Actional Mechanisms of Active Ingredients in Functional Food Adlay for Human Health
Abstract
1. Introduction
2. Functional Ingredients in Adlay
3. Action Pathway against Disease of Adlay
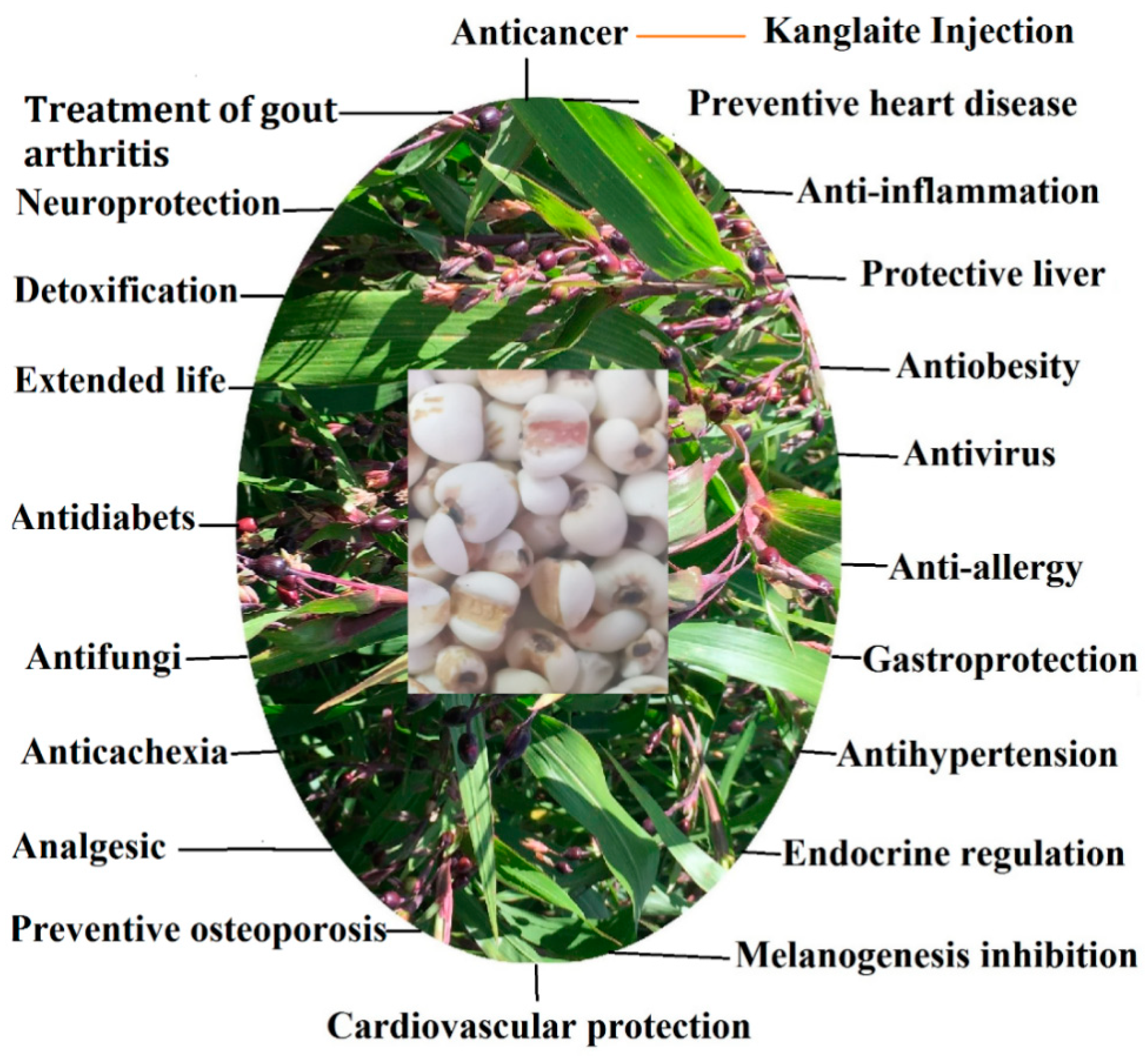
3.1. Anti-Cancer Effects
3.2. Anti-Inflammation Effects
3.3. Anti-Obesity Effects
3.4. Protective Liver Effects
3.5. Anti-Virus Effects
3.6. Cardiovascular Protection Effects
3.7. Gastroprotection Effects
3.8. Anti-Hypertension Effects
3.9. Preventative of Heart Disease
3.10. Anti-Allergy Effects
3.11. Melanogenesis Inhibition
3.12. Endocrine Regulation
3.13. Anti-Diabetes Effects
3.14. Anti-Cachexia Effects
3.15. Preventive of Osteoporosis
3.16. Analgesic Effects
3.17. Neuroprotection Effects
3.18. Treatment of Gout Arthritis
3.19. Extended Life
3.20. Ant-Fungi Effects
3.21. Detoxification Effects
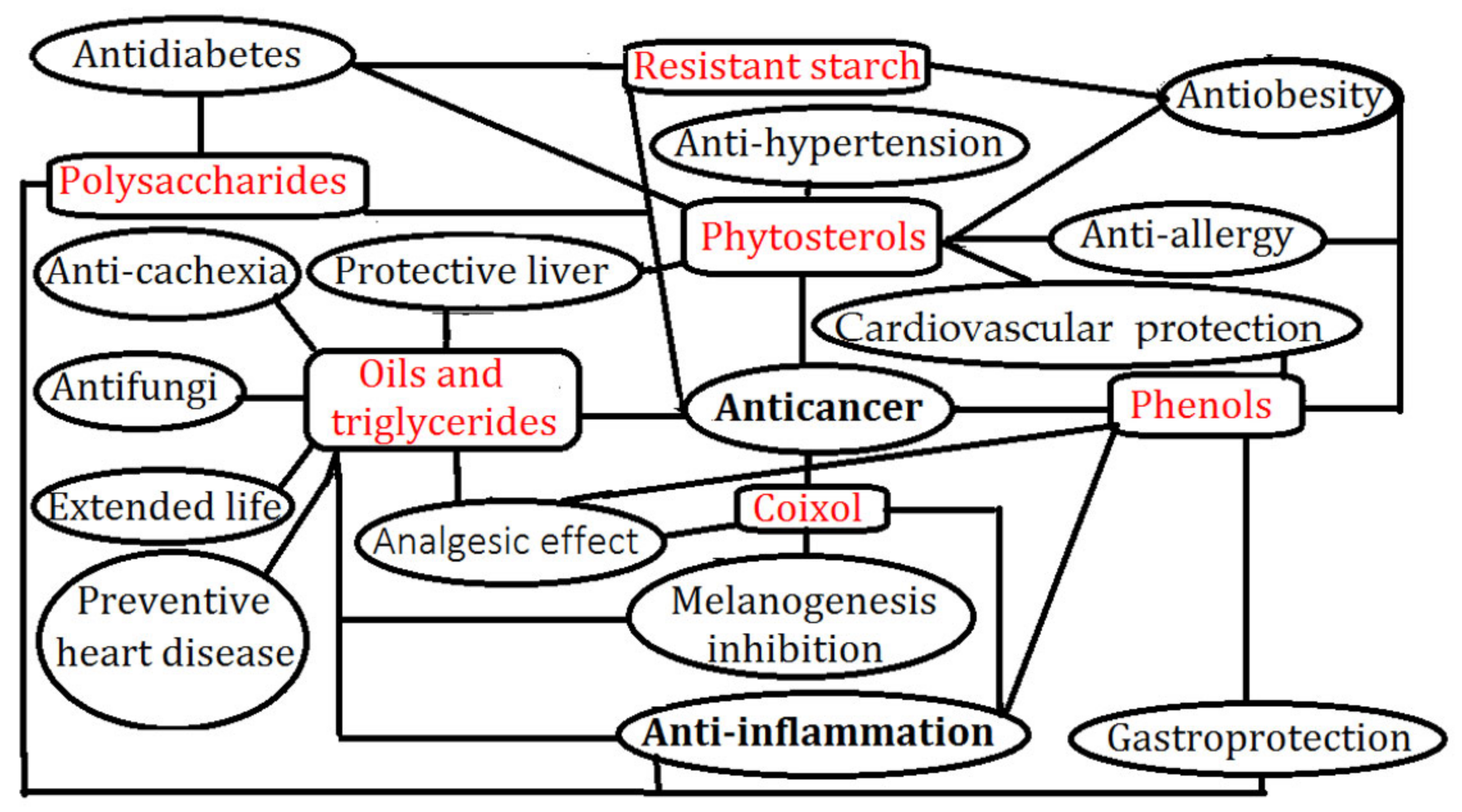
4. Functional Ingredients Mechanisms in Adlay to Combat Disease
4.1. Oils
4.2. Polysaccharides
4.3. Phenols
4.4. Coixol
4.5. Phytosterols
4.6. Resistant Starch
5. Anti-Oxidants of Functional Ingredients in Adlay
5.1. Anti-Oxidants in Adlay
5.2. Anti-Oxidants to Prevent Chronic Diseases
6. The Food and Pharmaceutical Industry
6.1. Food Industry
6.2. Kanglaite Injection of Anti-Cancer Drugs
6.3. Other Drugs
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steed, G.; Ramirez, D.C.; Hannah, M.A.; Webb, A.A.R. Chronoculture, harnessing the circadian clock to improve crop yield and sustainability. Science 2021, 372, eabc9141. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.W.; Yang, J.Z.; Du, J.; Li, X.; Yang, X.M.; Pu, X.Y. Regular functional rice to combat human diabetes. BMJ 2019, 364, 1969. Available online: https://www.bmj.com/content/364/bmj.l969/rr-0 (accessed on 28 March 2019).
