Abstract
HIF means hypoxia-inducible factor gene family, and it could regulate various biological processes, including tumor development. In 2021, the FDA approved the new drug Welireg for targeting HIF-2a, and it is mainly used to treat von Hippel-Lindau syndrome, which demonstrated its good prospects in tumor therapy. As the fourth deadliest cancer worldwide, gastric cancer endangers the health of people all across the world. Currently, there are various treatment methods for patients with gastric cancer, but the five-year survival rate of patients with advanced gastric cancer is still not high. Therefore, here we reviewed the regulatory role and target role of HIF in gastric cancer, and provided some references for the treatment of gastric cancer.
1. Introduction
The hypoxia-inducible factor (HIF) gene family consists of HIF1, HIF2 and HIF3: HIF1, the most important member of the HIF family, is mainly composed of two subunits, namely HIF1α and HIF1β. When the oxygen concentration is normal, HIF1α is degraded and cannot exist stably. In the case of hypoxia, HIF1α enters the nucleus and combines with HIF1β to promote downstream genes transcription [1]. The specific mechanism is: under normoxia conditions, HIF1α undergoes hydroxylation under the action of prolyl hydroxylase (PHD), and it is recognized and bound by the von Hippel-Lindau tumor suppressor (VHL), when the HIF1α binds to VHL, then it is ubiquitinated and degraded. Under hypoxia, the oxygen-dependent proline hydroxylation reaction is blocked due to the inactivation of PHD, HIF1α is not degraded, and the accumulated HIF1α enters the nucleus and combines with HIF1β to form a dimer, and the dimer regulates the expression of related genes under hypoxic conditions with the participation of transcriptional co-activators such as histone acetyltransferase p300, and finally realizes the adaptation of cells to hypoxia conditions [2,3,4,5,6] (Figure 1). HIF2 is composed of HIF2α and HIF2β. HIF2α is the main functional subunit, and it is rich in tissues such as vascular endothelial cells and fetal lung fibroblasts. After HIF2α is activated, it binds to ARNT to form heterodimerization. Then, it specifically binds to the hypoxia response element of hypoxia-inducible factor (5′-TACGTGCG-3′), thereby upregulating the expression of these genes [7]. It is currently known that HIF3, a less-studied member of the HIF gene family, is composed of HIF3α and HIF3β. The HIF3α gene produces a variety of HIF3α variants, and it is expressed and differentially regulated by hypoxia and other factors. Full-length HIF3α protein functions as an oxygen-regulated transcriptional activator [8]. The HIF gene family plays a very important regulatory role in a variety of diseases, including cancer [9,10,11,12,13,14,15]. For example, Kimberly J Briggs et al. found that HIFα promotes adaptation to hypoxia and stimulates growth in triple-negative breast cancer [16]. Joo-Yun Byun et al. found that HIF-1α promotes cancer stem-like cell phenotype and chemotherapy resistance in head and neck squamous cell carcinoma [17].
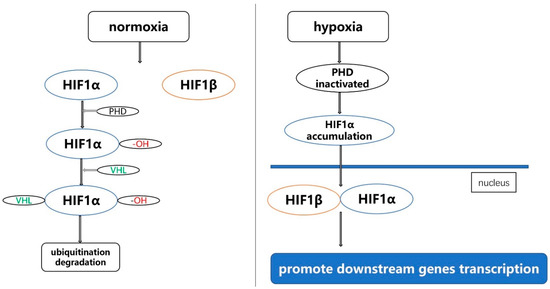
Figure 1.
The mechanism of function of HIF1 under normoxia and hypoxia.
According to the data of the World Health Organization, the number of new gastric cancer patients in the world in 2020 is 1.09 million, ranking fifth, and the number of gastric cancer deaths in the world in 2020 is 770,000, ranking fourth. Therefore, it can be said that gastric cancer seriously harms the health of people around the world [18]. Current treatments for gastric cancer include systemic chemotherapy, radiotherapy, surgery, immunotherapy and targeted therapy [19]. Even with so many treatments, the median survival for advanced gastric cancer is less than 1 year, and the treatments for advanced gastric cancer include chemotherapy, radiotherapy, immunotherapy and targeted therapy [20]. Targeted therapy for advanced gastric cancer includes anti-HER2, anti-EGFR, anti-VEGF, anti-mTOR, anti-HFG and PARP inhibitors. Representative drugs and specific schematic diagrams are shown in Figure 2 [21]. The above-mentioned targeted therapy drugs have certain curative effects, but the survival period of advanced gastric cancer is always relatively short, so the development of new targeted drugs to prolong the survival period of advanced gastric cancer is a top priority.
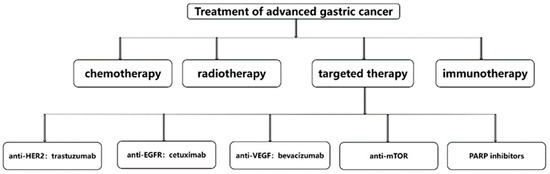
Figure 2.
Treatments for advanced gastric cancer.
In 2016, some scholars found that the antagonist PT2399 targeting HIF2α has good antitumor efficacy in VHL-deficient clear cell renal cell carcinoma [22,23,24]. The discovery of PT2399 suggested that targeting HIF may be a promising target for cancer therapy. Jung-Hyun Park et al. were firstly discover that HIF1α is stably expressed in gastric cancer and may be involved in the progression of gastric cancer [25]. Since then, many studies have proved that HIF plays a regulatory role in the occurrence and development of gastric cancer, and the development of targeted drugs for HIF may be a promising treatment for advanced gastric cancer. Therefore, this review discussed the role of HIF in gastric cancer from its regulation of proliferation, metastasis, apoptosis, drug resistance, angiogenesis, stemness and metabolism of gastric cancer cells, and discussed some HIF-targeted therapies drugs for gastric cancer as well.
2. The Regulatory Role of HIF in Gastric Cancer
From the abstract, we can know that HIF could regulate the occurrence and development of gastric cancer by proliferation, metastasis, apoptosis, drug resistance, angiogenesis, stemness and metabolism of gastric cancer cells (Figure 3). Therefore, we will discuss the progress of HIF regulation of gastric cancer from the above seven aspects.
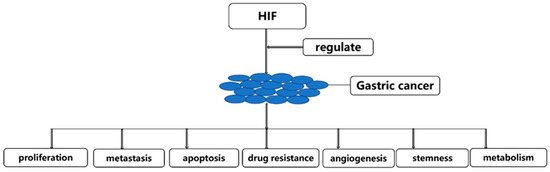
Figure 3.
HIF could regulate the occurrence and development of gastric cancer.
2.1. HIF Regulates Gastric Cancer Progression by Gastric Cancer Cell Proliferation
We know that tumor cell proliferation plays a huge role in tumor development [26]. HIF also regulates gastric cancer progression by regulating tumor cell proliferation (Table 1). Hai-Yan Piao et al. revealed that HIF1α can bind to the promoter of Hypoxia Yield Proliferation Associated LncRNA (HYPAL) and promote its transcription, which activates the Wnt/β-catenin signaling pathway through HYPAL/miR-431-5p/CDK14 and induces gastric cancer cell proliferation [27]. Jiayu Zhao et al. also discovered that HIF1α could bind to the promoter region of miR-17-5p to activate the transcription of pre-miR-17-5p and miR-17-5p, and miR-17-5p binds to the untranslated region of the gastric cancer suppressor gene programmed cell death 4 (PDCD4), the role of PDCD4 in gastric cancer mainly includes inhibition of cell proliferation, thus leading to the degradation of its mRNA. Finally, HIF1α promotes the proliferation of gastric cancer cells [28] (Figure 4). In addition, according to Lei Hong et al., the overexpression of HIF1α can promote the proliferation of gastric cancer cells, and the tumor suppressor gene Linc-pint can inhibit the proliferation of gastric cancer cells by down-regulating the expression of HIF1α [29].

Table 1.
