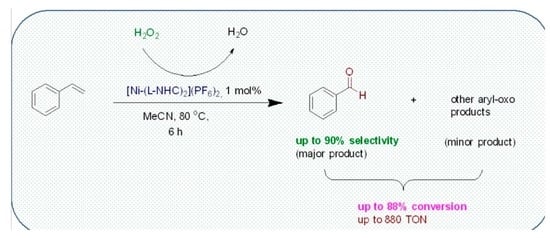
Oxidation of Styrene to Benzaldehyde Catalyzed by Schiff Base Functionalized Triazolylidene Ni(II) Complexes
Abstract
1. Introduction
2. Materials and Methods
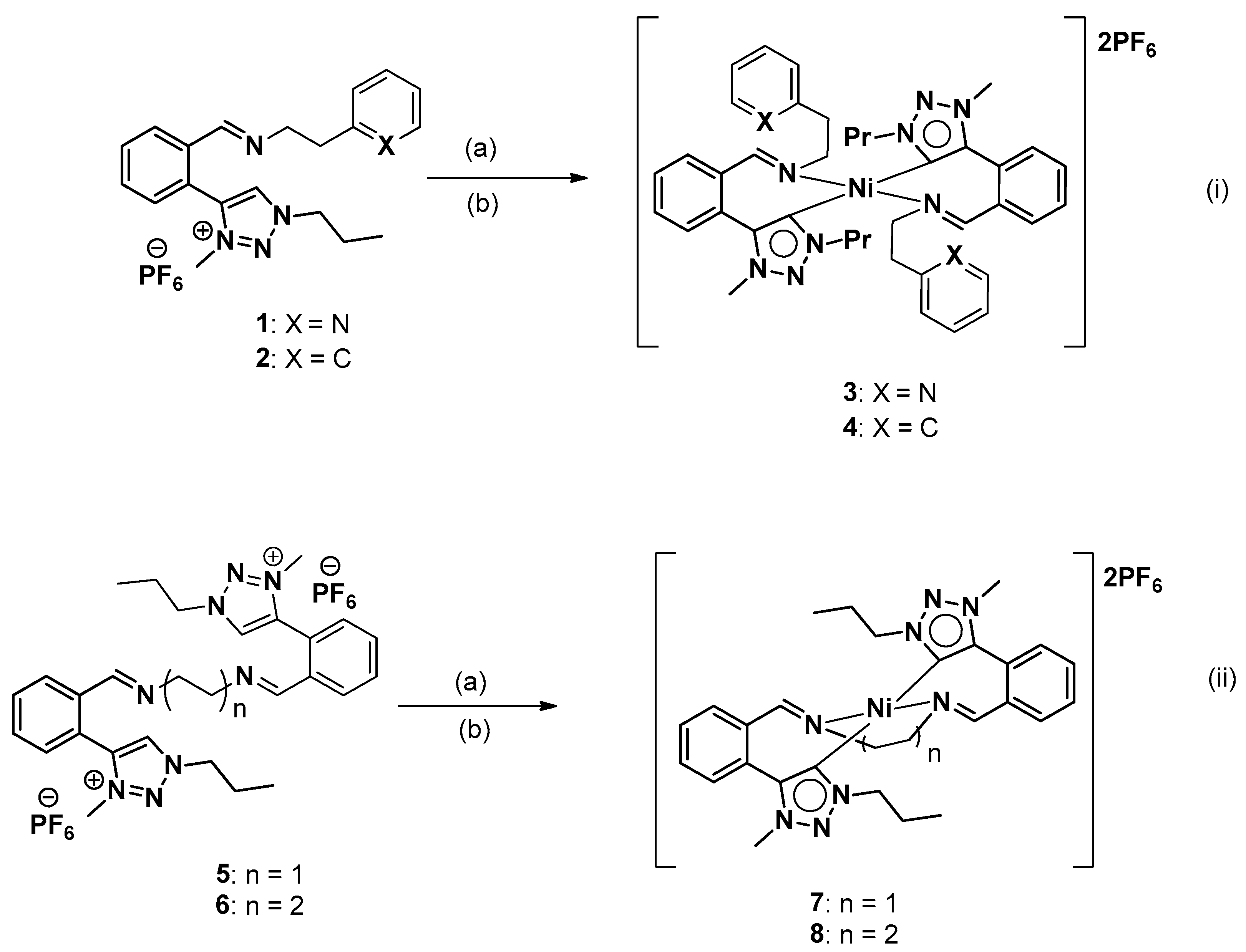
2.1. General Synthesis of Nickel Triazolylidene Complexes