- Zeng, Y.W. Regular staple food with low glycaemic index to combat human diabetes. BMJ 2022, 376, e067516. Available online: https://www.bmj.com/content/376/bmj-2021-067516/rr (accessed on 23 January 2022).
- Zeng, Y.W.; Ahmed, A.G.M.-D. Barley functional foods and human genes affect obesity. Science 2021, 373, eabf8683. Available online: https://www.science.org/do/10.1126/comment.763056/full/ (accessed on 4 July 2021).
- Liu, H.; Shi, J.; Cai, Z.; Huang, Y.; Lv, M.; Du, H.; Gao, Q.; Zuo, Y.; Dong, Z.; Huang, W.; et al. Evolution and domestication footprints uncovered from the genomes of Coix. Mol. Plant 2020, 13, 295–308. [Google Scholar] [CrossRef]
- Kuo, C.C.; Chen, H.H.; Chiang, W. Adlay (yì yĭ; “soft-shelled job’s tears”; the seeds of Coix lachryma-jobi L. var. ma-yuen Stapf) is a potential cancer chemopreventive agent toward multistage carcinogenesis processes. J. Tradit. Complement. Med. 2012, 2, 267–275. [Google Scholar] [CrossRef]
- Li, X.K.; Gu, K.; Liang, M.W.; Zhang, Y.T.; Wang, Y.M.; Li, Y.B. Research progress on chemical constituents and pharmacological effects of Coicis Semen. Chin. Tradit. Her. Drugs 2020, 51, 5045–5657. [Google Scholar]
- Diningrat, D.S.; Sari, A.N.; Harahap, N.S.; Kusdianti, K. Potential of Hanjeli (Coix lacryma-jobi) essential oil in preventing SARS-CoV-2 infection via blocking the Angiotensin Converting Enzyme 2(ACE2) receptor. J. Plant Biotechnol. 2021, 48, 289–303. [Google Scholar] [CrossRef]
- Diningrat, D.S.; Harahap, N.S.; Risfandi, M.Z.; Sari, A.N.; Kusdianti, K. Antioxidant and antibacterial activities of Coix lacryma-jobi seed and root oil potential for meningitis treatment. Jordan J. Biolog. Sci. 2021, 14, 881–887. [Google Scholar]
- Jinnouchi, M.; Miyahara, T.; Suzuki, Y. Coix seed consumption affects the gut microbiota and the peripheral lymphocyte subset profiles of healthy male adults. Nutrients 2021, 13, 4079. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.J.; Ko, J.; Cheng, W.Y.; Yang, H.Y. Dehulled adlay consumption modulates blood pressure in spontaneously hypertensive rats and overweight and obese young adults. Nutrients 2021, 13, 2305. [Google Scholar] [CrossRef]
- Devaraj, R.D.; Jeepipalli, S.P.K.; Xu, B.J. Phytochemistry and health promoting effects of Job’s tears (Coix lacryma-jobi)—A critical review. Food Biosci. 2020, 34, 100537. [Google Scholar] [CrossRef]
- Igbokwe, C.J.; Wei, M.; Feng, Y.Q.; Duan, Y.Q.; Ma, H.L.; Zhang, H.H. Coix seed: A review of its physicochemical composition, bioactivity, processing, application, functionality, and safety aspects. Food Rev. Int. 2021. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.E.; Wang, W.; Kan, J.Q.; Yu, Y.J. Analysis and evaluation of nutritional components in different tissues of Coix lacryma-jobi. Food Sci. 2013, 34, 255–259. [Google Scholar]
- Chen, M.H.; May, B.H.; Zhou, I.W.; Zhang, A.L.; Xue, C.C. Integrative medicine for relief of nausea and vomiting in the treatment of colorectal cancer using oxaliplatin-based chemotherapy: A systematic review and meta-analysis. Phytother. Res. 2016, 30, 741–753. [Google Scholar] [CrossRef]
- Kuo, C.C.; Chiang, W.; Liu, G.P.; Chien, Y.L.; Chang, J.Y.; Lee, C.K.; Lo, J.M.; Huang, S.L.; Shih, M.C.; Kuo, Y. 2,2′-Diphenyl-1-picrylhydrazyl radical-scavenging active components from adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) hulls. J. Agric. Food Chem. 2002, 50, 5850–5855. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Chiang, W.; Chang, J.Y.; Chien, Y.L.; Lee, C.K.; Liu, K.J.; Cheng, Y.T.; Chen, T.F.; Kuo, Y.H.; Kuo, C.C. Antimutagenic constituents of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) with potential cancer chemopreventive activity. J. Agric. Food Chem. 2011, 59, 6444–6452. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ryu, J.; Park, S.H.; Seo, E.K.; Han, A.R.; Lee, S.K.; Kim, Y.S.; Hong, J.H.; Seok, J.H.; Le, C.J. Suppressive effects of coixol, glyceryl trilinoleate and natural products derived from Coix Lachryma-Jobi var. ma-yuen on gene expression, production and secretion of airway MUC5AC mucin. Arch. Pharm. Res. 2015, 38, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gao, W.; Peng, C.; He, C.; Zhang, Q.; Bi, W. Characteristics of germplasm resources in Coix from xishuangbanna. China J. Chin. Mater. Med. 2010, 35, 415–418. [Google Scholar]
- Wang, L.; Chen, C.; Su, A.; Zhang, Y.; Yuan, J.; Ju, X. Structural characterization of phenolic compounds and antioxidant activity of the phenolic-rich fraction from defatted adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) seed meal. Food Chem. 2016, 196, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Pan, H.; Lu, X.J.; Wei, X.; Qin, L. Characteristics and comprehensive assessment of principal nutritional components in adlay landraces. Sci. Agric. Sin. 2018, 51, 835–842. [Google Scholar]
- Yang, Y.; Du, S.Y.; Sun, Y.Q.; Han, T.; Jia, M.; Qin, L.P. Determination of effective contents triolein and coixol in Coix lacrymajobi var. mayuen from different origins. Chin. Trad. Her. Drugs 2017, 48, 578–581. [Google Scholar]
- Wu, T.T.; Charles, A.L.; Huang, T.C. Determination of the contents of the main biochemical compounds of Adlay (Coxi lachrymal-jobi). Food Chem. 2007, 104, 1509–1515. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.H.; Jung, J.T.; Lee, Y.J.; Oh, M.W.; Chang, J.K.; Jeong, H.S.; Park, C.G. Changes in free sugar, coixol contents and antioxidant activities of Adlay sprout (Coix lacryma-jobi L. var. ma-yuen Stapf.) according to different growth stage. Korean J. Med. Crop Sci. 2019, 27, 339–347. [Google Scholar] [CrossRef]
- Xu, L.; Chen, L.; Ali, B.; Yang, N.; Chen, Y.; Wu, F.; Jin, Z.; Xu, X. Impact of germination on nutritional and physicochemical properties of adlay seed (Coix lachryma-jobi L.). Food Chem. 2017, 229, 312–318. [Google Scholar] [CrossRef]
- Deng, S.F.; Ying, Z.Y.; Yang, Y.Q.; Lin, Z.G.; Chen, M. Research progress of functional ingredients on adlay. Chin. Agri. Sci. Bullet. 2017, 33, 123–128. [Google Scholar]
- Xiong, L.; Han, Z.J.; Sun, Q.J. The comparison of Job’s-tears powder and Job’s-tears starch on their physicochemical properties and digestibility. J. Chin. Cereals Oils Assoc. 2012, 27, 32–37. [Google Scholar]
- He, C.J.; Li, Z.Y.; Liu, H.X.; Zhang, H.N.; Wang, L.Y.; Chen, H. Chemical compositions and antioxidant activity of adlay seed (Coixlachryma-jobi L.) oil extracted from four main producing areas in China. J. Food Sci. 2020, 85, 123–131. [Google Scholar] [CrossRef]
- Xu, L. Effect of Germination on the Nutritional Components, Physicochemical Properties and Biological Activities of Adlay. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2017. [Google Scholar]
- Wen, A.; Xie, C.; Mazhar, M.; Wang, C.; Zeng, H.; Qin, L.; Zhu, Y. Tetramethylpyrazine from adlay (Coix lacryma-jobi) biotransformation by Bacillus subtilis and its quality characteristics. J. Food Sci. Technol. 2020, 57, 4092–4102. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, H.; Mizukami, H.; Nagatsu, A.; Ohno, T.; Tanabe, H.; Inoue, M. Peroxisome proliferator-activated receptor gamma ligands isolated from adlay seed (Coix lacryma-jobi L. var. ma-yuen STAPF.). Bio. Pharmaceut. Bullet. 2009, 32, 735–740. [Google Scholar] [CrossRef]
- Manosroi, A.; Sainakham, M.; Chankhampan, C.; Abe, M.; Manosroi, W.; Manosroi, J. Potent in vitro anti-proliferative, apoptotic and anti-oxidative activities of semi-purified Job’s tears (Coix lachryma-jobi Linn.) extracts from different preparation methods on 5 human cancer cell lines. J. Ethnopharmacol. 2016, 187, 281–292. [Google Scholar] [CrossRef]
