Comparison of Constituents and Antioxidant Activity of Above-Ground and Underground Parts of Dryopteris crassirhizoma Nakai Based on HS-SPME-GC-MS and UPLC/Q-TOF-MS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Volatile Components of D. crassirhizoma Determined with HS-SPME-GC-MS
2.2. Nonvolatile Components of D. crassirhizoma Determined with UPLC-Q-TOF-MS
| No. | RT (Min) | Compound Formula | Molar Mass | Experimental [M − H]− m/z | ppm | UV (nm) λmax | Identification | Type | Part | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.884 | C11H14O6 | 242.0791 | 241.0718 | −0.19 | 250 | Elenolic acid | Phenolic acid | AB | [40] |
| 2 | 16.111 | C16H18O9 | 354.0954 | 353.0877 | −0.9 | 248,255,283 | Chlorogenic acid | Phenolic acid | AB | [41] |
| 3 | 16.795 | C16H18O9 | 354.0954 | 353.0884 | −1.53 | 245,285,330 | Neochlorogenic acid | Phenolic acid | AB | [42] |
| 4 | 20.9 | C16H18O8 | 338.1014 | 337.0941 | −3.68 | 245,310 | Coumaroylquinic acid | Phenolic acid | AB | [29] |
| 5 | 25.657 | C21H22O11 | 450.1178 | 449.1105 | −3.45 | 285 | Marein | Flavonoids | A | [36] |
| 6 | 26.5 | C14H24O8 | 320.1481 | 319.1408 | −2.92 | 258 | Unknown | _ | A | — |
| 7 | 27.524 | C22H32O14 | 520.179 | 519.1718 | 0.31 | 245,280 | 6,7-Dihydroxy-2-oxo-1-benzopyran-4-carboxylic acid | Phenolic acid | AB | [33] |
| 8 | 34.8 | C21H20O11 | 448.1022 | 447.0949 | −3.56 | 265 | Luteolin-7-O-glucoside | Flavonoids | A | [33] |
| 9 | 37 | C11H12O4 | 208.0743 | 207.0670 | −3.33 | 245 | Unknown | _ | A | — |
| 10 | 38.152 | C10H12O4 | 196.0734 | 195.0661 | 0.93 | 248,280 | Methyl veratrate | Phenolic acid | B | [49] |
| 11 | 40.021 | C10H12O4 | 196.0736 | 195.0663 | −0.2 | 248,280 | Hydroxyconiferyl alcohol | Phenolic acid | B | [50] |
| 12 | 59.042 | C20H22O8 | 390.1319 | 389.1246 | −1.03 | 283 | 7-Hydroxyl-4,3,5,6,8-pentamethoxyflavone | Flavonoids | B | [44] |
| 13 | 62.146 | C21H24O8 | 404.1479 | 403.1406 | –1.91 | 290 | Flavaspidic acid AP | Phloroglucinol | B | [4] |
| 14 | 65.032 | C22H26O8 | 418.1635 | 417.1562 | 0 | 245 | Flavaspidic acid AB | Phloroglucinol | AB | [4] |
| 15 | 65.8 | C12H26O8 | 398.1619 | 397.1546 | 2.99 | 250 | Unknown | _ | A | — |
| 16 | 66.55 | C18H32O3 | 296.2366 | 295.2293 | −4.91 | 245 | Hydroxyoctadecadienoic acid | Fatty acid | A | [35] |
