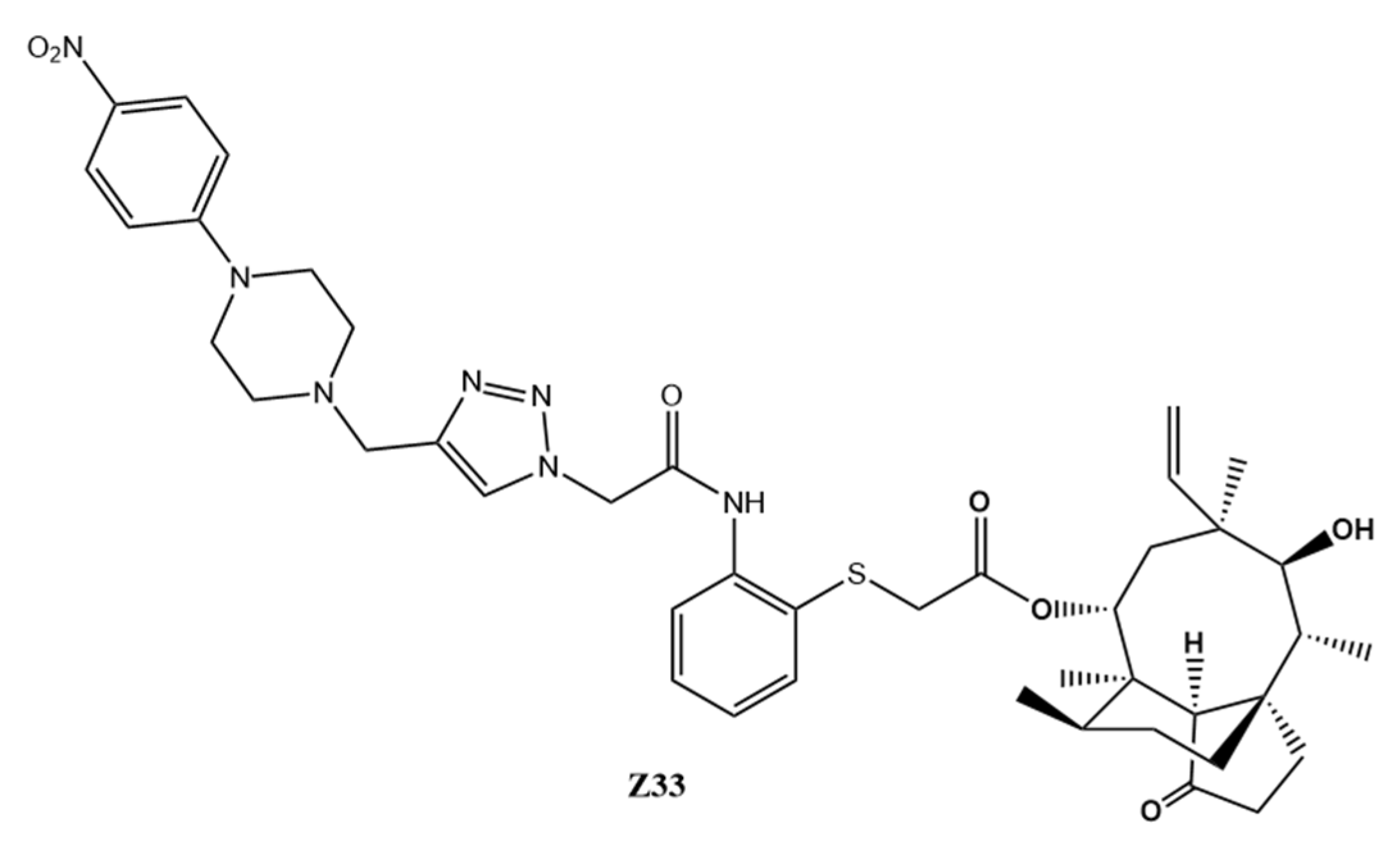
In Vitro and In Vivo Antibacterial Activity, Toxicity and Resistance Analysis of Pleuromutilin Derivative Z33 against Methicillin-Resistant Staphylococcus aureus
Abstract
:1. Introduction
2. Results
2.1. In Vitro Antibacterial Activity
2.2. In Vitro Drug-Resistance Test
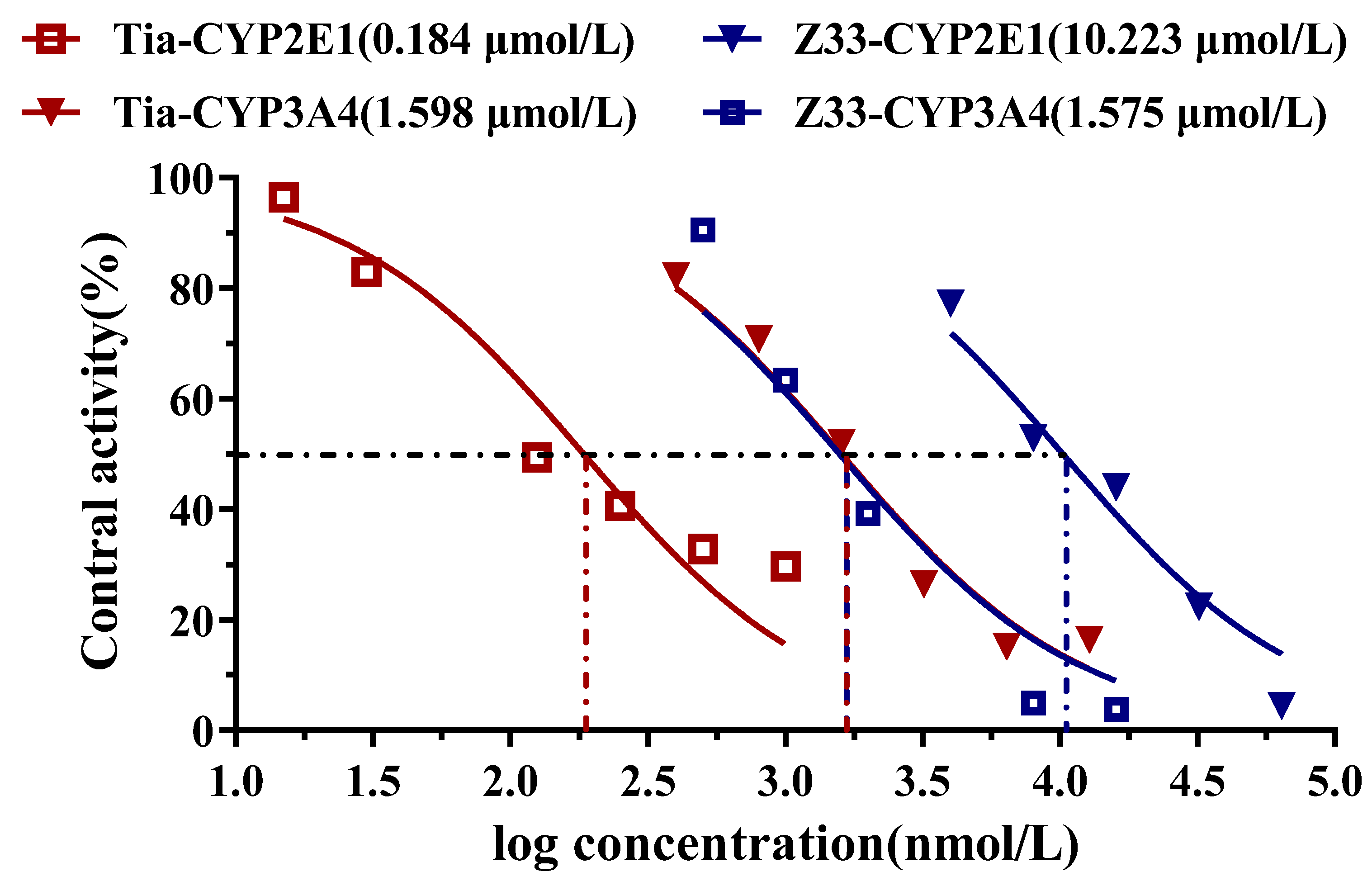
2.3. Inhibitory Effects on Cytochrome P450
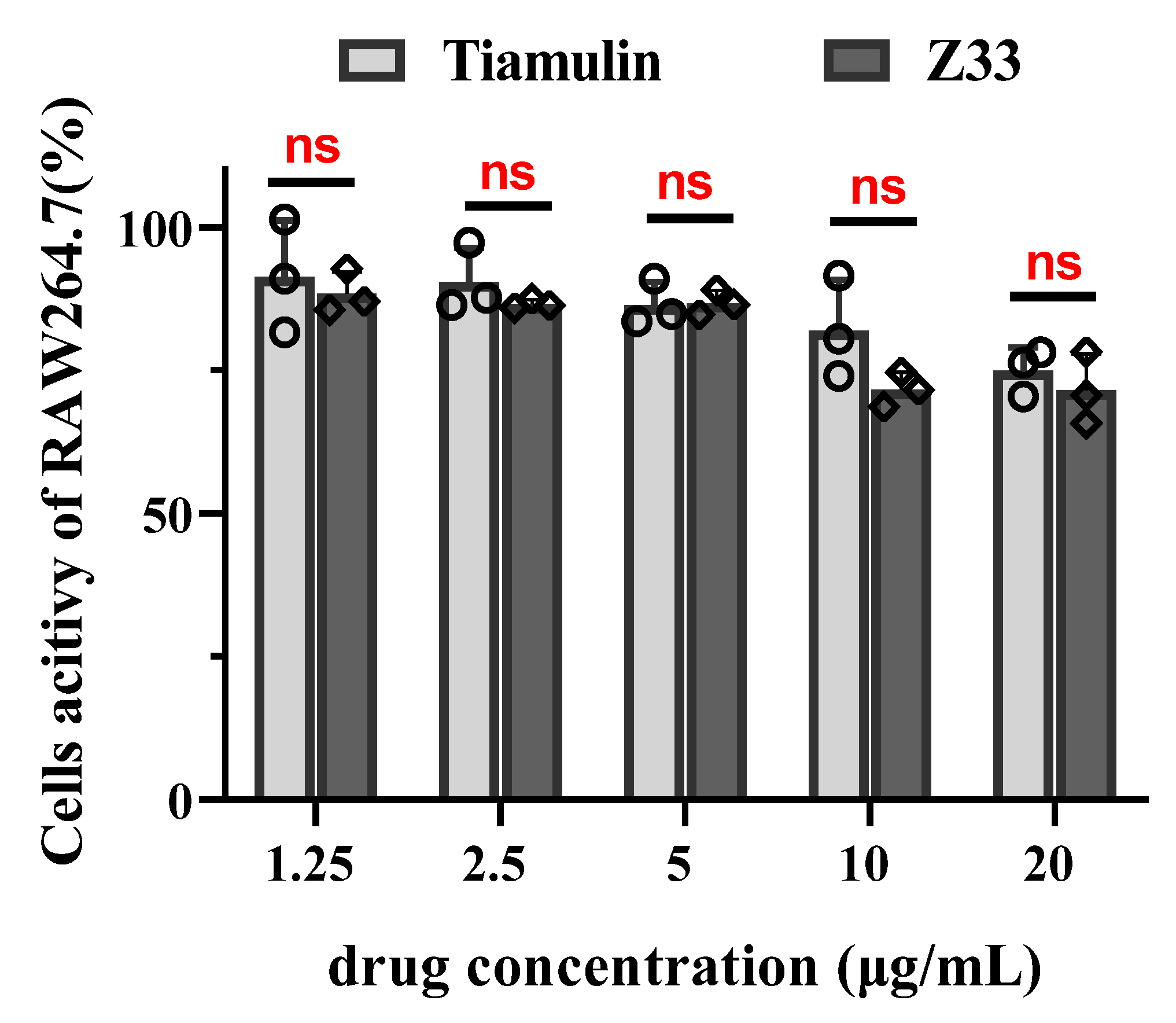
2.4. Cytotoxicity
2.5. Acute Oral Toxicity Study
2.6. In Vivo Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Microorganisms
4.3. Animals
4.4. Evaluation of In Vitro Antibacterial Activity
4.4.1. Determination of MIC and MBC
4.4.2. Time–Kill Kinetics Assay
4.4.3. Post-antibiotic Effect Assay
4.5. Drug-Resistance Test
4.5.1. Determination of MPC
4.5.2. Development of Bacterial Resistance
4.6. CYP450 Inhibition Assay
4.7. Cytotoxicity Test
4.8. Acute Toxicity in Mice
4.9. Neutropenic Mouse Thigh Infection Model
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kong, B.; Johnson, J.K.; Jabrarizk, M.A. Community-Associated Methicillin-Resistant Staphylococcus aureus: An Enemy amidst Us. PLoS Pathogens 2016, 12, e1005837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalaby, M.; Dokla, E.; Serya, R.; Abouzid, K. Penicillin binding protein 2a: An overview and a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 199, 112312. