An Exploration of the Effect of the Kleier Model and Carrier-Mediated Theory to Design Phloem-Mobile Pesticides Based on Researching the N-Alkylated Derivatives of Phenazine-1-Carboxylic Acid-Glycine
Abstract
1. Introduction
2. Results and Discussion
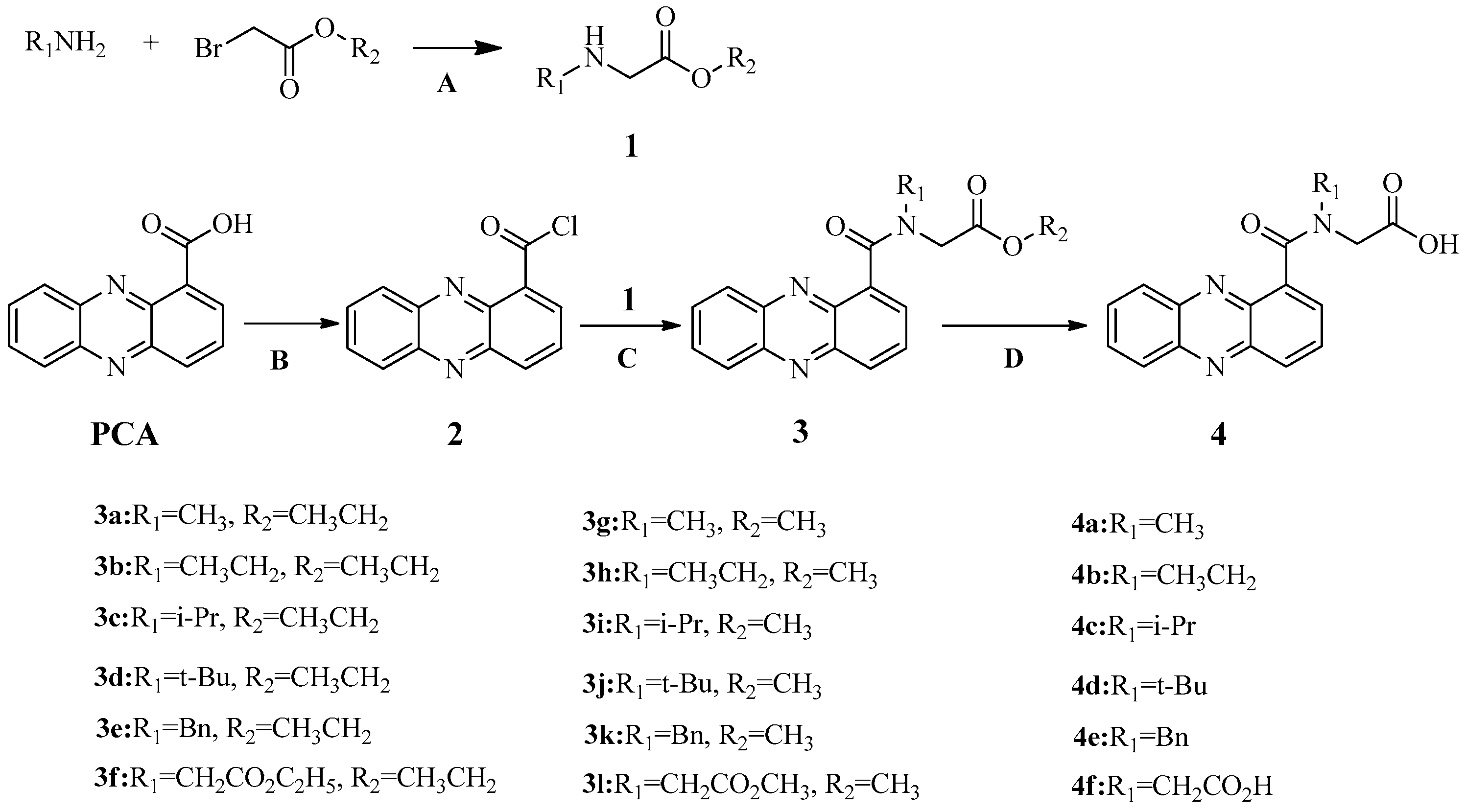
2.1. Synthesis
2.2. Phloem Mobility in R. communis Seedlings
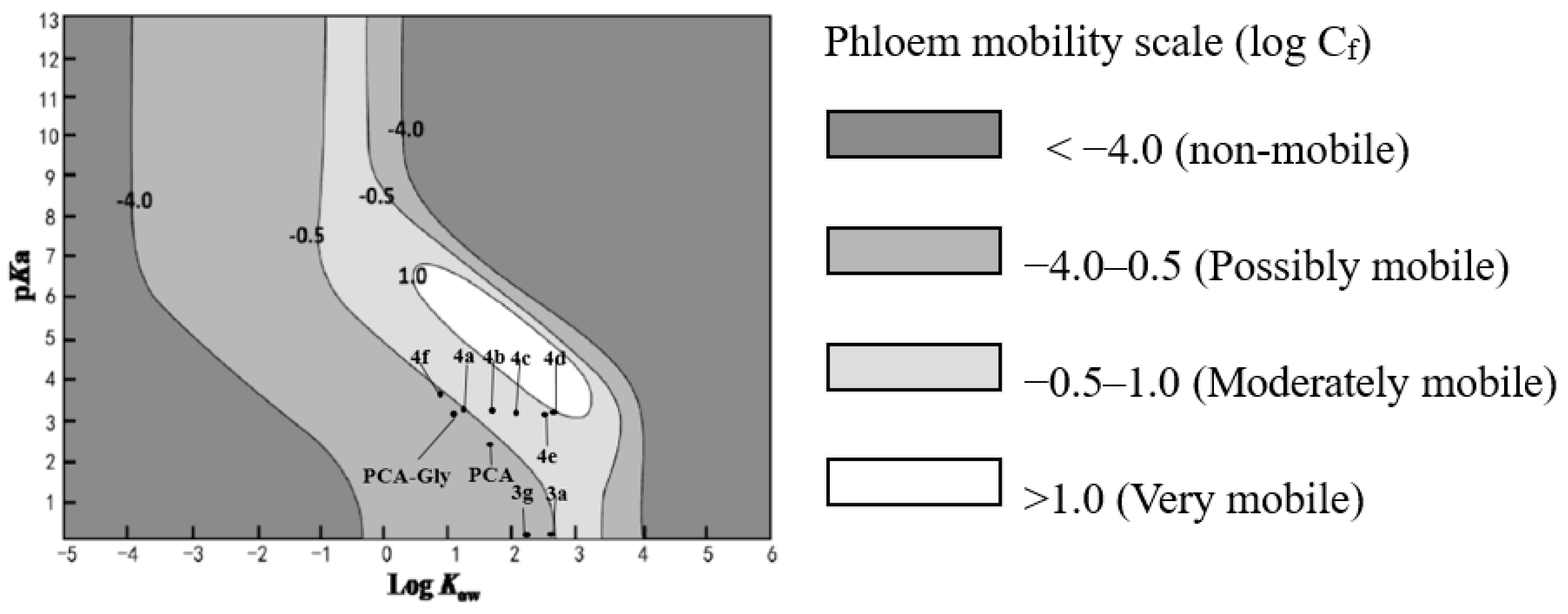
2.3. Prediction of Phloem Mobility Using the Kleier Model
2.4. Phloem Mobility in Adult Castor Bean Plants
3. Materials and Methods
3.1. Chemicals
3.2. Plant Materials
3.3. General Synthesis Procedure for Title Compounds 3a–3l and 4a–4f
3.3.1. General Procedure for Glycine Ester Derivatives 1
3.3.2. Synthesis of Phenazine-1-Carbonyl Chloride 2
3.3.3. General Procedure for PCA-Glycine Ester Derivatives 3a–3l
3.3.4. General Procedure for PCA-Glycine Derivatives 4a–4f
3.4. Sap Collection from R. communis L. Seedlings
3.5. Phloem Mobility in Adult Castor Bean Plants
3.6. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xu, H.H.; Zhang, Z.X.; Zha, Y.G. The prospect of botanical pesticides in China. Pesticides 2003, 42, 1–10. [Google Scholar]
- Delétage-Grandon, C.; Chollet, J.F.; Faucher, M.; Rocher, F.; Komor, E.; Bonnemain, J.-L. Carrier-Mediated Uptake and Phloem Systemy of a 350-Dalton Chlorinated Xenobiotic with an α-Amino Acid Function. Plant Physiol. 2001, 125, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Reckmann, U.; Thomzik, J.; Thielert, W. Biological profile of spirotetramat (Movento®)—A new two-way systemic (ambimobile) insecticide against sucking pest species. Bayer Crop Sci. J. 2008, 2, 245–278. [Google Scholar]
- Smith, K.; Evans, D.A.; El-Hiti, G.A. Role of modern chemistry in sustainable arable crop protection. Philos. Trans. R. Soc. B 2008, 1491, 623–637. [Google Scholar] [CrossRef]
- Kleier, D.A. Phloem mobility of xenobiotics. I. Mathematicalmodel unifying the weak acid and intermediate permeabilitytheories. Plant Physiol. 1988, 86, 803–810. [Google Scholar] [CrossRef]
- Hsu, F.C.; Sun, K.; Kleier, D.A.; Fielding, M.J. Phloem mobility of xenobiotics VI. A phloem-mobile pro-nematicide based on oxamyl exhibiting root-specific activation in transgenic tobacco. Pestic. Sci. 1995, 44, 9–19. [Google Scholar] [CrossRef]
