Effects of Submerged Macrophytes on the Growth, Morphology, Nutritional Value, and Flavor of Cultured Largemouth Bass (Micropterus salmoides)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sampling
2.2. Water Quality Determination
2.3. Biological, Color and Muscle Nutrients Measurements
2.4. Comparison of Fish Muscle Volatile Substances
2.5. Statistical Methods and Data Processing
3. Results
3.1. Growth Performance and Morphological Characteristics
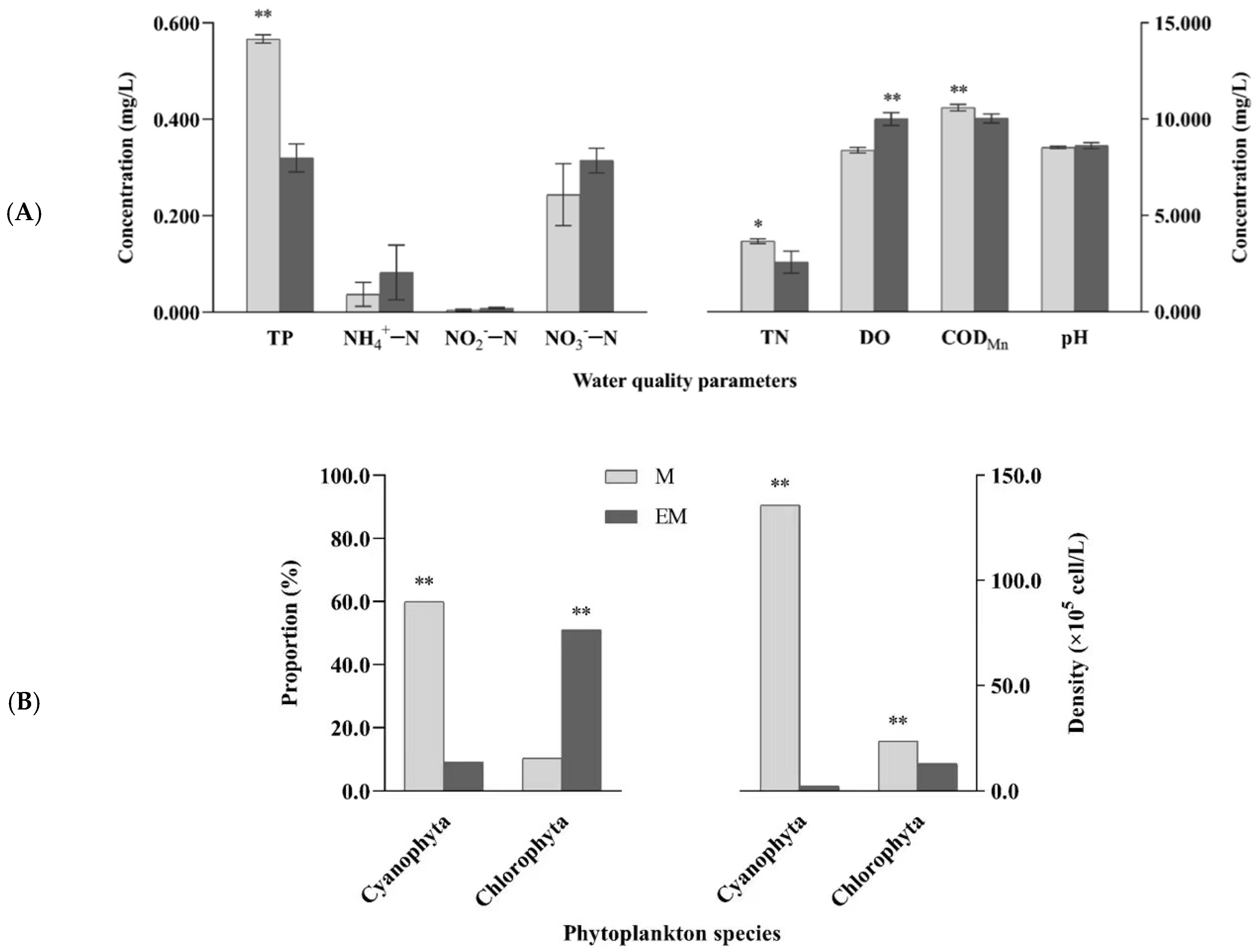
3.2. Water Quality and Dominant Phytoplankton
3.3. Nutritional Components
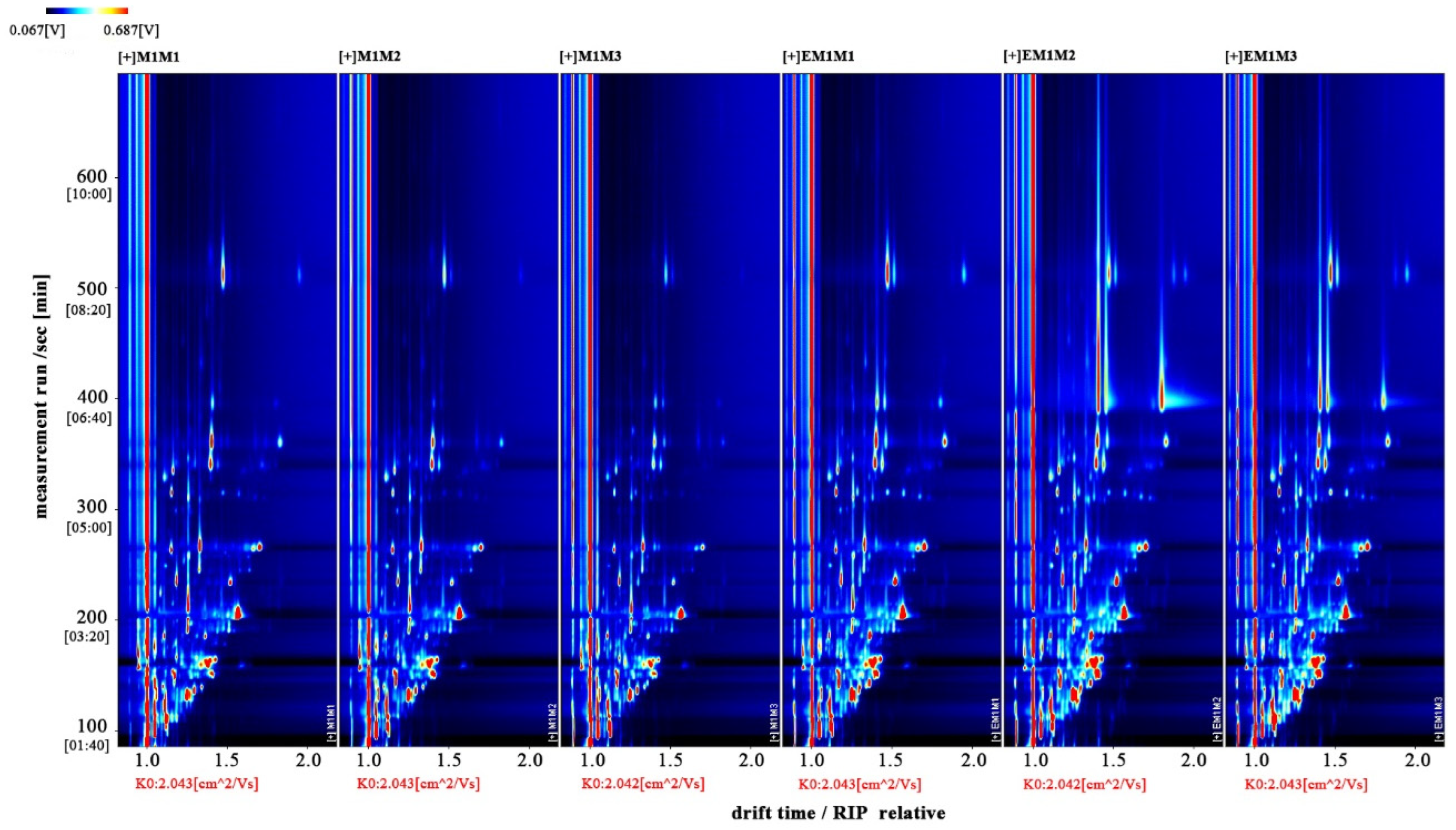
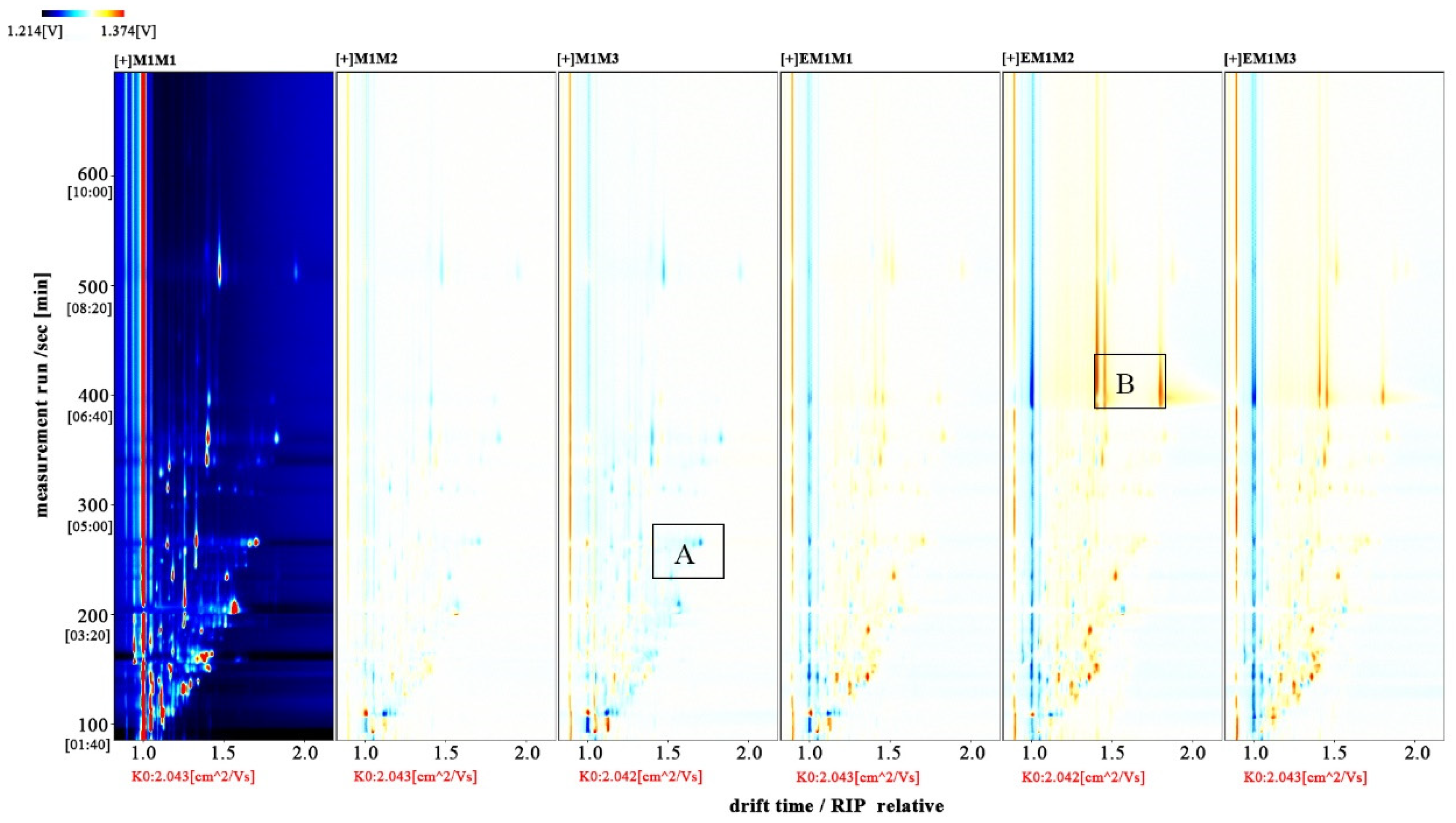
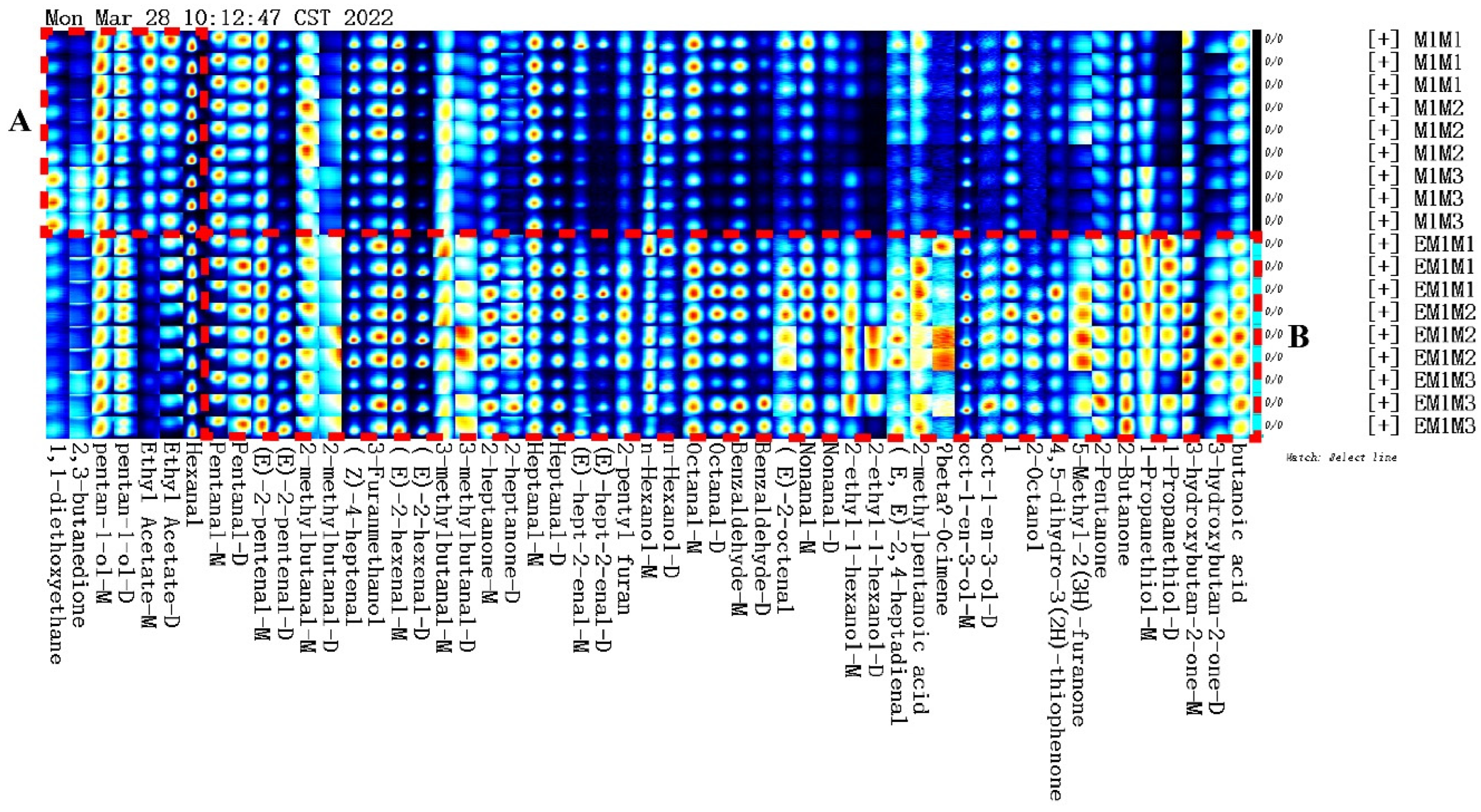
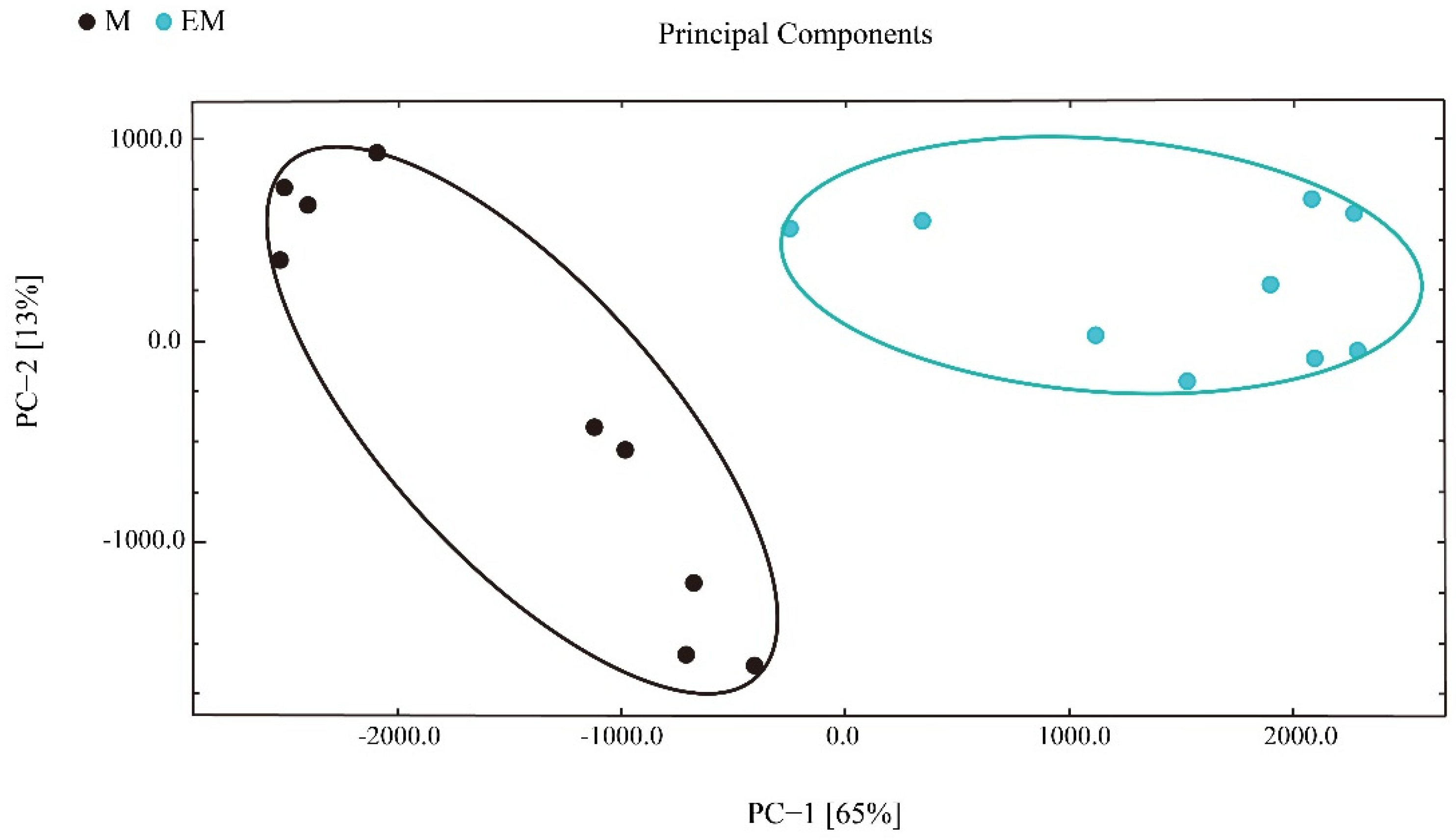
3.4. Volatile Compounds
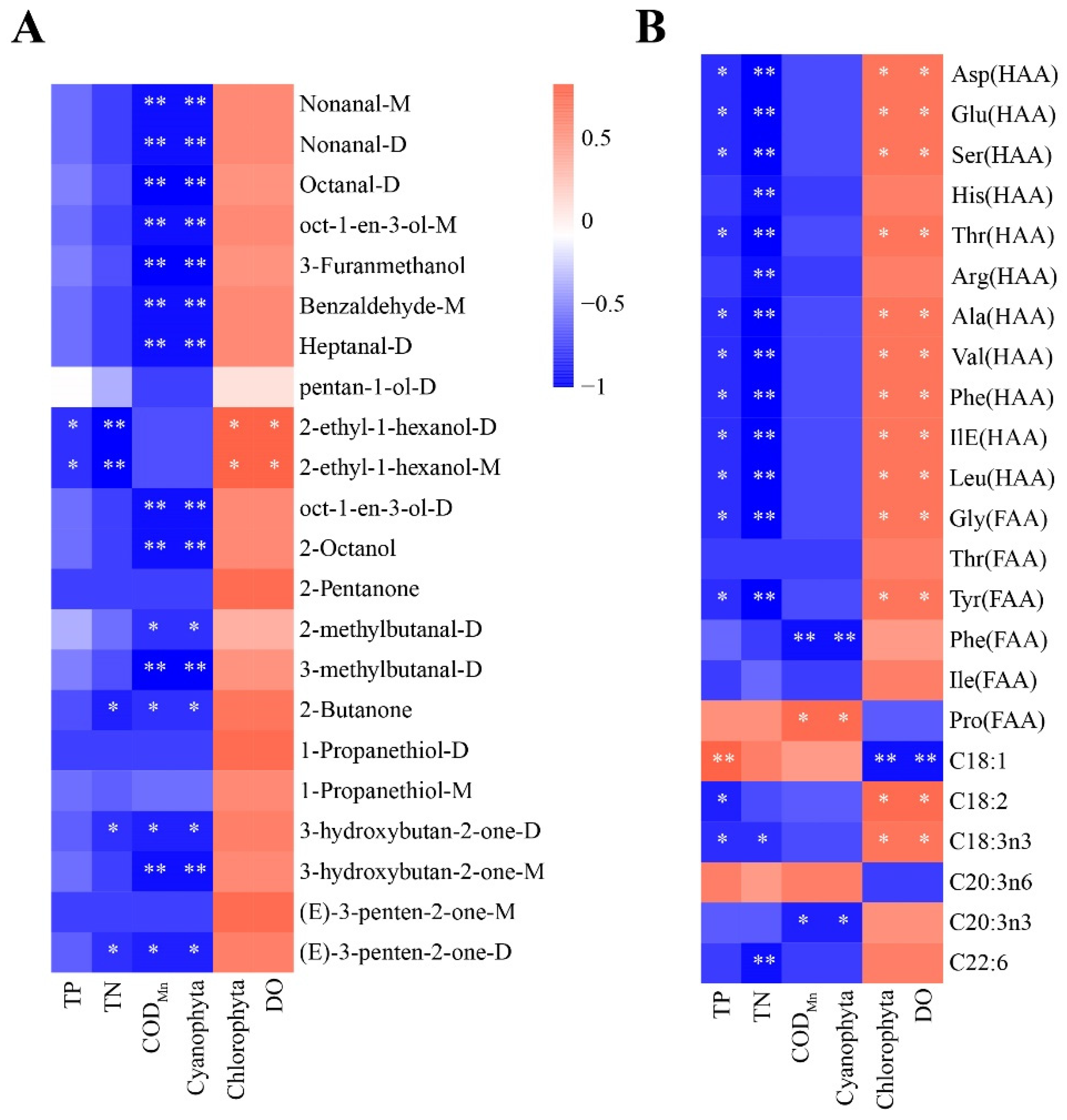
3.5. Correlation Analysis
4. Discussion
4.1. Effects of Submerged Macrophytes on the Growth and Morphology of Largemouth Bass
4.2. Effects of sSubmerged Macrophytes on the Nutrient Composition of Largemouth Bass Muscles
4.3. Effects of Submerged Macrophytes on Volatile Compounds in Largemouth Bass Muscles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, W.; Shen, H. A statistical analysis of China’s fisheries in the 12th five-year period. Aquac. Fish. 2016, 1, 41–49. [Google Scholar] [CrossRef]
- Bai, J.J.; Li, S.J.; Dong, G.C.; Xie, J. Culture Status and Technology of Micropterus salmoides in China. Sci. Fish Farming 2009, 6, 20–21. [Google Scholar]
- Yearbook, C.F.S. Fishery Bureau of Ministry of Agriculture of the People’s Republic of China; China Agriculture Press: Beijing, China, 2020; p. 24. [Google Scholar]
- Coyle, S.D.; Tidwell, J.H.; Webster, C.D. Response of largemouth bass Micropterus slmoides to dietary supplementation of lysine, methionine, and highly unsaturated fatty acids. J. World Aquac. Soc. 2000, 31, 89–95. [Google Scholar] [CrossRef]
- Harimana, Y.; Tang, X.; Xu, P.; Xu, G.; Karangwa, E.; Zhang, K.; Sun, Y.; Li, Y.; Ma, S.; Uriho, A.; et al. Effect of long-term moderate exercise on muscle cellularity and texture, antioxidant activities, tissue composition, freshness indicators and flavor characteristics in largemouth bass (Micropterus salmoides). Aquaculture 2019, 510, 100–108. [Google Scholar] [CrossRef]
- Jia, S.P.; Wang, L.; Zhang, J.M.; Zhang, L.; Ma, F.R.; Huang, M.L.; Liu, S.-S.; Gong, J.-H.; Zhang, M.; Yu, M.; et al. Comparative study on the morphological characteristics and nutritional quality of largemouth bass (Micropterus salmoides) cultured in an aquaculture system using land-based container with recycling water and a traditional pond system. Aquaculture 2022, 549, 737721. [Google Scholar] [CrossRef]
- Munni, M.A.; Fardus, Z.; Mia, M.Y.; Afrin, R. Assessment of pond water quality for fish culture: A case study of Santosh region in Tangail, Bangladesh. J. Environ. Sci. Nat. Resour. 2013, 6, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Costa, S.M.; Appel, E.; Macedo, C.F.; Huszar, V.L. Low water quality in tropical fishponds in southeastern Brazil. An. Acad. Bras. Ciências 2014, 86, 1181–1195. [Google Scholar] [CrossRef]
- Liu, Y.T.; Zhang, W.; Chen, Q.S.; Zhang, Q.; Yin, P.; Guo, Y.Z. On the Quality and Safety of Agricultural Products in Tianjin. J. Agric. 2021, 11, 97–100. [Google Scholar] [CrossRef]
