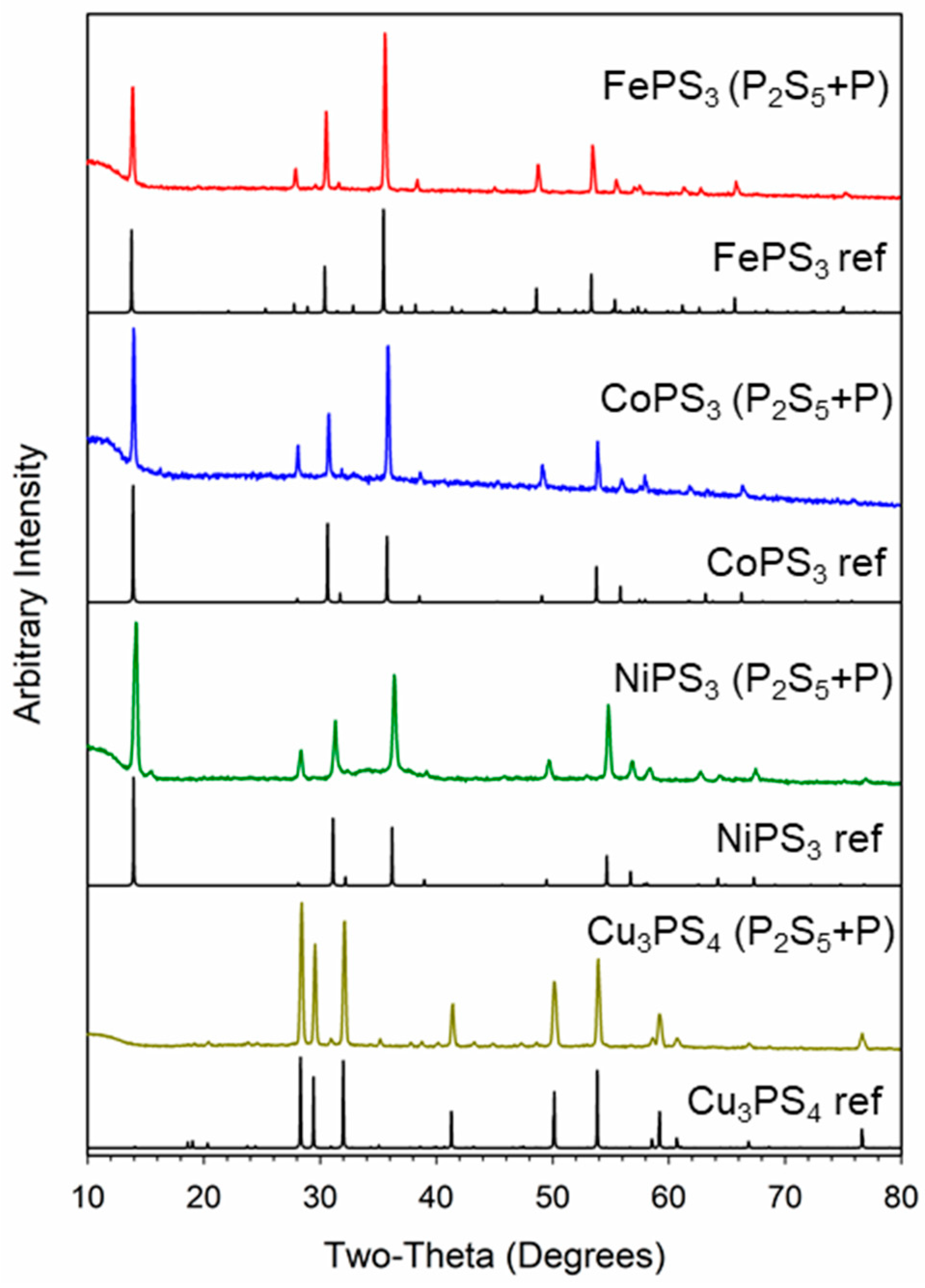
2.2. M-P-S Synthesis from Elemental P and P2S5
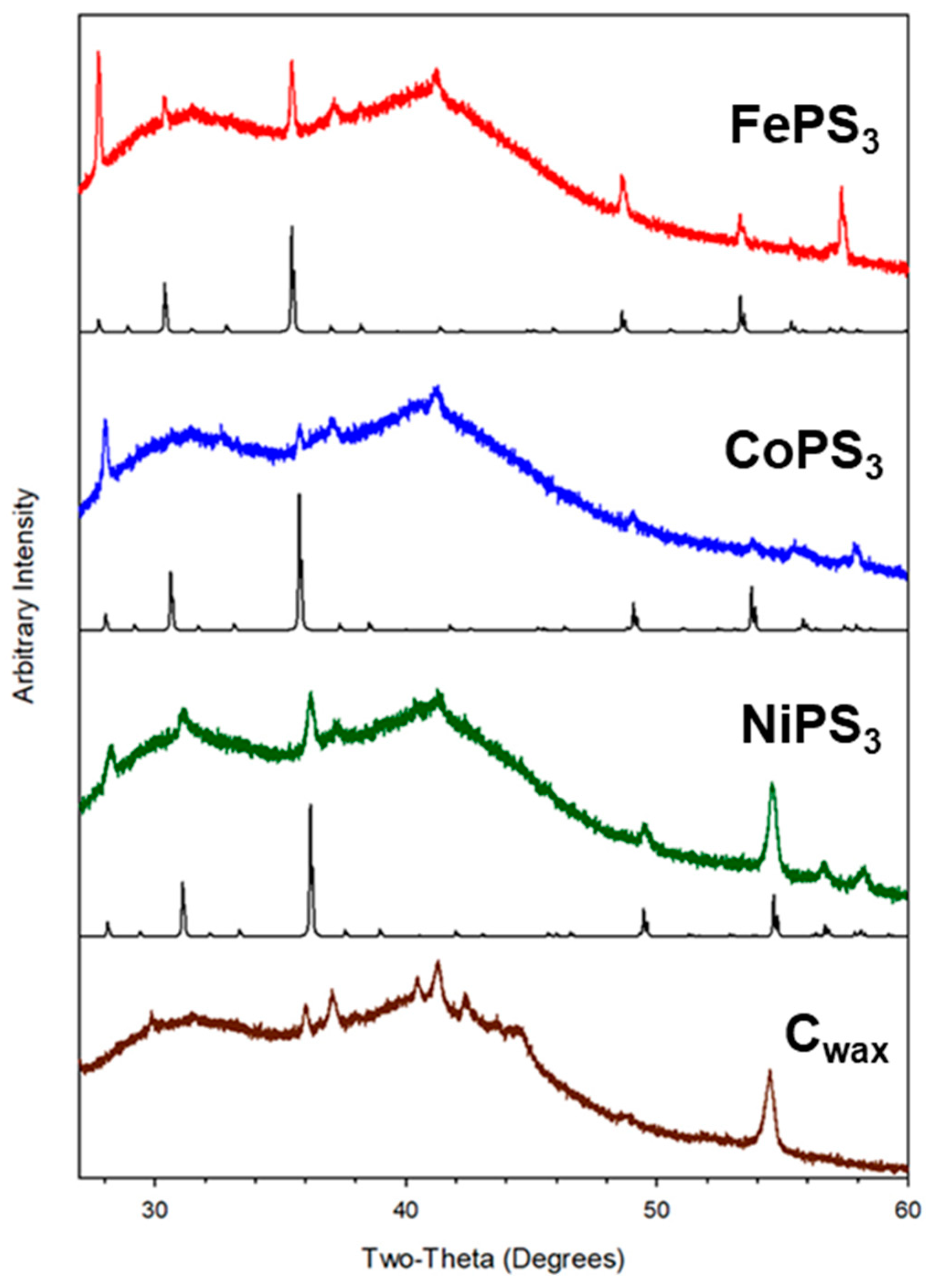
2.3. Compositional Analysis of M-P-S Products
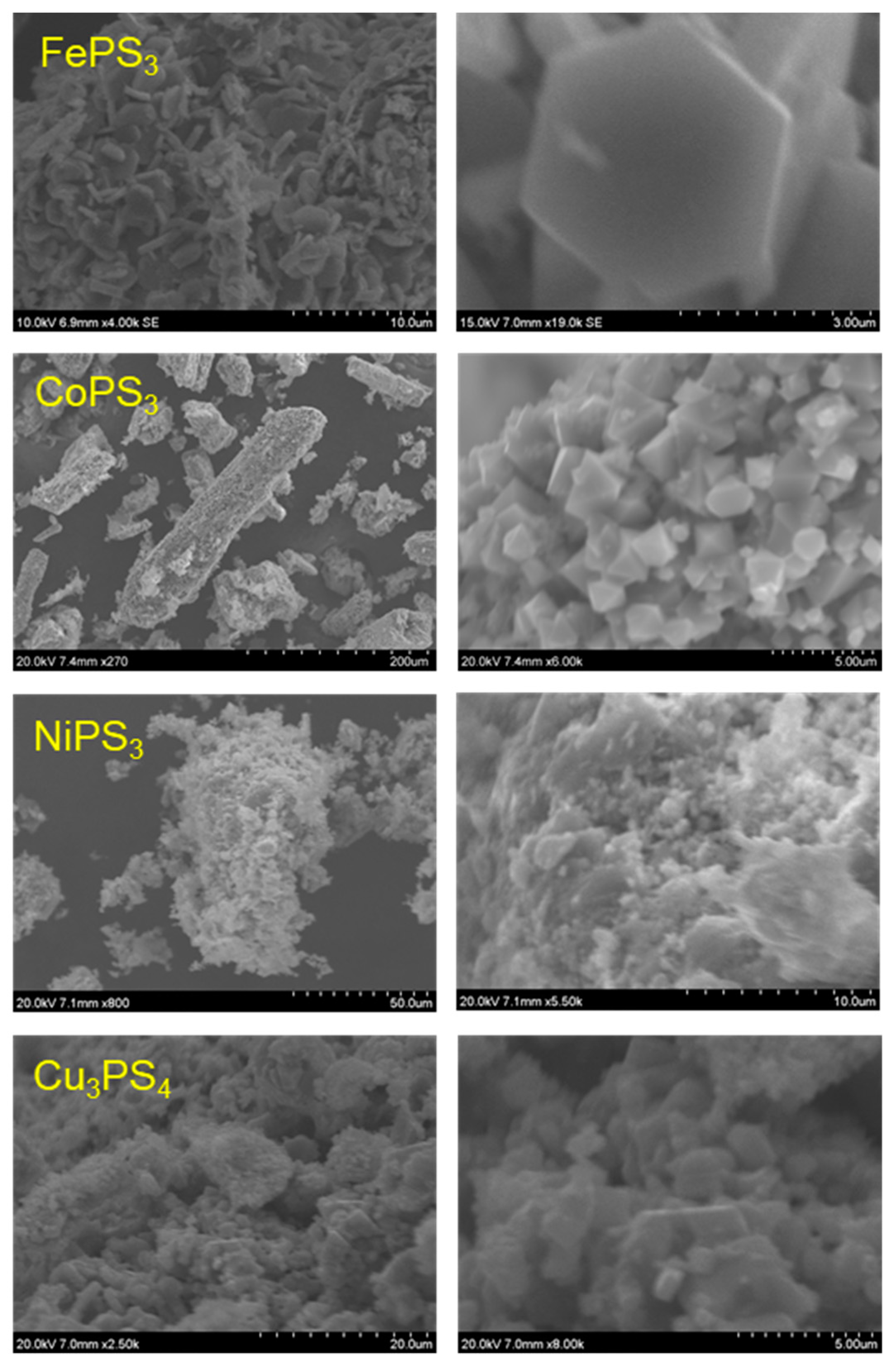
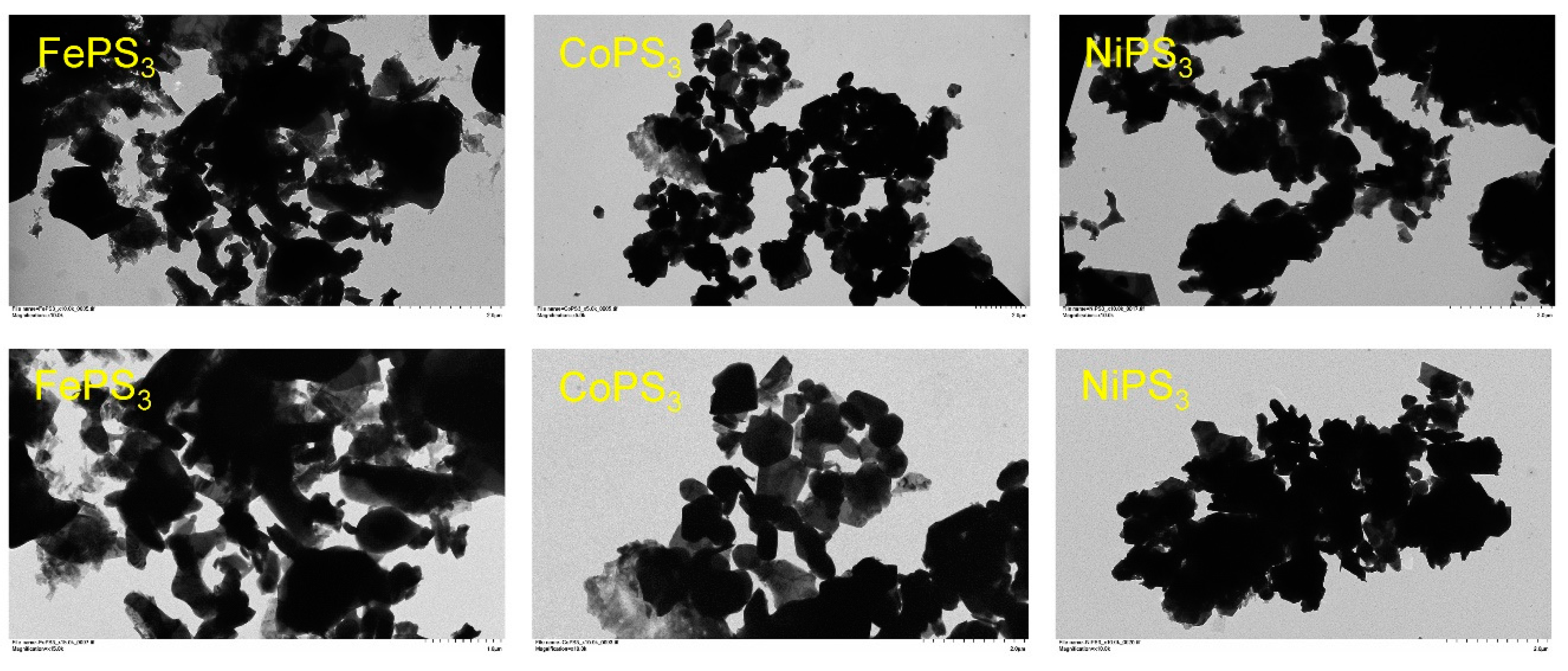
2.4. Particulate Morphologies of M-P-S Materials
2.5. Spectroscopic and Magnetic Analysis of M-P-S Products
2.6. Thermochemical Comparison of MPS3 Materials to MPx and MSy Counterparts
2.7. Examination of Electrocatalytic HER Activity for MPS3 Products
3. Materials and Methods
3.1. Starting Materials
3.2. Synthesis of M-P-S Materials in Sealed Ampoules
3.3. Sample Characterization
3.4. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Van Den Berg, A.W.C.; Areán, C.O. Materials for hydrogen storage: Current research trends and perspectives. Chem. Commun. 2008, 6, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.-P.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and hydrogen evolution reactions on Ru, RuO2, Ir, and IrO2 thin film electrodes in acidic and alkaline electrolytes: A comparative study on activity and stability. Catal. Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Marković, N.M.; Sarraf, S.T.; Gasteiger, H.A.; Ross, P.N. Hydrogen electrochemistry on platinum low-index single-crystal surfaces in alkaline solution. J. Chem. Soc. Faraday Trans. 1996, 92, 3719–3725. [Google Scholar] [CrossRef]
- Sheng, W.; Zhuang, Z.; Gao, M.; Zheng, J.; Chen, J.G.; Yan, Y. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nature Commun. 2015, 6, 5848. [Google Scholar] [CrossRef]
- Dinh, K.N.; Liang, Q.H.; Du, C.F.; Zhao, J.; Tok, A.L.Y.; Mao, H.; Yan, Q.Y. Nanostructured metallic transition metal carbides, nitrides, phosphides, and borides for energy storage and conversion. Nano Today 2019, 25, 99–121. [Google Scholar] [CrossRef]
- Xu, H.M.; Ci, S.Q.; Ding, Y.C.; Wang, G.X.; Wen, Z.H. Recent advances in precious metal-free bifunctional catalysts for electrochemical conversion systems. J. Mater. Chem. A 2019, 7, 8006–8029. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, X.; Wei, W.; Wang, S.; Zhang, Z.; Ni, B.-J. Boride-based electrocatalysts: Emerging candidates for water splitting. Nano Res. 2020, 13, 293–314. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Sun, X.M. Recent progress on earth abundant electrocatalysts for oxygen evolution reaction (OER) in alkaline medium to achieve efficient water splitting—A review. J. Power Sources 2018, 400, 31–68. [Google Scholar] [CrossRef]
