Psycho-Neuro-Endocrine-Immunology: A Role for Melatonin in This New Paradigm
Abstract
1. Introduction
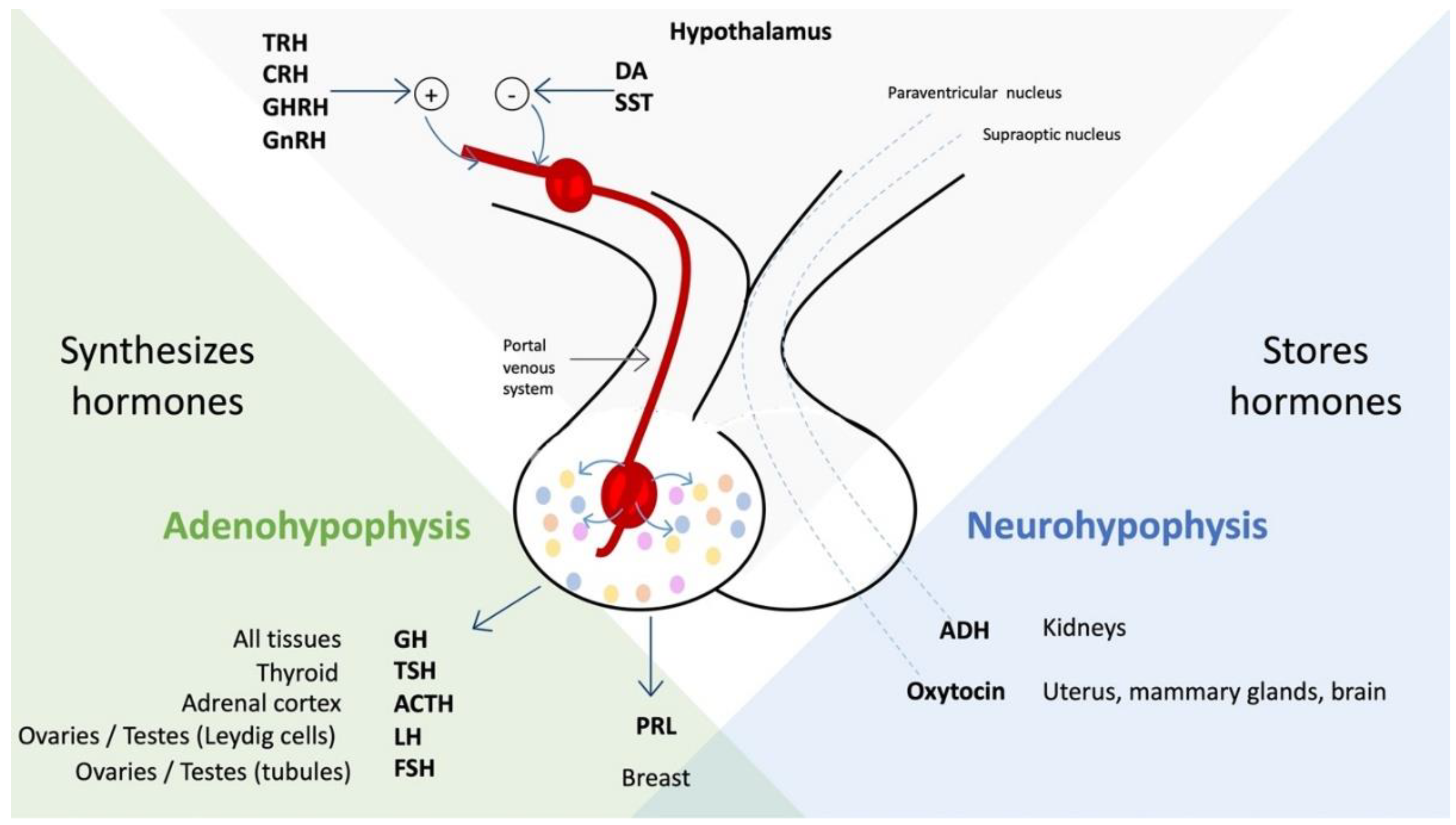
- (1)
- By direct secretion, passing through the neurohypophysis, of hormones arginine vasopressin and oxytocin, which affect the kidney, the gravid uterus, the postpartum mammary gland, and the brain [3].
- (2)
- Hormones that influence the adenohypophysis to regulate the activity of the gonads, uterus, thyroid, adrenal cortex, liver, and bones [3].
- (3)
2. Development of Psychoneuroendocrinoimmunology
3. Scopes and Limitations of Psychoneuroendocrinoimmunology
- (1)
- The identity between phytochemistry and the spiritual expansion of consciousness, which the culture of the West had opposed each other [12].
- (2)