HIF regulates gastric cancer progression by regulating tumor cell proliferation.
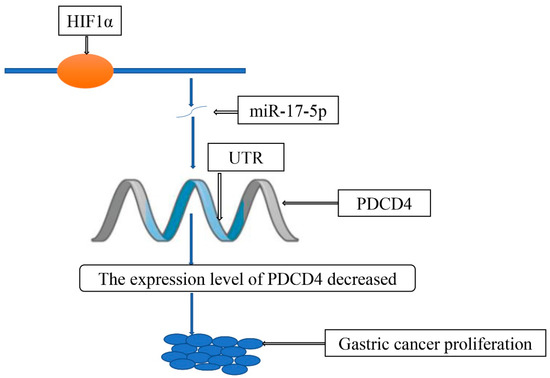
Figure 4.
HIF1α/miR-17-5p/PDCD4 axis contributes to the tumor growth of gastric cancer.
Many other studies have confirmed that HIF regulates the proliferation of gastric cancer cells [30,31,32,33,34]. Some tumor-targeted drugs such as crizotinib can exert their anti-tumor effects by inhibiting cell proliferation [35]. Maybe, we can find drugs that inhibit the proliferation of gastric cancer cells by targeting HIF in the future, which will help the clinical treatment of gastric cancer.
2.2. HIF Regulates Gastric Cancer Progression by Gastric Cancer Cell Metastasis
Metastasis causes most cancer deaths [36]. Extensive evidence indicated that HIF plays an important role in gastric cancer metastasis (Table 2). Deng Guan et al. found that HIF1α can promote the epithelial-mesenchymal transition of gastric cancer cells to promote the metastasis of gastric cancer [37]. R Guo et al. proved that HIF1α can directly bind to the promoter of LXRα to promote its transcription, and the increased content of LXRα activates the epithelial–mesenchymal transition of gastric cancer cells, so the metastatic ability of gastric cancer is greatly increased [38] (Figure 5). Xiang Xia et al. revealed that HIF1α could induce gastric cancer cells to release miR-301a-3p-enriched exosomes and promote the metastasis of gastric cancer cells through the MiR-301a-3p/PHD3/HIF-1α positive feedback loop [39]. Furthermore, many studies have found that HIF is involved in the metastasis of gastric cancer cells [40,41,42,43,44,45,46,47,48,49,50,51,52].

Table 2.
HIF regulates gastric cancer progression by gastric cancer cell metastasis.
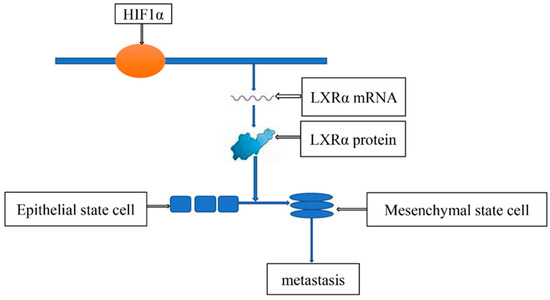
Figure 5.
HIF1α-Induced LXRα contributes to the metastasis of gastric cancer cells.
Inhibiting tumor metastasis by targeting certain genes is also a major strategy for anti-tumor therapy. For example, Entrectinib inhibits the metastasis of non-small cell lung cancer by targeting ROS proto-oncogene 1(ROS1) and neurotrophic receptor tyrosine kinase (NTRK), ROS1 is a proto-oncogene highly expressed in various tumor cells, and ROS1 protein is a type I integral membrane protein with tyrosine kinase activity, and has achieved satisfactory clinical efficacy [53,54,55,56]. From what we have stated above, we can know that HIF has a certain role in the metastasis of gastric cancer. In the future, it may be a good choice to develop drugs to inhibit the metastasis of gastric cancer by targeting HIF.
2.3. HIF Regulates Gastric Cancer Progression by Gastric Cancer Cell Apoptosis
Apoptosis refers to the autonomous and orderly death of cells controlled by genes in order to maintain the stability of the internal environment. It is not a phenomenon of autologous injury under pathological conditions, but a kind of actively striving death process for better adaptation to the living environment. Apoptosis plays a certain role in the occurrence and development of gastric cancer [57] (Table 3). Lili Liu et al. found that HIF1 can promote the expression of the adhesion molecule MGr1-Ag/37LRP by activating ERK and inhibiting the apoptosis of gastric cancer cells [58]. Nadine Rohwer et al. also found that HIF can inhibit the apoptosis of gastric cancer cells by up-regulating alpha5 [59]. However, in fact, the role of HIF in gastric cancer cell apoptosis may be controversial, and some scholars have revealed that HIF can promote gastric cancer cell apoptosis [60,61].

Table 3.
HIF regulates gastric cancer progression by gastric cancer cell apoptosis.
In summary, currently the role of HIF in gastric cancer cell apoptosis is controversial, and more studies are required to clarify the role of HIF in gastric cancer.
2.4. HIF Regulates Gastric Cancer Progression by Gastric Cancer Cell Drug Resistance
Drug resistance limits the efficacy of cancer treatment, and addressing drug resistance may be a key issue in cancer treatment [62,63,64,65] (Table 4). Mitsuyoshi Okazaki et al. found that HIF1 promotes drug resistance in gastric cancer cells by affecting the expression of pyruvate kinase muscle 1 (PKM1), PKM1 is a gene associated with chemotherapy resistance in gastric cancer [66]. Based on Yunna Chen et al., using siRNA to knock down HIF1α can reduce the drug resistance of gastric cancer cells and increase the killing effect of 5-fluorouracil on gastric cancer cells [67]. Qun Zhao et al. discovered that HIF1α directly binds miR-27a to promote its expression, and miR-27a promotes drug resistance of gastric cancer cells by inhibiting the expression of MDR1/P-gp, LRP and Bcl-2 [68].

Table 4.
HIF regulates gastric cancer progression by gastric cancer cell drug resistance.
The role of HIF in gastric cancer drug resistance is relatively certain: HIF can promote gastric cancer drug resistance, and many other studies have also confirmed this [69,70,71,72,73,74,75]. HIF promotes drug resistance of gastric cancer cells, and the idea of new drug design revolves around this point.
2.5. HIF Regulates Gastric Cancer Progression by Gastric Cancer Cell Angiogenesis
Anti-angiogenesis has always been an important method in designing anti-tumor drugs. For example, bevacizumab can combine with VEGF to inhibit tumor angiogenesis and achieve the effect of inhibiting tumors [76,77]. Zheng Li et al. illustrated that HIF1α can promote angiogenesis in gastric cancer, and this process can be promoted by Natriuretic peptide receptor A (NPRA), NPRA is the most important receptor of atrial natriuretic peptide (ANP), NPRA functions significantly in promoting GC development and progression [78] (Table 5). Ganggang Mu et al. proposed that HIF1α can promote the angiogenesis of gastric cancer by promoting the expression of VEGF-A [79]. E Tang et al. revealed that HIF1α promotes gastric angiogenesis through β-catenin/VEGF signaling, thus promoting gastric cancer progression [80].

Table 5.
HIF regulates gastric cancer progression by gastric cancer cell angiogenesis.
Many other studies have confirmed that HIF may be a key factor in gastric cancer angiogenesis [81,82,83,84,85,86]. Inhibition of tumor angiogenesis by developing targeted HIF-related drugs is a promising approach for the clinical treatment of gastric cancer.
2.6. HIF Regulates Gastric Cancer Progression by Gastric Cancer Cell Stemness
Cancer stem cells are defined by the American Cancer Society: A cancer stem cell is a small subset of cells present in a tumor that produces heterogeneous tumor cells with the ability to self-renew [87]. Cancer stem cells are considered as important factors in tumor progression [88,89,90,91].
Zhenqin Luo et al. found that HIF1α can promote the progression of gastric cancer by promoting the stemness of gastric cancer cells [92] (Table 6). On the other hand, Zhi-Feng Miao et al. proved that HIF1α promotes peritoneal dissemination by promoting the stemness of gastric cancer cells [93].

