NMR spectra were acquired with a 300 MHz Bruker Avance spectrometer (300.13 MHz for 1H and 75.5 MHz for 13C) in CDCl3 or DMSO-d6 and were referenced to residual solvent proton signals (δH = 7.26 and 2.50, respectively) and solvent carbon signals (δC = 77.16 and 39.52, respectively). Multiplicities are abbreviated as follows: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad, dd = doublet of doublets, dt = doublet of triplets, ddd = doublet/doublets of doublets; coupling constants, J, are reported in Hz. Mass spectra were acquired with an HRMS-ESI-qTOF spectrometer Nexera LCMS9030 or MaXis II Bruker Daltonic GmbH (electrospray ionization mode, positive ions detection). Flash column chromatography on silica (Merck, 230–400 mesh) was performed with a Biotage Isolera Prime instrument. TLC was performed on aluminum-backed pre-coated plates (0.25 mm) with silica gel 60 F254 with a suitable solvent system and was visualized using UV fluorescence.
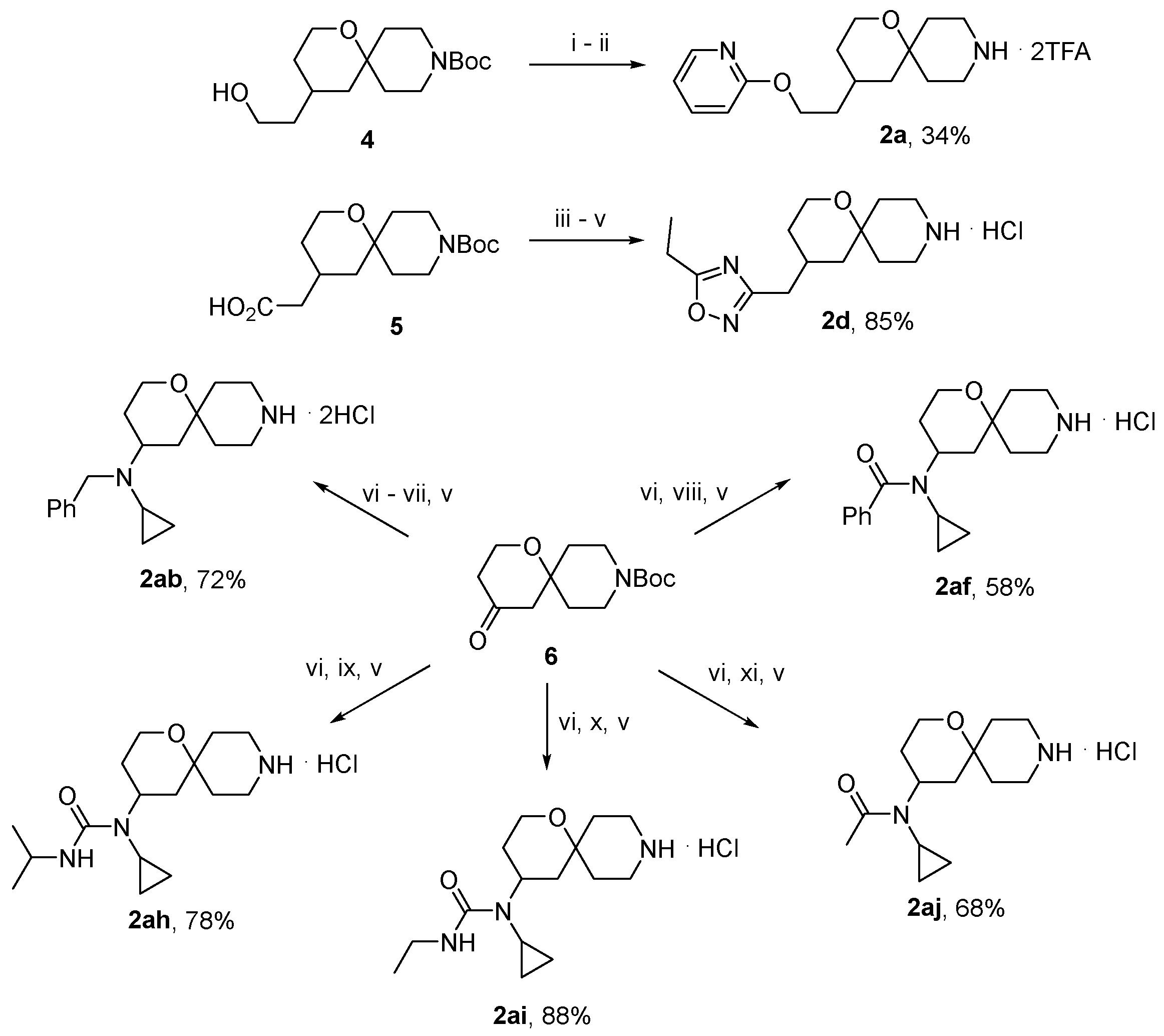
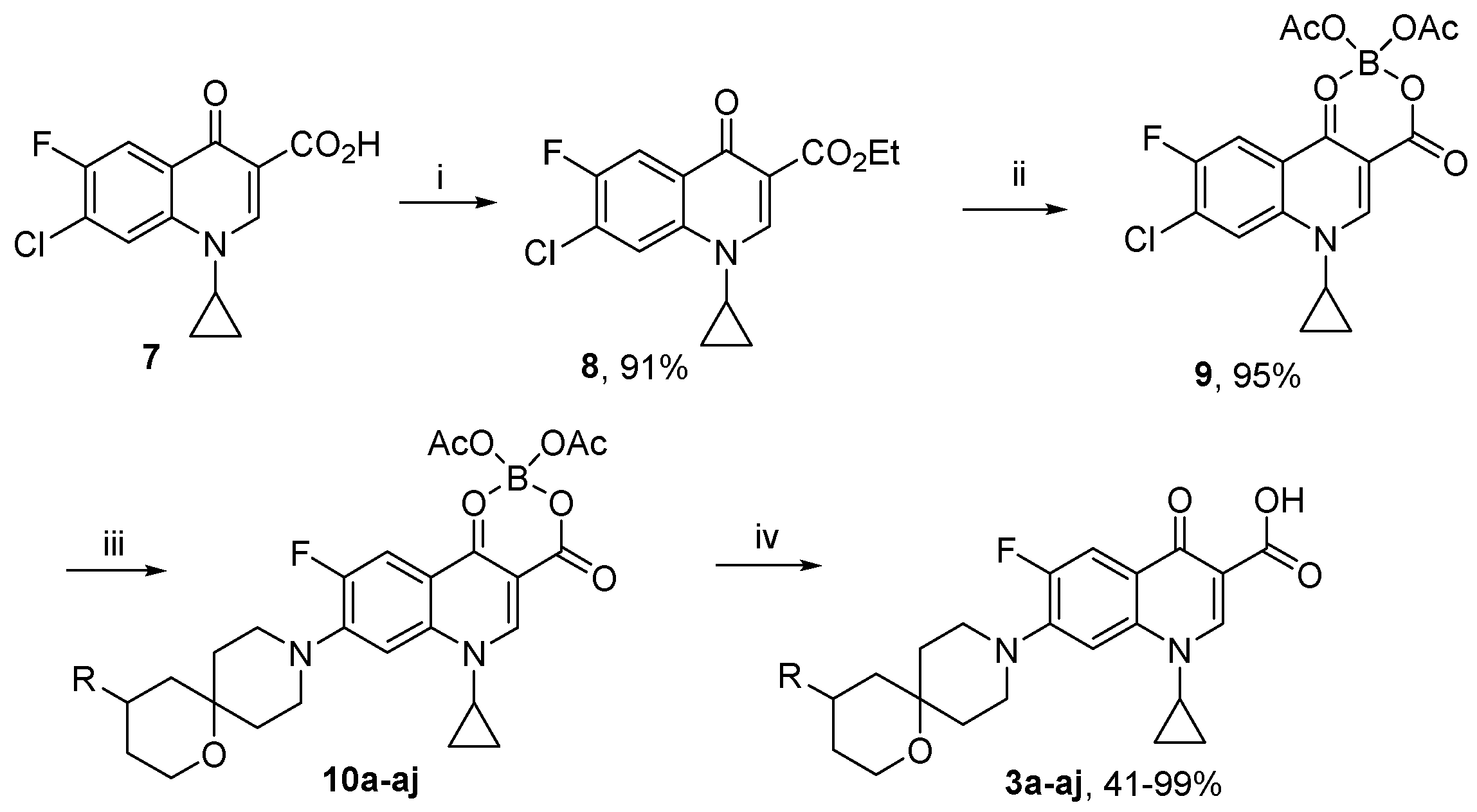
3.1.10. General Procedure for the Preparation of Compounds 3a–aj
Compound 9 (0.24 mmol) was dissolved in acetonitrile (10 mL) and treated, with stirring, with spirocyclic amine 2 (0.47 mmol) and triethylamine (0.28 mmol). The stirring continued at 60 °C for 10 h. The volatiles were removed in vacuo. The residue was fractionated on a silica gel column eluted with 0 → 20% methanol in dichloromethane. Fractions containing the product (by TLC analysis) were pooled and concentrated in vacuo. The residues was dissolved in 2% aqueous NaOH and left to stir at r. t. overnight. The reaction mixture was acidified with 5% aqueous citric acid to pH 4–5. The resulting precipitate was filtered off, washed with water and air-dried.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-(2-(pyridine-2-yloxy)-ethyl)-1-oxa-9-azaspiro[5.5]undec-9-yl)-1,4-dihydroquinoline-3-carboxylic Acid (3a)
Yield—100 mg (44%), white solid, m.p. 170–172 °C. 1H-NMR (300 MHz, CDCl3) δ 15.10 (s, 1H), 8.74 (s, 1H), 8.14 (d, J = 3.6 Hz, 1H), 7.97 (d, J = 13.1 Hz, 1H), 7.61–7.53 (m, 1H), 7.37 (d, J = 7.1 Hz, 1H), 6.93–6.82 (m, 1H), 6.72 (d, J = 8.3 Hz, 1H), 4.35 (t, J = 6.4 Hz, 2H), 3.80 (dd, J = 11.5, 4.6 Hz, 1H), 3.64 (t, J = 11.4 Hz, 1H), 3.55–3.39 (m, 3H), 3.32 (td, J = 11.7, 2.7 Hz, 1H), 3.11 (t, J = 11.3 Hz, 1H), 2.40 (br.d, J = 13.1 Hz, 1H), 2.04–1.93 (m, 1H), 1.92–1.76 (m, 2H), 1.74–1.69 (m, 3H), 1.67–1.52 (m, 2H), 1.42–1.34 (m, 2H), 1.33–1.27 (m, 1H), 1.26–1.16 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 177.0 (d, J = 2.5 Hz), 167.2, 163.9, 153.7 (d, J = 251.3 Hz), 147.2, 146.9, 146.5 (d, J = 10.3 Hz), 139.2, 138.7, 119.2 (d, J = 7.9 Hz), 116.8, 112.0 (d, J = 23.4 Hz), 111.1, 107.8, 104.9 (d, J = 3,3 Hz), 69.8, 63.1, 60.8, 45.7, 45.7, 45.6, 43.1, 39.2, 36.3, 35.4, 32.9, 29.3, 27.4, 8.3, 8.2; HRMS (ESI) m/z calculated for C29H32FN3O5 [M + H+] 522.2399, found 522.2422.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-(4-methylbenzyl)-1-oxa-9-azaspiro[5.5]undec-9-yl)-1,4-dihydroquinoline-3-carboxylic Acid (3b)
Yield—47 mg (22%), yellow solid, m.p. 215–217 °C. 1H-NMR (300 MHz, CDCl3) δ 15.05 (s, 1H), 8.71 (s, 1H), 7.93 (d, J = 13.1 Hz, 1H), 7.37 (d, J = 6.7 Hz, 1H), 7.06 (dd, J = 19.7, 7.4 Hz, 4H), 3.82–3.71 (m, 1H), 3.63–3.28 (m, 5H), 3.11 (t, J = 11.6 Hz, 1H), 2.48 (d, J = 6.6 Hz, 2H), 2.40–2.25 (m, 4H), 1.95–1.69 (m, 3H), 1.65–1.50 (m, 3H), 1.42–1.32 (m, 2H), 1.30–1.14 (m, 4H); 13C-NMR (75 MHz, CDCl3) δ 177.1 (d, J = 2.5 Hz), 167.3, 153.8 (d, J = 251.4 Hz), 147.4, 146.5 (d, J = 10.3 Hz), 139.2, 136.7, 135.6, 129.1, 119.4 (d, J = 7.9 Hz), 112.2 (d, J = 23.5 Hz), 108.0, 105.0 (d, J = 2.3 Hz), 69.9, 60.9, 45.9, 45.8, 45.8, 45.7, 43.4, 43.1, 39.2, 35.4, 32.7, 32.5, 29.3, 21.1, 8.4, 8.3; HRMS (ESI) m/z calculated for C30H33FN2O4 [M + H+] 505.2497, found 505.2517.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-(2-(6-methylpyridin-2-yloxy)-ethyl)-1-oxa-9-azaspiro[5.5]undec-9-yl)-1,4-dihydroquinoline-3-carboxylic Acid (3c)