- Yokoi, H.; Mizukami, H.; Nagatsu, A.; Tanabe, H.; Inoue, M. Hydroxy monounsaturated fatty acids as agonists for peroxisome proliferator-activated receptors. Bio. Pharmaceut. Bullet. 2010, 33, 854–861. [Google Scholar] [CrossRef]
- Leite, A.; Ottoboni, L.M.; Targon, M.L.; Silva, M.J.; Turcinelli, S.R.; Arruda, P. Phylogenetic relationship of zeins and coixins as determined by immunological cross-reactivity and Southern blot analysis. Plant Molecul. Bio. 1990, 14, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Son, E.S.; Kim, S.H.; Kim, Y.O.; Lee, Y.E.; Kyung, S.Y.; Jeong, S.H.; Kim, Y.J.; Park, J.W. Coix lacryma-jobi var. ma-yuen Stapf sprout extract induces cell cycle arrest and apoptosis in human cervical carcinoma cells. BMC Complement. Altern. Med. 2019, 19, 312. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Zhang, J.; Liu, C. Coix lacryma-jobi L. var. ma-yuen (Roman.) Stapf (Yiyiren, Jobstears). In Dietary Chinese Herbs; Liu, Y., Wang, Z., Zhang, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Wang, L.; Sun, J.; Yi, Q.; Wang, X.; Ju, X. Protective effect of polyphenols extract of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) on hypercholesterolemia-induced oxidative stress in rats. Molecules 2012, 17, 8886–8897. [Google Scholar] [CrossRef]
- Zeng, Y.W.; Pu, X.Y.; Du, J.; Yang, X.M.; Li, X.; Mandal, M.S.N.; Yang, T.; Yang, J.Z. Molecular mechanism of functional ingredients in barley to combat human chronic diseases. Oxid. Med. Cell. Longev. 2020, 2020, 3836172. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Y.; Xu, X.; Yuan, P. Effect of paclitaxel and carboplatin combined with Kanglaite on angiogenesis and invasion in patients with cervical cancer. Hebei Med. 2018, 24, 2020–2025. [Google Scholar]
- Guo, M.; Qu, D.; Qin, Y.; Chen, Y.; Liu, Y.; Huang, M.; Chen, Y. Transferrin- functionalized microemulsions coloaded with Coix seed oil and tripterine deeply penetrate to improve cervical cancer therapy. Mol. Pharm. 2019, 16, 4826–4835. [Google Scholar] [CrossRef]
- Huang, Y.J.; Chang, C.C.; Wang, Y.Y.; Chiang, W.C.; Shih, Y.H.; Shieh, T.M.; Wang, K.L.; Ali, M.; Hsia, S.M. Adlay testa (Coix lachryma-jobi L. var. ma-yuen Stapf.) ethanolic extract and its active components exert anti-proliferative effects on endometrial cancer cells via cell cycle arrest. Molecules 2021, 26, 1966. [Google Scholar] [CrossRef]
- Qu, D.; Liu, M.; Huang, M.; Wang, L.; Chen, Y.; Liu, C.; Liu, Y. Octanoyl galactose ester- modified microemulsion system self- assembled by coix seed components to enhance tumor targeting and hepatoma therapy. Int. J. Nanomed. 2017, 12, 2045–2059. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Arena, F.P.; Bienvenu, B.J.; Kaplan, E.H.; Camacho, L.H.; Campos, L.T.; Waymack, J.P.; Tagliaferri, M.A.; Chen, M.M.; Li, D. A randomized, open-label, safety and exploratory efficacy study of kanglaite injection (KLTi) plus gemcitabine versus gemcitabine in patients with advanced pancreatic cancer. J. Cancer 2017, 8, 1872–1883. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Yang, B.; Xiong, Y.; Gu, M. Coix seed emulsion synergistically enhances the antitumor activity of gemcitabine in pancreatic cancer through abrogation of NF-κB signaling. Oncol. Rep. 2016, 36, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Du, Q.H.; Xie, X.B. Effect of coixenolide on the proliferation and apoptosis of human HL-60 leukemic cells. China J. Cancer Prev. Treat. 2014, 21, 502–505. [Google Scholar]
- Lu, X.; Liu, W.; Wu, J.; Li, M.; Wang, J.; Wu, J.; Luo, C. A polysaccharide fraction of adlay seed (Coix lachryma-jobi L.) induces apoptosis in human non-small cell lung cancer A549 cells. Biochem. Biophys. Res. Commun. 2013, 430, 846–851. [Google Scholar] [CrossRef]
- Shi, G.; Zheng, X.; Zhang, S.; Wu, X.; Yu, F.; Wang, Y.; Xing, F. Kanglaite inhibits EMT caused by TNF-α via NF-κB inhibition in colorectal cancer cells. Oncotarget 2017, 9, 6771–6779. [Google Scholar] [CrossRef]
- Son, E.S.; Kim, Y.O.; Park, C.G.; Park, K.H.; Jeong, S.H.; Park, J.W.; Kim, S.H. Coix lacryma-jobi var. ma-yuen Stapf sprout extract has anti-metastatic activity in colon cancer cells in vitro. BMC Complement. Altern. Med. 2017, 17, 486. [Google Scholar] [CrossRef]
- Luo, C.; Wang, X.; An, C.; Hwang, C.F.; Miao, W.; Yang, L.; Xu, M.; Bai, A.; Deng, S. Molecular inhibition mechanisms of cell migration and invasion by coix polysaccharides in A549 NSCLC cells via targeting S100A4. Mol. Med. Rep. 2017, 15, 309–316. [Google Scholar] [CrossRef]
- Jiang, G.Q.; Fang, F. Effects of kanglaite and erlotinib combination on proliferation and inva-sion and JAK2/STAT3 signaling pathway of lung cancer A549 cells. J. Zhengzhou Univ. Med. l Sci. 2019, 54, 418–422. [Google Scholar]
- Xiong, M.H.; Chen, J.P.; Chao, R.Q.; Wang, J.H.; Chao, J. The effect of coix seed oil on migration and invasion of laryngeal cancer. Contempor. Med. 2018, 24, 15–18. [Google Scholar]
- Fang, T.; Jiang, Y.X.; Chen, L.; Huang, L.; Tian, X.H.; Zhou, Y.D.; Nagle, D.G.; Zhang, D.D. Coix seed oil exerts an anti-triple-negative breast cancer effect by disrupting miR-205/S1PR1 axis. Front. Pharmacol. 2020, 11, 529962. [Google Scholar] [CrossRef]
- Zeng, H.; Qin, L.; Liu, X.; Miao, S. Increases of lipophilic antioxidants and anticancer activity of Coix seed fermented by Monascus purpureus. Foods 2021, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Shih, C.K.; Yen, Y.T.; Chiang, W.; Hsia, S.M. Adlay (Coix lachryma-jobi L. var. ma-yuen Stapf.) hull extract and active compounds inhibit proliferation of primary human leiomyoma cells and protect against sexual hormone-induced mice smooth muscle hyperproliferation. Molecules 2019, 24, 1556. [Google Scholar] [CrossRef] [PubMed]
- Su, W.X.; Zhu, G.H.; Xiao, H.J.; Li, S.H. Effect of Kanglaite on the proliferation and apoptosis capacity of gastric cancer cells. J. Clin. Exper. Med. 2008, 7, 89–90. [Google Scholar]
- Qu, D.; He, J.; Liu, C.; Zhou, J.; Chen, Y. Triterpene-loaded microemulsion using Coix lacryma-jobi seed extract as oil phase for enhanced antitumor efficacy: Preparation and in vivo evaluation. Int. J. Nanomed. 2014, 9, 109–119. [Google Scholar]
- Chang, H.; Huang, Y.C.; Hung, W. Antiproliferative and chemopreventive effects of adlay seed on lung cancer In Vitro and In Vivo. J. Agric. Food Chem. 2003, 51, 3656–3660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Meng, X.; Tang, X.; Ren, L.; Liang, J. The effect of a coix seed oil injection on cancer pain relief. Support Care Cancer 2019, 27, 461–465. [Google Scholar] [CrossRef]
- Lee, M.Y.; Lin, H.Y.; Cheng, F.; Chiang, W.; Kuo, Y.H. Isolation and characterization of new lactam compounds that inhibit lung and colon cancer cells from adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) bran. Food Chem. Toxicol. 2008, 46, 1933–1939. [Google Scholar] [CrossRef]
- Wang, L.; Gao, S.; Jiang, W.; Luo, C.; Xu, M.; Bohlin, L.; Rosendahl, M.; Huang, W. Antioxidative dietary compounds modulate gene expression associated with apoptosis, DNA repair, inhibition of cell proliferation and migration. Int. J. Mol. Sci. 2014, 15, 16226–16245. [Google Scholar] [CrossRef]