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.F. MRSA—what is it and how do we deal with the problem? Expert Opin. Ther. Tar. 2005, 9, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Hsueh, P.R.; Chung, D.R.; Ko, K.S.; Kang, C.I.; Peck, K.R.; Yeom, J.S.; Kim, S.W.; Chang, H.H.; Kim, Y.S.; et al. Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: An ANSORP study. J. Antimicrob. Chemother. 2011, 66, 1061–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balm, M.; Lover, A.A.; Salmon, S.; Tambyah, P.A.; Fisher, D.A. Progression from new methicillin-resistant Staphylococcus aureus colonisation to infection: An observational study in a hospital cohort. BMC Infect. Dis. 2013, 13, 491. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, F.; Hervey, A.; Robbins, W.J. Antibiotic substances from basidiomycetes: VIII. Pleurotus multilus (Fr.) sacc. And pleurotus passeckerianus pilat. Proc. Natl. Acad. Sci. USA 1951, 37, 570–574. [Google Scholar] [CrossRef] [Green Version]
- Davidovich, C.; Bashan, A.; Auerbach-Nevo, T.; Yaggie, R.D.; Gontarek, R.R.; Yonath, A. Induced-fit tightens pleuromutilins binding to ribosomes and remote interactions enable their selectivity. Proc. Natl. Acad. Sci. USA 2007, 104, 4291–4296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stresser, D.M.; Broudy, M.I.; Ho, T.; Cargill, C.E.; Blanchard, A.P.; Sharma, R.; Dandeneau, A.A.; Goodwin, J.J.; Turner, S.D.; Erve, J.; et