Yield—60 mg (27%), white solid, m.p. 150–152 °C. 1H-NMR (300 MHz, CDCl3) δ 15.14 (s, 1H), 8.74 (s, 1H), 7.97 (d, J = 13.1 Hz, 1H), 7.46 (t, J = 7.7 Hz, 1H), 7.37 (d, J = 7.2 Hz, 1H), 6.71 (d, J = 7.2 Hz, 1H), 6.51 (d, J = 8.2 Hz, 1H), 4.31 (t, J = 6.3 Hz, 2H), 3.80 (dd, J = 11.7, 4.3 Hz, 1H), 3.64 (t, J = 11.5 Hz, 1H), 3.56–3.38 (m, 3H), 3.37–3.26 (m, 1H), 3.10 (t, J = 11.2 Hz, 1H), 2.43 (s, 3H), 2.41 (br.d, J = 15.7 Hz, 1H), 2.07–1.94 (m, 1H), 1.92–1.81 (m, 1H), 1.77–1.65 (m, 5H), 1.62–1.52 (m, 1H), 1.42–1.34 (m, 2H), 1.33–1.25 (m, 1H), 1.24–1.15 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 177.1 (d, J = 1.9 Hz), 167.3, 163.4, 156,4, 153.8 (d, J = 251.3 Hz), 147.3, 146.6 (d, J = 10.4 Hz), 139.2, 138.9, 119.3 (d, J = 7.1 Hz), 115.9, 112.1 (d, J = 23.6 Hz), 107.0, 104.9 (d, J = 3.5 Hz), 69.8, 62.9, 60.9, 45.8, 45.7, 45.7, 43.2, 39.3, 36.5, 35.4, 32.7, 29.3, 27.3, 24.3, 8.3, 8.3; HRMS (ESI) m/z calculated for C30H34FN3O5 [M + H+] 536.2555, found 536.2578.
1-Cyclopropyl-7-(4-(3-ethyl(1,2,4)oxadiazol-5-ylmethyl)-1-oxa-9-azaspiro[5.5]undec-9-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3d)
Yield—125 mg (59%), white solid, m.p. 141–143 °C. 1H-NMR (300 MHz, CDCl3) δ 15.03 (s, 1H), 8.71 (s, 1H), 7.94 (d, J = 13.1 Hz, 1H), 7.42 (d, J = 6.3 Hz, 1H), 3.81 (dd, J = 11.8, 4.4 Hz, 1H), 3.64 (t, J = 11.6 Hz, 1H), 3.55–3.25 (m, 4H), 3.12 (t, J = 11.3 Hz, 1H), 2.84–2.67 (m, 4H), 2.44–2.24 (m, 2H), 1.94–1.81 (m, 1H), 1.78–1.56 (m, 4H), 1.43–1.24 (m, 7H), 1.23–1.13 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ 177.7, 176.8 (d, J = 2.5 Hz), 171.6, 167.0, 153.6 (d, J = 251.4 Hz), 147.2, 146.2 (d, J = 10.3 Hz), 139.1, 119.1 (d, J = 7.9 Hz), 111.8 (d, J = 23.6 Hz), 107.7, 104.9 (d, J = 2.8 Hz), 69.7, 60.3, 45.5, 45.5, 42.4, 39.0, 35.4, 33.7, 32.2, 29.3, 29.1, 19.7, 11.3, 8.2; HRMS (ESI) m/z calculated for C27H31FN4O5 [M + H+] 511.2351, found 511.2369.
1-Cyclopropyl-6-fluoro-7-(1-oxa-9-azaspiro[5.5]undec-9-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3e)
Yield—83 mg (50%), yellow solid, m.p. 190–192 °C. 1H-NMR (300 MHz, CDCl3) δ 15.06 (s, 1H), 8.70 (s, 1H), 7.92 (d, J = 13.2 Hz, 1H), 7.36 (d, J = 7.2 Hz, 1H), 3.69 (t, J = 5.1 Hz, 2H), 3.56–3.39 (m, 3H), 3.22 (t, J = 11.3 Hz, 2H), 2.15–2.03 (m, 2H), 1.76–1.63 (m, 4H), 1.61–1.49 (m, 4H), 1.42–1.31 (m, 2H), 1.23–1.14 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ 177.2 (d, J = 2.7 Hz), 167.2, 153.9 (d, J = 251.3 Hz), 147.3, 146.6 (d, J = 10.4 Hz), 139.3, 119.4 (d, J = 7.9 Hz), 112.2 (d, J = 23.6 Hz), 108.1, 104.9 (d, J = 3.7 Hz), 69.4, 61.1, 45.8, 45.7, 36.2, 35.4, 34.1, 26.2, 18.9, 8.3; HRMS (ESI) m/z calculated for C22H25FN2O4 [M + H+] 401.1871, found 401.1889.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-(pyridin-4-yloxy)-1-oxa-9-azaspiro[5.5]undec-9-yl)-1,4-dihydroquinoline-3-carboxylic Acid (3f)
Yield—120 mg (59%), beige solid, m.p. 138–140 °C. 1H-NMR (300 MHz, CDCl3) δ 15.09 (s, 1H), 8.71 (s, 1H), 8.42 (d, J = 4.6 Hz, 2H), 7.93 (d, J = 13.0 Hz, 1H), 7.36 (d, J = 7.1 Hz, 1H), 6.81 (d, J = 5.4 Hz, 2H), 4.83–4.69 (m, 1H), 4.04–3.94 (m, 1H), 3.80–3.69 (m, 1H), 3.58–3.40 (m, 3H), 3.33–3.13 (m, 2H), 2.53 (br.s, 1H), 2.20–2.09 (m, 3H), 2.02–1.94 (m, 1H), 1.87–1.75 (m, 3H), 1.43–1.32 (m, 2H), 1.22–1.14 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ 177.1 (d, J = 2.6 Hz), 167.0, 163.8, 153.7 (d, J = 251.3 Hz), 150.5, 147.3, 146.3 (d, J = 10.4 Hz), 139.1, 119.5 (d, J = 7.9 Hz), 112.2 (d, J = 23.6 Hz), 111.1, 108.1, 104.8 (d, J = 3.5 Hz), 70.4, 70.2, 58.1, 45.6, 45.6, 45.5, 45.4, 40.5, 36.2, 35.3, 33.1, 31.1, 8.2; HRMS (ESI) m/z calculated for C27H28FN3O5 [M + H+] 494.2086, found 494.2107.
