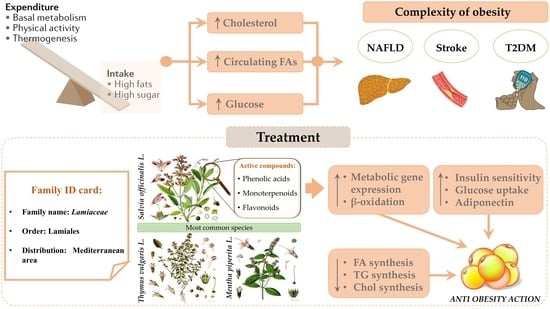
The Potential of Lamiaceae Herbs for Mitigation of Overweight, Obesity, and Fatty Liver: Studies and Perspectives
Abstract
1. Introduction
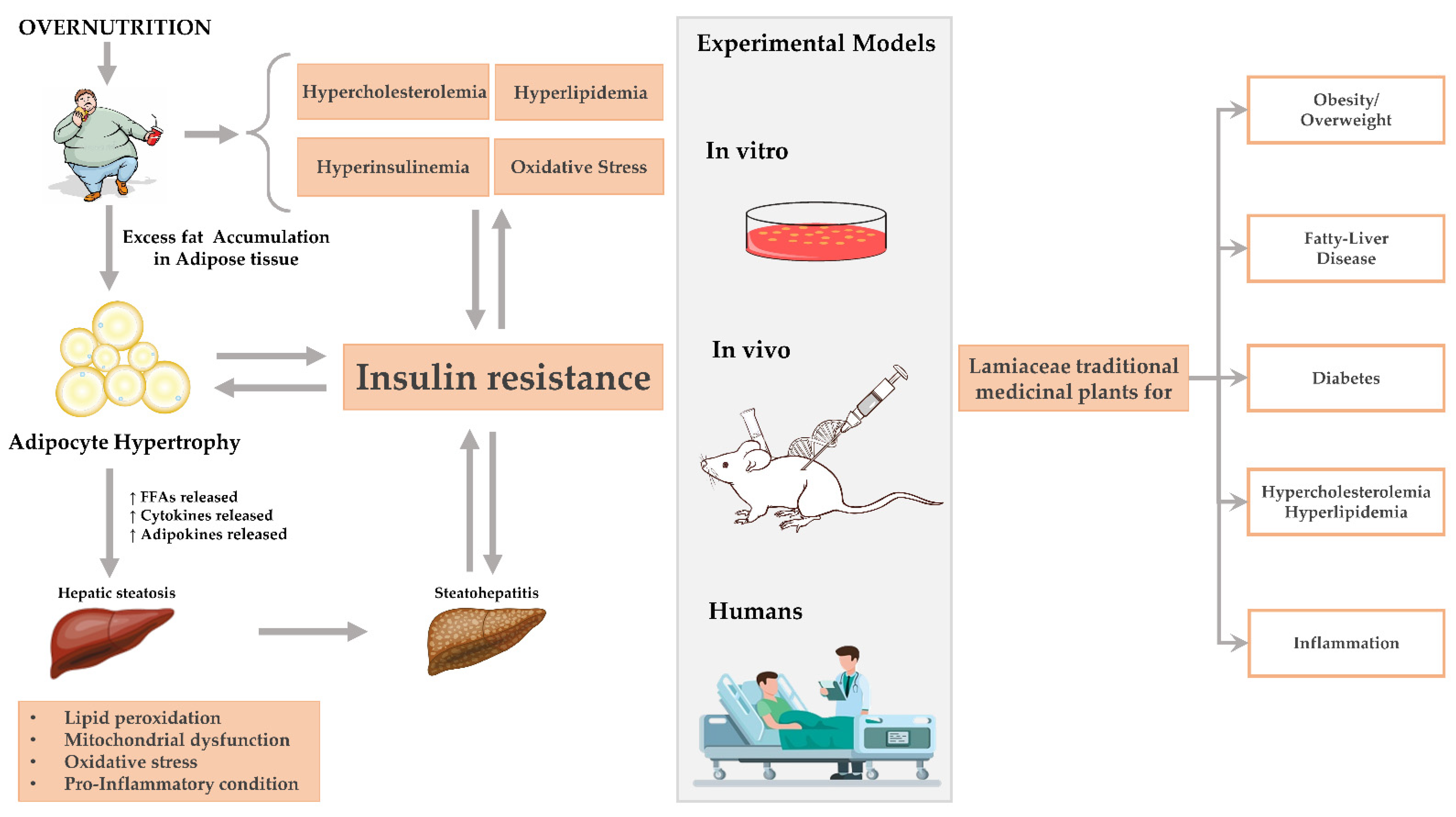
Obesity and Non-Alcoholic Fatty Liver Disease
2. Methodology
2.1. Data Extraction
2.2. Studies Selection Process and Distribution among Authors
2.3. Result Report
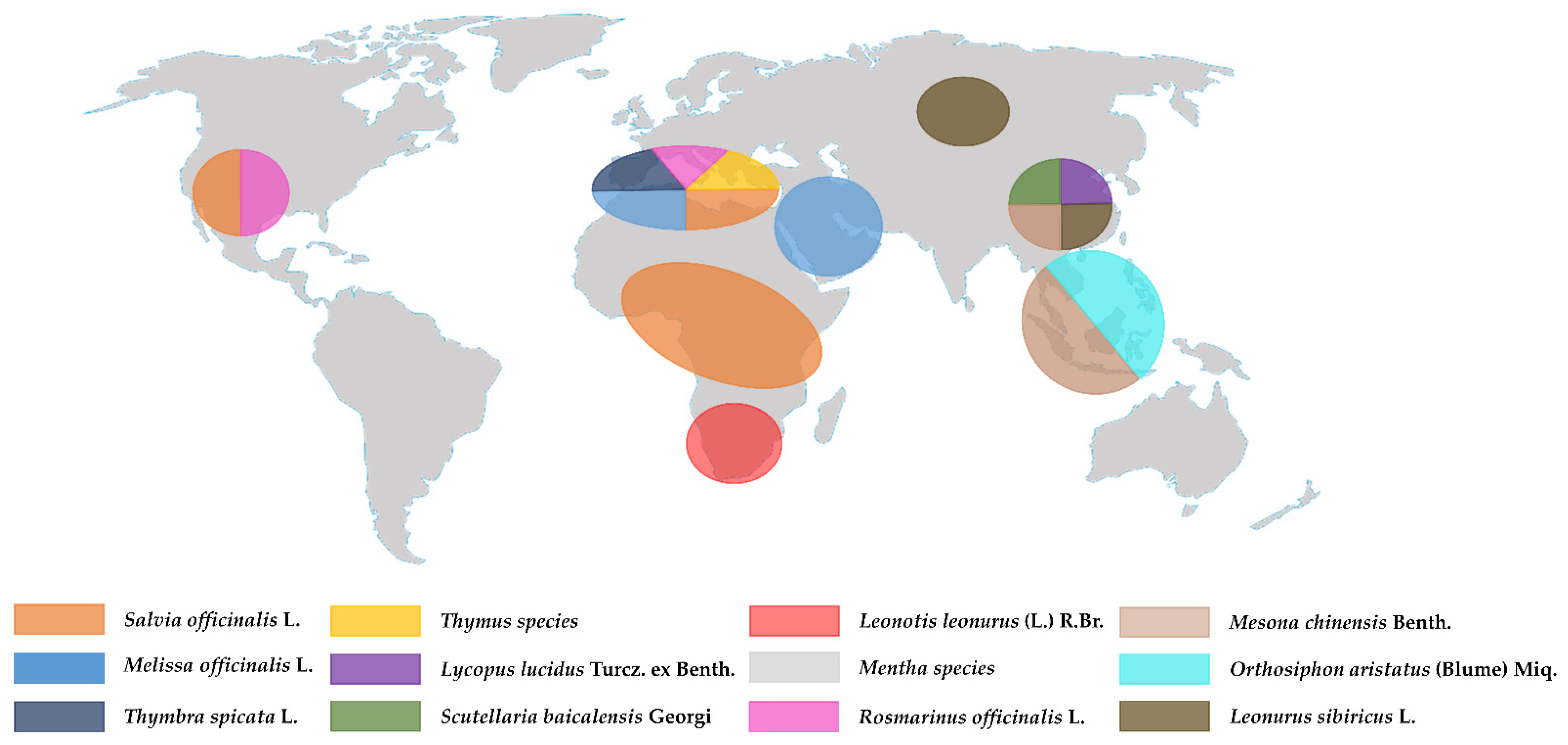
3. Lamiaceae: General Aspects of the Family
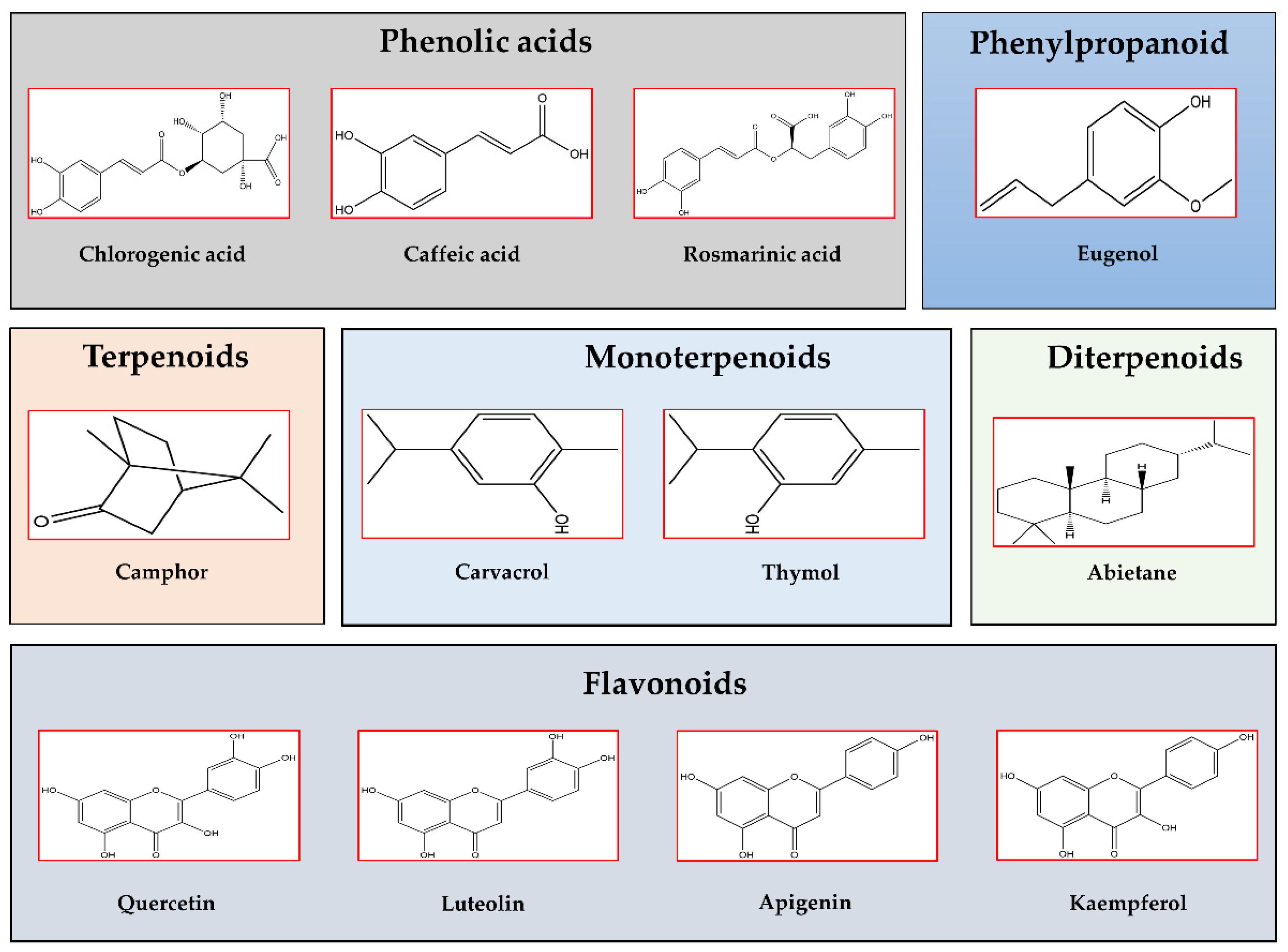
4. Lamiaceae: Bioactive Properties
4.1. Salvia Species
| List of Plants | Most Abundant Bioactive Compounds | Applications |
|---|---|---|
| Salvia officinalis L. | Diterpenoids: abietane and labdane Phenolic compounds: caffeic acid derivatives | Folk medicine |
| Salvia hispanica L. | Caffeic acid, chlorogenic acid, and quercetin | Uses in food, folk medicine, primary cosmetics, and a part of religious rituals |
| Thymus species | Thymol, carvacrol apigenin, luteolin, thymusin, rosmarinic, and caffeic acid and derivatives | Traditional phytomedicine, food, food additive, spicy, and herbal tea |
| Rosmarinus officinalis L. | Carnosic acid, rosmarinic acid, camphor, caffeic acid, ursolic acid, betulinic acid, and carnosol | Traditional phytomedicine, food additives, and herbal tea |
| Mentha species | Menthol, luteolin, rosmarinic acid, Kaempferol, and hesperidin | Traditional phytomedicine, food, food additive, spicy, herbal tea |
| Melissa officinalis L. | Rosmarinic acid, geranial, neral, luteolin, naringin, hesperidin, and caffeic acid and derivatives | Traditional phytomedicine, food flavoring, and herbal tea |
| Leonurus sibiricus L. | Chlorogenic acid, caffeic acid, and quercetin | Herbal medicine |
| Thymbra spicata L. | Carvacrol and rosmarinic acid | Culinary ingredient: in salad and tea infusion Herbal medicine |
| Orthosiphon aristatus (Blume) Miq. | Rosmarinic acid | Folk medicine |
| Lycopus lucidus Turcz. ex Benth | Rosmarinic acid and derivatives Flavonoid: chrysoeriol, luteolin, quercetin, isoquercitrin, and rutin | Traditional phytomedicine |
| Scutellaria baicalensis Georgi | Flavonoid: Baicalein, wogonoside, and wogonin | Traditional phytomedicine |
| Ocimum species | Eugenol, rosmarinic acid, apigenin, luteolin, β-sitosterol, and carnosic acid | Traditional phytomedicine, food additive, spicy, and fragrance agent |
| Mesona chinensis Benth. | Caffeic acid | Traditional phytomedicine, gelatin-type dessert, and herbal beverage |
| Leonotis leonurus (L.) R.Br. | Marrubin and premarrubin | Traditional phytomedicine |

4.2. Thymus Species
4.3. Rosmarinus officinalis L.
| Plants | Models | Treated Disorders | Proposed Mechanisms | Ref. | |
|---|---|---|---|---|---|
| Salvia officinalis L. | In vivo | Male Wistar rats | Diabetes Hypoglycemia | ↑Insulin secretion ↓Serum GLU, TG, TC, urea, uric acid, creatinine, AST, and ALT | [25] |
| In vitro In vivo | 3T3-L1 pre-adipocyte cell line HFD-fed mice (C57Bl6) | Diabetes Hyperlipidemia Obesity | ↓Blood GLU, TNF-α, KC/GRO, and IL-12 ↑ IL-2, IL-4, and IL-10 Improvement in HOMA-IR, TG, and NEFA ↓Body weight and LDs | [28] | |
| In vivo | Male Wistar rats | Diabetes Hyperlipidemia Obesity | Improvement in serum creatinine and UA concentrations ↓α-amylase and lipase activities ↓Serum AST, ALT, and LDH ↓Body weight | [27] | |
| In vivo | Female Balb/c mice and male Wistar rats | Diabetes | ↓Gluconeogenesis Inhibition of hepatic GLU production by glucagon | [29] | |
| In vivo | Female Wistar rats | Hyperlipidemia Obesity | ↓plasma Chol, HDL-Chol, LDL-Chol, TG, total lipids, and VLDL ↓Body weight | [30] | |
| Salvia hispanica L. | In vivo | Male Wistar rats | Dyslipidemia NAFLD/NASH | Prevention of cholestasis elevation (AP, GGTP, and TB) ↓ALT ↓Liver and plasma TNF-α ↓TG and total Chol ↓LP and CAT activities | [39] |
| In vivo | Adult female Wistar rats | Hyperlipidemia | ↑SOD and CAT activities ↑PPAR-α expression ↑HDL-Chol ↓TC, IL-1β, VLDL-Chol, & LDL-Chol ↓NFκB expression | [40] | |
| In vivo | Male Wistar rats | Obesity Dyslipidemia | Improvements in insulin sensitivity and plasma lipid profile (TG, FFA, & Chol) ↓FAT/CD 36 plasma membrane levels ↓ Fat synthesis enzyme activities (ATP CL, FAS, G-6-P DH, and PEPCK) ↓PKCβ and SREBP-1 protein levels | [42] | |
| In vivo | Wistar rats SRD-fed | Dyslipidemia Insulin resistance | ↓Body weight ↑CAT, SOD, & GPx activities ↑SOD and GPx mRNA ↑ PPAR-α protein level ↑Nrf2 expression ↑n-3/n-6 FA ratio of membrane phospholipid ↓IL-6 and TNF-α | [43] | |