| 17 | 67.819 | C23H28O8 | 432.1795 | 431.1723 | −2.6 | 245,295 | Flavaspidic acid PB | Phloroglucinol | B | [4] |
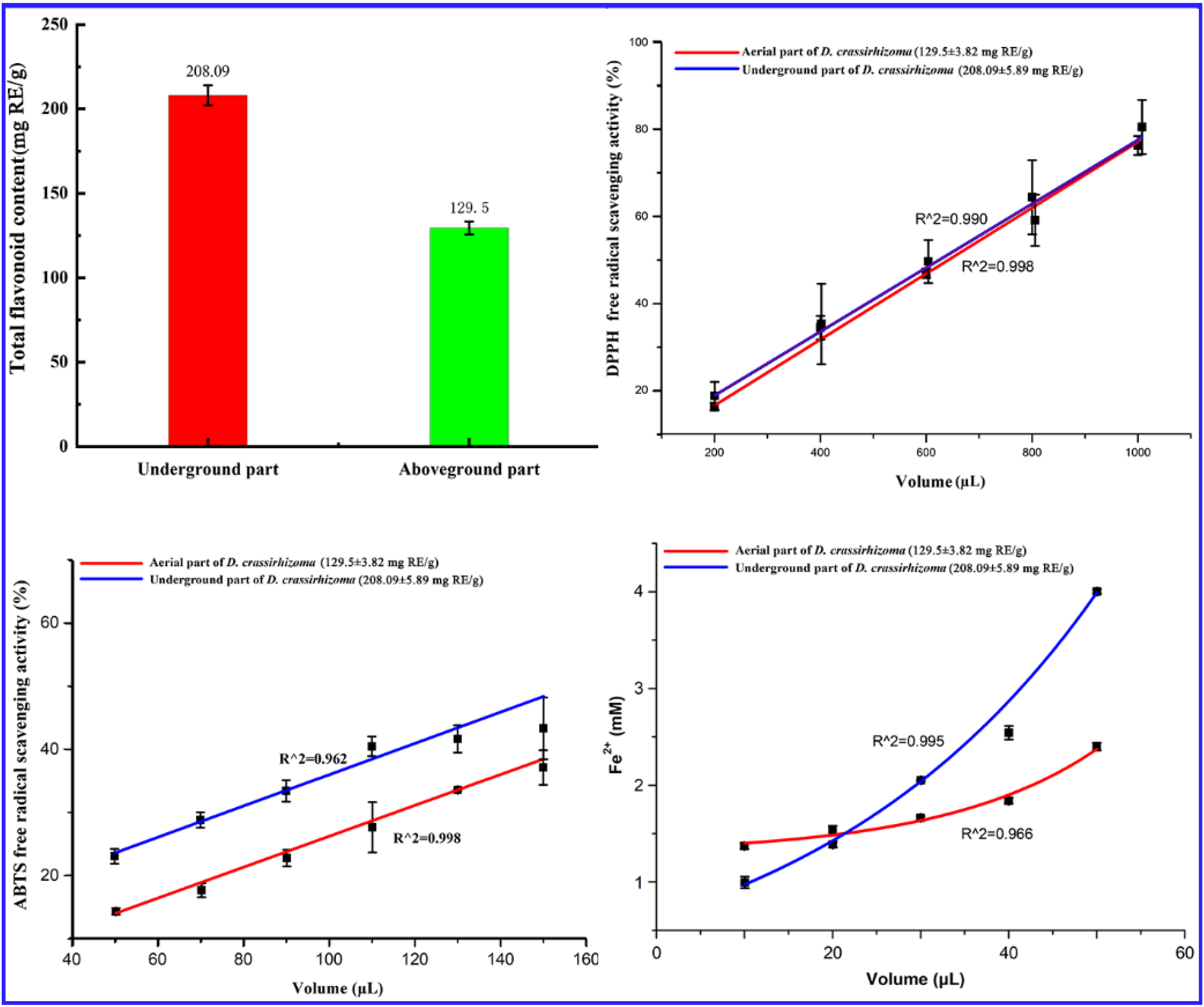
2.3. Comparison of Total Flavonoid Content and Antioxidant Activity of Above-Ground and Underground Parts of Dryopteris crassirhizoma
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals and Reagents
3.3. Preparation of Plant Extracts
3.4. Headspace Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry (HS-SPME-GC-MS) Analysis
3.5. Ultra-Performance Liquid Chromatography Quadrupole Time of Flight Mass Spectrometry (UPLC-Q-TOF-MS) Analysis
3.6. Determination of Total Flavonoids Content and Antioxidant Activity
3.6.1. Determination of Total Flavonoids Content
3.6.2. DPPH Assay
3.6.3. ABTS Assay
3.6.4. FRAP Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Na, M.K.; Jang, J.P.; Min, B.S.; Lee, S.J.; Lee, M.S.; Kim, B.Y.; Oh, W.K.; Ahn, J.S. Fatty acid synthase inhibitory activity of acylphloroglucinols isolated from Dryopteris crassirhizoma. Bioorg. Med. Chem. Lett. 2006, 16, 4738–4742. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Giri, S.S.; Jun, J.W.; Kim, H.J.; Yun, S.; Kim, S.G.; Park, S.C. Immunomodulatory effects of a bioactive compound isolated from Dryopteris crassirhizoma on the grass carp Ctenopharyngodon idella. J. Immunol. Res. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Xu, W.T.; Zhou, L.R.; Mai, B.W.; Zhu, N.Y.; Zhao, X.L.; Lei, Z.X. The complete chloroplast genome of Dryopteris crassirhizoma Nakai. Mitochondrial DNA 2021, 6, 1779–1780. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.J.; Kim, J.Y.; Sung, Y.Y.; Kim, D.S. Phloroglucinol derivatives from Dryopteris crassirhizoma as potent xanthine oxidase inhibitors. Molecules 2020, 26, 122. [Google Scholar] [CrossRef]
- Yim, N.H.; Lee, J.J.; Lee, B.H.; Li, W.; Ma, J.Y. Antiplatelet activity of acylphloroglucinol derivatives isolated from Dryopteris crassirhizoma. Molecules 2019, 24, 2212. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Nho, Y.H.; Yun, S.K.; Hwang, Y.S. Anti-invasive and anti-tumor effects of Dryopteris crassirhizoma extract by disturbing actin polymerization. Integr. Cancer Ther. 2019, 18, 1534735419851197. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.Q.; Hu, W.C.; Zhang, H.F.; Ding, C.B.; Huang, Y.; Liao, J.Q.; Zhang, Z.W.; Yuan, S.; Chen, Y.E.; Yuan, M. Antioxidant and immunomodulatory activities of polysaccharides from the rhizome of Dryopteris crassirhizoma Nakai. Int. J. Biol. Macromol. 2019, 130, 238–244. [Google Scholar] [CrossRef]
