Neutrophil Immunomodulatory Activity of (−)-Borneol, a Major Component of Essential Oils Extracted from Grindelia squarrosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Materials
2.3. Essential Oil Extraction
2.4. Gas Chromatography–Flame Ionization Detector (GC-FID) and Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
2.5. Analysis of Enantiomer Composition on Chiral Columns
2.6. Isolation of Human Neutrophils
2.7. Cell Culture
2.8. Ca2+ Mobilization Assay
2.9. Chemotaxis Assay
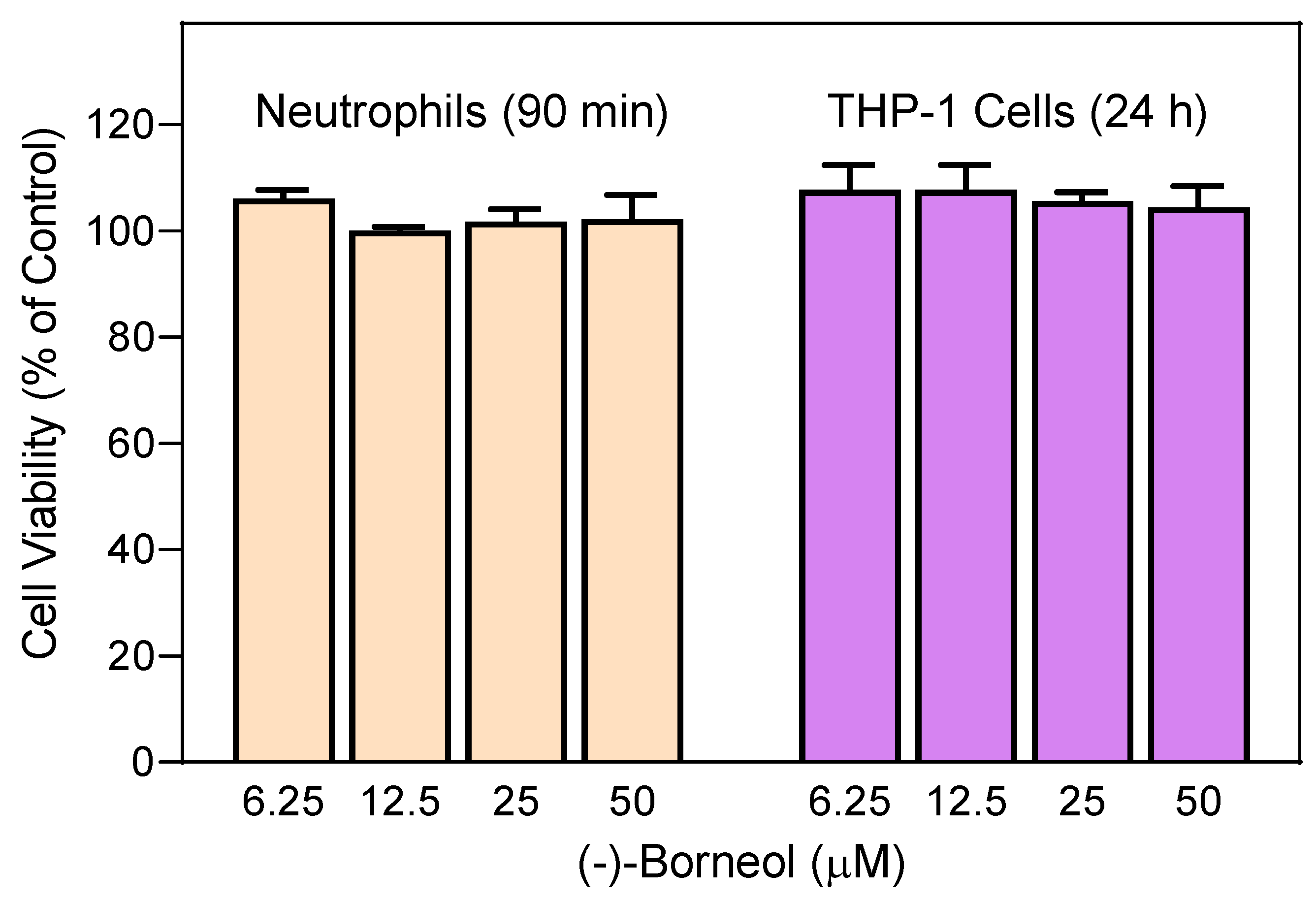
2.10. Cytotoxicity Assay
2.11. Physiochemical Properties of Compounds
2.12. Statistical Analysis
3. Results and Discussion