2.2. General Procedure for Styrene Oxidation
- (i)
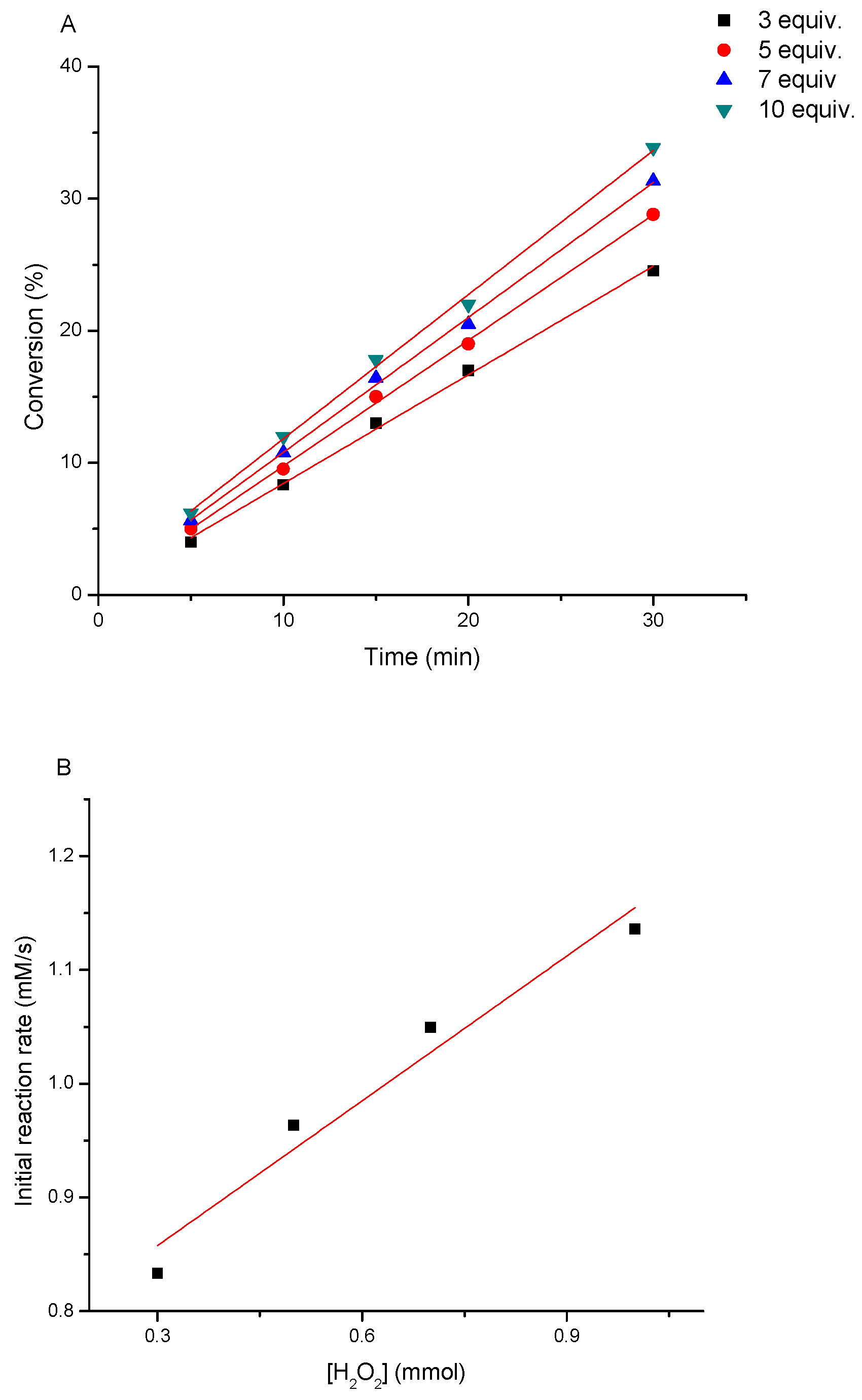
- Styrene: hydrogen peroxide molar ratios from 1:3 to 1:10,
- (ii)
- Reaction temperatures between 40 and 80 °C,
- (iii)
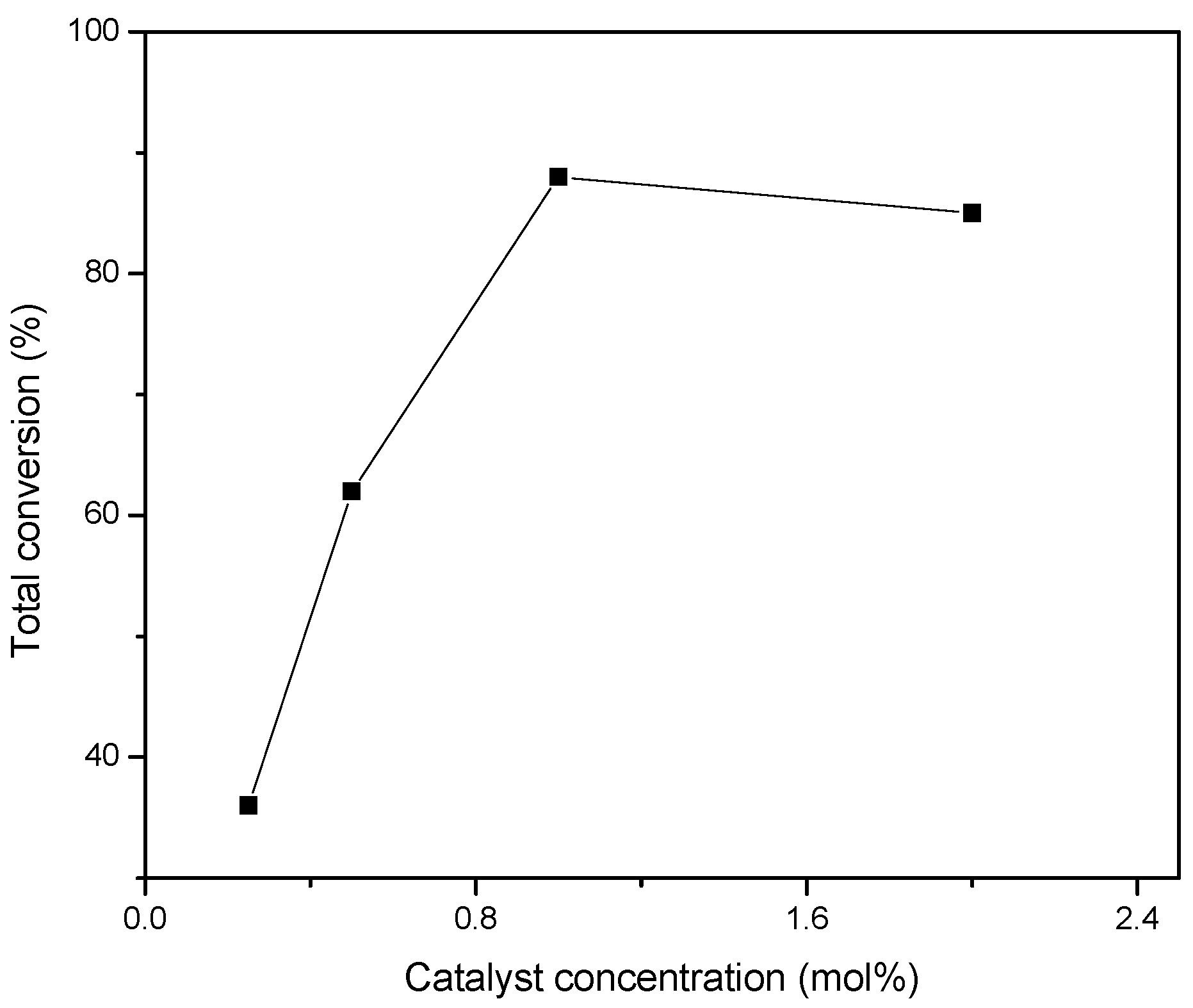
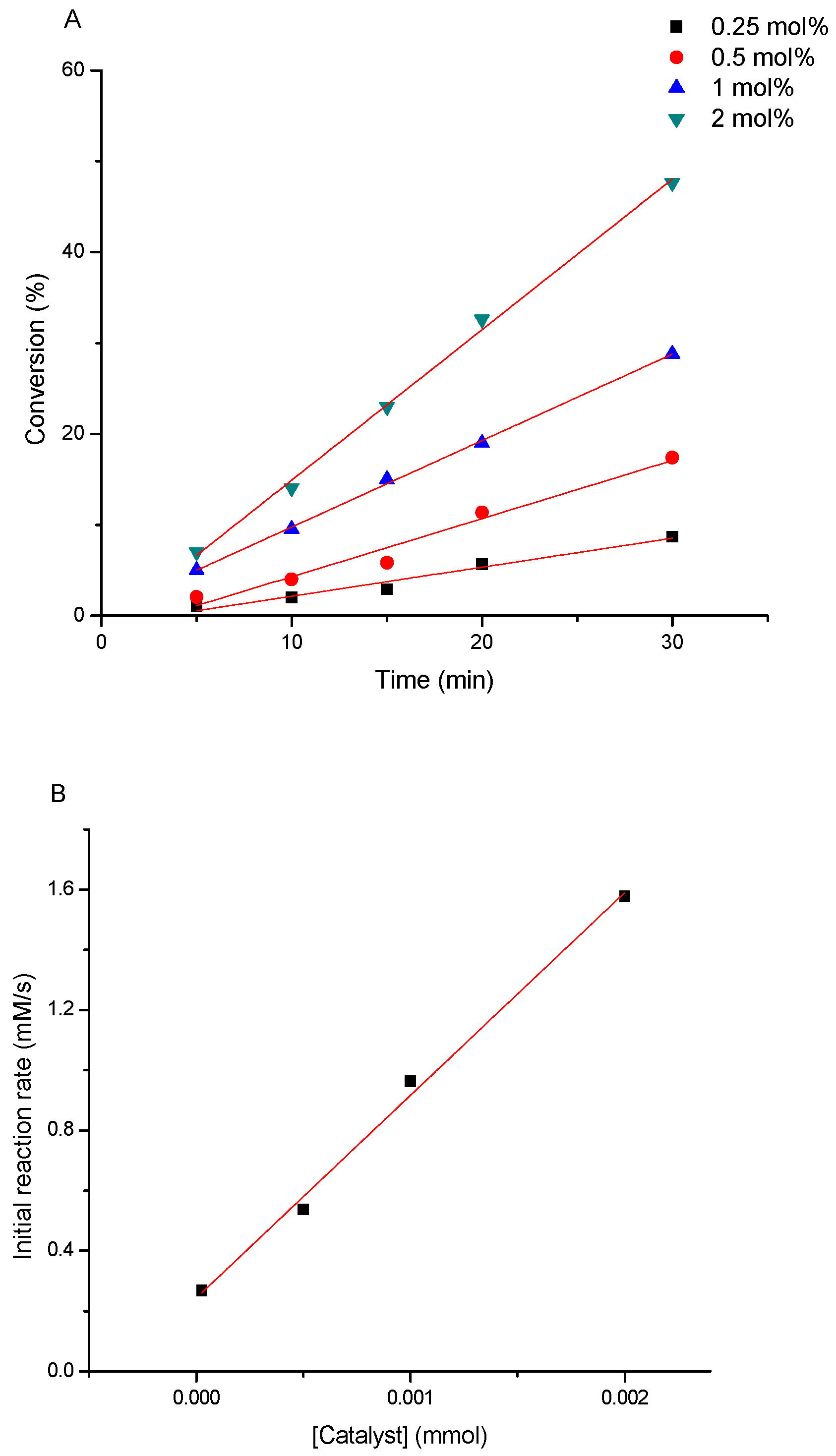
- Catalyst concentration from 0.5 to 2 mol%.