- Jiang, B.; Chi, C.; Fu, Y.W.; Zhang, Q.Z. In vivo anthelmintic effect of flavonol rhamnosides from Dryopteris crassirhizoma against Dactylogyrus intermedius in goldfish (Carassius auratus). Parasitol. Res. 2013, 112, 4097–4104. [Google Scholar] [CrossRef]
- Kim, E.J.; Choi, J.Y.; Yu, M.; Kim, M.Y.; Lee, S.; Lee, B.H. Total polyphenols, total flavonoid contents, and antioxidant activity of Korean natural and medicinal plants. Korean J. Food Sci. Technol. 2012, 44, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Crifò, T.; Puglisi, I.; Petrone, G.; Recupero, G.R.; Piero, A.R.L. Expression analysis in response to low temperature stress in blood oranges: Implication of the flavonoid biosynthetic pathway. Gene 2011, 476, 1–9. [Google Scholar] [CrossRef]
- Ríos, J.L. Effects of triterpenes on the immune system. J. Ethnopharmacol. 2010, 128, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Miyashiro, H.; Nakamura, N.; Hattori, M. Two new triterpenes from the rhizome of Dryopteris crassirhizoma, and inhibitory activities of its constituents on human immunodeficiency virus-1 protease. Chem. Pharm. Bull. 2008, 56, 711–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, V.C.; Kim, O.; Lee, J.H.; Min, B.S.; Kim, J.A. Inhibitory effects of phloroglucinols from the roots of Dryopteris crassirhizoma on melanogenesis. Phytochem. Lett. 2017, 21, 51–56. [Google Scholar] [CrossRef]
- Lee, S.M.; Na, M.K.; An, R.B.; Min, B.S.; Lee, H.K. Antioxidant activity of two phloroglucinol derivatives from Dryopteris crassirhizoma. Biol. Pharm. Bull. 2003, 26, 1354–1356. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yan, Y.T.; Fu, S.Z.; Peng, B.; Bao, L.L.; Zhang, Y.L.; Hu, J.H.; Zeng, Z.P.; Geng, D.H.; Gao, Z.P. Anti-influenza virus (H5N1) activity screening on the phloroglucinols from rhizomes of Dryopteris crassirhizoma. Molecules 2017, 22, 431. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.B.; Kim, J.C.; Lee, S.M. Antibacterial activity of two phloroglucinols, flavaspidic acids AB and PB, from Dryopteris crassirhizoma. Arch. Pharmacal Res. 2009, 32, 655–659. [Google Scholar] [CrossRef]