3.1. Composition of Essential Oils from G. squarrosa
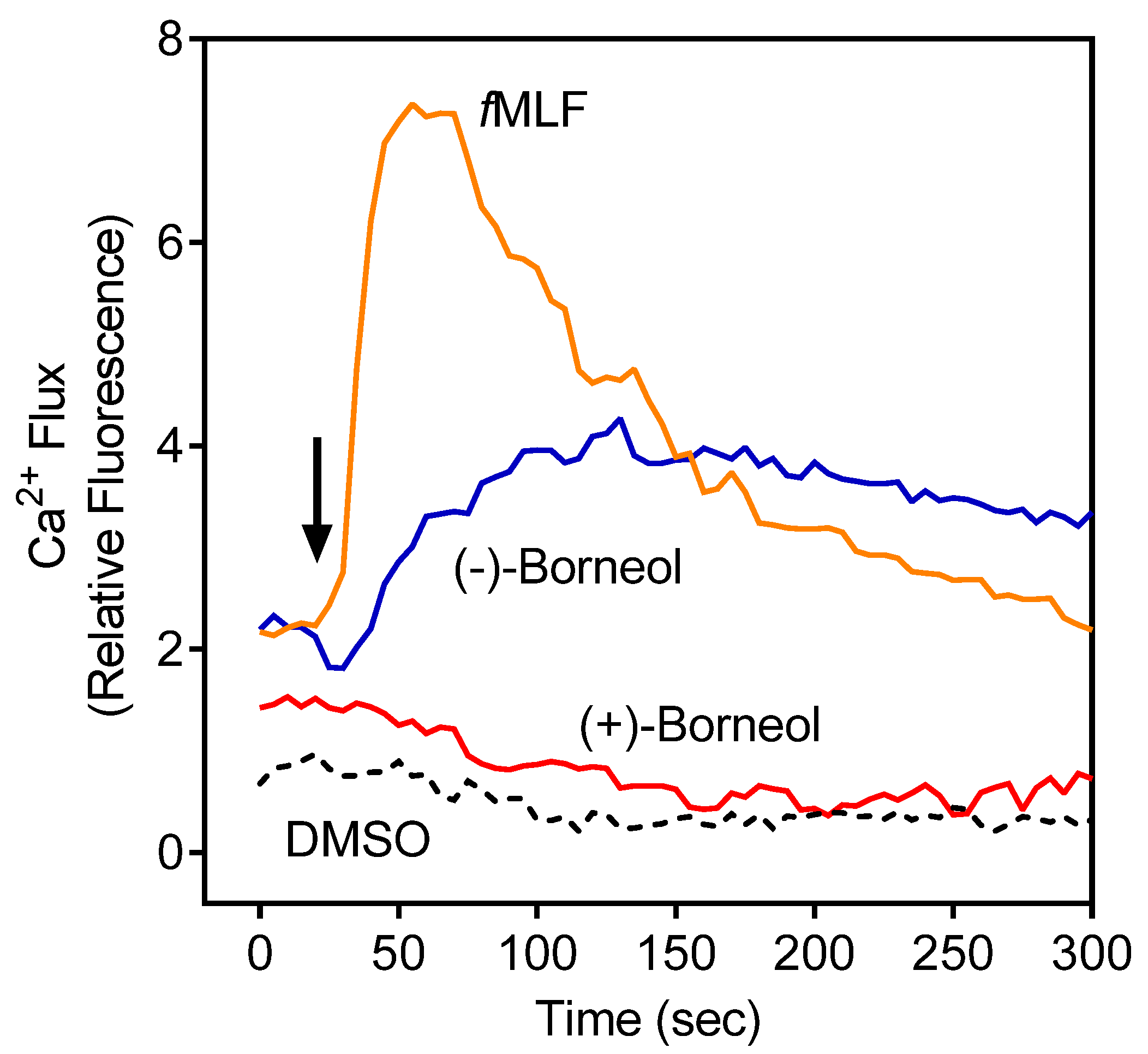
3.2. Effect of the Essential Oils from G. squarrosa and (−)-Borneol on Neutrophil Ca2+ Influx
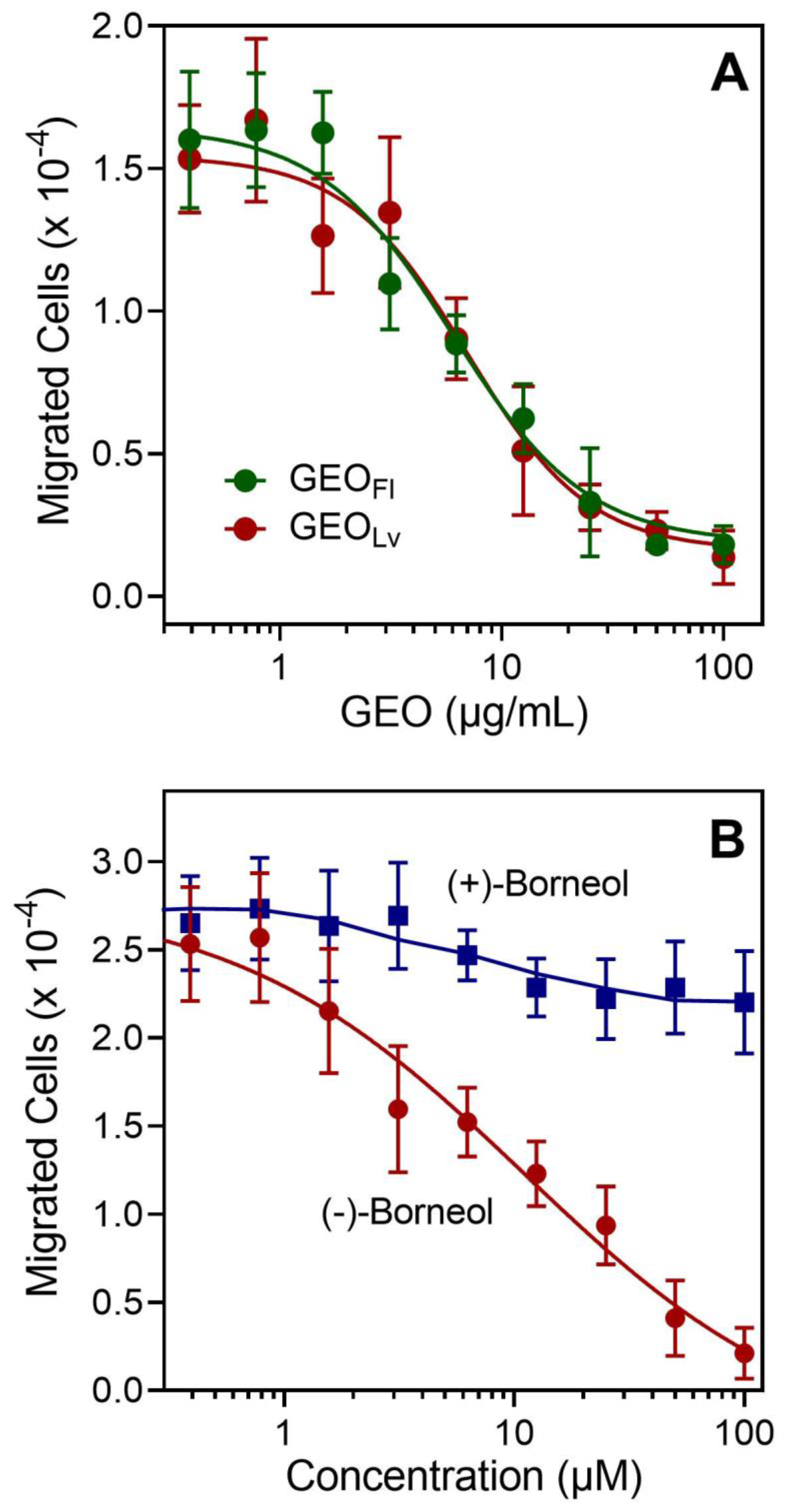
3.3. Effect of Essential Oils from G. squarrosa and Borneol on Neutrophil Chemotaxis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Steyermark, J.A. Studies in Grindelia. II. A monograph of the North American species of the genus Grindelia. Ann. Missouri Botan Gard. 1934, 21, 433–608. [Google Scholar] [CrossRef]
- The Plant List. Version 1.1. Available online: http://www.theplantlist.org (accessed on 21 April 2022).
