Asteraceae Seeds as Alternative Ingredients in a Fibre-Rich Diet: Protein Quality and Metabolic Effects in Rats
Abstract
1. Introduction
2. Results
2.1. Dietary Intake, Body Weight and Body Composition of Rats
2.2. Nitrogen Balance, Protein Digestibility and Nitrogen Retention in Rats
2.3. Caecal Metabolism in Rats after 4 Weeks of Feeding
2.4. Function of Internal Organs and Lipid Metabolism in Rats after 4 Weeks of Feeding
3. Discussion
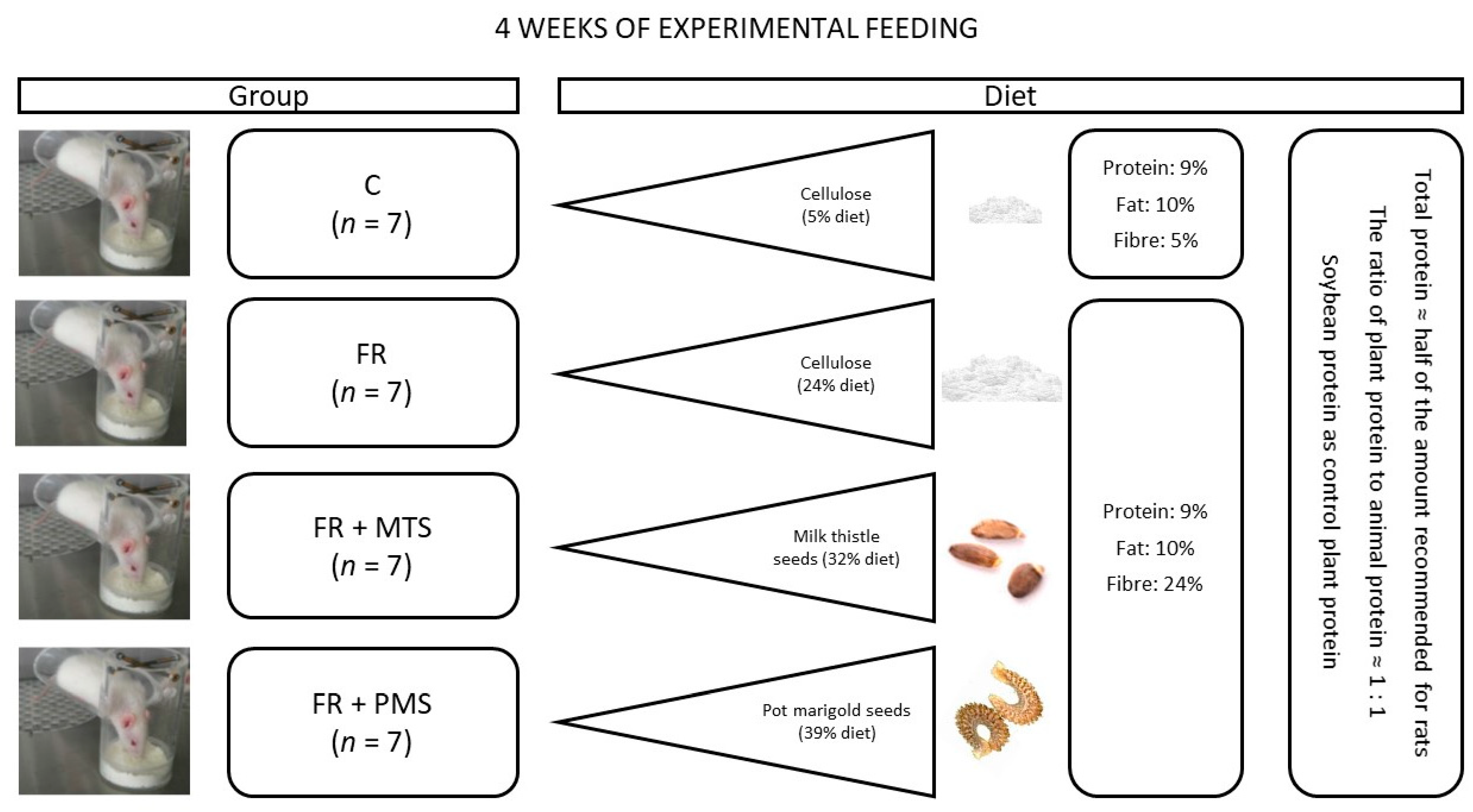
4. Materials and Methods
| Ingredient (g/100 g) | Group 1 | ||||
|---|---|---|---|---|---|
| C | FR | FR + MTS | FR + PMS | ||
| Casein 2 | 5.13 | 5.13 | 5.13 | 5.13 | |
| Rapeseed oil (canola type) | 9.8 | 9.8 | 2.1 | 6.2 | |
| Soybean protein isolate 3 | 4.87 | 4.87 | - | - | |
| Milk thistle seeds (MTSs) 4 | - | - | 31.91 | - | |
| Pot marigold seeds (PMSs) 5 | - | - | - | 38.57 | |
| Corn starch | 60.50 | 41.50 | 36.45 | 35.16 | |
| Sucrose | 10 | 10 | 10 | 10 | |
| Cellulose | 5 | 24 | 9.71 | 0.24 | |
| Mineral mix 6 | 3.5 | 3.5 | 3.5 | 3.5 | |
| Vitamin mix 6 | 1 | 1 | 1 | 1 | |
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 | |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marceddu, R.; Dinolfo, L.; Carrubba, A.; Sarno, M.; Di Miceli, G. Milk thistle (Silybum marianum L.) as a novel multipurpose crop for agriculture in marginal environments: A review. Agronomy 2022, 12, 729. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.)—Chemistry, bioavailability, and metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef]
- Arampatzis, D.A.; Karkanis, A.C.; Tsiropoulos, N.G. Silymarin content and antioxidant activity of seeds of wild Silybum marianum populations growing in Greece. Ann. Appl. Biol. 2019, 174, 61–73. [Google Scholar] [CrossRef]
- Theodosiou, E.; Purchartova, K.; Stamatis, H.; Kolisis, F.; Kren, V. Bioavailability of silymarin flavonolignans: Drug formulations and biotransformation. Phytochem. Rev. 2014, 13, 1–18. [Google Scholar] [CrossRef]
- Mukhtar, S.; Xiaoxiong, Z.; Qamer, S.; Saad, M.; Mubarik, M.S.; Mahmoud, A.H.; Mohammed, O.B. Hepatoprotective activity of silymarin encapsulation against hepatic damage in albino rats. Saudi J. Biol. Sci. 2021, 28, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Bortlikova, V.; Kolaric, L.; Simko, P. Application of milk thistle (Silybum marianum) in functional biscuits formulation. Acta Chim. Slov. 2019, 2, 192–199. [Google Scholar] [CrossRef]
- Li, F.; Li, F.; Wu, X.; Zhao, T.; Li, D.; Zhao, J.; Yang, l. Extraction, physicochemical, and functional properties of proteins from milk thistle Silybum marianum L. Gaernt seeds. Int. J. Food Prop. 2013, 16, 1750–1763. [Google Scholar] [CrossRef]