- Kleier, D.A.; Hsu, F.C. Phloem mobility of xenobiotics. VII. The design of phloem systemic pesticides. Weed Sci. 1996, 44, 749–756. [Google Scholar] [CrossRef]
- Hsu, F.C.; Kleier, D.A. Phloem mobility of xenobiotics VIII. A short review. Exp. Bot. 1996, 47, 1265–1271. [Google Scholar] [CrossRef]
- Grimm, E.; Grube, A.; Jahnke, S.; Neumann, S. Retention of xenobiotics along the phloem path. Planta 1995, 197, 11–18. [Google Scholar] [CrossRef]
- Chollet, J.F.; Rocher, F.; Jousse, C.; Delétage-Grandon, C.; Bashiardes, G.; Bonnemain, J.L. Synthesis and phloem mobility of acidic derivatives of the fungicide fenpiclonil. Pest Manag. Sci. 2004, 60, 1063–1072. [Google Scholar] [CrossRef]
- Leconte, F.; Bonnemain, J.L.; Decormis, L.; Barchietto, T. Metabolic fate, compartmentation and forms of transport (xylem and phloem mobility) of fosethyl-Al in Lycopersicon-esculentum Mill. CR Acad. Sci. Ser. III Sci. Vie/Life Sci. 1988, 307, 221–227. [Google Scholar]
- Fujita, M.; Fujita, Y.; Iuchi, S.; Yamada, K.; Kobayashi, Y.; Urano, K.; Iuchi, S.; Kobayashi, M.; Yamagucho-Shinozaki, K.; Shinozaki, K. Natural variation in a polyamine transporter determines paraquat tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 6343–6347. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, R. Phloem loading: How leaves gain their independence. Bioscience 2006, 56, 15–24. [Google Scholar] [CrossRef]
- Fischer, W.N.; Loo, D.D.; Koch, W.; Ludewig, U.; Boorer, K.J.; Tegeder, M.; Frommer, W.B. Low and high affinity amino acid H+-cotransporters for cellular import of neutral and charged amino acids. Plant 2002, 29, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.X.; Lu, X.L.; Hu, S.; Zhang, X.-B.; Xu, H.-H. A new derivative of fipronil: Effect of adding a glycinyl group to the 5-amine of pyrazole on phloem mobility and insecticidal activity. Pestic. Biochem. Physiol. 2009, 95, 126–130. [Google Scholar] [CrossRef]