- Nešović, M.; Gašić, U.; Tosti, T.; Horvacki, N.; Nedić, N.; Sredojević, M.; Blagojević, S.; Ignjatović, L.; Tešić, Ž. Distribution of polyphenolic and sugar compounds in different buckwheat plant parts. RSC Adv. 2021, 11, 25816–25829. [Google Scholar] [CrossRef]
- Pan, J.; Zheng, W.; Pang, X.; Zhang, J.; Chen, X.J.; Yuan, M.; Yu, K.; Guo, B.L.; Ma, B.P. Comprehensive investigation on ginsenosides in different parts of a garden-cultivated ginseng root and rhizome. Molecules 2021, 26, 1696. [Google Scholar] [CrossRef]
- Huang, H.H.; Lin, L.Y.; Chiang, H.M.; Lay, S.J.; Wu, C.S.; Chen, H.C. Analysis of volatile compounds from different parts of Citrus grandis (L.) Osbeck flowers by headspace solid-phase microextraction-gas chromatography-mass spectrometry. J. Essent. Oil Bear. Plants 2017, 20, 1057–1065. [Google Scholar] [CrossRef]
- Qi, S.; Zha, L.Y.; Peng, Y.Z.; Luo, W.; Chen, K.L.; Li, X.; Huang, D.F.; Yin, D.M. Quality and metabolomics analysis of Houttuynia cordata based on HS-SPME/GC-MS. Molecules 2022, 27, 3921. [Google Scholar] [CrossRef]
- Karrar, E.; Ahmed, I.A.M.; Wei, W.; Sarpong, F.; Proestos, C.; Amarowicz, R.; Oz, E.; Sheikha, A.F.E.; Allam, A.Y.; Oz, F.; et al. Characterization of volatile flavor compounds in supercritical fluid separated and identified in Gurum (Citrulluslanatus var. colocynthoide) seed oil using HSME and GC-MS. Molecules 2022, 27, 3905. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Bai, Z.L.; Jia, W.J.; Sun, J.H.; Wang, W.W.; Chen, S.Z.; Wang, H. Quantitative analysis of polysaccharide composition in Polyporus umbellatus by HPLC-ESI-TOF-MS. Molecules 2019, 24, 2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brochot, A.; Guilbot, A.; Haddioui, L.; Roques, C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiologyopen 2017, 6, e00459. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.Z.; Chen, H.M.; Wang, X.L.; Hu, Y.Y.; Yun, Y.H.; Zhong, Q.P.; Chen, W.J.; Chen, W.X. Antimicrobial activity and proposed action mechanism of 3-Carene against Brochothrix thermosphacta and Pseudomonas fluorescens. Molecules 2019, 24, 3246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, J.; Yang, H.; Yoon, M.; Gadhe, C.G.; Pae, A.N.; Cho, S.; Lee, C.J. 3-Carene, a phytoncide from pine tree has a sleep-enhancing effect by targeting the GABAA-benzodiazepine receptors. Exp. Neurobiol. 2019, 28, 593. [Google Scholar] [CrossRef] [Green Version]