- Apostol, L.; Iorga, C.S.; Mosoiu, C.; Mustatea, G.; Cucu, S. Nutrient composition of partially defatted milk thistle seeds. Sci. Bull. Ser. F. Biotechnol. 2017, 21, 165–172. [Google Scholar]
- Dabbour, I.R.; Al-Ismail, K.M.; Takruri, H.R.; Azzeh, F.S. Chemical characteristics and antioxidant content properties of cold pressed seed oil of wild milk thistle plant grown in Jordan. Pak. J. Nutr. 2014, 13, 67–78. [Google Scholar] [CrossRef]
- Meddeb, W.; Rezig, L.; Zarrouk, A.; Nury, T.; Vejux, A.; Prost, M.; Bretillon, L.; Mejri, M.; Lizard, G. Cytoprotective activities of milk thistle seed oil used in traditional Tunisian medicine on 7-ketocholesterol and 24S-hydroxycholesterol—Induced toxicity on 158N murine oligodendrocytes. Antioxidants 2018, 7, 95. [Google Scholar] [CrossRef]
- Gaca, A.; Kludska, E.; Hradecky, J.; Hajslova, J.; Jeleń, H.H. Changes in volatile compound profiles in cold-press oils obtained from various seeds during accelerated storage. Molecules 2021, 26, 285. [Google Scholar] [CrossRef]
- Nicolaus, C.; Junghanns, S.; Hartmann, A.; Murillo, R.; Ganzera, M.; Merfort, I. In vitro studies to evaluate the wound healing properties of calendula officinalis extracts. J. Ethnopharmacol. 2017, 196, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, G.; Dhakad, P.; Tanwar, S. Review on phytochemical constituents and pharmacological activities of plant Calendula officinalis Linn. Biol. Sci. 2022, 2, 216–228. [Google Scholar] [CrossRef]
- Szopa, A.; Klimek-Szczykutowicz, M.; Jafernik, K.; Koc, K.; Ekiert, H. Pot marigold (Calendula officinalis L.)—A position in classical phytotherapy and newly documented activities. Acta Sci. Pol. Hortorum Cultus 2020, 19, 47–61. [Google Scholar] [CrossRef]
- Savic Gajic, I.M.; Savic, I.M.; Skrba, M.; Dosić, A.; Vujadinovic, D. Food additive based on the encapsulated pot marigold (Calendula officinalis L.) flowers extract in calcium alginate microparticles. J. Food Process. Preserv. 2022, 46, e15792. [Google Scholar] [CrossRef]
- Dulf, F.V.; Pamfil, D.; Baciu, A.D.; Pintea, A. Fatty acid composition of lipids in pot marigold (Calendula officinalis L.) seed genotypes. Chem. Centr. J. 2013, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Król, B.; Paszko, T.; Król, A. Conjugated linolenic acid content in seeds of some pot marigold (Calendula officinalis L.) cultivars grown in Poland. Farmacia 2016, 64, 881–886. [Google Scholar]
- Hennessy, A.A.; Ross, R.P.; Devery, R.; Stanton, C. The health promoting properties of the conjugated isomers of alpha-linolenic acid. Lipids 2011, 46, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.A.F.E.; Martins, B.A.; Oliveira, G.P.; Takahashi, J.A. Alternative protein sources of plant, algal, fungal and insect origins for dietary diversification in search of nutrition and health. Crit. Rev. Food Sci. Nutr. 2022, 14, 1–18. [Google Scholar] [CrossRef]
