Antimicrobial and Cytotoxic Activities of Constituents from the Fruit of Albizia lebbeck L. Benth (Fabaceae)
Abstract
1. Introduction
2. Results
2.1. Structure Elucidation of Compounds 1 and 2
2.2. Physicochemical Characteristics of Compounds 1 and 2
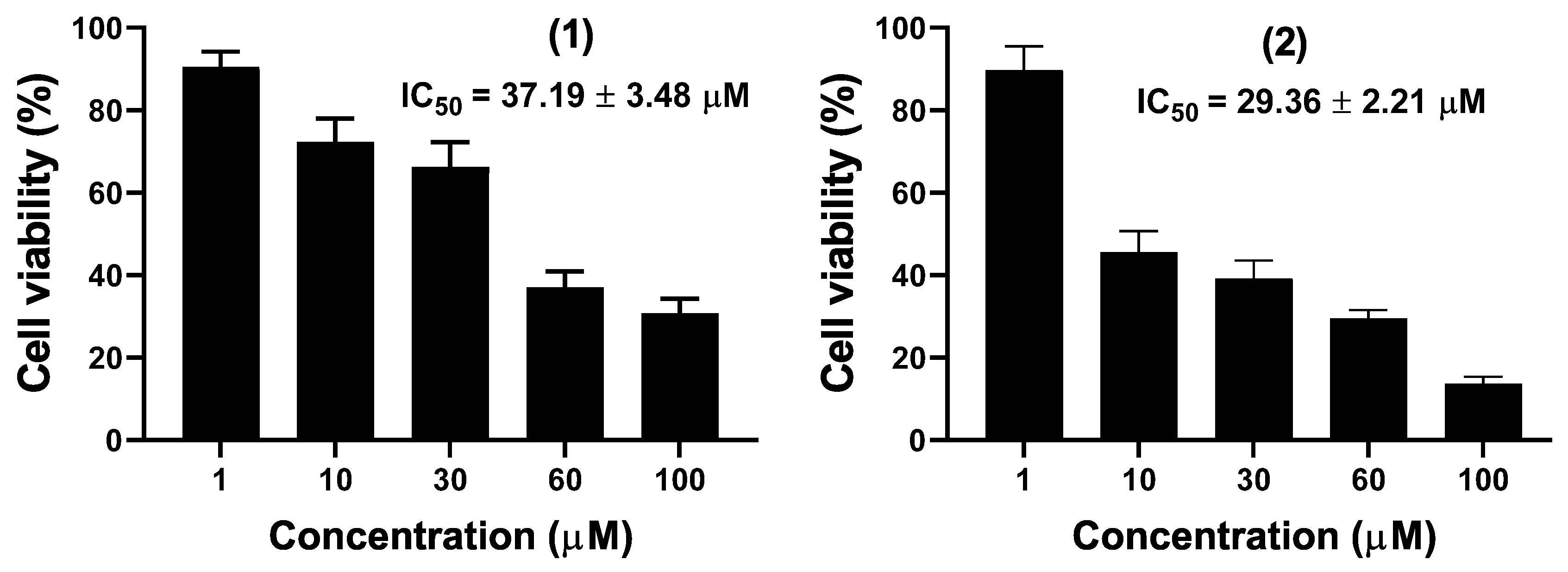
2.3. Biological Assays
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Antimicrobial Assay
4.5. Cytotoxicity Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Al-Massarani, S.M.; El Gamal, A.A.; Abd El Halim, M.F.; Al-Said, M.S.; Abdel-Kader, M.S.; Basudan, O.A.; Alqasoumi, S.I. New acyclic secondary metabolites from the biologically active fraction of Albizia lebbeck flowers. Saudi Pharm. J. 2017, 25, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mou, Y.; Zhao, J.; Wang, J.; Zhou, L.; Wang, M.; Yang, F. Flavonoids from Halostachys caspica and their antimicrobial and antioxidant activities. Molecules 2010, 15, 7933–7945. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Gao, Y.; Du, K.Y.; Luo, X.Y. Screening of 17 Chinese medicine plants against phytopathogenic fungi and active component in Syzygium aromaticum. J. Plant Dis. Prot. 2020, 127, 237–244. [Google Scholar] [CrossRef]
- Abd El-Ghany, A.E.S.; Dora, G.; Abdallah, R.H.; Hassan, W.; El-Salam, E.A. Phytochemical and biological study of Albizia lebbeck stem bark. J. Chem. Pharma. Res. 2015, 7, 29–43. [Google Scholar]
- Tchinda, C.F.; Sonfack, G.; Simo, I.K.; Çelik, İ.; Voukeng, I.K.; Nganou, B.K.; Kuete, V. Antibacterial and antibiotic-modifying activities of fractions and compounds from Albizia adianthifolia against MDR Gram-negative enteric bacteria. BMC Complement. Altern. Med. 2019, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Lim, K. Sterols from Cucurbita maxima. Philipp. J. Sci. 2005, 134, 83. [Google Scholar]
