Elecampane (Inula helenium) Root Extract and Its Major Sesquiterpene Lactone, Alantolactone, Inhibit Adipogenesis of 3T3-L1 Preadipocytes
Abstract
:1. Introduction
2. Material and Methods
2.1. Antibodies, Reagents, Plasmids, and Luciferase Reporter Assay
2.2. Preparation of IHE Extracts
2.3. High-Performance Liquid Chromatography (HPLC) Analysis
2.4. Cell Viability Assay and Oil Red O Staining
2.5. Western Blot, Quantitative Real-Time PCR, and Bromodeoxyuridine (BrdU) Cell Proliferation Assays
2.6. Statistical Analyses
3. Results
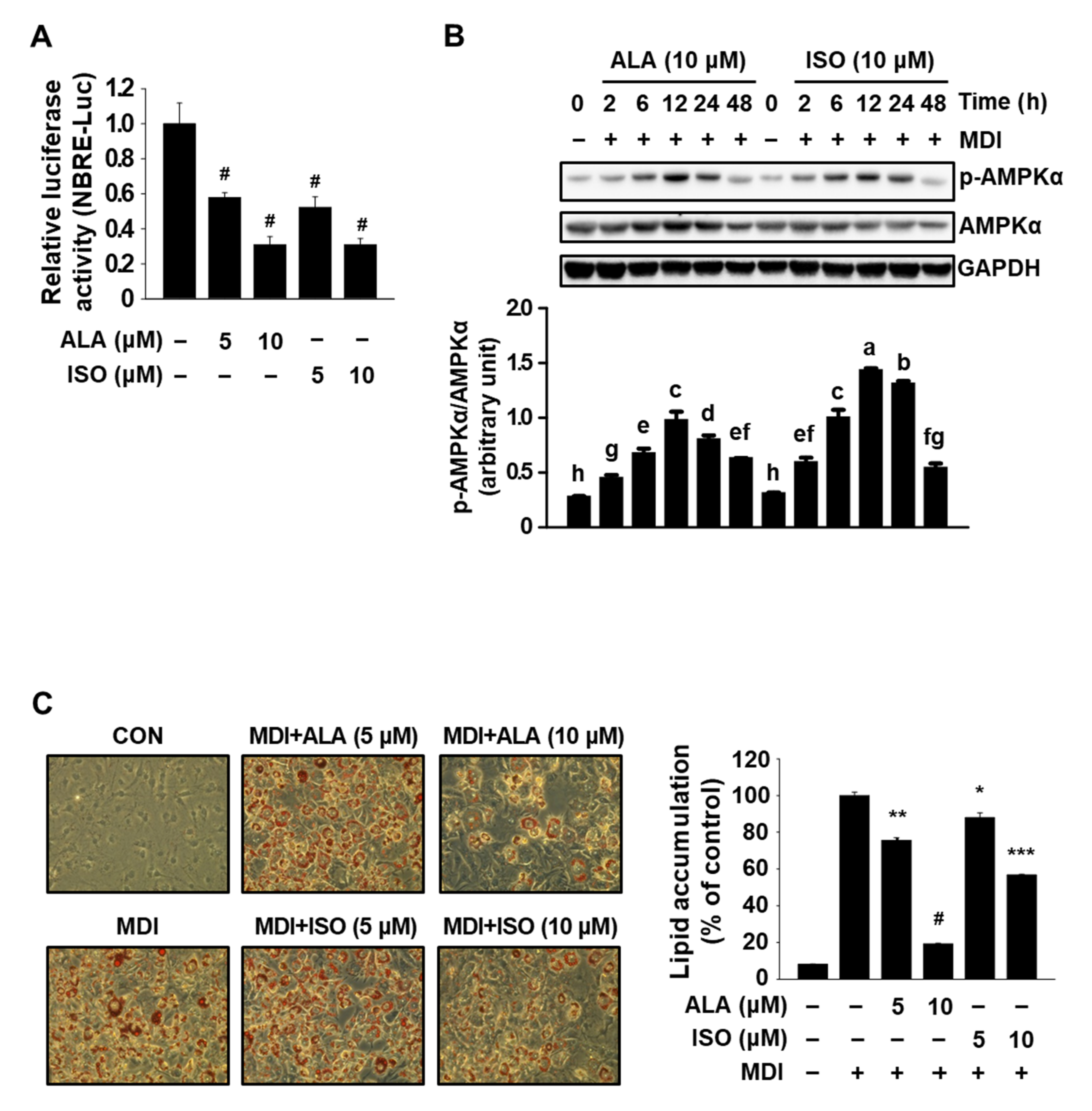