| In vivo | Wistar rats SRD-fed | Dyslipidemia Insulin resistance | ↓Adipocyte hypertrophy, cell volume, and size distribution ↓Lipogenic enzyme activities (ACC, FAS, ME, and G-6-PDH) ↑Hexokinase and PDHc activities ↑GLUT-4 protein mass ↑Glycogen storage, G-6-P concentration, and GSa activity | [44] | |
| In vivo | Wistar rats SRD-fed | Dyslipidemia Insulin resistance | ↓Systolic blood pressure ↑GIR ↓Lipid storage (TG, LC ACoA, and DAG) and plasma FAs ↑PDHa ↑FAT/CD36 protein mass level ↓M-CPT1 and PPARα activity | [45] | |
| Thymus vulgaris L. | In vivo | Sodium nitrite-treated mice | Liver damage | ↓ AST, ALT, MDA, IL-1β, IL-6, TNF-α, ↑ GSH and SOD activities | [48] |
| In vivo | Gentamicin-treated rats | Liver damage | ↓ AST, ALT, bilirubin, total lipids, ROS | [49] | |
| Thymus saturejoides Coss. | In vivo | Streptozotocin-treated rats | T2DM | ↓Blood GLU and weight Improve GLU tolerance | [51] |
| Thymus schimperi Ronniger | In vivo | Alloxan-induced Diabetic Mice | Diabetes | ↓Fasting blood GLU | [52] |
| Thymus praecox Opiz | In vivo | Streptozotocin/nicotinamide-induced type 2 diabetic rats | Diabetes | ↓Blood GLU ↑α-glucosidase, PEPCK, GLUT-2 and SGLTs | [53] |
| Rosmarinus officinalis L. | In vitro | Hela cells | Oxidative stress | ↓ROS | [58] |
| In vitro | HepG2 cells | Obesity | ↑AMPK, ACC, LDLR and PPARα ↓G6Pase | [59] | |
| In vitro | Preadipocytes | Obesity | ↓TG ↓ CDK4, CCND1 and CDKN1A ↑ GATA3 and WNT3A | [60] | |
| In vitro | L6 myotubes | Insulin resistance | Restored insulin-simulated GLU uptake ↓ palmitate induced phosphorylation in IRS-1 ↑AMPK ↓ JNK and mTOR | [61] | |
| In vivo | Male Wistar rats | Oxidative stress | ↓TBARS, H2O2 ↑ GSH ↑SOD, CAT, GPx and GST activities | [62] | |
| In vivo | Rats | Liver toxicity | ↑SOD, CAT and GPx activities ↓ MDA ↓neutrophils and macrophages ↓ hepatocytes necrosis and fibrosis | [63] | |
| In vivo | Rats | Hypercholesterolemia | ↓Chol, HDL and TBARS ↑SOD, CAT and GPx activities | [64] | |
| In vivo | Mice | Inflammation | ↓ COX2, PGE-2, IL- 1b, MMP2 and NO | [65] | |
| Mentha spicata L. | In vivo | Nicotine-induced liver damage in Wistar rats | Liver damage | ↓AST, ALP, ALT, LDH and MDA | [66] |
| Mentha pipertia L. | In vivo | Rats | Liver damage | ↓ALT, AST, ALP, and LDH ↓Lipid peroxidation ↓gamma glutamyl transferase, urea and creatinine | [67] |
| In vivo | Rats | Liver damage | ↓p53 Improve TGF-β1 expression | [68] | |
| In vivo | Rats | Liver damage | ↓ALT, AST, ALP, and LDH ↓Lipid peroxidation ↓gamma glutamyl transferase, urea and creatinine | [67] | |
| Mentha villosa Huds | In vivo | HFD-fed mice | NAFLD Obesity | ↓blood GLU, insulin, leptin and TG ↑ adiponectin ↓ IL-6, TNF-α and SEBP 1c ↑ AMPK | [69] |
| Melissa officinalis L. | In vitro | HUVECs | Oxidative stress | ↑ cell viability ↓ [hydroperoxide] | [70] |
| In vitro In vivo | HUVECs mice | Obesity | ↓ body weight gain, adipose tissue mass and adipocyte size ↓VEGF-A, FGF-2 and MMPs mRNAs | [71] | |
| In vitro In vivo | HepG2 cells mice | Obesity | ↓ body weight gain ↓ visceral fat mass ↓ adipocyte size ↓ hepatic lipid accumulation ↑ expression of PPARα target genes | [72] | |
| In vitro In vivo | HepG2 cells mice | NASH | ↑ SOD, CAT and GPx activities ↑ AMPK, PPARα and CPT-1L ↓ α-SMA and COL1A1 | [73] | |
| In vivo | Mice | Oxidative stress | ↓Mn-induced TBARS levels ↑ SOD, CAT, GPx | [74] | |
| In vivo | Rats | Diabetes | ↓ weight, hyper-glycemia, hypo-insulinemia and hepatic lipid accumulation ↑ AMPKα2, ACOX, MCAD, VLCAD ↓ IL-6 and CD68 restored β-cell mass | [75] | |
| Leonurus sibiricus L. | In vitro | INS-1E cells | Diabetes | ↑ Insulin secretion ↑ Insulinoma cell proliferation | [76] |
| In vivo | C57BL/6 mice | Hypercholesterolemia | ↓ Plasma cholesterol ↑HDL-Chol ↑SOD, CAT, GR & GPx activities ↓TBARS and protein carbonyls | [77] | |
| In vivo | C57BL/6 mice | Obesity | ↓ Serum TG, TC, and LDL-Chol ↑ HSL and ATGL expression | [78] | |
| Thymbra spicata L. | In vitro | Rat hepatocytes FaO cells Human endothelial HECV cells | Steatosis Endothelial dysfunction | ↓ Hepatic lipid accumulation ↓ ROS and lipid peroxidation | [10] |
| In vivo | HFD-fed mice | NAFLD | ↓ TC, LDL-Chol, TG, and MDA ↑ HDL-Chol ↑GSH, SOD, and CAT activities | [79,80] | |
| Thymbra capitata (L.) Cav. | In vivo | Paracetamol-insulted rats | Liver damage | ↑ SOD and GPx | [81] |
| Orthosiphon aristatus (Blume) Miq. | In vivo | C57BL/6 mice | Hyperlipidemia Obesity | ↓ Body weight, TG, TC, and LDL-Chol ↓ Hepatic LDs ↓ MDA ↑ SOD activity | [82] |