- Dzoyem, J.P.; Melong, R.; Tsamo, A.T.; Tchinda, A.T.; Kapche, D.G.; Ngadjui, B.T.; Eloff, J.N. Cytotoxicity, antimicrobial and antioxidant activity of eight compounds isolated from Entada abyssinica (Fabaceae). BMC Res. Notes 2017, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Leutcha, B.P.; Sema, D.K.; Dzoyem, J.P.; Ayimele, G.A.; Nyongbela, K.D.; Delie, F.; Alléman, E.; Sewald, N.; Lannang, A.M. Cytotoxicity of a new tirucallane derivative isolated from Stereospermum acuminatissimum K. Schum stem bark. Nat. Prod. Res. 2020, 35, 4417–4422. [Google Scholar] [CrossRef] [PubMed]
- Lannang, A.M.; Sema, D.K.; Tatsimo, S.J.; Tankeu, V.T.; Tegha, H.F.; Wansi, J.D.; Shiono, Y.; Sewald, N. A new depsidone derivative from the leaves of Garcinia polyantha. Nat. Prod. Res. 2018, 32, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Misra, L.N.; Dixit, A.K.; Wagner, H. N-demethylbudmunchiamines from Albizzia lebbeck seeds. Phytochemistry 1995, 39, 247–249. [Google Scholar] [CrossRef]
- Schaefer, H.W. Reaction of primary and secondary amines to form carbamic acid glucuronides. Curr. Drug Metab. 2006, 7, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Coggins, J.A.; Powner, W.M. Prebiotic synthesis of phosphoenol pyruvate by α-phosphorylation-controlled triose glycolysis. Nat. Chem. 2017, 9, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Koagne, R.R.; Annang, F.; Cautain, B.; Martín, J.; Pérez-Moreno, G.; Bitchagno, G.T.M.; González-Pacanowska, D.; Vicente, F.; Simo, I.K.; Reyes, F.; et al. Cytotoxycity and antiplasmodial activity of phenolic derivatives from Albizia zygia (DC.) J.F. Macbr. (Mimosaceae). BMC Complement. Med. Ther. 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.K. NMR spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef]