al. Highly selective inhibition of human CYP3A in vitro by azamulin and evidence that inhibition is irreversible. Drug Metab. Dispos. 2004, 32, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Fu, L.; Gao, S.; Chu, W.; Wang, H.; Huang, Y.; Chen, X.; Yang, Y. Design, synthesis, and Structure-Activity relationship studies of novel thioether pleuromutilin derivatives as potent antibacterial agents. J. Med. Chem. 2014, 57, 4772–4795. [Google Scholar] [CrossRef]
- Goethe, O.; Heuer, A.; Ma, X.; Wang, Z.; Herzon, S.B. Antibacterial properties and clinical potential of pleuromutilins. Nat. Prod. Rep. 2019, 36, 220–247. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, K.; Zhang, G.; Tang, Y.; Jin, Z. Design, synthesis and biological activities of novel pleuromutilin derivatives with a substituted triazole moiety as potent antibacterial agents. Eur. J. Med. Chem. 2020, 204, 112604. [Google Scholar] [CrossRef] [PubMed]
- Ooi, N.; Eady, E.A.; Cove, J.H.; O’Neill, A.J. Redox-active compounds with a history of human use: Antistaphylococcal action and potential for repurposing as topical antibiofilm agents. J. Antimicrob. Chemother. 2015, 70, 479–488. [Google Scholar] [CrossRef]
