A Comparison of Conventional and Ultrasound-Assisted BCR Sequential Extraction Methods for the Fractionation of Heavy Metals in Sewage Sludge of Different Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Sampling
2.2. Sample Preparation Procedure
2.3. Determination of the Total Heavy Metal Content
2.4. Quality Control—Precision and Accuracy
2.5. Chemical Reagents Used in the Study
2.6. Sequential Extraction Methods
2.6.1. Conventional Chemical Sequential Extraction Method
2.6.2. Ultrasound-Assisted Chemical Sequential Extraction Method
3. Results and Discussion
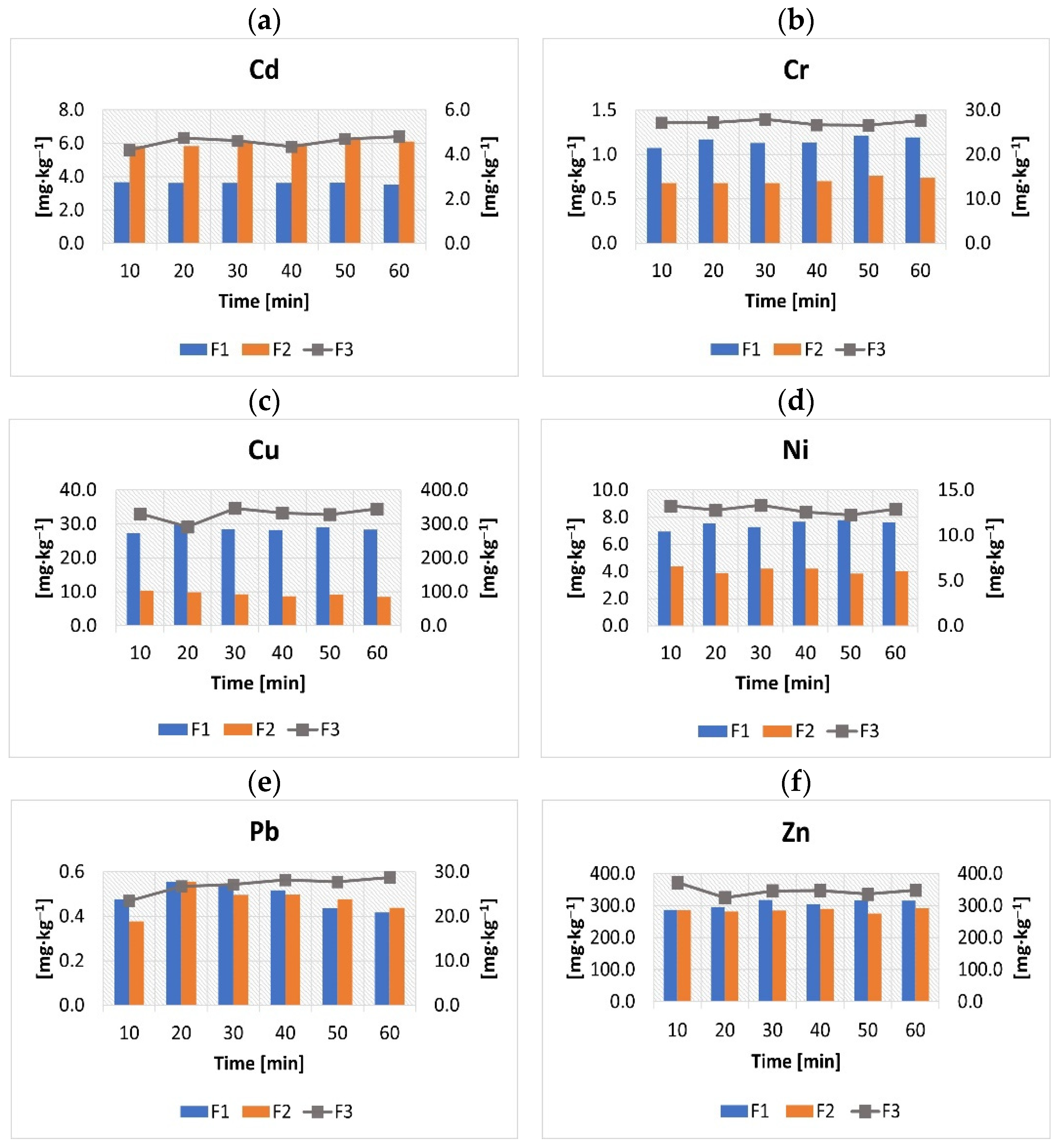
3.1. The Effect of the Sonication Time on the Extraction of Heavy Metals
3.2. Comparison of the Conventional and Ultrasound-Assisted BCR Sequential Extraction
3.2.1. Heavy Metals in the ERM-CC144 (JRC)
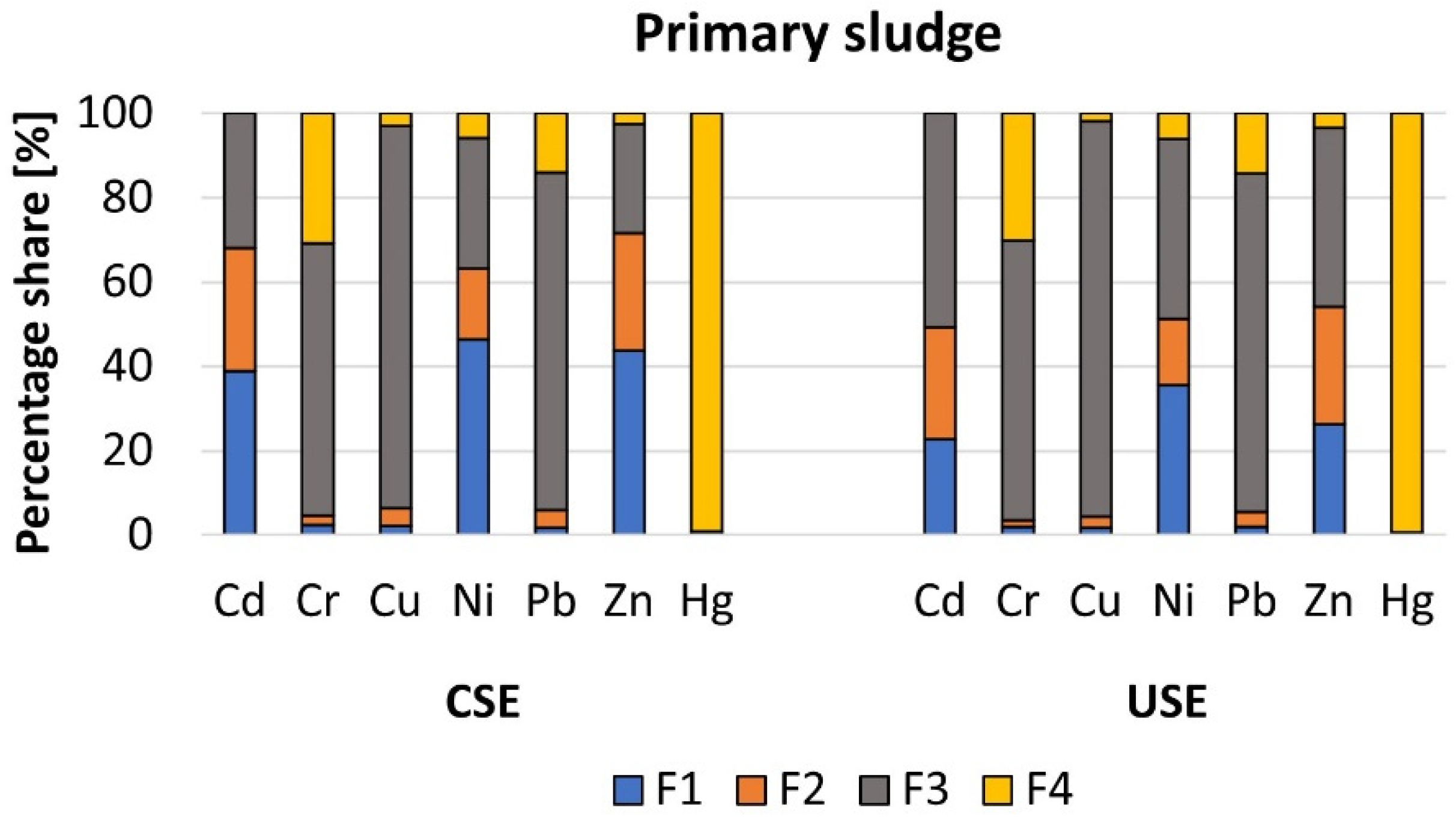
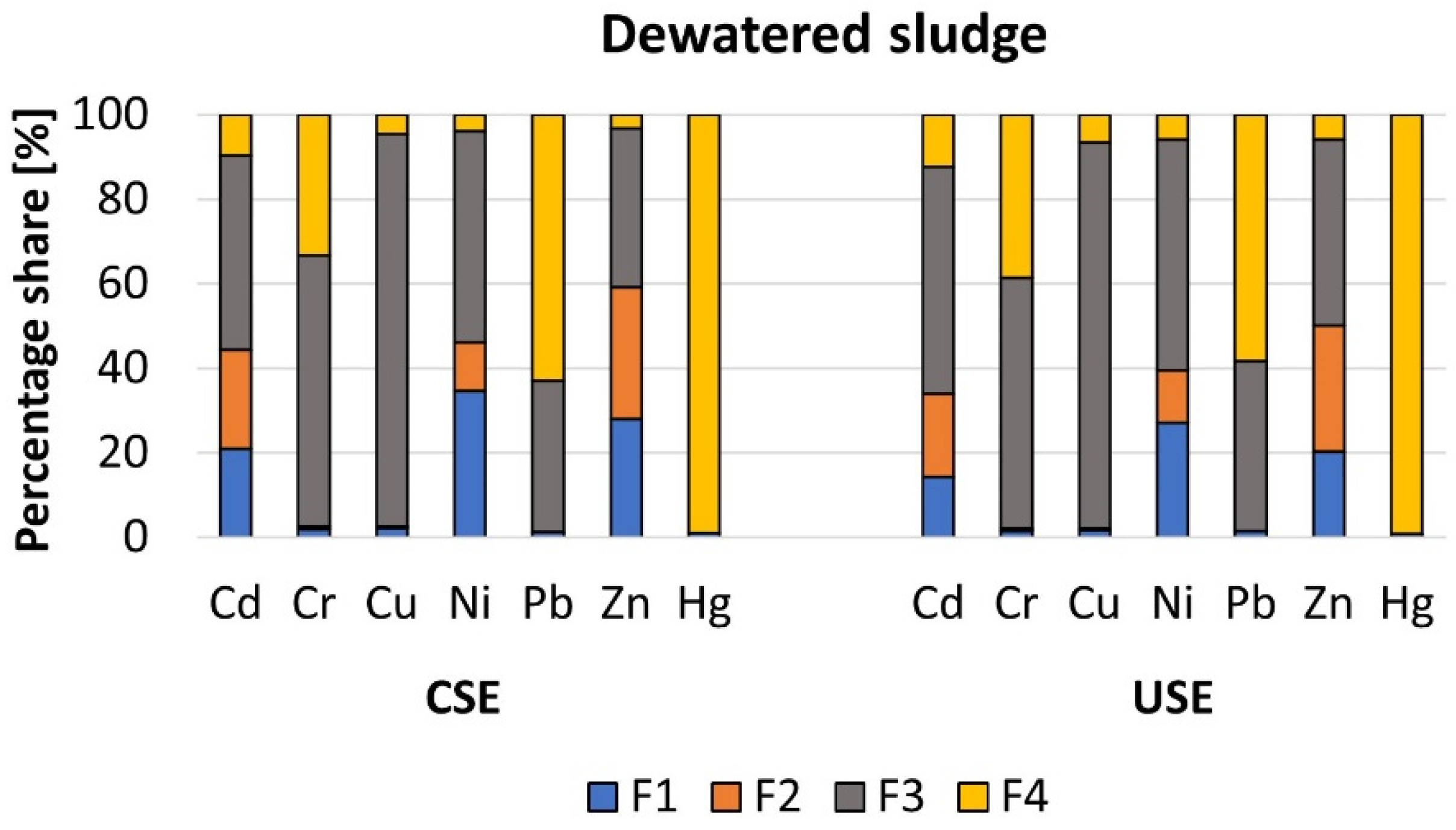
3.2.2. Heavy Metals in the Primary and Dewatered Sewage Sludge
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lin, X.; Li, S.; Wei, Z.; Chen, Y.; Hei, L.; Wu, Q.T. Indirect application of sludge for recycling in agriculture to minimize heavy metal contamination of soil. Resour. Conserv. Recycl. 2021, 166, 105358. [Google Scholar] [CrossRef]
- You, M.; Hu, Y.; Yan, Y.; Yao, J. Speciation characteristics and ecological risk assessment of heavy metals in municipal sludge of Huainan, China. Molecules 2021, 26, 6711. [Google Scholar] [CrossRef] [PubMed]
- Tytła, M.; Widziewicz-Rzońca, K. Heavy metals in municipal sewage sludge—A brief characteristic of potential threats and methods used to assess the ecological risk. Environ. Earth Ecol. 2021, 5, 18–25. [Google Scholar] [CrossRef]
- Cieślik, B.M.; Świerczek, L.; Konieczka, P. Analytical and legislative challenges of sewage sludge processing and management. Monatsh. Chem. 2018, 149, 1635–1645. [Google Scholar] [CrossRef]