| In vivo | Sprague Dawley rats | Diabetes | ↑ GLP-1 and ghrelin levels | [83] | |
| Lycopus lucidus Turcz. ex Benth | In vitro In vivo | HepG2 cells HFD-fed mice | NAFLD | ↓Intracellular lipid accumulation ↓lipogenesis ↑ β-oxidation ↓body weight, relative liver weight, serum ALT, total Chol, LDL, serum GLU, insulin, leptin, and TNF-α ↓SREBP-1 ↑ PPAR-α | [84] |
| In vitro | Human umbilical vein endothelial cells (HUVEC) | High glucose- induced Vascular inflammation | ↓ cell adhesion molecules (CAMs) ↓ ROS production ↓ NFκB expression | [85] | |
| In vivo | Mice | Liver injury | ↓ serum ALT, AST, ALP, TG, total Chol, and total bilirubin ↑ hepatic GSH contents ↑ SOD and CAT activities ↓ hepatic MDA ↓ DNA fragmentation | [86] | |
| Scutellaria baicalensis Georgi | In vitro In vivo | HepG2 cells Mice | NAFLD | ↓TG and Chol ↑AMPK | [87] |
| In vivo | Type 2 diabetic db/db mice | Obesity | ↓ weight gain, TG, ALT and hyperinsulinemia ↓p-AMPK | [88] | |
| In vivo | Mice | Insulin-resistance | ↓Fasting and postprandial GLU, fasting insulin, HOMA-IR, TG and LDL-Chol ↓adipose tissue macrophages, CD11b+; Kupffer cells, TNF-α | [89] | |
| Ocimum gratissimum L. | In vivo | Ovariectomized rats | Obesity | ↓Body weight ↓Adipocyte size | [90] |
| Ocimum tenuiflorum L. | In vivo | Rats | Dyslipidaemia | ↓Lipogenesis ↑Mitochondrial fatty acid uptake ↓Insulin resistance ↑GSH, GPx, CAT | [91] |
| In vivo | Rats | Hyperlipidemia | ↓Lipid accumulation ↓Oxidative stress | [92] | |
| In vivo | Rats | Diabetes | ↓Glucose ↓TG ↓creatinine ↓ Carbohydrate metabolism enzymes | [93,94] | |
| Mesona chinensis Benth. | In vitro In vivo | RAW 264.7 cells Male mice | Immune deficiency | ↑SOD, CAT and GPx activities ↓MDA | [95] |
| Leonotis leonurus (L.) R.Br. | In vivo | Rats | Diabetes mellitus | inhibited fresh egg albumin-induced paw edema and hypoglycemic effects in rats | [96] |
| In vitro In vivo | INS-1 cells Obese rats | Hyperglycemia | ↑ GLUT2 expression ↑RR and MM potential ↑ insulin | [97] | |
| In vitro In vivo | 3T3, Chang, C2C12, INS-1 Obese Wistar rats | Obesity | ↑ PPAR ↑glucokinase ↑FAS and UCP2 ↓leptin | [98] | |
4.4. Mentha Species
4.5. Melissa officinalis L.
| Plants | Sample Size | Gender (Age) | Participants | Format, Dose | Duration of Study | Action | Ref. |
|---|---|---|---|---|---|---|---|
| Salvia officinalis L. | n = 80 | Men and women | T2DM patients | tablets (150 mg extract/three times/day) | 90 days | ↓2hpp blood sugar and Chol | [31] |
| n = 86 | Men and women | Hyperlipidemic T2DM patients | extract capsules (500 mg/8 h) | 90 days | ↓GLU, HbA1c, total Chol, TG, and LDL-Chol ↑HDL-Chol | [32] | |
| n = 6 | Women (40–50 years) | Diabetic patients | Tea, (300 mL/twice a day) | 28 days | ↓Plasma LDL-Chol and total Chol ↑HDL-Chol ↑Hsp70 expression ↑SOD and CAT activities | [26] | |
| Salvia hispanica L. | n = 25 | Men and women | NAFLD patients | Milled chia (25 g/day) | 70 days | ↓Body weight, total Chol, FFA, and non-HDL-Chol | [38] |
| n = 77 | Men and women (35–75 years) | Overweight and obese patients with T2DM | ground chia (30 g/1000 kcal daily) | 180 days | Weight loss ↓CRP ↑Plasma adiponectin | [35] | |
| n = 20 | Men and women (64 ± 8 years) | T2DM patients | 37 ± 4 g/day | 84 days | ↓SBP ↓hs-CRP ↓ vWF | [37] | |
| n = 42 | Men and women (21–65 years) | T2DM patients | Chia seeds (40 g/day) | 84 days | ↓SBP | [36] | |
| Thyme spp. | n = 12 | Men and women (46–67 years) | Hypercholesterolemic patients | 25 mL/day | 3 weeks | ↓ox-LDL ↑bifidobacteria | [54] |
| n = 22 | Men and women (46–64 years) | Hypercholesterolemic patients | 25 mL/day | 3 weeks | ↑expression of key cholesterol efflux regulators | [55] | |
| Rosmarinus officinalis L. | n = 40 | Men and women (mean age 56) | Type 2 diabetes | Tea, 2 g/L/day | 90 days | ↓ body mass index ↓ waist-hip ratio ↓ HbA1c ↓insulin resistance ↓ lipid peroxide levels | [106] |
| Mentha longifolia (L.) L. | n = 29 | Men and women (40–65 years) | Blood hypertension patients | 300 mL/day | 16 weeks | ↓Systolic (SBP), diastolic blood pressures (DBP), mean arterial blood pressures (MAP) | [113] |
| Melissa officinalis L. | n = 58 | Men and women (25–65 years) | Hyperlipidemic patients | Two capsules (500 mg each) after meals, 3 times/day | 2 months | ↓ LDL | [114] |
| n = 62 | Men and women (20–65 years) | T2DM patients | two capsules (each 350 mg)/day | 12 weeks | ↓HbA1c, TG and hs-CRP ↑HDL | [116] | |
| Mesona chinensis Benth. | n = 40 | Men (20–40 years) | overweight | HC meal + 0.5 or 1 g of extract | 4 h | ↓MDA and serum TG ↑antioxidant status | [117] |