- Gupta, S.; Patel, M.K.; Miotello, A.; Patel, N. Metal Boride-Based Catalysts for Electrochemical Water-Splitting: A Review. Adv. Funct. Mater. 2020, 30, 1906481. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; He, Y.J.; Zhu, H.W. Recent advances in transition-metal-sulfide-based bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2021, 9, 5320–5363. [Google Scholar] [CrossRef]
- Callejas, J.F.; Read, C.G.; Roske, C.W.; Lewis, N.S.; Schaak, R.E. Synthesis, Characterization, and Properties of Metal Phosphide Catalysts for the Hydrogen-Evolution Reaction. Chem. Mater. 2016, 28, 6017–6044. [Google Scholar] [CrossRef]
- Faber, M.S.; Lukowski, M.A.; Ding, Q.; Kaiser, N.S.; Jin, S. Earth-Abundant Metal Pyrites (FeS2, CoS2, NiS2, and Their Alloys) for Highly Efficient Hydrogen Evolution and Polysulfide Reduction Electrocatalysis. J. Phys. Chem. C Nanomater. Interfaces 2014, 118, 21347–21356. [Google Scholar] [CrossRef] [PubMed]
- Chia, X.; Eng, A.Y.; Ambrosi, A.; Tan, S.M.; Pumera, M. Electrochemistry of Nanostructured Layered Transition-Metal Dichalcogenides. Chem. Rev. 2015, 115, 11941–11966. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.S.; Dziedzic, R.; Lukowski, M.A.; Kaiser, N.S.; Ding, Q.; Jin, S. High-performance electrocatalysis using metallic cobalt pyrite (CoS(2)) micro- and nanostructures. J. Am. Chem. Soc. 2014, 136, 10053–10061. [Google Scholar] [CrossRef]
- Coleman, N., Jr.; Lovander, M.D.; Leddy, J.; Gillan, E.G. Phosphorus-Rich Metal Phosphides: Direct and Tin Flux-Assisted Synthesis and Evaluation as Hydrogen Evolution Electrocatalysts. Inorg. Chem. 2019, 58, 5013–5024. [Google Scholar] [CrossRef]
- Xiao, P.; Chen, W.; Wang, X. A Review of Phosphide-Based Materials for Electrocatalytic Hydrogen Evolution. Adv. Energy Mater. 2015, 5, 1500985. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Tsai, C.; Chan, K.; Benck, J.D.; Norskov, J.K.; Abild-Pedersen, F.; Jaramillo, T.F. Designing an improved transition metal phosphide catalyst for hydrogen evolution using experimental and theoretical trends. Energy Environ. Sci. 2015, 8, 3022–3029. [Google Scholar] [CrossRef]
- Wexler, R.B.; Martirez, J.M.P.; Rappe, A.M. Stable Phosphorus-Enriched (0001) Surfaces of Nickel Phosphides. Chem. Mater. 2016, 28, 5365–5372. [Google Scholar] [CrossRef]
- Wexler, R.B.; Martirez, J.M.P.; Rappe, A.M. Active Role of Phosphorus in the Hydrogen Evolving Activity of Nickel Phosphide (0001) Surfaces. ACS Catal. 2017, 7, 7718–7725. [Google Scholar] [CrossRef]
- Itthibenchapong, V.; Kokenyesi, R.S.; Ritenour, A.J.; Zakharov, L.N.; Boettcher, S.W.; Wager, J.F.; Keszler, D.A. Earth-abundant Cu-based chalcogenide semiconductors as photovoltaic absorbers. J. Mater. Chem. C 2013, 1, 657–662. [Google Scholar] [CrossRef]