Table 6.
HIF regulates gastric cancer progression by gastric cancer cell stemness.
At present, there are not many studies on HIF in gastric cancer stem cells, and there is no successful clinical application of drugs targeting cancer stem cells. Therefore, the role of HIF in gastric cancer stem cells needs more research.
2.7. HIF Regulates Gastric Cancer Progression by Gastric Cancer Cell Metabolism
Metabolism is closely related to tumors, the metabolism of glucose, lipid and protein in tumors and it is different from normal cells [94,95,96,97,98,99,100,101,102]. Tao Wu et al. discovered that HIF1α promotes gastric cancer progression by promoting glycolysis in gastric cancer cells [103] (Table 7). Xiao-Hong Wang et al. displayed that HIF1α regulates gastric cancer cell glycolysis through the FOXO4/LDHA axis, thereby affecting the progression of gastric cancer cells [104]. According to Jia Liu et al., HIF1α can promote the glycolysis of gastric cancer cells through the circ-MAT2B/miR-515-5p axis, and promote the occurrence and development of gastric cancer cells [105].

Table 7.
HIF regulates gastric cancer progression by gastric cancer cell metabolism.
Many studies have suggested that HIF1α plays a key role in the metabolism of gastric cancer [106,107,108,109,110].
We know that 5-FU can exert an anti-tumor effect by inhibiting nucleic acid metabolism [111,112]. HIF is closely related to the metabolism of gastric cancer. As a good choice to design drugs for gastric cancer based on this, HIF can promote the progression of gastric cancer by promoting glycolysis under hypoxic conditions.
3. Small Molecule Drugs Targeting HIF to Inhibit Gastric Cancer
Small-molecule drugs mainly refer to organic compounds with molecular weights less than 1000, and they have been widely used and mature in theory [113,114,115,116,117]. Apixaban, widely used in clinical practice, is a small molecule drug whose main mechanism is to inhibit the expression of FXa [118,119,120,121,122]. Researchers have discovered many small-molecule drugs that can inhibit gastric cancer progression by targeting HIF (Table 8). Tae Woo Kim et al. found that apigenin, a flavonoid found in traditional medicine, fruits and vegetables, inhibits HIF1α-induced autophagy-related cell death [123]. Noriyuki Egawa et al. demonstrated that low-dose tipifarnib inhibits tumors by inhibiting the expression of HIF1α [124]. Yun-Ning Huang et al. exhibited that dextran sulfate (DS) could inhibit EMT in gastric cancer cells by inhibiting the expression of HIF [125].

Table 8.
Small molecule drugs targeting HIF to inhibit gastric cancer.
There are many small molecule drugs that inhibit the progression of gastric cancer by targeting HIF [74,126,127,128,129,130,131,132,133,134,135,136,137]. Unfortunately, although so many small molecule drugs have been found to inhibit the progression of gastric cancer through HIF, none of them can be used clinically, so more basic and clinical researches are needed.
However, there is a piece of exciting news that the US FDA has approved Merck’s innovative oncology drug Welireg, the first HIF2α inhibitor, for the treatment of VHL syndrome-related tumors. VHL syndrome is a rare and serious genetic disorder associated with a high risk of developing cancer in multiple organs. Prior to Welireg, no systemic therapies were approved for the treatment of VHL-related tumors. Patients suffering from VHL-related tumors treated with Welireg demonstrated high response rates and durable responses [138,139,140,141,142,143,144,145].
Given the success of Welireg, a drug targeting HIF2α in treating VHL syndrome-related tumors, HIF plays a huge role in gastric cancer. Can we look forward to the future that scientists discover that HIF-targeting drugs for the treatment of gastric cancer will benefit patients in the clinic?
4. Conclusions
HIF affects the progression of gastric cancer by regulating the proliferation, metastasis, apoptosis, drug resistance, angiogenesis, stemness and metabolism of gastric cancer cells. Many small molecule drugs that inhibit the progression of gastric cancer through HIF have been found in basic experiments, while these drugs have not yet been clinically applied. Given the success of Welireg, a drug targeting HIF2α in treating VHL syndrome-related tumors, HIF plays a huge role in gastric cancer. We look forward to the future where scientists discover that HIF-targeting drugs for the treatment of gastric cancer will benefit patients in the clinic.
Author Contributions
M.L. and G.L. (Guan Li) performed the literature research and drafted the article. X.Y. edited and revised the article. W.Y., G.L. (Guoqing Lv) and S.W. were involved in the design of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shenzhen Sanming Project (SZSM201612041) and Shenzhen Science and Technology Innovation Commission Project (GJHZ20180420180754917, ZDSYS20190902092855097).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by Shenzhen Sanming Project (SZSM201612041) andShenzhen Science and Technology Innovation Commission Project (GJHZ20180420180754917, ZDSYS20190902092855097).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Knutson, A.K.; Williams, A.L.; Boisvert, W.A.; Shohet, R.V. HIF in the heart: Development, metabolism, ischemia, and atherosclerosis. J. Clin. Investig. 2021, 131, e137557. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Kang, J.B.; Yang, X.; Li, N.; Chang, L.; Ji, J.; Meng, X.K.; Zhang, H.Q.; Zhong, Y.; Yu, S.P.; et al. An efficient strategy for digging protein-protein interactions for rational drug design—A case study with HIF-1α/VHL. Eur. J. Med. Chem. 2022, 227, 113871. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Batty-Stuart, S.; Lee, J.E.; Ohh, M. HIF-1α Hydroxyprolines Modulate Oxygen-Dependent Protein Stability Via Single VHL Interface With Comparable Effect on Ubiquitination Rate. J. Mol. Biol. 2021, 433, 167244. [Google Scholar] [CrossRef]
- Yang, Y.; Zou, P.; He, L.; Shao, J.; Tang, Y.; Li, J. CBL aggravates Ang II-induced cardiac hypertrophy via the VHL/HIF-1α pathway. Exp. Cell Res. 2021, 405, 112730. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.A. Coalescing lessons from oxygen sensing, tumor metabolism, and epigenetics to target VHL loss in kidney cancer. Semin. Cancer Biol. 2020, 67 Pt 2, 34–42. [Google Scholar] [CrossRef]
- Cargill, K.; Hemker, S.L.; Clugston, A.; Murali, A.; Mukherjee, E.; Liu, J.; Bushnell, D.; Bodnar, A.J.; Saifudeen, Z.; Ho, J.; et al. Von Hippel-Lindau Acts as a Metabolic Switch Controlling Nephron Progenitor Differentiation. J. Am. Soc. Nephrol. 2019, 30, 1192–1205. [Google Scholar] [CrossRef]
