Novel 1,3-Thiazole Analogues with Potent Activity against Breast Cancer: A Design, Synthesis, In Vitro, and In Silico Study
Abstract
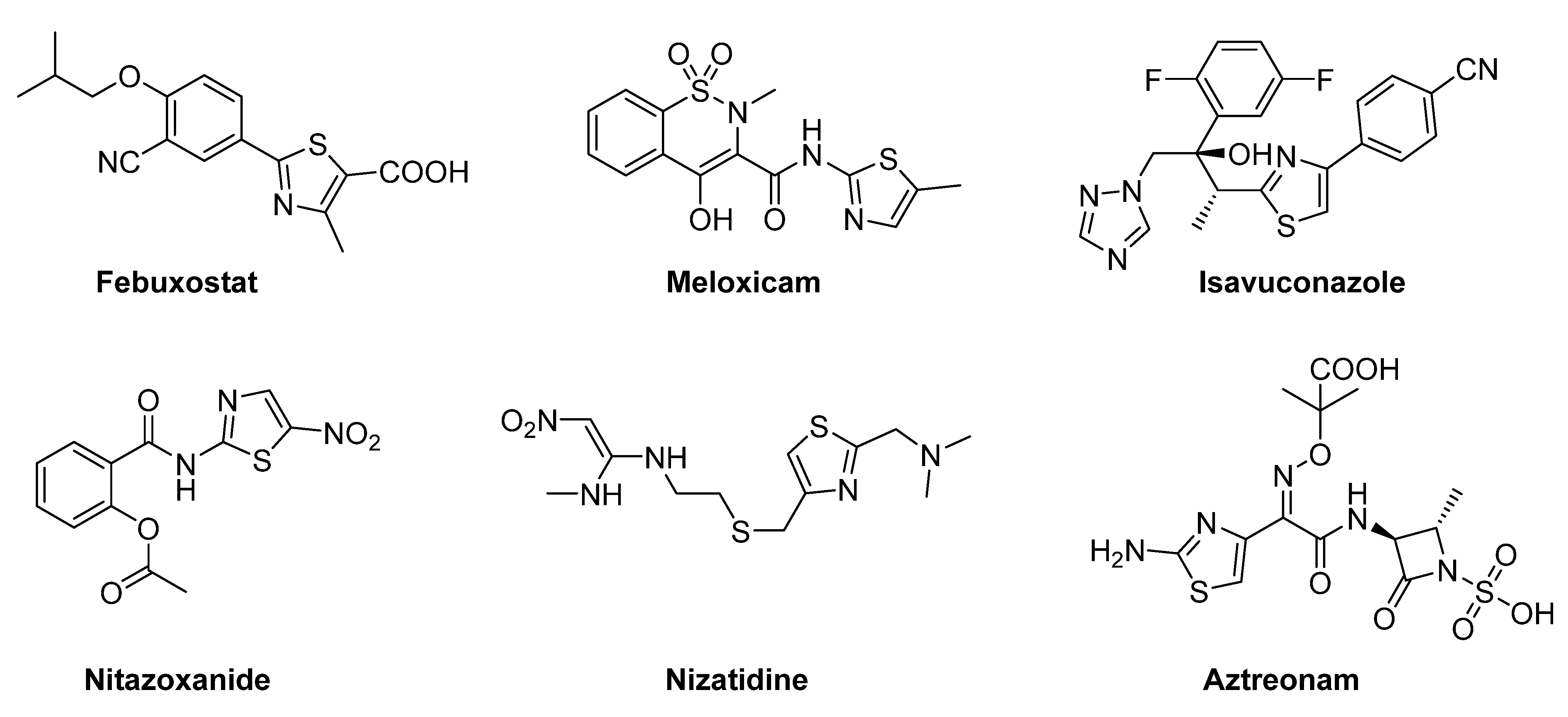
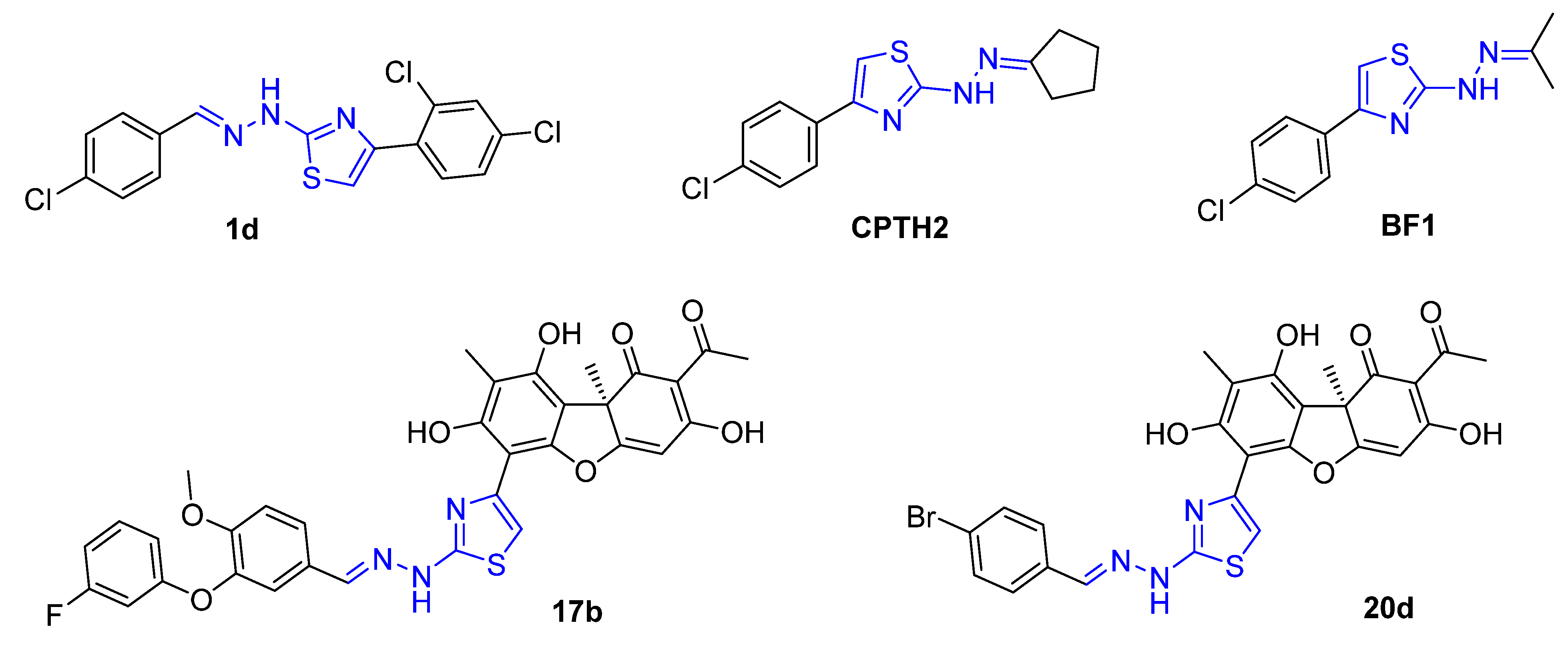
:1. Introduction
2. Results and Discussion
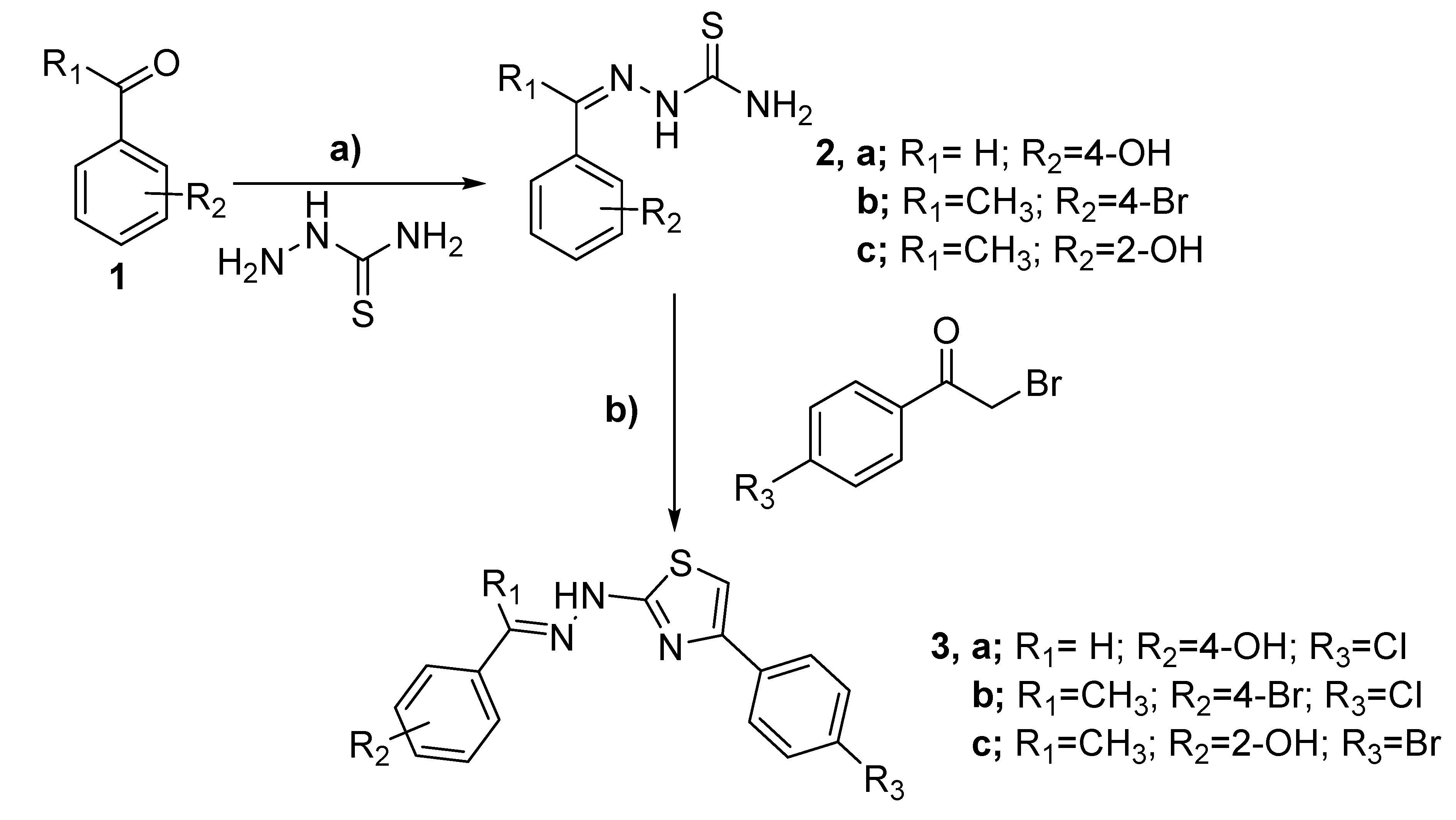
2.1. Chemistry
2.1.1. Investigation of the NMR Spectra of Synthesized 1,3-Thiazole Derivatives
4-[(2-(4-(4-Chlorophenyl) thiazol-4-yl) hydrazineylidene) methyl]phenol (3a)
2-(2-(1-(4-Bromophenyl)ethylidene)hydrazineyl)-4-(4-chlorophenyl)thiazole (3b)
2-[1-(2-(4-(4-Bromophenyl)thiazol-2-yl)hydrazineylidene)ethyl]-phenol (3c)
2-[5-(4-Chlorophenyl)-1,3-thiazol-2-yl]-1-acetylhydrazine (4)
N-Substituted-1,3-thiazole Derivatives (6a and 6b)
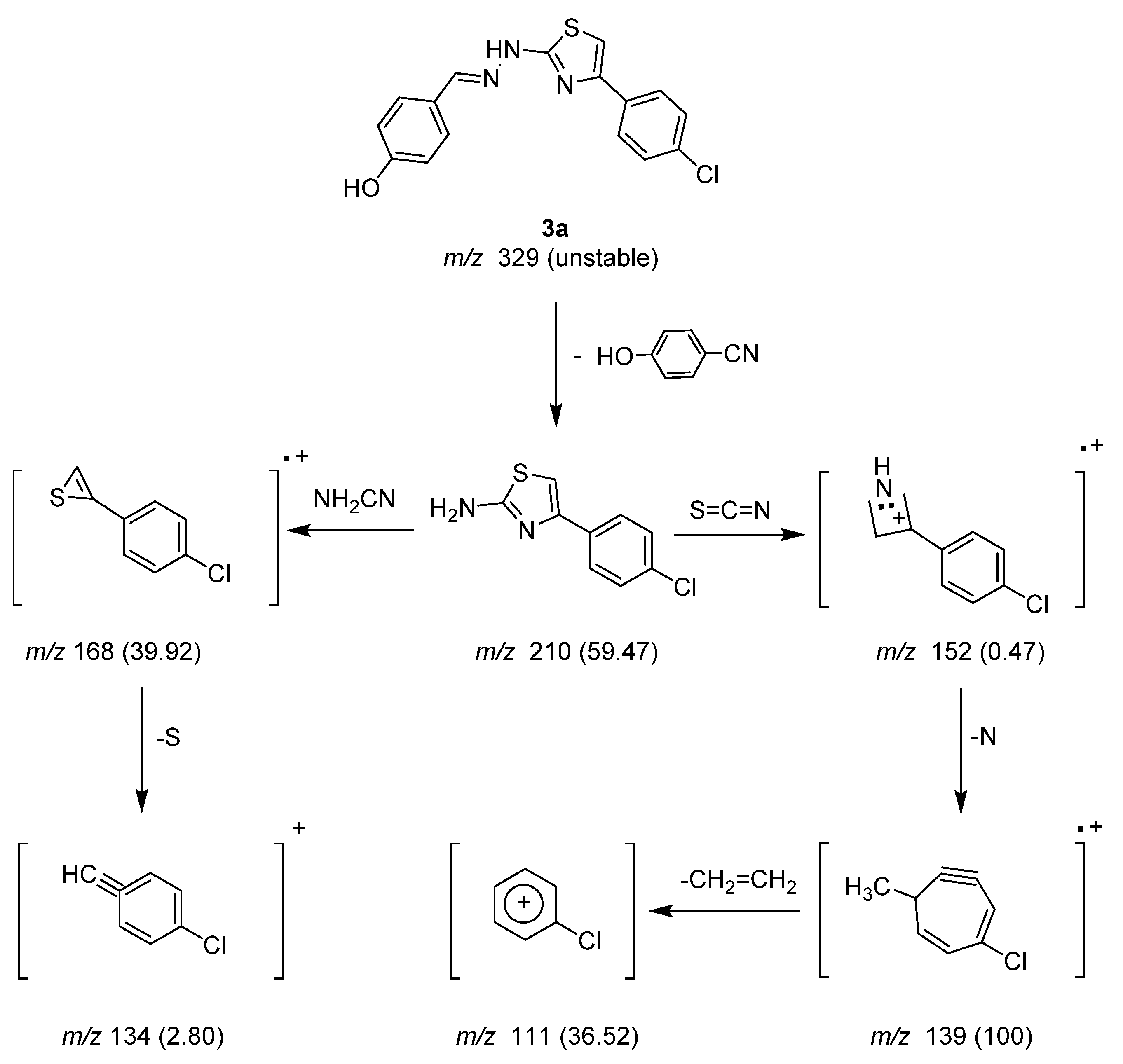
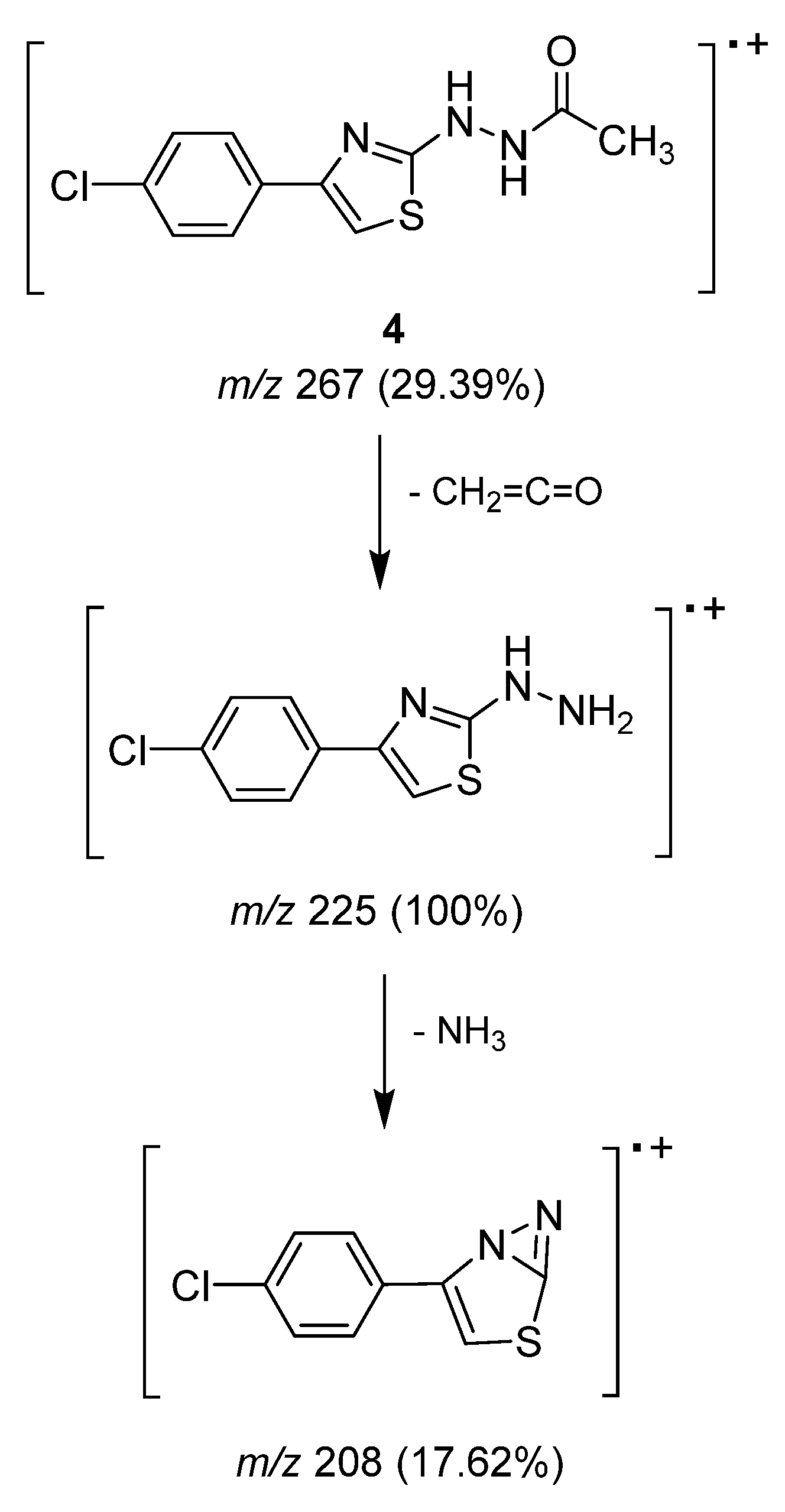
2.1.2. Mass Spectrometry Studies
2.2. Assessment of Anti-Proliferative Activity against Breast Cancer
2.3. Assessment of VEGFR-2 Kinase Activity
2.4. Cell Cycle Analysis
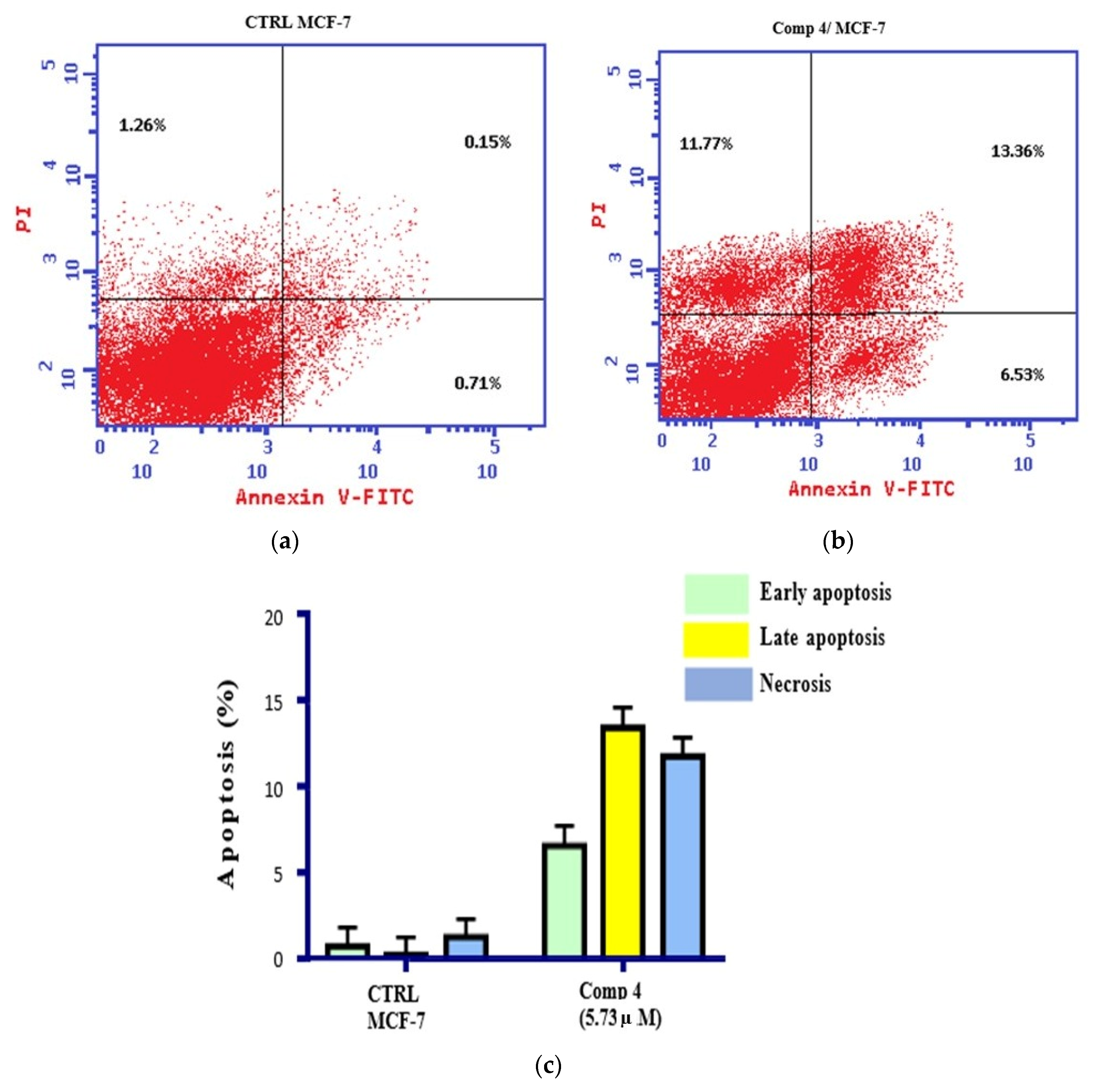
2.5. Apoptosis and Necrosis Analysis
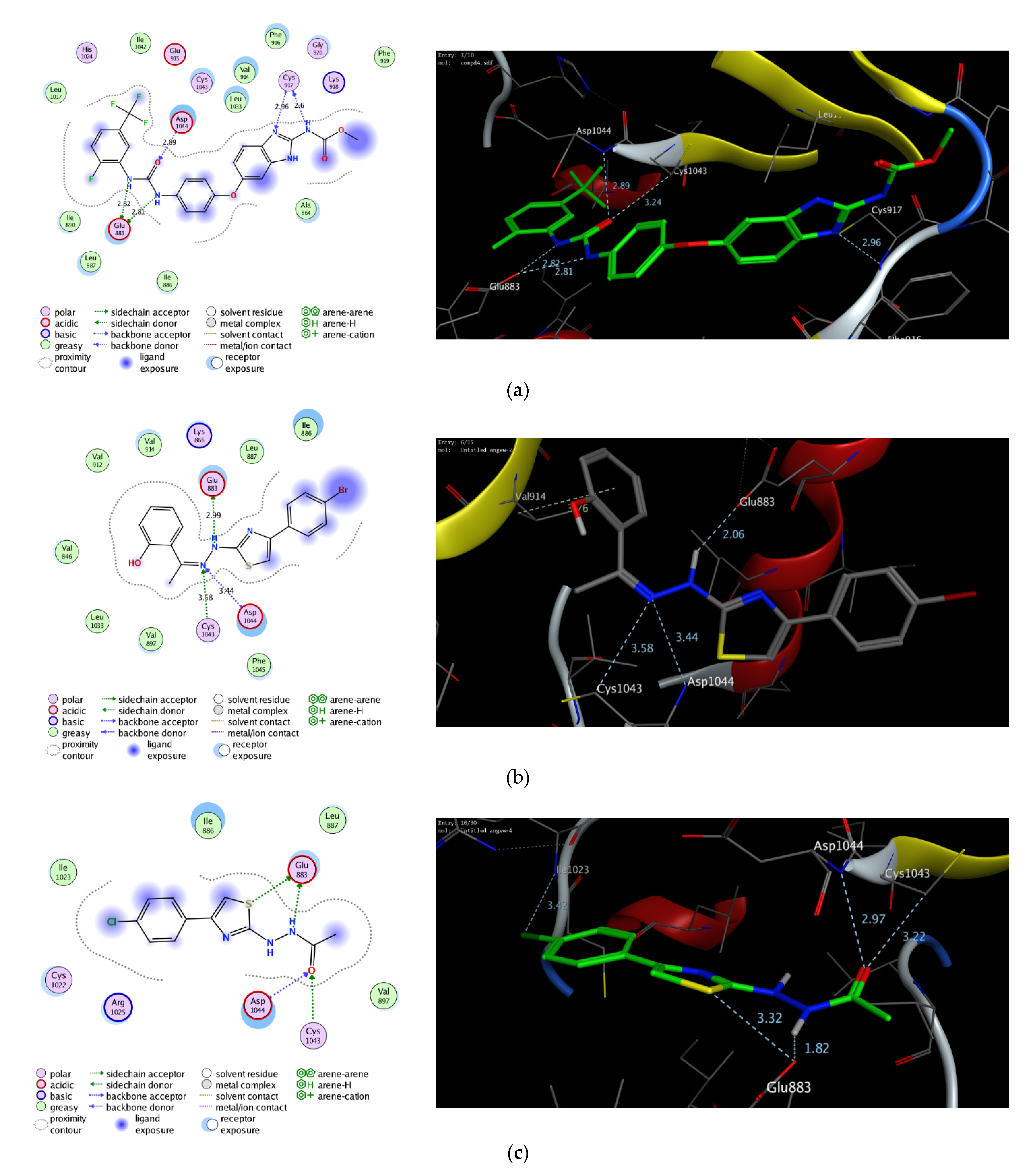
2.6. In Silico Molecular Docking Study
3. Materials and Methods
3.1. General Description of Materials and Instrumentation
3.2. Synthetic Procedures and Analytic Data of Compounds
3.2.1. General Procedure for the Synthesis of 4-substituted-2-Hydrazinyl-1,3-Thiazole Derivatives (3a–c)
(E)-4-((2-(4-(4-Chlorophenyl)thiazol-2-yl)hydrazineylidene)methyl)-phenol (3a)
(E)-2-(2-(1-(4-bromophenyl)ethylidene)hydrazineyl)-4-(4-chlorophenyl)thiazole (3b)
(E)-2-(1-(2-(4-(4-Bromophenyl)thiazol-2-yl)hydrazineylidene)ethyl)-phenol (3c)
3.2.2. Synthesis of N-(4-(4-Chlorophenyl)-Thiazol-2-yl)Acetamide (4)
3.2.3. General Procedure for the Preparation of 4-[(2-(1-(4- Bromophenyl)-Ethylidene]-1-[4-(4-Chlorophenyl)Thiazole-2-yl)Hydrazinyl](Hydroxy)Methyl]-Phenols (5a,b)
(E)-4-((2-(1-(4-Bromophenyl)ethylidene)-1-(4-(4-chlorophenyl)thiazol-2-yl)hydrazineyl)(hydroxy)methyl)phenol (5a)
(E)-4-((2-(1-(4-Bromophenyl)ethylidene)-1-(4-(4-chlorophenyl)thiazol-2-yl)hydrazineyl)(hydroxy)methyl)-2-methoxyphenol (5b)
3.2.4. Synthesis of Compounds 6a,b
(E)-4-(Acetoxy(2-(1-(4-bromophenyl)ethylidene)-1-(4-(4-chlorophenyl)thiazol-2-yl)hydrazineyl)methyl)phenyl acetate (6a)
(E)-4-(Acetoxy(2-(1-(4-bromophenyl)ethylidene)-1-(4-(4-chlorophenyl)thiazol-2-yl)hydrazineyl)methyl)-2-methoxyphenyl acetate (6b)
3.3. Assessment of Anti-Proliferative Activity against Breast Cancer
3.4. Assessment of VEGFR-2 Enzyme Activity
3.5. Cell Cycle Analysis
3.6. Annexin V-FITC/PI Dual Staining Analysis
3.7. In Silico Molecular Modeling Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast Cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primer 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.W.S.; Wu, M.; Cho, W.C.S.; To, K.K.W. Recent Advances in the Treatment of Breast Cancer. Front. Oncol. 2018, 8, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAndrew, N.P.; Finn, R.S. Clinical Review on the Management of Hormone Receptor–Positive Metastatic Breast Cancer. JCO Oncol. Pract. 2022, 18, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Narayan, P.; Osgood, C.L.; Wedam, S.; Prowell, T.M.; Gao, J.J.; Shah, M.; Krol, D.; Wahby, S.; Royce, M.; et al. U.S. FDA Drug Approvals for Breast Cancer: A Decade in Review. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Peng, C.; Xie, X.; Peng, F. New Advances in Targeted Therapy of HER2-Negative Breast Cancer. Front. Oncol. 2022, 12, 828438. [Google Scholar] [CrossRef]