- Wu, H.X.; Marhadour, S.; Lei, Z.W.; Yang, W.; Marivingt-Mounir, C.; Bonnemain, J.-L.; Chollet, J.-F. Vectorization of agrochemicals: Amino acid carriers are more efficient than sugar carriers to translocate phenylpyrrole conjugates in the Ricinus system. Environ. Sci. Pollut. Res. 2018, 25, 14336–14349. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, J.L.; Wang, C.W.; Yu, A.-X.; Liu, N.; Chen, L.; Lin, F.; Xu, H.-H. Glycinergic-fipronil uptake is mediated by an amino acid carrier system and induces the expression of amino acid transporter genes in Ricinus communis seedlings. Agric. Food Chem. 2016, 64, 3810–3818. [Google Scholar] [CrossRef]
- Wu, H.; Xu, H.; Marivingt-Mounir, C.; Bonneiman, J.-L.; Chollet, J.-F. Vectorizing agrochemicals: Enhancing bioavailability via carrier ediated transport. Pest Manag. Sci. 2018, 75, 1507–1516. [Google Scholar] [CrossRef]
- Ge, Y.; Huang, X.; Wang, S.; Zhang, X.; Xu, Y. Phenazine-1-carboxylic acid is negatively regulated and pyoluteorin positively regulated by gacA in Pseudomonas sp. M18. FEMS Microbiol. Lett. 2004, 237, 41–47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shao, J.; Fan, L.Y.; Zhang, W.; Guo, C.G.; Li, S.; Xu, Y.Q.; Cao, C.X. Purification of low-concentration phenazine-1-carboxylic acid from fermentation broth of Pseudomonas sp. M18 via free flow electrophoresis with gratis gravity. Electrophoresis 2010, 31, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, L.S.; Weller, D.M. Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. Bacteriology 1988, 170, 3499–3508. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Moon, S.S.; Hwang, B.K. Isolation and in vitro and in vivo activity against Phytophthora capsici and Colletotrichum orbiculare of phenazine-1-carboxylic acid from Pseudomonas aeruginosa strain GC-B26. Pest Manag. Sci. 2003, 59, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Nie, D.; Yu, D.; Wu, Q.; Yu, L.; Yao, Z.; Li, J. Synthesis, fungicidal activity and phloem mobility of phenazine-1-carboxylic acid-alanine conjugates. Pest. Biochem. Physiol. 2017, 143, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Huang, D.; Zhu, X.; Zhang, M.; Yao, Z.; Wu, Q.; Li, J. Design, Synthesis, Phloem Mobility, and Bioactivities of a Series of Phenazine-1-Carboxylic Acid-Amino Acid Conjugates. Molecules 2018, 23, 2139. [Google Scholar] [CrossRef]