- Kumari, R.; Mishra, R.C.; Sheoran, R.; Yadav, J.P. Fractionation of antimicrobial compounds from Acacia nilotica twig extract against oral pathogens. Biointerface Res. Appl. Chem. 2020, 10, 7097–7105. [Google Scholar]
- Cheng, S.S.; Lin, H.Y.; Chang, S.T. Chemical composition and antifungal activity of essential oils from different tissues of Japanese cedar (Cryptomeria japonica). J. Agric. Food Chem. 2005, 53, 614–619. [Google Scholar] [CrossRef]
- Saranya, J.; Eganathan, P.; Sujanapal, P.; Parida, A. Chemical composition of leaf essential oil of Syzygium densiflorum wall. ex wt. & arn.-a vulnerable tree species. J. Essent. Oil Bear. Plants 2012, 15, 283–287. [Google Scholar]
- Somkuwar, R.G.; Sharma, A.K.; Kambale, N.; Banerjee, K.; Bhange, M.A.; Oulkar, D.P. Volatome finger printing of red wines made from grapes grown under tropical conditions of India using thermal-desorption gas chromatography-mass spectrometry (TD-GC/MS). J. Food Sci. Technol. 2020, 57, 1119–1130. [Google Scholar] [CrossRef]
- Day, W.C.; Erdman, J.G. Ionene: A thermal degradation product of β-carotene. Science 1963, 141, 808. [Google Scholar] [CrossRef]
- Kammakakam, I.O.; Harra, K.E.; Dennis, G.P.; Jackson, E.M.; Bara, J.E. Self-healing imidazolium-based ionene-polyamide membranes: An experimental study on physical and gas transport properties. Polym. Int. 2019, 68, 1123–1129. [Google Scholar] [CrossRef]
- Liu, M.; Fan, P.; Hu, Q.; Russell, T.P.; Liu, Y. Naphthalene-diimide-based ionenes as universal interlayers for efficient organic solar cells. Angew. Chem. 2020, 132, 18288–18292. [Google Scholar] [CrossRef]
- Menotti, A.R. Detection and quantitative determination of 4-amino-2-methyl-1-naphthol. A synthetic vitamin K. Ind. Eng. Chem. Anal. Ed. 1942, 14, 601–602. [Google Scholar] [CrossRef]
- Wapenaar, H.; Bosch, T.; Leus, N.G.J.; Wouden, P.E.; Eleftheriadis, N.; Hermans, J.; Hailu, G.S.; Rotili, S.; Mai, A.; Dömling, A.; et al. The relevance of Ki calculation for bi-substrate enzymes illustrated by kinetic evaluation of a novel lysine (K) acetyltransferase 8 inhibitor. Eur. J. Med. Chem. 2017, 136, 480–486. [Google Scholar] [CrossRef]