1-Cyclopropyl-6-fluoro-7-(4-ethoxy-1-oxa-9-azaspiro[5.5]undec-9-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3g)
Yield—160 mg (86%), white solid, m.p. 172–174 °C. 1H-NMR (300 MHz, CDCl3) δ 15.06 (s, 1H), 8.71 (s, 1H), 7.93 (d, J = 13.1 Hz, 1H), 7.38 (d, J = 6.9 Hz, 1H), 3.96–3.83 (m, 1H), 3.73–3.60 (m, 2H), 3.58–3.39 (m, 5H), 3.28 (t, J = 10.7 Hz, 1H), 3.15 (t, J = 11.5 Hz, 1H), 2.18–2.07 (m, 1H), 2.05–1.91 (m, 2H), 1.91–1.79 (m, 2H), 1.78–1.66 (m, 1H), 1.61–1.44 (m, 2H), 1.42–1.33 (m, 2H), 1.26–1.16 (m, 5H); 13C-NMR (75 MHz, CDCl3) δ 177.2 (d, J = 2.5 Hz), 167.2, 153.9 (d, J = 251.4 Hz), 147.4, 146.3 (d, J = 10.1 Hz), 139.2, 119.6 (d, J = 7.9 Hz), 112.3 (d, J = 23.6 Hz), 108.1, 105.2 (d, J = 2.4 Hz), 71.3, 70.7, 63.3, 59.1, 45.9, 45.9, 45.8, 45.8, 42.0, 37.4, 35.4, 32.4, 32.1, 15.7, 8.4; HRMS (ESI) m/z calculated for C24H29FN2O5 [M + H+] 445.2133, found 445.2148.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-(2-(pyrimidin-2-yloxy)-ethyl)-1-oxa-9-azaspiro[5.5]undec-9-yl)-1,4-dihydroquinoline-3-carboxylic Acid (3i)
Yield—135 mg (62%), white solid, m.p. 182–184 °C. 1H-NMR (300 MHz, CDCl3) δ 15.11 (s, 1H), 8.73 (s, 1H), 8.51 (d, J = 4.7 Hz, 2H), 7.95 (d, J = 13.1 Hz, 1H), 7.36 (d, J = 7.1 Hz, 1H), 6.94 (t, J = 4.7 Hz, 1H), 4.42 (t, J = 6.2 Hz, 2H), 3.80 (dd, J = 11.6, 4.5 Hz, 1H), 3.63 (t, J = 11.5 Hz, 1H), 3.55–3.39 (m, 3H), 3.38–3.26 (m, 1H), 3.11 (t, J = 11.1 Hz, 1H), 2.39 (br.d, J = 13.7 Hz, 1H), 2.04 (dd, J = 11.9, 6.7 Hz, 1H), 1.83 (dd, J = 11.7, 4.0 Hz, 1H), 1.79–1.72 (m, 3H), 1.71–1.62 (m, 2H), 1.61–1.53 (m, 1H), 1.41–1.34 (m, 2H), 1.33–1.25 (m, 1H), 1.26–1.21 (m, 1H), 1.22–1.15 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ 176.4 (d, J = 2.6 Hz), 166.5, 164.5, 158.6, 153.0 (d, J = 251.3 Hz), 146.5, 145.7 (d, J = 10.4 Hz), 138.5, 118.6 (d, J = 7.9 Hz), 114.3, 111.5 (d, J = 23.6 Hz), 107.3, 104.1 (d, J = 3.5 Hz), 69.0, 64.0, 60.0, 45.0, 45.0, 44.9, 42.3, 38.5, 35.4, 34.6, 31.8, 28.6, 26.4, 7.5; HRMS (ESI) m/z calculated for C28H31FN4O5 [M + Na+] 545.2171, found 545.2190.
1-Cyclopropyl-7-(4-(2-(3,6-dimethylpyrazin-2-yloxy)-ethyl)-1-oxa-9-azaspiro[5.5]undec-9-yl) -6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3h)
Yield—50 mg (53%), pale brown solid, m.p. 87–89 °C. 1H-NMR (300 MHz, CDCl3) δ 15.10 (s, 1H), 8.71 (s, 1H), 7.93 (d, J = 13.1 Hz, 1H), 7.83 (s, 1H), 7.36 (d, J = 7.2 Hz, 1H), 4.37 (t, J = 6.4 Hz, 2H), 3.81 (dd, J = 11.8, 4.6 Hz, 1H), 3.69–3.60 (m, 1H), 3.55–3.47 (m, 2H), 3.45–3.38 (m, 1H), 3.31 (td, J = 11.6, 2.8 Hz, 1H), 3.16–3.06 (m, 1H), 2.40 (s, 3H), 2.45–2.36 (m, 1H), 2.38 (s, 3H), 2.00–1.94 (m, 1H), 1.84 (dd, J = 11.7, 4.7 Hz, 1H), 1.78–1.73 (m, 2H), 1.73–1.69 (m, 2H), 1.65 (d, J = 8.9 Hz, 1H), 1.61–1.53 (m, 1H), 1.39–1.35 (m, 2H), 1.32–1.22 (m, 2H), 1.20–1.17 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ 177.1 (d, J = 2.7 Hz), 167.3, 157.8, 153.8 (d, J = 251.3 Hz), 147.8, 147.4, 146.6 (d, J = 10.4 Hz), 140.8, 139.2, 134.1, 119.4 (d, J = 7.9 Hz), 112.2 (d, J = 23.6 Hz), 108.0, 104.9 (d, J = 3.4 Hz), 69.8, 63.4, 60.9, 45.8, 45.8, 45.7, 45.7, 43.2, 39.3, 36.2, 35.4, 32.8, 29.3, 27.6, 20.8, 18.8, 8.4, 8.3; HRMS (ESI) m/z calculated for C30H35FN4O5 [M + H+] 551.2664, found 551.2673.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-(2-(5-trifluoromethylpyridin-2-yloxy)-ethyl)-1-oxa-9-azaspiro[5.5]undec-9-yl)-1,4-dihydroquinoline-3-carboxylic Acid (3j)
Yield—65 mg (29%), white solid, m.p. 95–97 °C. 1H-NMR (300 MHz, CDCl3) δ 15.09 (s, 1H), 8.74 (s, 1H), 8.42 (s, 1H), 7.97 (d, J = 13.2 Hz, 1H), 7.77 (d, J = 8.0 Hz, 1H), 7.37 (d, J = 7.1 Hz, 1H), 6.81 (d, J = 8.7 Hz, 1H), 4.42 (t, J = 6.2 Hz, 2H), 3.87–3.76 (m, 1H), 3.64 (t, J = 11.7 Hz, 1H), 3.56–3.40 (m, 3H), 3.32 (t, J = 10.9 Hz, 1H), 3.11 (t, J = 11.6 Hz, 1H), 2.40 (br.d, J = 14.2 Hz, 1H), 2.00–1.91 (m, 1H), 1.89–1.81 (m, 1H), 1.77–1.69 (m, 4H), 1.66–1.62 (m, 1H), 1.61–1.53 (m, 1H), 1.42–1.30 (m, 3H), 1.26–1.15 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 177.2 (d, J = 2.6 Hz), 167.3, 165.9 (q, J = 0.8 Hz), 153.9 (d, J = 251.3 Hz), 147.4, 146.6 (d, J = 10.4 Hz), 145.1 (q, J = 4.4 Hz), 139.3, 135.8 (q, J = 6.0, 2.9 Hz), 124.1 (q, J = 542.2, 271.1 Hz), 120.1 (q, J = 65.1, 32.0 Hz), 119.5 (d, J = 7.9 Hz), 112.3 (d, J = 23.6 Hz), 111.4, 108.1, 105.0 (d, J = 3.5 Hz), 69.8, 64.1, 60.8, 45.9, 45.8, 45.7, 45.7, 43.2, 39.3, 36.2, 35.4, 32.8, 29.4, 27.4, 8.4, 8.3; HRMS (ESI) m/z calculated for C30H31FN4O5 [M + H+] 547.2351, found 547.2039.