- Gehrmann, B. Grindelia sp. Willd—Grindelie, Gummikraut, Teerkraut. Z. Phytother. 2020, 41, 218–224. [Google Scholar]
- Gierlikowska, B.; Gierlikowski, W.; Bekier, K.; Skalicka-Woźniak, K.; Czerwińska, M.E.; Kiss, A.K. Inula helenium and Grindelia squarrosa as a source of compounds with anti-inflammatory activity in human neutrophils and cultured human respiratory epithelium. J. Ethnopharmacol. 2020, 249, 112311. [Google Scholar] [CrossRef]
- Nowak, S.; Magiera, A.; Kopka, K.; Kicel, A.; Olszewska, M.A. Rodzaj Grindelia Willd. źródłem cennych roślin leczniczych. Farm. Pol. 2018, 74, 521–530. [Google Scholar] [CrossRef]
- Gruenwald, J.; Brendler, T.; Jaenicke, C. (Eds.) PDR for Herbal Medicines, 2nd ed.; Medical Economics Company: Montvale, NJ, USA, 2000; p. 1108. [Google Scholar]
- Hoffmann, D. Medical Herbalism: The Science and Practice of Herbal Medicine; Healing Arts Press: Rochester, VT, USA, 2003; p. 675. [Google Scholar]
- Pliszko, A.; Górecki, A. First observation of true vivipary in Grindelia squarrosa (Asteraceae). Biologia 2021, 76, 1147–1151. [Google Scholar] [CrossRef]
- Moerman, D.E. Native American Medicinal Plants. An Ethnobotanical Dictionary; Timber Press: Portland, OR, USA, 2009; p. 799. [Google Scholar]
- El-Shamy, A.; El-Hawary, S.; El-Shabrawy, A.; El-Hefnawy, H.; Glasl, H. Essential oil composition of three Grindelia species. J. Essent. Oil Res. 2000, 12, 631–634. [Google Scholar] [CrossRef]
- Newton, M.N.; Espinar, L.A.; Grosso, N.R.; Zunino, M.P.; Maestri, D.M.; Zygadlo, J.A. Analysis of the essential oil of Grindelia discoidea. Planta Med. 1998, 64, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Nowak, S.; Lisiecki, P.; Tomaszczak-Nowak, A.; Grudzińska, E.; Olszewska, M.A.; Kicel, A. Chemical composition and antimicrobial activity of the essential oils from flowers and leaves of Grindelia integrifolia DC. Nat. Prod. Res. 2019, 33, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Schimmer, O.; Schultze, W. Relative amounts of essential oil components in the flowers, leaves, and stems of four Grindelia species. Planta Med. 1993, 59, A634. [Google Scholar] [CrossRef]
- Schäfer, M.; Schimmer, O. Composition of the essential oils from flowers, leaves and stems of Grindelia robusta and G. squarrosa. J. Essent. Oil Res. 2000, 12, 547–552. [Google Scholar] [CrossRef]
- Veres, K.; Roza, O.; Laczkó-Zöld, E.; Hohmann, J. Chemical composition of essential oils of Grindelia squarrosa (Pursh) Dunal and Grindelia oregana A. Gray. Planta Med. 2013, 79, PI45. [Google Scholar] [CrossRef]
- Veres, K.; Roza, O.; Laczkó-Zöld, E.; Hohmann, J. Chemical composition of essential oils of Grindelia squarrosa and G. hirsutula. Nat. Prod. Commun. 2014, 9, 573–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmermann, B.N.; Hoffmann, J.J.; Jolad, S.D.; Bates, R.B.; Siahaan, T.J. Diterpenoids and flavonoids from Grindelia discoidea. Phytochem 1986, 25, 723–727. [Google Scholar] [CrossRef]
- Zhou, L. Bioactive Agents from Grindelia tarapacana Phil. (Asteraceae); The University of Arizona Graduate College: Tucson, AZ, USA, 1994. [Google Scholar]
- Alza, N.P.; Pferschy-Wenzig, E.M.; Ortmann, S.; Kretschmer, N.; Kunert, O.; Rechberger, G.N.; Bauer, R.; Murray, A.P. Inhibition of NO production by Grindelia Argentina and isolation of three new cytotoxic saponins. Chem. Biodiver 2014, 11, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Krenn, L.; Wollenweber, E.; Steyrleuthner, K.; Görick, C.; Melzig, M.F. Contribution of methylated exudate flavonoids to the anti-inflammatory activity of Grindelia robusta. Fitoterapia 2009, 80, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Blaschek, W.; Hänsel, R.; Keller, K.; Reichling, J.; Rimpler, H.; Schneider, G. (Eds.) Hagers Handbuch der Pharmazeutischen Praxis. Folgeband 2: Drogen, 5th ed.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 2, pp. 812–821. [Google Scholar]
- Nowak, S.; Rychlińska, I. Phenolic acids in the flowers and leaves of Grindelia robusta Nutt. and Grindelia squarrosa Dun. (Asteraceae). Acta Pol. Pharm. 2012, 69, 693–698. [Google Scholar] [PubMed]
- La, V.D.; Lazzarin, F.; Ricci, D.; Fraternale, D.; Genovese, S.; Epifano, F.; Grenier, D. Active principles of Grindelia robusta exert antiinflammatory properties in a macrophage model. Phytother. Res. 2010, 24, 1687–1692. [Google Scholar] [CrossRef]