- Cui, W.; Aouidate, A.; Wang, S.; Yu, Q.; Li, Y.; Yuan, S. Discovering Anti-Cancer Drugs via Computational Methods. Front. Pharmacol. 2020, 11, 733. [Google Scholar] [CrossRef]
- Quijia, C.R.; Chorilli, M. Piperine for Treating Breast Cancer: A Review of Molecular Mechanisms, Combination with Anticancer Drugs, and Nanosystems. Phytother. Res. PTR 2022, 36, 147–163. [Google Scholar] [CrossRef]
- Sabir, S.; Alhazza, M.I.; Ibrahim, A.A. A Review on Heterocyclic Moieties and Their Applications. Catal. Sustain. Energy 2016, 2, 99–115. [Google Scholar] [CrossRef]
- Ramsden, C.A. Chapter One—Heterocyclic Mesomeric Betaines: An Overview. In Advances in Heterocyclic Chemistry; Ramsden, C.A., Ed.; Heterocyclic Mesomeric Betaines and Mesoionic Compounds; Academic Press: New York, NY, USA, 2022; Volume 137, pp. 1–24. [Google Scholar]
- Kalaria, P.N.; Karad, S.C.; Raval, D.K. A Review on Diverse Heterocyclic Compounds as the Privileged Scaffolds in Antimalarial Drug Discovery. Eur. J. Med. Chem. 2018, 158, 917–936. [Google Scholar] [CrossRef] [PubMed]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef]
- Taylor, A.P.; Robinson, R.P.; Fobian, Y.M.; Blakemore, D.C.; Jones, L.H.; Fadeyi, O. Modern Advances in Heterocyclic Chemistry in Drug Discovery. Org. Biomol. Chem. 2016, 14, 6611–6637. [Google Scholar] [CrossRef]
- Petrou, A.; Fesatidou, M.; Geronikaki, A. Thiazole Ring-A Biologically Active Scaffold. Mol. Basel Switz. 2021, 26, 3166. [Google Scholar] [CrossRef]
- Scott, K.A.; Njardarson, J.T. Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Top. Curr. Chem. 2018, 376, 5. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Bansal, K.K.; Sharma, A.; Sharma, D.; Deep, A. Thiazole-Containing Compounds as Therapeutic Targets for Cancer Therapy. Eur. J. Med. Chem. 2020, 188, 112016. [Google Scholar] [CrossRef] [PubMed]
- Pola, S. Significance of Thiazole-Based Heterocycles for Bioactive Systems; IntechOpen: Rijeka, Croatia, 2016; ISBN 978-953-51-2504-4. [Google Scholar]
- Ali, S.H.; Sayed, A.R. Review of the Synthesis and Biological Activity of Thiazoles. Synth. Commun. 2021, 51, 670–700. [Google Scholar] [CrossRef]
- Makam, P.; Thakur, P.K.; Kannan, T. In Vitro and in Silico Antimalarial Activity of 2-(2-Hydrazinyl)Thiazole Derivatives. Eur. J. Pharm. Sci. 2014, 52, 138–145. [Google Scholar] [CrossRef]
- Jadav, S.S.; Kaptein, S.; Timiri, A.; De Burghgraeve, T.; Badavath, V.N.; Ganesan, R.; Sinha, B.N.; Neyts, J.; Leyssen, P.; Jayaprakash, V. Design, Synthesis, Optimization and Antiviral Activity of a Class of Hybrid Dengue Virus E Protein Inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 1747–1752. [Google Scholar] [CrossRef]
- Alsharekh, M.M.; Althagafi, I.I.; Shaaban, M.R.; Farghaly, T.A. Microwave-Assisted and Thermal Synthesis of Nanosized Thiazolyl-Phenothiazine Derivatives and Their Biological Activities. Res. Chem. Intermed. 2019, 45, 127–154. [Google Scholar] [CrossRef]
- Yurttaş, L.; Çavuşoğlu, B.K.; Cantürk, Z. Novel 2-(2-Hydrazinyl)Thiazole Derivatives as Chemotherapeutic Agents. Synth. Commun. 2020, 50, 3072–3079. [Google Scholar] [CrossRef]
- de Santana, T.I.; Barbosa, M.D.O.; Gomes, P.A.T.D.M.; da Cruz, A.C.N.; da Silva, T.G.; Leite, A.C.L. Synthesis, Anticancer Activity and Mechanism of Action of New Thiazole Derivatives. Eur. J. Med. Chem. 2018, 144, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Filimonov, A.S.; Chepanova, A.A.; Luzina, O.A.; Zakharenko, A.L.; Zakharova, O.D.; Ilina, E.S.; Dyrkheeva, N.S.; Kuprushkin, M.S.; Kolotaev, A.V.; Khachatryan, D.S.; et al. New Hydrazinothiazole Derivatives of Usnic Acid as Potent Tdp1 Inhibitors. Molecules 2019, 24, 