- Gwebu, S.; Tavengwa, N.T.; Klink, M.J.; Mtunzi, F.M.; Modise, S.J.; Pakade, V.E. Quantification of Cd, Cu, Pb and Zn from sewage sludge by modified-BCR and ultrasound assisted-modified BCR sequential extraction methods. Afr. J. Pure Appl. Chem. 2017, 11, 9–18. [Google Scholar] [CrossRef][Green Version]
- Tytła, M. Identification of the chemical forms of heavy metals in municipal sewage sludge as a critical element of ecological risk assessment in terms of its agricultural or natural use. Int. J. Environ. Res. Public Health 2020, 17, 4640. [Google Scholar] [CrossRef]
- Yakamercan, E.; Aygün, A. Ecological risk assessment of domestic sewage sludge: A case study. Sigma J. Eng. Nat. Sci. 2021, 39, 422–433. [Google Scholar] [CrossRef]
- Danish, M.; Hu, J.; Zhou, P.; Lou, Z.Y.; Qian, P.S. A new drying kinetic model for sewage sludge drying in presence of CaO and NaClO. Appl. Therm. Eng. 2016, 106, 141–152. [Google Scholar] [CrossRef]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The presence of contaminants in sewage sludge—The current situation. J. Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef]
- Tytła, M. Assessment of heavy metal pollution and potential ecological risk in sewage sludge from municipal wastewater treatment plant located in the most industrialized region in Poland—Case study. Int. J. Environ. Res. Public Health 2019, 16, 2430. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Ure, A.M.; Quevauviller, P.; Mantau, H.; Griepink, B. Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. Int. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- Álvarez, E.A.; Callejón Mochón, M.; Jiménez Sánchez, J.C.; Ternero Rodríguez, M. Heavy metal extractable forms in sludge from wastewater treatment plants. Chemosphere 2002, 47, 765–775. [Google Scholar] [CrossRef]