- Zhong, W.; Hartung, W.; Komor, E.; Schobert, C. Phloem transport of abscisic acid in Ricinus communis L. seedlings. Plant Cell Environ. 1996, 19, 471–477. [Google Scholar] [CrossRef]
- Kleier, D.A.; Grayson, B.T.; Hsu, F.C. The phloem mobility of pesticides. Pestic. Outlook 1998, 9, 26–30. [Google Scholar]
- Niu, J.; Chen, J.; Xu, Z.; Zhu, X.; Wu, Q.; Li, J. Synthesis and bioactivities of amino acid ester conjugates of phenazine-1-carboxylic acid. Bioorg. Med. Chem. Lett. 2016, 26, 5384–5386. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, M.; Yu, L.; Xu, Z.; Yang, D.; Du, X.; Li, J. Synthesis and bioactivities of diamide derivatives containing a phenazine-1-carboxamide scaffold. Nat. Prod. Res. 2018, 33, 2453–2460. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, L.; Hsiang, T.; Huang, D.; Xu, Z.; Wu, Q.; Du, X.; Li, J. The influence of steric configuration of phenazine-1-carboxylic acid-amino acid conjugates on fungicidal activity and systemicity. Pest Manag. Sci. 2019, 75, 3323–3330. [Google Scholar] [CrossRef]
- Xiong, Y.T.; Zhu, X.; Hu, J.Y.; Wang, Y.; Du, X.; Li, J.; Wu, Q. Effect of introducing amino acids into phenazine-1-carboxylic acid on phloem mobility. Nat. Prod. Res. 2020, 35, 4373–4379. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, M.; Xiao, Y.; Hsiang, T.; Hu, C.; Li, J. Systemic fungicidal activity of phenazine-1-carboxylic acid-valine conjugate against tobacco sore shin and its translocation and accumulation in tobacco (Nicotiana tabacum L.). Pest Manag. Sci. 2022, 78, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wu, H.-X.; Xu, H.-H.; Hu, A.-L.; Lu, M.-L. Synthesis of glucose-fipronil conjugate and its phloem mobility. Agric. Food Chem. 2011, 59, 12534–12542. [Google Scholar] [CrossRef] [PubMed]
- Aurelio, L.; Brownlee, R.T.; Hughes, A.B. Synthetic preparation of N-methyl-α-amino acids. Chem. Rev. 2004, 104, 5823–5846. [Google Scholar] [CrossRef] [PubMed]
- Parmar, N.J.; Pansuriya, B.R.; Labana, B.M.; Kant, R.; Gupta, V.K. A convenient 1,3-dipolar cycloaddition–reduction synthetic sequence from 2-allyloxy-5-nitro-salicylaldehyde to aminobenzopyran-annulated heterocycles. RSC Adv. 2013, 3, 17527–17539. [Google Scholar] [CrossRef]