4.6. Leonurus sibiricus L.
4.7. Thymbra Species
4.8. Orthosiphon aristatus (Blume) Miq.
4.9. Lycopus lucidus Turcz. ex Benth.
4.10. Scutellaria baicalensis Georgi
4.11. Ocimum Species
4.12. Mesona chinensis Benth
4.13. Lenotis leonurus (L.) R.Br.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khoury, M.; Stien, D.; Eparvier, V.; Ouaini, N.; El Beyrouthy, M. Report on the medicinal use of eleven Lamiaceae species in Lebanon and rationalization of their antimicrobial potential by examination of the chemical composition and antimicrobial activity of their essential oils. Evid.-Based Complement. Altern. Med. 2016, 2016, 2547169. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Antunes-Ricardo, M.; Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Basilio Heredia, J. Cellular antioxidant activity and in vitro inhibition of α-glucosidase, α-amylase and pancreatic lipase of oregano polyphenols under simulated gastrointestinal digestion. Food Res. Int. 2019, 116, 676–686. [Google Scholar] [CrossRef]
- Ghaeni Pasavei, A.; Mohebbati, R.; Boroumand, N.; Ghorbani, A.; Hosseini, A.; Taraz Jamshidi, S.; Soukhtanloo, M. Anti-hypolipidemic and anti-oxidative effects of hydroalcoholic extract of Origanum majorana on the hepatosteatosis induced with high-fat diet in rats. Malays. J. Med. Sci. 2020, 27, 57–69. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Berkay Yılmaz, Y.; Antika, G.; Salehi, B.; Tumer, T.B.; Kulandaisamy Venil, C.; Das, G.; Patra, J.K.; Karazhan, N.; Akram, M.; et al. Phytochemical constituents, biological activities, and health-promoting effects of the genus Origanum. Phyther. Res. 2021, 35, 95–121. [Google Scholar] [CrossRef]
- Grondona, E.; Gatti, G.; López, A.G.; Sánchez, L.R.; Rivero, V.; Pessah, O.; Zunino, M.P.; Ponce, A.A. Bio-efficacy of the essential oil of oregano (Origanum vulgare Lamiaceae. Ssp. Hirtum). Plant Foods Hum. Nutr. 2014, 69, 351–357. [Google Scholar] [CrossRef]
- Hernández-Hernández, E.; Ponce-Alquicira, E.; Jaramillo-Flores, M.E.; Guerrero Legarreta, I. Antioxidant effect rosemary (Rosmarinus officinalis L.) and oregano (Origanum vulgare L.) extracts on TBARS and colour of model raw pork batters. Meat Sci. 2009, 81, 410–417. [Google Scholar] [CrossRef]
- Suanarunsawat, T.; Boonnak, T.; Na Ayutthaya, W.D.; Thirawarapan, S. Anti-hyperlipidemic and cardioprotective effects of Ocimum sanctum L. fixed oil in rats fed a high fat diet. J. Basic Clin. Physiol. Pharmacol. 2010, 21, 387–400. [Google Scholar] [CrossRef]
- de Paula Dias Moreira, L.; Enes, B.N.; de São José, V.P.B.; Toledo, R.C.L.; Ladeira, L.C.M.; Cardoso, R.R.; da Silva Duarte, V.; Hermsdorff, H.H.M.; de Barros, F.A.R.; Martino, H.S.D. Chia (Salvia hispanica L.) flour and oil ameliorate metabolic disorders in the liver of rats fed a high-fat and high fructose diet. Foods 2022, 11, 285. [Google Scholar] [CrossRef]
- Farid, O.; Zeggwagh, N.A.; Ouadi, F.E.L.; Eddouks, M. Mentha pulegium aqueous extract exhibits antidiabetic and hepatoprotective effects in streptozotocin-induced diabetic rats. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2019, 19, 292–301. [Google Scholar] [CrossRef]
- Khalil, M.; Khalifeh, H.; Baldini, F.; Salis, A.; Damonte, G.; Daher, A.; Voci, A.; Vergani, L. Antisteatotic and antioxidant activities of Thymbra spicata L. extracts in hepatic and endothelial cells as in vitro models of non-alcoholic fatty liver disease. J. Ethnopharmacol. 2019, 239, 111919. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Wong, V.W.S.; Nobili, V.; Day, C.P.; Sookoian, S.; Maher, J.J.; Bugianesi, E.; Sirlin, C.B.; Neuschwander-Tetri, B.A.; Rinella, M.E. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Prim. 2015, 1, 15080. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Q.H.; Portincasa, P.; Neuschwander-Tetri, B.A. Steatosis in the Liver. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2013; Volume 3, pp. 1493–1532. [Google Scholar]
- Lonardo, A.; Ballestri, S.; Marchesini, G.; Angulo, P.; Loria, P. Nonalcoholic fatty liver disease: A precursor of the metabolic syndrome. Dig. Liver Dis. 2015, 47, 181–190. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Gusdon, A.M.; Qu, S. Nonalcoholic fatty liver disease: Molecular pathways and therapeutic strategies. Lipids Health Dis. 2013, 12, 171. [Google Scholar] [CrossRef]
- Khalil, M.; Rita Caponio, G.; Diab, F.; Shanmugam, H.; Di Ciaula, A.; Khalifeh, H.; Vergani, L.; Calasso, M.; De Angelis, M.; Portincasa, P. Unraveling the beneficial effects of herbal Lebanese mixture “Za’atar”. History, studies, and properties of a potential healthy food ingredient. J. Funct. Foods 2022, 90, 104993. [Google Scholar] [CrossRef]
- Napoli, E.; Siracusa, L.; Ruberto, G. New Tricks for Old Guys: Recent Developments in the Chemistry, Biochemistry, Applications and Exploitation of Selected Species from the Lamiaceae Family. Chem. Biodivers. 2020, 17, e1900677. [Google Scholar] [CrossRef] [PubMed]
- Carović-Stanko, K.; Petek, M.; Grdiša, M.; Pintar, J.; Bedeković, D.; Herak Ćustić, M.; Satovic, Z. Medicinal plants of the family Lamiaceae as functional foods—A review. Czech J. Food Sci. 2016, 34, 377–390. [Google Scholar] [CrossRef]
- Bekut, M.; Brkić, S.; Kladar, N.; Dragović, G.; Gavarić, N.; Božin, B. Potential of selected Lamiaceae plants in anti(retro)viral therapy. Pharmacol. Res. 2018, 133, 301–314. [Google Scholar] [CrossRef]
- Vukovic, N.; Sukdolak, S.; Solujic, S.; Niciforovic, N. Antimicrobial activity of the essential oil obtained from roots and chemical composition of the volatile constituents from the roots, stems, and leaves of Ballota nigra from Serbia. J. Med. Food 2009, 12, 435–441. [Google Scholar] [CrossRef]
- Askari, S.F.; Avan, R.; Tayarani-Najaran, Z.; Sahebkar, A.; Eghbali, S. Iranian Salvia species: A phytochemical and pharmacological update. Phytochemistry 2021, 183, 112619. [Google Scholar] [CrossRef] [PubMed]
- Hamidpour, M.; Hamidpour, R.; Hamidpour, S.; Shahlari, M. Chemistry, pharmacology, and medicinal property of sage (Salvia) to prevent and cure illnesses such as obesity, diabetes, depression, dementia, lupus, autism, heart disease, and cancer. J. Tradit. Complement. Med. 2014, 4, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Edwards, K.D.; Margetts, G.; Kleidonas, S.; Zaibi, N.S.; Clapham, J.C.; Zaibi, M.S. Effects of Salvia officinalis L. and Chamaemelum nobile (L.) extracts on inflammatory responses in two models of human cells: Primary subcutaneous adipocytes and neuroblastoma cell line (SK-N-SH). J. Ethnopharmacol. 2021, 268, 113614. [Google Scholar] [CrossRef] [PubMed]
- Eidi, A.; Eidi, M. Antidiabetic effects of sage (Salvia officinalis L.) leaves in normal and streptozotocin-induced diabetic rats. Diabetes Metab. Syndr. Clin. Res. Rev. 2009, 3, 40–44. [Google Scholar] [CrossRef]
- Sá, C.; Ramos, A.; Azevedo, M.; Lima, C.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Sage tea drinking improves lipid profile and antioxidant defences in humans. Int. J. Mol. Sci. 2009, 10, 3937–3950. [Google Scholar] [CrossRef]
- Belhadj, S.; Hentati, O.; Hammami, M.; Ben Hadj, A.; Boudawara, T.; Dammak, M.; Zouari, S.; El Feki, A. Metabolic impairments and tissue disorders in alloxan-induced diabetic rats are alleviated by Salvia officinalis L. essential oil. Biomed. Pharmacother. 2018, 108, 985–995. [Google Scholar] [CrossRef]
- Ben Khedher, M.R.; Hammami, M.; Arch, J.R.S.; Hislop, D.C.; Eze, D.; Wargent, E.T.; Kępczyńska, M.A.; Zaibi, M.S. Preventive effects of Salvia officinalis leaf extract on insulin resistance and inflammation in a model of high fat diet-induced obesity in mice that responds to rosiglitazone. PeerJ 2018, 6, e4166. [Google Scholar] [CrossRef]
- Lima, C.F.; Azevedo, M.F.; Araujo, R.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Metformin-like effect of Salvia officinalis (common sage): Is it useful in diabetes prevention? Br. J. Nutr. 2006, 96, 326–333. [Google Scholar] [CrossRef]