- Najjar, R.S.; Feresin, R.G. Plant-based diets in the reduction of body fat: Physiological effects and biochemical insights. Nutrients 2019, 11, 2712. [Google Scholar]
- Rolnik, A.; Olas, B. The plants of the Asteraceae family as agents in the protection of human health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef]
- Waddell, S.I.; Orfila, C. Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms. Crit. Rev. Food Sci. Nutr. 2022, 26, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Opyd, P.M.; Jurgoński, A. Intestinal, liver and lipid disorders in genetically obese rats are more efficiently reduced by dietary milk thistle seeds than their oil. Sci. Rep. 2021, 11, 20895. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Wang, T.; Luo, Y. A review on plant-based proteins from soybean: Health benefits and soy product development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Porwal, O.; Mohammed Ameen, M.S.; Anwer, E.T.; Uthirapathy, S.; Ahamad, J.; Tahsin, A. Silybum marianum (Milk thistle): Review on its chemistry, morphology, ethno-medical uses, phytochemistry and pharmacological activities. J. Drug Deliv. Ther. 2019, 9, 199–206. [Google Scholar] [CrossRef]
- Knudsen, K.E.B.; Wolstrup, J.; Eggum, B.O. The influence of dietary crude fibre and microbial activity in the digestive tract on true protein digestibility and biological value in rats. Z. Tierphysiol. Tierernaehr. Futtermittelkd. 1983, 49, 173–180. [Google Scholar] [CrossRef]
- Rios-Covian, D.; González, S.; Nogacka, A.M.; Arboleya, S.; Salazar, N.; Gueimonde, M.; de Los Reyes-Gavilán, C.G. An overview on fecal branched short-chain fatty acids along human life and as related with body mass index: Associated dietary and anthropometric factors. Front. Microbiol. 2020, 11, 973. [Google Scholar] [CrossRef]
- Shah, N.; Atallah, M.T.; Mahoney, R.R.; Pellett, P.L. Effect of dietary fiber components on fecal nitrogen excretion and protein utilization in growing rats. J. Nutr. 1982, 112, 658–666. [Google Scholar] [CrossRef]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef]
- Li, Y.; Xia, D.; Chen, J.; Zhang, X.; Wang, H.; Huang, L.; Shen, J.; Wang, S.; Feng, Y.; He, D.; et al. Dietary fibers with different viscosity regulate lipid metabolism via AMPK pathway: Roles of gut microbiota and short-chain fatty acid. Poult. Sci. 2022, 101, 101742. [Google Scholar] [CrossRef]