3711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakharenko, A.L.; Luzina, O.A.; Sokolov, D.N.; Kaledin, V.I.; Nikolin, V.P.; Popova, N.A.; Patel, J.; Zakharova, O.D.; Chepanova, A.A.; Zafar, A.; et al. Novel Tyrosyl-DNA Phosphodiesterase 1 Inhibitors Enhance the Therapeutic Impact of Topotecan on in Vivo Tumor Models. Eur. J. Med. Chem. 2019, 161, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, F.; Bizzarri, B.; Maccioni, E.; Secci, D.; Bolasco, A.; Chimenti, P.; Fioravanti, R.; Granese, A.; Carradori, S.; Tosi, F.; et al. A Novel Histone Acetyltransferase Inhibitor Modulating Gcn5 Network: Cyclopentylidene-[4-(4′-Chlorophenyl)Thiazol-2-Yl)Hydrazone. J. Med. Chem. 2009, 52, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Secci, D.; Carradori, S.; Bizzarri, B.; Bolasco, A.; Ballario, P.; Patramani, Z.; Fragapane, P.; Vernarecci, S.; Canzonetta, C.; Filetici, P. Synthesis of a Novel Series of Thiazole-Based Histone Acetyltransferase Inhibitors. Bioorg. Med. Chem. 2014, 22, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-D.; Liu, Y.; Zhang, Z.-Y.; Liu, G.-Y.; Xu, J.-H.; Liu, L.-Y.; Hu, Y.-M. Expression and Prognostic Significance of VEGFR-2 in Breast Cancer. Pathol.-Res. Pract. 2015, 211, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sullivan, C.A.W.; Zerkowski, M.P.; Molinaro, A.M.; Rimm, D.L.; Camp, R.L.; Chung, G.G. High Levels of Vascular Endothelial Growth Factor and Its Receptors (VEGFR-1, VEGFR-2, Neuropilin-1) Are Associated with Worse Outcome in Breast Cancer. Hum. Pathol. 2008, 39, 1835–1843. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, Y.; Murase, K.; Saito, M.; Imamura, M.; Oh, K. Mechanisms of Estrogen Receptor-α Upregulation in Breast Cancers. Med. Mol. Morphol. 2010, 43, 193–196. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, H.; Kong, Q.; Jiang, Y. Mechanisms for Estrogen Receptor Expression in Human Cancer. Exp. Hematol. Oncol. 2018, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, C.; Shi, W.; Yang, L.; Zhang, Q.; Cui, J.; Fang, Y.; Li, Y.; Ren, G.; Yang, S.; et al. The Novel VEGF Receptor 2 Inhibitor YLL545 Inhibits Angiogenesis and Growth in Breast Cancer. Oncotarget 2016, 7, 41067–41080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, H.; Guo, M.; Zhang, X.; Jiang, L.; Tan, S.; Yuan, J.; Cui, H.; Min, Y.; Zhang, J.; Schlisio, S.; et al. VEGFR2 Inhibition Hampers Breast Cancer Cell Proliferation via Enhanced Mitochondrial Biogenesis. Cancer Biol. Med. 2021, 18, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Gaber, A.; Refat, M.; Belal, A.; El-Deen, I.; Hassan, N.; Zakaria, R.; Alhomrani, M.; Alamri, A.; Alsanie, W.; Saied, E.M. New Mononuclear and Binuclear Cu(II), Co(II), Ni(II), and Zn(II) Thiosemicarbazone Complexes with Potential Biological Activity: Antimicrobial and Molecular Docking Study. Molecules 2021, 26, 2288. [Google Scholar] [CrossRef]
- Gaber, A.; Alsanie, W.F.; Kumar, D.N.; Refat, M.S.; Saied, E.M. Novel Papaverine Metal Complexes with Potential Anticancer Activities. Molecules 2020, 25, 5447. [Google Scholar] [CrossRef] [PubMed]
- Saied, E.M.; Banhart, S.; Bürkle, S.E.; Heuer, D.; Arenz, C. A Series of Ceramide Analogs Modified at the 1-Position with Potent Activity against the Intracellular Growth of Chlamydia Trachomatis. Future Med. Chem. 2015, 7, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Banhart, S.; Saied, E.M.; Martini, A.; Koch, S.; Aeberhard, L.; Madela, K.; Arenz, C.; Heuer, D. Improved Plaque Assay Identifies a Novel Anti-Chlamydia Ceramide Derivative with Altered Intracellular Localization. Antimicrob. Agents Chemother. 2014, 58, 5537–5546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saied, E.M.; Diederich, S.; Arenz, C. Facile Synthesis of the CERT Inhibitor HPA-12 and Some Novel Derivatives. Chem.-Asian J. 2014, 9, 2092–2094. [Google Scholar] [CrossRef]