- Foot, P.J.S.; Suradi, J.; Lee, P.A. Optical and Electronic-Properties of the Layered Semiconductors Nips3 and Feps3. Mater. Res. Bull. 1980, 15, 189–193. [Google Scholar] [CrossRef]
- Takano, Y.; Arai, A.; Takahashi, Y.; Takase, K.; Sekizawa, K. Magnetic properties and specific heat of new spin glass Mn0.5Fe0.5PS3. J. Appl. Phys. 2003, 93, 8197–8199. [Google Scholar] [CrossRef]
- Clement, R.; Girerd, J.J.; Morgensternbadarau, I. Dramatic modification of the magnetic-properties of lamellar mnps3 upon intercalation. Inorg. Chem. 1980, 19, 2852–2854. [Google Scholar] [CrossRef]
- Nahigian, H.; Steger, J.; Arnott, R.J.; Wold, A. Preparation and properties of the system CoPxS2−x. J. Phys. Chem. Solids 1974, 35, 1349–1354. [Google Scholar] [CrossRef]
- Clement, R.; Garnier, O.; Mathey, Y. Intercalation-Induced Modifications of the Optical and Magnetic-Properties of Feps3 and Nips3 Layer Phases. Nouv. J. De Chim. -New J. Chem. 1982, 6, 13–17. [Google Scholar]
- Chittari, B.L.; Park, Y.; Lee, D.; Han, M.; MacDonald, A.H.; Hwang, E.; Jung, J. Electronic and magnetic properties of single-layerMPX3metal phosphorous trichalcogenides. Phys. Rev. B 2016, 94, 184428. [Google Scholar] [CrossRef]
- Lemehaute, A.; Ouvrard, G.; Brec, R.; Rouxel, J. Intercalation of Lithium in Nips3 Lamellar Structure. Mater. Res. Bull. 1977, 12, 1191–1197. [Google Scholar] [CrossRef]
- Manriquez, V.; Barahona, P.; Ruiz, D.; Avila, R.E. Intercalation of polyethylene oxide PEO in layered MPS3 (M = Ni, Fe) materials. Mater. Res. Bull. 2005, 40, 475–483. [Google Scholar] [CrossRef]
- Oriakhi, C.O.; Lerner, M.M. Rapid and quantitative displacement of poly(ethylene oxide) from MnPS3 and other layered hosts. Chem. Mater. 1996, 8, 2016–2022. [Google Scholar] [CrossRef]
- Aruchamy, A.; Berger, H.; Levy, F. Photoelectronic properties of the p-type layered trichalcogenophosphates FePS3 and FePSe3. J. Solid State Chem. 1988, 72, 316–323. [Google Scholar] [CrossRef]
- Ichimura, K.; Sano, M. Electrical-conductivity of layered transition-metal phosphorus trisulfide crystals. Synth. Met. 1991, 45, 203–211. [Google Scholar] [CrossRef]
- Choi, C.; Ashby, D.; Rao, Y.; Anber, E.; Hart, J.L.; Butts, D.; Wilson, C.; Levin, E.; Taheri, M.; Ghazisaeidi, M.; et al. Mechanistic Insight and Local Structure Evolution of NiPS3 upon Electrochemical Lithiation. ACS Appl. Mater. Interfaces 2022, 14, 3980–3990. [Google Scholar] [CrossRef]