2.3. UV–Vis Analysis
2.4. Cyclic Voltammetry (CV) Analysis
3. Results and Discussion
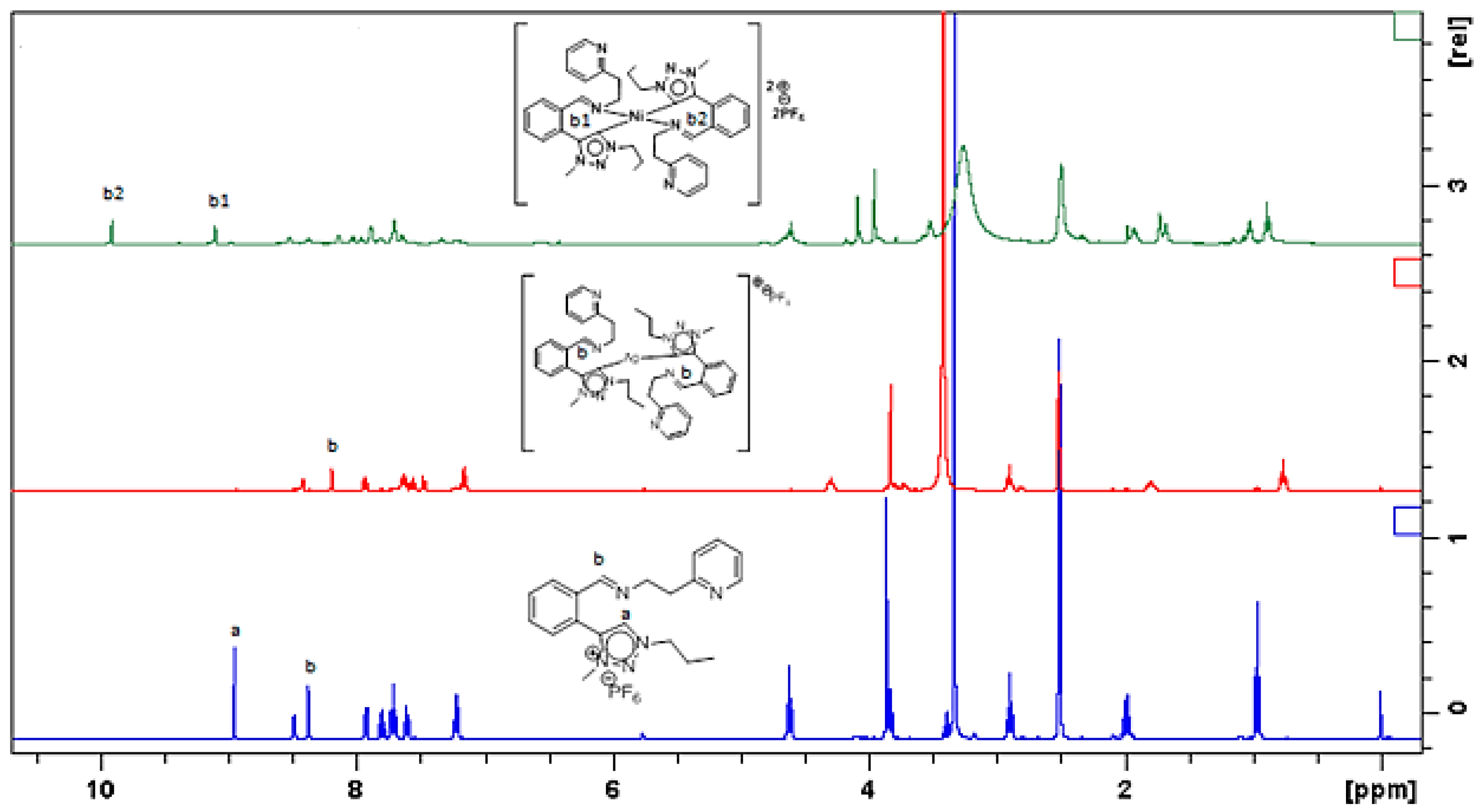
3.1. Synthesis and Spectroscopic Characterization
3.2. Peroxidative Conversion of Styrene Catalyzed by Complexes 3–4 and 7–8
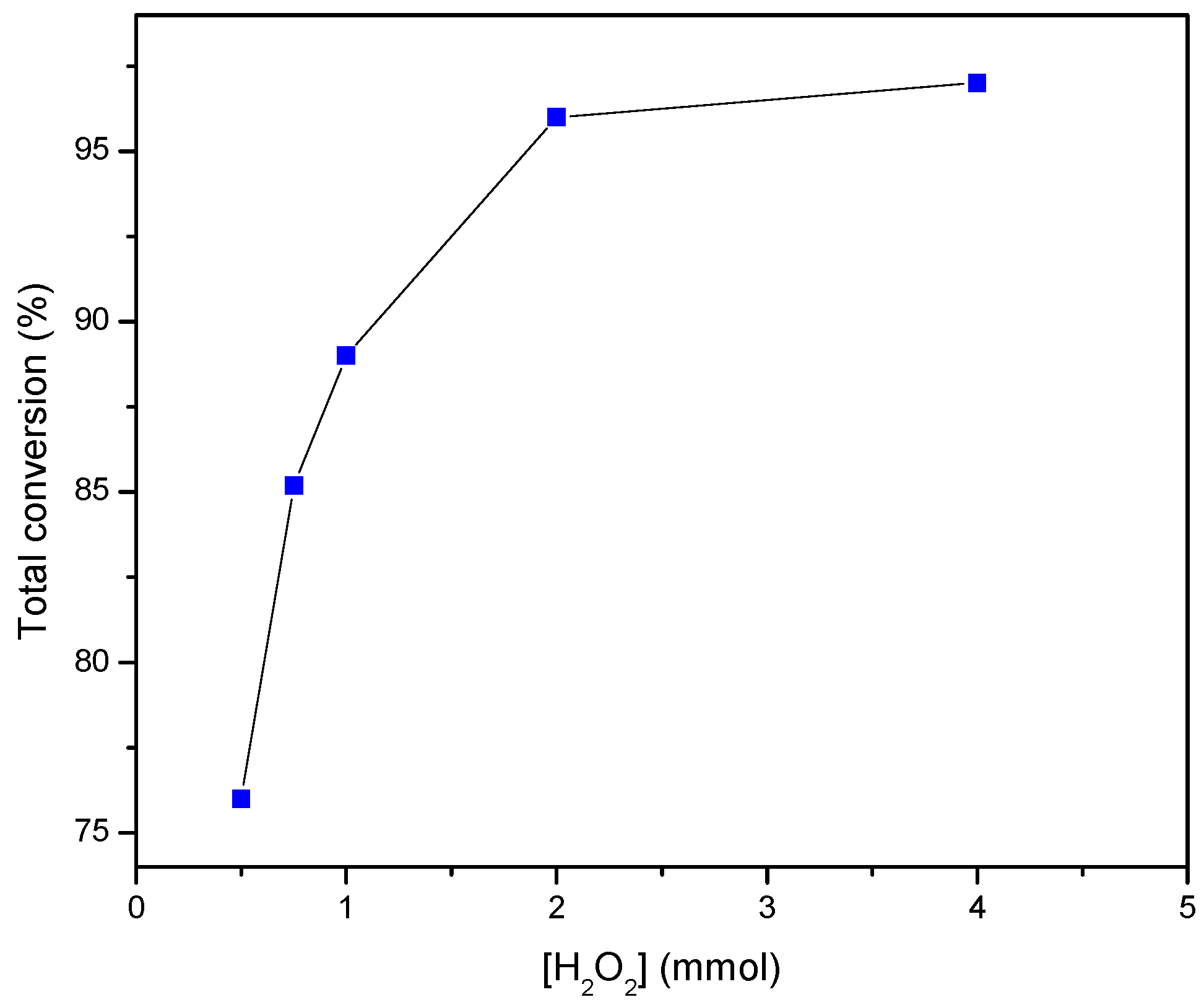
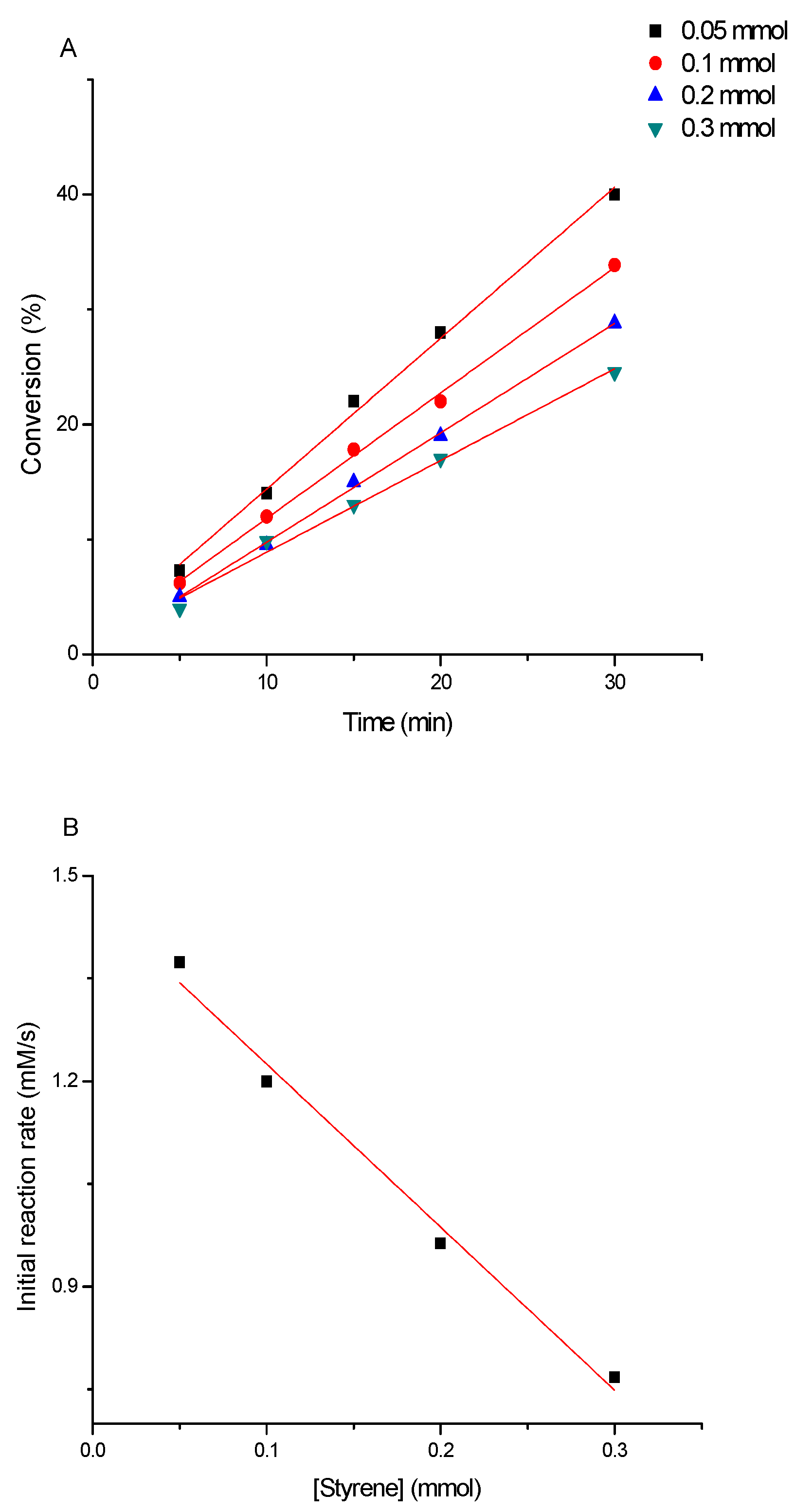
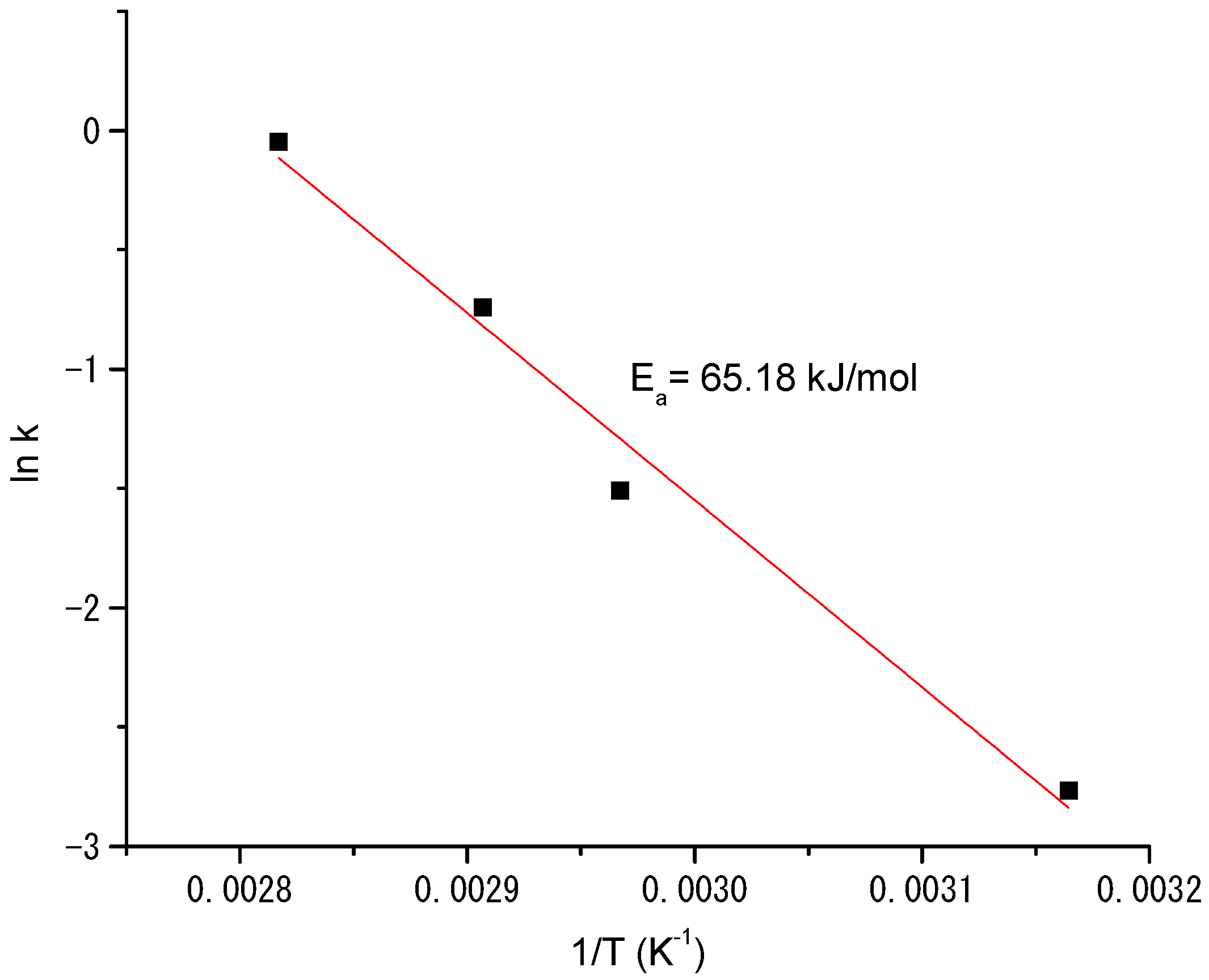
3.3. Determination of the Kinetics of Styrene Oxidation Catalyzed by Complex 3
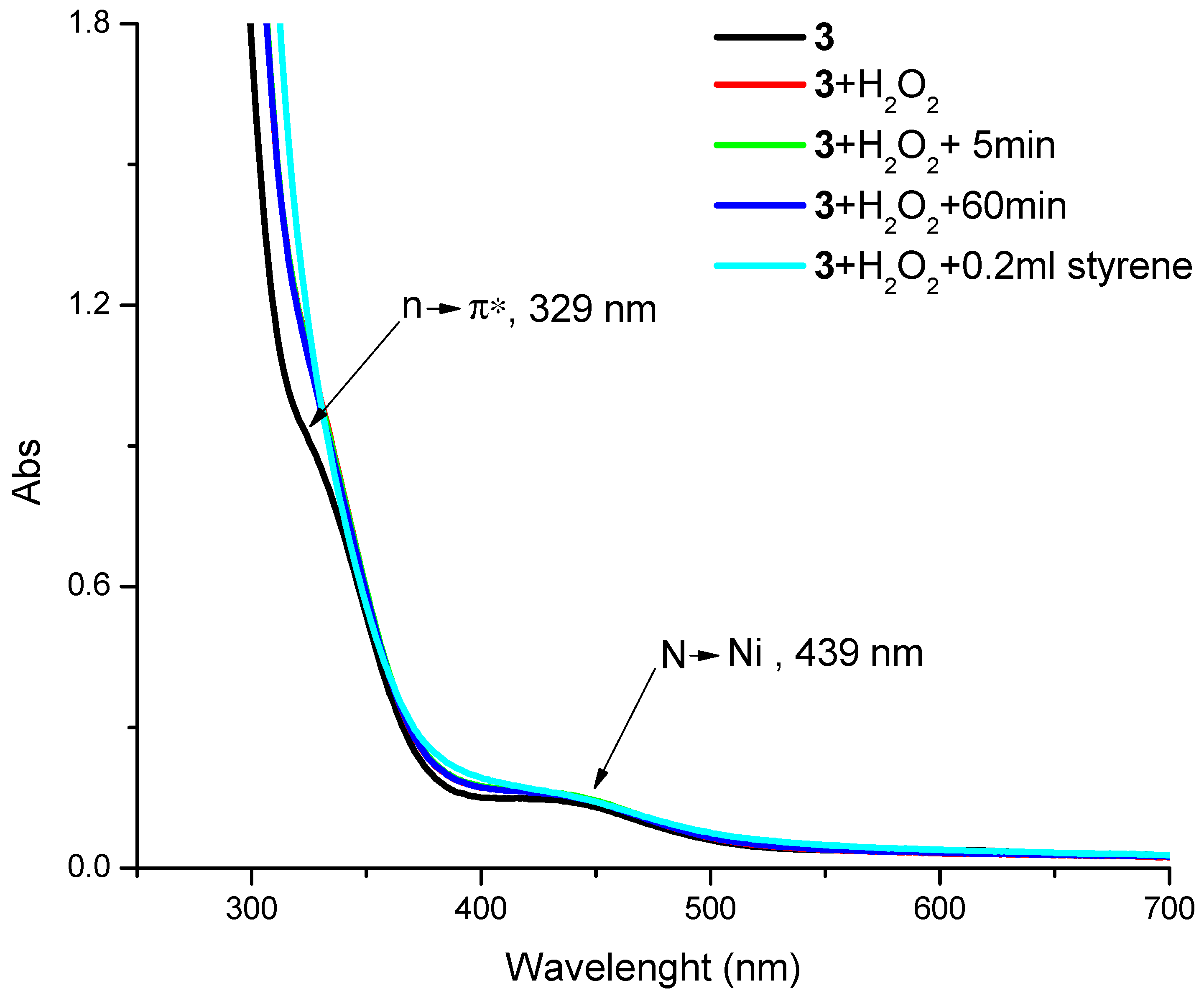
3.4. UV Study of the Reaction
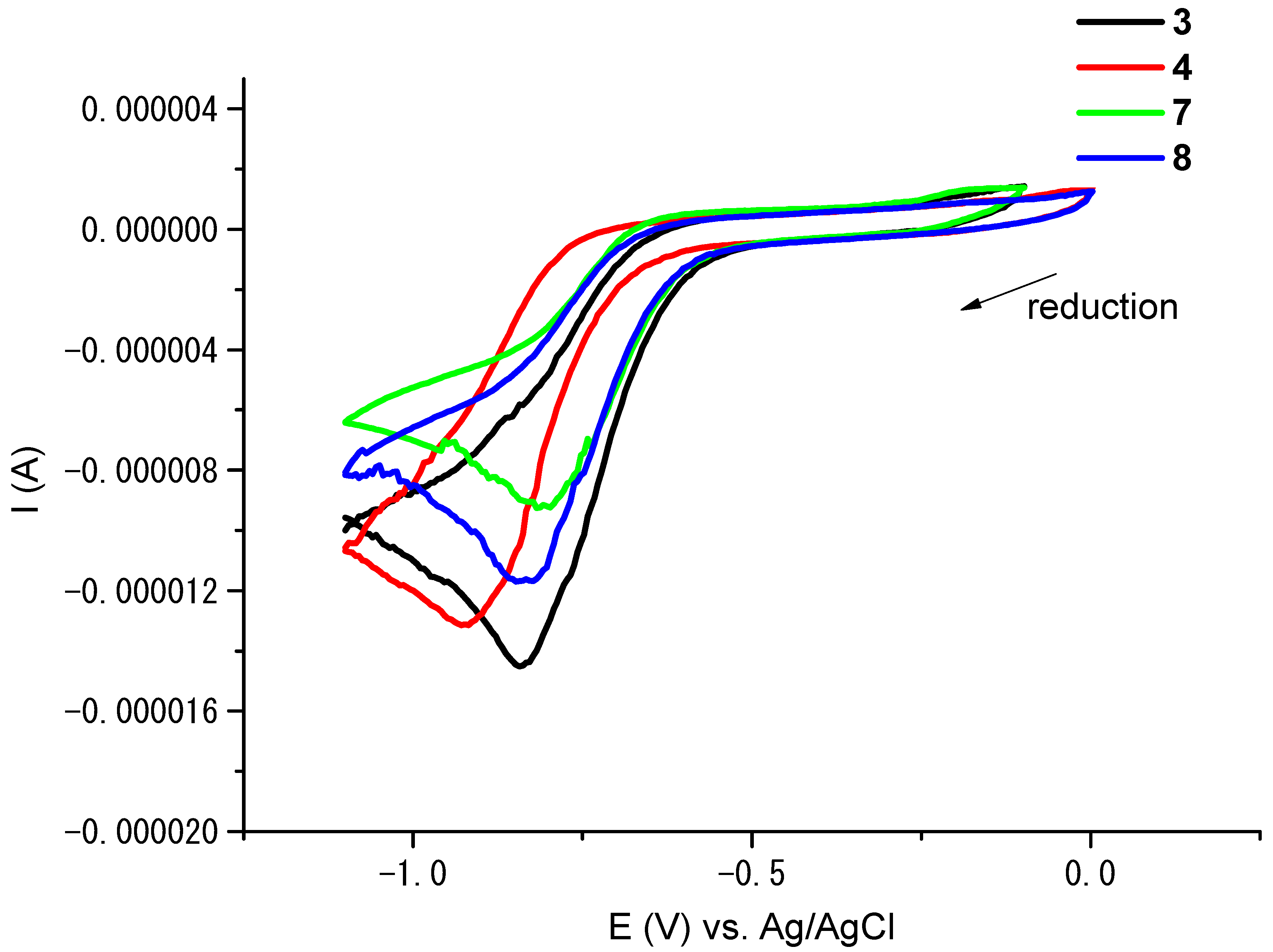
3.5. Cyclic Voltammetry (CV) Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kroschwitz, J.I. Kirk Othmer Encyclopedia of Chemical Technology, 4th ed.; Wiley-Interscience Publications: New York, NY, USA, 1992; Volume 4. [Google Scholar]
- Choudhary, R.V.; Dumbre, D.K. Solvent-free selective oxidation of benzyl alcohol to benzaldehyde by tert-butylhydroperoxide over U3O8-supported nano-gold catalysts. Appl. Catal. A 2010, 375, 252. [Google Scholar] [CrossRef]
- Wu, X.F.; Gong, J.L.; Qi, X. A powerful combination: Recent achievements on using TBAI and TBHP as oxidation system. Org. Biomol. Chem. 2014, 12, 5807. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wang, H.; Lu, B.; Zhao, J.; Cai, Q. Highly selective oxidation of styrene to benzaldehyde over Fe3O4 using H2O2 aqueous solution as oxidant. Catal. Sci. Technol. 2018, 125, 743. [Google Scholar] [CrossRef]
- Keith, J.A.; Henry, P.M. The Mechanism of the Wacker Reaction: A Tale of Two Hydroxypalladations. Angew. Chem. Int. Ed. 2009, 48, 9038. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Wu, W.; Jiang, H. Palladium-Catalyzed Oxidation Reactions of Alkenes with Green Oxidants. ChemSusChem 2009, 12, 2911. [Google Scholar] [CrossRef]
- Dong, J.J.; Browne, W.R.; Feringa, B.L. Palladium-Catalyzed anti-Markovnikov Oxidation of Terminal Alkenes. Angew. Chem. Int. Ed. 2015, 54, 734. [Google Scholar] [CrossRef]
- Abubakar, S.; Bala, M.D. Transfer Hydrogenation of Ketones Catalyzed by Symmetric Imino-N-heterocyclic Carbene. Co(III) Complexes. ACS Omega 2020, 5, 2670. [Google Scholar] [CrossRef]
- Reshi, N.U.D.; Kathuria, L.; Samuelson, A.G. Reduction of imines catalysed by NHC substituted group 6 metal carbonyls. Inorg. Chim. Acta 2019, 486, 119. [Google Scholar] [CrossRef]
- Zsigmond, A.; Horváth, A.; Notheisz, F. Effect of substituents on the Mn(III)Salen catalyzed oxidation of styrene. J. Mol. Catal. A Chem. 2001, 171, 95. [Google Scholar] [CrossRef]