- Janas, M.; Zawadzka, A.; Cichowicz, R. The influence of selected factors on leaching of metals from sewage sludge. Environ. Sci. Pollut. Res. 2018, 25, 33240–33248. [Google Scholar] [CrossRef]
- Li, S.; Li, R.; Tang, Y.; Chen, G. Microwave-induced heavy metal removal from dewatered biosolids for cost-effective composting. J. Clean. Prod. 2019, 241, 118342. [Google Scholar] [CrossRef]
- Qiu, C.; Xie, S.; Liu, N.; Meng, K.; Wang, C.; Wang, D.; Wang, S. Removal behavior and chemical speciation distributions of heavy metals in sewage sludge during bioleaching and combined bioleaching/Fenton-like processes. Sci. Rep. 2021, 11, 14879. [Google Scholar] [CrossRef]
- Cherfouh, R.; Lucas, Y.; Derridj, A.; Merdy, P. Metal speciation in sludges: A tool to evaluate risks of land application and to track heavy metal contamination in sewage network. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kazi, T.G.; Jamali, M.K.; Siddiqui, A.; Kazi, G.H.; Arain, M.B.; Afridi, H.I. An ultrasonic assisted extraction method to release heavy metals from untreated sewage sludge samples. Chemosphere 2006, 63, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Leśniewska, B.; Krymska, M.; Świerad, E.; Wiater, J. An ultrasound-assisted procedure for fast screening of mobile fractions of Cd, Pb and Ni in soil. Insight into method optimization and validation. Environ. Sci. Pollut. Res. 2016, 23, 25093–25104. [Google Scholar] [CrossRef] [PubMed]