- The distinction between the psyche and Spirit, thus laying the foundations for the elaboration of a Clinic of the Spirit, thus reappropriating as science the dimension of the Spirit, which had been hostage to Religion for centuries and centuries, having reached the self-conscious awareness of being a being of a spiritual nature finally.
- (3)
- The reinterpretation of the human being in terms of the unity of a trinity, composed of the biological body, the psyche, and the Spirit [13].
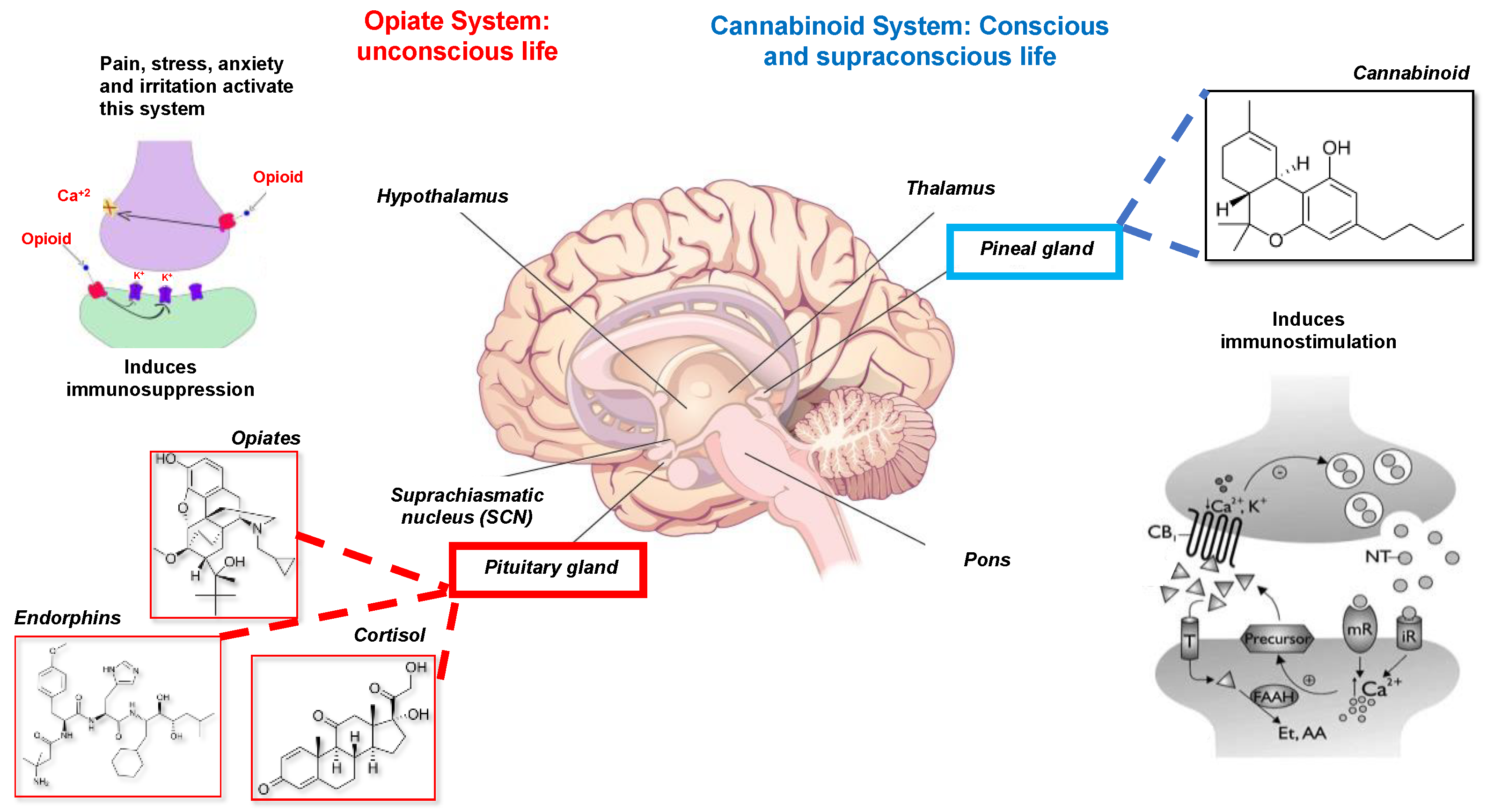
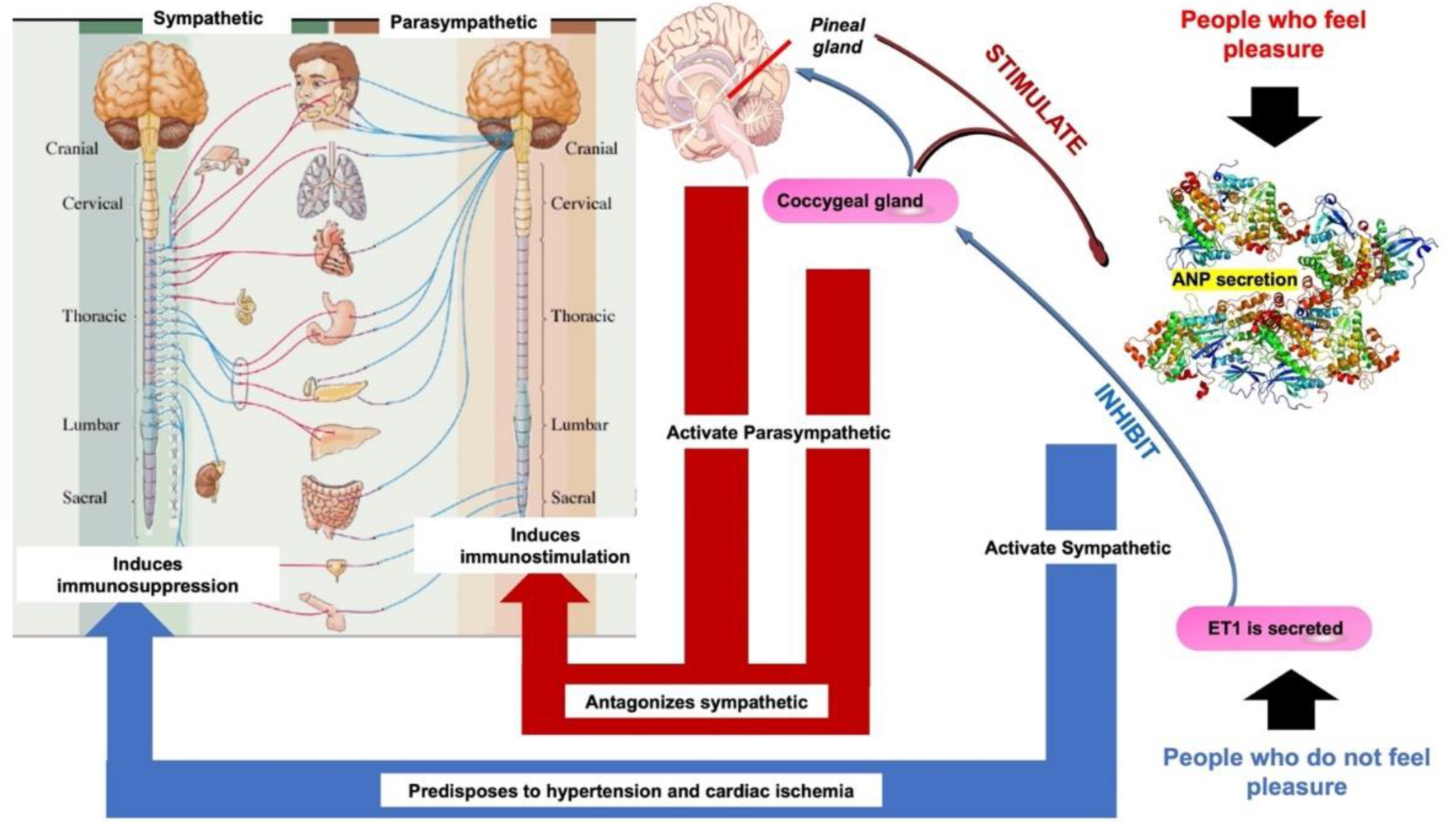
- The opiate system is connected to the unconscious life, to the pituitary gland or hypophysis located in the brain’s center. This system is activated under stress, pain, anxiety, and irritated ability and induces immunosuppression or disease. It is mediated by catecholamines, adrenal steroids, opioids, endorphins, and dynorphins [21].