- Refat, M.S.; Ibrahim, H.K.; Sowellim, S.Z.A.; Soliman, M.H.; Saeed, E.M. Spectroscopic and Thermal Studies of Mn(II), Fe(III), Cr(III) and Zn(II) Complexes Derived from the Ligand Resulted by the Reaction Between 4-Acetyl Pyridine and Thiosemicarbazide. J. Inorg. Organomet. Polym. Mater. 2009, 19, 521. [Google Scholar] [CrossRef]
- Saied, E.M.; Le, T.L.-S.; Hornemann, T.; Arenz, C. Synthesis and Characterization of Some Atypical Sphingoid Bases. Bioorg. Med. Chem. 2018, 26, 4047–4057. [Google Scholar] [CrossRef]
- Saied, E.M.; Arenz, C. Stereoselective Synthesis of Novel Sphingoid Bases Utilized for Exploring the Secrets of Sphinx. Int. J. Mol. Sci. 2021, 22, 8171. [Google Scholar] [CrossRef]
- El Azab, I.H.; Saied, E.M.; Osman, A.A.; Mehana, A.E.; Saad, H.A.; Elkanzi, N.A. Novel N-Bridged Pyrazole-1-Carbothioamides with Potential Antiproliferative Activity: Design, Synthesis, in Vitro and in Silico Studies. Future Med. Chem. 2021, 13, 1743–1766. [Google Scholar] [CrossRef] [PubMed]
- Ignat, A.; Lovasz, T.; Vasilescu, M.; Fischer-Fodor, E.; Tatomir, C.B.; Cristea, C.; Silaghi-Dumitrescu, L.; Zaharia, V. Heterocycles 27. Microwave Assisted Synthesis and Antitumour Activity of Novel Phenothiazinyl-Thiazolyl-Hydrazine Derivatives. Arch. Pharm. 2012, 345, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, V.; Ignat, A.; Palibroda, N.; Ngameni, B.; Kuete, V.; Fokunang, C.N.; Moungang, M.L.; Ngadjui, B.T. Synthesis of Some P-Toluenesulfonyl-Hydrazinothiazoles and Hydrazino-Bis-Thiazoles and Their Anticancer Activity. Eur. J. Med. Chem. 2010, 45, 5080–5085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-N.; Li, J.-T.; Song, Y.-L.; Liu, H.-M.; Li, H.-Y. Efficient One-Pot Three-Component Synthesis of N-(4-Arylthiazol-2-Yl) Hydrazones in Water under Ultrasound Irradiation. Ultrason. Sonochem. 2012, 19, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Borcea, A.-M.; Ionuț, I.; Crișan, O.; Oniga, O. An Overview of the Synthesis and Antimicrobial, Antiprotozoal, and Antitumor Activity of Thiazole and Bisthiazole Derivatives. Molecules 2021, 26, 624. [Google Scholar] [CrossRef]
- Grozav, A.; Găină, L.I.; Pileczki, V.; Crisan, O.; Silaghi-Dumitrescu, L.; Therrien, B.; Zaharia, V.; Berindan-Neagoe, I. The Synthesis and Antiproliferative Activities of New Arylidene-Hydrazinyl-Thiazole Derivatives. Int. J. Mol. Sci. 2014, 15, 22059–22072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Pepinosa, N.Y.; Cardona-Trujillo, M.C.; Garzón-Castaño, S.C.; Veloza, L.A.; Sepúlveda-Arias, J.C. Antiproliferative Activity of Thiazole and Oxazole Derivatives: A Systematic Review of in vitro and in vivo Studies. Biomed. Pharmacother. 2021, 138, 111495. [Google Scholar] [CrossRef]
- Herrera-España, A.D.; Us-Martín, J.; Quintal-Novelo, C.; Mirón-López, G.; Quijano-Quiñones, R.F.; Cáceres-Castillo, D.; Graniel-Sabido, M.; Moo-Puc, R.E.; Mena-Rejón, G.J. Cytotoxic and Antiproliferative Activity of Thiazole Derivatives of Ochraceolide A. Nat. Prod. Res. 2021, 1–5. [Google Scholar] [CrossRef]
- Sun, M.; Xu, Q.; Xu, J.; Wu, Y.; Wang, Y.; Zuo, D.; Guan, Q.; Bao, K.; Wang, J.; Wu, Y.; et al. Synthesis and Bioevaluation of N,4-Diaryl-1,3-Thiazole-2-Amines as Tubulin Inhibitors with Potent Antiproliferative Activity. PLoS ONE 2017, 12, e0174006. [Google Scholar] [CrossRef]