- Jorge, F.; Paulo, V.; Câmara, J.S. From aquaculture production to consumption: Freshness, safety, traceability and authentication, the four pillars of quality. Aquaculture 2019, 518, 734857. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Cornet, J.; Donnay-Moreno, C.; Gouygou, J.P.; Bergé, J.P.; Bacelar, M.; Escórcio, C.; Rocha, E.; Malhão, F.; Cardinal, M. Quality differences of gilthead sea bream from distinct production systems in Southern Europe: Intensive, integrated, semi-intensive or extensive systems. Food Control. 2011, 22, 708–717. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Tong, F.; Tang, T.; Ao, Z.; Wei, Z.; Yang, F.; Shu, Y.; Liu, S.; Mai, K. Comparative evaluation of nutritional value and flavor quality of muscle in triploid and diploid common carp: Application of genetic improvement in fish quality. Aquaculture 2021, 541, 736780. [Google Scholar] [CrossRef]
- Sobczak, M.; Panicz, R.; Eljasik, P.; Sadowski, J.; Tórz, A.; Żochowska-Kujawska, J.; Barbosa, V.L.; Domingues, V.; Marques, A.; Dias, J. Quality improvement of common carp (Cyprinus carpio L.) meat fortified with n-3 PUFA. Food Chem. Toxicol. 2020, 139, 111261. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Sun, D.W.; Han, Z.; Zeng, X.A. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 52–61. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Tang, R.; He, X.; Li, L.; Takagi, Y.; Li, D. Improvement of muscle quality of grass carp (Ctenopharyngodon idellus) with a bio-floating bed in culture ponds. Front. Physiol. 2019, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhou, J.; Qiu, H.; Lai, X.; Li, J.; Wu, D.; Sheng, J.; Hong, Y. Comparison of nutritional quality and volatile flavor compounds among bighead carp from three aquaculture systems. Saudi J. Biol. Sci. 2021, 28, 4291–4299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.H.; Xia, J.G.; Zhang, X.; He, X.G.; Li, L.; Tang, R.; Chi, W.; Li, D. Diet affects muscle quality and growth traits of grass carp (Ctenopharyngodon idellus): A comparison between grass and artificial feed. Front. Physiol. 2018, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Z.; Zhang, Y.; Liu, G.Y.; Huang, C. Effects of Two Kinds of Submerged Plant on the Quality of Waters Cultured with Pseudobagrus fulvidraco Fry. J. Lake Sci. 2003, 15, 184–188. [Google Scholar]
- Zhan, J.; Yang, X. Standard Aquaculture Techniques for Chinese Mitten Crab; Chemical Industry Press: Beijing, China, 2015. [Google Scholar]
- Zhai, Z.; Feng, J.X.; Gao, S.S.; Chen, J.F.; Huang, J.R.; Li, Z.F. Effects of ecological floating bed on content of heavy metals and nutritional quality in aquatic products. J. Fish. China 2017, 41, 88–98. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Xu, X.; Sun, C.; Liu, B.; Zhou, Q.; Xu, P.; Liu, M.; Wang, A.; Tian, H.; Luo, W.; Jiang, Q. Flesh flavor of red swamp crayfish (Procambarus clarkii Girard, 1852) processing by GS-IMS and electronic tongue is changed by dietary animal and plant protein. Food Chem. 2022, 373, 131453. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Zhang, J.; Wang, J.; Wang, X.; Du, D. Recent development of HS-GC-IMS technology in rapid and non-destructive detection of quality and contamination in agri-food products. TrAC Trends Anal. Chem. 2021, 144, 116435. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.; Fu, Y.; Shi, Y.G.; Chen, F.L.; Guan, H.N.; Liu, L.-L.; Zhang, C.-Y.; Zhu, P.-Y.; Liu, Y.; et al. HS-GC-IMS with PCA to analyze volatile flavor compounds across different production stages of fermented soybean whey tofu. Food Chem. 2021, 346, 128880. [Google Scholar] [CrossRef] [PubMed]