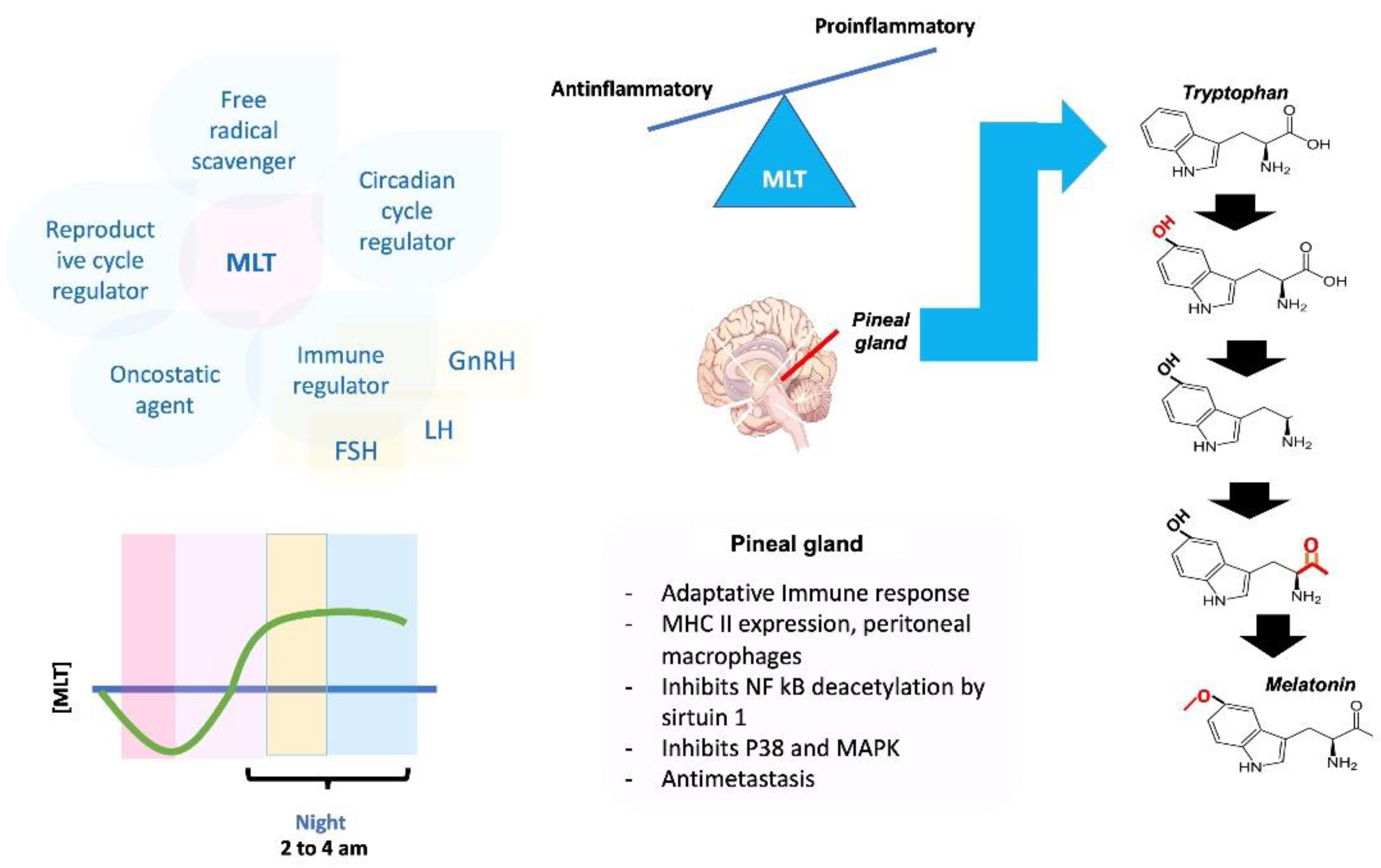
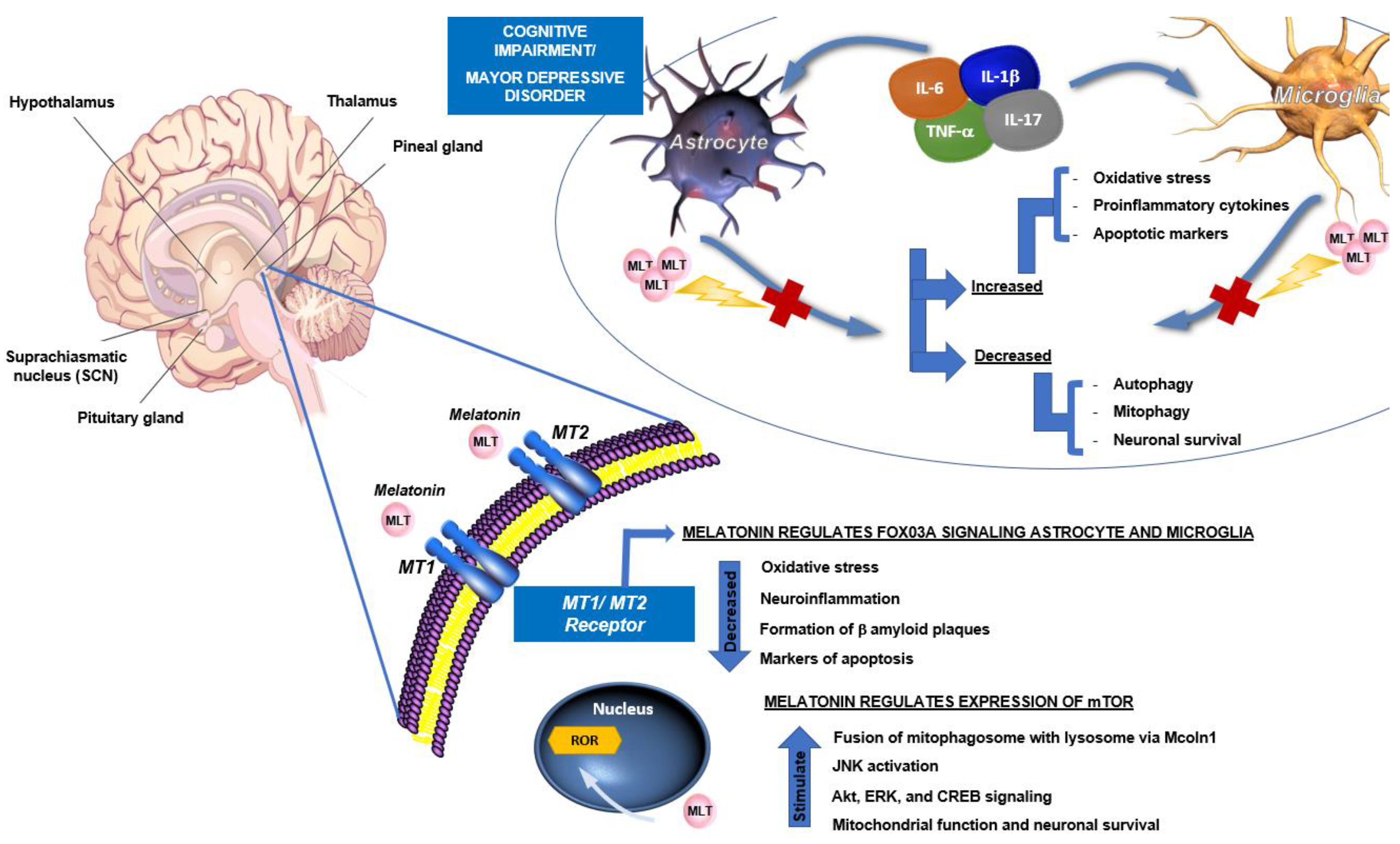
4. Role of Melatonin in the Neuroendocrine and Immune Systems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzalez-Diaz, S.N.; Arias-Cruz, A.; Elizondo-Villarreal, B.; Monge-Ortega, O.P. Psychoneuroimmunoendocrinology: Clinical implications. World Allergy Organ. J. 2017, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Bottaccioli, F.; Bottaccioli, A.G. Nervous regulation. In Psycho-Neuro-Endocrine-Immunology and Science of the Integrated Care the Manual; Bottaccioli, F., Bottaccioli, A.G., Eds.; Edra S.p.A.: Milano, Italy, 2020; pp. 103–126. [Google Scholar]
- Daniel, P.M. Anatomy of the hypothalamus and pituitary gland. J. Clin. Pathol. Suppl. (Assoc. Clin. Pathol.) 1976, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bottaccioli, F.; Bottaccioli, A.G. Neuroendocrine regulation. In Psycho-Neuro-Endocrine-Immunology and Science of the Integrated Care the Manual; Bottaccioli, F., Bottaccioli, A.G., Eds.; Edra S.p.A.: Milano, Italy, 2020; pp. 131–151. [Google Scholar]
- Feldt-Rasmussen, U.; Effraimidis, G.; Klose, M. The hypothalamus-pituitary-thyroid (HPT)-axis and its role in physiology and pathophysiology of other hypothalamus-pituitary functions. Mol. Cell. Endocrinol. 2021, 525, 111173. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; McCarthy, M.M. Organized for sex–steroid hormones and the developing hypothalamus. Eur. J. Neurosci. 2010, 32, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.R.; Prokosch, M.L.; Airington, Z. PsychoBehavioroimmunology: Connecting the Behavioral Immune System to Its Physiological Foundations. Front. Psychol. 2019, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Bottaccioli, F.; Bottaccioli, A.G. The crisis of the reductionist model in medicine and in psychology: PNEI as a new paradigm. In Psycho-Neuro-Endocrine-Immunology and Science of the Integrated Care the Manual; Bottaccioli, F., Bottaccioli, A.G., Eds.; Edra S.p.A.: Milano, Italy, 2020; pp. 25–33. [Google Scholar]
- Kautz, M.M. Applications of psychoneuroimmunology models of toxic stress in prevention and intervention efforts across early development. Brain Behav. Immun. Health 2021, 16, 100322. [Google Scholar] [CrossRef]
- Lu, S.; Wei, F.; Li, G. The evolution of the concept of stress and the framework of the stress system. Cell Stress 2021, 5, 76–85. [Google Scholar] [CrossRef]
- Caulfield, J.I. Anxiety, depression, and asthma: New perspectives and approaches for psychoneuroimmunology research. Brain Behav. Immun. Health 2021, 18, 100360. [Google Scholar] [CrossRef]
- Cordell, G.A. Phytochemistry and traditional medicine—A revolution in process. Phytochem. Lett. 2011, 4, 391–398. [Google Scholar] [CrossRef]
- Taheri, M.A.; Biriya, A. Definition of ‘Psyche’, Psychological or Emotional Body as Approached by Psymentology. Procedia Soc. Behav. Sci. 2013, 84, 1651–1659. [Google Scholar] [CrossRef][Green Version]
- Aulinas, A. Physiology of the Pineal Gland and Melatonin. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Berridge, K.C.; Kringelbach, M.L. Pleasure systems in the brain. Neuron 2015, 86, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.M.; Paradiso, S.; Marvel, C.L.; Pierson, R.; Boles Ponto, L.L.; Hichwa, R.D.; Robinson, R.G. The cerebellum and emotional experience. Neuropsychologia 2007, 45, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Geenen, V. The thymus and the science of self. Semin. Immunopathol. 2021, 43, 5–14. [Google Scholar] [CrossRef]
- Dal Lin, C.; Tona, F.; Osto, E. The Heart as a Psychoneuroendocrine and Immunoregulatory Organ. In Sex-Specific Analysis of Cardiovascular Function; Kerkhof, P.L.M., Miller, V.M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 225–239. [Google Scholar] [CrossRef]
- Bechter, K.; Brown, D.; Najjar, S. Editorial: Recent Advances in Psychiatry From Psycho-Neuro-Immunology Research: Autoimmune Encephalitis, Autoimmune Encephalopathy, and Mild Encephalitis. Front. Psychiatry 2019, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Sehlen, S.; Lenk, M.; Herschbach, P.; Aydemir, U.; Dellian, M.; Schymura, B.; Hollenhorst, H.; Duhmke, E. Depressive symptoms during and after radiotherapy for head and neck cancer. Head Neck 2003, 25, 1004–1018. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Messina, G.; Lissoni, A.; Franco, R. The psychoneuroendocrine-immunotherapy of cancer: Historical evolution and clinical results. J. Res. Med. Sci. 2017, 22, 45. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.; Salazar, J.; Galban, N.; Rojas, M.; Ariza, D.; Chavez-Castillo, M.; Nava, M.; Riano-Garzon, M.E.; Diaz-Camargo, E.A.; Medina-Ortiz, O.; et al. Psycho-Neuro-Endocrine-Immunological Basis of the Placebo Effect: Potential Applications beyond Pain Therapy. Int. J. Mol. Sci. 2022, 23, 4196. [Google Scholar] [CrossRef]
- Lissoni, P.; Tassoni, S.; Messina, G.; Tomasi, M.; Porro, G.; Di Fede, G. Neuroimmune Therapy of Autoimmune Diseases with the Pineal Hormone Melatonin Plus Cannabidiol and its Effects on Auto-Antibody Secretion. Clin. Res. Neurol. 2022, 4, 4. [Google Scholar] [CrossRef]
- Satsangi, A.K.; Brugnoli, M.P. Anxiety and psychosomatic symptoms in palliative care: From neuro-psychobiological response to stress, to symptoms’ management with clinical hypnosis and meditative states. Ann. Palliat. Med. 2018, 7, 75–111. [Google Scholar] [CrossRef] [PubMed]
- Ashley, N.T.; Demas, G.E. Neuroendocrine-immune circuits, phenotypes, and interactions. Horm. Behav. 2017, 87, 25–34. [Google Scholar] [CrossRef]