- Tytła, M.; Widziewicz, K.; Zielewicz, Z. Heavy metals and its chemical speciation in sewage sludge at different stages of processing. Environ. Technol. 2016, 37, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Tytła, M.; Zielewicz, E. The impact of temporal variability of excess sludge characteristics on the effects obtained in the process of its ultrasonic disintegration. Environ. Technol. 2018, 39, 3020–3032. [Google Scholar] [CrossRef] [PubMed]
- Tytła, M. The effects of ultrasonic disintegration as a function of waste activated sludge characteristics and technical conditions of conducting the process—Comprehensive Analysis. Int. J. Environ. Res. Public Health 2018, 15, 2311. [Google Scholar] [CrossRef]
- Leśniewska, B.; Kisielewska, K.; Wiater, J.; Godlewska-Żyłkiewicz, B. Fast and simple procedure for fractionation of zinc in soil using an ultrasound probe and FAAS detection. Validation of the analytical method and evaluation of the uncertainty budget. Environ. Monit. Assess. 2016, 188, 29. [Google Scholar] [CrossRef] [PubMed]
- Pérez Cid, B.; Lavilla, I.; Bendicho, C. Speeding up of a three-stage sequential extraction method for metal speciation using focused ultrasound. Anal. Chim. Acta 1998, 360, 35–41. [Google Scholar] [CrossRef]
- Pérez Cid, B.; Lavilla, I.; Bendicho, C. Comparison between conventional and ultrasound accelerated Tessier sequential extraction schemes for metal fractionation in sewage sludge. Fresenius J. Anal. Chem. 1999, 363, 667–672. [Google Scholar] [CrossRef]
- Hristozov, D.; Domini, C.E.; Kmetov, V.; Stefanova, V.; Georgieva, D.; Canals, A. Direct ultrasound-assisted extraction of heavy metals from sewage sludge samples for ICP-OES analysis. Anal. Chim. Acta 2004, 516, 187–196. [Google Scholar] [CrossRef]
- Regulation of the Minister of Environment of 6th February 2015 on the Municipal Sewage Sludge (J. L. 2015, Item. 257). Available online: http://prawo.sejm.gov.pl/isap.nsf/download.xsp/WDU20150000257/O/D20150257.pdf (accessed on 4 April 2022).