- Chardigny, J.M.; Hasselwander, O.; Genty, M.; Kraemer, K.; Ptock, A.; Sébédio, J.L. Effect of conjugated FA on feed intake, body composition, and liver FA in mice. Lipids 2003, 38, 895–902. [Google Scholar] [CrossRef]
- Pintea, A.; Bara, A.; Andrei, S.; Bele, C. The evaluation of hepatoprotective effect of Calendula officinalis L. seeds oil. Bull. Univ. Agric. Sci. Vet. Med. 2003, 60, 125–130. [Google Scholar]
- Zandani, G.; Anavi-Cohen, S.; Yudelevich, T.; Nyska, A.; Dudai, N.; Madar, Z.; Gorelick, J. Chiliadenus iphionoides reduces body weight and improves parameters related to hepatic lipid and glucose metabolism in a high-fat-diet-induced mice model of NAFLD. Nutrients 2022, 14, 4552. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.P.; Dias, D.M.; de Castro Moreira, M.E.; Toledo, R.C.; da Matta, S.L.; Lucia, C.M.; Martino, H.S.; Pinheiro-Sant’Ana, H.M. Chia seed shows good protein quality, hypoglycemic effect and improves the lipid profile and liver and intestinal morphology of Wistar rats. Plant Foods Hum. Nutr. 2016, 71, 225–230. [Google Scholar] [CrossRef]
- Reeves, P.C. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Hofirek, B.; Haas, D. Comparative studies of ruminal fluid collected by oral tube or by puncture of the caudoventral ruminal sac. Acta Vet. Brno 2001, 70, 27–33. [Google Scholar] [CrossRef]
- Barczyńska, R.; Jurgoński, A.; Śliżewska, K.; Juśkiewicz, J.; Kapuśniak, J. Effects of potato dextrin on the composition and metabolism of the gut microbiota in rats fed standard and high-fat diets. J. Funct. Foods 2017, 34, 398–407. [Google Scholar]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantys, A.J.; Trakatellis, A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Opyd, P.M.; Jurgoński, A.; Juśkiewicz, J.; Fotschki, B.; Koza, J. Comparative effects of native and defatted flaxseeds on intestinal enzyme activity and lipid metabolism in rats fed a high-fat diet containing cholic acid. Nutrients 2018, 10, 1181. [Google Scholar] [CrossRef]
| Group 2 | |||||
|---|---|---|---|---|---|
| C | FR | FR + MTS | FR + PMS | ||
| Initial body weight (g) | 142 ± 2.62 | 141 ± 4.01 | 141 ± 2.05 | 142 ± 3.71 | |
| Initial fat (%) | 13.5 ± 0.578 | 12.2 ± 0.563 | 12.4 ± 0.586 | 13.5 ± 0.702 | |
| Initial lean (%) | 69.6 ± 0.620 | 70.3 ± 0.495 | 70.0 ± 0.628 | 69.3 ± 0.663 | |
| Initial free fluids (%) | 3.83 ± 0.108 | 4.20 ± 0.139 | 3.95 ± 0.125 | 3.92 ± 0.074 | |
| Dietary intake (g/day) | 18.6 ± 0.362 | 18.9 ± 0.301 ab | 19.4 ± 0.115 a | 15.7 ± 0.901 b | |
| Final body weight (g) | 281 ± 4.55 * | 266 ± 3.09 a | 267 ± 1.52 a | 222 ± 10.8 b | |
| Final fat (%) | 21.4 ± 1.02 | 19.1 ± 0.892 | 17.3 ± 1.02 | 19.1 ± 0.560 | |
| Final lean (%) | 63.1 ± 0.805 | 64.4 ± 0.746 | 65.5 ± 0.869 | 64.4 ± 0.558 | |