- Koubaa-Ghorbel, F.; Chaâbane, M.; Jdidi, H.; Turki, M.; Makni-Ayadi, F.; El Feki, A. Salvia officinalis mitigates uterus and liver damages induced by an estrogen deficiency in ovariectomized rats. J. Food Biochem. 2021, 45, e13542. [Google Scholar] [CrossRef]
- Behradmanesh, S.; Derees, F.; Rafieian-Kopaei, M. Effect of Salvia officinalis on diabetic patients. J. Ren. Inj. Prev. 2013, 2, 51–54. [Google Scholar] [CrossRef]
- Kianbakht, S.; Dabaghian, F.H. Improved glycemic control and lipid profile in hyperlipidemic type 2 diabetic patients consuming Salvia officinalis L. leaf extract: A randomized placebo. Controlled clinical trial. Complement. Ther. Med. 2013, 21, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Knez Hrnčič, M.; Ivanovski, M.; Cör, D.; Knez, Ž. Chia Seeds (Salvia hispanica L.): An overview—Phytochemical profile, isolation methods, and application. Molecules 2019, 25, 11. [Google Scholar] [CrossRef] [PubMed]
- Teoh, S.L.; Lai, N.M.; Vanichkulpitak, P.; Vuksan, V.; Ho, H.; Chaiyakunapruk, N. Clinical evidence on dietary supplementation with chia seed (Salvia hispanica L.): A systematic review and meta-analysis. Nutr. Rev. 2018, 76, 219–242. [Google Scholar] [CrossRef]
- Vuksan, V.; Jenkins, A.L.; Brissette, C.; Choleva, L.; Jovanovski, E.; Gibbs, A.L.; Bazinet, R.P.; Au-Yeung, F.; Zurbau, A.; Ho, H.V.T.; et al. Salba-chia (Salvia hispanica L.) in the treatment of overweight and obese patients with type 2 diabetes: A double-blind randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Alwosais, E.Z.M.; Al-Ozairi, E.; Zafar, T.A.; Alkandari, S. Chia seed (Salvia hispanica L.) supplementation to the diet of adults with type 2 diabetes improved systolic blood pressure: A randomized controlled trial. Nutr. Health 2021, 27, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Vuksan, V.; Whitham, D.; Sievenpiper, J.L.; Jenkins, A.L.; Rogovik, A.L.; Bazinet, R.P.; Vidgen, E.; Hanna, A. Supplementation of conventional therapy with the novel grain Salba (Salvia hispanica L.) improves major and emerging cardiovascular risk factors in type 2 diabetes. Diabetes Care 2007, 30, 2804–2810. [Google Scholar] [CrossRef]
- Medina-Urrutia, A.; Lopez-Uribe, A.R.; El Hafidi, M.; González-Salazar, M.D.C.; Posadas-Sánchez, R.; Jorge-Galarza, E.; del Valle-Mondragón, L.; Juárez-Rojas, J.G. Chia (Salvia hispanica)-supplemented diet ameliorates non-alcoholic fatty liver disease and its metabolic abnormalities in humans. Lipids Health Dis. 2020, 19, 96. [Google Scholar] [CrossRef]
- Fernández-Martínez, E.; Lira-Islas, I.G.; Cariño-Cortés, R.; Soria-Jasso, L.E.; Pérez-Hernández, E.; Pérez-Hernández, N. Dietary chia seeds (Salvia hispanica) improve acute dyslipidemia and steatohepatitis in rats. J. Food Biochem. 2019, 43, e12986. [Google Scholar] [CrossRef]
- da Silva, B.P.; Toledo, R.C.L.; Mishima, M.D.V.; Moreira, M.E.D.C.; Vasconcelos, C.M.; Pereira, C.E.R.; Favarato, L.S.C.; Costa, N.M.B.; Martino, H.S.D. Effects of chia (Salvia hispanica L.) on oxidative stress and inflammation in ovariectomized adult female Wistar rats. Food Funct. 2019, 10, 4036–4045. [Google Scholar] [CrossRef]
- da Silva Marineli, R.; Lenquiste, S.A.; Moraes, É.A.; Maróstica, M.R. Antioxidant potential of dietary chia seed and oil (Salvia hispanica L.) in diet-induced obese rats. Food Res. Int. 2015, 76, 666–674. [Google Scholar] [CrossRef]
- Oliva, M.E.; del Rosario Ferreira, M.; Vega Joubert, M.B.; D’Alessandro, M.E. Salvia hispanica L. (chia) seed promotes body fat depletion and modulates adipocyte lipid handling in sucrose-rich diet-fed rats. Food Res. Int. 2021, 139, 109842. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.R.; Alvarez, S.M.; Illesca, P.; Giménez, M.S.; Lombardo, Y.B. Dietary Salba (Salvia hispanica L.) ameliorates the adipose tissue dysfunction of dyslipemic insulin-resistant rats through mechanisms involving oxidative stress, inflammatory cytokines and peroxisome proliferator-activated receptor γ. Eur. J. Nutr. 2018, 57, 83–94. [Google Scholar] [CrossRef]
- Oliva, M.E.; Ferreira, M.R.; Chicco, A.; Lombardo, Y.B. Prostaglandins, Leukotrienes and Essential Fatty Acids Dietary Salba (Salvia hispanica L.) seed rich in α-linolenic acid improves adipose tissue dysfunction and the altered skeletal muscle glucose and lipid metabolism in dyslipidemic insulin-resistant. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Creus, A.; Ferreira, M.; Oliva, M.; Lombardo, Y. Mechanisms involved in the improvement of lipotoxicity and impaired lipid metabolism by dietary α-linolenic acid rich Salvia hispanica L. (Salba) seed in the heart of dyslipemic insulin-resistant rats. J. Clin. Med. 2016, 5, 18. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.d.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phyther. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef] [PubMed]
- Vigo, E.; Cepeda, A.; Perez-Fernandez, R.; Gualillo, O. In-vitro anti-inflammatory effect of Eucalyptus globulus and Thymus vulgaris: Nitric oxide inhibition in J774A.1 murine macrophages. J. Pharm. Pharmacol. 2010, 56, 257–263. [Google Scholar] [CrossRef]
- Soliman, M.M.; Aldhahrani, A.; Metwally, M.M.M. Hepatoprotective effect of Thymus vulgaris extract on sodium nitrite-induced changes in oxidative stress, antioxidant and inflammatory marker expression. Sci. Rep. 2021, 11, 5747. [Google Scholar] [CrossRef]
- Hegazy, A.; Abdel-Azeem, A.; Zeidan, H.; Ibrahim, K.; Sayed, E. El Hypolipidemic and hepatoprotective activities of rosemary and thyme in gentamicin-treated rats. Hum. Exp. Toxicol. 2018, 37, 420–430. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Chebat, A.; Fikri-Benbrahim, K. Ethnopharmacology, Phytochemistry, and Pharmacological Properties of Thymus satureioides Coss. Evid.-Based Complement. Altern. Med. 2021, 2021, 6673838. [Google Scholar] [CrossRef]
- Kabbaoui, M.E.L.; Chda, A.; Mejrhit, N.; Farah, A.; Aarab, L.; Bencheikh, R.; Tazi, A. Antidiabetic effect of Thymus satureioides aqueous extract in streptozotocin-induced diabetic rats. Int. J. Pharm. Pharm. Sci. 2016, 8, 140. [Google Scholar] [CrossRef][Green Version]
- Melesie Taye, G.; Bule, M.; Alemayehu Gadisa, D.; Teka, F.; Abula, T. In vivo antidiabetic activity evaluation of aqueous and 80% methanolic extracts of leaves of Thymus schimperi (Lamiaceae) in alloxan-induced diabetic mice. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Cam, M.E.; Hazar-Yavuz, A.N.; Yildiz, S.; Ertas, B.; Ayaz Adakul, B.; Taskin, T.; Alan, S.; Kabasakal, L. The methanolic extract of Thymus praecox subsp. skorpilii var. skorpilii restores glucose homeostasis, ameliorates insulin resistance and improves pancreatic β-cell function on streptozotocin/nicotinamide-induced type 2 diabetic rats. J. Ethnopharmacol. 2019, 231, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Mosele, J.I.; Pizarro, N.; Farràs, M.; de la Torre, R.; Subirana, I.; Pérez-Cano, F.J.; Castañer, O.; Solà, R.; Fernandez-Castillejo, S.; et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: Implications of human gut microbiota. Eur. J. Nutr. 2017, 56, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Farràs, M.; Arranz, S.; Carrión, S.; Subirana, I.; Muñoz-Aguayo, D.; Blanchart, G.; Kool, M.; Solà, R.; Motilva, M.J.; Escolà-Gil, J.C. A functional virgin olive oil enriched with olive oil and thyme phenolic compounds improves the expression of cholesterol efflux-related genes: A randomized, crossover, controlled trial. Nutrients 2019, 11, 1732. [Google Scholar] [CrossRef] [PubMed]
- Rahbardar, M.G.; Hosseinzadeh, H. Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders. Iran. J. Basic Med. Sci. 2020, 23, 1100–1112. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A novel insight on an ancient aromatic plant: The rosemary (Rosmarinus officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Nie, J.; Li, R.; Wang, Y.; Tan, J.; Tang, S.; Jiang, Z. Antioxidant activity evaluation of rosemary ethanol extract and their cellular antioxidant activity toward HeLa cells. J. Food Biochem. 2019, 43, e12851. [Google Scholar] [CrossRef]