- Brinksma, J.; Schmieder, L.; Vliet, G.V.; Boaron, R.; Hage, R.; Vos, D.E.D.; Alsters, P.L.; Feringa, B.L. Homogeneous cis-dihydroxylation and epoxidation of olefins with high H2O2 efficiency by mixed manganese/activated carbonyl catalyst system. Tetrahedron Lett. 2002, 43, 2619. [Google Scholar] [CrossRef]
- Kureshy, I.R.; Khan, H.N.; Abdi, H.R.S.; Patel, T.S.; Iyer, K.P.; Subramanian, S.P.; Jasra, R.V. A Highly Potential Analogue of Jacobsen Catalyst with In-built Phase Transfer Capability in Enantioselective Epoxidation of Nonfunctionalized Alkenes. J. Catal. 2002, 209, 99. [Google Scholar] [CrossRef]
- Rayatia, S.; Koliaei, M.; Ashouri, F.; Mohebbi, S.; Wojtczak, A.; Kozakiewicz, A. Oxovanadium(IV) Schiff base complexes derived from 2,2’-dimethylpropandiamine: A homogeneous catalyst for cyclooctene and styrene oxidation. Appl. Catal. A 2008, 346, 65. [Google Scholar] [CrossRef]
- Adam, M.S.S. Catalytic activity of nickel(II), copper(II) and oxovanadium(II)-dihydroindolone complexes towards homogeneous oxidation reactions. Appl. Organomet. Chem. 2018, 32, e4232. [Google Scholar] [CrossRef]
- Duarte, A.G.T.; Estrada, C.A.; Simões, M.Q.M.; Santos, C.M.S.I.; Cavaleiro, M.V.A.; Neves, P.M.S.G.M.; Cavaleiroa, J.A.S. Homogeneous catalytic oxidation of styrene and styrene derivatives with hydrogen peroxide in the presence of transition metal-substituted polyoxotungstates. Catal. Sci. Technol. 2015, 5, 351. [Google Scholar] [CrossRef]
- Neacşu, A.V.; Maxim, C.; Mădălan, M.A.; Hillebrand, M.; González-Arellano, C.M.; Soriano, S.; Rentschler, E.; Andruh, M. New complexes of Ni(II) and Co(III) with a Schiff-base ligand derived from o-vanillin. Crystal structure, magnetic and catalytic properties of a dissymmetric binuclear nickel(II) complex. Polyhedron 2018, 150, 77. [Google Scholar] [CrossRef]
- Heshmatpour, F.; Rayati, S.; Hajiabbas, A.M.; Neumüller, B. Synthesis, Characterization, and Catalytic Performance in Oxidation of Styrene and α-Methyl Styrene of a Nickel(II) Complex Derived from a Schiff Base Ligand. Z. Anorg. Allg. Chem. 2011, 637, 1224. [Google Scholar] [CrossRef]
- Liu, D.; Lu, X.Q.; Lu, R. Homogeneous and heterogeneous styrene epoxidation catalyzed by copper(II) and nickel(II) Schiff base complexes. Trans. Met. Chem. 2014, 39, 705. [Google Scholar] [CrossRef]
- Chishiro, T.; Kon, Y.; Nakashima, T.; Goto, M.; Sato, K. Practical Iron–Catalyzed Hydrogen Peroxide Epoxidation of Aromatic Olefins Using a Combination of Two Kinds of Simple Picolinate Ligands under Halide–Free Reaction Conditions. Adv. Synth. Catal. 2014, 356, 623. [Google Scholar] [CrossRef]