- Kosuge, T.; Ishida, H.; Satoh, T. Studies on Antihemorrhagic Substances in Herbs Classified as Hemostatics in Chinese Medicine. V. On Antihemorrhagic Principle in Biota orientalis (L.) ENDL. Chem. Pharm. Bull. 1985, 33, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Al-Amin, M.D.; Siddiqi, M.M.A.; Akter, S.; Haque, M.M.; Sultana, N.; Chowdhury, A.S. Isolation of Quercetin-3-O-β-D-glucopyranoside from the Leaves of AzadirachtaIndica and Antimicrobial and Cytotoxic screening of the Crude Extracts. Dhaka Univ. J. Sci. 2012, 60, 11–14. [Google Scholar] [CrossRef]
- Taewoong, R.; Kee, D.Y. Chemical Constituents of Nelumbonucifera Seeds. Nat. Prod. Sci. 2017, 23, 253–257. [Google Scholar]
- Hu, J.; Feng, X. Triterpenoids, p-coumaric acid esters and flavonoids from Artemisia igniaria. Planta Med. 2000, 66, 684–686. [Google Scholar]
- Kamaya, R.; Tanaka, Y.; Hiyama, R.; Ageta, H. Fern constituents: Triterpenoids isolated from the leaves of Cheiropleuria bicuspis. Chem. Pharm. Bull. 1990, 38, 2130–2132. [Google Scholar] [CrossRef][Green Version]
- You, Y.J.; Nam, N.H.; Kim, Y.; Bae, K.H.; Ahn, B.Z. Antiangiogenic activity of lupeol from Bombax ceiba. Phytotherapy Res. 2003, 17, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Tijjani, A.; Ndukwe, I.G.; Ayo, R.G. Isolation and characterization of lup-20(29)-ene-3, 28-diol (Betulin) from the stem-bark of Adeniumobesum (Apocynaceae). Trop. J. Pharm. Res. 2012, 11, 259–262. [Google Scholar] [CrossRef]
- Seebacher, W.; Simic, N.; Weis, R.; Saf, R.; Kunert, O. Complete assignments of 1H and 13C-NMR resonances of oleanolic acid, 18α-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn. Reason. Chem. 2003, 41, 636–638. [Google Scholar] [CrossRef]