1-Cyclopropyl-7-(4-(2-(2-cyclopropyl-6,7-dihydro-5H-cyclopentapyrimidin-4-yloxy)-ethyl)-1-oxa-9-azaspiro[5.5]undec-9-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3k)
Yield—97 mg (39%), white solid, m.p. 97–99 °C. 1H-NMR (300 MHz, CDCl3) δ 15.13 (s, 1H), 8.74 (s, 1H), 7.97 (d, J = 13.1 Hz, 1H), 7.37 (d, J = 7.1 Hz, 1H), 4.39 (t, J = 6.2 Hz, 2H), 3.81 (dd, J = 11.4, 3.9 Hz, 1H), 3.64 (t, J = 11.7 Hz, 1H), 3.57–3.38 (m, 3H), 3.31 (t, J = 10.6 Hz, 1H), 3.09 (t, J = 11.5 Hz, 1H), 2.90 (t, J = 7.6 Hz, 2H), 2.76 (t, J = 7.3 Hz, 2H), 2.40 (br.d, J = 13.3 Hz, 1H), 2.16–2.04 (m, 3H), 1.93–1.82 (m, 2H), 1.76–1.54 (m, 6H), 1.37 (br.d, J = 5.8 Hz, 2H), 1.33–1.23 (m, 2H), 1.23–1.17 (m, 2H), 1.11–1.03 (m, 2H), 0.98 (br.d, J = 7.6 Hz, 2H); 13C-NMR (75 MHz, CDCl3) δ 176.9 (d, J = 2.3 Hz), 174.4, 170.6, 167.1, 165.6, 153.7 (d, J = 251.4 Hz), 147.2, 146.4 (d, J = 10.4 Hz), 139.2, 119.1 (d, J = 7.6 Hz), 116.3, 111.9 (d, J = 23.8 Hz), 107.8, 104.8 (d, J = 2.9 Hz), 69.7, 63.0, 60.8, 45.7, 45.6, 45.6, 43.1, 39.2, 36.1, 35.4, 34.1, 32.8, 29.3, 27.5, 26.4, 21.9, 17.8, 10.0, 8.2; HRMS (ESI) m/z calculated for C34H39FN4O5 [M + H+] 603.2977, found 603.3007.
1-Cyclopropyl-6-fluoro-7-(4-(2-(3-methylpyrazin-2-yloxy)-ethyl)-1-oxa-9-azaspiro[5.5]undec-9-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3l)
Yield—55 mg (25%), white solid, m.p. 86–88 °C. 1H-NMR (300 MHz, CDCl3) δ 15.08 (s, 1H), 8.72 (s, 1H), 8.01–7.87 (m, 3H), 7.36 (d, J = 7.2 Hz, 1H), 4.38 (t, J = 6.6 Hz, 2H), 3.81 (dd, J = 11.8, 4.4 Hz, 1H), 3.65 (t, J = 11.3 Hz, 1H), 3.56–3.47 (m, 2H), 3.41 (br.s, 1H), 3.32 (td, J = 11.7, 2.9 Hz, 1H), 3.18–3.05 (m, 1H), 2.46 (s, 3H), 2.50–2.35 (m, 1H), 1.98–1.81 (m, 2H), 1.77–1.66 (m, 5H), 1.63–1.55 (m, 1H), 1.41–1.35 (m, 2H), 1.35–1.29 (m, 1H), 1.28–1.23 (m, 1H), 1.21–1.16 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ 177.1 (d, J = 2.6 Hz), 167.2, 158.5, 153.7 (d, J = 251.3 Hz), 147.3, 146.5 (d, J = 10.4 Hz), 144.8, 139.1, 138.1, 135.5, 119.4 (d, J = 7.9 Hz), 112.2 (d, J = 23.6 Hz), 108.0, 104.8 (d, J = 3.5 Hz), 69.7, 63.6, 60.7, 45.7, 45.7, 45.6, 45.6, 43.0, 39.2, 36.0, 35.3, 32.7, 29.2, 27.6, 19.4, 8.3, 8.2; HRMS (ESI) m/z calculated for C29H33FN4O5 [M + H+] 537.2508, found 537.2531.
7-(4-tert-Butoxycarbonylamino-1-oxa-9-azaspiro[5.5]undec-9-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3m)
Yield—110 mg (51%), white solid, m.p. 246–248 °C. 1H-NMR (300 MHz, CDCl3) δ 15.01 (s, 1H), 8.73 (s, 1H), 7.97 (d, J = 13.1 Hz, 1H), 7.42 (d, J = 6.9 Hz, 1H), 4.38 (br.s, 1H), 3.93–3.77 (m, 2H), 3.68 (t, J = 11.6 Hz, 1H), 3.56–3.41 (m, 3H), 3.40–3.28 (m, 1H), 3.15 (t, J = 11.2 Hz, 1H), 2.34 (br.d, J = 13.8 Hz, 1H), 2.01–1.86 (m, 3H), 1.80–1.69 (m, 2H), 1.44 (s, 9H), 1.43–1.35 (m, 3H), 1.26–1.16 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 177.1 (d, J = 2.6 Hz), 167.2, 155.2, 153.8 (d, J = 251.4 Hz), 147.4, 146.3 (d, J = 10.2 Hz), 139.2, 119.5 (d, J = 7.8 Hz), 112.2 (d, J = 23.6 Hz), 108.0, 105.1 (d, J = 2.5 Hz), 79.7, 60.1, 45.8, 45.7, 45.6, 45.6, 43.6, 43.1, 39.0, 35.4, 33.5, 29.5, 28.5, 8.3; HRMS (ESI) m/z calculated for C27H34FN3O6 [M + H+] 516.2504, found 516.2486.
7-(4-Amino-1-oxa-9-azaspiro[5.5]undec-9-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3n)
Yield—60 mg (35%), brown solid, m.p. 131–133 °C. 1H-NMR (300 MHz, D2O) δ 8.53 (s, 1H), 7.32 (br.s, 1H), 7.15 (d, J = 6.3 Hz, 1H), 4.00 (br.s, 1H), 3.97–3.87 (m, 1H), 3.82 (br.s, 1H), 3.70–3.36 (m, 3H), 3.33–3.09 (m, 2H), 2.51–2.35 (m, 1H), 2.25–2.11 (m, 2H), 2.00 (br.s, 1H), 1.92–1.73 (m, 3H), 1.70–1.60 (m, 1H), 1.46 (br.s, 2H), 1.15 (br.s, 2H); 13C-NMR (75 MHz, D2O) δ 175.8, 169.4, 153.6 (d, J = 251.4 Hz), 148.1, 146.2 (d, J = 7.5 Hz), 139.3, 117.6 (d, J = 3.5 Hz), 110.6 (d, J = 24.3 Hz), 106.1, 105.9, 72.1, 59.6, 45.8, 45.8, 45.7, 45.7, 44.8, 39.6, 38.3, 36.6, 30.5, 29.1, 8.0; HRMS (ESI) m/z calculated for C22H26FN3O4 [M + H+] 416.1980, found 416.1996.
7-(4-(Benzyloxycarbonylaminomethyl)-1-oxa-9-azaspiro[5.5]undec-9-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3o)
Yield—220 mg (93%), white solid, m.p. 102–104 °C. 1H-NMR (300 MHz, CDCl3) δ 15.09 (s, 1H), 8.73 (s, 1H), 7.96 (d, J = 13.1 Hz, 1H), 7.42–7.31 (m, 6H), 5.10 (s, 2H), 4.92 (br.s, 1H), 3.81 (dd, J = 11.6, 4.3 Hz, 1H), 3.61 (t, J = 11.9 Hz, 1H), 3.56–3.39 (m, 3H), 3.37–3.26 (m, 1H), 3.17–3.02 (m, 3H), 2.34 (br.d, J = 13.2 Hz, 1H), 1.93 (d, J = 13.8 Hz, 1H), 1.84 (dd, J = 11.5, 3.9 Hz, 1H), 1.71 (d, J = 14.6 Hz, 2H), 1.61 (d, J = 13.8 Hz, 2H), 1.42–1.33 (m, 2H), 1.28–1.10 (m, 4H); 13C-NMR (75 MHz, CDCl3) δ 176.9 (d, J = 2.6 Hz), 167.1, 156.6, 153.6 (d, J = 251.3 Hz), 147.2, 146.3 (d, J = 10.3 Hz), 139.1, 136.5, 128.5, 128.1, 128.0, 119.1 (d, J = 7.9 Hz), 111.9 (d, J = 23.6 Hz), 107.8, 104.8 (d, J = 3.4 Hz), 69.6, 66.7, 60.4, 47.1, 45.6, 45.6, 45.6, 40.2, 39.1, 35.3, 31.2, 30.2, 29.2, 8.2; HRMS (ESI) m/z calculated for C31H34FN3O6 [M + H+] 564.2504, found 564.2488.