- Roman, M.; Irwin, M.R. Novel neuroimmunologic therapeutics in depression: A clinical perspective on what we know so far. Brain Behav. Immun. 2020, 83, 7–21. [Google Scholar] [CrossRef]
- Luo, F.; Sandhu, A.F.; Rungratanawanich, W.; Williams, G.E.; Akbar, M.; Zhou, S.; Song, B.J.; Wang, X. Melatonin and Autophagy in Aging-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 7174. [Google Scholar] [CrossRef] [PubMed]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: Molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef]
- Gengatharan, A.; Malvaut, S.; Marymonchyk, A.; Ghareghani, M.; Snapyan, M.; Fischer-Sternjak, J.; Ninkovic, J.; Gotz, M.; Saghatelyan, A. Adult neural stem cell activation in mice is regulated by the day/night cycle and intracellular calcium dynamics. Cell 2021, 184, 709–722.e13. [Google Scholar] [CrossRef] [PubMed]
- Prada, C.; Udin, S.B. Melatonin decreases calcium levels in retinotectal axons of Xenopus laevis by indirect activation of group III metabotropic glutamate receptors. Brain Res. 2005, 1053, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Rahman, S.U.; Hao, Q.; Li, W.; Liu, Z.; Ali Shah, F.; Murtaza, I.; Zhang, Z.; Yang, X.; Liu, G.; et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J. Pineal Res. 2020, 69, e12667. [Google Scholar] [CrossRef]
- Chen, C.; Yang, C.; Wang, J.; Huang, X.; Yu, H.; Li, S.; Li, S.; Zhang, Z.; Liu, J.; Yang, X.; et al. Melatonin ameliorates cognitive deficits through improving mitophagy in a mouse model of Alzheimer’s disease. J. Pineal Res. 2021, 71, e12774. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Voiculescu, S.E.; Zygouropoulos, N.; Zahiu, C.D.; Zagrean, A.M. Role of melatonin in embryo fetal development. J. Med. Life 2014, 7, 488–492. [Google Scholar]
- Kennaway, D.J. Melatonin and development: Physiology and pharmacology. Semin Perinatol 2000, 24, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Olcese, J.M. Melatonin and Female Reproduction: An Expanding Universe. Front. Endocrinol. 2020, 11, 85. [Google Scholar] [CrossRef]
- Balik, A.; Kretschmannova, K.; Mazna, P.; Svobodova, I.; Zemkova, H. Melatonin action in neonatal gonadotrophs. Physiol. Res. 2004, 53 (Suppl. S1), S153–S166. [Google Scholar] [PubMed]
- Luo, J.; Zhang, Z.; Sun, H.; Song, J.; Chen, X.; Huang, J.; Lin, X.; Zhou, R. Effect of melatonin on T/B cell activation and immune regulation in pinealectomy mice. Life Sci. 2020, 242, 117191. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhao, Y.; Xu, L.; Gao, L.; Su, Y.; Lin, N.; Pu, J. The nuclear melatonin receptor RORα is a novel endogenous defender against myocardial ischemia/reperfusion injury. J. Pineal Res. 2016, 60, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Morrey, K.M.; McLachlan, J.A.; Serkin, C.D.; Bakouche, O. Activation of human monocytes by the pineal hormone melatonin. J. Immunol. 1994, 153, 2671–2680. [Google Scholar]
- Currier, N.L.; Sun, L.Z.; Miller, S.C. Exogenous melatonin: Quantitative enhancement in vivo of cells mediating non-specific immunity. J. Neuroimmunol. 2000, 104, 101–108. [Google Scholar] [CrossRef]
- Barjavel, M.J.; Mamdouh, Z.; Raghbate, N.; Bakouche, O. Differential expression of the melatonin receptor in human monocytes. J. Immunol. 1998, 160, 1191–1197. [Google Scholar] [PubMed]
- Lai, S.W.; Liu, Y.S.; Lu, D.Y.; Tsai, C.F. Melatonin Modulates the Microenvironment of Glioblastoma Multiforme by Targeting Sirtuin 1. Nutrients 2019, 11, 1343. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, S.; Zeng, S.; Zhao, Y.; Zhu, C.; Deng, B.; Zhu, G.; Yin, Y.; Wang, W.; Hardeland, R.; et al. Melatonin in macrophage biology: Current understanding and future perspectives. J. Pineal Res. 2019, 66, e12547. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and Microglia. Int. J. Mol. Sci. 2021, 22, 8296. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cui, K.; Li, J.; Tang, X.; Lin, J.; Lu, X.; Huang, R.; Yang, B.; Shi, Y.; Ye, D.; et al. Melatonin attenuates choroidal neovascularization by regulating macrophage/microglia polarization via inhibition of RhoA/ROCK signaling pathway. J. Pineal Res. 2020, 69, e12660. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Tan, D.X.; Hardeland, R.; Leon, J.; Rodriguez, C.; Reiter, R.J. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 2005, 165, 139–149. [Google Scholar] [CrossRef]
- Markus, R.P.; Cecon, E.; Pires-Lapa, M.A. Immune-pineal axis: Nuclear factor kappaB (NF-kB) mediates the shift in the melatonin source from pinealocytes to immune competent cells. Int. J. Mol. Sci. 2013, 14, 10979–10997. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.J.; Kim, T.S. Melatonin protects mice against stress-induced inflammation through enhancement of M2 macrophage polarization. Int. Immunopharmacol. 2017, 48, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Xu, M.M.; Sun, Y.; Ding, Z.X.; Wei, Y.Y.; Zhang, D.W.; Wang, Y.G.; Shen, J.L.; Wu, H.M.; Fei, G.H. Melatonin attenuates LPS-induced pyroptosis in acute lung injury by inhibiting NLRP3-GSDMD pathway via activating Nrf2/HO-1 signaling axis. Int. Immunopharmacol. 2022, 109, 108782. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Zhang, T.; Ren, Q.; Sun, C. Melatonin alleviates adipose inflammation through elevating alpha-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J. Pineal Res. 2018, 64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, X.; Yan, J.; Li, M.; Liu, T.; Zhu, C.; Pan, G.; Guo, Q.; Yang, H.; Pei, M.; et al. Melatonin at pharmacological concentrations suppresses osteoclastogenesis via the attenuation of intracellular ROS. Osteoporos. Int. 2017, 28, 3325–3337. [Google Scholar] [CrossRef] [PubMed]