- Edwards, J.C.; Hunter, J.M.; Nemzer, B.V. Multinuclear NMR of calcium fructoborate complex-structure, stability, and quantitation in the presence of other ingredients, excipients or adulterants. J. Food Res. 2014, 3, 115–131. [Google Scholar] [CrossRef]
- Jones, A.J.; Hanisch, P.; McPhail, A.K. Sucrose: An assignment of the 13C-NMR parameters by selective decoupling. Aust. J. Chem. 1979, 32, 2763–2766. [Google Scholar] [CrossRef]
- Kamboj, A.; Saluja, A.K. Isolation of stigmasterol and β-sitosterol from petroleum ether extract of aerial parts of Ageratum conyzoides (Asteraceae). Int. J. Pharm. Pharm. Sci. 2011, 3, 94–96. [Google Scholar]
- Ramaiarantsoa, H.; Koffi, A.B.; Assi, A.M.; Djakoure, L.A. Les 3-O-β-D-glucoside du β-sitostérol isolés des feuilles de Ravenala madagascariensis. J. Soc. Ouest-Afr. Chim. 2008, 26, 99–103. [Google Scholar]
- Freire, C.S.; Silvestre, A.J.; Neto, C.P. Demonstration of long-chain n-alkyl caffeates and Δ7-steryl glucosides in the bark of Acacia species by gas chromatography-mass spectrometry. Phytochem. Anal. Int. J. Plant. Chem. Biochem. Tech. 2007, 18, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, G.E.; Lewis, D. 1H nuclear magnetic resonance spectra and conformations of alditols in deuterium oxide. J. Chem. Soc. Perkin Trans. 1984, 2, 2073–2078. [Google Scholar] [CrossRef]
- Koay, Y.C.; Wong, K.C.; Osman, H.; Eldeen, I.; Asmawi, M.Z. Chemical constituents and biological activities of Strobilanthescrispus L. Rec. Nat. Prod. 2013, 7, 59–64. [Google Scholar]
- Melong, R.; Kapche, D.G.; Feussi, M.T.; Laatsch, H. A new aggreceride analogue and a peltogynoid isolated from the stem bark of Entadaabyssinica (Fabaceae). Nat. Prod. Commun. 2014, 9, 1934578X1400901024. [Google Scholar]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef]