- Liang, Q.; Zheng, Y.; Du, C.; Luo, Y.; Zhang, J.; Li, B.; Zong, Y.; Yan, Q. General and Scalable Solid-State Synthesis of 2D MPS3 (M = Fe, Co, Ni) Nanosheets and Tuning Their Li/Na Storage Properties. Small Methods 2017, 1, 1700304. [Google Scholar] [CrossRef]
- Glass, D.E.; Jones, J.P.; Shevade, A.V.; Bugga, R.V. Transition Metal Phosphorous Trisulfides as Cathode Materials in High Temperatures Batteries. J. Electrochem. Soc. 2020, 167, 110512. [Google Scholar] [CrossRef]
- Byvik, C.E.; Smith, B.T.; Reichman, B. Layered transition metal thiophosphates (MPX3) as photoelectrodes in photoelectrochemical cells. Solar Energy Mater. 1982, 7, 213–223. [Google Scholar] [CrossRef]
- Ismail, N.; Temerk, Y.M.; El-Meligi, A.A.; Badr, M.A.; Madian, M. Synthesis and characterization of MnPS3 for hydrogen sorption. J. Solid State Chem. 2010, 183, 984–987. [Google Scholar] [CrossRef]
- Ismail, N.; Madian, M.; El-Meligi, A.A. Synthesis of NiPS3 and CoPS and its hydrogen storage capacity. J. Alloy. Compd. 2014, 588, 573–577. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Sofer, Z.; Sedmidubsky, D.; Huber, S.; Eng, A.Y.; Pumera, M. Layered Metal Thiophosphite Materials: Magnetic, Electrochemical, and Electronic Properties. ACS Appl. Mater. Interfaces 2017, 9, 12563–12573. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.M.; Pastika, J.; Mazanek, V.; Melle-Franco, M.; Sofer, Z.; Gusmao, R. Cobalt Phosphorous Trisulfide as a High-Performance Electrocatalyst for the Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2021, 13, 23638–23646. [Google Scholar] [CrossRef] [PubMed]
- Du, C.F.; Liang, Q.; Dangol, R.; Zhao, J.; Ren, H.; Madhavi, S.; Yan, Q. Layered Trichalcogenidophosphate: A New Catalyst Family for Water Splitting. Nanomicro. Lett. 2018, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Dangol, R.; Dai, Z.; Chaturvedi, A.; Zheng, Y.; Zhang, Y.; Dinh, K.N.; Li, B.; Zong, Y.; Yan, Q. Few-layer NiPS3 nanosheets as bifunctional materials for Li-ion storage and oxygen evolution reaction. Nanoscale 2018, 10, 4890–4896. [Google Scholar] [CrossRef] [PubMed]
- Samal, R.; Sanyal, G.; Chakraborty, B.; Rout, C.S. Two-dimensional transition metal phosphorous trichalcogenides (MPX3): A review on emerging trends, current state and future perspectives. J. Mater. Chem. A 2021, 9, 2560–2591. [Google Scholar] [CrossRef]
- Wang, F.M.; Shifa, T.A.; Yu, P.; He, P.; Liu, Y.; Wang, F.; Wang, Z.X.; Zhan, X.Y.; Lou, X.D.; Xia, F.; et al. New Frontiers on van der Waals Layered Metal Phosphorous Trichalcogenides. Adv. Funct. Mater. 2018, 28, 1802151. [Google Scholar] [CrossRef]