- Bimoussa, A.; Oubella, A.; El Mansouri, A.; Fawzi, M.; Laamari, Y.; Auhmani, A.; Itto, M.Y.A.; Morjani, H.; Auhmani, A. Synthesis and Biological Evaluation of Novel Thiazole Analogs with Both Anti-Proliferative and Mechanistic Analyses and Molecular Docking Studies. ChemistrySelect 2022, 7, e202104270. [Google Scholar] [CrossRef]
- Ramos-Inza, S.; Aydillo, C.; Sanmartín, C.; Plano, D. Thiazole Moiety: An Interesting Scaffold for Developing New Antitumoral Compounds; IntechOpen: Rijeka, Croatia, 2019; ISBN 978-1-83880-624-8. [Google Scholar]
- Senger, D.R.; Galli, S.J.; Dvorak, A.M.; Perruzzi, C.A.; Harvey, V.S.; Dvorak, H.F. Tumor Cells Secrete a Vascular Permeability Factor That Promotes Accumulation of Ascites Fluid. Science 1983, 219, 983–985. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.-K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF Receptor Signalling—In Control of Vascular Function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Colbert, L.S.; Fuller, M.; Zhang, Y.; Gonzalez-Perez, R.R. Vascular Endothelial Growth Factor Receptor-2 in Breast Cancer. Biochim. Biophys. Acta 2010, 1806, 108–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, K.J.; Liu, S.; Abdelrahim, M.; Yoon, K.; Vanderlaag, K.; Porter, W.; Metz, R.P.; Safe, S. Vascular Endothelial Growth Factor Receptor-2 Expression Is Induced by 17β-Estradiol in ZR-75 Breast Cancer Cells by Estrogen Receptor α/Sp Proteins. Endocrinology 2006, 147, 3285–3295. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.-W.; Liu, D.-K.; Zhang, Q.-W.; Xu, Y.-G.; Shi, L. VEGFR-2 Inhibitors and the Therapeutic Applications Thereof: A Patent Review (2012-2016). Expert Opin. Ther. Pat. 2017, 27, 987–1004. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, M.M.; Eissa, I.H.; Alsaif, N.A.; Obaidullah, A.J.; Alanazi, W.A.; Alasmari, A.F.; Albassam, H.; Elkady, H.; Elwan, A. Design, Synthesis, Docking, ADMET Studies, and Anticancer Evaluation of New 3-Methylquinoxaline Derivatives as VEGFR-2 Inhibitors and Apoptosis Inducers. J. Enzym. Inhib. Med. Chem. 2021, 36, 1760–1782. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, M.M.; Elwan, A.; Alsaif, N.A.; Obaidullah, A.J.; Alkahtani, H.M.; Al-Mehizia, A.A.; Alsubaie, S.M.; Taghour, M.S.; Eissa, I.H. Discovery of New 3-Methylquinoxalines as Potential Anti-Cancer Agents and Apoptosis Inducers Targeting VEGFR-2: Design, Synthesis, and in Silico Studies. J. Enzym. Inhib. Med. Chem. 2021, 36, 1732–1750. [Google Scholar] [CrossRef] [PubMed]
- Alsaif, N.A.; Taghour, M.S.; Alanazi, M.M.; Obaidullah, A.J.; Al-Mehizia, A.A.; Alanazi, M.M.; Aldawas, S.; Elwan, A.; Elkady, H. Discovery of New VEGFR-2 Inhibitors Based on Bis([1, 2, 4]Triazolo)[4,3-a:3’,4’-c]Quinoxaline Derivatives as Anticancer Agents and Apoptosis Inducers. J. Enzym. Inhib. Med. Chem. 2021, 36, 1093–1114. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Alsaid, M.S.; Soliman, A.M.; Ragab, F.A. VEGFR-2 Inhibitors and Apoptosis Inducers: Synthesis and Molecular Design of New Benzo[g]Quinazolin Bearing Benzenesulfonamide Moiety. J. Enzym. Inhib. Med. Chem. 2017, 32, 893–907. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, A.M.; Zidan, A.; Elkaeed, E.B.; Taghour, M.S.; Badawi, W.A. Design, Synthesis and Docking Studies of New Hydrazinyl-Thiazole Derivatives as Anticancer and Antimicrobial Agents. J. Saudi Chem. Soc. 2022, 26, 101488. [Google Scholar] [CrossRef]