3.1. ALA Targets Both Nur77 and AMPKα and Inhibits MDI-Induced Adipogenesis in 3T3-L1 Cells
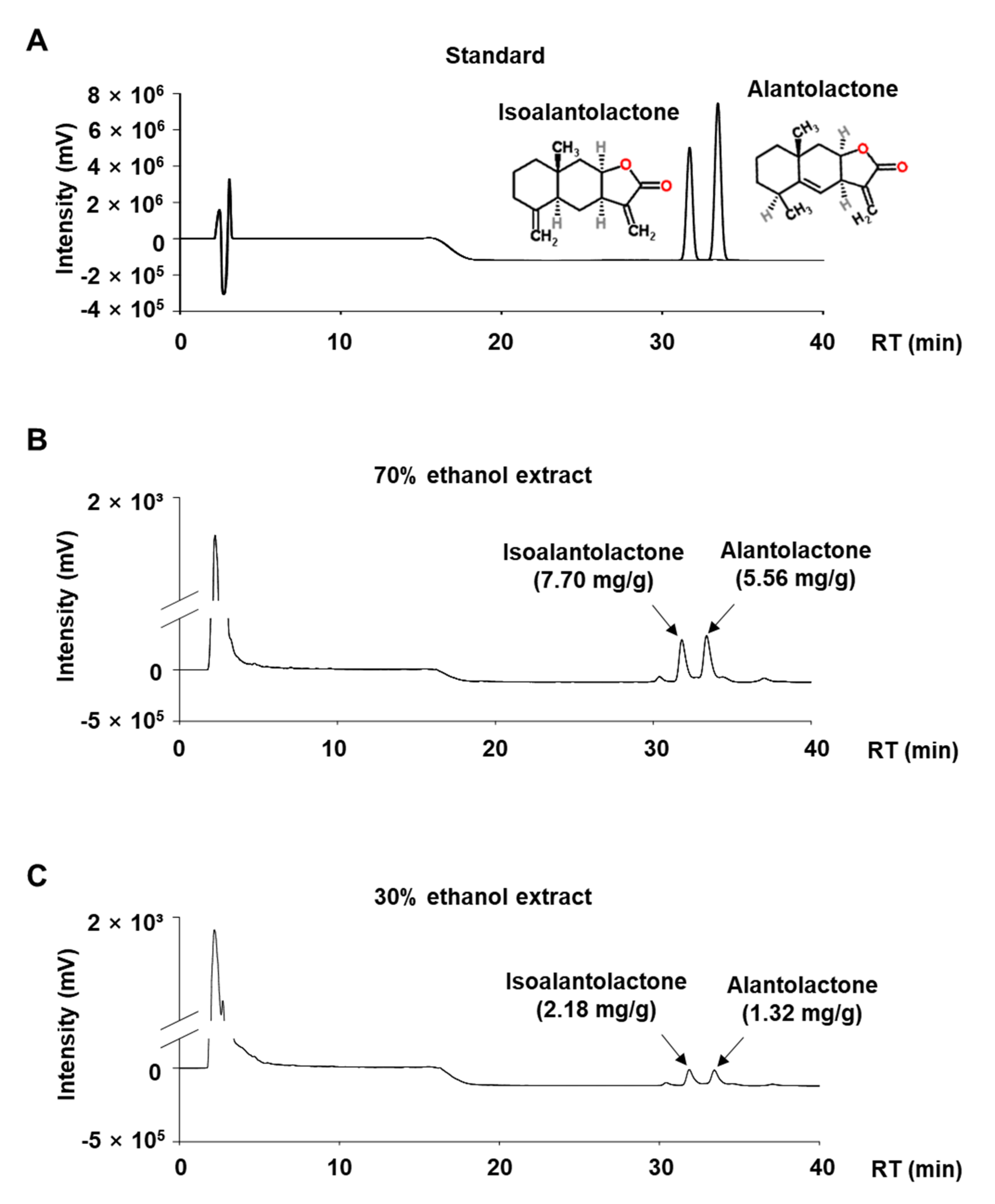
3.2. Quantitative Analysis of ALA and ISO in IHE
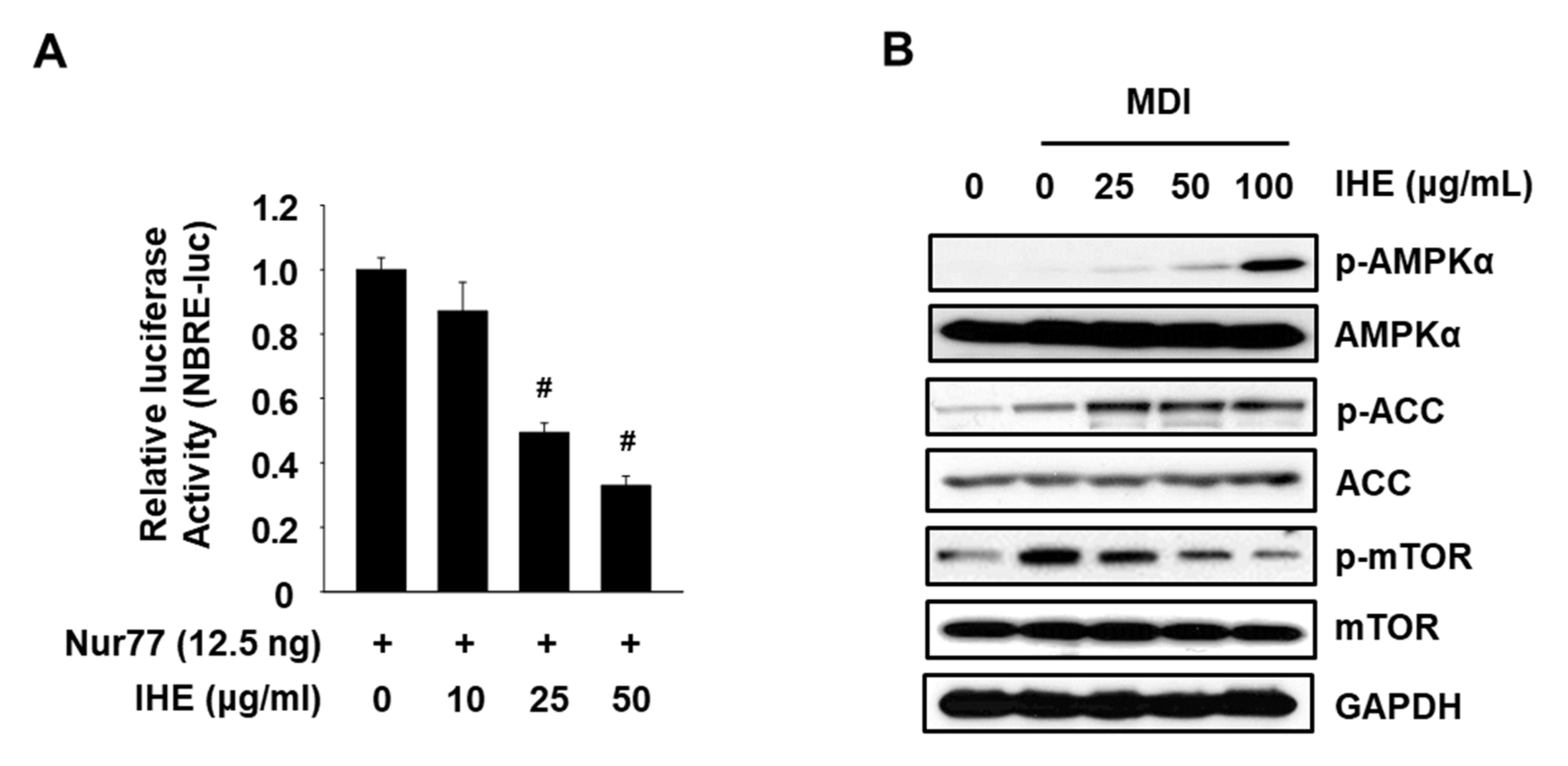