- Kuthati, Y.; Goutham Davuluri, V.N.; Yang, C.P.; Chang, H.C.; Chang, C.P.; Wong, C.S. Melatonin MT2 receptor agonist IIK-7 produces antinociception by modulation of ROS and suppression of spinal microglial activation in neuropathic pain rats. J. Pain Res. 2019, 12, 2473–2485. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Chen, J.; Feng, J.; Zhang, R.; Fan, M.; Han, D.; Li, X.; Li, C.; Ren, J.; Wang, Y.; et al. Melatonin Ameliorates the Progression of Atherosclerosis via Mitophagy Activation and NLRP3 Inflammasome Inhibition. Oxid. Med. Cell. Longev. 2018, 2018, 9286458. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Bi, Y.; McClements, D.J.; Lip, G.Y.H.; Richardson, D.R.; Reiter, R.J.; Klionsky, D.J.; Ren, J. Melatonin-based therapeutics for atherosclerotic lesions and beyond: Focusing on macrophage mitophagy. Pharmacol. Res. 2022, 176, 106072. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liu, M.; Xu, Z.; Li, Y.; Guo, H.; Ge, Y.; Liu, Y.; Zheng, D.; Shi, J. A double feedback loop mediated by microRNA-23a/27a/24-2 regulates M1 versus M2 macrophage polarization and thus regulates cancer progression. Oncotarget 2016, 7, 13502–13519. [Google Scholar] [CrossRef]
- Xiao, W.Z.; Zhao, L.; Cao, L.; Zhu, X.X.; Zou, H.J. Melatonin Alleviates Acute Gouty Inflammation In Vivo and In Vitro. Curr. Med. Sci. 2021, 41, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Pires-Lapa, M.A.; Carvalho-Sousa, C.E.; Cecon, E.; Fernandes, P.A.; Markus, R.P. beta-Adrenoceptors Trigger Melatonin Synthesis in Phagocytes. Int. J. Mol. Sci. 2018, 19, 2182. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhafeez, H.H.; Hassan, A.H.S.; Hussein, M.T. Melatonin administration provokes the activity of dendritic reticular cells in the seminal vesicle of Soay ram during the non-breeding season. Sci. Rep. 2021, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.T.; Mokhtar, D.M.; Hassan, A.H.S. Melatonin activates the vascular elements, telocytes, and neuroimmune communication in the adrenal gland of Soay rams during the non-breeding season. Protoplasma 2020, 257, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, Y.P.; Yu, Y.X.; Zhou, T.; Liu, C.; Fei, E.K.; Gao, F.; Mu, C.C.; Ren, H.G.; Wang, G.H. Dendritic cell nuclear protein-1 regulates melatonin biosynthesis by binding to BMAL1 and inhibiting the transcription of N-acetyltransferase in C6 cells. Acta Pharmacol. Sin. 2018, 39, 597–606. [Google Scholar] [CrossRef]
- NaveenKumar, S.K.; Hemshekhar, M.; Jagadish, S.; Manikanta, K.; Vishalakshi, G.J.; Kemparaju, K.; Girish, K.S. Melatonin restores neutrophil functions and prevents apoptosis amid dysfunctional glutathione redox system. J. Pineal Res. 2020, 69, e12676. [Google Scholar] [CrossRef]
- Chang, E.J.; Mun, K.C. Effect of melatonin on the malondialdehyde level of neutrophils in cyclosporine-treated rats. Transplant. Proc. 2004, 36, 2165–2166. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, W.; Kwak, M.; Zhang, L.; Lee, P.C.W.; Jin, J.O. Protective Effect of Melatonin Against Polymicrobial Sepsis Is Mediated by the Anti-bacterial Effect of Neutrophils. Front. Immunol. 2019, 10, 1371. [Google Scholar] [CrossRef]
- Lu, H.; Wu, B.; Ma, G.; Zheng, D.; Song, R.; Huang, E.; Mao, M.; Lu, B. Melatonin represses oral squamous cell carcinoma metastasis by inhibiting tumor-associated neutrophils. Am. J. Transl. Res. 2017, 9, 5361–5374. [Google Scholar]
- Silva, S.O.; Rodrigues, M.R.; Ximenes, V.F.; Bueno-da-Silva, A.E.; Amarante-Mendes, G.P.; Campa, A. Neutrophils as a specific target for melatonin and kynuramines: Effects on cytokine release. J. Neuroimmunol. 2004, 156, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Cernysiov, V.; Mauricas, M.; Girkontaite, I. Melatonin inhibits granulocyte adhesion to ICAM via MT3/QR2 and MT2 receptors. Int. Immunol. 2015, 27, 599–608. [Google Scholar] [CrossRef]
- Ren, D.L.; Sun, A.A.; Li, Y.J.; Chen, M.; Ge, S.C.; Hu, B. Exogenous melatonin inhibits neutrophil migration through suppression of ERK activation. J. Endocrinol. 2015, 227, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.O.; Carvalho, S.R.; Ximenes, V.F.; Okada, S.S.; Campa, A. Melatonin and its kynurenin-like oxidation products affect the microbicidal activity of neutrophils. Microbes Infect. 2006, 8, 420–425. [Google Scholar] [CrossRef]
- Wongsena, W.; Charoensuk, L.; Dangtakot, R.; Pinlaor, P.; Intuyod, K.; Pinlaor, S. Melatonin suppresses eosinophils and Th17 cells in hamsters treated with a combination of human liver fluke infection and a chemical carcinogen. Pharmacol. Rep. 2018, 70, 98–105. [Google Scholar] [CrossRef]
- Dair, E.L.; Simoes, R.S.; Simoes, M.J.; Romeu, L.R.; Oliveira-Filho, R.M.; Haidar, M.A.; Baracat, E.C.; Soares, J.M., Jr. Effects of melatonin on the endometrial morphology and embryo implantation in rats. Fertil. Steril. 2008, 89, 1299–1305. [Google Scholar] [CrossRef]
- Wang, Y.T.; Chen, S.L.; Xu, S.Y. Effect of melatonin on the expression of nuclear factor-kappa B and airway inflammation in asthmatic rats. Zhonghua Er Ke Za Zhi 2004, 42, 94–97. [Google Scholar] [PubMed]