1-Cyclopropyl-6-fluoro-7-(4-(3-fluorobenzyloxy)-1-oxa-9-azaspiro[5.5]undec-9-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3p)
Yield—58 mg (27%), brown solid, m.p. 78–80 °C. 1H-NMR (300 MHz, CDCl3) δ 15.01 (s, 1H), 8.73 (s, 1H), 7.97 (d, J = 13.1 Hz, 1H), 7.44 (d, J = 7.0 Hz, 1H), 7.35–7.27 (m, 1H), 7.13–7.03 (m, 2H), 7.03–6.92 (m, 1H), 4.56 (s, 2H), 3.93 (dt, J = 11.9, 4.5 Hz, 1H), 3.85–3.72 (m, 1H), 3.68–3.58 (m, 1H), 3.56–3.40 (m, 3H), 3.36–3.25 (m, 1H), 3.25–3.13 (m, 1H), 2.09 (br.d, J = 12.9 Hz, 2H), 2.03–1.95 (m, 1H), 1.95–1.90 (m, 1H), 1.90–1.86 (m, 1H), 1.85–1.74 (m, 1H), 1.72–1.64 (m, 1H), 1.64–1.57 (m, 1H), 1.42–1.34 (m, 2H), 1.24–1.15 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ 177.1 (d, J = 2.6 Hz), 167.1, 163.1 (d, J = 245.9 Hz), 153.8 (d, J = 251.4 Hz), 147.4, 146.3 (d, J = 10.2 Hz), 141.4 (d, J = 7.1 Hz), 139.2, 130.1 (d, J = 8.2 Hz), 122.8 (d, J = 2.9 Hz), 119.6 (d, J = 7.9 Hz), 114.5 (d, J = 19.8 Hz), 114.2 (d, J = 20.3 Hz), 112.3 (d, J = 23.6 Hz), 108.1, 105.2 (d, J = 2.9 Hz), 71.6, 70.6, 69.3, 69.3, 58.9, 45.9, 45.9, 45.8, 45.7, 41.8, 37.1, 35.4, 32.5, 32.1, 8.3; HRMS (ESI) m/z calculated for C29H30F2N2O5 [M + H+] 525.2196, found 525.2212.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-(pyridin-2-yloxy)-1-oxa-9-azaspiro[5.5]undec-9-yl)-1,4-dihydroquinoline-3-carboxylic Acid (3q)
Yield—110 mg (54%), white solid, m.p. 118–120 °C. 1H-NMR (300 MHz, CDCl3) δ 15.12 (s, 1H), 8.73 (s, 1H), 8.12 (d, J = 3.7 Hz, 1H), 7.95 (d, J = 13.1 Hz, 1H), 7.64–7.49 (m, 1H), 7.37 (d, J = 7.1 Hz, 1H), 6.94–6.79 (m, 1H), 6.70 (d, J = 8.3 Hz, 1H), 5.48–5.35 (m, 1H), 4.05–3.89 (m, 1H), 3.83–3.72 (m, 1H), 3.56–3.39 (m, 3H), 3.34–3.14 (m, 2H), 2.24 (d, J = 12.9 Hz, 1H), 2.15–1.99 (m, 3H), 1.91–1.76 (m, 3H), 1.73–1.68 (m, 1H), 1.43–1.32 (m, 2H), 1.25–1.14 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ 177.0 (d, J = 2.6 Hz), 167.1, 162.9, 153.7 (d, J = 251.3 Hz), 147.2, 146.9, 146.4 (d, J = 10.3 Hz), 139.2, 138.8, 119.2 (d, J = 7.9 Hz), 116.8, 112.0 (d, J = 23.6 Hz), 111.7, 107.8, 104.9 (d, J = 3.4 Hz), 70.8, 67.5, 58.9, 45.8, 45.7, 45.6, 45.5, 41.2, 37.1, 35.4, 32.4, 31.9, 8.3; HRMS (ESI) calculated for C27H28FN3O5 [M + H+] 494.2086, found 494.2108.
1-Cyclopropyl-6-fluoro-7-(4-methoxy-1-oxa-9-azaspiro[5.5]undec-9-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3r)
Yield—84 mg (47%), white solid, m.p. 182–184 °C. 1H-NMR (300 MHz, CDCl3) δ 15.01 (s, 1H), 8.71 (s, 1H), 7.94 (d, J = 13.1 Hz, 1H), 7.41 (d, J = 6.7 Hz, 1H), 3.95–3.85 (m, 1H), 3.67–3.60 (m, 1H), 3.57–3.39 (m, 4H), 3.35 (s, 3H), 3.33–3.24 (m, 1H), 3.18 (t, J = 11.4 Hz, 1H), 2.14–1.93 (m, 3H), 1.91–1.81 (m, 2H), 1.75 (t, J = 11.7 Hz, 1H), 1.61–1.45 (m, 2H), 1.43–1.32 (m, 2H), 1.19 (br.s, 2H); 13C-NMR (75 MHz, CDCl3) δ 177.2 (d, J = 2.6 Hz), 167.2, 153.9 (d, J = 251.5 Hz), 147.5, 146.1 (d, J = 10.8 Hz), 139.2, 119.8 (d, J = 5.4 Hz), 112.4 (d, J = 23.5 Hz), 108.1, 105.4 (d, J = 2.1 Hz), 73.1, 70.6, 59.0, 55.7, 46.0, 45.9, 45.8, 41.4, 37.1, 35.5, 32.2, 31.8, 8.4; HRMS (ESI) m/z calculated for C23H27FN2O5 [M + H+] 431.1977, found 431.1995.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-(pyrazin-2-yloxy)-1-oxa-9-azaspiro[5.5]undec-9-yl)-1,4-dihydroquinoline-3-carboxylic Acid (3s)
Yield—170 mg (83%), white solid, m.p. 128–130 °C. 1H-NMR (300 MHz, CDCl3) δ 15.08 (s, 1H), 8.72 (s, 1H), 8.18 (s, 1H), 8.11 (d, J = 2.4 Hz, 1H), 8.05 (br.s, 1H), 7.94 (d, J = 13.1 Hz, 1H), 7.37 (d, J = 6.8 Hz, 1H), 5.50–5.31 (m, 1H), 4.03–3.89 (m, 1H), 3.78 (t, J = 9.9 Hz, 1H), 3.58–3.41 (m, 3H), 3.29 (t, J = 11.1 Hz, 1H), 3.18 (t, J = 11.4 Hz, 1H), 2.24 (br.d, J = 14.7 Hz, 1H), 2.17–2.06 (m, 2H), 2.03 (br.s, 1H), 1.91–1.77 (m, 3H), 1.76–1.66 (m, 1H), 1.38 (br.d, J = 5.2 Hz, 2H), 1.19 (br.s, 2H); 13C-NMR (75 MHz, CDCl3) δ 176.8 (d, J = 0.8 Hz), 167.0, 159.4, 153.6 (d, J = 251.5 Hz), 147.2, 146.2 (d, J = 10.4 Hz), 140.5, 139.1, 136.6, 136.3, 119.1 (d, J = 7.9 Hz), 111.9 (d, J = 23.4 Hz), 107.9, 104.9 (d, J = 1.9 Hz), 70.8, 68.7, 58.7, 45.6, 45.5, 41.0, 37.1, 35.3, 32.2, 31.6, 8.2; HRMS (ESI) m/z calculated for C26H27FN4O5 [M + Na+] 517.1858, found 517.1881.
1-Cyclopropyl-6-fluoro-7-(4-methyl-1-oxa-9-azaspiro[5.5]undec-9-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3t)
Yield—113 mg (66%), yellow solid, m.p. 131–133 °C. 1H-NMR (300 MHz, CDCl3) δ 15.09 (s, 1H), 8.72 (s, 1H), 7.95 (d, J = 13.1 Hz, 1H), 7.36 (d, J = 7.1 Hz, 1H), 3.77 (dd, J = 12.1, 4.7 Hz, 1H), 3.61 (t, J = 12.2 Hz, 1H), 3.55–3.37 (m, 3H), 3.36–3.25 (m, 1H), 3.10 (t, J = 11.1 Hz, 1H), 2.37 (br.d, J = 13.9 Hz, 1H), 1.91–1.64 (m, 4H), 1.64–1.48 (m, 3H), 1.42–1.31 (m, 2H), 1.26–1.15 (m, 3H), 0.93 (d, J = 6.4 Hz, 3H); 13C-NMR (75 MHz, CDCl3) δ 176.8 (d, J = 2.0 Hz), 167.0, 153.6 (d, J = 251.2 Hz), 147.1, 146.4 (d, J = 10.3 Hz), 139.1, 119.0 (d, J = 8.0 Hz), 111.8 (d, J = 23.7 Hz), 107.8, 104.8 (d, J = 3.2 Hz), 69.7, 60.9, 45.7, 45.7, 45.6, 44.9, 39.2, 35.3, 34.7, 29.3, 25.1, 22.5, 8.2, 8.1; HRMS (ESI) m/z calculated for C23H27FN2O4 [M + Na+] 437.1847, found 437.1861.