- Silagi, E.S.; Schipani, E.; Shapiro, I.M.; Risbud, M.V. The role of HIF proteins in maintaining the metabolic health of the intervertebral disc. Nat. Rev. Rheumatol. 2021, 17, 426–439. [Google Scholar] [CrossRef]
- Duan, C. Hypoxia-inducible factor 3 biology: Complexities and emerging themes. Am. J. Physiol. Cell Physiol. 2016, 310, C260–C269. [Google Scholar] [CrossRef] [Green Version]
- Fehsel, K.; Christl, J. Comorbidity of osteoporosis and Alzheimer’s disease: Is ‘AKT’ -ing on cellular glucose uptake the missing link? Ageing Res. Rev. 2022, 76, 101592. [Google Scholar] [CrossRef]
- Singh, C. Metabolism and Vascular Retinopathies: Current Perspectives and Future Directions. Diagnostics 2022, 12, 903. [Google Scholar] [CrossRef]
- Ma, Z.; Xiang, X.; Li, S.; Xie, P.; Gong, Q.; Goh, B.C.; Wang, L. Targeting hypoxia-inducible factor-1, for cancer treatment: Recent advances in developing small-molecule inhibitors from natural compounds. Semin. Cancer Biol. 2022, 80, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Passirani, C.; Vessières, A.; La Regina, G.; Link, W.; Silvestri, R. Modulating undruggable targets to overcome cancer therapy resistance. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer Chemother. 2022, 60, 100788. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Liu, C.; Qin, M.; Zhang, X.; Xi, T.; Yuan, S.; Hao, H.; Xiong, J. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharm. Sinica. B 2022, 12, 558–580. [Google Scholar] [CrossRef] [PubMed]
- Cramer, T.; Vaupel, P. Severe hypoxia is a typical characteristic of human hepatocellular carcinoma: Scientific fact or fallacy? J. Hepatol. 2022, 76, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, X.; Song, Y.; Xie, G.; Jiao, S.; Shi, L.; Cao, X.; Han, X.; Qu, A. The role of hypoxia-inducible factors in cardiovascular diseases. Pharmacol. Ther. 2022, 238, 108186. [Google Scholar] [CrossRef] [PubMed]
- Briggs, K.J.; Koivunen, P.; Cao, S.; Backus, K.M.; Olenchock, B.A.; Patel, H.; Zhang, Q.; Signoretti, S.; Gerfen, G.J.; Richardson, A.L.; et al. Paracrine Induction of HIF by Glutamate in Breast Cancer: EglN1 Senses Cysteine. Cell 2016, 166, 126–139. [Google Scholar] [CrossRef] [Green Version]
- Byun, J.Y.; Huang, K.; Lee, J.S.; Huang, W.; Hu, L.; Zheng, X.; Tang, X.; Li, F.; Jo, D.G.; Song, X.; et al. Targeting HIF-1α/NOTCH1 pathway eliminates CD44(+) cancer stem-like cell phenotypes, malignancy, and resistance to therapy in head and neck squamous cell carcinoma. Oncogene 2022, 41, 1352–1363. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA A Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Patel, T.H.; Cecchini, M. Targeted Therapies in Advanced Gastric Cancer. Curr. Treat. Options Oncol. 2020, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, C.J.; Crooks, D.R.; Linehan, W.M. Targeting HIF2α in Clear-Cell Renal Cell Carcinoma. Cancer Cell 2016, 30, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Du, X.; Rizzi, J.P.; Liberzon, E.; Chakraborty, A.A.; Gao, W.; Carvo, I.; Signoretti, S.; Bruick, R.K.; Josey, J.A.; et al. On-target efficacy of a HIF-2α antagonist in preclinical kidney cancer models. Nature 2016, 539, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Hill, H.; Christie, A.; Kim, M.S.; Holloman, E.; Pavia-Jimenez, A.; Homayoun, F.; Ma, Y.; Patel, N.; Yell, P.; et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 2016, 539, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Kim, T.Y.; Jong, H.S.; Kim, T.Y.; Chun, Y.S.; Park, J.W.; Lee, C.T.; Jung, H.C.; Kim, N.K.; Bang, Y.J. Gastric epithelial reactive oxygen species prevent normoxic degradation of hypoxia-inducible factor-1alpha in gastric cancer cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 433–440. [Google Scholar]
- Zhu, J.; Sammons, M.A.; Donahue, G.; Dou, Z.; Vedadi, M.; Getlik, M.; Barsyte-Lovejoy, D.; Al-awar, R.; Katona, B.W.; Shilatifard, A.; et al. Gain-of-function p53 mutants co-opt chromatin pathways to drive cancer growth. Nature 2015, 525, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Piao, H.Y.; Liu, Y.; Kang, Y.; Wang, Y.; Meng, X.Y.; Yang, D.; Zhang, J. Hypoxia associated lncRNA HYPAL promotes proliferation of gastric cancer as ceRNA by sponging miR-431-5p to upregulate CDK14. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2022, 25, 44–63. [Google Scholar] [CrossRef]
- Zhao, J.; Xiao, A.; Liu, C.; Ye, C.; Yin, K.; Lu, M.; Jiao, R.; Chen, X.; Zhang, C.; Liu, M. The HIF-1A/miR-17-5p/PDCD4 axis contributes to the tumor growth and metastasis of gastric cancer. Signal Transduct. Target. Ther. 2020, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.; Wang, J.; Wang, H.; Wei, S.; Zhang, F.; Han, J.; Liu, Y.; Ma, M.; Liu, C.; Xu, Y.; et al. Linc-pint overexpression inhibits the growth of gastric tumors by downregulating HIF-1α. Mol. Med. Rep. 2019, 20, 2875–2881. [Google Scholar] [CrossRef]
- Huang, R.; Jin, X.; Gao, Y.; Yuan, H.; Wang, F.; Cao, X. DZNep inhibits Hif-1α and Wnt signalling molecules to attenuate the proliferation and invasion of BGC-823 gastric cancer cells. Oncol. Lett. 2019, 18, 4308–4316. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Gao, H.; Liu, K.; Gao, B.; Ren, H.; Li, Z.; Liu, F. The lncRNA ZEB2-AS1 is upregulated in gastric cancer and affects cell proliferation and invasion via miR-143-5p/HIF-1α axis. OncoTargets Ther. 2019, 12, 657–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Xu, J.; Dong, Y.; Huang, B. Down-regulation of HIF-1α inhibits the proliferation, migration, and invasion of gastric cancer by inhibiting PI3K/AKT pathway and VEGF expression. Biosci. Rep. 2018, 38, BSR20180741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Wang, L.; Zhang, J.; Xu, H. Hypoxia-inducible microRNA-224 promotes the cell growth, migration and invasion by directly targeting RASSF8 in gastric cancer. Mol. Cancer 2017, 16, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoeltzing, O.; McCarty, M.F.; Wey, J.S.; Fan, F.; Liu, W.; Belcheva, A.; Bucana, C.D.; Semenza, G.L.; Ellis, L.M. Role of hypoxia-inducible factor 1alpha in gastric cancer cell growth, angiogenesis, and vessel maturation. J. Natl. Cancer Inst. 2004, 96, 946–956. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, W.; Okamoto, I.; Arao, T.; Kuwata, K.; Hatashita, E.; Yamaguchi, H.; Sakai, K.; Yanagihara, K.; Nishio, K.; Nakagawa, K. Antitumor action of the MET tyrosine kinase inhibitor crizotinib (PF-02341066) in gastric cancer positive for MET amplification. Mol. Cancer Ther. 2012, 11, 1557–1564. [Google Scholar] [CrossRef] [Green Version]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Guan, D.; Li, C.; Li, Y.; Li, Y.; Wang, G.; Gao, F.; Li, C. The DpdtbA induced EMT inhibition in gastric cancer cell lines was through ferritinophagy-mediated activation of p53 and PHD2/hif-1α pathway. J. Inorg. Biochem. 2021, 218, 111413. [Google Scholar] [CrossRef]