- Huang, D.W.; Chung, C.P.; Kuo, Y.H.; Lin, Y.L.; Chiang, W. Identification of compounds in adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) seed hull extracts that inhibit lipopolysaccharide-induced inflammation in RAW 264.7 macrophages. J. Agric. Food Chem. 2009, 57, 10651–10657. [Google Scholar] [CrossRef]
- Liu, S.; Li, F.; Zhang, X. Structural modulation of gut microbiota reveals Coix seed contributes to weight loss in mice. Appl. Microbiol. Biotechnol. 2019, 103, 5311–5321. [Google Scholar] [CrossRef]
- Ha do, T.; Nam Trung, T.; Bich Thu, N.; Van On, T.; Hai Nam, N.; Van Men, C.; Thi Phuong, T.; Bae, K. Adlay seed extract (Coix lachryma-jobi L.) decreased adipocyte differentiation and increased glucose uptake in 3T3-L1 cells. J. Med. Food 2010, 13, 1331–1339. [Google Scholar] [PubMed]
- Hidaka, Y.; Kaneda, T.; Amino, N.; Miyai, K. Chinese medicine, Coix seeds increase peripheral cytotoxic T and NK cells. Biotherapy 1992, 5, 201–203. [Google Scholar] [CrossRef]
- Wang, Q.; Du, Z.; Zhang, H.; Zhao, L.; Sun, J.; Zheng, X.; Ren, F. Modulation of gut microbiota by polyphenols from adlay (Coix lacryma-jobi L. var. ma-yuen Stapf.) in rats fed a high-cholesterol diet. Int. J. Food Sci. Nutr. 2015, 66, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, Z.; Lu, Z.; Feng, Z.; Chen, Q.; Deng, S.; Li, Z.; Yan, Y.; Ying, Z. Coix seed improves growth performance and productivity in post-weaning pigs by reducing gut pH and modulating gut microbiota. AMB Express 2019, 9, 115. [Google Scholar] [CrossRef]
- Li, B.; Qiao, L.; Li, L.; Zhang, Y.; Li, K.; Wang, L.; Qiao, Y. A novel antihypertensive derived from Adlay (Coix larchryma-jobi L. var. ma-yuen Stapf) glutelin. Molecules 2017, 22, 123. [Google Scholar] [CrossRef]
- Chen, P.Y.; Li, L.L.; Huo, X.Q.; Qiao, L.S.; Zhang, Y.L.; Chen, Z.J.; Wang, L.Z. New angiotensin-converting enzyme inhibitory peptide from Coix prolamin and its influence on the gene expression of renin-angiotensin system in vein endothelial cells. J. Cereal Sci. 2020, 96, 103099. [Google Scholar] [CrossRef]
- Yu, F.; Gao, J.; Zeng, Y.; Liu, C.X. Effects of adlay seed oil on blood lipids and antioxidant capacity in hyperlipidemic rats. J. Sci. Food Agric. 2011, 91, 1843–1848. [Google Scholar] [CrossRef]
- Kang, S.H.; Lee, J.Y.; Lee, T.H.; Park, S.Y.; Kim, C.K. De novo transcriptome assembly of the Chinese pearl barley, adlay, by full-length isoform and short-read RNA sequencing. PLoS ONE 2018, 13, e0208344. [Google Scholar] [CrossRef]
- Chen, H.J.; Lo, Y.C.; Chiang, W. Inhibitory effects of adlay bran (Coix lachryma-jobi L. var. ma-yuen Stapf) on chemical mediator release and cytokine production in rat basophilic leukemia cells. J. Ethnopharmacol. 2012, 141, 119–127. [Google Scholar] [CrossRef]
- Chen, H.J.; Shih, C.K.; Hsu, H.Y.; Chiang, W. Mast cell-dependent allergic responses are inhibited by ethanolic extract of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) testa. J. Agric. Food Chem. 2010, 58, 2596–2601. [Google Scholar] [CrossRef]
- Ting, Y.W.; Hu, Y.T.; Hu, J.Y.; Chang, W.C.; Huang, Q.R.; Hsieh, S.C. Nanoemulsified adlay bran oil reduces tyrosinase activity and melanin synthesis in B16F10 cells and zebrafish. Food Sci. Nutr. 2019, 7, 3216–3223. [Google Scholar] [CrossRef] [PubMed]
- Amen, Y.; Arung, E.T.; Afifi, M.S.; Halim, A.F.; Ashour, A.; Fujimoto, R.; Goto, T.; Shimizu, K. Melanogenesis inhibitors from Coix lacryma-jobi seeds in B16-F10 melanoma cells. Nat. Prod. Res. 2017, 31, 2712–2718. [Google Scholar] [CrossRef]
- Hsia, S.M.; Chiang, W.; Kuo, Y.H.; Wang, P.S. Downregulation of progesterone biosynthesis in rat granulosa cells by adlay (Coix lachryma-jobi L. var. ma-yuen Stapf.) bran extracts. Int. J. Impot. Res. 2006, 18, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Amen, Y.; Zhu, Q.; Tran, H.B.; Afifi, M.S.; Halim, A.F.; Ashour, A.; Fujimoto, R.; Goto, T.; Shimizu, K. Rho-kinase inhibitors from adlay seeds. Nat. Prod. Res. 2018, 32, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Hsia, S.M.; Tseng, Y.W.; Wang, S.W.; Kuo, Y.H.; Huang, D.W.; Wang, P.S.; Chiang, W. Effect of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf.) hull extracts on testosterone release from rat Leydig cells. Phytother. Res. 2009, 23, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Fan, Z.Y.; Wang, H.Y.; Wen, D.C.; Zjhang, S.Y. Effect of polysaccharides from adlay seed on anti-diabetic and gut microbiota. Food Funct. 2019, 10, 4372–4380. [Google Scholar] [CrossRef]
- Yuan, H.B.; Zhu, Y.D.; Wang, S.S.; Meng, L.N. Anti-inflammatory effect of adlay seed protein in diabetic mice. Cur. Topics. Nutraceut. Res. 2019, 17, 380–387. [Google Scholar]
- Wang, H.W.; Ding, J.T.; Xiao, N.Y.; Liu, X.L.; Zhang, Y.Y.; Zhang, H. Insights into the hierarchical structure and digestibility of starch in heat- moisture treated adlay seeds. Food Chem. 2020, 318, 126489. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, L.; Zou, J.; Zhou, T.; Wang, B.; Sun, H.; Yu, S. Coix seed oil ameliorates cancer cachexia by counteracting muscle loss and fat lipolysis. BMC Complement. Altern. Med. 2019, 19, 267. [Google Scholar] [CrossRef]
- Tsay, G.J.; Lin, Y.T.; Hsu, C.H.; Tang, F.Y.; Kuo, Y.H.; Chao, C.Y. Adlay hull extracts attenuate β-amyloid-induced neurotoxicity and oxidative stress in PC12 cells through antioxidative, anti-inflammatory, and antiapoptotic activities. Biochem. Biophys. Rep. 2021, 26, 101020. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, B.Y.; Jia, Z.X.; Wu, W.J.; Lu, Z.Q. Hepatocellular carcinoma HepG2 cell apoptosis and caspase-8 and Bcl-2 expression induced by injectable seed extract of Coix lacryma-jobi. Hepatobil. Pancr. Dis. Int. 2011, 10, 303–307. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, Y.; Zhang, J.; Feng, Y.; Shen, L. Formulation, preparation and evaluation of nanostructured lipid carrier containing naringin and coix seed oil for anti-tumor application based on “unification of medicines and excipients”. Drug Des. Develop. Ther. 2020, 14, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Xiong, Y.; Feng, D.; Wu, Y.; Zhang, X.; Chen, L.; Gu, M. Coix seed extract enhances the anti-pancreatic cancer efficacy of gemcitabine through regulating ABCB1- and ABCG2-mediated drug efflux: A bioluminescent pharmacokinetic and pharmacodynamic study. Int. J. Mol. Sci. 2019, 20, 5250. [Google Scholar] [CrossRef]
- Sainakham, M.; Manosroi, A.; Abe, M.; Manosroi, W.; Manosroi, J. Potent in vivo anticancer activity and stability of liposomes encapsulated with semi-purified Job’s tear (Coix lacryma-jobi Linn.) extracts on human colon adenocarcinoma (HT-29) xenografted mice. Drug Deliv. 2016, 23, 3399–3407. [Google Scholar] [CrossRef]
- Manosroi, A.; Sainakham, M.; Abe, M.; Sakai, K.; Sinchaipanid, N.; Manosroi, W.; Manosroi, J. Potent anti-proliferation on the colon cancer cell line (HT-29) of liposomal formulations entrapped with semi-purified Job’s tears (Coix lacryma-jobi Linn.) fractions. J. Nanosci. Nanotechnol. 2019, 19, 1996–2007. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Ming, Q.L.; Zhang, Q.Y.; Chen, Y.; Cheng, N.; Wu, W.W.; Han, T.; Qin, L.P. Gibberella moniliformis AH13 with antitumor activity, an endophytic fungus strain producing triolein isolated from Adlay (Coix lacryma-jobi: Poaceae). Curr. Microbiol. 2014, 69, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.W.; Yang, X.M.; Li, X.; Pu, X.Y.; Yang, L.E.; Yang, J.Z. Barley functional foods control human type I diabetes. Science 2021, 373, 522–527. Available online: https://www.science.org/do/10.1126/comment.764482/full/ (accessed on 7 August 2021).