| Compd. | Concentration in Phloem Sap a (uM) | |||||
|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | Average Concentration | |
| 3a | NDb | ND | ND | ND | ND | 0 |
| 3g | ND | ND | ND | ND | ND | 0 |
| 4a | ND | 14.06 ± 1.04 b | 16.69 ± 0.79 b | 19.41 ± 1.33 b | 22.96 ± 2.01 c | 14.62 |
| 4b | ND | ND | 17.44 ± 0.55 ab | 22.56 ± 0.21 ab | 29.90 ± 0.49 a | 13.98 |
| 4c | ND | 21.52 ± 1.77 a | 18.58 ± 1.27 a | 22.79 ± 0.96 a | 25.26 ± 0.39 b | 17.63 |
| 4d | ND | 5.29 ± 0.25 c | 4.65 ± 0.67 cd | ND | ND | 1.99 |
| 4e | 0.74 ± 0.23 a | 2.30 ± 0.32 d | 0.96 ± 0.11 e | 0.95 ± 0.19 c | 1.09 ± 0.07 d | 1.21 |
| 4f | 0.54 ± 0.18 a | 0.56 ± 0.21 d | 0.83 ± 0.18 e | 1.18 ± 0.22 c | 1.56 ± 0.13 d | 0.93 |
| PCA-Gly | ND | 2.10 ± 0.12 d | 2.51 ± 0.22 d | 2.08 ± 0.16 c | 1.88 ± 0.27 d | 1.714 |
| PCA | ND | ND | ND | ND | ND | 0 |
| Compound | Molecular Formula | Molecular Weight (g/mol) | pKa | Log Kow |
|---|---|---|---|---|
| 3a | C18H17N3O3 | 323.35 | 0.05 | 2.62 |
| 3g | C17H15N3O3 | 309.32 | 0.04 | 2.22 |
| 4a | C16H13N3O3 | 295.29 | 3.31 | 1.23 |
| 4b | C17H15N3O3 | 309.32 | 3.31 | 1.73 |
| 4c | C18H17N3O3 | 323.35 | 3.28 | 2.09 |
| 4d | C19H19N3O3 | 337.37 | 3.31 | 2.61 |
| 4e | C22H17N3O3 | 371.39 | 3.30 | 2.58 |
| 4f | C17H13N3O5 | 339.30 | 3.56 | 0.98 |
| PCA-Gly | C15H11N3O3 | 281.27 | 3.29 | 1.06 |
| PCA | C13H8N2O2 | 224.21 | 2.34 | 1.59 |
| Concentration (mmol/L) | Content (μg/Kg) | |||||
|---|---|---|---|---|---|---|
| 3 h | 6 h | 12 h | 18 h | 24 h | ||
| root | 1 | 0.14 ± 0.02 d | 9.36 ± 0.11 a | 3.13 ± 0.14 c | 2.96 ± 0.09 cd | 4.18 ± 0.21 b |
| 2 | 0.58 ± 0.04 d | 11.05 ± 0.38 a | 4.15 ± 0.19 c | 3.87 ± 0.22 c | 7.16 ± 0.18 b | |
| 5 | 5.44 ± 0.17 b | 29.92 ± 0.77 a | 34.38 ± 6.91 a | 19.54 ± 4.35 ab | 17.98 ± 3.82 ad | |
| 4a | 4b | 4c | 4d |
|---|---|---|---|
| 95.35 ± 3.27 a | 10.32 ± 0.74 c | 34.38 ± 1.96 b | ND |
| 4e | 4f | PCA-Gly | PCA |
| 0.72 ± 0.11 d | 0.03 ± 0.01 d | 7.01 ± 0.72 d | ND |
| Compound | Linear Equations | R2 | LOQ (mg/L) |
|---|---|---|---|