- State EPA of China. Monitoring and Determination Methods for Water and Wastewater, 4th ed.; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Ninwichian, P.; Phuwan, N.; Limlek, P. Effects of tank color on the growth, survival rate, stress response, and skin color of juvenile hybrid catfish (Clarias macrocephalus × Clarias gariepinus). Aquaculture 2022, 554, 738129. [Google Scholar] [CrossRef]
- Cunniff, P. Official methods of analysis of AOAC International, Method 923.03. Trends Food Sci. Technol. 1995, 6, 382. [Google Scholar]
- Gao, J.; Tai, X.; Shao, N.; Sun, Y.; Nie, Z.; Wang, Y.; Li, Q.; Xu, P.; Xu, G. Effects of effective microorganisms on the growth performance, nutritional composition and flavour quality of the pond-cultured Eriocheir sinensis. Aquaculture 2021, 52, 871–880. [Google Scholar] [CrossRef]
- Sun, C.; Zou, X.; Yao, Y.; Jin, J.; Xia, Y.; Huang, J.; Jin, Q.; Wang, X. Evaluation of fatty acid composition in commercial infant formulas on the Chinese market: A comparative study based on fat source and stage. Int. Dairy J. 2016, 63, 42–51. [Google Scholar] [CrossRef]
- Liu, D.; Bai, L.U.; Feng, X.I.; Chen, Y.P.; Zhang, D.; Yao, W.; Zhang, H.; Chen, G.; Liu, Y. Characterization of Jinhua ham aroma profiles in specific to aging time by gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2020, 168, 108178. [Google Scholar] [CrossRef] [PubMed]
- Viadero, R.C. Factors affecting fish growth and production. Water Encycl. 2005, 3, 129–133. [Google Scholar] [CrossRef]
- Shang, W.; Jin, S.; He, Y.; Zhang, Y.; Li, J. Spatial–Temporal Variations of Total Nitrogen and Phosphorus in Poyang, Dongting and Taihu Lakes from Landsat-8 Data. Water 2021, 13, 1704. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Y.L.; Liu, X.H.; Zhu, G.W.; Qin, B.Q.; Shi, Z.Q.; Feng, L. Temporal and spatial variations of chemical oxygen demand in lake taihu, China, from 2005 to 2009. Hydrobiologia 2011, 665, 129–141. [Google Scholar] [CrossRef]
- Zhao, C.S.; Shao, N.F.; Yang, S.T.; Ren, H.; Ge, Y.R.; Zhang, Z.S.; Feng, P.; Liu, W. Quantitative assessment of the effects of human activities on phytoplankton communities in lakes and reservoirs. Sci. Total Environ. 2019, 665, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Boyer, G.L.; Zimba, P.V. A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture. Aquaculture 2008, 280, 5–20. [Google Scholar] [CrossRef]
- Drobac, D.; Tokodi, N.; Lujić, J.; Marinović, Z.; Subakov-Simić, G.; Dulić, T.; Važić, T.; Nybom, S.; Meriluoto, J.; Codd, G.A.; et al. Cyanobacteria and cyanotoxins in fishponds and their effects on fish tissue. Harmful Algae 2016, 55, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.L.; Zhao, Y.; Wang, H.; Chen, H.J.; Ji, X.S. Growth promotion and dietary contribution assessment of three submerged macrophytes to Macrobrachium nipponense. Aquaculture 2019, 504, 70–80. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Wu, G. Nutrition and functions of amino acids in fish. Amino Acids Nutr. Health 2021, 1285, 133–168. [Google Scholar] [CrossRef]