- Numata, M.; Yamamoto, A.; Moribayashi, A.; Yamada, H. Antitumor components isolated from the Chinese herbal medicine Coix lachryma-jobi. Planta Med. 1994, 60, 356–359. [Google Scholar] [CrossRef]
- Xi, X.J.; Zhu, Y.G.; Tong, Y.P.; Yang, X.L.; Tang, N.N.; Ma, S.M.; Li, S.; Cheng, Z. Assessment of the genetic diversity of different Job’s tears (Coix lacryma-jobi L.) accessions and the active composition and anticancer effect of its seed oil. PLoS ONE 2016, 11, e0153269. [Google Scholar] [CrossRef]
- Seo, W.G.; Pae, H.O.; Chai, K.Y.; Yun, Y.G.; Kwon, T.H.; Chung, H.T. Inhibitory effects of methanol extract of seeds of Job’s Tears (Coix lachryma-jobi L. var. ma-yuen) on nitric oxide and superoxide production in RAW 264.7 macrophages. Immunopharmacol. Immunotoxicol. 2000, 22, 545–554. [Google Scholar] [CrossRef]
- Choi, G.; Han, A.R.; Lee, J.H.; Park, J.Y.; Kang, U.; Hong, J.; Kim, Y.S.; Seo, E.K. A comparative study on hulled adlay and unhulled adlay through evaluation of their LPS-induced anti-inflammatory effects, and isolation of pure compounds. Chem. Biodivers. 2015, 12, 380–387. [Google Scholar] [CrossRef]
- Otsuka, H.; Hirai, Y.; Nagao, T.; Yamasaki, K. Anti-inflammatory activity of benzoxazinoids from roots of Coix lachryma-jobi var. ma-yuen. J. Nat. Prod. 1988, 51, 174–179. [Google Scholar] [CrossRef]
- Huang, D.W.; Kuo, Y.H.; Lin, F.Y.; Lin, Y.L.; Chiang, W. Effect of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) Testa and its phenolic components on Cu2+-treated low-density lipoprotein (LDL) oxidation and lipopolysaccharide (LPS)-induced inflammation in RAW 264.7 macrophages. J. Agric. Food Chem. 2009, 57, 2259–2266. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Yeh, W.J.; Ko, J.; Huang, Y.L.; Yang, H.Y. Consumption of dehulled adlay improved lipid metabolism and inflammation in overweight and obese individuals after a 6-week single-arm pilot study. Nutrients 2022, 14, 2250. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Yun, S.J.; Jung, B.; Lee, E.H.; Hahm, D.H.; Shim, I.; Lee, H.J. Hypolipidemic effects of crude extract of adlay seed (Coix lachryma-jobi var. mayuen) in obesity rat fed high fat diet: Relations of TNF-alpha and leptin mRNA expressions and serum lipid levels. Life Sci. 2004, 75, 1391–1404. [Google Scholar] [CrossRef]
- Kim, S.O.; Yun, S.J.; Lee, E.H. The water extract of adlay seed (Coix lachrymajobi var. mayuen) exhibits anti-obesity effects through neuroendocrine modulation. Am. J. Chin. Med. 2007, 35, 297–308. [Google Scholar] [CrossRef]
- Chiang, H.; Lu, H.F.; Chen, J.C.; Chen, Y.H.; Sun, H.T.; Huang, H.C.; Tien, H.H.; Huang, C. Adlay seed (Coix lacryma-jobi L.) extracts exhibit a prophylactic effect on diet-induced metabolic dysfunction and nonalcoholic fatty liver disease in mice. Evid. Based Complement. Alternat. Med. 2020, 2020, 9519625. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, Y.; Zhang, S.; Zhao, H.; Wang, Y.; Kan, D.; Zhang, Y.; Guo, L.; Lv, J.; Hao, Q.; et al. Coix lacryma-jobi seed oil reduces fat accumulation in nonalcoholic fatty liver gisease by inhibiting the activation of the p-AMPK/SePP1/apoER2 pathway. J. Oleo Sci. 2021, 75, 685–696. [Google Scholar] [CrossRef]
- Chen, L.C.; Zhang, S.Y.; Zi, Y.; Zhao, H.M.; Wang, H.Y.; Zhang, Y. Functional coix seed protein hydrolysates as a novel agent with potential hepatoprotective effect. Food Funct. 2020, 11, 9495–9502. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, H.; Yan, H.; Wang, P.Z.; Rong, R.; Zhang, Y.Y.; Zhang, C.B.; Du, R.K.; Rong, L.J. A cell-based high- throughput protocol to screen entry inhibitors of highly pathogenic viruses with Traditional Chinese Medicines. J. Med. Virol. 2017, 89, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ye, J.; Xuan, Z.; Li, L.; Wang, H.; Wang, S.; Liu, H.; Wang, S. Development and validation of a rapid and efficient method for simultaneous determination of mycotoxins in coix seed using one-step extraction and UHPLC-HRMS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2021, 38, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Z.; Ma, N.N.; Li, L.; Jiang, D. Efficacy of traditional Chinese medicine on COVID-19: Two case reports. Med. Acupunct. 2021, 33, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.W.; Du, J.; Pu, X.Y.; Yang, X.M.; Li, X.; Yang, J.Z. Remarkable efficacy of COVID-19 treatment by traditional Chinese medicine. Science 2020, 367, 962–963. Available online: https://www.science.org/do/10.1126/comment.740317/full/ (accessed on 28 February 2020).
- Check, J.B.; K’Ombut, F.O. The effect on fibrinolytic system of blood plasma of Wister rats after feeding them with Coix mixed diet. East Afr. Med. J. 1995, 72, 51–55. [Google Scholar] [PubMed]
- Chung, C.P.; Hsia, S.M.; Lee, M.Y.; Chen, H.J.; Cheng, F.; Chan, L.C.; Kuo, Y.H.; Lin, Y.L.; Chiang, W. Gastroprotective activities of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) on the growth of the stomach cancer AGS cell line and indomethacin -induced gastric ulcers. J. Agric. Food Chem. 2011, 59, 6025–6033. [Google Scholar] [CrossRef]
- Hsia, S.M.; Yeh, C.L.; Kuo, Y.H.; Wang, P.S.; Chiang, W. Effects of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf.) hull extracts on the secretion of progesterone and estradiol in vivo and in vitro. Exper. Bio. Med. 2007, 232, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kato, M.; Ayugase, J. Anti-diabetic effects of adlay protein in type 2 diabetic db/db mice. Food Sci. Technol. Res. 2012, 18, 383–390. [Google Scholar] [CrossRef]
- Yang, R.S.; Lu, Y.H.; Chiang, W.; Liu, S.H. Osteoporosis prevention by adlay (Yì Yǐ: The seeds of Coix lachryma-jobi L. var. ma-yuen Stapf) in a mouse model. J. Trad. Complement. Med. 2013, 3, 134–138. [Google Scholar] [CrossRef]
- Sreekeesoon, D.P.; Mahomoodally, M.F. Ethnopharmacological analysis of medicinal plants and animals used in the treatment and management of pain in Mauritius. J. Ethnopharm. 2014, 157, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Chen, Y.C.; Chen, H.Y.; Chiang, Y.F.; Ali, M.; Chiang, W.; Chung, C.P.; Hsia, S.M. Ethanolic extracts of adlay testa and hull and their active biomolecules exert relaxing effect on uterine muscle contraction through blocking extracellular calcium influx in ex vivo and in vivo studies. Biomolecules 2021, 11, 887. [Google Scholar] [CrossRef]
- Hsia, S.M.; Kuo, Y.H.; Chiang, W.; Wang, P.S. Effects of adlay hull extracts on uterine contraction and Ca2+ mobilization in the rat. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E719–E726. [Google Scholar] [CrossRef]
- Tao, J.T.; Zhang, M.; Liu, F.; Ma, Y. Effects of coix seed paste recipe on curative effect, joint function and serum proinflammatory factors in patients with gout arthritis (Damp-heat bizu syndrome). Inform. Trad. Chin. Med. 2021, 38, 72–76. [Google Scholar]
- Taejarernwiriyakul, O.; Anzai, N.; Jutabha, P.; Kruanamkam, W.; Chanluang, S. Hypouricemia and nephroprotection of Coix lacryma-jobi L. seed extract. Songklanakarin J. Sci. Technol. 2015, 37, 441–447. [Google Scholar]
- Chen, X.Y.; Liao, D.C.; Yu, Y.T.; Wei, C.M.; Xuan, L.Y.; Li, S.; Wang, H.B. Coix seed oil prolongs lifespan and enhances stress resistance in Caenorhabditis elegans. Biogerontology 2020, 21, 245–256. [Google Scholar] [CrossRef]
- Ruan, J.J.; Weng, W.F.; Yan, J.; Zhou, Y.X.; Chen, H.; Ren, M.J.; Cheng, J.P. Coix lacryma-jobi chymotrypsin inhibitor displays antifungal activity. Pestic. Biochem. Physiol. 2019, 160, 49–57. [Google Scholar] [CrossRef]
- Diningrat, D.S.; Risfandi, M.; Harahap, N.S.; Sari, A.N.; Kusdianti, K.; Siregar, H.K. Phytochemical screening and antibacterial activity Coix lacryma-jobi oil. J. Plant Biotechnol. 2020, 47, 100–106. [Google Scholar] [CrossRef]
- Rajesh, K.S.; Bharath, B.R.; Rao, C.V.; Bhat, K.I.; Bhat, K.S.C.; Bhat, P. Neutralization of Naja naja venom induced lethality, edema and myonecrosis by ethanolic root extract of Coix lacryma-jobi. Toxicol. Rep. 2017, 4, 637–645. [Google Scholar] [CrossRef]