- Zhu, M.; Kou, H.; Wang, K.; Wu, H.; Ding, D.; Zhou, G.; Ding, S. Promising functional two-dimensional lamellar metal thiophosphates: Synthesis strategies, properties and applications. Mater. Horiz. 2020, 7, 3131–3160. [Google Scholar] [CrossRef]
- Cheng, M.; Lee, Y.S.; Iyer, A.K.; Chica, D.G.; Qian, E.K.; Shehzad, M.A.; Dos Reis, R.; Kanatzidis, M.G.; Dravid, V.P. Mixed Metal Thiophosphate Fe2-xCoxP2S6: Role of Structural Evolution and Anisotropy. Inorg. Chem. 2021, 60, 17268–17275. [Google Scholar] [CrossRef]
- Binnewies, M.; Milke, E. Thermochemical Data of Elements and Compounds; Weinheim: New York, NY, USA, 1999; pp. 88–344. [Google Scholar]
- The Materials Project. Available online: https://materialsproject.org (accessed on 15 May 2022).
- Kubaschewski, O.; Alcock, C.B. Metallurgical Thermochemistry, 5th ed.; Pergamon Press Inc.; Maxwell House: Elmsford, NY, USA, 1979. [Google Scholar]
- Marzik, J.V.; Hsieh, A.K.; Dwight, K.; Wold, A. Photoelectronic properties of cu3ps4 and cu3ps3se single-crystals. J. Solid State Chem. 1983, 49, 43–50. [Google Scholar] [CrossRef]
- Susner, M.A.; Chyasnavichyus, M.; McGuire, M.A.; Ganesh, P.; Maksymovych, P. Metal Thio- and Selenophosphates as Multifunctional van der Waals Layered Materials. Adv. Mater. 2017, 29, 1602852. [Google Scholar] [CrossRef]
- Brec, R. Review on Structural and Chemical-Properties of Transition-Metal Phosphorus Trisulfides Mps3. Solid State Ion. 1986, 22, 3–30. [Google Scholar] [CrossRef]
- Schenk, P.W.; Brauer, G. Preparative Methods. In Handbook of Preparative Inorganic Chemistry; Brauer, G., Ed.; Academic Press: Cambridge, MA, USA, 1963; pp. 3–107. [Google Scholar] [CrossRef]
- Yu, L.; Ren, Z. Systematic study of the influence of iR compensation on water electrolysis. Mater. Today Phys. 2020, 14, 100253. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Karthick, K.; Sankar, S.S.; Sangeetha, K.; Karthik, P.E.; Kundu, S. Precision and correctness in the evaluation of electrocatalytic water splitting: Revisiting activity parameters with a critical assessment. Energy Environ. Sci 2018, 11, 744–771. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S. iR drop correction in electrocatalysis: Everything one needs to know! J. Mater. Chem. A 2022, 10, 9348–9354. [Google Scholar] [CrossRef]
- Chen, R.; Yang, C.; Cai, W.; Wang, H.-Y.; Miao, J.; Zhang, L.; Chen, S.; Liu, B. Use of Platinum as the Counter Electrode to Study the Activity of Nonprecious Metal Catalysts for the Hydrogen Evolution Reaction. ACS Energy Lett. 2017, 2, 1070–1075. [Google Scholar] [CrossRef]