- Council Directive of 12th June 1986 on the Protection of the Environment, and in Particular of the Soil, When Sewage Sludge Is Used in Agriculture (86/278/EEC). Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/PDF/?uri=CELEX:31986L0278&from=EN (accessed on 4 April 2022).
- PN-EN 3696:1999; Water Used in Analytical Laboratories—Requirements and test methods (in Polish: Woda stosowana w laboratoriach analitycznych—Wymagania i metody badań). Polish Committee for Standardization (in Polish: Polski Komitet Normalizacyjny—PKN): Warsaw, Poland, 1999.
- Cole, T.J.; Altman, D.G. Statistics notes: What is a percentage difference? BMJ 2017, 358, j3663. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Medeiros, D.C.C.; Piechontcoski, F.; Da Rocha Watanabe, E.R.L.; Chaves, E.S.; Inglez, S.D. Fast and effective simultaneous determination of metals in soil samples by ultrasound-assisted extraction and flame atomic absorption spectrometry: Assessment of trace elements contamination in agricultural and native forest soils from Paraná-Brazil. Environ. Monit. Assess. 2020, 192, 111. [Google Scholar] [CrossRef]
- Frentiu, T.; Pintican, B.P.; Butaciu, S.; Mihaltan, A.I.; Ponta, M.; Frentiu, M. Determination, speciation and distribution of mercury in soil in the surroundings of a former chlor-alkali plant: Assessment of sequential extraction procedure and analytical technique. Chem. Cent. J. 2013, 7, 178. [Google Scholar] [CrossRef]
- Relić, D.; Đorđević, D.; Sakan, S.; Anđelković, I.; Pantelić, A.; Stanković, R.; Radojičić, A.; Popović, A. An appraisal of conventional, microwave and ultrasound BCR extraction methods for the analysis of metals in sediments of Pančevo, Serbia. E3S Web Conf. 2013, 1, 39002. [Google Scholar] [CrossRef]
- Kovács, K.; Halász, G.; Takács, A.; Heltai, G.; Széles, E.; Győri, Z.; Horváth, M. Study of ultrasound-assisted sequential extraction procedure for potentially toxic element content of soils and sediments. Microchem. J. 2018, 136, 80–84. [Google Scholar] [CrossRef]
- Relić, D.; Héberger, K.; Sakan, S.; Škrbić, B.; Popović, A.; Đorđević, D. Ranking and similarity of conventional, microwave and ultrasound element sequential extraction methods. Chemosphere 2018, 198, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Wiater, J. Content of Heavy Metals and their fractions in organic soils of Podlasie. J. Ecol. Eng. 2019, 20, 179–184. [Google Scholar] [CrossRef]
- Pérez, G.; Valiente, M.; Bendicho, C. A Comparative Study of Metal Readsorption in the Application of a Three-Stage Sequential Extraction Scheme and Two Accelerated Versions (Ultrasonic and Single Extractions). Open Anal. Chem. J. 2008, 2, 40–46. [Google Scholar] [CrossRef]
- Perin, G.; Craboledda, L.; Lucchese, M.; Cirillo, R.; Dotta, L.; Zanette, M.L.; Orio, A.A. Heavy metal speciation in the sediments of Northern Adriatic Sea—A new approach for environmental toxicity determination. In Heavy Metal in the Environment 2; Lakkas, T.D., Ed.; CEP Consultants Limited: Edinburgh, UK, 1985; pp. 454–456. [Google Scholar]