- Gierlikowska, B.; Filipek, A.; Gierlikowski, W.; Kania, D.; Stefanska, J.; Demkow, U.; Kiss, A.K. Grindelia squarrosa extract and grindelic acid modulate pro-inflammatory functions of respiratory epithelium and human macrophages. Front. Pharmacol. 2020, 11, 534111. [Google Scholar] [CrossRef] [PubMed]
- Malburet, P.; Ragažinskienė, O.; Maruška, A. Introduction and chemical analysis of Grindelia squarrosa (Purch) Dunal in Vytautas Magnus univesity. In Proceedings of the Vital Nature Sign [Elektroninis Išteklius]: 10th International Scientific Conference, Vilnius, Lithuania, 19–20 May 2016. [Google Scholar]
- Sandner, G.; Heckmann, M.; Weghuber, J. Immunomodulatory activities of selected essential oils. Biomolecules 2020, 10, 1139. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Haran, G.H.; Calisto, V.; Jothi, G.; Quintans, J.S.S.; Cuevas, L.E.; Narain, N.; Junior, L.J.Q.; Cipolotti, R.; et al. Essential oils and its bioactive compounds modulating cytokines: A systematic review on anti-asthmatic and immunomodulatory properties. Phytomedicine 2020, 73, 152854. [Google Scholar] [CrossRef] [Green Version]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozek, G.; Schepetkin, I.A.; Yermagambetova, M.; Ozek, T.; Kirpotina, L.N.; Almerekova, S.S.; Abugalieva, S.I.; Khlebnikov, A.I.; Quinn, M.T. Innate Immunomodulatory activity of cedrol, a component of essential oils isolated from Juniperus species. Molecules 2021, 26, 7644. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Ozek, G.; Ozek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Chemical composition and immunomodulatory activity of essential oils from Rhododendron albiflorum. Molecules 2021, 26, 3652. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Ozek, G.; Ozek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Chemical composition and immunomodulatory activity of Hypericum perforatum essential oils. Biomolecules 2020, 10, 916. [Google Scholar] [CrossRef]
- Ozek, G.; Schepetkin, I.A.; Utegenova, G.A.; Kirpotina, L.N.; Andrei, S.R.; Ozek, T.; Baser, K.H.C.; Abidkulova, K.T.; Kushnarenko, S.V.; Khlebnikov, A.I.; et al. Chemical composition and phagocyte immunomodulatory activity of Ferula iliensis essential oils. J. Leukoc. Biol. 2017, 101, 1361–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schepetkin, I.A.; Kushnarenko, S.V.; Ozek, G.; Kirpotina, L.N.; Sinharoy, P.; Utegenova, G.A.; Abidkulova, K.T.; Ozek, T.; Baser, K.H.; Kovrizhina, A.R.; et al. Modulation of human neutrophil responses by the essential oils from Ferula akitschkensis and their constituents. J. Agric. Food Chem. 2016, 64, 7156–7170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schepetkin, I.A.; Kushnarenko, S.V.; Ozek, G.; Kirpotina, L.N.; Utegenova, G.A.; Kotukhov, Y.A.; Danilova, A.N.; Ozek, T.; Baser, K.H.; Quinn, M.T. Inhibition of human neutrophil responses by the essential oil of Artemisia kotuchovii and its constituents. J. Agric. Food. Chem. 2015, 63, 4999–5007. [Google Scholar] [CrossRef] [Green Version]
- Campra, N.A.; Montironi, I.D.; Reinoso, E.B.; Raviolo, J.; Moreno, F.R.; Maletto, B.; Cariddi, L.N. A natural oil increases specific anti-OVA IgG levels and induces a cellular immune response combined with aluminum hydroxide. J. Leukoc. Biol. 2021, 109, 223–232. [Google Scholar] [CrossRef]
- Siveen, K.S.; Kuttan, G. Augmentation of humoral and cell mediated immune responses by Thujone. Int. Immunopharmacol. 2011, 11, 1967–1975. [Google Scholar] [CrossRef]
- Khlebnikov, A.I.; Schepetkin, I.A.; Kishkentaeva, A.S.; Shaimerdenova, Z.R.; Atazhanova, G.A.; Adekenov, S.M.; Kirpotina, L.N.; Quinn, M.T. Inhibition of T cell receptor activation by semi-synthetic sesquiterpene lactone derivatives and molecular modeling of their interaction with glutathione and tyrosine kinase ZAP-70. Molecules 2019, 24, 350. [Google Scholar] [CrossRef] [Green Version]