- Guo, R.; Yang, B. Hypoxia-Induced LXRα Contributes to the Migration and Invasion of Gastric Cancer Cells. Folia Biol. 2021, 67, 91–101. [Google Scholar]
- Xia, X.; Wang, S.; Ni, B.; Xing, S.; Cao, H.; Zhang, Z.; Yu, F.; Zhao, E.; Zhao, G. Hypoxic gastric cancer-derived exosomes promote progression and metastasis via MiR-301a-3p/PHD3/HIF-1α positive feedback loop. Oncogene 2020, 39, 6231–6244. [Google Scholar] [CrossRef]
- Jin, Y.; Che, X.; Qu, X.; Li, X.; Lu, W.; Wu, J.; Wang, Y.; Hou, K.; Li, C.; Zhang, X.; et al. CircHIPK3 Promotes Metastasis of Gastric Cancer via miR-653-5p/miR-338-3p-NRP1 Axis Under a Long-Term Hypoxic Microenvironment. Front. Oncol. 2020, 10, 1612. [Google Scholar] [CrossRef]
- Ding, X.; Huang, R.; Zhong, Y.; Cui, N.; Wang, Y.; Weng, J.; Chen, L.; Zang, M. CTHRC1 promotes gastric cancer metastasis via HIF-1α/CXCR4 signaling pathway. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 123, 109742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, H.Y.; Wu, Y.; Zheng, Z.C.; Guo, S.; Wang, Y.; Yang, D.; Meng, X.Y.; Xu, X.; Zhao, Y. Hypoxia-induced LncRNA PCGEM1 promotes invasion and metastasis of gastric cancer through regulating SNAI1. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2019, 21, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, S.; Wu, Y.; Zheng, Z.C.; Wang, Y.; Zhao, Y. P4HB, a Novel Hypoxia Target Gene Related to Gastric Cancer Invasion and Metastasis. BioMed Res. Int. 2019, 2019, 9749751. [Google Scholar] [CrossRef] [Green Version]
- Ou, X.W.; Wang, R.X.; Kang, M.F.; Shi, J.Q. Hypoxia promotes migration and invasion of gastric cancer cells by activating HIF-1α and inhibiting NDRG2 associated signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8237–8247. [Google Scholar] [PubMed]
- Liu, X.; Wang, Y.; Sun, L.; Min, J.; Liu, J.; Chen, D.; Zhang, H.; Zhang, H.; Zhang, H.; Zhou, Y.; et al. Long noncoding RNA BC005927 upregulates EPHB4 and promotes gastric cancer metastasis under hypoxia. Cancer Sci. 2018, 109, 988–1000. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhao, X.; Zou, H.; Bai, R.; Yang, K.; Tian, Z. Hypoxia Promotes Gastric Cancer Malignancy Partly through the HIF-1α Dependent Transcriptional Activation of the Long Non-coding RNA GAPLINC. Front. Physiol. 2016, 7, 420. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.L.; Liu, D.; Ding, G.R.; Liao, P.F.; Zhang, J.W. Hypoxia-inducible factor-1α and Wnt/β-catenin signaling pathways promote the invasion of hypoxic gastric cancer cells. Mol. Med. Rep. 2015, 12, 3365–3373. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Wang, Y.; Zhou, Y.; Pang, H.; Zhou, J.; Qian, P.; Liu, L.; Zhang, H. Krüppel-like factor 8 involved in hypoxia promotes the invasion and metastasis of gastric cancer via epithelial to mesenchymal transition. Oncol. Rep. 2014, 32, 2397–2404. [Google Scholar] [CrossRef] [Green Version]
- Miyake, S.; Kitajima, Y.; Nakamura, J.; Kai, K.; Yanagihara, K.; Tanaka, T.; Hiraki, M.; Miyazaki, K.; Noshiro, H. HIF-1α is a crucial factor in the development of peritoneal dissemination via natural metastatic routes in scirrhous gastric cancer. Int. J. Oncol. 2013, 43, 1431–1440. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Li, K.; Gu, Y.; Feng, B.; Ren, G.; Zhang, L.; Wang, Y.; Nie, Y.; Fan, D. Transcriptional up-regulation of RhoE by hypoxia-inducible factor (HIF)-1 promotes epithelial to mesenchymal transition of gastric cancer cells during hypoxia. Biochem. Biophys. Res. Commun. 2011, 415, 348–354. [Google Scholar] [CrossRef]
- Liu, L.; Sun, L.; Zhao, P.; Yao, L.; Jin, H.; Liang, S.; Wang, Y.; Zhang, D.; Pang, Y.; Shi, Y.; et al. Hypoxia promotes metastasis in human gastric cancer by up-regulating the 67-kDa laminin receptor. Cancer Sci. 2010, 101, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Rohwer, N.; Lobitz, S.; Daskalow, K.; Jöns, T.; Vieth, M.; Schlag, P.M.; Kemmner, W.; Wiedenmann, B.; Cramer, T.; Höcker, M. HIF-1alpha determines the metastatic potential of gastric cancer cells. Br. J. Cancer 2009, 100, 772–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardini, E.; Menichincheri, M.; Banfi, P.; Bosotti, R.; De Ponti, C.; Pulci, R.; Ballinari, D.; Ciomei, M.; Texido, G.; Degrassi, A.; et al. Entrectinib, a Pan-TRK, ROS1, and ALK Inhibitor with Activity in Multiple Molecularly Defined Cancer Indications. Mol. Cancer Ther. 2016, 15, 628–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiyama, A.; Yamada, T.; Kita, K.; Wang, R.; Arai, S.; Fukuda, K.; Tanimoto, A.; Takeuchi, S.; Tange, S.; Tajima, A.; et al. Foretinib Overcomes Entrectinib Resistance Associated with the NTRK1 G667C Mutation in NTRK1 Fusion-Positive Tumor Cells in a Brain Metastasis Model. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 2357–2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brastianos, P.K.; Ippen, F.M.; Hafeez, U.; Gan, H.K. Emerging Gene Fusion Drivers in Primary and Metastatic Central Nervous System Malignancies: A Review of Available Evidence for Systemic Targeted Therapies. Oncologist 2018, 23, 1063–1075. [Google Scholar] [CrossRef] [Green Version]
- Ou, S.I.; Zhu, V.W. CNS metastasis in ROS1+ NSCLC: An urgent call to action, to understand, and to overcome. Lung Cancer 2019, 130, 201–207. [Google Scholar] [CrossRef]
- Petrocca, F.; Visone, R.; Onelli, M.R.; Shah, M.H.; Nicoloso, M.S.; de Martino, I.; Iliopoulos, D.; Pilozzi, E.; Liu, C.G.; Negrini, M.; et al. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell 2008, 13, 272–286. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhang, H.; Sun, L.; Gao, Y.; Jin, H.; Liang, S.; Wang, Y.; Dong, M.; Shi, Y.; Li, Z.; et al. ERK/MAPK activation involves hypoxia-induced MGr1-Ag/37LRP expression and contributes to apoptosis resistance in gastric cancer. Int. J. Cancer 2010, 127, 820–829. [Google Scholar] [CrossRef]
- Rohwer, N.; Welzel, M.; Daskalow, K.; Pfander, D.; Wiedenmann, B.; Detjen, K.; Cramer, T. Hypoxia-inducible factor 1alpha mediates anoikis resistance via suppression of alpha5 integrin. Cancer Res. 2008, 68, 10113–10120. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Kitajima, Y.; Miyake, S.; Yanagihara, K.; Hara, H.; Nishijima-Matsunobu, A.; Baba, K.; Shida, M.; Wakiyama, K.; Nakamura, J.; et al. The Apoptotic Effect of HIF-1α Inhibition Combined with Glucose plus Insulin Treatment on Gastric Cancer under Hypoxic Conditions. PLoS ONE 2015, 10, e0137257. [Google Scholar] [CrossRef]