1-Cyclopropyl-6-fluoro-7-(4-hydroxy-1-oxa-9-azaspiro[5.5]undec-9-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3u)
Yield—143 mg (83%), yellow solid, m.p. 250–252 °C. 1H-NMR (300 MHz, D2O) δ 8.43 (s, 1H), 7.68 (d, J = 13.6 Hz, 1H), 7.32 (d, J = 7.3 Hz, 1H), 4.08–3.93 (m, 1H), 3.86–3.75 (m, 1H), 3.62 (t, J = 11.5 Hz, 1H), 3.49–3.35 (m, 1H), 3.24–2.99 (m, 3H), 2.86 (t, J = 10.8 Hz, 1H), 2.06 (br.d, J = 14.2 Hz, 1H), 1.98–1.86 (m, 2H), 1.75 (br.s, 2H), 1.66–1.54 (m, 1H), 1.53–1.39 (m, 1H), 1.36–1.24 (m, 3H), 1.01 (br.s, 2H); 13C-NMR (75 MHz, D2O) δ 173.8 (d, J = 2.0 Hz), 170.8, 151.6 (d, J = 247.2 Hz), 145.5, 142.9 (d, J = 10.9 Hz), 136.9, 120.3 (d, J = 7.2 Hz), 115.1, 109.9 (d, J = 23.0 Hz), 104.6 (d, J = 1.9 Hz), 70.7, 62.1, 57.8, 44.4, 41.9, 36.1, 33.2, 32.8, 28.4, 6.0; HRMS (ESI) m/z calculated for C22H25FN2O5 [M + Na+] 439.1640, found 439.1652.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-(4-trifluoromethylbenzyloxy)-1-oxa-9-azaspiro[5.5]undec-9-yl)-1,4-dihydroquinoline-3-carboxylic Acid (3v)
Yield—62 mg (26%), white solid, m.p. 86–88 °C. 1H-NMR (300 MHz, CDCl3) δ 15.00 (s, 1H), 8.74 (s, 1H), 7.99 (d, J = 13.1 Hz, 1H), 7.61 (d, J = 8.0 Hz, 2H), 7.46 (d, J = 7.8 Hz, 3H), 4.63 (s, 2H), 3.99–3.89 (m, 1H), 3.86–3.75 (m, 1H), 3.70–3.59 (m, 1H), 3.56–3.40 (m, 3H), 3.32 (t, J = 11.2 Hz, 1H), 3.20 (t, J = 11.4 Hz, 1H), 2.13–1.90 (m, 5H), 1.79–1.55 (m, 3H), 1.39 (d, J = 5.5 Hz, 2H), 1.20 (s, 2H); 13C-NMR (75 MHz, CDCl3) δ 177.2 (d, J = 2.2 Hz), 167.3, 153.9 (d, J = 251.5 Hz), 147.5, 146.2 (d, J = 6.0 Hz), 142.7, 139.2, 129.9 (q, J = 65.1, 32.7 Hz), 127.5, 125.5 (q, J = 3.7 Hz), 124.2 (q, J = 272.0 Hz), 119.8 (d, J = 5.5 Hz), 112.4 (d, J = 23.5 Hz), 108.2, 105.3 (d, J = 1.5 Hz), 71.8, 70.6, 69.3, 58.9, 45.9, 45.8, 41.8, 37.1, 35.5, 32.4, 32.1, 8.4; HRMS (ESI) m/z calculated for C30H30F4N2O5 [M + Na+] 597.1983, found 597.2006.
1-Cyclopropyl-6-fluoro-7-(4-(4-fluorobenzyl)-1-oxa-9-azaspiro[5.5]undec-9-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3w)
Yield—93 mg (44%), white solid, m.p. 87–89 °C. 1H-NMR (300 MHz, CDCl3) δ 15.05 (s, 1H), 8.74 (s, 1H), 7.96 (d, J = 12.9 Hz, 1H), 7.42 (br.s, 1H), 7.14–6.93 (m, 4H), 3.88–3.73 (m, 1H), 3.62–3.29 (m, 5H), 3.20–3.05 (m, 1H), 2.58–2.45 (m, 2H), 2.41–2.28 (m, 1H), 1.97–1.82 (m, 2H), 1.77–1.68 (m, 1H), 1.62–1.48 (m, 3H), 1.43–1.32 (m, 2H), 1.29–1.13 (m, 4H); 13C-NMR (75 MHz, CDCl3) δ 177.2 (d, J = 2.4 Hz), 167.2, 161.6 (d, J = 243.9 Hz), 153.9 (d, J = 251.5 Hz), 147.4, 146.3 (d, J = 11.3 Hz), 139.3, 135.5 (d, J = 3.2 Hz), 130.5 (d, J = 7.7 Hz), 119.7 (d, J = 7.8 Hz), 115.2 (d, J = 21.1 Hz), 112.4 (d, J = 23.7 Hz), 108.2, 105.2 (d, J = 1.7 Hz), 69.8, 60.9, 46.1, 46.0, 45.9, 45.9, 43.0, 43.0, 39.3, 35.5, 32.6, 29.4, 8.4, 8.4; HRMS (ESI) m/z calculated for C29H30F2N2O4 [M + Na+] 531.2066, found 531.2084.
1-Cyclopropyl-6-fluoro-7-(4-(4-fluorobenzyloxy)-1-oxa-9-azaspiro[5.5]undec-9-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3x)
Yield—88 mg (38%), white solid, m.p. 89–91 °C. 1H-NMR (300 MHz, CDCl3) δ 15.04 (s, 1H), 8.74 (s, 1H), 7.98 (d, J = 13.1 Hz, 1H), 7.42 (d, J = 6.1 Hz, 1H), 7.31 (dd, J = 8.3, 5.5 Hz, 2H), 7.03 (t, J = 8.6 Hz, 2H), 4.52 (s, 2H), 3.98–3.87 (m, 1H), 3.84–3.71 (m, 1H), 3.68–3.57 (m, 1H), 3.55–3.38 (m, 3H), 3.36–3.24 (m, 1H), 3.17 (t, J = 11.7 Hz, 1H), 2.13–2.02 (m, 2H), 1.99–1.82 (m, 3H), 1.80–1.72 (m, 1H), 1.71–1.63 (m, 1H), 1.60–1.53 (m, 2H), 1.43–1.34 (m, 2H), 1.20 (br.s, 2H); 13C-NMR (75 MHz, CDCl3) δ 177.2 (d, J = 2.5 Hz), 167.2, 162.4 (d, J = 245.6 Hz), 153.9 (d, J = 251.5 Hz), 147.5, 146.1 (d, J = 6.5 Hz), 139.2, 134.3 (d, J = 3.1 Hz), 129.3 (d, J = 8.1 Hz), 119.9 (d, J = 6.9 Hz), 115.4 (d, J = 21.4 Hz), 112.5 (d, J = 23.4 Hz), 108.2, 105.5 (d, J = 2.6 Hz), 71.3, 70.6, 69.4, 59.0, 45.9, 45.9, 41.8, 37.1, 35.5, 32.3, 32.2, 8.4; HRMS (ESI) m/z calculated for C32H34FN3O5 [M + Na+] 547.2020, found 547.2018.
1-Cyclopropyl-7-(4-cyclopropylmethoxy-1-oxa-9-azaspiro[5.5]undec-9-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3y)
Yield—86 mg (44%), yellow solid, m.p. 86–88 °C. 1H-NMR (300 MHz, CDCl3) δ 15.05 (s, 1H), 8.72 (s, 1H), 7.94 (d, J = 13.1 Hz, 1H), 7.40 (d, J = 7.0 Hz, 1H), 3.89 (dt, J = 11.9, 4.2 Hz, 1H), 3.71–3.64 (m, 1H), 3.63–3.55 (m, 1H), 3.54–3.40 (m, 3H), 3.34–3.23 (m, 3H), 3.21–3.10 (m, 1H), 2.13 (d, J = 13.8 Hz, 1H), 2.05–1.93 (m, 2H), 1.91–1.83 (m, 2H), 1.77–1.67 (m, 1H), 1.64–1.53 (m, 1H), 1.53–1.45 (m, 1H), 1.42–1.34 (m, 2H), 1.22–1.15 (m, 2H), 1.12–0.97 (m, 1H), 0.66–0.48 (m, 2H), 0.29–0.14 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ 176.8, (d, J = 2.4 Hz), 166.9, 153.6 (d, J = 251.4 Hz), 147.1, 146.2 (d, J = 10.3 Hz), 139.1, 119.0 (d, J = 7.9 Hz), 111.7 (d, J = 23.7 Hz), 107.7, 104.9 (d, J = 3.2 Hz), 72.6, 71.1, 70.6, 59.0, 45.7, 45.6, 45.5, 45.5, 41.8, 37.4, 35.4, 32.3, 31.9, 10.9, 8.1, 3.0; HRMS (ESI) m/z calculated for C26H31FN2O5 [M + H+] 471.2290, found 471.2310.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-(3-pyridin-3-yl(1,2,4)oxadiazol-5-ylmethyl)-1-oxa-9-azaspiro[5.5]undec-9-yl)-1,4-dihydroquinoline-3-carboxylic Acid (3z)
Yield—180 mg (77%), beige solid, m.p. 238–240 °C. 1H-NMR (300 MHz, CDCl3) δ 15.07 (s, 1H), 9.34–9.23 (m, 1H), 8.73 (dd, J = 4.9, 1.7 Hz, 1H), 8.70 (s, 1H), 8.33 (dt, J = 8.0, 1.9 Hz, 1H), 7.92 (d, J = 13.1 Hz, 1H), 7.43 (ddd, J = 8.0, 4.9, 0.7 Hz, 1H), 7.35 (d, J = 7.2 Hz, 1H), 3.84 (dd, J = 11.9, 4.3 Hz, 1H), 3.74–3.62 (m, 1H), 3.56–3.38 (m, 3H), 3.31 (td, J = 11.6, 2.9 Hz, 1H), 3.18–3.05 (m, 1H), 2.91 (d, J = 7.0 Hz, 2H), 2.42 (br.d, J = 13.6 Hz, 2H), 1.94–1.57 (m, 5H), 1.52–1.31 (m, 4H), 1.22–1.14 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ 178.7, 176.7 (d, J = 2.4 Hz), 166.8, 166.3, 153.5 (d, J = 249.7 Hz), 151.9, 148.4, 147.0, 146.1 (d, J = 10.3 Hz), 139.1, 134.6, 123.6, 123.1, 118.9 (d, J = 7.8 Hz), 111.7 (d, J = 23.6 Hz), 107.7, 104.8 (d, J = 3.3 Hz), 69.8, 60.3, 45.5, 45.5, 42.3, 39.0, 35.3, 33.6, 32.3, 29.3, 29.2, 8.1; HRMS (ESI) m/z calculated for C30H30FN5O5 [M + H+] 560.2304, found 560.2323.