- Rayati, S.; Zakavi, S.; Koliaei, M.; Wojtczak, A.; Kozakiewicz, A. Electron-rich salen-type Schiff base complexes of Cu(II) as catalysts for oxidation of cyclooctene and styrene with tert-butylhydroperoxide: A comparison with electron-deficient ones. Inorg. Chem. Commun. 2010, 13, 203. [Google Scholar] [CrossRef]
- Duarte, A.G.T.; Carvalho, P.A.; Martins, L.M.D.R.S. Ultra-fast and selective oxidation of styrene to benzaldehyde catalyzed by a C-scorpionate Cu(II) complex. Catal. Sci. Technol. 2018, 8, 2285. [Google Scholar] [CrossRef]
- Singh, J.; Hundal, G.; Gupta, R. Studies on Nickel(II) Complexes with Amide-Based Ligands: Syntheses, Structures, Electrochemistry and Oxidation Chemistry. Eur. J. Inorg. Chem. 2008, 2008, 2052. [Google Scholar] [CrossRef]
- Sankaralingam, M.; Balamurugan, M.; Palaniandavar, M. Alkane and alkene oxidation reactions catalyzed by nickel(II) complexes: Effect of ligand factors. Coord. Chem. Rev. 2020, 403, 213085. [Google Scholar] [CrossRef]
- Kon, Y. Metal-catalyzed Hydrogen Peroxide Oxidation for Synthesis of Value-added Chemicals. J. Jpn. Petrol. Inst. 2017, 60, 159. [Google Scholar] [CrossRef][Green Version]
- Wójtowicz-Młochowska, H. Synthetic utility of metal catalyzed hydrogen peroxide oxidation of C-H, C-C and C=C bonds in alkanes, arenes and alkenes: Recent advances. Arkivoc 2017, ii, 12. [Google Scholar] [CrossRef]
- Vivancos, A.; Segarra, C.; Albrecht, M. Mesoionic and Related Less Heteroatom-Stabilized N-Heterocyclic Carbene Complexes: Synthesis, Catalysis, and Other Applications. Chem. Rev. 2018, 118, 9493. [Google Scholar] [CrossRef]
- Lawal, N.S.; Bala, M.D. Click synthesis and characterization of 1,2,3-triazolium salts. J. Mol. Struc. 2020, 1200, 127124. [Google Scholar] [CrossRef]
- Lawal, N.S.; Ibrahim, H.; Bala, M.D. Facile peroxidation of cyclohexane catalysed by in situ generated triazole-functionalised Schiff base copper complexes. Catal. Lett. 2022, 152, 1264. [Google Scholar] [CrossRef]
- Lawal, N.S.; Ibrahim, H.; Bala, M.D. Cu(I) mediated hydrogen borrowing strategy for the α-alkylation of aryl ketones with aryl alcohols. Monatsh. Chem. 2021, 152, 275. [Google Scholar] [CrossRef]
- Lin, J.B.I.; Vasam, C.S. Preparation and application of N-heterocyclic carbene complexes of Ag(I). Coord. Chem. Rev. 2007, 251, 642. [Google Scholar] [CrossRef]
- Abubakar, S.; Ibrahim, H.; Bala, M.D. Transfer hydrogenation of ketones catalyzed by a trinuclear Ni(II) complex of a Schiff base functionalized N-heterocyclic carbene ligand. Inorg. Chim. Acta 2019, 484, 276. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Sun, H.; Fuhr, O.; Fenske, D. Nickel(II) complexes of amine functionalized N-heterocyclic carbenes (NHCs), synthesis and catalysis in Kumada coupling of aryl chlorides. J. Organomet. Chem. 2016, 820, 41. [Google Scholar] [CrossRef]