- Mitsushima, S.; Kawahara, S.; Ota, K.-I.; Kamiya, N. Consumption Rate of Pt under Potential Cycling. J. Electrochem. Soc. 2007, 154, B153. [Google Scholar] [CrossRef]
- Mitsushima, S.; Koizumi, Y.; Ota, K.-I.; Kamiya, N. Solubility of Platinum in Acidic Media (I)-in Sulfuric Acid. Electrochemistry 2007, 75, 155–158. [Google Scholar] [CrossRef][Green Version]
- Mitsushima, S.; Koizumi, Y.; Uzuka, S.; Ota, K. Dissolution Mechanism of Platinum in Acidic Media. ECS Trans. 2007, 11, 1195–1201. [Google Scholar] [CrossRef]
- Dong, G.; Fang, M.; Wang, H.; Yip, S.; Cheung, H.-Y.; Wang, F.; Wong, C.-Y.; Chu, S.T.; Ho, J.C. Insight into the electrochemical activation of carbon-based cathodes for hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 13080–13086. [Google Scholar] [CrossRef]
- Bard, A.J.; Parsons, R.; Jordan, J. Standard Potentials in Aqueous Solution; Routledge: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Abeysinghe, J.P.; Kölln, A.F.; Gillan, E.G. Rapid and Energetic Solid-State Metathesis Reactions for Iron, Cobalt, and Nickel Boride Formation and Their Investigation as Bifunctional Water Splitting Electrocatalysts. ACS Mater. Au 2022, 2, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Wei, B.; Sun, R.; Yu, W.; Hoh, H.Y.; Xu, H.; Li, J.; Ge, X.; Chen, Z.; et al. Activating Basal Planes of NiPS3 for Hydrogen Evolution by Nonmetal Heteroatom Doping. Adv. Funct. Mater. 2020, 30, 1908708. [Google Scholar] [CrossRef]
- Liang, Q.; Zhong, L.; Du, C.; Zheng, Y.; Luo, Y.; Xu, J.; Li, S.; Yan, Q. Mosaic-Structured Cobalt Nickel Thiophosphate Nanosheets Incorporated N-doped Carbon for Efficient and Stable Electrocatalytic Water Splitting. Adv. Funct. Mater. 2018, 28, 1805075. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, R.; Li, X.a.; Liu, X.; Huang, W. A nanohybrid consisting of NiPS3 nanoparticles coupled with defective graphene as a pH-universal electrocatalyst for efficient hydrogen evolution. J. Mater. Chem. A 2017, 5, 23536–23542. [Google Scholar] [CrossRef]
- Song, B.; Li, K.; Yin, Y.; Wu, T.; Dang, L.; Cabán-Acevedo, M.; Han, J.; Gao, T.; Wang, X.; Zhang, Z.; et al. Tuning Mixed Nickel Iron Phosphosulfide Nanosheet Electrocatalysts for Enhanced Hydrogen and Oxygen Evolution. ACS Catal. 2017, 7, 8549–8557. [Google Scholar] [CrossRef]
- Li, K.; Rakov, D.; Zhang, W.; Xu, P. Improving the intrinsic electrocatalytic hydrogen evolution activity of few-layer NiPS3 by cobalt doping. Chem. Commun. 2017, 53, 8199–8202. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiao, B.; Shen, S.; Song, K.; Lin, Z.; Wang, Z.; Chen, Y.; Zhong, W. Cobalt doping of FePS3 promotes intrinsic active sites for the efficient hydrogen evolution reaction. Nanoscale 2020, 12, 14459–14464. [Google Scholar] [CrossRef]