| Final free fluids (%) | 4.35 ± 0.037 | 4.32 ± 0.062 | 4.49 ± 0.104 | 4.19 ± 0.102 | |
| Body weight gain (g) | 139 ± 5.99 * | 125 ± 1.46 a | 126 ± 2.85 a | 79.3 ± 8.97 b | |
| Fat gain (g) | 40.9 ± 3.47 | 33.5 ± 1.86 a | 28.8 ± 2.89 ab | 23.0 ± 2.34 b | |
| Lean gain (g) | 78.9 ± 3.21 | 72.5 ± 2.06 a | 76.5 ± 1.89 a | 44.0 ± 5.65 b | |
| Free fluid gain (g) | 6.83 ± 0.267 | 5.60 ± 0.152 a | 6.42 ± 0.441 a | 3.67 ± 0.416 b | |
| Group 2 | |||||
|---|---|---|---|---|---|
| C | FR | FR + MTS | FR + PMS | ||
| Nitrogen | |||||
| Intake (mg/5 days) | 1361 ± 49.5 | 1376 ± 10.5 | 1510 ± 1.04 | 1373 ± 78.5 | |
| In faeces (mg/5 days) | 121 ± 8.22 * | 157 ± 2.27 b | 326 ± 4.62 a | 370 ± 18.5 a | |
| In faeces (%N intake) | 8.93 ± 0.646 * | 11.4 ± 0.204 c | 21.6 ± 0.307 b | 27.0 ± 0.633 a | |
| In urine (mg/5 days) | 397 ± 9.81 * | 494 ± 24.9 | 468 ± 30.0 | 473 ± 12.3 | |
| In urine (%N intake) | 29.6 ± 1.70 * | 35.9 ± 1.74 | 31.0 ± 1.98 | 35.1 ± 1.98 | |
| Total digested protein (g/5 days) | 7.75 ± 0.311 | 7.62 ± 0.070 a | 7.40 ± 0.030 ab | 6.27 ± 0.386 b | |
| Apparent protein digestibility 3 (%) | 91.1 ± 0.646 * | 88.6 ± 0.204 a | 78.4 ± 0.307 b | 73.0 ± 0.633 c | |
| Total retained nitrogen (mg/5 days) | 8463 ± 56.5 | 725 ± 23.7 a | 716 ± 31.4 a | 530 ± 60.1 b | |
| Apparent nitrogen retention 4 (%) | 61.5 ± 2.04 * | 52.7 ± 1.68 a | 47.4 ± 2.09 a | 37.9 ± 2.46 b | |
| Group 2 | |||||
|---|---|---|---|---|---|
| C | FR | FR + MTS | FR + PMS | ||
| Mass of empty segment (g/100 bw) | 0.165 ± 0.005 * | 0.209 ± 0.020 | 0.222 ± 0.008 | 0.241 ± 0.011 | |
| Digesta mass (g/g tissue) | 3.25 ± 0.119 * | 4.08 ± 0.226 b | 4.24 ± 0.319 b | 6.08 ± 0.540 a | |
| pH of digesta | 7.72 ± 0.057 | 7.80 ± 0.136 | 7.56 ± 0.041 | 7.61 ± 0.153 | |
| Ammonia (mg/g digesta) | 0.283 ± 0.008 * | 0.140 ± 0.018 | 0.170 ± 0.007 | 0.195 ± 0.029 | |
| SCFA concentration (µmol/g digesta) | |||||
| Acetate | 51.9 ± 2.16 * | 27.9 ± 3.68 b | 45.4 ± 2.02 a | 44.1 ± 3.17 a | |
| Propionate | 10.8 ± 0.583 * | 5.89 ± 0.242 c | 9.91 ± 0.428 a | 7.20 ± 0.237 b | |
| Isobutyrate | 1.06 ± 0.050 * | 0.605 ± 0.074 | 0.741 ± 0.043 | 0.622 ± 0.045 | |
| Butyrate | 8.79 ± 1.24 * | 2.62 ± 0.560 b | 8.14 ± 0.643 a | 7.10 ± 1.68 a | |
| Isovalerate | 0.988 ± 0.061 * | 0.511 ± 0.081 | 0.591 ± 0.038 | 0.581 ± 0.067 | |
| Valerate | 1.06 ± 0.155 * | 0.553 ± 0.046 b | 0.838 ± 0.038 a | 0.778 ± 0.074 a | |
| Total SCFA 3 | 74.6 ± 3.31 * | 38.1 ± 4.48 b | 65.6 ± 2.66 a | 60.3 ± 5.12 a | |
| Total PSCFA 4 | 3.11 ± 0.188 * | 1.67 ± 0.186 | 2.17 ± 0.085 | 1.98 ± 0.159 | |
| SCFA proportion (% total conc.) | |||||