7-(4-((N-Benzyl-N-methylcarbamoyl)methyl)-1-oxa-9-azaspiro[5.5]undec-9-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3aa)
Yield—143 mg (61%), white solid, m.p. 97–99 °C. 1H-NMR (300 MHz, CDCl3) δ 15.07 (s, 1H), 8.73 (s, 1H), 7.97 (d, J = 13.2 Hz, 1H), 7.40–7.20 (m, 5H), 7.18–7.11 (m, 1H), 4.66–4.52 (m, 2H), 3.81–3.63 (m, 2H), 3.56–3.39 (m, 3H), 3.38–3.26 (m, 1H), 3.21–3.09 (m, 1H), 2.98–2.91 (2s, 3H), 2.47–2.35 (m, 2H), 2.32–2.22 (m, 2H), 1.87–1.62 (m, 6H), 1.37 (br.d, J = 5.4 Hz, 2H), 1.24–1.14 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 176.8, 171.8, 171.4, (d, J = 1.7 Hz), 153.6 (d, J = 251.8 Hz), 147.3, 146.2 (d, J = 4.7 Hz), 138.1, 137.4, 136.6, 129.0, 128.6, 128.0, 127.7, 127.4, 126.2, 119.2 (d, J = 2.7 Hz), 111.9 (d, J = 24.6 Hz), 104.7 (d, J = 2.7 Hz), 69.9, 69.9, 60.8, 60.7, 53.3, 50.8, 45.7, 45.7, 42.8, 42.7, 40.4, 40.1, 39.2, 35.3, 34.9, 34.1, 32.9, 32.8, 29.3, 27.5, 27.5, 8.2; HRMS (ESI) m/z calculated for C32H36FN3O5 [M + H+] 562.2712, found 562.2697.
7-(4-((N-Benzyl-N-cyclopropyl)amino)-1-oxa-9-azaspiro[5.5]undec-9-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3ab)
Yield—66 mg (29%), brown solid, m.p. 241–243 °C. 1H-NMR (300 MHz, DMSO-d6) δ 15.25 (s, 1H), 8.64 (s, 1H), 7.87 (dd, J = 13.3, 3.0 Hz, 1H), 7.78–7.62 (m, 2H), 7.57 (t, J = 7.2 Hz, 1H), 7.46 (d, J = 1.8 Hz, 3H), 4.42 (br.s, 2H), 3.83 (br.s, 2H), 3.70–3.55 (m, 2H), 3.52–3.43 (m, 2H), 3.36–3.20 (m, 2H), 3.12–2.96 (m, 1H), 2.76–2.53 (m, 1H), 2.30–2.12 (m, 2H), 2.01–1.90 (m, 1H), 1.90–1.83 (m, 1H), 1.82–1.67 (m, 2H), 1.66–1.42 (m, 1H), 1.31 (br.s, 3H), 1.19 (br.s, 2H), 0.96–0.76 (m, 2H), 0.74–0.57 (m, 1H); 13C-NMR (75 MHz, DMSO-d6) δ 176.8 (d, J = 2.7 Hz), 166.3, 153.5 (d, J = 249.1 Hz), 148.3, 145.8 (d, J = 10.2 Hz), 139.8, 132.4, 132.2, 129.8, 129.0, 118.9 (d, J = 7.8 Hz), 111.4 (d, J = 23.4 Hz), 107.4, 106.7 (d, J = 3.5 Hz), 71.2, 59.5, 55.9, 55.3. 45.8, 45.8, 45.7, 45.7, 45.6, 36.3, 29.1, 8.1, 8.0; HRMS (ESI) m/z calculated for C32H36FN3O4 [M + H+] 546.2763, found 546.2771.
1-Cyclopropyl-7-(4-(3-cyclopropyl[1,2,4]oxadiazol-5-ylmethyl)-1-oxa-9-azaspiro[5.5]undec-9-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3ac)
Yield—102 mg (47%), white solid, m.p. 106–108 °C. 1H-NMR (300 MHz, CDCl3) δ 15.07 (s, 1H), 8.73 (s, 1H), 7.96 (d, J = 13.1 Hz, 1H), 7.36 (d, J = 6.2 Hz, 1H), 3.81 (dd, J = 11.5, 3.9 Hz, 1H), 3.64 (t, J = 11.8 Hz, 1H), 3.57–3.38 (m, 3H), 3.31 (t, J = 10.6 Hz, 1H), 3.10 (t, J = 11.1 Hz, 1H), 2.74 (d, J = 6.7 Hz, 2H), 2.43–2.20 (m, 2H), 2.11–2.03 (m, 1H), 1.90–1.80 (m, 1H), 1.77–1.58 (m, 4H), 1.41–1.17 (m, 6H), 1.08–0.96 (m, 4H); 13C-NMR (75 MHz, CDCl3) δ 175.5, 175.0 (d, J = 2.6 Hz), 170.4, 165.1, 151.7 (d, J = 251.3 Hz), 145.3, 144.4 (d, J = 10.4 Hz), 137.1, 117.4 (d, J = 8.0 Hz), 110.1 (d, J = 23.6 Hz), 105.9, 102.8 (d, J = 3.4 Hz), 67.7, 58.3, 43.6, 43.5, 43.5, 40.4, 37.0, 33.3, 31.7, 30.2, 27.3, 27.1, 6.2, 6.2, 5.8, 4.8; HRMS (ESI) m/z calculated for C28H31FN4O5 [M + Na+] 545.2171, found 545.2187.
1-Cyclopropyl-6-fluoro-7-(4-(3-(2-methoxyethyl)[1,2,4]oxadiazol-5-ylmethyl)-1-oxa-9-azaspiro[5.5]undec-9-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3ad)
Yield—138 mg (61%), brown solid, m.p. 101–103 °C. 1H-NMR (300 MHz, CDCl3) δ 15.03 (s, 1H), 8.70 (s, 1H), 7.93 (d, J = 13.1 Hz, 1H), 7.39 (d, J = 6.5 Hz, 1H), 3.80–3.73 (m, 2H), 3.70–3.58 (m, 1H), 3.55–3.44 (m, 2H), 3.43–3.30 (m, 4H), 3.12 (t, J = 11.5 Hz, 1H), 2.99 (t, J = 6.3 Hz, 2H), 2.79 (d, J = 6.7 Hz, 2H), 2.44–2.24 (m, 2H), 2.15–1.79 (m, 3H), 1.77–1.54 (m, 4H), 1.40–1.15 (m, 6H); 13C-NMR (75 MHz, CDCl3) δ 178.0, 177.1 (d, J = 2.5 Hz), 168.4, 167.2, 153.8 (d, J = 251.4 Hz), 147.4, 146.2 (d, J = 10.2 Hz), 139.2, 119.6 (d, J = 7.8 Hz), 112.3 (d, J = 23.6 Hz), 108.1, 105.2 (d, J = 2.0 Hz), 69.8, 69.2, 60.5, 58.8, 45.8, 45.8, 45.7, 42.6, 39.1, 35.4, 33.8, 32.4, 29.4, 29.3, 26.9, 8.4; HRMS (ESI) m/z calculated for C28H33FN4O6 [M + H+] 541.2457, found 541.2472.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-(4-phenylpiperazin-1-yl)-1-oxa-9-azaspiro[5.5]undec-9-yl)-1,4-dihydroquinoline-3-carboxylic Acid (3ae)
Yield—170 mg (73%), pale brown solid, m.p. 117–119 °C. 1H-NMR (300 MHz, DMSO-d6) δ 15.23 (s, 1H), 8.64 (s, 1H), 7.86 (d, J = 12.8 Hz, 1H), 7.56 (d, J = 5.2 Hz, 1H), 7.21 (br.s, 2H), 6.93 (d, J = 7.2 Hz, 2H), 6.77 (br.s, 1H), 3.87–3.75 (m, 2H), 3.69–3.55 (m, 2H), 3.47–3.39 (m, 3H), 3.33–3.27 (m, 2H), 3.19–3.11 (m, 4H), 2.80–2.67 (m, 4H), 2.35–2.24 (m, 1H), 1.91–1.76 (m, 3H), 1.71–1.57 (m, 2H), 1.36–1.27 (m, 3H), 1.23–1.15 (m, 2H); 13C-NMR (75 MHz, DMSO-d6) δ 176.4 (d, J = 2.4 Hz), 166.1, 153.1 (d, J = 249.5 Hz), 150.7, 148.0, 145.6 (d, J = 10.2 Hz), 139.3, 129.0, 119.2, 118.3 (d, J = 7.0 Hz), 115.5, 110.9 (d, J = 22.8 Hz), 106.7, 106.4 (d, J = 4.0 Hz), 76.8, 71.8, 70.4, 59.4, 56.3, 48.4, 47.9, 45.5, 45.4, 45.3, 43.7, 38.0, 35.9, 29.1, 28.2, 7.6; HRMS (ESI) m/z calculated for C32H37FN4O4 [M + H+] 561.2872, found 561.2859.