- Mukherjee, D.; Austeria, P.M.; Sampath, S. Two-Dimensional, Few-Layer Phosphochalcogenide, FePS3: A New Catalyst for Electrochemical Hydrogen Evolution over Wide pH Range. ACS Energy Lett. 2016, 1, 367–372. [Google Scholar] [CrossRef]
- Barry, B.M.; Gillan, E.G. A General and Flexible Synthesis of Transition-Metal Polyphosphides via PCl3 Elimination. Chem. Mater. 2009, 21, 4454–4461. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. Powder cell—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Almeida, C.M.V.B.; Giannetti, B.F. A new and practical carbon paste electrode for insoluble and ground samples. Electrochem. Commun. 2002, 4, 985–988. [Google Scholar] [CrossRef]
- Long, J.W.; Ayers, K.E.; Rolison, D.R. Electrochemical characterization of high-surface-area catalysts and other nanoscale electroactive materials at sticky-carbon electrodes. J. Electroanal. Chem. 2002, 522, 58–65. [Google Scholar] [CrossRef]
- McCrory, C.C.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Ouvrard, G.; Brec, R.; Rouxel, J. Structural determination of some MPS3 layered phases (M = Mn, Fe, Co, Ni and Cd). Mater. Res. Bull. 1985, 20, 1181–1189. [Google Scholar] [CrossRef]
- Taylor, B.E.; Steger, J.; Wold, A. Preparation and properties of some transition metal phosphorus trisulfide compounds. J. Solid State Chem. 1973, 7, 461–467. [Google Scholar] [CrossRef]
- Brec, R.; Schleich, D.M.; Ouvrard, G.; Louisy, A.; Rouxel, J. Physical properties of lithium intercalation compounds of the layered transition-metal chalcogenophosphites. Inorg. Chem. 1979, 18, 1814–1818. [Google Scholar] [CrossRef]
- Brec, R.; Ouvrard, G.; Louisy, A.; Rouxel, J. The Influence, on Lithium Electrochemical Intercalation, of Bond Ionicity in Layered Chalcogenophosphates of Transition-Metals. Solid State Ion. 1982, 6, 185–190. [Google Scholar] [CrossRef]
- Fuentealba, P.; Cortes, C.; Audebrand, N.; Le Fur, E.; Paredes-Garcia, V.; Venegas-Yazigi, D.; Manzur, J.; Spodine, E. First copper(ii) phase M’0.2Mn0.8PS3.0.25H2O and analogous M’ = Co(II), Ni(II) and Zn(II) materials obtained by microwave assisted synthesis. Dalton Trans. 2015, 44, 12493–12496. [Google Scholar] [CrossRef]



| Reaction | Target | Yield 1 | XRD Phases 2 | M:P:S:Cl Atomic Ratio (EDS) | M:P:S Atomic Ratio (ICP) |
|---|---|---|---|---|---|