- Tu, Z.; Moss-Pierce, T.; Ford, P.; Jiang, T.A. Rosemary (Rosmarinus officinalis L.) extract regulates glucose and lipid metabolism by activating AMPK and PPAR pathways in HepG2 cells. J. Agric. Food Chem. 2013, 61, 2803–2810. [Google Scholar] [CrossRef]
- Stefanon, B.; Pomari, E.; Colitti, M. Effects of Rosmarinus officinalis extract on human primary omental preadipocytes and adipocytes. Exp. Biol. Med. 2015, 240, 884–895. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Tsiani, E. Attenuation of free fatty acid-induced muscle insulin resistance by rosemary extract. Nutrients 2018, 10, 1623. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; El-Sayed, R.A.; Abdel-Daim, M.M. Hepatoprotective potential of Rosmarinus officinalis essential oil against hexavalent chromium-induced hematotoxicity, biochemical, histological, and immunohistochemical changes in male rats. Environ. Sci. Pollut. Res. 2021, 28, 17445–17456. [Google Scholar] [CrossRef] [PubMed]
- Ielciu, I.; Sevastre, B.; Olah, N.-K.; Turdean, A.; Chișe, E.; Marica, R.; Oniga, I.; Uifălean, A.; Sevastre-Berghian, A.C.; Niculae, M.; et al. Evaluation of hepatoprotective activity and oxidative stress reduction of Rosmarinus officinalis L. shoots tincture in rats with experimentally induced hepatotoxicity. Molecules 2021, 26, 1737. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.S.; de O. Silva, A.M.; Carvalho, E.B.; Rivelli, D.P.; Barros, S.B.; Rogero, M.M.; Lottenberg, A.M.; Torres, R.P.; Mancini-Filho, J. Phenolic compounds from Rosemary (Rosmarinus officinalis L.) attenuate oxidative stress and reduce blood cholesterol concentrations in diet-induced hypercholesterolemic rats. Nutr. Metab. 2013, 10, 19. [Google Scholar] [CrossRef]
- Ghasemzadeh Rahbardar, M.; Amin, B.; Mehri, S.; Mirnajafi-Zadeh, S.J.; Hosseinzadeh, H. Anti-inflammatory effects of ethanolic extract of Rosmarinus officinalis L. and rosmarinic acid in a rat model of neuropathic pain. Biomed. Pharmacother. 2017, 86, 441–449. [Google Scholar] [CrossRef]
- Ben Saad, A.; Rjeibi, I.; Alimi, H.; Ncib, S.; Bouhamda, T.; Zouari, N. Protective effects of Mentha spicata against nicotine-induced toxicity in liver and erythrocytes of Wistar rats. Appl. Physiol. Nutr. Metab. 2018, 43, 77–83. [Google Scholar] [CrossRef]
- Bellassoued, K.; Ben Hsouna, A.; Athmouni, K.; van Pelt, J.; Makni Ayadi, F.; Rebai, T.; Elfeki, A. Protective effects of Mentha piperita L. leaf essential oil against CCl4 induced hepatic oxidative damage and renal failure in rats. Lipids Health Dis. 2018, 17, 9. [Google Scholar] [CrossRef]
- Ogaly, H.A.; Eltablawy, N.A.; Abd-Elsalam, R.M. Antifibrogenic influence of Mentha piperita L. essential oil against CCl4-induced liver fibrosis in rats. Oxid. Med. Cell. Longev. 2018, 2018, 4039753. [Google Scholar] [CrossRef]
- Naowaboot, J.; Nanna, U.; Chularojmontri, L.; Songtavisin, T.; Tingpej, P.; Sattaponpan, C.; Jansom, C.; Wattanapitayakul, S. Mentha cordifolia Leaf Extract Improves Hepatic Glucose and Lipid Metabolism in Obese Mice Fed with High-Fat Diet. Prev. Nutr. Food Sci. 2021, 26, 157–165. [Google Scholar] [CrossRef]
- Safaeian, L.; Sajjadi, S.E.; Javanmard, S.H.; Montazeri, H.; Samani, F. Protective effect of Melissa officinalis extract against H2O2-induced oxidative stress in human vascular endothelial cells. Res. Pharm. Sci. 2016, 11, 383–389. [Google Scholar] [CrossRef]
- Park, B.Y.; Lee, H.; Woo, S.; Yoon, M.; Kim, J.; Hong, Y.; Lee, H.S.; Park, E.K.; Hahm, J.C.; Kim, J.W.; et al. Reduction of adipose tissue mass by the angiogenesis inhibitor ALS-L1023 from Melissa officinalis. PLoS ONE 2015, 10, e0141612. [Google Scholar] [CrossRef]
- Lee, D.; Shin, Y.; Roh, J.S.; Ahn, J.; Jeoong, S.; Shin, S.S.; Yoon, M. Lemon balm extract ALS-L1023 regulates obesity and improves insulin sensitivity via activation of hepatic PPARα in high-fat diet-fed obese C57BL/6J mice. Int. J. Mol. Sci. 2020, 21, 4256. [Google Scholar] [CrossRef]
- Kim, M.; Yoo, G.; Randy, A.; Son, Y.-J.; Hong, C.R.; Kim, S.M.; Nho, C.W. Lemon balm and its constituent, rosmarinic acid, alleviate liver damage in an animal model of nonalcoholic steatohepatitis. Nutrients 2020, 12, 1166. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.N.; Pessano, N.T.C.; Leal, L.; Roos, D.H.; Folmer, V.; Puntel, G.O.; Rocha, J.B.T.; Aschner, M.; Ávila, D.S.; Puntel, R.L. Protective effect of Melissa officinalis aqueous extract against Mn-induced oxidative stress in chronically exposed mice. Brain Res. Bull. 2012, 87, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Lee, D.; Ahn, J.; Lee, M.; Shin, S.S.; Yoon, M. The herbal extract ALS-L1023 from Melissa officinalis reduces weight gain, elevated glucose levels and β-cell loss in Otsuka Long-Evans Tokushima fatty rats. J. Ethnopharmacol. 2021, 264, 113360. [Google Scholar] [CrossRef]
- Schmidt, S.; Jakab, M.; Jav, S.; Streif, D.; Pitschmann, A.; Zehl, M.; Purevsuren, S.; Glasl, S.; Ritter, M. Extracts from Leonurus sibiricus L. increase insulin secretion and proliferation of rat INS-1E insulinoma cells. J. Ethnopharmacol. 2013, 150, 85–94. [Google Scholar] [CrossRef]
- LEE, M.-J.; LEE, H.-S.; PARK, S.-D.; MOON, H.-I.; PARK, W.-H. Leonurus sibiricus herb extract suppresses oxidative stress and ameliorates hypercholesterolemia in C57BL/6 mice and TNF-α induced expression of adhesion molecules and lectin-like oxidized LDL receptor-1 in human umbilical vein endothelial cells. Biosci. Biotechnol. Biochem. 2010, 74, 279–284. [Google Scholar] [CrossRef][Green Version]
- Kim, J.; Kim, M.H.; Choi, Y.Y.; Hong, J.; Yang, W.M. Inhibitory effects of Leonurus sibiricus on weight gain after menopause in ovariectomized and high-fat diet-fed mice. J. Nat. Med. 2016, 70, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Akkol, E.K.; Avcı, G.; Küçükkurt, I.; Keleş, H.; Tamer, U.; Ince, S.; Yesilada, E. Cholesterol-reducer, antioxidant and liver protective effects of Thymbra spicata L. var. spicata. J. Ethnopharmacol. 2009, 126, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Avcı, G.; Kupeli, E.; Eryavuz, A.; Yesilada, E.; Kucukkurt, I. Antihypercholesterolaemic and antioxidant activity assessment of some plants used as remedy in Turkish folk medicine. J. Ethnopharmacol. 2006, 107, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Banna, H.; Soliman, M.; Wabel, N. Hepatoprotective effects of Thymus and Salvia essential oils on paracetamol-induced toxicity in rats. J. Physiol. Pharmacol. Adv. 2013, 3, 41. [Google Scholar] [CrossRef]
- Seyedan, A.; Alshawsh, M.; Alshagga, M.; Mohamed, Z. Antiobesity and lipid lowering effects of Orthosiphon stamineus in high-fat diet-induced obese mice. Planta Med. 2016, 83, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Lokman, E.F.; Saparuddin, F.; Muhammad, H.; Omar, M.H.; Zulkapli, A. Orthosiphon stamineus as a potential antidiabetic drug in maternal hyperglycemia in streptozotocin-induced diabetic rats. Integr. Med. Res. 2019, 8, 173–179. [Google Scholar] [CrossRef]
- Lee, M.R.; Yang, H.J.; Park, K.I.L.; Ma, J.Y. Lycopus lucidus Turcz. ex Benth. Attenuates free fatty acid-induced steatosis in HepG2 cells and non-alcoholic fatty liver disease in high-fat diet-induced obese mice. Phytomedicine 2019, 55, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kang, D.G.; Kim, J.S.; Lee, H.S. Lycopus lucidus inhibits high glucose-induced vascular inflammation in human umbilical vein endothelial cells. Vascul. Pharmacol. 2008, 48, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-H.; Tian, C.-R.; Gao, C.-Y.; Wang, W.-J.; Yang, W.-Y.; Kong, X.; Chen, Y.-X.; Liu, Z.-Z. Protective effect of free phenolics from Lycopus lucidus Turcz. root on carbon tetrachloride-induced liver injury in vivo and in vitro. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, M.; Yu, H.; Li, J.; Wang, S.; Zhang, Y.; Qiu, F.; Wang, T. Scutellaria baicalensis regulates FFA metabolism to ameliorate NAFLD through the AMPK-mediated SREBP signaling pathway. J. Nat. Med. 2018, 72, 655–666. [Google Scholar] [CrossRef]