- Hao, Y.X.; Zhong, H.; Yu, P.W.; Zhang, C.; Zeng, D.Z.; Shi, Y.; Tang, B. Effects of HIF-1alpha on human gastric cancer cell apoptosis at different CO(2) pressures. Clin. Exp. Med. 2009, 9, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Crisafulli, G.; Sogari, A.; Reilly, N.M.; Arena, S.; Lamba, S.; Bartolini, A.; Amodio, V.; Magrì, A.; Novara, L.; et al. Adaptive mutability of colorectal cancers in response to targeted therapies. Science 2019, 366, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Huangyang, P.; Wang, Y.; Xue, L.; Devericks, E.; Nguyen, H.G.; Yu, X.; Oses-Prieto, J.A.; Burlingame, A.L.; Miglani, S.; et al. ERα is an RNA-binding protein sustaining tumor cell survival and drug resistance. Cell 2021, 184, 5215–5229.e17. [Google Scholar] [CrossRef]
- Okazaki, M.; Fushida, S.; Tsukada, T.; Kinoshita, J.; Oyama, K.; Miyashita, T.; Ninomiya, I.; Harada, S.; Ohta, T. The effect of HIF-1α and PKM1 expression on acquisition of chemoresistance. Cancer Manag. Res. 2018, 10, 1865–1874. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Sun, L.; Guo, D.; Wu, Z.; Chen, W. Co-delivery of hypoxia inducible factor-1α small interfering RNA and 5-fluorouracil to overcome drug resistance in gastric cancer SGC-7901 cells. J. Gene Med. 2017, 19, e2998. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.; Tan, B.B.; Fan, L.Q.; Yang, P.G.; Tian, Y. HIF-1α Induces Multidrug Resistance in Gastric Cancer Cells by Inducing MiR-27a. PLoS ONE 2015, 10, e0132746. [Google Scholar] [CrossRef]
- Xuan, Y.; Hur, H.; Ham, I.H.; Yun, J.; Lee, J.Y.; Shim, W.; Kim, Y.B.; Lee, G.; Han, S.U.; Cho, Y.K. Dichloroacetate attenuates hypoxia-induced resistance to 5-fluorouracil in gastric cancer through the regulation of glucose metabolism. Exp. Cell Res. 2014, 321, 219–230. [Google Scholar] [CrossRef]
- Sun, X.P.; Dong, X.; Lin, L.; Jiang, X.; Wei, Z.; Zhai, B.; Sun, B.; Zhang, Q.; Wang, X.; Jiang, H.; et al. Up-regulation of survivin by AKT and hypoxia-inducible factor 1α contributes to cisplatin resistance in gastric cancer. FEBS J. 2014, 281, 115–128. [Google Scholar] [CrossRef]
- Rohwer, N.; Dame, C.; Haugstetter, A.; Wiedenmann, B.; Detjen, K.; Schmitt, C.A.; Cramer, T. Hypoxia-inducible factor 1alpha determines gastric cancer chemosensitivity via modulation of p53 and NF-kappaB. PLoS ONE 2010, 5, e12038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Sun, L.; Zhang, H.; Li, Z.; Ning, X.; Shi, Y.; Guo, C.; Han, S.; Wu, K.; Fan, D. Hypoxia-mediated up-regulation of MGr1-Ag/37LRP in gastric cancers occurs via hypoxia-inducible-factor 1-dependent mechanism and contributes to drug resistance. Int. J. Cancer 2009, 124, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ning, X.; Sun, L.; Zhang, H.; Shi, Y.; Guo, C.; Han, S.; Liu, J.; Sun, S.; Han, Z.; et al. Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced chemoresistance in gastric cancer. Cancer Sci. 2008, 99, 121–128. [Google Scholar] [PubMed]
- Zhou, X.; Ma, W.; Li, X.; Xu, J. Glaucocalyxin a prevents hypoxia-induced epithelial-mesenchymal transition in human gastric cancer cells through the PI3K/Akt signaling pathway. J. Recept. Signal Transduct. Res. 2020, 42, 109–116. [Google Scholar] [CrossRef]
- Chen, M.; Lu, J.; Wei, W.; Lv, Y.; Zhang, X.; Yao, Y.; Wang, L.; Ling, T.; Zou, X. Effects of proton pump inhibitors on reversing multidrug resistance via downregulating V-ATPases/PI3K/Akt/mTOR/HIF-1α signaling pathway through TSC1/2 complex and Rheb in human gastric adenocarcinoma cells in vitro and in vivo. OncoTargets Ther. 2018, 11, 6705–6722. [Google Scholar] [CrossRef] [Green Version]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef] [Green Version]
- Lieu, C.H.; Messersmith, W.A. Cetuximab or Bevacizumab With First-Line Chemotherapy in Advanced KRAS Wild-Type Colorectal Cancer: No Difference, but Not the Same. JAMA 2017, 317, 2376–2378. [Google Scholar] [CrossRef]
- Li, Z.; Fan, H.; Cao, J.; Sun, G.; Sen, W.; Lv, J.; Xuan, Z.; Xia, Y.; Wang, L.; Zhang, D.; et al. Natriuretic peptide receptor a promotes gastric malignancy through angiogenesis process. Cell Death Dis. 2021, 12, 968. [Google Scholar] [CrossRef]
- Mu, G.; Zhu, Y.; Dong, Z.; Shi, L.; Deng, Y.; Li, H. Calmodulin 2 Facilitates Angiogenesis and Metastasis of Gastric Cancer via STAT3/HIF-1A/VEGF-A Mediated Macrophage Polarization. Front. Oncol. 2021, 11, 727306. [Google Scholar] [CrossRef]
- Tang, E.; Wang, Y.; Liu, T.; Yan, B. Gastrin promotes angiogenesis by activating HIF-1α/β-catenin/VEGF signaling in gastric cancer. Gene 2019, 704, 42–48. [Google Scholar] [CrossRef]
- Fang, S.; Hong, H.; Li, L.; He, D.; Xu, Z.; Zuo, S.; Han, J.; Wu, Q.; Dai, Z.; Cai, W.; et al. Plasminogen kringle 5 suppresses gastric cancer via regulating HIF-1α and GRP78. Cell Death Dis. 2017, 8, e3144. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.S.; Cho, S.J.; Park, J.; Choi, Y.; Lee, J.S.; Youn, H.D.; Kim, W.H.; Kim, M.A.; Park, J.W.; Lee, B.L. Hypoxic inactivation of glycogen synthase kinase-3β promotes gastric tumor growth and angiogenesis by facilitating hypoxia-inducible factor-1 signaling. Acta Pathol. Microbiol. Immunol. Scand. 2016, 124, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.K.; Lee, S.H.; Kim, M.J.; Lee, Y.M. MicroRNA-382 induced by HIF-1α is an angiogenic miR targeting the tumor suppressor phosphatase and tensin homolog. Nucleic Acids Res. 2014, 42, 8062–8072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Bae, S.C.; Kim, K.W.; Lee, Y.M. RUNX3 inhibits hypoxia-inducible factor-1α protein stability by interacting with prolyl hydroxylases in gastric cancer cells. Oncogene 2014, 33, 1458–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.L.; Kim, W.H.; Jung, J.; Cho, S.J.; Park, J.W.; Kim, J.; Chung, H.Y.; Chang, M.S.; Nam, S.Y. A hypoxia-independent up-regulation of hypoxia-inducible factor-1 by AKT contributes to angiogenesis in human gastric cancer. Carcinogenesis 2008, 29, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Lang, S.A.; Klein, D.; Moser, C.; Gaumann, A.; Glockzin, G.; Dahlke, M.H.; Dietmaier, W.; Bolder, U.; Schlitt, H.J.; Geissler, E.K.; et al. Inhibition of heat shock protein 90 impairs epidermal growth factor-mediated signaling in gastric cancer cells and reduces tumor growth and vascularization in vivo. Mol. Cancer Ther. 2007, 6, 1123–1132. [Google Scholar] [CrossRef] [Green Version]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [Green Version]
- Pastushenko, I.; Mauri, F.; Song, Y.; de Cock, F.; Meeusen, B.; Swedlund, B.; Impens, F.; Van Haver, D.; Opitz, M.; Thery, M.; et al. Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis. Nature 2021, 589, 448–455. [Google Scholar] [CrossRef]
- Boumahdi, S.; Driessens, G.; Lapouge, G.; Rorive, S.; Nassar, D.; Le Mercier, M.; Delatte, B.; Caauwe, A.; Lenglez, S.; Nkusi, E.; et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature 2014, 511, 246–250. [Google Scholar] [CrossRef]
- Miao, Y.; Yang, H.; Levorse, J.; Yuan, S.; Polak, L.; Sribour, M.; Singh, B.; Rosenblum, M.D.; Fuchs, E. Adaptive Immune Resistance Emerges from Tumor-Initiating Stem Cells. Cell 2019, 177, 1172–1186.e14. [Google Scholar] [CrossRef]