- Ikem, A.; Egiebor, N.O.; Nyavor, K. Trace elements in water fish and sediment from Tuskegee Lake, Southeastern USA. Water Air Soil Pollut. 2003, 149, 51–75. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, C.; Yang, Y.; Niu, J.; Shen, Z. Risk assessment of sedimentary metals in the Yangtze Estuary: New evidence of the relationships between two typical index methods. J. Hazard. Mater. 2012, 241–242, 164–172. [Google Scholar] [CrossRef]

| Heavy Metal | This Study | ERM-CC144 | R | RSD |
|---|---|---|---|---|
| mg·kg−1 | mg·kg−1 | % | % | |
| Cd | 12.67 ± 0.18 | 14.5 | 87.4 | 1.4 |
| Cr | 151.23 ± 0.52 | 168.0 | 90.0 | 0.3 |
| Cu | 351.60 ± 2.58 | 348.0 | 101.0 | 0.7 |
| Ni | 83.51 ± 0.23 | 91.0 | 91.8 | 0.3 |
| Pb | 151.60 ± 1.64 | 157.0 | 96.6 | 1.1 |
| Zn | 927.58 ± 9.02 | 980.0 | 94.7 | 1.0 |
| Hg | 5.16 ± 0.15 | 5.9 | 87.4 | 2.9 |
| Chemical Reagent | Chemical Formula | Purity | Commercial Brand | Country of Origin |
|---|---|---|---|---|
| Acetic acid (V); 65% | HNO3 | analysis-pure (a.p.) | POCH | Poland |
| Hydrochloric acid; 35–38% | HCl | analysis-pure (a.p.) | POCH | Poland |
| Acetic acid; 99.5–99.9% | CH3COOH | analysis-pure (a.p.) | POCH | Poland |
| Hydroxylamine hydrochloride | NH2OH·HCl | analysis-pure (a.p.) | Chempur | Poland |
| Hydrogen peroxide; 30% | H2O2 | analysis-pure (a.p.) | Chempur | Poland |
| Fraction | Extraction Agent | Conventional BCR (CSE) | Ultrasound-Assisted BCR (USE) |
|---|---|---|---|
| Extraction Time | |||
| Acid soluble/exchangeable fraction; bound to carbonates (F1) | 20 mL CH3COOH (0.11 M) | Shake for 16 h | Shake for 30 min and sonicate for 30 min |
| Reducible fraction; bound to Mn and Fe oxides (F2) | 20 mL NH2OH·HCl (0.1 M, pH = 2) | Shake for 16 h | Shake for 30 min and sonicate for 30 min |
| Oxidizable fraction; bound to organic matter and sulfides (F3) | 5 mL H2O2 (8.8 M, pH = 2), heat to 85 °C for 1 h (repeat twice); 25 mL CH3COONH4 (1 M, pH = 2) | Shake for 16 h | Sonicate for 30 min |
| Residual fraction (F4) | 15 mL HCl/5 mL HNO3 (3:1) (aqua regia; microwave digestion). | - | - |
| Fraction | HM | Conventional Extraction (CSE) | Ultrasound-Assisted Extraction (USE) |
|---|---|---|---|
| mg·kg−1 | |||
| F1 | Cd | 3.69 ± 0.12 | 3.63 ± 0.14 |
| F2 | 6.96 ± 0.17 | 6.06 ± 0.26 | |
| F3 | 4.03 ± 0.13 | 4.62 ± 0.35 | |
| F4 | 0.90 ± 0.10 | 1.09 ± 0.00 | |
| Total content | 12.67 ± 0.18 | ||
| RM; % | 123.0 | 121.6 | |
| F1 | Cr | 1.29 ± 0.02 | 1.13 ± 0.03 |
| F2 | 0.84 ± 0.00 | 0.68 ± 0.00 | |
| F3 | 28.18 ± 0.28 | 27.92 ± 0.92 | |
| F4 | 107.17 ± 4.61 | 113.37 ± 12.45 | |
| Total content | 151.23 ± 0.52 | ||
| RM; % | 90.9 | 94.6 | |
| F1 | Cu | 31.93 ± 0.90 | 28.43 ± 0.43 |
| F2 | 10.17 ± 0.33 | 9.27 ± 0.54 | |
| F3 | 334.24 ± 5.70 | 346.14 ± 1.32 | |
| F4 | 13.83 ± 0.40 | 16.33 ±0.22 | |
| Total content | 351.60 ± 2.58 | ||
| RM; % | 111.0 | 113.8 | |
| F1 | Ni | 8.75 ± 0.24 | 7.29 ± 0.02 |
| F2 | 5.23 ± 0.10 | 4.21 ± 0.28 | |
| F3 | 10.31 ± 0.27 | 13.33 ± 0.26 | |
| F4 | 52.56 ± 1.66 | 54.35 ± 5.59 | |
| Total content | 83.51 ± 0.23 | ||
| RM; % | 92.0 | 94.8 | |
| F1 | Pb | 0.38 ± 0.02 | 0.54 ± 0.03 |
| F2 | 0.40 ± 0.07 | 0.50 ± 0.03 | |
| F3 | 41.05 ± 0.82 | 27.15 ± 2.36 | |