- Sarma, D.; Akhtar, M.S.; Das, P.; Das, P.; Shahi, N.; Ciji, A.; Mahanta, P.C.; Yengkokpam, S.; Debnath, D. Nutritional quality in terms of amino acid and fatty acid of five coldwater fish species: Implications to human health. Natl. Acad. Sci. Lett. 2013, 36, 385–391. [Google Scholar] [CrossRef]
- Fuentes, A.; Fernández-Segovia, I.; Serra, J.A.; Barat, J.M. Comparison of wild and cultured sea bass (Dicentrarchus labrax) quality. Food Chem. 2010, 119, 1514–1518. [Google Scholar] [CrossRef]
- Grammes, F.; Reveco, F.E.; Romarheim, O.H.; Landsverk, T.; Mydland, L.T.; Øverland, M. Candida utilis and Chlorella vulgaris counteract intestinal inflammation in Atlantic salmon (Salmo salar L.). PLoS ONE 2013, 8, e83213. [Google Scholar] [CrossRef] [Green Version]
- Pasupuleti, R.R.; Tsai, P.C.; Ponnusamy, V.K.; Chen, N.C. Green sample pre-treatment technique coupled with UHPLC-MS/MS for the rapid biomonitoring of dietary poly-unsaturated (omega) fatty acids to predict health risks. Chemosphere 2022, 291, 132685. [Google Scholar] [CrossRef]
- Strobel, C.; Jahreis, G.; Kuhnt, K. Survey of n-3 and n-6 polyunsaturated fatty acids in fish and fish products. Lipids Health Dis. 2012, 11, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candiloro, F.; Borioli, V.; Borsellino, G.; Picozza, M.; Pellini, R.; Cereda, E.; Gargano, F.; Caraccia, M.; Nardi, M.T.; Bellu, L.; et al. Influence of different lipid emulsions on specific immune cell functions in head and neck cancer patients receiving supplemental parenteral nutrition: An exploratory analysis. Nutrition 2021, 86, 111178. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J. Intake or blood levels of n-3 polyunsaturated fatty acids and risk of colorectal cancer: A systematic review and meta-analysis of prospective studies. Cancer Epidemiol. Prev. Biomark. 2020, 29, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.; Ciappolino, V.; Agostoni, C. DHA effects in brain development and function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yu, Y.; Dong, L.; Gan, J.; Mao, T.; Liu, T.; Li, X.; He, L. Effects of moderate exercise on hepatic amino acid and fatty acid composition, liver transcriptome, and intestinal microbiota in channel catfish (Ictalurus punctatus). Comp. Biochem. Physiol. Part D 2021, 40, 100921. [Google Scholar] [CrossRef]
- Soler, P.; Faria, M.; Barata, C.; García-Galea, E.; Lorente, B.; Vinyoles, D. Improving water quality does not guarantee fish health: Effects of ammonia pollution on the behaviour of wild-caught pre-exposed fish. PLoS ONE 2021, 16, e0243404. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Qian, Y.L.; Alcazar Magana, A.; Xiong, S.; Qian, M.C. Comparative characterization of aroma compounds in silver carp (Hypophthalmichthys molitrix), pacific whiting (Merluccius productus), and alaska pollock (Theragra chalcogramma) surimi by aroma extract dilution analysis, odor activity value, and aroma recombination studies. J. Agric. Food Chem. 2020, 68, 10403–10413. [Google Scholar] [CrossRef]
- Varlet, V.; Prost, C.; Serot, T. Volatile aldehydes in smoked fish: Analysis methods, occurence and mechanisms of formation. Food Chem. 2007, 105, 1536–1556. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Y.; Cao, W.; Zhou, L.; Zhang, C.; Qin, X.; Zheng, H.; Lin, H.; Gao, J. Novel insight into the role of processing stages in nutritional components changes and characteristic flavors formation of noble scallop Chlamys nobilis adductors. Food Chem. 2022, 378, 132049. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, J.; Zhang, W.; Wæhrens, S.S.; Tøstesen, M.; Hansen, E.T.; Bredie, W.L.; Lametsch, R. Exopeptidase treatment combined with Maillard reaction modification of protein hydrolysates derived from porcine muscle and plasma: Structure–taste relationship. Food Chem. 2020, 306, 125613. [Google Scholar] [CrossRef]