- Zhu, R.; Xu, X.; Shan, Q.; Wang, K.; Cao, G.; Wu, X. Determination of differentiating markers in coicis semen from multi-sources based on structural similarity classification coupled with UPCC-Xevo G2-XS QTOF. Front. Pharmacol. 2020, 11, 549181. [Google Scholar] [CrossRef]
- Hou, J.J.; Cao, C.M.; Xu, Y.W.; Yao, S.; Cai, L.Y.; Long, H.L.; Bi, Q.R.; Zhen, Y.Y.; Wu, W.Y.; Guo, D.A. Exploring lipid markers of the quality of coix seeds with different geographical origins using supercritical fluid chromatography mass spectrometry and chemometrics. Phytomedicine 2018, 45, 1–7. [Google Scholar] [CrossRef]
- Tseng, H.; Chang, C.W.; Chiang, W.C.; Hsieh, S.H. Gluconeogenesis and attenuates hyperlipidemia in type 2 diabetes rats. J. Med. Food 2019, 22, 22–28. [Google Scholar] [CrossRef]
- Lu, X.; Liu, W.; Luo, C. Apoptotic effect of coix polysaccharides on A549 lung cancer cells in vitro. Chin. J. Lung Cancer 2012, 15, 624–629. [Google Scholar]
- Li, Y.; Tian, X.; Li, S.; Chang, L.; Sun, P.; Lu, Y.; Yu, X.; Chen, S.; Wu, Z.; Xu, Z.; et al. Total polysaccharides of adlay bran (Coix lachryma-jobi L.) improve TNF-α induced epithelial barrier dysfunction in Caco-2 cells via inhibition of the inflammatory response. Food Funct. 2019, 10, 2906–2913. [Google Scholar] [CrossRef]
- Chen, J.C.; Chen, Y.Z.; Ge, H.F.; Wu, C.H.; Pang, J.; Miao, S. Multi-scale structure, pasting and digestibility of adlay (Coix lachryma-jobi L.) seed starch. Food Hydrocoll. 2019, 89, 885–891. [Google Scholar] [CrossRef]
- Chen, L.C.; Jiang, B.K.; Zheng, W.H.; Zhang, S.Y.; Li, J.J.; Fan, Z.Y. Preparation, characterization and anti-diabetic activity of polysaccharides from adlay seed. Int. J. Biol. Macromol. 2019, 139, 605–613. [Google Scholar] [CrossRef]
- Zhao, M.M.; Yang, Q.Y.; Lin, L.Z.; Sun, B.G.; Wang, Y. Intracellular antioxidant activities of selected cereal phenolic extracts and mechanisms underlying the protective effects of adlay phenolic extracts on H2O2-induced oxidative stress in human erythrocytes. J. Funct. Foods 2017, 31, 160–171. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, Q.; Liu, T.; Liu, Z. Coixol suppresses NF-κB, MAPK pathways and NLRP3 inflammasome activation in lipopolysaccharide-induced RAW 264.7 cells. Molecules 2020, 25, 894. [Google Scholar] [CrossRef]
- Huang, B.W.; Chiang, M.T.; Yao, H.T.; Chiang, W. The effect of adlay oil on plasma lipids, insulin and leptin in rat. Phytomedicine 2005, 12, 433–439. [Google Scholar] [CrossRef]
- Chung, C.P.; Lee, M.Y.; Hsia, S.M.; Chiang, W.; Kuo, Y.H.; Hsu, H.Y.; Lin, Y.L. Suppression on allergic airway inflammation of dehulled adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) in mice and anti-degranulation phytosterols from adlay bran. Food Funct. 2021, 12, 12788–12799. [Google Scholar] [CrossRef]
- Yeh, C.C.; Chiang, W.; Chiang, M.T. Effects of dehulled adlay on plasma glucose and lipid concentrations in Streptozotocin-induced diabetic rats fed a diet enriched in cholesterol. Int. J. Vitam. Nutr. Res. 2006, 76, 299–305. [Google Scholar] [CrossRef]
- Kim, E.; Kim, H.; Choi, S.; Park, C.S.; Moon, T.W. Low digestion property of amylosucrase -modified waxy adlay starch. Food Sci. Biotechnol. 2016, 25, 457–460. [Google Scholar] [CrossRef]
- Lin, L.Z.; Yang, Q.Y.; Zhao, K.; Zhao, M.M. Identification of the free phenolic profile of Adlay bran by UPLC-QTOF-MS/MS and inhibitory mechanisms of phenolic acids against xanthine oxidase. Food Chem. 2018, 253, 108–118. [Google Scholar] [CrossRef]
- Nagai, E.; Iwai, M.; Koketsu, R.; Sogabe, R.; Morimoto, R.; Suzuki, Y.; Ohta, Y.; Okuno, Y.; Ohshima, A.; Enomoto, T.; et al. Inhibition of influenza virus replication by adlay tea. J. Sci. Food. Agric. 2018, 98, 1899–1905. [Google Scholar] [CrossRef]
- El-Missiry, M.A.; Fekri, A.; Kesar, L.A.; Othman, A.I. Polyphenols are potential nutritional adjuvants for targeting COVID-19. Phytother. Res. 2021, 35, 2879–2889. [Google Scholar] [CrossRef]
- Yao, Y.J.; Wang, H.L.; Xu, F.R.; Zhang, Y.; Li, Z.; Ju, X.; Wang, L. Insoluble-bound polyphenols of adlay seed ameliorate H2O2-induced oxidative stress in HepG2 cells via Nrf2 signalling. Food Chem. 2020, 325, 126865. [Google Scholar] [CrossRef]
- Marahatha, R.; Gyawali, K.; Sharma, K.; Gyawali, N.; Tandan, P.; Adhikari, A.; Timilsina, G.; Bhattarai, S.; Lamichhane, G.; Acharya, A.; et al. Pharmacologic activities of phytosteroids in inflammatory diseases: Mechanism of action and therapeutic potentials. Phytother. Res. 2021, 35, 5103–5124. [Google Scholar] [CrossRef]
- Xu, L.; Wang, P.; Ali, B.; Yang, N.; Chen, Y.; Wu, F.; Xu, X. Changes of the phenolic compounds and antioxidant activities in germinated adlay seeds. J. Sci. Food Agric. 2017, 97, 4227–4234. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Xie, H.; Ju, X.; Liu, R.H. Phytochemical profiles and antioxidant activity of adlay varieties. J. Agric. Food Chem. 2013, 61, 5103–5113. [Google Scholar] [CrossRef]
- Xiong, W.; Li, Y.; Yao, Y.; Xu, Q.; Wang, L. Antioxidant mechanism of a newly found phenolic compound from adlay (NDPS) in HepG2 cells via Nrf2 signalling. Food Chem. 2022, 378, 132034. [Google Scholar] [CrossRef]
- Mu, S.; Yang, W.; Huang, G. Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 2021, 97, 628–632. [Google Scholar] [CrossRef]
- Feng, S.; Wang, L.; Shao, P.; Sun, P.; Yang, C.S. A review on chemical and physical modifications of phytosterols and their influence on bioavailability and safety. Crit. Rev. Food Sci. Nutr. 2021, 62, 5638–5657. [Google Scholar] [CrossRef]
- Zeng, Y.W.; Ali, M.K.; Du, J.; Li, X.; Yang, X.M.; Yang, J.Z.; Pu, X.Y.; Yang, L.E.; Hong, J.A.; Mou, B.; et al. Resistant starch in rice: Its biosynthesis and mechanism of action against diabetes-related diseases. Food Rev. Int. 2022. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Ball, T.; Yu, L.; Li, Y.; Xing, F. Revealing a 5,000-y-old beer recipe in China. Proc. Natl. Acad. Sci. USA 2016, 113, 6444–6448. [Google Scholar] [CrossRef]
- Na, H.Y.; Seol, M.H.; Kim, M.; Lee, B.C. Effect of Seyoeum on obesity, insulin resistance, and nonalcoholic fatty liver disease of high-fat diet-fed C57BL/6 mice. Evid. Based. Complement. Alternat. Med. 2017, 2017, 4658543. [Google Scholar] [CrossRef]
- Chen, L.C.; Kong, Y.P.; Zheng, Y.; Zhang, S.Y.; Zhang, L.Y.; Wang, J.Y. Preparation of coix seed oil bioactive delivery systems based on homologous polysaccharides and proteins. Int. J. Biol. Macromol. 2020, 151, 376–383. [Google Scholar] [CrossRef]
- Yin, H.; Zhong, Y.; Xia, S.; Hu, J.; Nie, S.; Xiong, T.; Xie, M. Effects of fermentation with Lactobacillus plantarum NCU137 on nutritional, sensory and stability properties of Coix (Coix lachryma-jobi L.) seed. Food Chem. 2020, 314, 126037. [Google Scholar] [CrossRef]
- Luo, X.; Li, H.; Jiang, D.; Meng, J.; Zhang, F.; Xu, Q.; Chen, X.; Liu, C.; Yang, Y. Analysis of fungi on coix (Coix lacryma-jobi) seed and the effect of its aqueous extract on the growth of Aspergillus flavus. J. Food Prot. 2019, 82, 1775–1782. [Google Scholar] [CrossRef]
- Xu, M.; He, D.; Teng, H.; Chen, L.; Song, H.; Huang, Q. Physiological and proteomic analyses of coix seed aging during storage. Food Chem. 2018, 260, 82–89. [Google Scholar] [CrossRef]
- Manosroi, J.; Khositsuntiwong, N.; Manosroi, A. Biological activities of fructooligosaccharide (FOS)-containing Coix lachryma -jobi Linn. Extract. J. Food Sci. Technol. 2014, 51, 341–346. [Google Scholar] [CrossRef]
- Bai, C.; Zheng, J.; Zhao, L.; Chen, L.; Xiong, H.; McClements, D.J. Development of oral delivery systems with enhanced antioxidant and anticancer activity: Coix seed oil and β-carotene coloaded liposomes. J. Agric. Food Chem. 2019, 67, 406–414. [Google Scholar] [CrossRef]
- Liu, M.J.; Qu, D.; Chen, Y.; Liu, C.Y.; Liu, Y.P.; Ding, X.F. Preparation of novel butyryl galactose ester-modified coix component microemulsions and evaluation on hepatoma -targeting in vitro and in vivo. Drug Deliv. 2016, 23, 3444–3451. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.J.; Ko, J.; Cheng, W.Y.; Yang, H.Y. Diet containing dehulled adlay ameliorates hepatic steatosis, inflammation and insulin resistance in rats with non-alcoholic fatty liver disease. Br. J. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Yang, L.; Xiong, J.; Xiong, L. Efficacy and safety of Kanglaite injection for gastric cancer: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e21619. [Google Scholar] [CrossRef]