| 4a | y = 4.6 × 10−7x + 0.3213 | 0.9996 | 0.1 |
| 4b | y = 1.7 × 10−7x + 0.2596 | 0.9993 | 0.1 |
| 4c | y = 9.1 × 10−8x + 0.0389 | 0.9998 | 0.3 |
| 4d | y = 1.8 × 10−7x − 0.0782 | 0.9999 | 0.1 |
| 4e | y = 1.1 × 10−10x − 0.0007 | 0.9983 | 0.2 |
| 4f | y = 8.4 × 10−7x − 0.3128 | 0.9988 | 0.1 |
| PCA-Gly | y = 6.7 × 10−7x + 0.1035 | 0.9993 | 0.4 |
| Compound | Linear Equations | R2 |
|---|---|---|
| 4a | y = 3.4 × 10−7x + 0.3054 | 0.9995 |
| 4b | y = 1.1 × 10−7x − 0.0864 | 0.9998 |
| 4c | y = 7.1 × 10−8x − 0.2330 | 0.9993 |
| 4d | y = 9.9 × 10−6x − 0.2282 | 0.9993 |
| 4e | y = 1.0 × 10−7x − 0.2599 | 0.9993 |
| 4f | y = 4.7 × 10−7x − 0.1863 | 0.9994 |
| PCA-Gly | y = 6.6 × 10−7x − 0.0723 | 0.9993 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Xiong, Y.; Zhu, X.; Hu, J.; Wang, Y.; Li, J.; Wu, J.; Wu, Q. An Exploration of the Effect of the Kleier Model and Carrier-Mediated Theory to Design Phloem-Mobile Pesticides Based on Researching the N-Alkylated Derivatives of Phenazine-1-Carboxylic Acid-Glycine. Molecules 2022, 27, 4999. https://doi.org/10.3390/molecules27154999
Cai J, Xiong Y, Zhu X, Hu J, Wang Y, Li J, Wu J, Wu Q. An Exploration of the Effect of the Kleier Model and Carrier-Mediated Theory to Design Phloem-Mobile Pesticides Based on Researching the N-Alkylated Derivatives of Phenazine-1-Carboxylic Acid-Glycine. Molecules. 2022; 27(15):4999. https://doi.org/10.3390/molecules27154999
Chicago/Turabian StyleCai, Jinlong, Yongtong Xiong, Xiang Zhu, Jinyu Hu, Yunping Wang, Junkai Li, Jianfeng Wu, and Qinglai Wu. 2022. "An Exploration of the Effect of the Kleier Model and Carrier-Mediated Theory to Design Phloem-Mobile Pesticides Based on Researching the N-Alkylated Derivatives of Phenazine-1-Carboxylic Acid-Glycine" Molecules 27, no. 15: 4999. https://doi.org/10.3390/molecules27154999
APA StyleCai, J., Xiong, Y., Zhu, X., Hu, J., Wang, Y., Li, J., Wu, J., & Wu, Q. (2022). An Exploration of the Effect of the Kleier Model and Carrier-Mediated Theory to Design Phloem-Mobile Pesticides Based on Researching the N-Alkylated Derivatives of Phenazine-1-Carboxylic Acid-Glycine. Molecules, 27(15), 4999. https://doi.org/10.3390/molecules27154999