| Acetate | 69.7 ± 1.16 | 72.5 ± 1.19 a | 69.2 ± 0.881 b | 73.4 ± 0.891 a | |
| Propionate | 14.5 ± 0.382 | 16.4 ± 1.35 a | 15.2 ± 0.648 a | 12.2 ± 0.638 b | |
| Butyrate | 11.7 ± 1.32 * | 6.55 ± 0.893 b | 12.4 ± 0.734 a | 11.0 ± 1.57 a | |
| SCFA pool (µmol/total digesta mass) | 114 ± 10.9 | 84.1 ± 10.4 b | 164 ± 12.8 a | 198 ± 28.5 a | |
| Group 2 | |||||
|---|---|---|---|---|---|
| C | FR | FR + MTS | FR + PMS | ||
| Liver | |||||
| Mass (g/100 bw) | 3.29 ± 0.104 | 3.24 ± 0.081 b | 2.94 ± 0.091 b | 3.87 ± 0.202 a | |
| Fat (% liver) | 10.4 ± 0.486 | 9.66 ± 0.330 a | 10.0 ± 0.573 a | 8.20 ± 0.255 b | |
| Triglycerides (mg/g liver) | 5.37 ± 0.588 | 5.12 ± 0.277 | 5.57 ± 0.382 | 4.78 ± 0.438 | |
| Cholesterol (mg/g liver) | 1.34 ± 0.091 | 1.39 ± 0.118 a | 1.65 ± 0.094 a | 1.11 ± 0.049 b | |
| MDA (µg/g liver) 3 | 0.600 ± 0.048 | 0.561 ± 0.016 | 0.570 ± 0.035 | 0.644 ± 0.052 | |
| Kidneys | |||||
| Mass (g/100 bw) | 0.647 ± 0.018 | 0.626 ± 0.011 b | 0.649 ± 0.021 b | 0.734 ± 0.018 a | |
| MDA (µg/g kidney) 3 | 3.39 ± 0.656 | 1.95 ± 0.662 | 2.42 ± 0.265 | 3.28 ± 0.185 | |
| Plasma | |||||
| AST (U/L) 4 | 50.5 ± 2.81 | 46.7 ± 3.60 b | 57.4 ± 4.51 b | 82.6 ± 10.1 a | |
| ALT (U/L) 4 | 26.9 ± 1.53 | 23.8 ± 2.13 b | 25.3 ± 1.24 b | 82.7 ± 13.3 a | |
| ALP (U/L) 4 | 255 ± 15.6 | 228 ± 23.9 b | 190 ± 11.4 b | 346 ± 39.0 a | |
| Uric acid (µmol/L) | 25.4 ± 1.09 | 25.4 ± 2.02 | 28.0 ± 3.32 | 49.4 ± 19.2 | |
| Creatinine (µmol/L) | 18.7 ± 2.91 | 12.4 ± 1.85 | 11.3 ± 3.59 | 18.4 ± 2.08 | |
| Urea (mmol/L) | 1.89 ± 0.245 | 1.85 ± 0.071 ab | 1.58 ± 0.153 b | 2.56 ± 0.322 a | |
| Cholesterol (mmol/L) | 1.78 ± 0.065 | 2.02 ± 0.132 | 1.79 ± 0.069 | 1.98 ± 0.057 | |
| Triglycerides (mmol/L) | 2.02 ± 0.254 | 2.47 ± 0.286 ab | 1.47 ± 0.263 b | 3.13 ± 0.534 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koza, J.; Jurgoński, A. Asteraceae Seeds as Alternative Ingredients in a Fibre-Rich Diet: Protein Quality and Metabolic Effects in Rats. Molecules 2023, 28, 3275. https://doi.org/10.3390/molecules28073275
Koza J, Jurgoński A. Asteraceae Seeds as Alternative Ingredients in a Fibre-Rich Diet: Protein Quality and Metabolic Effects in Rats. Molecules. 2023; 28(7):3275. https://doi.org/10.3390/molecules28073275
Chicago/Turabian StyleKoza, Jarosław, and Adam Jurgoński. 2023. "Asteraceae Seeds as Alternative Ingredients in a Fibre-Rich Diet: Protein Quality and Metabolic Effects in Rats" Molecules 28, no. 7: 3275. https://doi.org/10.3390/molecules28073275
APA StyleKoza, J., & Jurgoński, A. (2023). Asteraceae Seeds as Alternative Ingredients in a Fibre-Rich Diet: Protein Quality and Metabolic Effects in Rats. Molecules, 28(7), 3275. https://doi.org/10.3390/molecules28073275