- Malan, F.P.; Singleton, E.; Bulling, B.W.; Cukrowski, I.; van Rooyen, P.H.; Landman, M. CpNiBr(NHC) complexes as pre-catalysts in the chemoselective anaerobic oxidation of secondary aryl alcohols: Experimental and DFT studies. Mol. Catal. 2017, 432, 47. [Google Scholar] [CrossRef]
- Bauer, L.; Anderson, H.J. Comments on the Treatment of Aromaticity and Acid-Base Character of Pyridine and Pyrrole in Contemporary Organic Chemistry Textbooks. J. Chem. Ed. 1999, 76, 1151. [Google Scholar] [CrossRef]
- Jhaumeer-Laulloo, S.B.; Bhowon, M.G. Synthesis, biological and catalytic properties of Ru (II) benzamides Schiff base complexes. Indian J. Chem. 2003, 42A, 2536. [Google Scholar]
- Bagihalli, G.B.; Patil, S.A.; Badami, P.S. Synthesis, physicochemical investigation and biological studies of zinc (II) complexes with 1, 2, 4-triazole schiff bases. J. Iran. Chem. Soc. 2009, 6, 259. [Google Scholar] [CrossRef]
- Singh, K.; Singh, P.D.; Barwa, S.M.; Tyagi, P.; Mirza, Y. Some bivalent metal complexes of Schiff bases containing N and S donor atoms. J. Enzyme Inhib. Med. Chem. 2006, 21, 749. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, Y.; Puri, P.; Sharma, C.; Aneja, K.R. Antimicrobial, spectral and thermal studies of divalent cobalt, nickel, copper and zinc complexes with triazole Schiff bases. Arab. J. Chem. 2017, 10, S978–S987. [Google Scholar] [CrossRef]
- Andrade, M.A.; Martins, L.M.D.R.S. Selective Styrene Oxidation to Benzaldehyde over Recently Developed Heterogeneous Catalysts. Molecules 2021, 26, 1680. [Google Scholar] [CrossRef]
- Tulloch, A.A.D.; Danopoulos, A.A.; Cafferkey, S.M.; Kleinhenz, S.; Hursthouse, M.B.; Tooze, R.P. Pyridine functionalised N-heterocyclic carbene complexes of palladium. Chem. Commun. 2000, 14, 1247–1248. [Google Scholar] [CrossRef]
- Danopoulos, A.A.; Tsoureas, N.; Macgregor, A.S.; Smith, C. Phosphine- and Pyridine-Functionalized N-Heterocyclic Carbene Methyl and Allyl Complexes of Palladium. Unexpected Regiospecificity of the Protonation Reaction of the Dimethyl Complexes. Organometallics 2007, 26, 253. [Google Scholar] [CrossRef]
- Danopoulos, A.A.; Tulloch, A.D.A.; Winston, S.; Eastham, G.; Hursthouse, M.B. Chelating and ‘pincer’ dicarbene complexes of palladium; synthesis and structural studies. Dalton Trans. 2003, 3, 1009. [Google Scholar]
- Ibrahim, H.; Bala, M.D. Earth abundant metal complexes of donor functionalised N-heterocyclic carbene ligands: Synthesis, characterisation and application as amination catalysts. New J. Chem. 2016, 40, 6986. [Google Scholar] [CrossRef]