- Zhou, M.Z.; Xiong, G.Q.; Qiao, Y.; Wang, C.; Wang, L.; Wu, W.J.; Li, X.; Shi, L.; Ding, A.Z.; Li, C. Analysis on flavor components of different edible parts of Procambarus clarkii. Meat Res. 2020, 34, 52–58. [Google Scholar] [CrossRef]
- Salum, P.; Guclu, G.; Selli, S. Comparative evaluation of key aromaactive compounds in raw and cooked red mullet (Mullus barbatus) by aroma extract dilution analysis. J. Agric. Food Chem. 2017, 65, 8402–8408. [Google Scholar] [CrossRef]
- Jiang, J.J.; Xie, P.H.; Ren, F.; Lai, Z.J.; Ou, A.F. Flavor analysis of Xinhui citrus Pu’er tea based on gas-phase ion migration spectrum and headspace solid phase microextraction. Sci. Technol. Food Ind. 2020, 41, 214–220. [Google Scholar] [CrossRef]
- Hsieh, R.J.; Kinsella, J.E. Lipoxygenase generation of specific volatile flavor carbonyl compounds in fish tissues. J. Agric. Food Chem. 1989, 37, 279–286. [Google Scholar] [CrossRef]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.S. Off-flavor problems in aquaculture. Rev. Fish. Sci. 2000, 8, 45–88. [Google Scholar] [CrossRef]
- Grigorakis, K. Compositional and organoleptic quality of farmed and wild gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) and factors affecting it: A review. Aquaculture 2007, 272, 55–75. [Google Scholar] [CrossRef]

| Item | Time/d | M | EM |
|---|---|---|---|
| BL/cm | 30 | 139.96 ± 8.19 | 142.69 ± 4.42 |
| 60 | 191.21 ± 9.00 | 192.38 ± 11.04 | |
| 90 | 207.13 ± 8.06 | 221.15 ± 7.67 * | |
| BT/cm | 30 | 21.91 ± 2.01 | 22.56 ± 1.80 |
| 60 | 31.22 ± 1.20 * | 30.43 ± 1.26 | |
| 90 | 34.51 ± 2.89 | 36.26 ± 2.00 * | |
| BW/g | 30 | 61.54 ± 8.38 | 63.32 ± 7.56 |
| 60 | 163.72 ± 16.71 | 174.07 ± 18.37 * | |
| 90 | 214.99 ± 25.37 | 252.86 ± 22.21 * | |
| WGR/% | 30 | 330.33 ± 58.60 | 342.77 ± 52.85 |
| 60 | 168.05 ± 20.72 | 175.42 ± 9.57 | |
| 90 | 31.23 ± 6.85 | 45.64 ± 5.36 * | |
| SGR/(%/d) | 30 | 4.83 ± 0.49 | 4.89 ± 0.40 |
| 60 | 3.28 ± 0.24 | 3.38 ± 0.12 * | |
| 90 | 0.90 ± 0.18 | 1.25 ± 0.56 * | |
| HSI/% | 30 | 2.82 ± 0.40 * | 2.49 ± 0.42 |
| 60 | 2.23 ± 0.28 * | 1.63 ± 0.38 | |
| 90 | 2.09 ± 0.20 * | 1.59 ± 0.15 |
| Item | Dorsal Skin | Abdominal Skin | ||
|---|---|---|---|---|
| M | EM | M | EM | |
| L* | 49.92 ± 5.81 ** | 37.78 ± 2.77 | 68.67 ± 6.11 | 64.08 ± 3.89 |
| a* | 1.07 ± 0.44 *** | −1.52 ± 0.73 | 2.81 ± 0.91 ** | 0.93 ± 0.81 |
| b* | 14.50 ± 1.13 *** | 5.97 ± 1.14 | 6.25 ± 0.71 *** | 2.15 ± 0.47 |
| Items | M | EM | p |
|---|---|---|---|
| Moisture | 72.63 ± 1.04 | 69.03 ± 0.40 | 0.005 ** |
| Ash | 1.43 ± 0.06 | 1.87 ± 0.35 | 0.103 |
| Crude lipid | 3.43 ± 0.71 | 3.87 ± 0.45 | 0.422 |
| Crude protein | 21.13 ± 0.67 | 23.07 ± 0.35 | 0.011 * |
| FAA (mg/g) | HAA (g/100 g) | |||
|---|---|---|---|---|
| M | EM | M | EM | |
| Aspartic acid (Asp) | 0.17 ± 0.00 | 0.16 ± 0.00 | 4.58 ± 0.24 | 5.29 ± 0.29 * |
| Glutamic acid (Glu) | 0.45 ± 0.01 | 0.46 ± 0.00 | 6.97 ± 0.41 | 8.08 ± 0.48 * |
| Serine (Ser) | 0.03 ± 0.00 | 0.03 ± 0.00 | 1.58 ± 0.09 | 1.86 ± 0.10 * |
| Histidine (His) | 3.02 ± 0.16 | 2.96 ± 0.08 | 1.19 ± 0.05 | 1.49 ± 0.11 * |