- Venkatesh, H.N.; Mohana, D.C. Antimicrobial activities of successive solvent extracts of Albizialebbeck and Solanumseaforthianum against some human pathogenic microorganisms. J. Pharm. Clin. Res. 2019, 12, 294–296. [Google Scholar]
- Tegha, H.F.; Jouda, J.B.; Dzoyem, J.P.; Sema, D.K.; Leutcha, B.P.; Allémann, E.; Delie, F.; Shiono, Y.; Sewald, N.; Lannang, A.M. A New Chromene Derivative and a New Polyalcohol Isolated from the Fungus Xylaria sp. 111A Associated with Garciniapolyantha Leaves. Nat. Prod. Commun. 2021, 16, 1934578X20987334. [Google Scholar]
- Kaoke, M.D.; Sema, D.K.; Dzoyem, J.P.; Leutcha, P.B.; Delie, F.; Allemann, E. A new cytotoxic glycocerebroside from RauvolfiamacrophyllaStapf. Nat. Prod. Chem. Res. 2020, 8, 1–5. [Google Scholar]
- Mishra, S.S.; Gothecha, V.K.; Sharma, A. Albizia lebbeck: A short review. J. Herb. Med. Toxicol. 2010, 4, 9–15. [Google Scholar]
- Tam, S.W. Flavonoid and Triterpenoid Constituents of the Ericaceae of Hong-Kong. Master’s Thesis, The University of Hong Kong, Hong Kong, China, 1961. [Google Scholar]
- Abdurrahman, I.; Cai-Xiab, Y. Isolation and characterization of fatty acid derivatives from the stem barks of Albizia Amara (Fabaceae), Sudanese Medicinal Plant. Chem. Methodol. 2020, 4, 369–377. [Google Scholar]
- Nehdi, I. Characteristics, chemical composition and utilisation of Albizia julibrissin seed oil. Ind. Crops Prod. 2011, 33, 30–34. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Tchamgoue, J.; Tchouankeu, J.C.; Kouam, S.F.; Choudhary, M.I.; Bakowsky, U. Antibacterial activity and cytotoxicity of flavonoids compounds isolated from Pseudarthriahookeri Wight &Arn. (Fabaceae). S. Afr. J. Bot. 2018, 114, 100–103. [Google Scholar]
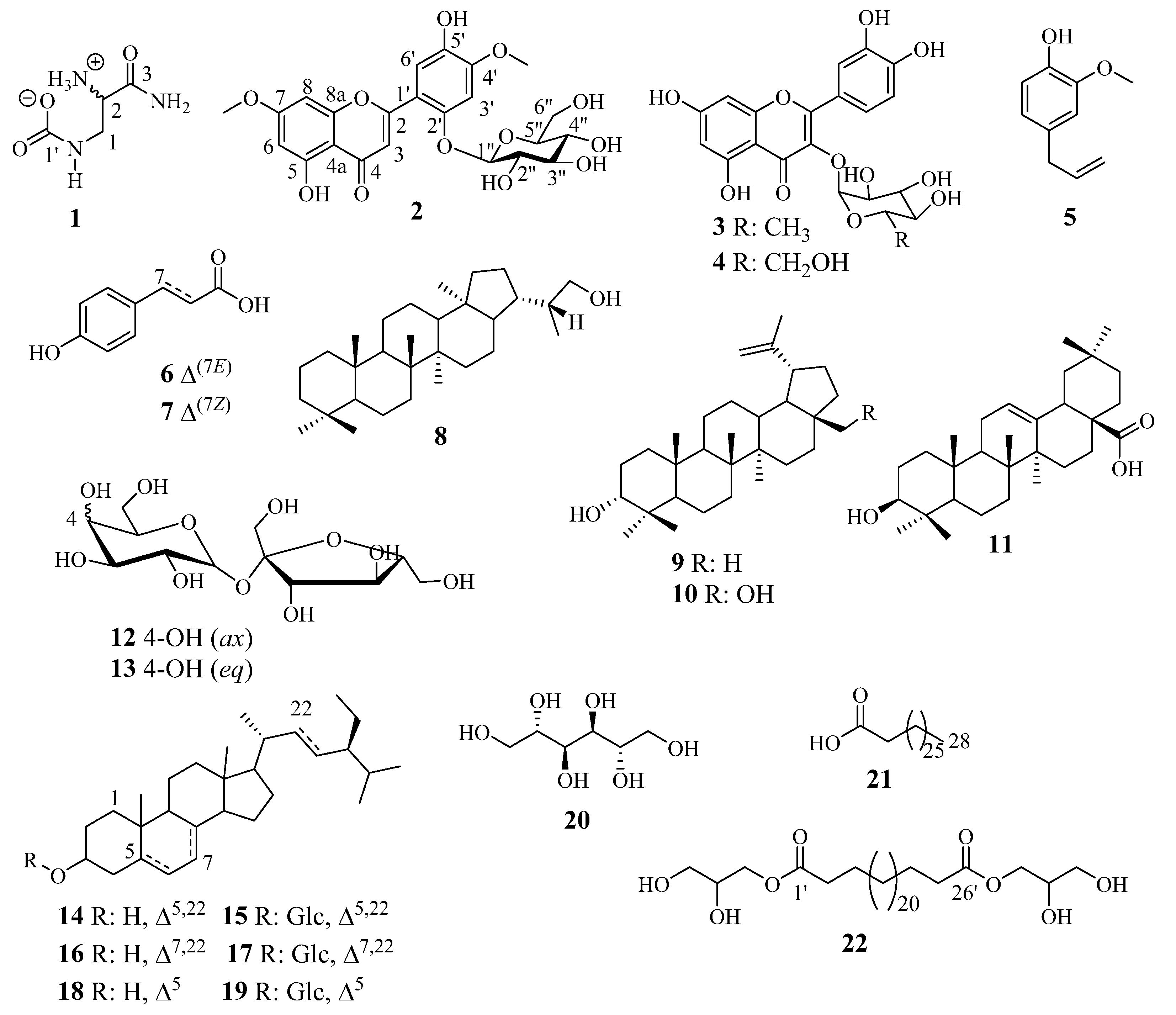
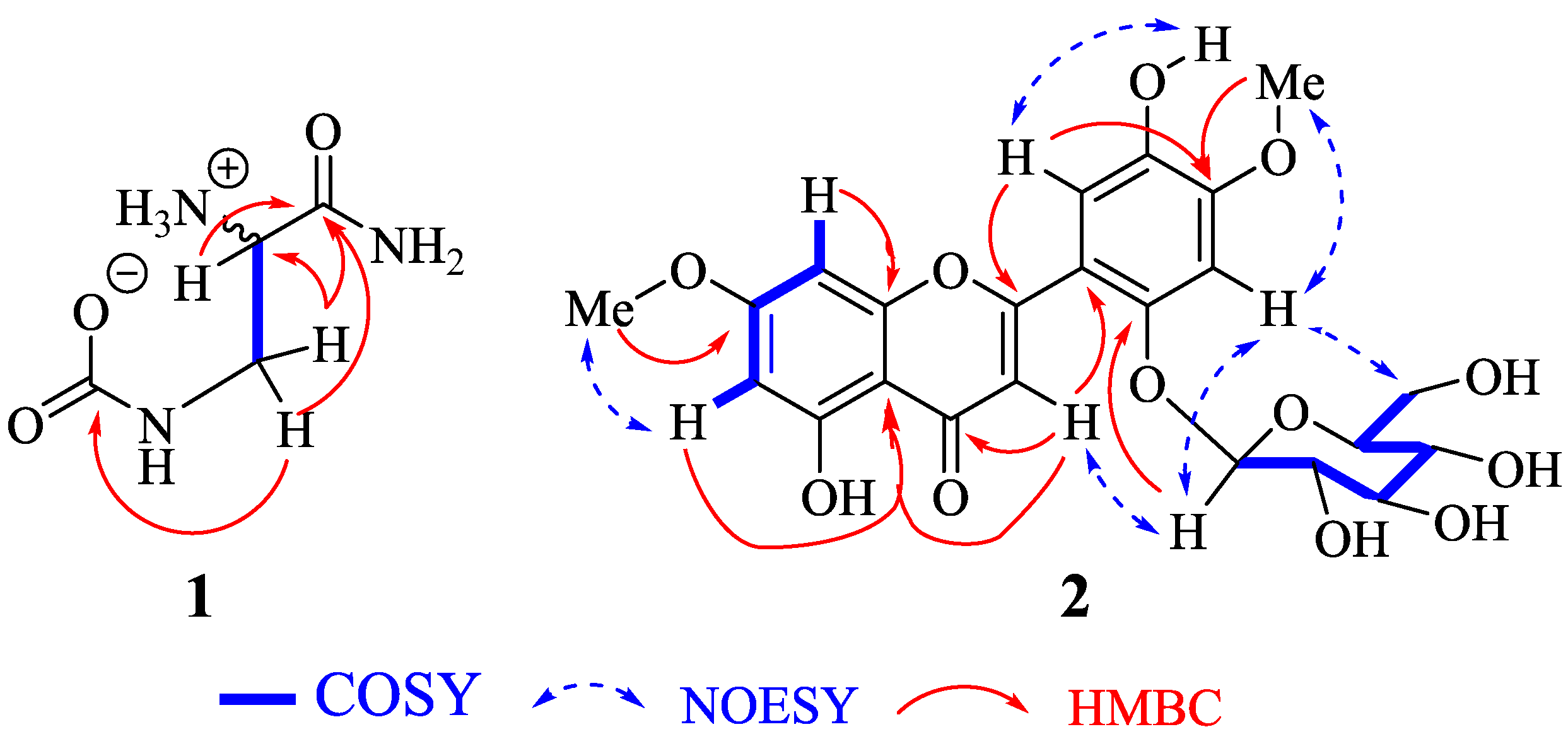

| N° | 1 | N° | 2 | ||||
|---|---|---|---|---|---|---|---|