- Ibrahim, H.; Bala, M.D. Air stable pincer (CNC) N-heterocyclic carbene–cobalt complexes and their application as catalysts for C–N coupling reactions. J. Organomet. Chem. 2015, 794, 301. [Google Scholar] [CrossRef]
- Sankaralingam, M.; Vadivelu, P.; Palaniandavar, M. Novel nickel(ii) complexes of sterically modified linear N4 ligands: Effect of ligand stereoelectronic factors and solvent of coordination on nickel(ii) spin-state and catalytic alkane hydroxylation. Dalton Trans. 2017, 46, 7181. [Google Scholar] [CrossRef] [PubMed]
- Villa de P, A.L.; Farlán Taborda, A.; de Correa, C.M. Kinetics of limonene epoxidation by hydrogen peroxide on PW-Amberlite. J. Mol. Catal. A Chem. 2002, 185, 269. [Google Scholar] [CrossRef]
- Sauxa, C.; Pierella, L.B. Studies on styrene selective oxidation to benzaldehyde catalyzed by Cr-ZSM-5: Reaction parameters effects and kinetics. Appl. Catal. A Gen. 2011, 400, 117. [Google Scholar] [CrossRef]
- Taffarel, R.S.; Lansarin, A.M.; Moro, C.C. Styrene Photocatalytic Degradation Reaction Kinetics. J. Braz. Chem. Soc. 2011, 22, 1872. [Google Scholar] [CrossRef]
- Tan, C.P.; Che Man, Y.B.; Selamat, J.; Yusoff, M.S.A. Application of Arrhenius Kinetics to Evaluate Oxidative Stability in Vegetable Oils by Isothermal Differential Scanning Calorimetry. J. Am. Oil Chem. Soc. 2001, 78, 1133. [Google Scholar] [CrossRef]
- Sun, Y.X.; Gao, X.H. Synthesis, Characterization, and Crystal Structure of a New Cu(II) Complex with Salen-Type Ligand. Synth. React. Inorg. Metal-Org. Nano-Met. Chem. 2011, 41, 973. [Google Scholar] [CrossRef]
- Unver, H.; Hayvali, Z. Synthesis, spectroscopic studies and structures of square-planar nickel(II) and copper(II) complexes derived from 2-{(Z)-[furan-2-ylmethyl]imino]methyl}-6-methoxyphenol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 782. [Google Scholar] [CrossRef]
- Rocha, F.S.; Gomes, A.J.; Lunardi, C.N.; Kaliaguine, S.; Patience, G.S. Experimental methods in chemical engineering: Ultraviolet visible spectroscopy—UV-Vis. Can. J. Chem. Eng. 2018, 96, 2512. [Google Scholar] [CrossRef]
- Chernyshev, V.M.; Denisova, E.A.; Eremin, D.B.; Ananikov, V.P. The key role of R–NHC coupling (R=C, H, heteroatom) and M–NHC bond cleavage in the evolution of M/NHC complexes and formation of catalytically active species. Chem. Sci. 2020, 11, 6957. [Google Scholar] [CrossRef] [PubMed]
- Ulm, F.; Shahane, S.; Truong-Phuoc, L.; Romero, T.; Papaefthimiou, V.; Chessé, M.; Chetcuti, M.J.; Pham-Huu, C.; Michon, C.; Ritleng, V. Half-Sandwich Nickel(II) NHC-Picolyl Complexes as Catalysts for the Hydrosilylation of Carbonyl Compounds: Evidence for NHC-Nickel Nanoparticles under Harsh Reaction Conditions. Eur. J. Inorg. Chem. 2021, 2021, 3074. [Google Scholar] [CrossRef]