| Glycine (Gly) | 1.69 ± 0.17 | 2.21 ± 0.07 ** | 2.26 ± 0.05 | 2.60 ± 0.21 |
| Threonine (Thr) | 0.61 ± 0.02 | 0.74 ± 0.02 ** | 1.80 ± 0.08 | 2.09 ± 0.14 * |
| Arginine (Arg) | 0.04 ± 0.00 | 0.03 ± 0.01 | 2.37 ± 0.10 | 2.73 ± 0.20 * |
| Alanine (Ala) | 1.00 ± 0.05 | 1.04 ± 0.03 | 2.57 ± 0.12 | 2.97 ± 0.18 * |
| Tyrosine (Tyr) | 0.13 ± 0.01 | 0.15 ± 0.00 * | 1.15 ± 0.22 | 1.35 ± 0.08 |
| Cysteine (Cys-s) | 0.04 ± 0.01 | 0.03 ± 0.00 | 0.07 ± 0.05 | 0.09 ± 0.01 |
| Valine (Val) | 0.27 ± 0.01 | 0.27 ± 0.01 | 2.46 ± 0.16 | 2.88 ± 0.19 * |
| Methionine (Met) | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.94 ± 0.31 | 1.13 ± 0.05 |
| Phenylalanine (Phe) | 0.08 ± 0.00 | 0.09 ± 0.00 ** | 1.82 ± 0.11 | 2.10 ± 0.12 * |
| Isoleucine (IIe) | 0.09 ± 0.00 | 0.10 ± 0.00 *** | 2.12 ± 0.12 | 2.48 ± 0.15 * |
| Leucine (Leu) | 0.12 ± 0.09 | 0.18 ± 0.00 | 3.41 ± 0.21 | 3.97 ± 0.25 * |
| Lysine (Lys) | 0.52 ± 0.01 | 0.54 ± 0.01 | 4.00 ± 0.29 | 4.69 ± 0.33 |
| Proline (Pro) | 0.40 ± 0.05 ** | 0.23 ± 0.03 | 1.60 ± 0.18 | 1.33 ± 0.36 |
| EAA | 1.81 ± 0.09 | 2.05 ± 0.04 * | 16.54 ± 1.19 | 19.33 ± 1.23 |
| NEAA | 6.95 ± 0.11 | 7.30 ± 0.18 * | 24.33 ± 1.42 | 27.79 ± 1.69 |
| TAA | 8.76 ± 0.02 | 9.35 ± 0.20 * | 40.87 ± 2.61 | 47.12 ± 2.89 * |
| Fatty Acid | M | EM |
|---|---|---|
| C12:0 | 0.06 ± 0.01 | 0.06 ± 0.01 |
| C14:0 | 3.30 ± 0.08 | 3.59 ± 0.27 |
| C15:0 | 0.36 ± 0.02 | 0.37 ± 0.01 |
| C16:0 | 22.82 ± 0.20 | 22.11 ± 0.47 |
| C17:0 | 0.31 ± 0.01 | 0.25 ± 0.07 |
| C18:0 | 3.78 ± 0.15 | 3.29 ± 0.37 |
| C20:0 | 0.17 ± 0.01 | 0.15 ± 0.01 |
| C22:0 | 0.17 ± 0.08 | 0.10 ± 0.01 |
| ΣSFA | 30.98 ± 0.20 ** | 29.92 ± 0.30 |
| C14:1 | 0.07 ± 0.04 | 0.06 ± 0.02 |
| C16:1 | 5.71 ± 0.19 | 5.97 ± 0.29 |
| C17:1 | 0.39 ± 0.03 | 0.35 ± 0.01 |
| C18:1 | 25.24 ± 0.10 ** | 23.33 ± 0.59 |
| C20:1 | 0.74 ± 0.08 | 0.66 ± 0.04 |
| ΣMUFA | 32.16 ± 0.25 ** | 30.37 ± 0.35 |
| C18:2 | 23.63 ± 0.25 | 25.64 ± 0.50 ** |
| C18:3n6 | 0.14 ± 0.02 | 0.13 ± 0.02 |
| C18:3n3 | 2.53 ± 0.02 | 2.64 ± 0.06 * |
| C20:2 | 0.48 ± 0.01 | 0.48 ± 0.00 |
| C20:3n6 | 0.15 ± 0.01 * | 0.13 ± 0.01 |
| C20:3n3 | 0.17 ± 0.00 | 0.19 ± 0.01 * |
| C20:4 | 0.53 ± 0.03 | 0.49 ± 0.04 |
| C20:5 | 1.48 ± 0.15 | 1.51 ± 0.05 |
| C22:6 | 7.76 ± 0.19 | 8.33 ± 0.07 ** |
| ΣPUFA | 36.87 ± 0.23 | 39.54 ± 0.47 ** |
| ΣPUFA/∑SFA | 1.19 ± 0.01 | 1.32 ± 0.02 *** |
| Count | Compound | CAS# | Formula | MW | RI | Rt [sec] | Dt [a.u.] | Peak Intensity | |
|---|---|---|---|---|---|---|---|---|---|
| M | EM | ||||||||
| 1 | Nonanal-M | C124196 | C9H18O | 142.2 | 1112.8 | 513.223 | 1.47528 | 1594.20 ± 569.27 | 2220.24 ± 352.87 * |
| 2 | Nonanal-D | C124196 | C9H18O | 142.2 | 1113.2 | 513.73 | 1.95085 | 222.52 ± 132.59 | 444.14 ± 156.23 ** |
| 3 | (E)-2-octenal | C2548870 | C8H14O | 126.2 | 1058.2 | 434.653 | 1.33775 | 230.10 ± 172.74 | 251.44 ± 100.42 |
| 4 | 2-methylpentanoic acid | C97610 | C6H12O2 | 116.2 | 1028.9 | 392.58 | 1.26318 | 138.11 ± 95.69 | 161.91 ± 26.07 |
| 5 | (E, E)-2,4-heptadienal | C4313035 | C7H10O | 110.2 | 1014.3 | 371.527 | 1.19175 | 195.11 ± 105.12 | 225.32 ± 80.96 |
| 6 | Octanal-M | C124130 | C8H16O | 128.2 | 1007.1 | 361.154 | 1.40395 | 1404.23 ± 435.87 | 1646.58 ± 185.32 |
| 7 | Octanal-D | C124130 | C8H16O | 128.2 | 1007.3 | 361.434 | 1.82706 | 542.77 ± 398.71 | 958.61 ± 320.32 * |
| 8 | 1 | unidentified | − | 0 | 990.4 | 340.757 | 1.39455 | 1201.18 ± 324.12 | 1280.64 ± 185.88 |