- Ayati, A.; Emami, S.; Moghimi, S.; Foroumadi, A. Thiazole in the Targeted Anticancer Drug Discovery. Future Med. Chem. 2019, 11, 1929–1952. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, W.; Fan, M.; He, M.; Li, Y.; Peng, Z. Design, Synthesis and Biological Evaluation of Novel Thiazole-Naphthalene Derivatives as Potential Anticancer Agents and Tubulin Polymerisation Inhibitors. J. Enzym. Inhib. Med. Chem. 2021, 36, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Oki, H.; Okada, K.; Takagi, T.; Tawada, M.; Miyazaki, Y.; Imamura, S.; Hori, A.; Lawson, J.D.; Hixon, M.S.; et al. A Back-to-Front Fragment-Based Drug Design Search Strategy Targeting the DFG-Out Pocket of Protein Tyrosine Kinases. ACS Med. Chem. Lett. 2012, 3, 342–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McTigue, M.; Murray, B.W.; Chen, J.H.; Deng, Y.-L.; Solowiej, J.; Kania, R.S. Molecular Conformations, Interactions, and Properties Associated with Drug Efficiency and Clinical Performance among VEGFR TK Inhibitors. Proc. Natl. Acad. Sci. USA 2012, 109, 18281–18289. [Google Scholar] [CrossRef] [Green Version]
- McAulay, K.; Hoyt, E.A.; Thomas, M.; Schimpl, M.; Bodnarchuk, M.S.; Lewis, H.J.; Barratt, D.; Bhavsar, D.; Robinson, D.M.; Deery, M.J.; et al. Alkynyl Benzoxazines and Dihydroquinazolines as Cysteine Targeting Covalent Warheads and Their Application in Identification of Selective Irreversible Kinase Inhibitors. J. Am. Chem. Soc. 2020, 142, 10358–10372. [Google Scholar] [CrossRef]
- Kettle, J.G.; Anjum, R.; Barry, E.; Bhavsar, D.; Brown, C.; Boyd, S.; Campbell, A.; Goldberg, K.; Grondine, M.; Guichard, S.; et al. Discovery of N-(4-{[5-Fluoro-7-(2-Methoxyethoxy)Quinazolin-4-Yl]Amino}phenyl)-2-[4-(Propan-2-Yl)-1H-1,2,3-Triazol-1-Yl]Acetamide (AZD3229), a Potent Pan-KIT Mutant Inhibitor for the Treatment of Gastrointestinal Stromal Tumors. J. Med. Chem. 2018, 61, 8797–8810. [Google Scholar] [CrossRef]
- Norman, M.H.; Liu, L.; Lee, M.; Xi, N.; Fellows, I.; D’Angelo, N.D.; Dominguez, C.; Rex, K.; Bellon, S.F.; Kim, T.-S.; et al. Structure-Based Design of Novel Class II c-Met Inhibitors: 1. Identification of Pyrazolone-Based Derivatives. J. Med. Chem. 2012, 55, 1858–1867. [Google Scholar] [CrossRef]
- Hasegawa, M.; Nishigaki, N.; Washio, Y.; Kano, K.; Harris, P.A.; Sato, H.; Mori, I.; West, R.I.; Shibahara, M.; Toyoda, H.; et al. Discovery of Novel Benzimidazoles as Potent Inhibitors of TIE-2 and VEGFR-2 Tyrosine Kinase Receptors. J. Med. Chem. 2007, 50, 4453–4470. [Google Scholar] [CrossRef]
- Hilberg, F.; Roth, G.J.; Krssak, M.; Kautschitsch, S.; Sommergruber, W.; Tontsch-Grunt, U.; Garin-Chesa, P.; Bader, G.; Zoephel, A.; Quant, J.; et al. BIBF 1120: Triple Angiokinase Inhibitor with Sustained Receptor Blockade and Good Antitumor Efficacy. Cancer Res. 2008, 68, 4774–4782. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.A.; Cheung, M.; Hunter, R.N.; Brown, M.L.; Veal, J.M.; Nolte, R.T.; Wang, L.; Liu, W.; Crosby, R.M.; Johnson, J.H.; et al. Discovery and Evaluation of 2-Anilino-5-Aryloxazoles as a Novel Class of VEGFR2 Kinase Inhibitors. J. Med. Chem. 2005, 48, 1610–1619. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Matsunaga, S.; Tang, J.; Maeda, Y.; Nakano, M.; Philippe, R.J.; Shibahara, M.; Liu, W.; Sato, H.; Wang, L.; et al. Novel 4-Amino-Furo[2,3-d]Pyrimidines as Tie-2 and VEGFR2 Dual Inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 2203–2207. [Google Scholar] [CrossRef] [PubMed]
- Oguro, Y.; Miyamoto, N.; Okada, K.; Takagi, T.; Iwata, H.; Awazu, Y.; Miki, H.; Hori, A.; Kamiyama, K.; Imamura, S. Design, Synthesis, and Evaluation of 5-Methyl-4-Phenoxy-5H-Pyrrolo[3,2-d]Pyrimidine Derivatives: Novel VEGFR2 Kinase Inhibitors Binding to Inactive Kinase Conformation. Bioorg. Med. Chem. 2010, 18, 7260–7273. [Google Scholar] [CrossRef] [PubMed]