- Schepetkin, I.A.; Kirpotina, L.N.; Mitchell, P.T.; Kishkentaeva capital, A.C.; Shaimerdenova, Z.R.; Atazhanova, G.A.; Adekenov, S.M.; Quinn, M.T. The natural sesquiterpene lactones arglabin, grosheimin, agracin, parthenolide, and estafiatin inhibit T cell receptor (TCR) activation. Phytochem 2018, 146, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Ozek, G.; Ishmuratova, M.; Tabanca, N.; Radwan, M.M.; Goger, F.; Ozek, T.; Wedge, D.E.; Becnel, J.J.; Cutler, S.J.; Can Baser, K.H. One-step multiple component isolation from the oil of Crinitaria tatarica (Less.) Sojak by preparative capillary gas chromatography with characterization by spectroscopic and spectrometric techniques and evaluation of biological activity. J. Sep. Sci. 2012, 35, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. High-throughput screening for small-molecule activators of neutrophils: Identification of novel N-formyl peptide receptor agonists. Mol. Pharmacol. 2007, 71, 1061–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- Pala-Paul, J.; García-Jiménez, R.; Pérez-Alonso, M.J.; Velasco-Negueruela, A.; Sanz, J. Essential oil composition of the leaves and stems of Meum athamanticum Jacq., from Spain. J. Chromatog. A 2004, 1036, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Wedge, D.E.; Klun, J.A.; Tabanca, N.; Demirci, B.; Ozek, T.; Baser, K.H.; Liu, Z.; Zhang, S.; Cantrell, C.L.; Zhang, J. Bioactivity-guided fractionation and GC/MS fingerprinting of Angelica sinensis and Angelica archangelica root components for antifungal and mosquito deterrent activity. J. Agric. Food Chem. 2009, 57, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Gonny, M.; Cavaleiro, C.; Salgueiro, L.; Casanova, J. Analysis of Juniperus communis subsp alpina needle, berry, wood and root oils by combination of GC, GC/MS and 13C-NMR. Flavour Fragr. J. 2006, 21, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Bonikowski, R.; Paoli, M.; Szymczak, K.; Krajewska, A.; Wajs‐Bonikowska, A.; Tomi, F.; Kalemba, D. Chromatographic and spectral characteristic of some esters of a common monoterpene alcohols. Flavour Fragr. J. 2016, 31, 290–292. [Google Scholar] [CrossRef]
- Castioni, P.; Kapetanidis, I. Volatile constituents from Brunfelsia grandiflora ssp. grandiflora: Qualitative analysis by GC-MS. Sci. Pharm. 1996, 64, 83–91. [Google Scholar]
- Hazzit, M.; Baaliouamer, A.; Faleiro, M.L.; Miguel, M.G. Composition of the essential oils of Thymus and Origanum species from Algeria and their antioxidant and antimicrobial activities. J. Agric. Food Chem. 2006, 54, 6314–6321. [Google Scholar] [CrossRef] [PubMed]
- Suleimenov, E.M.; Ozek, T.; Demirci, F.; Demirci, B.; Baser, K.H.C.; Adekenov, S.M. Component composition of essential oils of Artemisia lercheana and A. sieversiana of the flora of Kazakhstan. Antimicrobial activity of A. sieversiana essential oil. Chem. Nat. Comp. 2009, 45, 120–123. [Google Scholar] [CrossRef]
- Weyerstahl, P.; Marschall, H.; Son, P.T.; Giang, P.M. Constituents of the flower essential oil of Aglaia odorata Lour. from Vietnam. Flavour Fragr. J. 1999, 14, 219–224. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Özek, G.; Özek, T.; Duran, A.; Duman, H. Composition of the essential oils of Rhabdosciadium oligocarpum (Post ex Boiss.) Hedge et Lamond and Rhabdosciadium microcalycinum Hand.-Mazz. Flavour Fragr. J. 2006, 21, 650–655. [Google Scholar] [CrossRef]
- Cavaleiro, C.; Pinto, E.; Gonçalves, M.J.; Salgueiro, L. Antifungal activity of Juniperus essential oils against dermatophyte, Aspergillus and Candida strains. J. Appl. Microbiol. 2006, 100, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Filho, J.; da Silva Brandi, J.; Ferreira Costa, H.; Carla de Paula Medeiros, K.; Alves Leite, J.; Pergentino de Sousa, D.; Regina Piuvezam, M. Carvone enantiomers differentially modulate IgE-mediated airway inflammation in mice. Int. J. Mol. Sci. 2020, 21, 9209. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Liao, J.; Qian, Z.; Wu, C.; Zhang, X.; Zhang, J.; Xie, Y.; Jiang, S. Synthesis and evaluation of enantiomers of hydroxychloroquine against SARS-CoV-2 in vitro. Bioorg. Med. Chem. 2022, 53, 116523. [Google Scholar] [CrossRef] [PubMed]
- Murai, T.; Kawashita, N.; Tian, Y.-S.; Takagi, T. In silico analysis of enantioselective binding of immunomodulatory imide drugs to cereblon. Springerplus 2016, 5, 1122. [Google Scholar] [CrossRef] [Green Version]
- Malech, H.L.; DeLeo, F.R.; Quinn, M.T. The Role of Neutrophils in the Immune System: An Overview. Methods Mol. Biol. 2020, 2087, 3–10. [Google Scholar] [CrossRef]
- Keir, H.R.; Chalmers, J.D. Neutrophil extracellular traps in chronic lung disease: Implications for pathogenesis and therapy. Eur. Respir. Rev. 2022, 31, 210241. [Google Scholar] [CrossRef]
- Stojkov, D.; Gigon, L.; Peng, S.; Lukowski, R.; Ruth, P.; Karaulov, A.; Rizvanov, A.; Barlev, N.A.; Yousefi, S.; Simon, H.U. Physiological and Pathophysiological Roles of Metabolic Pathways for NET Formation and Other Neutrophil Functions. Front. Immunol. 2022, 13, 826515. [Google Scholar] [CrossRef]