- Huang, X.; Wang, J.; Lin, W.; Zhang, N.; Du, J.; Long, Z.; Yang, Y.; Zheng, B.; Zhong, F.; Wu, Q.; et al. Kanglaite injection plus platinum-based chemotherapy for stage III/IV non-small cell lung cancer: A meta-analysis of 27 RCTs. Phytomedicine 2020, 67, 153154. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Zhang, S.; Zhao, Z.; Wang, J.; Song, J.; Wang, Y.; Liu, J.; Hou, S. Kanglaite sensitizes colorectal cancer cells to Taxol via NF-κΒ inhibition and connexin 43 upregulation. Sci. Rep. 2017, 7, 1280. [Google Scholar] [CrossRef]
- Chen, Q.; Ai, D.; Wei, Y.H. Kanglaite enhances the efficacy of cisplatin in suppression of hepatocellular carcinoma via inhibiting CKLF1 mediated NF-κB pathway and regulating transporter mediated drug efflux. J. Ethnopharm. 2021, 264, 113388. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Ye, H.; Zhang, C.; Ye, L.; Lin, G. Effect of kanglaite on rat cytochrome P450. Pharm. Bio. 2015, 53, 995–1001. [Google Scholar] [CrossRef]
- Zhan, Y.P.; Huang, X.E.; Cao, J.; Lu, Y.Y.; Wu, X.Y.; Liu, J.; Xu, X.; Xiang, J.; Ye, L.H. Clinical safety and efficacy of Kanglaite® (Coix Seed Oil) injection combined with chemotherapy in treating patients with gastric cancer. Asian Pac. J. Cancer Prev. 2012, 13, 5319–5321. [Google Scholar] [CrossRef]
- Fang, J.; Chen, J.; Peng, J.W.; Li, H.L.; Jiang, C.F. Mechanism of action of Yi Fuzi Baijiangsan on ulcerative colitis on Nrf2 pathway. Chin. J. Exper. Trad. Med. Form. 2018, 24, 346–350. [Google Scholar]
- Wang, D.; Yang, C.; Wang, Z.; Yang, Y.; Li, D.; Ding, X.; Xu, W.; Zheng, Q. Norcantharidin combined with Coix seed oil synergistically induces apoptosis and inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation. Sci. Rep. 2017, 7, 9373. [Google Scholar] [CrossRef]
- Wen, A.; Zhu, Y.; Mazhar, M.; Qin, L.; Zeng, H.; Zhu, Y. Enhancement of anti-proliferative activity of the extracts from dehulled adlay by fermentation with Bacillus subtilis. Foods 2021, 10, 2959. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.C.; Chang, B.H.; Tien, H.H.; Cai, Y.L.; Fan, Y.C.; Chen, W.J.; Chu, H.F.; Chen, Y.H.; Huang, C. Synbiotic intervention with an adlay-based prebiotic and probiotics improved diet-induced metabolic disturbance in mice by modulation of the gut microbiota. Nutrients 2021, 13, 3161. [Google Scholar] [CrossRef]
- Huang, C.C.; Lin, T.C.; Liu, C.H.; Hu, H.C.; Yu, S.Y.; Wu, S.J.; Yen, M.H.; Tsai, Y.H.; Chang, F.R. Lipid metabolism and its mechanism triggered by supercritical CO2 extract of adlay (Coix lacryma-jobi var. ma-yuen (Rom. Caill.) Stapf) bran in high-fat diet induced hyperlipidemic hamsters. Front. Pharmacol. 2021, 12, 785944. [Google Scholar] [CrossRef]
- Carpena, M.; Caleja, C.; Nuñez-Estevez, B.; Pereira, E.; Fraga-Corral, M.; Reis, F.S.; Simal-Gandara, J.; Ferreira, I.C.F.R.; Prieto, M.A.; Barros, L. Flavonoids: A Group of Potential Food Additives with Beneficial Health Effects. Food Addit. 2021. Available online: https://www.intechopen.com/online-first/79721 (accessed on 19 December 2021). [CrossRef]
- Chang, C.C.; Huang, L.H.; Chiang, W.; Hsia, S.M. Hexane fraction of adlay (Coix lachryma-jobi L.) testa ethanolic extract inhibits human uterine sarcoma cancer cells growth and chemosensitizes human uterine sarcoma cells to doxorubicin. Phytomedicine 2018, 47, 69–80. [Google Scholar] [CrossRef] [PubMed]
| Components | Adlay Seeds | Adlay Coat | Adlay Hull | Adlay Root | Adlay Stem | Adlay Leaves |
|---|---|---|---|---|---|---|
| Total Ash (%) | 2.65 | 15.68 | 21.32 | 5.89 | 1.83 | 14.70 |
| Protein (%) | 19.33 | 10.85 | 7.56 | 19.06 | 12.07 | 18.29 |
| Crude fiber (%) | 2.05 | 53.56 | 45.43 | 28.78 | 43.44 | 18.95 |
| Crude fat (%) | 4.70 | 5.31 | 4.52 | 7.15 | 2.44 | 0.38 |
| Polysaccharides (%) | 2.26 | 0.65 | 0.52 | 0.53 | 0.28 | 0.47 |
| Starch (%) | 48.58 | 3.55 | 4.56 | 6.78 | 8.89 | 3.21 |
| VE (mg/kg) | 86.61 | 106.85 | 119.27 | 146.5 | 109.32 | 112.36 |
| VC (mg/kg) | 0.11 | 5 0.08 | 0.12 | 8 0.26 | 0.28 | 0.31 |
| VB1 (mg/kg) | 0.51 | 0.56 | 0.42 | 0.61 | 0.44 | 0.58 |
| VB2 (mg/kg) | 0.62 | 0.78 | 1.28 | 2.96 | 1.66 | 0.99 |
| VB6 (mg/kg) | 8.38 | 9.09 | 12.66 | 21.64 | 7.72 | 40.07 |
| VB12 (mg/kg) | 0.54 | 0.66 | — | 1.32 | 0.43 | 0.89 |
| Folciate acid (mg/kg) | 0.12 | — | 0.05 | 2.51 | 1.97 | 8.85 |
| Nicic acid (mg/kg) | 12.36 | 14.97 | 13.21 | 4.92 | — | 3.27 |
| K (mg/kg) | 2165.50 | 2294.61 | 1987.05 | 3714.79 | 2468.96 | 4175.83 |
| Ca (mg/kg) | 305.39 | 609.51 | 875.69 | 1845.33 | 1374.30 | 15,645.02 |
| Na (mg/kg) | 1020.42 | 1231.67 | 1393.59 | 877.92 | 1060.83 | 1904.38 |
| Mg (mg/kg) | 1212.29 | 1481.48 | 1567.37 | 2385.63 | 2283.13 | 5845.83 |
| Zn (mg/kg) | 146.83 | 88.93 | 91.36 | 84.01 | 20.91 | 41.22 |
| Fe (mg/kg) | 116.23 | 107.89 | 90.47 | 1459.28 | 98.92 | 367.18 |
| P (mg/kg) | 1933.20 | 323.93 | 318.92 | 849.51 | 391.89 | 1405.92 |
| Se (mg/kg) | 0.04 | 0.09 | 0.07 | 0.11 | — | 0.04 |
| Total amino acids (%) | 14.400 | 3.804 | 1.654 | 6.612 | 3.394 | 8.278 |
| Palmitate * (%) | 0.68 | 8.42 | 10.52 | 8.42 | 9.53 | 7.93 |
| Palm-linoleic acid * (%) | 0.59 | — | — | 1.36 | — | — |
| Stearic acid * (%) | 5.64 | 2.35 | 1.96 | 3.35 | 2.12 | 2.93 |
| Oleic acid * (%) | 40.59 | 43.55 | 21.17 | 26.74 | 43.87 | 46.11 |
| Linoleic acid * (%) | 26.53 | 31.18 | 14.49 | 18.78 | 29.88 | 33.47 |
| Decoanic acid * (%) | 1.10 | 1.04 | 0.90 | 0.75 | 0.87 | 1.24 |
| Eoixol (mg/g) | 0.14 | 0.25 | 0.23 | 1.26 | 0.34 | 0.34 |
| Flavonoids (mg/g) | 2.26 | 0.13 | 0.09 | 0.21 | 0.44 | 2.13 |
| Total phenol (mg/g) | 0.27 | 1.89 | 1.04 | 4.53 | 3.07 | 3.15 |
| Composition | Mean ± SD | Range | References |
|---|---|---|---|
| Polysaccharides (%) | 1.59 ± 0.95 | 0.92–2.26 | [14,26] |
| Total starch (%) | 65.54 ± 3.28 | 57.82–71.51 | [21] |
| Amylase (%) | 4.87 ± 5.69 | 0.00–25.48 | [21] |
| Resistant starch (%) | 5.57 ± 0.08 | 5.49–5.65 | [27] |
| Lipid (fat+oil) (%) | 7.82 ± 0.60 | 6.32–9.13 | [21] |
| Triolein (%) | 0.82 ± 0.19 | 0.53–1.04 | [22] |
| Protein (%) | 18.18 ± 1.36 | 14.77–21.78 | [21] |
| Total amino acid (%) | 17.38 ± 1.21 | 14.02–20.67 | [21] |
| Flavonoids (mg/g) | 1.24 ± 1.44 | 0.22–2.26 | [14,28] |
| Polyphenols (mg/g) | 0.81 ± 0.76 | 0.27–1.35 | [14] |
| Eoixol (mg/g) | 29.92 ± 42.12 | 0.14–59.70 | [14,24] |
| γ-oryzanol (μg/g) | 271.64 ± 101.36 | 176.52–375.35 | [29] |
| γ-Tocopherol (μg/g) | 29.72 ± 10.34 | 22.41–37.03 | [29] |
| Pharmaco- Logical Action | Anti- Cancer Type | Action Pathway | Functional Ingredients (Study Types) | References |
|---|---|---|---|---|
| Inhibiting tumor micro- angiogenesis | Cervical cancer | Inhibiting vascular endothelial factor secretion and its receptor activity | Besterol, β-glusterol (human clinical trials) | [6,39] |
| Induced the apoptosis of cancer cells | Cervical cancers | Inactivation of the PI3K/AKT pathway in HeLa cells inhibited tumor cell proliferation, enhanced anti-angiogenesis, and induced apoptosis by regulating bax/ bcl-2 and the activating caspase-3 pathway. | Transferrin-functionalized microemulsions coloaded; Coix seed oil (human clinical trials) | [35,40] |
| Hepatoma cancer | Regulating the expression of caspase-8; the half- maximal inhibitory effect against HepG2 cells was 46.5 ± 2.4μg/mL | Octanoyl galactose ester; adlay extract (in vitro, in vivo) | [41,42] | |
| Pancreatic Cancer | Downregulation of BCL-2 protein expression, increased expression of the Fas gene, apoptosis by activating caspase-3 and increasing the Bax/Bcl-2 ratio, and inhibition of NF-κB activity and downstream target genes. | Triglycerides, Coixenolide, Coixol, coix seed emulsion (human clinical trials) | [6,43,44] | |
| Early mature leukemia | Activated apoptotic protein Caspase-3, induced mitochondrial apoptosis, and promoted cell apoptosis. | Coixenolide (human clinical trials) | [45] | |
| Non-small cell lung cancer | Activating the endogenous mitochondrial apoptosis pathway and activating the apoptotic proteins Caspase-6 and Caspase-9 and promotes apoptosis. | Polysaccharides (human clinical trials) | [6,46] | |