- Xu, B.; Chen, B.Y.; Hsueh, C.C.; Qin, L.J.; Chang, C.T. Deciphering characteristics of bicyclic aromatics-mediators for reductive decolorization and bioelectricity generation. Bioresour. Technol. 2014, 163, 280–286. [Google Scholar] [CrossRef]
- Kabbour, M.; Luque, R. Furfural as a platform chemical: From production to applications. Biomass Biofuels Biochem. 2020, 10, 283–297. [Google Scholar]
- Al-Taher, F.; Nemzer, B. Identification of aroma compounds in freeze-dried strawberries and raspberries by HS-SPME-GC-MS. J. Food Res. 2020, 9, 30–40. [Google Scholar] [CrossRef]
- Rodriguez-Kabana, R.; Kloepper, J.W.; Weaver, C.F.; Robertson, D.G. Control of plant parasitic nematodes with furfural-a naturally occurring fumigant. Nematropica 1993, 23, 63–73. [Google Scholar]
- Anthonia, E.E.; Philip, H.S. An overview of the applications of furfural and its derivatives. Int. J. Adv. Chem. 2015, 3, 42–47. [Google Scholar]
- Garcia-Aloy, M.; Groff, N.; Masuero, D.; Nisi, M.; Franco, A.; Battelini, F.; Vrhovsek, U.; Mattivi, F. Exploratory analysis of commercial olive-based dietary supplements using untargeted and targeted metabolomics. Metabolites 2020, 10, 516. [Google Scholar] [CrossRef]
- Hidayat, A.F.A.; Chan, C.K.; Mohamad, J.; Kadir, H.A. Leptospermum flavescens Sm. protect pancreatic β cell function from streptozotocin involving apoptosis and autophagy signaling pathway in in vitro and in vivo case study. J. Ethnopharmacol. 2018, 226, 120–131. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Yu, X.A.; Liu, W.; Li, J.; Chang, Y.X. Identification and screening of natural neuraminidase inhibitors from reduning injection via one-step high-performance liquid chromatography-fraction collector and UHPLC/Q-TOF-MS. Int. J. Anal. Chem. 2020, 2020, 8838025. [Google Scholar] [CrossRef]
- López-Ramírez, Y.; Cabañas-García, E.; Areche, C.; Trejo-Tapia, G.; Pérez-Molphe-Balch, E.; Gómez-Aguirre, Y.A. Callus induction and phytochemical profiling of Yucca carnerosana (Trel.) McKelvey obtained from in vitro cultures. Rev. Mex. Ing. Quim. 2021, 20, 823–837. [Google Scholar] [CrossRef]
- Lin, Z.T.; Wang, H.; Xu, Y.; Dong, J.; Hashi, Y.; Chen, S.Z. Identification of antioxidants in Fructus aurantii and its quality evaluation using a new on-line combination of analytical techniques. Food Chem. 2012, 134, 1181–1191. [Google Scholar] [CrossRef]