- Cikler, E.; Ercan, F.; Cetinel, S.; Contuk, G.; Sener, G. The protective effects of melatonin against water avoidance stress-induced mast cell degranulation in dermis. Acta Histochem. 2005, 106, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Izzo, G.; d’Istria, M.; Serino, I.; Minucci, S. Inhibition of the increased 17beta-estradiol-induced mast cell number by melatonin in the testis of the frog Rana esculenta, in vivo and in vitro. J. Exp. Biol. 2004, 207, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.P.; Windschuettl, S.; Matzkin, M.E.; Terradas, C.; Ponzio, R.; Puigdomenech, E.; Levalle, O.; Calandra, R.S.; Mayerhofer, A.; Frungieri, M.B. Melatonin in testes of infertile men: Evidence for anti-proliferative and anti-oxidant effects on local macrophage and mast cell populations. Andrology 2014, 2, 436–449. [Google Scholar] [CrossRef]
- Ozkanlar, S.; Kara, A.; Sengul, E.; Simsek, N.; Karadeniz, A.; Kurt, N. Melatonin Modulates the Immune System Response and Inflammation in Diabetic Rats Experimentally-Induced by Alloxan. Horm. Metab. Res. 2016, 48, 137–144. [Google Scholar] [CrossRef][Green Version]
- Yildiz, A.; Vardi, N.; Karaaslan, M.G.; Ates, B.; Taslidere, E.; Esrefoglu, M. The protective effect of melatonin in lungs of newborn rats exposed to maternal nicotine. Biotech. Histochem. 2018, 93, 442–452. [Google Scholar] [CrossRef]
- Pham, L.; Baiocchi, L.; Kennedy, L.; Sato, K.; Meadows, V.; Meng, F.; Huang, C.K.; Kundu, D.; Zhou, T.; Chen, L.; et al. The interplay between mast cells, pineal gland, and circadian rhythm: Links between histamine, melatonin, and inflammatory mediators. J. Pineal Res. 2021, 70, e12699. [Google Scholar] [CrossRef]
- Maldonado, M.D.; Garcia-Moreno, H.; Calvo, J.R. Melatonin protects mast cells against cytotoxicity mediated by chemical stimuli PMACI: Possible clinical use. J. Neuroimmunol. 2013, 262, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.D.; Garcia-Moreno, H.; Gonzalez-Yanes, C.; Calvo, J.R. Possible Involvement of the Inhibition of NF-kappaB Factor in Anti-Inflammatory Actions That Melatonin Exerts on Mast Cells. J. Cell. Biochem. 2016, 117, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.D.; Mora-Santos, M.; Naji, L.; Carrascosa-Salmoral, M.P.; Naranjo, M.C.; Calvo, J.R. Evidence of melatonin synthesis and release by mast cells. Possible modulatory role on inflammation. Pharmacol. Res. 2010, 62, 282–287. [Google Scholar] [CrossRef]
- Markus, R.P.; Sousa, K.S.; da Silveira Cruz-Machado, S.; Fernandes, P.A.; Ferreira, Z.S. Possible Role of Pineal and Extra-Pineal Melatonin in Surveillance, Immunity, and First-Line Defense. Int. J. Mol. Sci. 2021, 22, 12143. [Google Scholar] [CrossRef] [PubMed]
- Bier, A.; Khashab, R.; Sharabi, Y.; Grossman, E.; Leibowitz, A. Melatonin Prevents T Lymphocyte Infiltration to the Kidneys of Hypertensive Rats, Induced by a High-Salt Diet, by Preventing the Expression of CXCR3 Ligand Chemokines. Nutrients 2021, 13, 3577. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, G.J. The photoperiod transducer melatonin and the immune-hematopoietic system. J. Photochem. Photobiol. B 1998, 43, 186–192. [Google Scholar] [CrossRef]
- Maestroni, G.J.; Hertens, E.; Galli, P.; Conti, A.; Pedrinis, E. Melatonin-induced T-helper cell hematopoietic cytokines resembling both interleukin-4 and dynorphin. J. Pineal Res. 1996, 21, 131–139. [Google Scholar] [CrossRef]
- Garcia-Maurino, S.; Gonzalez-Haba, M.G.; Calvo, J.R.; Rafii-El-Idrissi, M.; Sanchez-Margalet, V.; Goberna, R.; Guerrero, J.M. Melatonin enhances IL-2, IL-6, and IFN-gamma production by human circulating CD4+ cells: A possible nuclear receptor-mediated mechanism involving T helper type 1 lymphocytes and monocytes. J. Immunol. 1997, 159, 574–581. [Google Scholar] [PubMed]
- Majewska, M.; Zajac, K.; Zemelka, M.; Szczepanik, M. Influence of melatonin and its precursor L-tryptophan on Th1 dependent contact hypersensitivity. J. Physiol. Pharmacol. 2007, 58 (Suppl. S6), 125–132. [Google Scholar] [PubMed]
- Srinivasan, V.; Spence, D.W.; Trakht, I.; Pandi-Perumal, S.R.; Cardinali, D.P.; Maestroni, G.J. Immunomodulation by melatonin: Its significance for seasonally occurring diseases. Neuroimmunomodulation 2008, 15, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Hao, H.; Gao, X.; Cai, Y.; Zhou, J.; Liang, P.; Lv, J.; He, Q.; Shi, C.; Hu, D.; et al. Melatonin ameliorates necrotizing enterocolitis by preventing Th17/Treg imbalance through activation of the AMPK/SIRT1 pathway. Theranostics 2020, 10, 7730–7746. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Niu, C.; Sun, C.; Ma, Y.; Guo, R.; Ruan, Z.; Gao, Y.; Lu, X.; Li, H.; Lin, Y.; et al. Melatonin exerts immunoregulatory effects by balancing peripheral effector and regulatory T helper cells in myasthenia gravis. Aging 2020, 12, 21147–21160. [Google Scholar] [CrossRef]
- Alvarez-Sanchez, N.; Cruz-Chamorro, I.; Lopez-Gonzalez, A.; Utrilla, J.C.; Fernandez-Santos, J.M.; Martinez-Lopez, A.; Lardone, P.J.; Guerrero, J.M.; Carrillo-Vico, A. Melatonin controls experimental autoimmune encephalomyelitis by altering the T effector/regulatory balance. Brain Behav. Immun. 2015, 50, 101–114. [Google Scholar] [CrossRef]
- Perfilyeva, Y.V.; Ostapchuk, Y.O.; Abdolla, N.; Tleulieva, R.; Krasnoshtanov, V.C.; Belyaev, N.N. Exogenous Melatonin Up-Regulates Expression of CD62L by Lymphocytes in Aged Mice under Inflammatory and Non-Inflammatory Conditions. Immunol. Investig. 2019, 48, 632–643. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Esquifino, A.I.; Srinivasan, V.; Pandi-Perumal, S.R. Melatonin and the immune system in aging. Neuroimmunomodulation 2008, 15, 272–278. [Google Scholar] [CrossRef]
- Srinivasan, V.; Spence, D.W.; Pandi-Perumal, S.R.; Trakht, I.; Cardinali, D.P. Therapeutic actions of melatonin in cancer: Possible mechanisms. Integr. Cancer Ther. 2008, 7, 189–203. [Google Scholar] [CrossRef]