| Inhibiting cancer cell metastasis | Colorectal cancer | Inhibits the tumor necrosis factor-α-mediated epithelial mesenchymal transition via the inhibition of NF-κΒ. | Resistant starch, coix seed oil (human clinical trials) | [38,47] |
| Colon cancer | HUVECs via repression of the ERK1/2 and AKT pathways. | Adlay sprout extract (human clinical trials) | [48] | |
| Non-small cell lung cancer | Downregulation of S100 calcium-binding protein and A4 expression and supports the expression of proliferative proteins, invasive proteins, matrix proteins, and the JAK2/STAT3 signaling pathway. | Polysaccharides, Triglycerides, Coixenolide, Coixol (human clinical trials) | [6,49,50] | |
| Laryngeal cancer | Inhibits the expression of invasion and transfer factors. | Triglycerides, Coixenolide, Coixol (human clinical trials) | [51] | |
| Suppress the cancer cell proliferation | Breast cancer | miR-205/S1PR1 regulate sphingomyelin metabolism, downstream STAT3/MAPK/AKT signal pathways. | Coix seed oil (animal study) | [52] |
| Endometr- ial cancer | Ethyl acetate showed dose-dependent cell cycle arrest of HEC-1A and RL95-2 cells at the sub G1 checkpoint and G2/M checkpoint. | Polyphenols, Flavonoids, Phytosterols, Fatty acid (in vitro) | [41] | |
| Laryngeal cancer | Meddling with protein phosphatase 2A in the sphingom-yelin cycle and blocking the cell cycle at the G0/G1 phase. | γ-oryzanol, Coixenolide (in vitro) | [53] | |
| Myterine oma | Inhibits ustrosphyperplasia induced by sex hormone hexene estrogol/methoxyprogesterone 17-acetate. | Besterol, β-glusterol (animal study) | [6,54] | |
| Gastric cancer | Inhibits Bcl-2 gene expression and blocks cancer cells in the G1 cycle. | Triglycerides, Coixenolide, Coixol (human clinical trials) | [6,55] | |
| Lung cancer | Suppresses cytocyclin A expression and blocks cancer cell proliferation during the cell cycle G1/S transition. | Adlay extract, Triterpene (in vitro, in vivo) | [56,57] | |
| Uterine leiomyoma | Reduced diethylstilbestrol/medroxyprogesterone 17-acetate-induced uterine myometrial hyperplasia. | Stigmasterol, β-sitosterol (animal study) | [54] | |
| Anti-cachexia | Lewis lung cancer | Adjusts the NF-κB-MuRF1 and AMPK-HSL pathways to inhibit elevated inflammatory factors and leads to phosphorylation of HSL to avoid fat and muscle loss. | Coix seed oil (human clinical trials) | [58,59] |
| Chronic Diseases | Action Pathway | Functional Ingredients | References |
|---|---|---|---|
| Anti- inflammation | Cell membrane into the cytosol and nucleus, triggering gene expression, changes in cell proliferation, and the induction of apoptosis; increased cellular production of nitric oxide and prostaglandin E2 by downregulating nitric oxide synthase and cyclooxygenase 2 expression. | Polyphenols, coixol, polysaccharides, eriodictyol, ceramide, p-coumaric acid | [6,60,61] |
| Anti-obesity | Glycerolipid metabolism, biosynthesis of unsaturated fatty acids, sulfur reduction, and glutathione transport system; AMP-activated protein kinase in adipose differentiation decrease adipogenesis expression and enhances binding protein α. | Polyphenols, dietary fiber, adlay extract, phytosterols, resistant starch | [38,62,63] |
| Protective liver | Inhibiting vascular endothelial factor secretion and its receptor activity. | Besterol, β-glusterol | [6,39] |
| Anti-virus | Increase in peripheral cytotoxic lymphocytes and enhanced cytotoxic activity. | Adlay extract | [64] |
| Cardiovascular protection | Reduced serum cholesterol and low-density lipoprotein cholesterol and improved high-density lipoprotein cholesterol. | Polyphenol | [37,65] |
| Gastro- protection | Reducing the pH value of gastric juice, increasing the density and length of gastrointestinal villi, and modulating gut microbiota. | Phenolic acids, caffeic, chlorogenic acids, polysaccharides | [66] |
| Anti- hypertension | Angiotensin converting enzyme inhibitory peptide GAAGGAF by glutelin and polypeptides produced by gluten; high angiotensin I conversion of enzyme inhibitory activity tetrapeptide downregulated expression. | Glutelin, gluten | [6,67,68] |
| Preventive heart disease | Assembled gene sets by the levels of prolamin and vitamin E biosynthesis-associated protein; blood lipid-reducing and anti-oxidant effects. | Vitamin E, adlay seed oil | [69,70] |
| Anti-allergy | Inhibit mast cell degranulation, suppress the production of Akt, influence the signal transduction in cells; inhibitory effect on allergic response via the ERK signaling transduction in RBL-2H3 cells. | Phenolic acids; hydroxyacetophenone, flavone p-coumaric acid | [71,72] |
| Melanogenesis inhibition | Reduce tyrosinase activity and melanin production in melanoma cells, which has a tyrosinase inhibitor and anti-hyperpigmentation agent. | Adlay bran oil, coixol | [73,74] |
| Endocrine regulation | Decrease testosterone release via the inhibition of the protein kinase A/C signal transduction pathways; GnRH-induced luteinizing hormone secretion; inhibited progesterone secretion and reduced cAMP, PKC, and post-cAMP pathway action. | Methanol extracts of adlay hull | [75,76,77] |
| Anti-diabetes | Improve insulin resistance by inhibition of the IKK/NF-κB inflammatory pathway; regulation of the intestinal microbiota and its metabolic pathways. | Protein, resistant starch; polysaccharides phytosterols | [38,78,79,80] |
| Anti-cachexia | Counteract muscle and adipose tissue loss through regulating the NF-κB-MuRF1 and AMPK-HSL pathways. | Coix seed oil | [81] |
| Preventive osteoporosis | Increase the proliferation of osteoblast cells via an extracellular signal-regulated kinase-regulated signaling pathway. | Water extract of adlay | [13,79] |
| Neuroprotect- ion | Aβ25-35-induced apoptosis in dPC12 cells was associated with the enhancement of the PI3K/Akt signaling pathway | Water extract of adlay hull | [82] |
| Functional Ingredients | Chronic Diseases | Efficacy Number |
|---|---|---|
| Oils and triglycerides | Anti-cancer [6,40,85,92], anti-inflammation [62], liver protection [101], preventive heart disease [69], melanogenesis inhibition [73], anti-cachexia [81], analgesic [6], extended life [118], anti-fungi [119] | 9 |
| Polysaccharides | Anti-cancer [49,60,124], anti-inflammation [61,125], gastroprotection [126], anti-diabetes [127] | 4 |
| Phenols | Anti-cancer [6,41], anti-inflammation [6,96], anti-obesity [38], cardiovascular protection [37,128], gastroprotection [108], anti-allergy [71], analgesic [113] | 7 |
| Coixol | Anti-cancer [17,44,59], anti-inflammation [129], melanogenesis inhibition [74], analgesic [5] | 4 |
| Phytosterols | Anti-cancer [41,54], anti-obesity [130], protective liver [100,101], cardiovascular protection [39,41], anti-hypertension [67,68], anti-allergy [131], anti-diabetes [132], analgesic [113] | 8 |
| Resistant starch | Anti-cancer [47], anti-obesity [62], anti-diabetes [126,133] | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Yang, J.; Chen, J.; Pu, X.; Li, X.; Yang, X.; Yang, L.; Ding, Y.; Nong, M.; Zhang, S.; et al. Actional Mechanisms of Active Ingredients in Functional Food Adlay for Human Health. Molecules 2022, 27, 4808. https://doi.org/10.3390/molecules27154808
Zeng Y, Yang J, Chen J, Pu X, Li X, Yang X, Yang L, Ding Y, Nong M, Zhang S, et al. Actional Mechanisms of Active Ingredients in Functional Food Adlay for Human Health. Molecules. 2022; 27(15):4808. https://doi.org/10.3390/molecules27154808
Chicago/Turabian StyleZeng, Yawen, Jiazhen Yang, Jia Chen, Xiaoying Pu, Xia Li, Xiaomeng Yang, Li’e Yang, Yumei Ding, Mingying Nong, Shibao Zhang, and et al. 2022. "Actional Mechanisms of Active Ingredients in Functional Food Adlay for Human Health" Molecules 27, no. 15: 4808. https://doi.org/10.3390/molecules27154808
APA StyleZeng, Y., Yang, J., Chen, J., Pu, X., Li, X., Yang, X., Yang, L., Ding, Y., Nong, M., Zhang, S., & He, J. (2022). Actional Mechanisms of Active Ingredients in Functional Food Adlay for Human Health. Molecules, 27(15), 4808. https://doi.org/10.3390/molecules27154808