- Dixit, N.; Kim, M.H.; Rossaint, J.; Yamayoshi, I.; Zarbock, A.; Simon, S.I. Leukocyte function antigen-1, kindlin-3, and calcium flux orchestrate neutrophil recruitment during inflammation. J. Immunol. 2012, 189, 5954–5964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schepetkin, I.A.; Ozek, G.; Ozek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Klein, R.A.; Quinn, M.T. Neutrophil immunomodulatory activity of farnesene, a component of Artemisia dracunculus essential oils. Pharmaceuticals 2022, 15, 642. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Richardson, R.M.; Haribabu, B.; Snyderman, R. Chemoattractant receptor cross-desensitization. J. Biol. Chem. 1999, 274, 6027–6030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherneva, E.; Pavlovic, V.; Smelcerovic, A.; Yancheva, D. The effect of camphor and borneol on rat thymocyte viability and oxidative stress. Molecules 2012, 17, 10258–10266. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, Q.; Lin, A.; Wu, Y.; Min, H.; Song, S.; Wang, Y.; Wang, H.; Yi, L.; Gao, Q. Borneol alleviates brain injury in sepsis mice by blocking neuronal effect of endotoxin. Life Sci. 2019, 232, 116647. [Google Scholar] [CrossRef] [PubMed]
- Sherkheli, M.A.; Schreiner, B.; Haq, R.; Werner, M.; Hatt, H. Borneol inhibits TRPA1, a proinflammatory and noxious pain-sensing cation channel. Pak. J. Pharm. Sci. 2015, 28, 1357–1363. [Google Scholar]
- Zhong, W.; Cui, Y.; Yu, Q.; Xie, X.; Liu, Y.; Wei, M.; Ci, X.; Peng, L. Modulation of LPS-stimulated pulmonary inflammation by Borneol in murine acute lung injury model. Inflammation 2014, 37, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.; Souza, G.R.; Silva, J.C.; Saraiva, S.R.; Junior, R.G.; Quintans Jde, S.; Barreto Rde, S.; Bonjardim, L.R.; Cavalcanti, S.C.; Quintans, L.J., Jr. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. Sci. World J. 2013, 2013, 808460. [Google Scholar] [CrossRef] [Green Version]
- Juhas, S.; Cikos, S.; Czikkova, S.; Vesela, J.; Il’kova, G.; Hajek, T.; Domaracka, K.; Domaracky, M.; Bujnakova, D.; Rehak, P.; et al. Effects of borneol and thymoquinone on TNBS-induced colitis in mice. Folia Biol. 2008, 54, 1–7. [Google Scholar]
- Wang, J.Y.; Dong, X.; Yu, Z.; Ge, L.; Lu, L.; Ding, L.; Gan, W. Borneol inhibits CD4 + T cells proliferation by down-regulating miR-26a and miR-142-3p to attenuate asthma. Int. Immunopharmacol. 2021, 90, 107223. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, F.; Liu, L.; Feng, L.; Wu, X.; Shen, Y.; Sun, Y.; Wu, X.; Xu, Q. (+)-Borneol improves the efficacy of edaravone against DSS-induced colitis by promoting M2 macrophages polarization via JAK2-STAT3 signaling pathway. Int. Immunopharmacol. 2017, 53, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Leite-Sampaio, N.F.; Gondim, C.; Martins, R.A.A.; Siyadatpanah, A.; Norouzi, R.; Kim, B.; Sobral-Souza, C.E.; Gondim, G.E.C.; Ribeiro-Filho, J.; Coutinho, H.D.M. Potentiation of the activity of antibiotics against ATCC and MDR bacterial strains with (+)-α-pinene and (−)-borneol. Biomed. Res. Int. 2022, 2022, 8217380. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Xie, Q.; Li, H.; Guo, X.; Wang, J.; Li, Y.; Ren, M.; Gong, D.; Gao, T. l-Borneol exerted the neuroprotective effect by promoting angiogenesis coupled with neurogenesis via Ang1-VEGF-BDNF Pathway. Front. Pharmacol. 2021, 12, 641894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wen, J.; Jiang, Y.; Hu, Q.; Wang, J.; Wei, S.; Li, H.; Ma, X. l-Borneol ameliorates cerebral ischaemia by downregulating the mitochondrial calcium uniporter-induced apoptosis cascade in pMCAO rats. J. Pharm. Pharmacol. 2021, 73, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.E.; Ribeiro, F.; Menezes, P.M.N.; Duarte-Filho, L.A.M.; Quintans, J.S.S.; Quintans-Junior, L.J.; Silva, F.S.; Ribeiro, L.A.A. New insights on relaxant effects of (−)-borneol monoterpene in rat aortic rings. Fundam Clin. Pharmacol. 2019, 33, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Luz, M.S.; Gadelha, D.D.A.; Andrade, K.J.S.; Travassos, R.A.; Ribeiro, J.D.; Carvalho-Galvao, A.; Cruz, J.C.; Balarini, C.M.; Braga, V.A.; Franca-Falcao, M.S. Borneol reduces sympathetic vasomotor hyperactivity and restores depressed baroreflex sensitivity in rats with renovascular hypertension. Hypertens Res. 2022, 45, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Silva-Filho, J.C.; Oliveira, N.N.; Arcanjo, D.D.; Quintans-Junior, L.J.; Cavalcanti, S.C.; Santos, M.R.; Oliveira Rde, C.; Oliveira, A.P. Investigation of mechanisms involved in (−)-borneol-induced vasorelaxant response on rat thoracic aorta. Basic Clin. Pharmacol. Toxicol. 2012, 110, 171–177. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yoshimaru, T.; Inoue, T.; Ra, C. Ca v 1.2 L-type Ca2+ channel protects mast cells against activation-induced cell death by preventing mitochondrial integrity disruption. Mol. Immunol. 2009, 46, 2370–2380. [Google Scholar] [CrossRef]