| F4 | 105.78 ± 1.79 | 118.18 ± 9.72 | |
| Total content | 151.60 ± 1.64 | ||
| RM; % | 97.4 | 96.5 | |
| F1 | Zn | 395.90 ± 3.10 | 318.16 ± 3.61 |
| F2 | 357.49 ± 6.99 | 284.35 ± 3.27 | |
| F3 | 203.90 ± 3.63 | 346.24 ± 3.45 | |
| F4 | 17.67±1.25 | 27.30 ± 0.54 | |
| Total content | 927.58 ± 9.02 | ||
| RM; % | 105.1 | 105.2 | |
| F1 | Hg | 0.003 ± 0.00 | 0.002 ± 0.00 |
| F2 | 0.000 ± 0.00 | 0.000 ± 0.00 | |
| F3 | 0.000 ± 0.00 | 0.000 ± 0.00 | |
| F4 | 1.282 ± 0.20 | 1.279 ± 0.20 | |
| Total content | 5.16 ± 0.15 | ||
| RM; % | 24.9 | 24.8 | |
| HM | Sum of the 4 Chemical Fractions | Percent Difference between CSE and USE | |
|---|---|---|---|
| Conventional Extraction (CSE) | Ultrasound-Assisted Extraction (USE) | ||
| mg·kg−1 | % | ||
| Cd | 15.57 ± 0.23 | 15.40 ± 0.23 | 1.1 |
| Cr | 137.48 ± 4.44 | 143.10 ± 11.50 | 4.0 |
| Cu | 390.17 ± 6.46 | 400.17 ± 0.98 | 2.5 |
| Ni | 76.85 ± 1.91 | 79.17 ± 6.11 | 3.0 |
| Pb | 147.61 ± 2.08 | 146.36 ± 7.41 | 0.9 |
| Zn | 974.97 ± 8.80 | 976.10 ± 3.96 | 0.1 |
| Fraction | HM | Conventional Extraction (CSE) | Ultrasound-Assisted Extraction (USE) | ||
|---|---|---|---|---|---|
| Primary Sludge | Dewatered Sludge | Primary Sludge | Dewatered Sludge | ||
| mg·kg−1 | mg·kg−1 | ||||
| F1 | Cd | 1.95 ± 0.08 | 0.97 ± 0.06 | 1.19 ± 0.07 | 0.70 ± 0.02 |
| F2 | 1.46 ± 0.02 | 1.08 ± 0.04 | 1.38 ± 0.06 | 0.96 ± 0.06 | |
| F3 | 1.59 ± 0.01 | 2.11 ± 0.14 | 2.64 ± 0.07 | 2.62 ± 0.16 | |
| F4 | 0.00 ± 0.00 | 0.45 ± 0.07 | 0.00 ± 0.00 | 0.60 ± 0.14 | |
| Total content | 3.84 ± 0.15 | 4.35 ± 0.16 | 3.84 ± 0.15 | 4.35 ± 0.16 | |
| RM; % | 130.2 | 105.7 | 135.7 | 112.2 | |
| F1 | Cr | 1.22 ± 0.04 | 1.44 ± 0.04 | 1.16 ± 0.02 | 1.16 ± 0.02 |
| F2 | 1.21 ± 0.02 | 0.41 ± 0.02 | 0.96 ± 0.04 | 0.41 ± 0.02 | |
| F3 | 33.22 ± 1.09 | 46.85 ± 1.39 | 39.23 ± 0.94 | 45.14 ± 1.01 | |
| F4 | 15.82 ± 0.70 | 24.33 ± 1.90 | 17.86 ± 0.20 | 29.33 ± 0.91 | |
| Total content | 53.17 ± 1.39 | 72.17 ± 1.35 | 53.17 ± 1.39 | 72.17 ± 1.35 | |
| RM; % | 96.8 | 101.2 | 111.4 | 105.4 | |
| F1 | Cu | 6.25 ± 0.24 | 5.15 ± 0.10 | 5.73 ± 0.16 | 4.11 ± 0.25 |
| F2 | 11.11 ± 0.66 | 0.97 ± 0.06 | 8.43 ± 0.54 | 0.86 ± 0.06 | |
| F3 | 246.74 ± 6.00 | 226.11 ± 10.94 | 294.97 ± 2.52 | 224.24 ± 3.86 | |
| F4 | 8.16 ± 1.08 | 10.92 ± 0.05 | 5.98 ± 0.19 | 15.78 ± 0.41 | |
| Total content | 281.85 ± 14.12 | 278.94 ± 4.21 | 281.85 ± 14.12 | 278.94 ± 4.21 | |
| RM; % | 96.6 | 87.2 | 111.8 | 87.8 | |
| F1 | Ni | 36.83 ± 0.56 | 54.54 ± 0.48 | 28.55 ± 0.50 | 41.4 3± 0.78 |
| F2 | 13.43 ± 0.13 | 18.34 ± 0.97 | 12.51 ± 0.15 | 18.95 ± 0.84 | |
| F3 | 24.60 ± 2.81 | 78.60 ± 2.36 | 34.18 ± 0.28 | 83.76 ± 4.45 | |
| F4 | 4.58 ± 0.40 | 6.17 ± 0.55 | 4.81 ± 0.06 | 9.13 ± 0.47 | |
| Total content | 73.18 ± 2.98 | 150.58 ± 2.04 | 73.18 ± 2.98 | 150.58 ± 2.04 | |
| RM; % | 108.5 | 104.7 | 109.4 | 101.8 | |
| F1 | Pb | 0.74 ± 0.06 | 0.71 ± 0.17 | 0.95 ± 0.18 | 1.06 ± 0.09 |
| F2 | 1.82 ± 0.13 | 0.00 ±0.00 | 1.66 ± 0.12 | 0.00 ± 0.00 | |
| F3 | 33.97 ± 0.65 | 22.30 ± 0.68 | 38.64 ± 0.99 | 27.14 ± 0.86 | |
| F4 | 5.94 ± 0.41 | 38.93 ± 2.71 | 6.87 ± 0.36 | 38.94 ± 0.86 | |