- Okaniwa, M.; Hirose, M.; Imada, T.; Ohashi, T.; Hayashi, Y.; Miyazaki, T.; Arita, T.; Yabuki, M.; Kakoi, K.; Kato, J.; et al. Design and Synthesis of Novel DFG-Out RAF/Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) Inhibitors. 1. Exploration of [5,6]-Fused Bicyclic Scaffolds. J. Med. Chem. 2012, 55, 3452–3478. [Google Scholar] [CrossRef]
- Bold, G.; Schnell, C.; Furet, P.; McSheehy, P.; Brüggen, J.; Mestan, J.; Manley, P.W.; Drückes, P.; Burglin, M.; Dürler, U.; et al. A Novel Potent Oral Series of VEGFR2 Inhibitors Abrogate Tumor Growth by Inhibiting Angiogenesis. J. Med. Chem. 2016, 59, 132–146. [Google Scholar] [CrossRef]
- Patterson, J.R.; Graves, A.P.; Stoy, P.; Cheung, M.; Desai, T.A.; Fries, H.; Gatto, G.J.; Holt, D.A.; Shewchuk, L.; Totoritis, R.; et al. Identification of Diarylurea Inhibitors of the Cardiac-Specific Kinase TNNI3K by Designing Selectivity Against VEGFR2, P38α, and B-Raf. J. Med. Chem. 2021, 64, 15651–15670. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid Colorimetric Assay for Cell Growth and Survival: Modifications to the Tetrazolium Dye Procedure Giving Improved Sensitivity and Reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Abou-Seri, S.M.; Eldehna, W.M.; Ali, M.M.; Abou El Ella, D.A. 1-Piperazinylphthalazines as Potential VEGFR-2 Inhibitors and Anticancer Agents: Synthesis and in Vitro Biological Evaluation. Eur. J. Med. Chem. 2016, 107, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.H.; Elsaadi, M.T.; Zaki, S.S.; Abdel-Rahman, H.M. Design, Synthesis and Molecular Modeling Studies of 2-Styrylquinazoline Derivatives as EGFR Inhibitors and Apoptosis Inducers. Bioorganic Chem. 2020, 105, 104358. [Google Scholar] [CrossRef]
- Kuroda, K.; Fukuda, T.; Isogai, H.; Okumura, K.; Krstic-Demonacos, M.; Isogai, E. Antimicrobial Peptide FF/CAP18 Induces Apoptotic Cell Death in HCT116 Colon Cancer Cells via Changes in the Metabolic Profile. Int. J. Oncol. 2015, 46, 1516–1526. [Google Scholar] [CrossRef] [Green Version]
- Abu Almaaty, A.H.; Elgrahy, N.A.; Fayad, E.; Abu Ali, O.A.; Mahdy, A.R.E.; Barakat, L.A.A.; El Behery, M. Design, Synthesis and Anticancer Evaluation of Substituted Cinnamic Acid Bearing 2-Quinolone Hybrid Derivatives. Molecules 2021, 26, 4724. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, F.Z.; Rizzk, Y.W.; El-Deen, I.M.; Gad, E.M.; El Behery, M.; Mahdy, A.R.E. Discovery of 2-Amino-4H-1, 3, 4-Thiadiazine-5(6H)-One Derivatives and Their In Vitro Antitumor Investigation. ChemistrySelect 2022, 7, e202104333. [Google Scholar] [CrossRef]
- Saied, E.M.; El-Maradny, Y.A.; Osman, A.A.; Darwish, A.M.G.; Abo Nahas, H.H.; Niedbała, G.; Piekutowska, M.; Abdel-Rahman, M.A.; Balbool, B.A.; Abdel-Azeem, A.M. A Comprehensive Review about the Molecular Structure of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Insights into Natural Products against COVID-19. Pharmaceutics 2021, 13, 1759. [Google Scholar] [CrossRef] [PubMed]
- Healey, R.D.; Saied, E.M.; Cong, X.; Karsai, G.; Gabellier, L.; Saint-Paul, J.; Del Nero, E.; Jeannot, S.; Drapeau, M.; Fontanel, S.; et al. Discovery and Mechanism of Action of Small Molecule Inhibitors of Ceramidases**. Angew. Chem. Int. Ed. 2022, 61, e202109967. [Google Scholar] [CrossRef]
- Mohamed, D.I.; Alaa El-Din Aly El-Waseef, D.; Nabih, E.S.; El-Kharashi, O.A.; Abd El-Kareem, H.F.; Abo Nahas, H.H.; Abdel-Wahab, B.A.; Helmy, Y.A.; Alshawwa, S.Z.; Saied, E.M. Acetylsalicylic Acid Suppresses Alcoholism-Induced Cognitive Impairment Associated with Atorvastatin Intake by Targeting Cerebral MiRNA155 and NLRP3: In Vivo, and In Silico Study. Pharmaceutics 2022, 14, 529. [Google Scholar] [CrossRef]