- Muhammad, N.; Veghar, H.; Muhammad, A.; Asghar, A.K.; Ghulam, J.K.; Muhammad, S.; Fawwad, A.; Daryoush, B.; Xia, F.F.; Faezeh, M.G.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar]
- Gao, X.H.; Zhang, S.D.; Wang, L.T.; Yu, L.; Zhao, X.L.; Ni, H.Y.; Wang, Y.Q.; Wang, J.D.; Shan, C.H.; Fu, Y.J. Anti-inflammatory effects of neochlorogenic acid extract from mulberry leaf (Morus alba L.) against LPS-stimulated inflammatory response through mediating the AMPK/Nrf2 signaling pathway in A549 cells. Molecules 2020, 25, 1385. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Choi, S.Y.; Lee, P.; Hur, J. Neochlorogenic acid inhibits lipopolysaccharide-induced activation and pro-inflammatory responses in BV2 microglial cells. Neurochem. Res. 2015, 40, 1792–1798. [Google Scholar] [CrossRef]
- Li, Y.L.; Yu, X.; Deng, L.C.; Wang, Y.X.; Zheng, X.D.; Chu, Q. Neochlorogenic acid anchors MCU-based calcium overload for cancer therapy. Food Funct. 2021, 12, 11387–11398. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.Q.; Chen, B.; Deng, X.F.; Luo, Q.; Zao, X.R. UPLC-Q-TOF-MS/MS analysis of phenolic compounds from the fruit of Cephalostachyum fuchsianum gamble and their antioxidant and cytoprotective activities. Molecules 2022, 27, 3767. [Google Scholar] [CrossRef]
- Leyva-Jimenez, F.J.; Lozano-Sanchez, J.; Borras-Linares, I.; Cadiz-Gurrea, M.L.; Mahmoodi-Khaledi, E. Potential antimicrobial activity of honey phenolic compounds against Gram positive and Gram negative bacteria. LWT 2019, 101, 236–245. [Google Scholar] [CrossRef]
- Xia, X.; Cao, J.G.; Zheng, Y.X.; Wang, Q.X.; Xiao, J.B. Flavonoid concentrations and bioactivity of flavonoid extracts from 19 species of ferns from China. Ind. Crops Prod. 2014, 58, 91–98. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.L.; Cao, J.; Wu, Y.H.; Xiao, J.B.; Wang, Q.X. Analysis of flavonoids and antioxidants in extracts of ferns from Tianmu Mountain in Zhejiang Province (China). Ind. Crops Prod. 2017, 97, 137–145. [Google Scholar] [CrossRef]
- Cao, J.G.; Zheng, Y.X.; Xia, X.; Wang, Q.X.; Xiao, J.B. Total flavonoid contents, antioxidant potential and acetylcholinesterase inhibition activity of the extracts from 15 ferns in China. Ind. Crops Prod. 2015, 75, 135–140. [Google Scholar] [CrossRef]