- Song, K.H.; Lee, S.H.; Kim, B.-Y.; Park, A.Y.; Kim, J.Y. Extracts of Scutellaria baicalensis reduced body weight and blood triglyceride in db/db mice. Phyther. Res. 2013, 27, 244–250. [Google Scholar] [CrossRef]
- Na, H.-Y.; Lee, B.-C. Scutellaria baicalensis Alleviates insulin resistance in diet-induced obese mice by modulating inflammation. Int. J. Mol. Sci. 2019, 20, 727. [Google Scholar] [CrossRef]
- Chao, P.-Y.; Chiang, T.-I.; Chang, I.-C.; Tsai, F.-L.; Lee, H.-H.; Hsieh, K.; Chiu, Y.-W.; Lai, T.-J.; Liu, J.-Y.; Hsu, L.-S.; et al. Amelioration of estrogen-deficiency-induced obesity by Ocimum gratissimum. Int. J. Med. Sci. 2017, 14, 896–901. [Google Scholar] [CrossRef][Green Version]
- Gamboa-Gómez, C.; Salgado, L.M.; González-Gallardo, A.; Ramos-Gómez, M.; Loarca-Piña, G.; Reynoso-Camacho, R. Consumption of Ocimum sanctum L. and Citrus paradisi infusions modulates lipid metabolism and insulin resistance in obese rats. Food Funct. 2014, 5, 927–935. [Google Scholar] [CrossRef]
- Suanarunsawat, T.; Ayutthaya, W.D.N.; Songsak, T.; Thirawarapan, S.; Poungshompoo, S. Lipid-lowering and antioxidative activities of aqueous extracts of Ocimum sanctum L. leaves in rats fed with a high-cholesterol diet. Oxid. Med. Cell. Longev. 2011, 2011, 962025. [Google Scholar] [CrossRef] [PubMed]
- Suanarunsawat, T.; Anantasomboon, G.; Piewbang, C. Anti-diabetic and anti-oxidative activity of fixed oil extracted from Ocimum sanctum L. leaves in diabetic rats. Exp. Ther. Med. 2016, 11, 832–840. [Google Scholar] [CrossRef]
- Suanarunsawat, T.; Devakul Na Ayutthaya, W.; Songsak, T.; Thirawarapan, S.; Poungshompoo, S. Antioxidant activity and lipid-lowering effect of essential oils extracted from Ocimum sanctum L. leaves in rats fed with a high cholesterol diet. J. Clin. Biochem. Nutr. 2009, 46, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shen, M.; Wu, T.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Mesona chinensis Benth polysaccharides protect against oxidative stress and immunosuppression in cyclophosphamide-treated mice via MAPKs signal transduction pathways. Int. J. Biol. Macromol. 2020, 152, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Ojewole, J.A.O. Antinociceptive, anti-inflammatory and antidiabetic effects of Leonotis leonurus (L.) R. Br. [Lamiaceae] leaf aqueous extract in mice and rats. Methods Find. Exp. Clin. Pharmacol. 2005, 27, 257. [Google Scholar] [CrossRef]
- Mnonopi, N.; Levendal, R.-A.; Mzilikazi, N.; Frost, C.L. Marrubiin, a constituent of Leonotis leonurus, alleviates diabetic symptoms. Phytomedicine 2012, 19, 488–493. [Google Scholar] [CrossRef]
- Odei-Addo, F.; Ramlugon, S.; Levendal, R.-A.; Frost, C.L. Leonotis Leonurus improves the crosstalk between peripheral tissues both in vivo and in vitro. J. Ethnopharmacol. 2021, 267, 113609. [Google Scholar] [CrossRef]
- Adımcılar, V.; Kalaycıoğlu, Z.; Aydoğdu, N.; Dirmenci, T.; Kahraman, A.; Erim, F.B. Rosmarinic and carnosic acid contents and correlated antioxidant and antidiabetic activities of 14 Salvia species from Anatolia. J. Pharm. Biomed. Anal. 2019, 175, 112763. [Google Scholar] [CrossRef]
- Park, M.-Y.; Sung, M.-K. Carnosic acid attenuates obesity-induced glucose intolerance and hepatic fat accumulation by modulating genes of lipid metabolism in C57BL/6J-ob/ob mice. J. Sci. Food Agric. 2015, 95, 828–835. [Google Scholar] [CrossRef]
- Wang, T.; Takikawa, Y.; Satoh, T.; Yoshioka, Y.; Kosaka, K.; Tatemichi, Y.; Suzuki, K. Carnosic acid prevents obesity and hepatic steatosis in ob/ob mice. Hepatol. Res. 2011, 41, 87–92. [Google Scholar] [CrossRef]
- Birtić, S.; Dussort, P.; Pierre, F.-X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef]
- Wang, S.-J.; Chen, Q.; Liu, M.-Y.; Yu, H.-Y.; Xu, J.-Q.; Wu, J.-Q.; Zhang, Y.; Wang, T. Regulation effects of rosemary (Rosmarinus officinalis Linn.) on hepatic lipid metabolism in OA induced NAFLD rats. Food Funct. 2019, 10, 7356–7365. [Google Scholar] [CrossRef] [PubMed]
- Bakirel, T.; Bakirel, U.; Keleş, O.U.; Ulgen, S.G.; Yardibi, H. In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. J. Ethnopharmacol. 2008, 116, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Romo-Vaquero, M.; Larrosa, M.; Yáñez-Gascón, M.J.; Issaly, N.; Flanagan, J.; Roller, M.; Tomás-Barberán, F.A.; Espín, J.C.; García-Conesa, M.-T. A rosemary extract enriched in carnosic acid improves circulating adipocytokines and modulates key metabolic sensors in lean Zucker rats: Critical and contrasting differences in the obese genotype. Mol. Nutr. Food Res. 2014, 58, 942–953. [Google Scholar] [CrossRef]
- Quirarte-Báez, S.M.; Zamora-Perez, A.L.; Reyes-Estrada, C.A.; Gutiérrez-Hernández, R.; Sosa-Macías, M.; Galaviz-Hernández, C.; Manríquez, G.G.G.; Lazalde-Ramos, B.P. A shortened treatment with rosemary tea (Rosmarinus officinalis) instead of glucose in patients with diabetes mellitus type 2 (TSD). J. Popul. Ther. Clin. Pharmacol. 2019, 26, e18–e28. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.D.; Peltoketo, A.; Hiltunen, R.; Tikkanen, M.J. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003, 83, 255–262. [Google Scholar] [CrossRef]
- Mahendran, G.; Verma, S.K.; Rahman, L.-U. The traditional uses, phytochemistry and pharmacology of spearmint (Mentha spicata L.): A review. J. Ethnopharmacol. 2021, 278, 114266. [Google Scholar] [CrossRef] [PubMed]
- Farid, O.; El Haidani, A.; Eddouks, M. Antidiabetic Effect of Spearmint in Streptozotocin-Induced Diabetic Rats. Endocr. Metab. Immune Disord.-Drug Targets 2018, 18, 581–589. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phyther. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef]
- Abdellatief, S.A.; Beheiry, R.R.; El-Mandrawy, S.A.M. Peppermint essential oil alleviates hyperglycemia caused by streptozotocin-nicotinamide-induced type 2 diabetes in rats. Biomed. Pharmacother. 2017, 95, 990–999. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, M.K.; Kumar, M. Protective effect of Mentha piperita against arsenic-induced toxicity in liver of Swiss Albino mice. Basic Clin. Pharmacol. Toxicol. 2007, 100, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Samaha, A.A.; Fawaz, M.; Salami, A.; Baydoun, S.; Eid, A.H. Antihypertensive indigenous lebanese plants: Ethnopharmacology and a clinical trial. Biomolecules 2019, 9, 292. [Google Scholar] [CrossRef]
- Jandaghi, P.; Noroozi, M.; Ardalani, H.; Alipour, M. Lemon balm: A promising herbal therapy for patients with borderline hyperlipidemia—A randomized double-blind placebo-controlled clinical trial. Complement. Ther. Med. 2016, 26, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Sipos, S.; Moacă, E.-A.; Pavel, I.Z.; Avram, Ş.; Crețu, O.M.; Coricovac, D.; Racoviceanu, R.-M.; Ghiulai, R.; Pană, R.D.; Şoica, C.M.; et al. Melissa officinalis L. aqueous extract exerts antioxidant and antiangiogenic effects and improves physiological skin parameters. Molecules 2021, 26, 2369. [Google Scholar] [CrossRef]
- Asadi, A.; Shidfar, F.; Safari, M.; Hosseini, A.F.; Fallah Huseini, H.; Heidari, I.; Rajab, A. Efficacy of Melissa officinalis L. (lemon balm) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: A randomized, double-blind, clinical trial. Phytother. Res. 2019, 33, 651–659. [Google Scholar] [CrossRef]
- Chusak, C.; Thilavech, T.; Adisakwattana, S. Consumption of Mesona chinensis attenuates postprandial glucose and improves antioxidant status induced by a high carbohydrate meal in overweight subjects. Am. J. Chin. Med. 2014, 42, 315–336. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.A.; Alam, M.A.; Islam, M.S.; Ali, M.T.; Ullah, M.E.; Shibly, A.Z.; Ali, M.A.; Hasan-Olive, M.M. Leonurus sibiricus L. (honeyweed): A review of its phytochemistry and pharmacology. Asian Pac. J. Trop. Biomed. 2016, 6, 1076–1080. [Google Scholar] [CrossRef]