- Stubbings, W.J.; Bostock, J.M.; Eileen, I.; Ian, C. Assessment of a microplate method for determining the post-antibiotic effect in Staphylococcus aureus and Escherichia coli. J. Antimicrob. Chemother. 2004, 54, 139–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.F.; Tao, M.T.; Huo, W.; Liao, X.P.; Sun, J.; Liu, Y.H. In Vivo Pharmacokinetic and Pharmacodynamic Profiles of Antofloxacin against Klebsiella pneumoniae in a Neutropenic Murine Lung Infection Model. Antimicrob. Agents Chemother. 2017, 61, e02691-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isvoran, A.; Louet, M.; Vladoiu, D.L.; Craciun, D.; Loriot, M.; Villoutreix, B.O.; Miteva, M.A. Pharmacogenomics of the cytochrome P450 2C family: Impacts of amino acid variations on drug metabolism. Drug Discov. Today 2017, 22, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yi, Y.; Chen, J.; Xin, R.; Yang, Z.; Guo, Z.; Liang, J.; Shang, R. In vivo efficacy and toxicity studies of a novel antibacterial agent: 14-O-[(2-Amino-1,3,4-thiadiazol-5-yl)Thioacetyl] mutilin. Molecules 2015, 20, 5299–5312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, X.; Gao, H.; Gao, M.; Jin, Z.; Tang, Y. Antibacterial activity of a promising antibacterial agent: 22-(4-(2-(4-Nitrophenyl-piperazin-1-yl)-acetyl)-piperazin-1-yl)-22-deoxypleuromutilin. Molecules 2021, 26, 3502. [Google Scholar] [CrossRef]
- CLSI M100-S25; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2015.