- Wang, X.; Cao, J.G.; Wu, Y.H.; Wang, Q.X.; Xiao, J.B. Flavonoids, antioxidant potential, and acetylcholinesterase inhibition activity of the extracts from the gametophyte and archegoniophore of Marchantia polymorpha L. Molecules 2016, 21, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.G.; Xia, X.; Dai, X.L.; Xiao, J.B.; Wang, Q.X.; Andrae-Marobela, K.; Okatch, H. Flavonoids profiles, antioxidant, acetylcholinesterase inhibition activities of extract from Dryoathyrium boryanum (Willd.) Ching. Food Chem. Toxicol. 2013, 55, 121–128. [Google Scholar] [CrossRef] [PubMed]
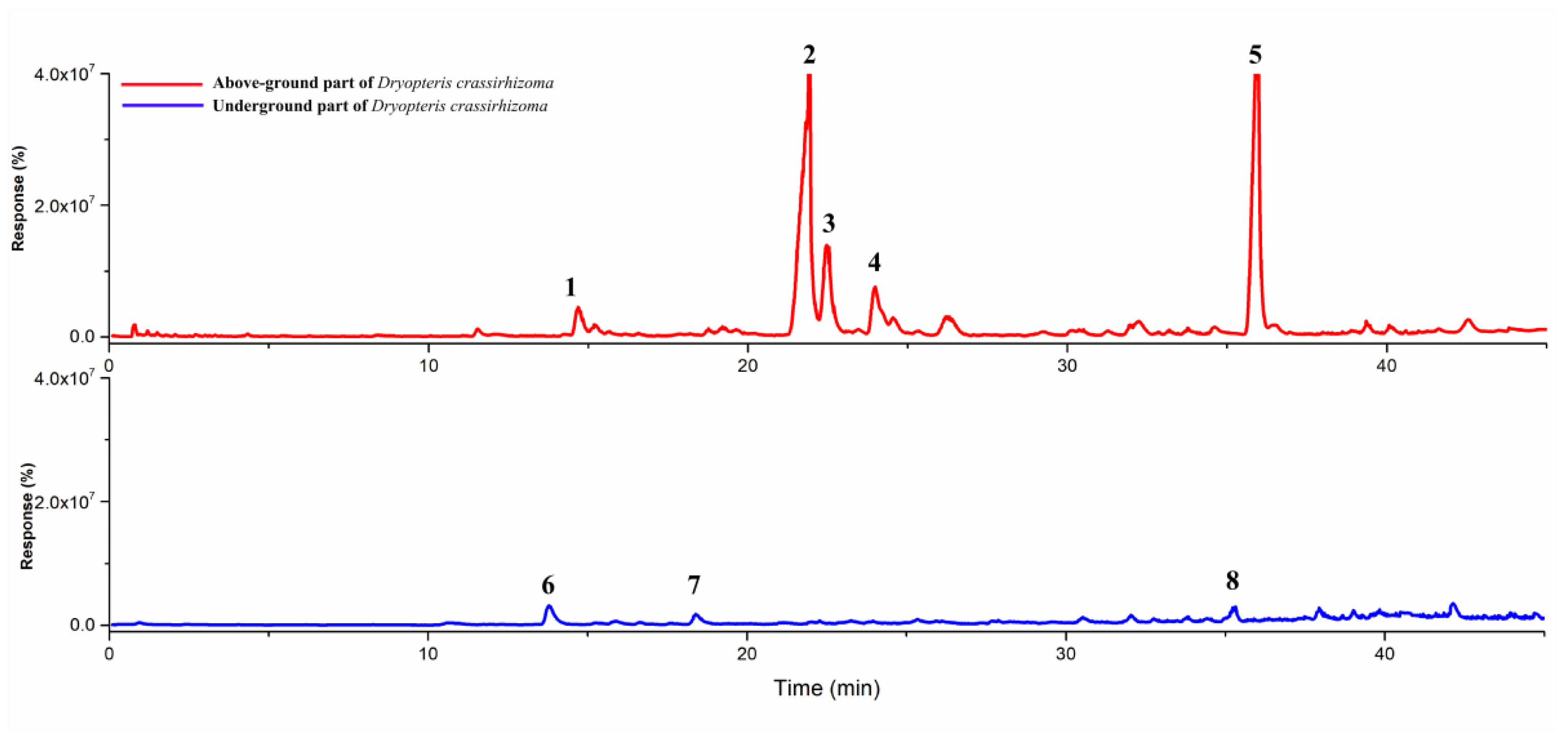


| No. | RT (min) | Volatile Component | Molecular Formula | Proportion (%) | PW 50% (min) |
|---|---|---|---|---|---|
| 1 | 14.649 | Furfural | C5H4O2 | 1.97 (AP) | 0.243 |
| 2 | 21.65 | Isoledene | C15H24 | 17.57 (AP) | 0.381 |
| 3 | 22.44 | 4-Amino-1-naphthol | C10H9NO | 5.87 (AP) | 0.19 |
| 4 | 23.978 | Ionene | C13H18 | 12.09 (AP) | 0.638 |
| 5 | 35.94 | 3-Carene | C10H16 | 62.50 (AP) | 0.695 |
| 6 | 13.783 | 3-Furaldehyde | C5H4O2 | 22.04 (UP) | 0.243 |
| 7 | 18.316 | 2-Acetyl-5-methylfuran | C7H8O2 | 3.03 (UP) | 0.11 |
| 8 | 35.888 | 2-Nonadecanol | C19H40O | 1.01 (UP) | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, B.; Wang, X.; Fan, Y. Comparison of Constituents and Antioxidant Activity of Above-Ground and Underground Parts of Dryopteris crassirhizoma Nakai Based on HS-SPME-GC-MS and UPLC/Q-TOF-MS. Molecules 2022, 27, 4991. https://doi.org/10.3390/molecules27154991
Wang Y, Liu B, Wang X, Fan Y. Comparison of Constituents and Antioxidant Activity of Above-Ground and Underground Parts of Dryopteris crassirhizoma Nakai Based on HS-SPME-GC-MS and UPLC/Q-TOF-MS. Molecules. 2022; 27(15):4991. https://doi.org/10.3390/molecules27154991
Chicago/Turabian StyleWang, Yanjia, Baodong Liu, Xin Wang, and Yawen Fan. 2022. "Comparison of Constituents and Antioxidant Activity of Above-Ground and Underground Parts of Dryopteris crassirhizoma Nakai Based on HS-SPME-GC-MS and UPLC/Q-TOF-MS" Molecules 27, no. 15: 4991. https://doi.org/10.3390/molecules27154991
APA StyleWang, Y., Liu, B., Wang, X., & Fan, Y. (2022). Comparison of Constituents and Antioxidant Activity of Above-Ground and Underground Parts of Dryopteris crassirhizoma Nakai Based on HS-SPME-GC-MS and UPLC/Q-TOF-MS. Molecules, 27(15), 4991. https://doi.org/10.3390/molecules27154991