- Ramírez-Delgado, V.; Osorio-Monreal, G.; Hernández-Ayala, L.F.; Reyes-Vidal, Y.; García-Ramos, C.J.; Ruiz-Azuara, L.; Ortiz-Frade, L. Electrochemical Behavior of Ni(II) Complexes with N2S2 and N6 Ligands as Potential Catalysts in Hydrogen Evolution Reaction. J. Mex. Chem. Soc. 2015, 59, 294. [Google Scholar]


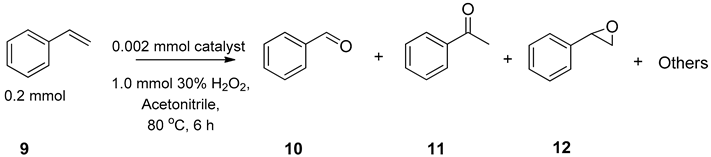
| Entry | Catalyst | Time (h) | Conversion (%) b | c Selectivity (%) | TON d | TOF e | |||
|---|---|---|---|---|---|---|---|---|---|
| Benzaldehyde | Acetophenone | Styrene Oxide | Others | ||||||
| 1 | H2O2 | 6 | 22 | 97 | - | - | 3 | - | - |
| 2 | 3 (no H2O2 added) | 6 | - | - | - | - | - | - | - |
| 3 | 3 | 3 | 77 | 74 | 18 | 3 | 5 | 770 | 260 |
| 4 | 3 | 6 | 88 | 70 | 22 | 3 | 5 | 880 | 150 |
| 5 | Ni(diglyme)Cl2 | 6 | 35 | 93 | - | - | 7 | 350 | 58 |
| 6 | 1 | 6 | 26 | 94 | - | - | 6 | 260 | 43 |
| 7 | 4 | 3 | 62 | 91 | 1 | 5 | 3 | 620 | 210 |
| 8 | 4 | 6 | 75 | 90 | 1 | 6 | 3 | 750 | 125 |
| 9 | 7 | 3 | 49 | 92 | 1 | 5 | 2 | 490 | 160 |
| 10 | 7 | 6 | 65 | 90 | 0 | 6 | 4 | 650 | 110 |
| 11 | 8 | 3 | 50 | 94 | 1 | 5 | 0 | 500 | 170 |
| 12 | 8 | 6 | 69 | 91 | 1 | 5 | 3 | 690 | 110 |
| Entry | Catalyst | Oxidant | Time (h) | Conversion (%) | Selectivity (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Benzaldehyde | Styrene Oxide | Others | ||||||
| 1 | 3 | H2O2 | 3 | 77 | 74 | 2 | 24 | This work |
| 2 | 3 | H2O2 | 6 | 88 | 70 | 3 | 27 | This work |
| 3 | NiLa a | TBHP | 6 | 58 | 49 | 9 | - | [17] |
| 4 | NiLb b | H2O2 | 4 | 57 | 26 | 14 | - | [18] |
| 5 | NiH2ID c | H2O2 | 3 | 52 | - | 61 | - | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawal, N.S.; Ibrahim, H.; Bala, M.D. Oxidation of Styrene to Benzaldehyde Catalyzed by Schiff Base Functionalized Triazolylidene Ni(II) Complexes. Molecules 2022, 27, 4941. https://doi.org/10.3390/molecules27154941
Lawal NS, Ibrahim H, Bala MD. Oxidation of Styrene to Benzaldehyde Catalyzed by Schiff Base Functionalized Triazolylidene Ni(II) Complexes. Molecules. 2022; 27(15):4941. https://doi.org/10.3390/molecules27154941
Chicago/Turabian StyleLawal, Nasir S., Halliru Ibrahim, and Muhammad D. Bala. 2022. "Oxidation of Styrene to Benzaldehyde Catalyzed by Schiff Base Functionalized Triazolylidene Ni(II) Complexes" Molecules 27, no. 15: 4941. https://doi.org/10.3390/molecules27154941
APA StyleLawal, N. S., Ibrahim, H., & Bala, M. D. (2022). Oxidation of Styrene to Benzaldehyde Catalyzed by Schiff Base Functionalized Triazolylidene Ni(II) Complexes. Molecules, 27(15), 4941. https://doi.org/10.3390/molecules27154941