- Lewinski, A.; Zelazowski, P.; Sewerynek, E.; Zerek-Melen, G.; Szkudlinski, M.; Zelazowska, E. Melatonin-induced suppression of human lymphocyte natural killer activity in vitro. J. Pineal Res. 1989, 7, 153–164. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Physiological crosstalk between the AC/PKA and PLC/PKC pathways modulates melatonin-mediated, monochromatic-light-induced proliferation of T-lymphocytes in chickens. Cell Tissue Res. 2017, 369, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Jiang, J.W.; Cao, X.D.; Wu, G.C. Melatonin enhances lymphocyte proliferation and decreases the release of pituitary pro-opiomelanocortin-derived peptides in surgically traumatized rats. Neurosci. Lett. 2003, 343, 109–112. [Google Scholar] [CrossRef]
- Pioli, C.; Caroleo, M.C.; Nistico, G.; Doria, G. Melatonin increases antigen presentation and amplifies specific and non specific signals for T-cell proliferation. Int. J. Immunopharmacol. 1993, 15, 463–468. [Google Scholar] [CrossRef]
- Pedrosa, A.M.; Weinlich, R.; Mognol, G.P.; Robbs, B.K.; Viola, J.P.; Campa, A.; Amarante-Mendes, G.P. Melatonin protects CD4+ T cells from activation-induced cell death by blocking NFAT-mediated CD95 ligand upregulation. J. Immunol. 2010, 184, 3487–3494. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin mediates monochromatic light-induced proliferation of T/B lymphocytes in the spleen via the membrane receptor or nuclear receptor. Poult. Sci. 2020, 99, 4294–4302. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. A Green and Blue Monochromatic Light Combination Therapy Reduces Oxidative Stress and Enhances B-Lymphocyte Proliferation through Promoting Melatonin Secretion. Oxid. Med. Cell. Longev. 2021, 2021, 5595376. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Miller, S.C.; Osmond, D.G. Melatonin inhibits apoptosis during early B-cell development in mouse bone marrow. J. Pineal Res. 2000, 29, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Cernysiov, V.; Gerasimcik, N.; Mauricas, M.; Girkontaite, I. Regulation of T-cell-independent and T-cell-dependent antibody production by circadian rhythm and melatonin. Int. Immunol. 2010, 22, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Miguez, M.P.; Morgado, S.; Sanchez-Correa, B.; Gordillo, J.J.; Casado, J.G.; Tarazona, R.; Regodon, S. Melatonin enhances responsiveness to Dichelobacter nodosus vaccine in sheep and increases peripheral blood CD4 T lymphocytes and IgG-expressing B lymphocytes. Vet. Immunol. Immunopathol. 2018, 206, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Steinhilber, D.; Brungs, M.; Werz, O.; Wiesenberg, I.; Danielsson, C.; Kahlen, J.P.; Nayeri, S.; Schrader, M.; Carlberg, C. The nuclear receptor for melatonin represses 5-lipoxygenase gene expression in human B lymphocytes. J. Biol. Chem. 1995, 270, 7037–7040. [Google Scholar] [CrossRef]
- del Gobbo, V.; Libri, V.; Villani, N.; Calio, R.; Nistico, G. Pinealectomy inhibits interleukin-2 production and natural killer activity in mice. Int. J. Immunopharmacol. 1989, 11, 567–573. [Google Scholar] [CrossRef]
- Libri, V.; Del Gobbo, V.; Villani, N.; Calio, R.; Nistico, G. Influence of pineal gland lesion on interleukin-2 production and natural killer activity in C57BL/6 mice. Pharmacol. Res. 1990, 22 (Suppl. S3), 52. [Google Scholar] [CrossRef]
- Ozcelik, F.; Hacimustafaoglu, F.; Tanoglu, A. Modulatory effect of resveratrol and melatonin on natural killer cell activity and adrenomedullin in diabetic rats. Turk J. Med. Sci. 2021, 52, 258–267. [Google Scholar] [CrossRef]
- Sapede, D.; Cau, E. The pineal gland from development to function. Curr. Top. Dev. Biol. 2013, 106, 171–215. [Google Scholar] [CrossRef] [PubMed]
- Orgeta, V.; Tabet, N.; Nilforooshan, R.; Howard, R. Efficacy of Antidepressants for Depression in Alzheimer’s Disease: Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2017, 58, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; Eatmon, C.V.; Slevin, J.T. Drug treatment strategies for depression in Parkinson disease. Expert Opin. Pharmacother. 2019, 20, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Reiter, R.J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. Linking the biological underpinnings of depression: Role of mitochondria interactions with melatonin, inflammation, sirtuins, tryptophan catabolites, DNA repair and oxidative and nitrosative stress, with consequences for classification and cognition. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.J.; Liu, C.; Yu, L.Z.; Zhou, J.H.; Li, Y.; Xiong, Y.; Guo, A.; Chao, L.M.; Qu, Q.; Wei, G.W.; et al. Melatonin Alleviates Neuroinflammation and Metabolic Disorder in DSS-Induced Depression Rats. Oxid. Med. Cell. Longev. 2020, 2020, 1241894. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Copes, N.; O’Neal-Moffitt, G.; Jin, J.; Buzzeo, R.; Mamcarz, M.; Tan, J.; Cao, C.; Olcese, J.M.; Arendash, G.W.; et al. Melatonin treatment restores mitochondrial function in Alzheimer’s mice: A mitochondrial protective role of melatonin membrane receptor signaling. J. Pineal Res. 2011, 51, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Qiu, X.; Wang, Y.; Liu, J.; Li, Q.; Jiang, H.; Li, S.; Song, C. Long-term oral melatonin alleviates memory deficits, reduces amyloid-beta deposition associated with downregulation of BACE1 and mitophagy in APP/PS1 transgenic mice. Neurosci. Lett. 2020, 735, 135192. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Wu, G.; Zhai, X.; Lu, B.; Meng, B.; Chen, J. Melatonin and Rapamycin Attenuate Isoflurane-Induced Cognitive Impairment Through Inhibition of Neuroinflammation by Suppressing the mTOR Signaling in the Hippocampus of Aged Mice. Front. Aging Neurosci. 2019, 11, 314. [Google Scholar] [CrossRef]