- Fu, T.; Coulter, S.; Yoshihara, E.; Oh, T.G.; Fang, S.; Cayabyab, F.; Zhu, Q.; Zhang, T.; Leblanc, M.; Liu, S.; et al. FXR Regulates Intestinal Cancer Stem Cell Proliferation. Cell 2019, 176, 1098–1112.e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; Luo, Y.; Xiao, K. A-Kinase Interacting Protein 1 Promotes Cell Invasion and Stemness via Activating HIF-1α and β-Catenin Signaling Pathways in Gastric Cancer Under Hypoxia Condition. Front. Oncol. 2021, 11, 798557. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.F.; Wang, Z.N.; Zhao, T.T.; Xu, Y.Y.; Gao, J.; Miao, F.; Xu, H.M. Peritoneal milky spots serve as a hypoxic niche and favor gastric cancer stem/progenitor cell peritoneal dissemination through hypoxia-inducible factor 1α. Stem Cells 2014, 32, 3062–3074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Leone, R.D.; Zhao, L.; Englert, J.M.; Sun, I.M.; Oh, M.H.; Sun, I.H.; Arwood, M.L.; Bettencourt, I.A.; Patel, C.H.; Wen, J.; et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 2019, 366, 1013–1021. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Yoon, H.; Ringel, A.E.; Jeanfavre, S.; Clish, C.B.; Haigis, M.C. Metabolic recycling of ammonia via glutamate dehydrogenase supports breast cancer biomass. Science 2017, 358, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Ringel, A.E.; Drijvers, J.M.; Baker, G.J.; Catozzi, A.; García-Cañaveras, J.C.; Gassaway, B.M.; Miller, B.C.; Juneja, V.R.; Nguyen, T.H.; Joshi, S.; et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell 2020, 183, 1848–1866.e26. [Google Scholar] [CrossRef]
- Sadik, A.; Somarribas Patterson, L.F.; Öztürk, S.; Mohapatra, S.R.; Panitz, V.; Secker, P.F.; Pfänder, P.; Loth, S.; Salem, H.; Prentzell, M.T.; et al. IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression. Cell 2020, 182, 1252–1270.e34. [Google Scholar] [CrossRef]
- Koundouros, N.; Karali, E.; Tripp, A.; Valle, A.; Inglese, P.; Perry, N.J.S.; Magee, D.J.; Anjomani Virmouni, S.; Elder, G.A.; Tyson, A.L.; et al. Metabolic Fingerprinting Links Oncogenic PIK3CA with Enhanced Arachidonic Acid-Derived Eicosanoids. Cell 2020, 181, 1596–1611.e27. [Google Scholar] [CrossRef]
- Baksh, S.C.; Finley, L.W.S. Metabolic diversity drives cancer cell invasion. Nature 2022, 605, 627–628. [Google Scholar] [CrossRef]
- Lien, E.C.; Westermark, A.M.; Zhang, Y.; Yuan, C.; Li, Z.; Lau, A.N.; Sapp, K.M.; Wolpin, B.M.; Vander Heiden, M.G. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature 2021, 599, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, G.; Longo, V.D. Diet comparison suggests a lipid imbalance can slow tumour growth. Nature 2021, 599, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yang, Y.; Zhang, B.; Zhang, Z.S.; Zhou, S.; Jia, G.Z.; Liu, S.Q.; He, X.L.; He, J.X.; Wang, N. EDDM3A drives gastric cancer progression by promoting HIF-1α-dependent aerobic glycolysis. Oncogenesis 2022, 11, 3. [Google Scholar] [CrossRef]
- Wang, X.H.; Jiang, Z.H.; Yang, H.M.; Zhang, Y.; Xu, L.H. Hypoxia-induced FOXO4/LDHA axis modulates gastric cancer cell glycolysis and progression. Clin. Transl. Med. 2021, 11, e279. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, H.; Zeng, Q.; Xu, P.; Liu, M.; Yang, N. Circular RNA circ-MAT2B facilitates glycolysis and growth of gastric cancer through regulating the miR-515-5p/HIF-1α axis. Cancer Cell Int. 2020, 20, 171. [Google Scholar] [CrossRef]
- Xu, G.; Li, M.; Wu, J.; Qin, C.; Tao, Y.; He, H. Circular RNA circNRIP1 Sponges microRNA-138-5p to Maintain Hypoxia-Induced Resistance to 5-Fluorouracil Through HIF-1α-Dependent Glucose Metabolism in Gastric Carcinoma. Cancer Manag. Res. 2020, 12, 2789–2802. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Yang, H.; Zhang, X.; Wang, Z.; Rong, R.; Wang, X. Histone deacetylase-1 as a prognostic factor and mediator of gastric cancer progression by enhancing glycolysis. Hum. Pathol. 2019, 85, 194–201. [Google Scholar] [CrossRef]
- Gan, L.; Meng, J.; Xu, M.; Liu, M.; Qi, Y.; Tan, C.; Wang, Y.; Zhang, P.; Weng, W.; Sheng, W.; et al. Extracellular matrix protein 1 promotes cell metastasis and glucose metabolism by inducing integrin β4/FAK/SOX2/HIF-1α signaling pathway in gastric cancer. Oncogene 2018, 37, 744–755. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Bai, R.; Yang, K.; Tian, Z. MiR-186 inhibited aerobic glycolysis in gastric cancer via HIF-1α regulation. Oncogenesis 2017, 6, e318. [Google Scholar] [CrossRef] [Green Version]
- Song, I.S.; Wang, A.G.; Yoon, S.Y.; Kim, J.M.; Kim, J.H.; Lee, D.S.; Kim, N.S. Regulation of glucose metabolism-related genes and VEGF by HIF-1alpha and HIF-1beta, but not HIF-2alpha, in gastric cancer. Exp. Mol. Med. 2009, 41, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Neoptolemos, J.P.; Moore, M.J.; Cox, T.F.; Valle, J.W.; Palmer, D.H.; McDonald, A.C.; Carter, R.; Tebbutt, N.C.; Dervenis, C.; Smith, D.; et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: The ESPAC-3 periampullary cancer randomized trial. JAMA 2012, 308, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Cadman, E.; Heimer, R.; Davis, L. Enhanced 5-fluorouracil nucleotide formation after methotrexate administration: Explanation for drug synergism. Science 1979, 205, 1135–1137. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, J.; Tan, S.; Liu, W.; Wang, R.; Chen, C. PLGA sustained-release microspheres loaded with an insoluble small-molecule drug: Microfluidic-based preparation, optimization, characterization, and evaluation in vitro and in vivo. Drug Deliv. 2022, 29, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Lao, Y.; Skiba, M.A.; Chun, S.W.; Narayan, A.R.H.; Smith, J.L. Structural Basis for Control of Methylation Extent in Polyketide Synthase Metal-Dependent C-Methyltransferases. ACS Chem. Biol. 2022. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, Y.; Cai, D.; Guo, Q.; Wang, J.; Lei, L.; Li, X.; Shi, S. Penetrating-peptide-mediated non-invasive Axitinib delivery for anti-neovascularisation. J. Control. Release Off. J. Control. Release Soc. 2022, 347, 449–459. [Google Scholar] [CrossRef]
- Ovens, A.J.; Gee, Y.S.; Ling, N.X.Y.; Yu, D.; Hardee, J.P.; Chung, J.D.; Ngoei, K.R.W.; Waters, N.J.; Hoffman, N.J.; Scott, J.W.; et al. Structure-function analysis of the AMPK activator SC4 and identification of a potent pan AMPK activator. Biochem. J. 2022, 479, 1181–1204. [Google Scholar] [CrossRef]
- Ahamad, S.; Mathew, S.; Khan, W.A.; Mohanan, K. Development of small-molecule PCSK9 inhibitors for the treatment of hypercholesterolemia. Drug Discov. Today 2022, 27, 1332–1349. [Google Scholar] [CrossRef]
- Lim, G.B. Novel factor XIa inhibitor reduces bleeding compared with apixaban in atrial fibrillation. Nat. Rev. Cardiol. 2022, 19, 350. [Google Scholar] [CrossRef]