| 9 | oct-1-en-3-ol-M | C3391864 | C8H16O | 128.2 | 984 | 335.282 | 1.16248 | 796.05 ± 170.35 | 1026.74 ± 115.11 ** |
| 10 | 3-Furanmethanol | C4412913 | C5H6O2 | 98.1 | 977 | 329.248 | 1.10918 | 332.53 ± 60.43 | 403.17 ± 56.14 * |
| 11 | Benzaldehyde-M | C100527 | C7H6O | 106.1 | 961.3 | 315.903 | 1.15223 | 466.75 ± 161.68 | 674.45 ± 169.11 * |
| 12 | Benzaldehyde-D | C100527 | C7H6O | 106.1 | 960.2 | 314.96 | 1.4733 | 116.41 ± 81.63 | 322.43 ± 328.21 |
| 13 | (E)-hept-2-enal-M | C18829555 | C7H12O | 112.2 | 956.1 | 311.424 | 1.25978 | 684.92 ± 388.43 | 753.34 ± 177.46 |
| 14 | (E)-hept-2-enal-D | C18829555 | C7H12O | 112.2 | 957.7 | 312.838 | 1.67575 | 204.13 ± 284.33 | 344.39 ± 361.19 |
| 15 | Heptanal-M | C111717 | C7H14O | 114.2 | 902.7 | 265.925 | 1.33254 | 1319.31 ± 149.13 | 1298.56 ± 92.38 |
| 16 | Heptanal-D | C111717 | C7H14O | 114.2 | 902.2 | 265.453 | 1.70106 | 1117.23 ± 546.80 | 1592.96 ± 307.47 * |
| 17 | (Z)-4-heptenal | C6728310 | C7H12O | 112.2 | 898.9 | 262.624 | 1.15065 | 706.22 ± 133.75 | 693.12 ± 113.33 |
| 18 | 2-heptanone-D | C110430 | C7H14O | 114.2 | 893.9 | 258.375 | 1.63475 | 127.34 ± 87.70 | 223.94 ± 109.79 |
| 19 | n-Hexanol-M | C111273 | C6H14O | 102.2 | 872.7 | 246.48 | 1.32617 | 564.98 ± 71.06 | 544.49 ± 104.32 |
| 20 | (E)-2-hexenal-M | C6728263 | C6H10O | 98.1 | 851.9 | 235.365 | 1.18317 | 1160.40 ± 329.61 | 1239.75 ± 158.51 |
| 21 | (E)-2-hexenal-D | C6728263 | C6H10O | 98.1 | 847.6 | 233.025 | 1.5206 | 1069.01 ± 1015.98 | 1662.32 ± 977.41 |
| 22 | Hexanal | C66251 | C6H12O | 100.2 | 790.8 | 202.605 | 1.55949 | 4991.75 ± 600.01 | 4903.75 ± 318.76 |
| 23 | pentan-1-ol-D | C71410 | C5H12O | 88.1 | 770 | 193.277 | 1.51613 | 434.40 ± 117.86 | 531.57 ± 69.93 * |
| 24 | 2-ethyl-1-hexanol-D | C104767 | C8H18O | 130.2 | 1032.7 | 398.037 | 1.80573 | 124.18 ± 43.72 | 846.93 ± 639.43 ** |
| 25 | 2-pentyl furan | C3777693 | C9H14O | 138.2 | 997.1 | 346.84 | 1.25753 | 164.73 ± 67.08 | 232.93 ± 82.24 |
| 26 | 2-ethyl-1-hexanol-M | C104767 | C8H18O | 130.2 | 1031.2 | 395.891 | 1.41016 | 615.64 ± 225.45 | 2346.12 ± 1172.02 ** |
| 27 | β-Ocimene | C13877913 | C10H16 | 136.2 | 1046.2 | 417.35 | 1.2186 | 32.92 ± 6.86 | 51.02 ± 17.46 * |
| 28 | pentan-1-ol-M | C71410 | C5H12O | 88.1 | 766.3 | 191.798 | 1.25475 | 653.56 ± 54.47 | 607.04 ± 67.51 |
| 29 | oct-1-en-3-ol-D | C3391864 | C8H16O | 128.2 | 983.8 | 335.066 | 1.60462 | 114.15 ± 29.48 | 185.28 ± 50.23 ** |
| 30 | n-Hexanol-D | C111273 | C6H14O | 102.2 | 870.6 | 245.347 | 1.6415 | 102.66 ± 26.47 | 112.21 ± 31.71 |
| 31 | 2-heptanone-M | C110430 | C7H14O | 114.2 | 893.8 | 258.277 | 1.26096 | 376.60 ± 68.28 | 426.02 ± 44.28 |
| 32 | 2-Octanol | C123966 | C8H18O | 130.2 | 993 | 342.983 | 1.80374 | 66.70 ± 20.87 | 109.93 ± 34.54 ** |
| 33 | 4,5-dihydro-3(2H)-thiophenone | C1003049 | C4H6OS | 102.2 | 940.8 | 298.387 | 1.18131 | 102.88 ± 47.04 | 186.07 ± 129.76 |
| 34 | 5-Methyl-2(3H)-furanone | C591128 | C5H6O2 | 98.1 | 878.9 | 249.833 | 1.11789 | 118.59 ± 40.71 | 171.50 ± 77.70 |
| 35 | (E)-2-pentenal-M | C1576870 | C5H8O | 84.1 | 751.9 | 185.969 | 1.10931 | 432.20 ± 106.45 | 454.48 ± 47.37 |
| 36 | (E)-2-pentenal-D | C1576870 | C5H8O | 84.1 | 753.4 | 186.582 | 1.36359 | 742.97 ± 779.75 | 1300.98 ± 769.26 |
| 37 | Pentanal-M | C110623 | C5H10O | 86.1 | 698.1 | 164.15 | 1.18501 | 371.17 ± 17.61 *** | 318.41 ± 31.78 |