- Ghosh, M.; Schepetkin, I.A.; Ozek, G.; Ozek, T.; Khlebnikov, A.I.; Damron, D.S.; Quinn, M.T. Essential Oils from Monarda fistulosa: Chemical Composition and Activation of Transient Receptor Potential A1 (TRPA1) Channels. Molecules 2020, 25, 4873. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Tetko, I.V.; Tanchuk, V.Y. Application of associative neural networks for prediction of lipophilicity in ALOGPS 2.1 program. J. Chem. Inf. Comp. Sci. 2002, 42, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Cao, L.; Ge, H.; Duanmu, W.; Tan, L.; Yuan, J.; Tunan, C.; Li, F.; Hu, R.; Gao, F.; et al. L-Borneol induces transient opening of the blood-brain barrier and enhances the therapeutic effect of cisplatin. Neuroreport 2017, 28, 506–513. [Google Scholar] [CrossRef] [PubMed]

| No | RRI | Pub RRI | Compound | GEOFl | GEOLv | No | RRI | Pub RRI | Compound | GEOFl | GEOLv |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1032 | 1008–1039 a | α-Pinene | 24.7 | 23.2 | 37 | 1648 | 1597–1648 a | Myrtenal | 0.8 | 1.0 |
| 2 | 1076 | 1043–1086 a | Camphene | 1.2 | 1.5 | 38 | 1650 | 1612–1654 a | δ-Elemene | t | 0.1 |
| 3 | 1118 | 1085–1130 a | β-Pinene | 4.0 | 3.8 | 39 | 1670 | 1643–1671 a | trans-Pinocarveol | 4.2 | 3.7 |
| 4 | 1132 | 1098–1140 a | Sabinene | t | 0.1 | 40 | 1674 | 1670–1740 a | p-Mentha-1,5-dien-8-ol | t | 0.1 |
| 5 | 1150 | 1140 b | Thuja-2,4(10)-diene | t | t | 41 | 1683 | 1665–1691 a | trans-Verbenol | 1.9 | 2.0 |
| 6 | 1159 | 1122–1169 a | δ-3-Carene | t | 42 | 1700 | 1681 d | p-Mentha-1,8-dien-4-ol | 0.2 | 0.1 | |
| 7 | 1174 | 1140–1175 a | Myrcene | 0.3 | 0.4 | 43 | 1706 | 1659–1724 a | α-Terpineol | 1.7 | 0.5 |
| 8 | 1176 | 1148–1186 a | α-Phellandrene | t | t | 44 | 1719 | 1653–1728 a | Borneol | 23.4 | 16.6 |
| 9 | 1188 | 1154–1195 a | α-Terpinene | t | t | 45 | 1726 | 1676–1726 a | Germacrene D | 0.2 | 0.3 |
| 10 | 1195 | 1167–1197 a | Dehydro-1,8-cineole | t | t | 46 | 1751 | 1699–1751 a | Carvone | 0.4 | |
| 11 | 1203 | 1178–1219 a | Limonened | 10.0 | 14.7 | 47 | 1763 | 1771 e | Isobornyl isovalerate | 0.3 | |
| 12 | 1213 | 1186–1231 a | 1,8-Cineole | t | t | 48 | 1773 | 1722–1774 a | δ-Cadinene | 0.3 | |
| 13 | 1218 | 1188–1233 a | β-Phellandrene | 0.2 | 0.2 | 49 | 1796 | 1750–1800 a | Selina-3,7(11)-diene | 0.3 | 0.3 |
| 14 | 1246 | 1211–1251 a | (Z)-β-Ocimene | 0.3 | 0.1 | 50 | 1797 | 1739–1797 a | p-Methyl acetophenone | 0.2 | 0.3 |
| 15 | 1255 | 1222–1266 a | γ-Terpinene | 0.1 | 51 | 1804 | 1743–1808 a | Myrtenol | 2.5 | 1.7 | |
| 16 | 1266 | 1232–1267 a | (E)-β-Ocimene | 0.2 | 0.6 | 52 | 1854 | 1778–1854 a | Germacrene B | 0.4 | 0.5 |
| 17 | 1280 | 1246–1291 a | p-Cymene | 0.5 | 0.6 | 53 | 1864 | 1813–1865 a | p-Cymen-8-ol | 6.1 | 5.8 |
| 18 | 1290 | 1260–1300 a | Terpinolene | 1.7 | 2.0 | 54 | 1882 | 1818–1882 a | cis-Carveol | t | |
| 19 | 1328 | 1328 f | 2,2,6-Trimethylcyclohexanone | t | 55 | 1949 | 1840–1949 a | Piperitenone | t | ||
| 20 | 1384 | 1331–1384 a | α-Pinene oxide | t | 56 | 1992 | 1954–1992 a | 2-Phenylethyl-3-methylbutyrate | t | ||
| 21 | 1452 | 1452 g | α,p-Dimethylstyrene | 0.1 | 57 | 2088 | 2026–2090 a | 1-epi-Cubenol | t | t | |
| 22 | 1477 | 1477 c | 4,8-Epoxyterpinolene | 0.3 | 0.5 | 58 | 2095 | 2033–2097 a | Hexyl benzoate | t | |
| 23 | 1494 | 1494 h | (Z)-3-Hexenyl 3-methylbutyrate | 0.2 | t | 59 | 2098 | 2049–2104 a | Globulol | 0.3 | 0.3 |
| 24 | 1495 | 1471–1495 a | Bicycloelemene | 0.1 | 60 | 2115 | 2115 i | 4-Hydroxy-4-methyl-cyclohex-2-enone | 0.9 | 0.7 | |
| 25 | 1499 | 1477–1511 a | α-Campholene aldehyde | 0.2 | 61 | 2144 | 2074–2150 a | Spathulenol | 3.0 | 2.0 | |
| 26 | 1522 | 1482–1522 a | Chrysanthenone | 0.1 | 62 | 2164 | 2154 j | Muurola-4,10(14)dien-1-ol | 0.3 | ||
| 27 | 1532 | 1481–1537 a | Camphor | 0.5 | 0.9 | 63 | 2187 | 2135–2219 a | τ-Cadinol | 0.2 | |
| 28 | 1549 | 1518–1560 a | β-Cubebene | t | 64 | 2247 | 2247 h | trans-α-Bergamotol | 0.2 | 0.3 | |