- Muhammad, T.; Ali, T.; Ikram, M.; Khan, A.; Alam, S.I.; Kim, M.O. Melatonin Rescue Oxidative Stress-Mediated Neuroinflammation/Neurodegeneration and Memory Impairment in Scopolamine-Induced Amnesia Mice Model. J. Neuroimmune Pharmacol. 2019, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Zhong, Y. Interleukin-17A: The Key Cytokine in Neurodegenerative Diseases. Front. Aging Neurosci. 2020, 12, 566922. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Lowell, J.A. Th17 cells in depression. Brain Behav. Immun. 2018, 69, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Kuklina, E.M.; Glebezdina, N.S.; Nekrasova, I.V. Role of Melatonin in the Regulation of Differentiation of T Cells Producing Interleukin-17 (Th17). Bull. Exp. Biol. Med. 2016, 160, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Liu, G.; Chen, S.; Yin, J.; Wang, J.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Li, T.; et al. Melatonin signaling in T cells: Functions and applications. J. Pineal Res. 2017, 62, 19. [Google Scholar] [CrossRef]

| Immune Cell | Effects of Melatonin | References |
|---|---|---|
| Innate System | ||
| Monocyte | Activates cells through PKC | [40] |
| Increases the number of cells | [41] | |
| Induces ROS production | [40] | |
| Induces the cytokine production: IL-1, IL-6, TNF-α | [42] | |
| Attenuates binding activity | [43] | |
| Macrophage | Influences the phenotype polarization | [44,45,46] |
| Preventes COX-2 and iNOS activation | [47] | |
| Regulates signaling pathways: NF-κB, STATs, NLRP3/ caspase-1 | [48,49,50] | |
| Regulates cellular metabolic pathways: α-KG, ROS | [51,52,53] | |
| Mitochondrial dynamics and mitophagy | [54] | |
| Regulates the expression of miRNAs: miR-155, miR34a, miR23a, miR27a, miR24-2 | [55,56] | |
| Expression of cytokines: IL-1b, IL-6 | [57] | |
| Could synthesize melatonin | [58] | |
| Dendritic cell | Increases the secretory activity through raised endosomal compartments | [59] |
| Increases in the number of cells and diameter | [59,60] | |
| Could synthesize melatonin | [58,61] | |
| Neutrophil | Protects neutrophils from oxidative stress-induced apoptosis | [62] |
| Reduces ROS generation | [62,63] | |
| Restores their functions: phagocytosis, degranulation, NETosis | [62,64] | |
| Suppresses the release of CXCL8, CCL2, CCL4, and MMP9 by blockage of p38 MAPK and Akt signaling | [65] | |
| Suppresses the release of cytokines: TNF-a, IL-8 | [66] | |
| Modulates the migration through the endothelial layer by blocking the ERK phosphorylation signal | [67,68] | |
| Reduces the microbicidal activity | [69] | |
| Eosinophil | Suppresses eosinophils activity | [70] |
| Decreases eosinophil number | [71,72] | |
| Mast cell | Avoid degranulation | [73] |
| Regulates differentiation and cellular proliferation | [74,75,76,77] | |
| Restores circadian rhythms | [78] | |
| Reduces the cytotoxicity | [79] | |
| Inhibits the expression of proinflammatory cytokines and COX2 | [75,80] | |
| Could synthesize melatonin | [81] | |
| Adaptative system | ||
| T cell | Decrease migration | [82,83] |
| Release cytokines: IL-2, IL-4, IL-6, INF-g | [84,85,86] | |
| Promotes the balance in subpopulations T cell-mediated immune responses | [87,88,89,90,91] | |
| Regulates the levels of surface CD62L on CD8+ cells | [92] | |
| Regulates cell proliferation by AC/ PKA and PLC/ PKC pathways | [41,93,94,95,96,97] | |
| Enhances antigen presentation by macrophages to T cells by increasing the expression of MHC-II | [98] | |
| Inhibits the expression of TLR3, p38 MAP, JNK, and MAPK/ NF-κ B pathways | [38] | |
| Regulates the expression of CD95 ligand | [99] | |
| B cell | Induces the cellular activation | [38] |
| Promotes the cell proliferation | [100,101,102] | |
| Promotes the antibody production | [103,104] | |
| Represses 5-lipoxygenase gene | [105] | |
| Natural killer | Increases the number of cells | [93] |
| Promotes the expression of cytokines: IL-2, IL-12, IL-6, INF-g | [94,106,107] | |
| Modulates cell activity | [108] | |
| Regulates the levels of surface CD62L | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bitzer-Quintero, O.K.; Ortiz, G.G.; Jaramillo-Bueno, S.; Ramos-González, E.J.; Márquez-Rosales, M.G.; Delgado-Lara, D.L.C.; Torres-Sánchez, E.D.; Tejeda-Martínez, A.R.; Ramirez-Jirano, J. Psycho-Neuro-Endocrine-Immunology: A Role for Melatonin in This New Paradigm. Molecules 2022, 27, 4888. https://doi.org/10.3390/molecules27154888
Bitzer-Quintero OK, Ortiz GG, Jaramillo-Bueno S, Ramos-González EJ, Márquez-Rosales MG, Delgado-Lara DLC, Torres-Sánchez ED, Tejeda-Martínez AR, Ramirez-Jirano J. Psycho-Neuro-Endocrine-Immunology: A Role for Melatonin in This New Paradigm. Molecules. 2022; 27(15):4888. https://doi.org/10.3390/molecules27154888
Chicago/Turabian StyleBitzer-Quintero, Oscar K., Genaro G. Ortiz, Socorro Jaramillo-Bueno, Elsy J. Ramos-González, María G. Márquez-Rosales, Daniela L. C. Delgado-Lara, Erandis D. Torres-Sánchez, Aldo R. Tejeda-Martínez, and Javier Ramirez-Jirano. 2022. "Psycho-Neuro-Endocrine-Immunology: A Role for Melatonin in This New Paradigm" Molecules 27, no. 15: 4888. https://doi.org/10.3390/molecules27154888
APA StyleBitzer-Quintero, O. K., Ortiz, G. G., Jaramillo-Bueno, S., Ramos-González, E. J., Márquez-Rosales, M. G., Delgado-Lara, D. L. C., Torres-Sánchez, E. D., Tejeda-Martínez, A. R., & Ramirez-Jirano, J. (2022). Psycho-Neuro-Endocrine-Immunology: A Role for Melatonin in This New Paradigm. Molecules, 27(15), 4888. https://doi.org/10.3390/molecules27154888