| 38 | Pentanal-D | C110623 | C5H10O | 86.1 | 699.6 | 164.751 | 1.42598 | 484.46 ± 115.69 | 543.81 ± 64.33 |
| 39 | 2-Pentanone | C107879 | C5H10O | 86.1 | 687.3 | 159.943 | 1.37898 | 630.06 ± 58.16 | 955.98 ± 114.13 *** |
| 40 | 2-methylbutanal-M | C96173 | C5H10O | 86.1 | 664.3 | 153.733 | 1.16001 | 130.22 ± 19.39 | 119.90 ± 13.07 |
| 41 | 2-methylbutanal-D | C96173 | C5H10O | 86.1 | 659.3 | 152.403 | 1.39552 | 84.85 ± 23.11 | 128.68 ± 36.05 ** |
| 42 | 3-methylbutanal-M | C590863 | C5H10O | 86.1 | 651.6 | 150.312 | 1.17203 | 262.76 ± 35.38 | 255.62 ± 28.59 |
| 43 | 3-methylbutanal-D | C590863 | C5H10O | 86.1 | 656.2 | 151.553 | 1.40848 | 162.28 ± 33.59 | 212.25 ± 31.97 ** |
| 44 | Ethyl Acetate-M | C141786 | C4H8O2 | 88.1 | 613.8 | 140.105 | 1.10042 | 232.82 ± 56.67 *** | 96.49 ± 12.85 |
| 45 | Ethyl Acetate-D | C141786 | C4H8O2 | 88.1 | 610.6 | 139.259 | 1.3406 | 242.03 ± 57.78 | 258.51 ± 72.43 |
| 46 | 2-Butanone | C78933 | C4H8O | 72.1 | 585.5 | 132.491 | 1.24941 | 2444.76 ± 456.05 | 3285.25 ± 393.55 *** |
| 47 | 1-Propanethiol-D | C107039 | C3H8S | 76.2 | 626.5 | 143.526 | 1.36157 | 106.78 ± 70.85 | 500.18 ± 146.62 *** |
| 48 | 1-Propanethiol-M | C107039 | C3H8S | 76.2 | 620.8 | 142.008 | 1.17085 | 358.20 ± 125.51 | 550.51 ± 67.43 ** |
| 49 | 3-hydroxybutan-2-one-D | C513860 | C4H8O2 | 88.1 | 719.1 | 172.675 | 1.33338 | 115.23 ± 30.90 | 212.55 ± 53.40 *** |
| 50 | 3-hydroxybutan-2-one-M | C513860 | C4H8O2 | 88.1 | 720.3 | 173.16 | 1.06435 | 296.69 ± 50.68 | 405.20 ± 71.00 ** |
| 51 | (E)-3-penten-2-one-M | C3102338 | C5H8O | 84.1 | 728.7 | 176.54 | 1.09091 | 119.00 ± 24.20 | 158.03 ± 14.62 *** |
| 52 | 1,1-diethoxyethane | C105577 | C6H14O2 | 118.2 | 726.7 | 175.748 | 1.13189 | 68.33 ± 31.71 | 45.99 ± 9.48 |
| 53 | (E)-3-penten-2-one-D | C3102338 | C5H8O | 84.1 | 725.1 | 175.11 | 1.34845 | 50.96 ± 19.65 | 99.06 ± 26.14 *** |
| 54 | butanoic acid | C107926 | C4H8O2 | 88.1 | 816 | 216.095 | 1.16109 | 42.25 ± 6.81 | 43.85 ± 4.03 |
| 55 | 2,3-butanedione | C431038 | C4H6O2 | 86.1 | 580.7 | 131.198 | 1.17158 | 103.28 ± 44.52 | 118.02 ± 18.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Nie, Z.; Zheng, Y.; Tang, X.; Sun, Y.; Zhu, H.; Gao, J.; Xu, P.; Xu, G. Effects of Submerged Macrophytes on the Growth, Morphology, Nutritional Value, and Flavor of Cultured Largemouth Bass (Micropterus salmoides). Molecules 2022, 27, 4927. https://doi.org/10.3390/molecules27154927
Zheng Z, Nie Z, Zheng Y, Tang X, Sun Y, Zhu H, Gao J, Xu P, Xu G. Effects of Submerged Macrophytes on the Growth, Morphology, Nutritional Value, and Flavor of Cultured Largemouth Bass (Micropterus salmoides). Molecules. 2022; 27(15):4927. https://doi.org/10.3390/molecules27154927
Chicago/Turabian StyleZheng, Zhaowei, Zhijuan Nie, Yao Zheng, Xue Tang, Yi Sun, Haojun Zhu, Jiancao Gao, Pao Xu, and Gangchun Xu. 2022. "Effects of Submerged Macrophytes on the Growth, Morphology, Nutritional Value, and Flavor of Cultured Largemouth Bass (Micropterus salmoides)" Molecules 27, no. 15: 4927. https://doi.org/10.3390/molecules27154927
APA StyleZheng, Z., Nie, Z., Zheng, Y., Tang, X., Sun, Y., Zhu, H., Gao, J., Xu, P., & Xu, G. (2022). Effects of Submerged Macrophytes on the Growth, Morphology, Nutritional Value, and Flavor of Cultured Largemouth Bass (Micropterus salmoides). Molecules, 27(15), 4927. https://doi.org/10.3390/molecules27154927