- Jin, Z.; Wang, L.; Gao, H.; Zhou, Y.; Liu, Y.; Tang, Y. Design, synthesis and biological evaluation of novel pleuromutilin derivatives possessing acetamine phenyl linker. Eur. J. Med. Chem. 2019, 181, 111594. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhao, X.; Domagala, J.; Drlica, K. Effect of fluoroquinolone concentration on selection of resistant mutants of Mycobacterium bovis BCG and Staphylococcus aureus. Antimicrob. Agents Chemother. 1999, 43, 1756–1758. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.Z.; Tang, Y.Z.; Liu, Y.H. Wogonoside displays anti-inflammatory effects through modulating inflammatory mediator expression using RAW264.7 cells. J. Ethnopharmacol. 2013, 148, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wei, M.; Luo, Y.; Jin, Z.; Tang, Y. Synergistic Effect of a Pleuromutilin Derivative with Tetracycline against Streptococcus suis in Vitro and in the Neutropenic Thigh Infection Model. Molecules 2020, 25, 3522. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lepak, A.J.; Marchillo, K.; Vanhecker, J.; Andes, D.R. In Vivo Pharmacodynamic Target Assessment of Eravacycline against Escherichia coli in a Murine Thigh Infection Model. Antimicrob. Agents Chemother. 2017, 61, e00250-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Compounds | MRSA | ATCC 29213 | AD3 | 144 |
|---|---|---|---|---|
| Z33 | 0.125/0.25 | 0.125/0.125 | 0.25/0.25 | 0.25/0.25 |
| tiamulin | 0.5/1 | 0.5/1 | 1/2 | 0.5/2 |
| valnemulin | 0.125/0.125 | 0.125/0.125 | 0.125/0.125 | 0.125/0.25 |
| Compound | Concentrations | PAE (h) | |
|---|---|---|---|
| Exposure for 1 h | Exposure for 2 h | ||
| Z33 | 2 × MIC | 2.24 | 2.18 |
| 4 × MIC | 3.89 | 3.93 | |
| Compounds | MRSA | ATCC 29213 | AD3 | 144 |
|---|---|---|---|---|
| Z33 | 0.5 | 0.5 | 0.5 | 0.5 |
| tiamulin | 1 | 1 | 2 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Chen, F.; Zhou, K.; Zhang, Z.; Li, F.; Zhang, J.; Tang, Y.; Jin, Z. In Vitro and In Vivo Antibacterial Activity, Toxicity and Resistance Analysis of Pleuromutilin Derivative Z33 against Methicillin-Resistant Staphylococcus aureus. Molecules 2022, 27, 4939. https://doi.org/10.3390/molecules27154939
Hu Y, Chen F, Zhou K, Zhang Z, Li F, Zhang J, Tang Y, Jin Z. In Vitro and In Vivo Antibacterial Activity, Toxicity and Resistance Analysis of Pleuromutilin Derivative Z33 against Methicillin-Resistant Staphylococcus aureus. Molecules. 2022; 27(15):4939. https://doi.org/10.3390/molecules27154939
Chicago/Turabian StyleHu, Yuhan, Fang Chen, Kexin Zhou, Zhe Zhang, Fei Li, Jianfeng Zhang, Youzhi Tang, and Zhen Jin. 2022. "In Vitro and In Vivo Antibacterial Activity, Toxicity and Resistance Analysis of Pleuromutilin Derivative Z33 against Methicillin-Resistant Staphylococcus aureus" Molecules 27, no. 15: 4939. https://doi.org/10.3390/molecules27154939
APA StyleHu, Y., Chen, F., Zhou, K., Zhang, Z., Li, F., Zhang, J., Tang, Y., & Jin, Z. (2022). In Vitro and In Vivo Antibacterial Activity, Toxicity and Resistance Analysis of Pleuromutilin Derivative Z33 against Methicillin-Resistant Staphylococcus aureus. Molecules, 27(15), 4939. https://doi.org/10.3390/molecules27154939