| δC, | δH (m, J in Hz) | HMBC | δC, | δH (m, J in Hz) | HMBC | ||
| 1′ | 161.6 | 1 | |||||
| 1 | 40.2 | 3.55 (ddd, 15.3, 3.6, 1.3) | 1, 2′, 3′ | 2 | 161.5 | ||
| 3.35 (ddd, 15.2, 6.5, 1.4) | |||||||
| 2 | 55.4 | 3.70 (ddd, 6.5, 3.5, 1.3) | 1′, 3′ | 3 | 109.6 | 7.17 (s) | 2, 4, 4a, 1′ |
| 3 | 172.4 | 4 | 182.6 | ||||
| 4a | 105.1 | ||||||
| 5 | 161.6 | ||||||
| 6 | 98.3 | 6.38 (d, 2.2) | 4a, 5, 7, 8 | ||||
| 7 | 165.6 | ||||||
| 8 | 92.8 | 6.71 (d, 2.2) | 4a, 6, 7, 8a | ||||
| 8a | 157.8 | ||||||
| 1′ | 111.6 | ||||||
| 2′ | 150.6 | ||||||
| 3′ | 101.1 | 6.99 (s) | 1′, 2′, 4′, 5′ | ||||
| 4′ | 151.9 | ||||||
| 5′ | 141.5 | ||||||
| 6′ | 114.4 | 7.37 (s) | 2, 2′, 4′, 5′ | ||||
| 7-OMe | 56.6 | 3.87 (s) | 7 | ||||
| 4′-OMe | 56.5 | 3.86 (s) | 4′ | ||||
| 1″ | 101.6 | 5.05 (d, 7.2) | 2′ | ||||
| 2″ | 73.9 | 3.35 (m) | |||||
| 3″ | 77.9 | 3.43 (dd, 9.6, 4.0) | |||||
| 4″ | 70.5 | 3.14 (td, 8.6, 4.8) | |||||
| 5″ | 77.5 | 3.29 (m) | |||||
| 6″ | 61.3 | 3.74 (dd, 9.7, 5.4) 3.43 (dd, 9.6, 4.0) | |||||
| 4′-OH | 9.09 (s) | 4′ | |||||
| 5-OH | 13.05 (s) | ||||||
| Samples | Microorganisms | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Candida albicans | Escherichia coli | Staphylococcus aureus | Pseudomonas aeruginosa | Enterococcus faecalis | ||||||
| MIC | MFC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| CH2Cl2-MeOH (1:1, v/v) extract | 512 | - | 128 | - | 512 | - | 256 | - | 256 | 1024 |
| n-hexane extract | - | - | - | - | 512 | - | - | - | 512 | - |
| ethyl acetate extract | 128 | - | 128 | 1024 | 256 | - | 512 | - | 256 | 1024 |
| n-butanol extract | - | - | - | - | - | - | - | - | - | - |
| 1 | 32 | 256 | 256 | - | 128 | - | 128 | - | - | - |
| 2 | 32 | 256 | 32 | 256 | 256 | 256 | 128 | 256 | 64 | - |
| 12 | 256 | - | - | - | 256 | - | - | - | 256 | - |