7-(4-((N-Benzoyl-N-cyclopropyl)amino)-1-oxa-9-azaspiro[5.5]undec-9-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3af)
Yield—188 mg (81%), white solid, m.p. 136–138 °C. 1H-NMR (300 MHz, CDCl3) δ 15.09 (s, 1H), 8.75 (s, 1H), 7.98 (d, J = 13.0 Hz, 1H), 7.52–7.43 (m, 3H), 7.42–7.35 (m, 3H), 4.66 (t, J = 9.7 Hz, 1H), 3.93 (dd, J = 11.1, 3.6 Hz, 1H), 3.77 (t, J = 11.8 Hz, 1H), 3.57–3.32 (m, 4H), 3.19 (t, J = 10.9 Hz, 1H), 2.58 (br.s, 1H), 2.47 (br.d, J = 13.2 Hz, 1H), 2.06–1.76 (m, 7H), 1.39 (br.s, 2H), 1.25–1.16 (m, 2H), 0.68–0.55 (m, 2H), 0.46 (s, 2H); 13C-NMR (75 MHz, CDCl3) δ 177.2 (d, J = 2.4 Hz), 173.2, 167.2, 153.8 (d, J = 251.7 Hz), 147.5, 145.9 (d, J = 8.6 Hz), 139.2, 137.9, 129.7, 128.1, 127.4, 119.9 (d, J = 7.2 Hz), 112.5 (d, J = 23.5 Hz), 108.2, 105.5, 71.2, 60.9, 51.4, 46.1, 46.0, 45.9, 45.9, 40.7, 39.4, 35.5, 31.3, 29.2, 28.7, 10.1, 10.1, 8.4; HRMS (ESI) m/z calculated for C32H34FN3O5 [M + H+] 560.2555, found 560.2567.
1-Cyclopropyl-6-fluoro-7-(4-morpholin-4-yl-1-oxa-9-azaspiro[5.5]undec-9-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3ag)
Yield—91 mg (45%), yellow solid, m.p. 187–189 °C. 1H-NMR (300 MHz, CDCl3) δ 14.97 (s, 1H), 8.72 (s, 1H), 7.96 (d, J = 13.2 Hz, 1H), 7.36 (d, J = 7.2 Hz, 1H), 3.94–3.84 (m, 1H), 3.78–3.69 (m, 4H), 3.68–3.58 (m, 1H), 3.54–3.39 (m, 3H), 3.39–3.28 (m, 1H), 3.19–3.06 (m, 1H), 2.70–2.52 (m, 5H), 2.32 (br.d, J = 14.0 Hz, 1H), 1.95–1.75 (m, 4H), 1.70–1.50 (m, 2H), 1.47–1.33 (m, 3H), 1.23–1.15 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ 177.0 (d, J = 2.7 Hz), 166.8, 153.7 (d, J = 251.1 Hz), 147.1, 146.3 (d, J = 10.4 Hz), 139.2, 119.3 (d, J = 7.9 Hz), 112.1 (d, J = 23.7 Hz), 108.1, 104.7 (d, J = 3.6 Hz), 70.5, 67.1, 60.2, 56.9, 49.6, 45.7, 45.7, 45.6, 45.6, 39.2, 39.1, 35.2, 30.0, 28.9, 8.1, 8.1; HRMS (ESI) m/z calculated for C26H32FN3O5 [M + H+] 486.2399, found 486.2420.
1-Cyclopropyl-7-(4-(1-cyclopropyl-3-isopropylureido)-1-oxa-9-azaspiro[5.5]undec-9-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3ah)
Yield—79 mg (35%), white solid, m.p. 130–132 °C. 1H-NMR (300 MHz, CDCl3) δ 15.11 (s, 1H), 8.74 (s, 1H), 7.97 (d, J = 13.1 Hz, 1H), 7.35 (d, J = 7.1 Hz, 1H), 5.17 (d, J = 7.6 Hz, 1H), 4.48 (tt, J = 12.1, 3.3 Hz, 1H), 4.02–3.91 (m, 1H), 3.86 (dd, J = 12.3, 5.0 Hz, 1H), 3.71 (t, J = 11.4 Hz, 1H), 3.56–3.49 (m, 1H), 3.49–3.39 (m, 2H), 3.38–3.27 (m, 1H), 3.14 (t, J = 10.9 Hz, 1H), 2.43 (br.d, J = 14.2 Hz, 1H), 2.33–2.24 (m, 1H), 2.13–1.96 (m, 1H), 1.86–1.68 (m, 7H), 1.38 (d, J = 7.0 Hz, 2H), 1.17 (d, J = 6.5 Hz, 7H), 0.91–0.83 (m, 2H), 0.82–0.73 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ 176.9 (d, J = 2.5 Hz), 167.1, 158.6, 153.6 (d, J = 251.4 Hz), 147.2, 146.3 (d, J = 10.3 Hz), 139.1, 119.1 (d, J = 7.9 Hz), 111.9 (d, J = 23.5 Hz), 107.8, 104.8 (d, J = 3.4 Hz), 71.2, 60.9, 50.2, 45.7, 45.6, 45.6, 45.5, 42.4, 41.2, 39.3, 35.3, 31.6, 29.2, 24.7, 23.5, 23.5, 8.8, 8.2; HRMS (ESI) m/z calculated for C29H37FN4O5 [M + Na+] 563.2640, found 563.2665.
1-Cyclopropyl-7-(4-(1-cyclopropyl-3-ethylureido)-1-oxa-9-azaspiro[5.5]undec-9-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3ai)
Yield—180 mg (82%), white solid, m.p. 151–153 °C. 1H-NMR (300 MHz, CDCl3) δ 15.14 (s, 1H), 8.71 (s, 1H), 7.92 (d, J = 13.1 Hz, 1H), 7.35 (d, J = 7.2 Hz, 1H), 5.32 (t, J = 5.4 Hz, 1H), 4.47 (tt, J = 12.3, 3.5 Hz, 1H), 3.85 (dd, J = 11.8, 4.1 Hz, 1H), 3.77–3.64 (m, 1H), 3.56–3.48 (m, 1H), 3.48–3.37 (m, 2H), 3.37–3.21 (m, 3H), 3.19–3.06 (m, 1H), 2.42 (br.d, J = 14.1 Hz, 1H), 2.35–2.24 (m, 1H), 1.99–1.91 (m, 2H), 1.83–1.75 (m, 2H), 1.75–1.65 (m, 3H), 1.44–1.32 (m, 2H), 1.21–1.16 (m, 2H), 1.14 (t, J = 7.2 Hz, 3H), 0.91–0.83 (m, 2H), 0.82–0.73 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ 177.2 (d, J = 2.7 Hz), 167.4, 159.4, 153.8 (d, J = 251.3 Hz), 147.4, 146.5 (d, J = 10.4 Hz), 139.3, 119.5 (d, J = 7.9 Hz), 112.3 (d, J = 23.5 Hz), 108.1, 104.9 (d, J = 3.6 Hz), 71.4, 61.1, 50.5, 45.9, 45.8, 45.7, 45.7, 41.3, 39.5, 35.6, 35.4, 31.7, 29.3, 25.0, 15.7, 8.8, 8.3; HRMS (ESI) m/z calculated for C28H35FN4O5 [M + Na+] 549.2484, found 549.2502.
7-(4-(N-Acetyl-N-cyclopropylamino)-1-oxa-9-azaspiro[5.5]undec-9-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (3aj)
Yield—80 mg (39%), beige solid, m.p. 120–122 °C. 1H-NMR (300 MHz, CDCl3) δ 15.07 (s, 1H), 8.74 (s, 1H), 7.96 (d, J = 13.1 Hz, 1H), 7.44 (d, J = 6.8 Hz, 1H), 4.55 (t, J = 11.9 Hz, 1H), 3.88 (dd, J = 11.7, 4.5 Hz, 1H), 3.73 (t, J = 11.8 Hz, 1H), 3.59–3.33 (m, 4H), 3.18 (t, J = 11.1 Hz, 1H), 2.58–2.41 (m, 2H), 2.23 (s, 3H), 2.16–2.03 (m, 1H), 1.97–1.85 (m, 2H), 1.84–1.67 (m, 4H), 1.41 (d, J = 6.6 Hz, 2H), 1.26–1.16 (m, 2H), 1.01–0.90 (m, 2H), 0.89–0.79 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ 177.2 (d, J = 2.7 Hz), 174.1, 167.1, 153.9 (d, J = 251.4 Hz), 147.5, 146.0 (d, J = 10.5 Hz), 139.3, 119.9 (d, J = 8.0 Hz), 112.5 (d, J = 23.6 Hz), 108.3, 105.3 (d, J = 2.3 Hz), 71.2, 61.0, 50.7, 46.1, 46.0, 46.0, 45.9, 40.7, 39.4, 35.5, 31.3, 29.3, 28.3, 23.8, 9.5, 9.4, 8.4; HRMS (ESI) m/z calculated for C27H32FN3O5 [M + Na+] 520.2218, found 520.2237.