| Total content | 45.04 ± 2.09 | 67.14 ± 2.60 | 45.04 ± 2.09 | 67.14 ± 2.60 | |
| RM; % | 94.3 | 92.3 | 106.8 | 100.7 | |
| F1 | Zn | 356.35 ± 2.67 | 325.17 ± 2.69 | 201.53 ± 3.24 | 224.72 ± 6.02 |
| F2 | 225.28 ± 4.17 | 363.49 ± 7.16 | 214.12 ± 5.69 | 331.61 ± 23.06 | |
| F3 | 210.91 ± 1.31 | 436.95 ± 3.66 | 325.92 ± 4.17 | 490.79 ± 8.03 | |
| F4 | 19.44 ± 0.35 | 36.58 ± 2.22 | 26.03 ± 0.60 | 65.24 ± 3.34 | |
| Total content | 696.62 ± 17.01 | 1026.90 ± 5.03 | 696.62 ± 17.01 | 1026.90 ± 5.03 | |
| RM; % | 116.6 | 113.2 | 110.2 | 108.3 | |
| F1 | Hg | 0.003 ± 0.01 | 0.003 ± 0.01 | 0.002 ± 0.01 | 0.003 ± 0.02 |
| F2 | 0.000 ± 0.00 | 0.000± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | |
| F3 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | |
| F4 | 0.303 ± 0.02 | 0.216 ± 0.01 | 0.387 ± 0.02 | 0.357 ± 0.03 | |
| Total content | 0.49 ± 0.10 | 2.51 ± 0.05 | 0.49 ± 0.10 | 2.51 ± 0.05 | |
| RM; % | 62.8 | 8.7 | 79.8 | 14.4 | |
| HM | Sum of the 4 Chemical Fractions | Percent Difference between CSE and USE | |
|---|---|---|---|
| Conventional Extraction (CSE) | Ultrasound-Assisted Extraction (USE) | ||
| mg·kg−1 | % | ||
| Cd | 4.99 ± 0.08 | 5.21 ± 0.09 | 4.3 |
| Cr | 51.47 ± 9.87 | 59.21 ± 1.08 | 14.0 |
| Cu | 272.26 ± 10.90 | 315.11 ± 9.48 | 14.6 |
| Ni | 79.44 ± 4.72 | 80.06 ± 0.93 | 0.8 |
| Pb | 42.47 ± 3.78 | 48.13 ± 1.51 | 12.5 |
| Zn | 811.97 ± 14.50 | 767.50 ± 12.31 | 5.6 |
| HM | Sum of the 4 Chemical Fractions | Percent Difference between CSE and USE | |
|---|---|---|---|
| Conventional Extraction (CSE) | Ultrasound-Assisted Extraction (USE) | ||
| mg·kg−1 | % | ||
| Cd | 4.60 ± 0.31 | 4.88 ± 0.14 | 5.9 |
| Cr | 73.04 ± 5.27 | 76.05 ± 1.82 | 4.0 |
| Cu | 243.15 ± 10.99 | 244.99 ± 3.99 | 0.8 |
| Ni | 157.65 ± 2.03 | 153.27 ± 3.04 | 2.8 |
| Pb | 61.95 ± 3.46 | 67.60 ± 0.10 | 8.7 |
| Zn | 1162.20 ± 12.37 | 1112.35 ± 16.00 | 4.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tytła, M.; Widziewicz-Rzońca, K.; Bernaś, Z. A Comparison of Conventional and Ultrasound-Assisted BCR Sequential Extraction Methods for the Fractionation of Heavy Metals in Sewage Sludge of Different Characteristics. Molecules 2022, 27, 4947. https://doi.org/10.3390/molecules27154947
Tytła M, Widziewicz-Rzońca K, Bernaś Z. A Comparison of Conventional and Ultrasound-Assisted BCR Sequential Extraction Methods for the Fractionation of Heavy Metals in Sewage Sludge of Different Characteristics. Molecules. 2022; 27(15):4947. https://doi.org/10.3390/molecules27154947
Chicago/Turabian StyleTytła, Malwina, Kamila Widziewicz-Rzońca, and Zuzanna Bernaś. 2022. "A Comparison of Conventional and Ultrasound-Assisted BCR Sequential Extraction Methods for the Fractionation of Heavy Metals in Sewage Sludge of Different Characteristics" Molecules 27, no. 15: 4947. https://doi.org/10.3390/molecules27154947
APA StyleTytła, M., Widziewicz-Rzońca, K., & Bernaś, Z. (2022). A Comparison of Conventional and Ultrasound-Assisted BCR Sequential Extraction Methods for the Fractionation of Heavy Metals in Sewage Sludge of Different Characteristics. Molecules, 27(15), 4947. https://doi.org/10.3390/molecules27154947