| 13 | - | - | - | - | 256 | - | - | - | - | - |
| Ketoconazole | 0.5 | 64 | - | - | - | - | - | - | - | - |
| Ciprofloxacin | - | - | 2 | 512 | 1 | 512 | 2 | 8 | 4 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leutcha, B.P.; Dzoyem, J.P.; Jouda, J.-B.; Sema, D.K.; Tsague Tankeu, V.F.; Bitchagno, G.T.M.; Tchegnitegni, B.T.; Essoung, F.R.E.; Ndjakou Lenta, B.; Fogue Kouam, S.; et al. Antimicrobial and Cytotoxic Activities of Constituents from the Fruit of Albizia lebbeck L. Benth (Fabaceae). Molecules 2022, 27, 4823. https://doi.org/10.3390/molecules27154823
Leutcha BP, Dzoyem JP, Jouda J-B, Sema DK, Tsague Tankeu VF, Bitchagno GTM, Tchegnitegni BT, Essoung FRE, Ndjakou Lenta B, Fogue Kouam S, et al. Antimicrobial and Cytotoxic Activities of Constituents from the Fruit of Albizia lebbeck L. Benth (Fabaceae). Molecules. 2022; 27(15):4823. https://doi.org/10.3390/molecules27154823
Chicago/Turabian StyleLeutcha, Bosco Peron, Jean Paul Dzoyem, Jean-Bosco Jouda, Denis Kehdinga Sema, Virginie Flaure Tsague Tankeu, Gabin Thierry Mbahbou Bitchagno, Billy Toussie Tchegnitegni, Flaure Rosette Ehawa Essoung, Bruno Ndjakou Lenta, Siméon Fogue Kouam, and et al. 2022. "Antimicrobial and Cytotoxic Activities of Constituents from the Fruit of Albizia lebbeck L. Benth (Fabaceae)" Molecules 27, no. 15: 4823. https://doi.org/10.3390/molecules27154823
APA StyleLeutcha, B. P., Dzoyem, J. P., Jouda, J.-B., Sema, D. K., Tsague Tankeu, V. F., Bitchagno, G. T. M., Tchegnitegni, B. T., Essoung, F. R. E., Ndjakou Lenta, B., Fogue Kouam, S., Delie, F., Meli Lannang, A., & Sewald, N. (2022). Antimicrobial and Cytotoxic Activities of Constituents from the Fruit of Albizia lebbeck L. Benth (Fabaceae). Molecules, 27(15), 4823. https://doi.org/10.3390/molecules27154823