| FeCl3 (P + S) | FePS3 | 93% | FePS3 | 1:1.29:3.04:0.08 | 1:0.94:3.01 |
| FeCl3 (P2S5 + P) | FePS3 | 84% | FePS3 | 1:1.36:3.24:<0.01 | 1:0.99:3.58 |
| CoCl2 (P + S) | CoPS3 | 62% | CoPS3, CoP0.5S1.5 | 1:1.95:4.52:<0.01 | 1:0.40:1.62 |
| CoCl2 (P2S5 + P) | CoPS3 | 79% | CoPS3 | 1:1.97:5.13:<0.01 | 1:0.73:2.42 |
| NiCl2 (P + S) | NiPS3 | 63% | NiPS3, NiS2 | 1:1.34:3.34:0.02 | 1:0.83:4.13 |
| NiCl2 (P2S5 + P) | NiPS3 | 87% | NiPS3 | 1:1.24:3.20:<0.01 | 1:0.79:4.23 |
| MP2 | MS2 | MPS | MPS3 | |
| FeP2 | FeS2 | FePS | FePS3 | |
| ΔHf (kJ/mol) | −221 | −172 | n/a | n/a |
| ΔHf (eV/atom) | −0.537 | −0.948 | −0.754 | −0.648 |
| CoP3 | CoS2 | CoPS | CoPS3 | |
| ΔHf (kJ/mol) | −280 | −153 | n/a | n/a |
| ΔHf (eV/atom) | −0.497 | −0.774 | −0.773 | −0.613 |
| NiP2 | NiS2 | NiPS | NiPS3 | |
| ΔHf (kJ/mol) | −129 | −134 | n/a | n/a |
| ΔHf (eV/atom) | −0.361 | −0.684 | −0.575 | −0.587 |
| Catalyst Material | Onset (mV) 2 | 10 mA/cm2 (mV) | 20 mA/cm2 (mV) | Tafel Slope (mV/dec) 3 | ECSA (cm2) 4 | Extended Stability 5 |
|---|---|---|---|---|---|---|
| CoPS3 | −96 ± 1 (−80 ± 1) | −222 ± 2 (−169 ± 1) | −289 ± 4 (−198 ± 2) | −71 ± 5 | 6/2 | 87% @ −241 mV |
| NiPS3 | −178 ± 9 (−174 ± 2) | −311 ± 8 (−261 ± 2) | −378 ± 10 (−290 ± 3) | −86 ± 4 | 2/1 | 84% @ −401 mV |
| FePS3 | ~500 (~270) | n/a | n/a | n/a | 2/1 | 29% @ −749 mV |
| 10% Pt on C | 47 ± 9 (122 ± 2) | −31 ± 4 (−8 ± 1) | −57 ± 8 (−33 ± 2) | −49 ± 2 | 27/44 | 22% @ −79 mV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coleman, N., Jr.; Liyanage, I.A.; Lovander, M.D.; Leddy, J.; Gillan, E.G. Facile Solvent-Free Synthesis of Metal Thiophosphates and Their Examination as Hydrogen Evolution Electrocatalysts. Molecules 2022, 27, 5053. https://doi.org/10.3390/molecules27165053
Coleman N Jr., Liyanage IA, Lovander MD, Leddy J, Gillan EG. Facile Solvent-Free Synthesis of Metal Thiophosphates and Their Examination as Hydrogen Evolution Electrocatalysts. Molecules. 2022; 27(16):5053. https://doi.org/10.3390/molecules27165053
Chicago/Turabian StyleColeman, Nathaniel, Jr., Ishanka A. Liyanage, Matthew D. Lovander, Johna Leddy, and Edward G. Gillan. 2022. "Facile Solvent-Free Synthesis of Metal Thiophosphates and Their Examination as Hydrogen Evolution Electrocatalysts" Molecules 27, no. 16: 5053. https://doi.org/10.3390/molecules27165053
APA StyleColeman, N., Jr., Liyanage, I. A., Lovander, M. D., Leddy, J., & Gillan, E. G. (2022). Facile Solvent-Free Synthesis of Metal Thiophosphates and Their Examination as Hydrogen Evolution Electrocatalysts. Molecules, 27(16), 5053. https://doi.org/10.3390/molecules27165053