| 29 | 1553 | 1507–1564 a | Linalool | 0.1 | 0.1 | 65 | 2255 | 2180–2255 a | α-Cadinol | t | 0.2 |
| 30 | 1562 | 1511–1562 a | Isopinocamphone | 0.7 | 1.6 | 66 | 2257 | 2196–2272 a | β-Eudesmol | t | 0.4 |
| 31 | 1571 | 1557–1625 a | trans-p-Menth-2-en-1-ol | t | 67 | 2260 | 2262 k | Alismol | 0.5 | 0.3 | |
| 32 | 1586 | 1545–1590 a | Pinocarvone | t | 68 | 2300 | 2300 | Tricosane | t | ||
| 33 | 1590 | 1549–1597 a | Bornyl acetate | 3.0 | 5.1 | 69 | 2308 | 2339 l | 13-epi-Manoyl oxide | 0.8 | 0.9 |
| 34 | 1611 | 1564–1630 a | Terpinen-4-ol | 0.9 | 0.5 | 70 | 2320 | - | Guaia-6,10(14)-dien-4-ol isomer # | 0.4 | |
| 35 | 1628 | 1583–1668 a | Aromadendrene | t | 71 | 2500 | 2500 | Pentacosane | 0.3 | ||
| 36 | 1639 | 1611–1688 a | trans-p-Mentha-2,8-dien-1-ol | 0.2 | 0.2 | ||||||
| Summary of the Oil Composition | |||||||||||
| Major Components | GEOFl | GEOLv | |||||||||
| Monoterpene hydrocarbons | 43.2 | 47.2 | |||||||||
| Oxygenated monoterpenes | 46.5 | 41.4 | |||||||||
| Sesquiterpene hydrocarbons | 1.2 | 1.3 | |||||||||
| Oxygenated sesquiterpenes | 4.4 | 4.0 | |||||||||
| Miscellaneous compounds | 2.1 | 2.3 | |||||||||
| Total | 97.4 | 96.2 | |||||||||
| Compound | GEOFl | GEOLv | |||
|---|---|---|---|---|---|
| (%) | ee (%) | (%) | ee (%) | ||
| α-Pinene | (1S,5S)-(−) | 100 | 100 | 100 | 100 |
| (1R,5R)-(+) | 0 | 0 | |||
| β-Pinene | (1S,5S)-(−) (1R,5R)-(+) | 91 9 | 82 | 89 11 | 78 |
| Borneol | (1S,2R,4S)-(−) | 100 | 100 | 100 | 100 |
| (1R,2S,4R)-(+) | 0 | 0 | |||
| Camphor | (1S,4S)-(−) | 95 | 90 | 97 | 94 |
| (1R,4R)-(+) | 5 | 3 | |||
| Limonene | (4S)-(−) | 3 | 2 | ||
| (4R)-(+) | 97 | 94 | 98 | 96 | |
| Essential Oil | Activation of [Ca2+]i | Inhibition of [Ca2+]i | |
|---|---|---|---|
| fMLF-Induced | WKYMVM-Induced | ||
| EC50 (μg/mL) | IC50 (μg/mL) | ||
| GEOFl | 22.3 ± 5.7 | 16.4 ± 3.9 | 1.6 ± 0.7 |
| GEOLv | 19.4 ± 5.3 | 16.9 ± 2.0 | 3.4 ± 1.2 |
| Pure Compound | EC50 (μM) | IC50 (μM) | |
| (−)-Borneol | 28.7 ± 2.6 | 36.1 ± 1.5 | 54.2 ± 11.2 |
| (+)-Borneol | N.A. | N.A. | N.A. |
| (±)-Bornyl acetate * | 50.1 ± 11.5 | 42.6 ± 9.7 | 19.1 ± 0.1 |
| Property | Borneol |
|---|---|
| Formula | C10H18O |
| M.W. | 154.25 |
| Heavy atoms | 11 |
| Fraction Csp3 | 1.00 |
| Rotatable bonds | 0 |
| H-bond acceptors | 1 |
| H-bond donors | 1 |
| MR | 46.60 |
| tPSA | 20.23 |
| LogP | 2.83 |
| BBB permeation | Yes |
| Compound | Chemical Class | IC50 (uM) | Reference |
|---|---|---|---|
| (−)-Borneol | Oxygenated monoterpene | 36.1 ± 1.5 | Present work |
| Bornyl Acetate | Oxygenated monoterpene | 42.6 ± 9.7 | [59] |
| Cedrol | Oxygenated sesquiterpene | 15.4 ± 4.3 | [29] |
| Curzerene | Oxygenated sesquiterpene | 11.0 ± 3.8 | [30] |
| Farnesene | Sesquiterpene hydrocarbone | 1.1 ± 0.2 | [59] |
| Germacrene D | Sesquiterpene hydrocarbone | 0.5 ± 0.1 | [31] |
| Germacrone | Oxygenated sesquiterpene | 27.9 ± 8.9 | [30] |
| 6-Methyl-3,5-heptadien-2-one | Enone | 8.2 ± 2.5 | [34] |
| Spathulenol | Oxygenated sesquiterpene | 36.2 ± 8.2 | [30] |
| Viridiflorol | Oxygenated sesquiterpene | 7.8 ± 2.3 | [30] |
| Xanthoxylin | Alkyl-phenylketone | 27.2 ± 6.6 | [59] |
| α-Humulene | Sesquiterpene hydrocarbone | 0.3 ± 0.1 | [31] |
| β-Caryophyllene | Sesquiterpene hydrocarbone | 0.33 ± 0.02 | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schepetkin, I.A.; Özek, G.; Özek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Neutrophil Immunomodulatory Activity of (−)-Borneol, a Major Component of Essential Oils Extracted from Grindelia squarrosa. Molecules 2022, 27, 4897. https://doi.org/10.3390/molecules27154897
Schepetkin IA, Özek G, Özek T, Kirpotina LN, Khlebnikov AI, Quinn MT. Neutrophil Immunomodulatory Activity of (−)-Borneol, a Major Component of Essential Oils Extracted from Grindelia squarrosa. Molecules. 2022; 27(15):4897. https://doi.org/10.3390/molecules27154897
Chicago/Turabian StyleSchepetkin, Igor A., Gulmira Özek, Temel Özek, Liliya N. Kirpotina, Andrei I. Khlebnikov, and Mark T. Quinn. 2022. "Neutrophil Immunomodulatory Activity of (−)-Borneol, a Major Component of Essential Oils Extracted from Grindelia squarrosa" Molecules 27, no. 15: 4897. https://doi.org/10.3390/molecules27154897
APA StyleSchepetkin, I. A., Özek, G., Özek, T., Kirpotina, L. N., Khlebnikov, A. I., & Quinn, M. T. (2022). Neutrophil Immunomodulatory Activity of (−)-Borneol, a Major Component of Essential Oils Extracted from Grindelia squarrosa. Molecules, 27(15), 4897. https://doi.org/10.3390/molecules27154897