- Collet, J.P.; Van Belle, E.; Thiele, H.; Berti, S.; Lhermusier, T.; Manigold, T.; Neumann, F.J.; Gilard, M.; Attias, D.; Beygui, F.; et al. Apixaban vs. standard of care after transcatheter aortic valve implantation: The ATLANTIS trial. Eur. Heart J. 2022. [Google Scholar] [CrossRef]
- Dawwas, G.K.; Cuker, A.; Connors, J.M.; Barnes, G.D. Apixaban has superior effectiveness and safety compared to rivaroxaban in patients with commercial healthcare coverage: A population-based analysis in response to CVS 2022 formulary changes. Am. J. Hematol. 2022, 97, E173–E176. [Google Scholar] [CrossRef]
- Wetmore, J.B.; Weinhandl, E.D.; Yan, H.; Reyes, J.L.; Herzog, C.A.; Roetker, N.S. Apixaban Dosing Patterns Versus Warfarin in Patients With Nonvalvular Atrial Fibrillation Receiving Dialysis: A Retrospective Cohort Study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2022. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Caso, V.; Connolly, S.J.; Fox, K.A.A.; Oldgren, J.; Jones, W.S.; Gorog, D.A.; Durdil, V.; Viethen, T.; Neumann, C.; et al. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): A multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet 2022, 399, 1383–1390. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, H.G. Apigenin Induces Autophagy and Cell Death by Targeting EZH2 under Hypoxia Conditions in Gastric Cancer Cells. Int. J. Mol. Sci. 2021, 22, 13455. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Tanaka, T.; Matsufuji, S.; Yamada, K.; Ito, K.; Kitagawa, H.; Okuyama, K.; Kitajima, Y.; Noshiro, H. Antitumor effects of low-dose tipifarnib on the mTOR signaling pathway and reactive oxygen species production in HIF-1α-expressing gastric cancer cells. FEBS Open Bio 2021, 11, 1465–1475. [Google Scholar] [CrossRef]
- Huang, Y.N.; Xu, Y.Y.; Ma, Q.; Li, M.Q.; Guo, J.X.; Wang, X.; Jin, X.; Shang, J.; Jiao, L.X. Dextran Sulfate Effects EMT of Human Gastric Cancer Cells by Reducing HIF-1α/ TGF-β. J. Cancer 2021, 12, 3367–3377. [Google Scholar] [CrossRef]
- Li, X.Y.; Shi, L.X.; Yao, X.M.; Jing, M.; Li, Q.Q.; Wang, Y.L.; Li, Q.S. Functional vinorelbine plus schisandrin B liposomes destroying tumor metastasis in treatment of gastric cancer. Drug Dev. Ind. Pharm. 2021, 47, 100–112. [Google Scholar] [CrossRef]
- Xu, Q.H.; Xiao, Y.; Li, X.Q.; Fan, L.; Zhou, C.C.; Cheng, L.; Jiang, Z.D.; Wang, G.H. Resveratrol Counteracts Hypoxia-Induced Gastric Cancer Invasion and EMT through Hedgehog Pathway Suppression. Anti-Cancer Agents Med. Chem. 2020, 20, 1105–1114. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Q.; Yang, W.; Wu, T.; Lu, X. Oleanolic acid reduces aerobic glycolysis-associated proliferation by inhibiting yes-associated protein in gastric cancer cells. Gene 2019, 712, 143956. [Google Scholar] [CrossRef]
- Li, B.; Qu, G. Inhibition of the hypoxia-induced factor-1α and vascular endothelial growth factor expression through ginsenoside Rg3 in human gastric cancer cells. J. Cancer Res. Ther. 2019, 15, 1642–1646. [Google Scholar]
- Fu, J.D.; Yao, J.J.; Wang, H.; Cui, W.G.; Leng, J.; Ding, L.Y.; Fan, K.Y. Effects of EGCG on proliferation and apoptosis of gastric cancer SGC7901 cells via down-regulation of HIF-1α and VEGF under a hypoxic state. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 155–161. [Google Scholar]
- Wang, S.J.; Zhao, J.K.; Ren, S.; Sun, W.W.; Zhang, W.J.; Zhang, J.N. Wogonin affects proliferation and the energy metabolism of SGC-7901 and A549 cells. Exp. Ther. Med. 2019, 17, 911–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Li, Z.; Xiong, R.; Liu, J.; Liu, R.; Peng, J.; Chen, Y.; Lei, X.; Cao, X.; Zheng, X.; et al. FS-7 inhibits MGC-803 cells growth in vitro and in vivo via down-regulating glycolysis. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 109, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zheng, N.; Wang, X.; Tang, C.; Yan, P.; Zhou, H.B.; Huang, J. A novel HDAC6 inhibitor exerts an anti-cancer effect by triggering cell cycle arrest and apoptosis in gastric cancer. Eur. J. Pharmacol. 2018, 828, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jin, X.; Huang, Y.; Dong, J.; Wang, H.; Wang, X.; Cao, X. Inhibition of peritoneal metastasis of human gastric cancer cells by dextran sulphate through the reduction in HIF-1α and ITGβ1 expression. Oncol. Rep. 2016, 35, 2624–2634. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Sung, B.; Kang, Y.J.; Hwang, S.Y.; Kim, M.J.; Yoon, J.H.; Im, E.; Kim, N.D. Sulforaphane inhibits hypoxia-induced HIF-1α and VEGF expression and migration of human colon cancer cells. Int. J. Oncol. 2015, 47, 2226–2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Liu, R.; Li, J.; Mao, J.; Lei, Y.; Wu, J.; Zeng, J.; Zhang, T.; Wu, H.; Chen, L.; et al. Quercetin induces protective autophagy in gastric cancer cells: Involvement of Akt-mTOR- and hypoxia-induced factor 1α-mediated signaling. Autophagy 2011, 7, 966–978. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, K.; Bai, F.; Zhai, H.; Xie, H.; Du, Y.; Liang, J.; Han, S.; Chen, Y.; Lin, T.; et al. Celecoxib could reverse the hypoxia-induced Angiopoietin-2 upregulation in gastric cancer. Cancer Lett. 2006, 242, 20–27. [Google Scholar] [CrossRef]
- Jonasch, E.; Donskov, F.; Iliopoulos, O.; Rathmell, W.K.; Narayan, V.K.; Maughan, B.L.; Oudard, S.; Else, T.; Maranchie, J.K.; Welsh, S.J.; et al. Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease. N. Engl. J. Med. 2021, 385, 2036–2046. [Google Scholar] [CrossRef]
- Kamihara, J.; Hamilton, K.V.; Pollard, J.A.; Clinton, C.M.; Madden, J.A.; Lin, J.; Imamovic, A.; Wall, C.B.; Wassner, A.J.; Weil, B.R.; et al. Belzutifan, a Potent HIF2α Inhibitor, in the Pacak-Zhuang Syndrome. N. Engl. J. Med. 2021, 385, 2059–2065. [Google Scholar] [CrossRef]
- Deeks, E.D. Belzutifan: First Approval. Drugs 2021, 81, 1921–1927. [Google Scholar] [CrossRef]
- FDA OK’s HIF2α Inhibitor Belzutifan. Cancer Discov. 2021, 11, 2360–2361. [CrossRef] [PubMed]
- Romero, D. Belzutifan has potential in RCC. Nat. Rev. Clin. Oncol. 2021, 18, 322. [Google Scholar] [CrossRef] [PubMed]
- The HIF2α Inhibitor Belzutifan Shows Signs of Efficacy in Kidney Cancer. Cancer Discov. 2021, 11, 1319. [CrossRef]
- Choueiri, T.K.; Bauer, T.M.; Papadopoulos, K.P.; Plimack, E.R.; Merchan, J.R.; McDermott, D.F.; Michaelson, M.D.; Appleman, L.J.; Thamake, S.; Perini, R.F.; et al. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: A phase 1 trial and biomarker analysis. Nat. Med. 2021, 27, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.W.; Lam, Y.H.; Ren, S.; Strotman, N.A. Late-Stage Carbon Isotope Exchange of Aryl Nitriles through Ni-Catalyzed C-CN Bond Activation. J. Am. Chem. Soc. 2021, 143, 4817–4823. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).