- Samaha, D.; Hamdo, H.H.; Cong, X.; Schumacher, F.; Banhart, S.; Aglar, Ö.; Möller, H.M.; Heuer, D.; Kleuser, B.; Saied, E.M.; et al. Liposomal FRET Assay Identifies Potent Drug-Like Inhibitors of the Ceramide Transport Protein (CERT). Chem. Eur. J. 2020, 26, 16616–16621. [Google Scholar] [CrossRef]
- Mohamed, D.I.; Abou-Bakr, D.A.; Ezzat, S.F.; El-Kareem, H.F.A.; Nahas, H.H.A.; Saad, H.A.; Mehana, A.E.; Saied, E.M. Vitamin D3 Prevents the Deleterious Effects of Testicular Torsion on Testis by Targeting MiRNA-145 and ADAM17: In Silico and In Vivo Study. Pharmaceuticals 2021, 14, 1222. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, D.I.; Ezzat, S.F.; Elayat, W.M.; El-Kharashi, O.A.; El-Kareem, H.F.A.; Nahas, H.H.A.; Abdel-Wahab, B.A.; Alshawwa, S.Z.; Saleh, A.; Helmy, Y.A.; et al. Hepatoprotective Role of Carvedilol against Ischemic Hepatitis Associated with Acute Heart Failure via Targeting MiRNA-17 and Mitochondrial Dynamics-Related Proteins: An In Vivo and In Silico Study. Pharmaceuticals 2022, 15, 832. [Google Scholar] [CrossRef] [PubMed]




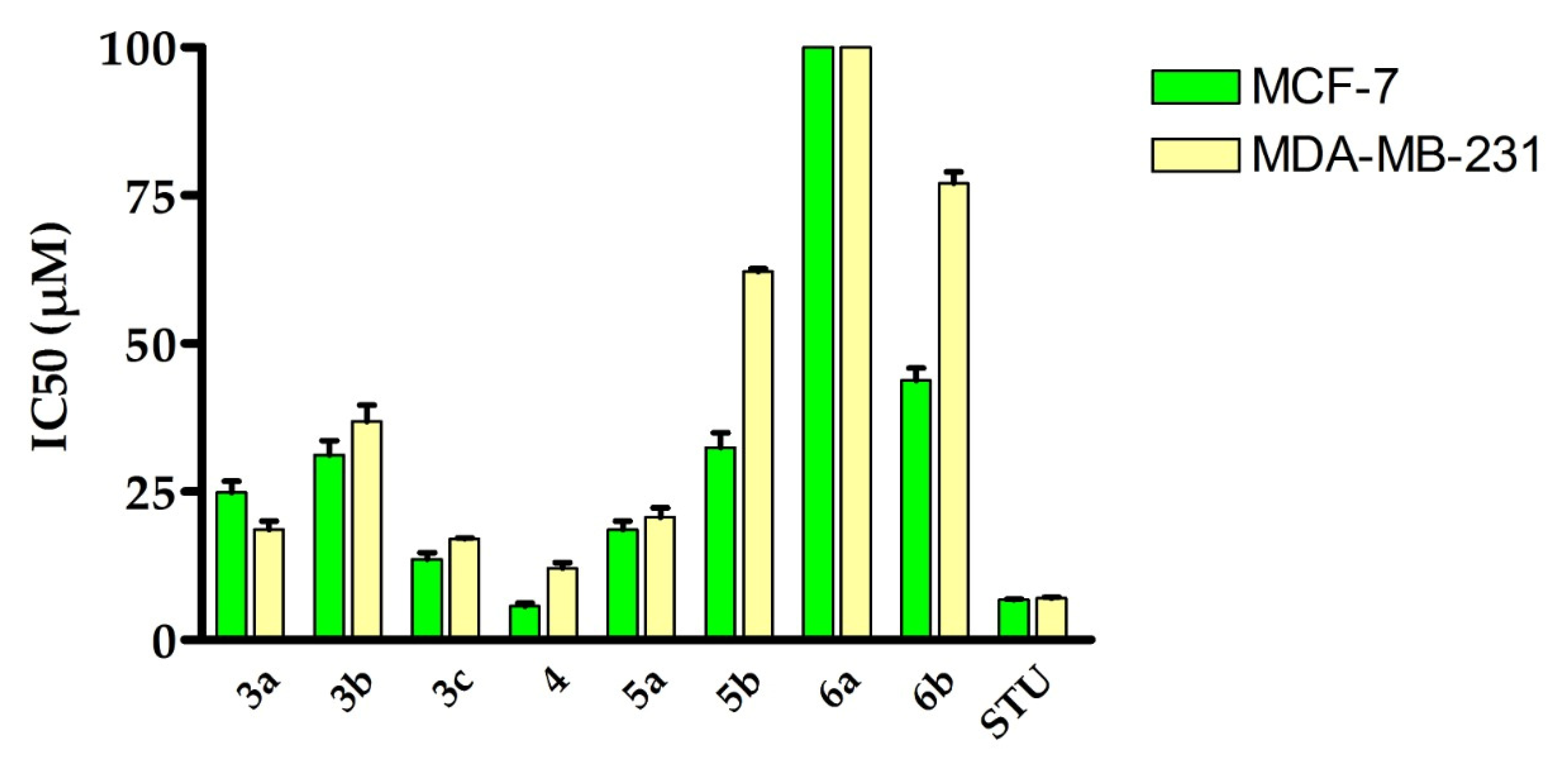

| Compound | IC50 (µM) | ||
|---|---|---|---|
| MCF-7 | MDA-MB-231 | MCF-10A | |
| 3a | 24.9 ± 1.9 | 18.65 ± 1.42 | NT |
| 3b | 31.22 ± 2.38 | 36.84 ± 2.81 | NT |
| 3c | 13.66 ± 1.04 | 17.08 ± 0.13 | NT |
| 4 | 5.73 ± 0.44 | 12.15 ± 0.93 | 36.83 ± 05 |
| 5a | 18.64 ± 1.42 | 20.72 ± 1.58 | NT |
| 5b | 32.48 ± 2.47 | 62.12 ± 0.47 | NT |
| 6a | >100 | >100 | NT |
| 6b | 43.81 ± 2.04 | 77.04 ± 1.95 | NT |
| STU | 6.77 ± 0.08 | 7.03 ± 0.19 | 26.72 ± 1.26 |
| Comp No. | IC50 Values (µM) |
|---|---|
| 3c | 0.253 ± 0.54 |
| 4 | 0.093 ± 0.22 |
| Sorafenib | 0.059 ± 0.35 |
| Protein (PDB Code) | Compound | Hydrophilic Interactions | Distance (A) | Hydrophobic Interactions | S (kcal/mol) |
|---|---|---|---|---|---|
| VEGFR2 (2oh4) | benzimidazole-urea ligand | Glu883 Glu883 Cys917 Cys917 Asp1044 | 2.82 2.81 2.6 2.96 2.89 | Ala864, Ile886, Leu887, Ile890, Leu1017, Phe919, Phe916, Val914, Leu1033, Ile1042 | −11.47 |
| 3c | Glu883 Cys1043 Asp1044 | 2.99 3.58 3.44 | Val846, Ile886, Leu887, Val897, Val912, Val914, Leu1033, Phe1045 | −8.21 | |
| 4 | Glu883 Glu883 Ile1023 Cys1043 Asp1044 | 1.82 3.32 3.42 3.22 2.97 | Val897, Leu887, Ile886, Ile1045 | −10.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salem, M.G.; El-Maaty, D.M.A.; El-Deen, Y.I.M.; Elesawy, B.H.; Askary, A.E.; Saleh, A.; Saied, E.M.; Behery, M.E. Novel 1,3-Thiazole Analogues with Potent Activity against Breast Cancer: A Design, Synthesis, In Vitro, and In Silico Study. Molecules 2022, 27, 4898. https://doi.org/10.3390/molecules27154898
Salem MG, El-Maaty DMA, El-Deen YIM, Elesawy BH, Askary AE, Saleh A, Saied EM, Behery ME. Novel 1,3-Thiazole Analogues with Potent Activity against Breast Cancer: A Design, Synthesis, In Vitro, and In Silico Study. Molecules. 2022; 27(15):4898. https://doi.org/10.3390/molecules27154898
Chicago/Turabian StyleSalem, Manar G., Dina M. Abu El-Maaty, Yassmina I. Mohey El-Deen, Basem H. Elesawy, Ahmad El Askary, Asmaa Saleh, Essa M. Saied, and Mohammed El Behery. 2022. "Novel 1,3-Thiazole Analogues with Potent Activity against Breast Cancer: A Design, Synthesis, In Vitro, and In Silico Study" Molecules 27, no. 15: 4898. https://doi.org/10.3390/molecules27154898
APA StyleSalem, M. G., El-Maaty, D. M. A., El-Deen, Y. I. M., Elesawy, B. H., Askary, A. E., Saleh, A., Saied, E. M., & Behery, M. E. (2022). Novel 1,3-Thiazole Analogues with Potent Activity against Breast Cancer: A Design, Synthesis, In Vitro, and In Silico Study. Molecules, 27(15), 4898. https://doi.org/10.3390/molecules27154898