- Sitarek, P.; Skała, E.; Wysokińska, H.; Wielanek, M.; Szemraj, J.; Toma, M.; Śliwiński, T. The effect of Leonurus sibiricus plant extracts on stimulating repair and protective activity against oxidative DNA damage in CHO cells and content of phenolic compounds. Oxid. Med. Cell. Longev. 2016, 2016, 5738193. [Google Scholar] [CrossRef]
- Ünlü, M.; Vardar-Ünlü, G.; Vural, N.; Dönmez, E.; Özbaş, Z.Y. Chemical composition, antibacterial and antifungal activity of the essential oil of Thymbra spicata L. from Turkey. Nat. Prod. Res. 2009, 23, 572–579. [Google Scholar] [CrossRef]
- Hancı, S.; Sahin, S.; Yılmaz, L. Isolation of volatile oil from thyme (Thymbra spicata) by steam distillation. Food/Nahrung 2003, 47, 252–255. [Google Scholar] [CrossRef]
- Verdeguer, M.; Torres-Pagan, N.; Muñoz, M.; Jouini, A.; García-Plasencia, S.; Chinchilla, P.; Berbegal, M.; Salamone, A.; Agnello, S.; Carrubba, A.; et al. Herbicidal activity of Thymbra capitata (L.) Cav. essential oil. Molecules 2020, 25, 2832. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.; Barroso, J.; Pedro, L.; Salgueiro, L.; Miguel, M.; Faleiro, M. Portuguese Thymbra and Thymus species volatiles: Chemical composition and biological activities. Curr. Pharm. Des. 2008, 14, 3120–3140. [Google Scholar] [CrossRef] [PubMed]
- Faleiro, L.; Miguel, G.; Gomes, S.; Costa, L.; Venâncio, F.; Teixeira, A.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Antibacterial and antioxidant activities of essential oils isolated from Thymbra capitata L. (Cav.) and Origanum vulgare L. J. Agric. Food Chem. 2005, 53, 8162–8168. [Google Scholar] [CrossRef]
- Aazza, S.; El-Guendouz, S.; Miguel, M.G.; Antunes, M.D.; Faleiro, M.L.; Correia, A.I.; Figueiredo, A.C. Antioxidant, anti-inflammatory and anti-hyperglycaemic activities of essential oils from Thymbra capitata, Thymus albicans, Thymus caespititius, Thymus carnosus, Thymus lotocephalus and Thymus mastichina from Portugal. Nat. Prod. Commun. 2016, 11, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Awale, S.; Tezuka, Y.; Banskota, A.H.; Kadota, S. Siphonols A–E: Novel nitric oxide inhibitors from Orthosiphon stamineus of Indonesia. Bioorg. Med. Chem. Lett. 2003, 13, 31–35. [Google Scholar] [CrossRef]
- Sumaryono, W.; Proksch, P.; Wray, V.; Witte, L.; Hartmann, T. Qualitative and quantitative analysis of the phenolic constituents from Orthosiphon aristatus. Planta Med. 1991, 57, 176–180. [Google Scholar] [CrossRef]
- Mohamed, E.A.H.; Mohamed, A.J.; Asmawi, M.Z.; Sadikun, A.; Ebrika, O.S.; Yam, M.F. Antihyperglycemic effect of Orthosiphon stamineus benth leaves extract and its bioassay-guided fractions. Molecules 2011, 16, 3787–3801. [Google Scholar] [CrossRef]
- Ren, Q.; Ding, L.; Sun, S.; Wang, H.; Qu, L. Chemical identification and quality evaluation of Lycopus lucidus Turcz by UHPLC-Q-TOF-MS and HPLC-MS/MS and hierarchical clustering analysis. Biomed. Chromatogr. 2017, 31, e3867. [Google Scholar] [CrossRef]
- Nan, J.-X.; Park, E.-J.; Kim, Y.-C.; Ko, G.; Sohn, D.H. Scutellaria baicalensis inhibits liver fibrosis induced by bile duct ligation or carbon tetrachloride in rats. J. Pharm. Pharmacol. 2010, 54, 555–563. [Google Scholar] [CrossRef]
- Han, Y.K.; Kim, H.; Shin, H.; Song, J.; Lee, M.K.; Park, B.; Lee, K.Y. Characterization of anti-inflammatory and antioxidant constituents from Scutellaria baicalensis using LC-MS coupled with a bioassay method. Molecules 2020, 25, 3617. [Google Scholar] [CrossRef]
- Das, S.; Barman, S.; Teron, R.; Bhattacharya, S.S.; Kim, K.-H. Secondary metabolites and anti-microbial/anti-oxidant profiles in Ocimum spp.: Role of soil physico-chemical characteristics as eliciting factors. Environ. Res. 2020, 188, 109749. [Google Scholar] [CrossRef] [PubMed]
- Vats, V.; Yadav, S.; Grover, J. Ethanolic extract of Ocimum sanctum leaves partially attenuates streptozotocin-induced alterations in glycogen content and carbohydrate metabolism in rats. J. Ethnopharmacol. 2004, 90, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Semwal, A.; Kumar, H.; Verma, H.C.; Kumar, A. In-vivo study for anti-hyperglycemic potential of aqueous extract of Basil seeds (Ocimum basilicum Linn) and its influence on biochemical parameters, serum electrolytes and haematological indices. Biomed. Pharmacother. 2016, 84, 2008–2013. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Takahashi-Muto, C.; Nagase, M.; Kassai, M.; Tanaka-Yachi, R.; Kiyose, C. Anti-inflammatory effects of extracts of sweet basil (Ocimum basilicum L.) on a co-culture of 3T3-L1 adipocytes and RAW264.7 macrophages. J. Oleo Sci. 2020, 69, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Genfi, A.K.A.; Larbie, C.; Emikpe, B.O.; Oyagbemi, A.A.; Firempong, C.K.; Adjei, C.O. Modulation of oxidative stress and inflammatory cytokines as therapeutic mechanisms of Ocimum americanum L extract in carbon tetrachloride and acetaminophen-induced toxicity in rats. J. Evid.-Based Integr. Med. 2020, 25, 2515690X2093800. [Google Scholar] [CrossRef]
- Lahon, K.; Das, S. Hepatoprotective activity of Ocimum sanctum alcoholic leaf extract against paracetamol-induced liver damage in Albino rats. Pharmacogn. Res. 2011, 3, 13. [Google Scholar] [CrossRef]
- Ogaly, H.; Eltablawy, N.; El-Behairy, A.; El-Hindi, H.; Abd-Elsalam, R. Hepatocyte growth factor mediates the antifibrogenic action of Ocimum bacilicum essential oil against CCl4-Induced liver fibrosis in rats. Molecules 2015, 20, 13518–13535. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.; Das, N.; Bandyopadhyay, D.; Mahapatra, S.C.; Sahu, D.S.; Meda, M. Effect of Tulsi (Ocimum sanctum Linn.) supplementation on metabolic parameters and liver enzymes in young overweight and obese subjects. Indian J. Clin. Biochem. 2017, 32, 357–363. [Google Scholar] [CrossRef]
- Akbarian, S.-A.; Asgary, S.; Feizi, A.; Iraj, B.; Askari, G. Comparative study on the effect of Plantago psyllium and Ocimum basilicum seeds on anthropometric measures in nonalcoholic fatty liver patients. Int. J. Prev. Med. 2016, 7, 114. [Google Scholar] [CrossRef]
- Tang, W.; Shen, M.; Xie, J.; Liu, D.; Du, M.; Lin, L.; Gao, H.; Hamaker, B.R.; Xie, M. Physicochemical characterization, antioxidant activity of polysaccharides from Mesona chinensis Benth and their protective effect on injured NCTC-1469 cells induced by H2O2. Carbohydr. Polym. 2017, 175, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Ouakouak, H.; Benchikha, N.; Hassani, A.; Ashour, M.L. Chemical composition and biological activity of Mentha citrata Ehrh., essential oils growing in southern Algeria. J. Food Sci. Technol. 2019, 56, 5346–5353. [Google Scholar] [CrossRef] [PubMed]
- Nammi, S.; Koka, S.; Chinnala, K.M.; Boini, K.M. Obesity: An overview on its current perspectives and treatment options. Nutr. J. 2004, 3, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diab, F.; Zbeeb, H.; Baldini, F.; Portincasa, P.; Khalil, M.; Vergani, L. The Potential of Lamiaceae Herbs for Mitigation of Overweight, Obesity, and Fatty Liver: Studies and Perspectives. Molecules 2022, 27, 5043. https://doi.org/10.3390/molecules27155043
Diab F, Zbeeb H, Baldini F, Portincasa P, Khalil M, Vergani L. The Potential of Lamiaceae Herbs for Mitigation of Overweight, Obesity, and Fatty Liver: Studies and Perspectives. Molecules. 2022; 27(15):5043. https://doi.org/10.3390/molecules27155043
Chicago/Turabian StyleDiab, Farah, Hawraa Zbeeb, Francesca Baldini, Piero Portincasa, Mohamad Khalil, and Laura Vergani. 2022. "The Potential of Lamiaceae Herbs for Mitigation of Overweight, Obesity, and Fatty Liver: Studies and Perspectives" Molecules 27, no. 15: 5043. https://doi.org/10.3390/molecules27155043
APA StyleDiab, F., Zbeeb, H., Baldini, F., Portincasa, P., Khalil, M., & Vergani, L. (2022). The Potential of Lamiaceae Herbs for Mitigation of Overweight, Obesity, and Fatty Liver: Studies and Perspectives. Molecules, 27(15), 5043. https://doi.org/10.3390/molecules27155043