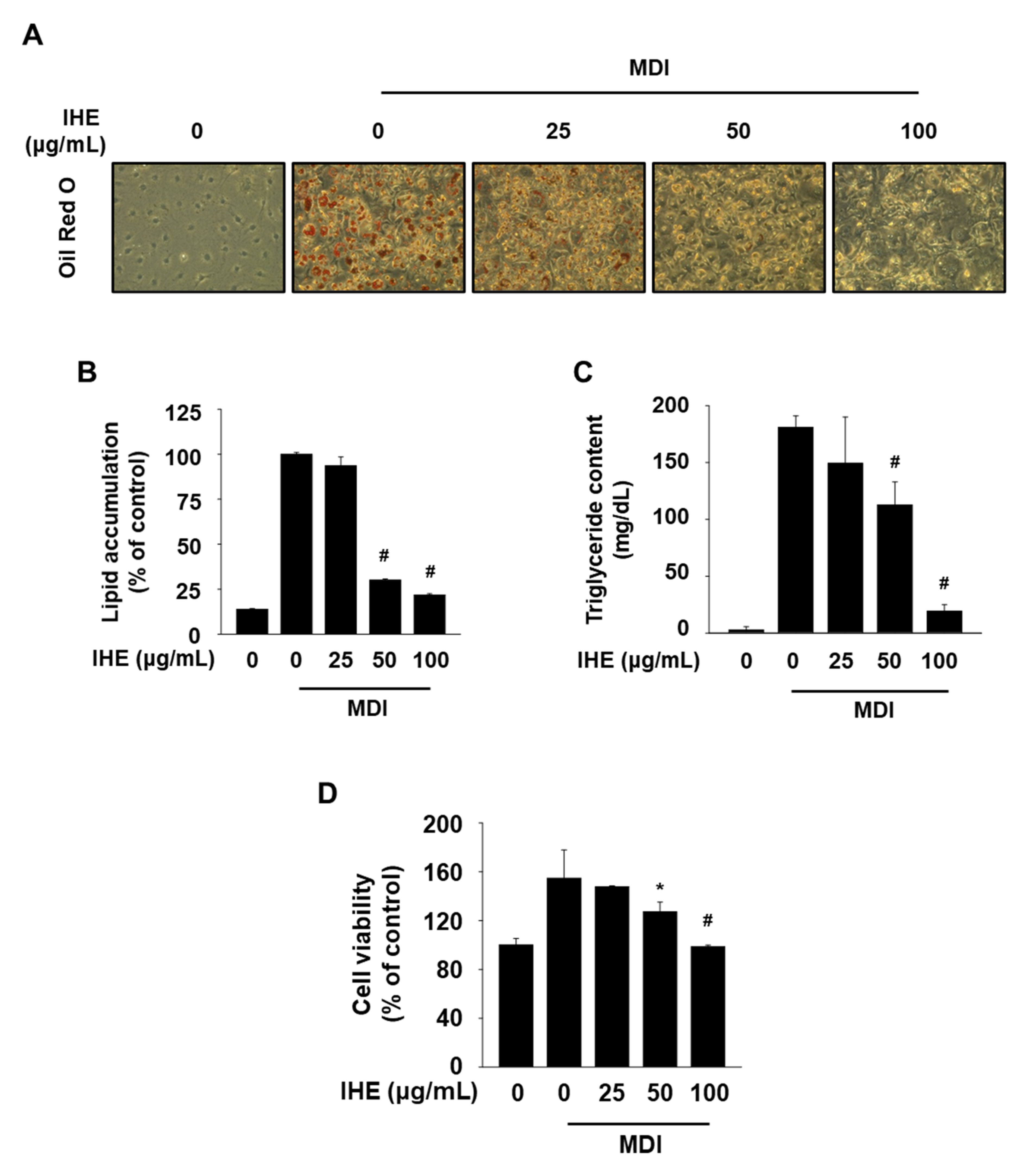
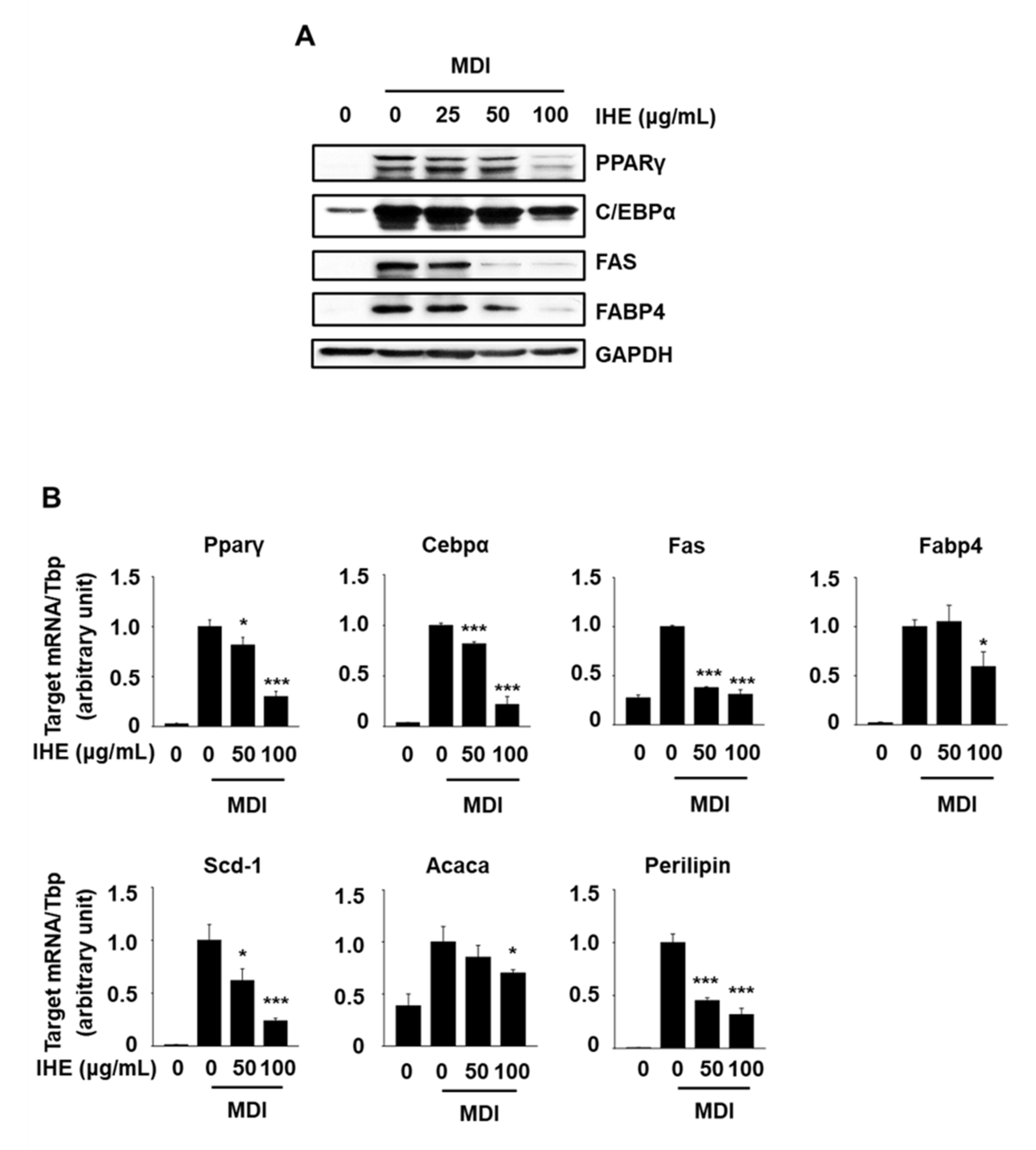
3.3. IHE Simultaneously Regulates Nur77 and AMPKα and Inhibits Adipogenesis in MDI-Treated 3T3-L1 Cells
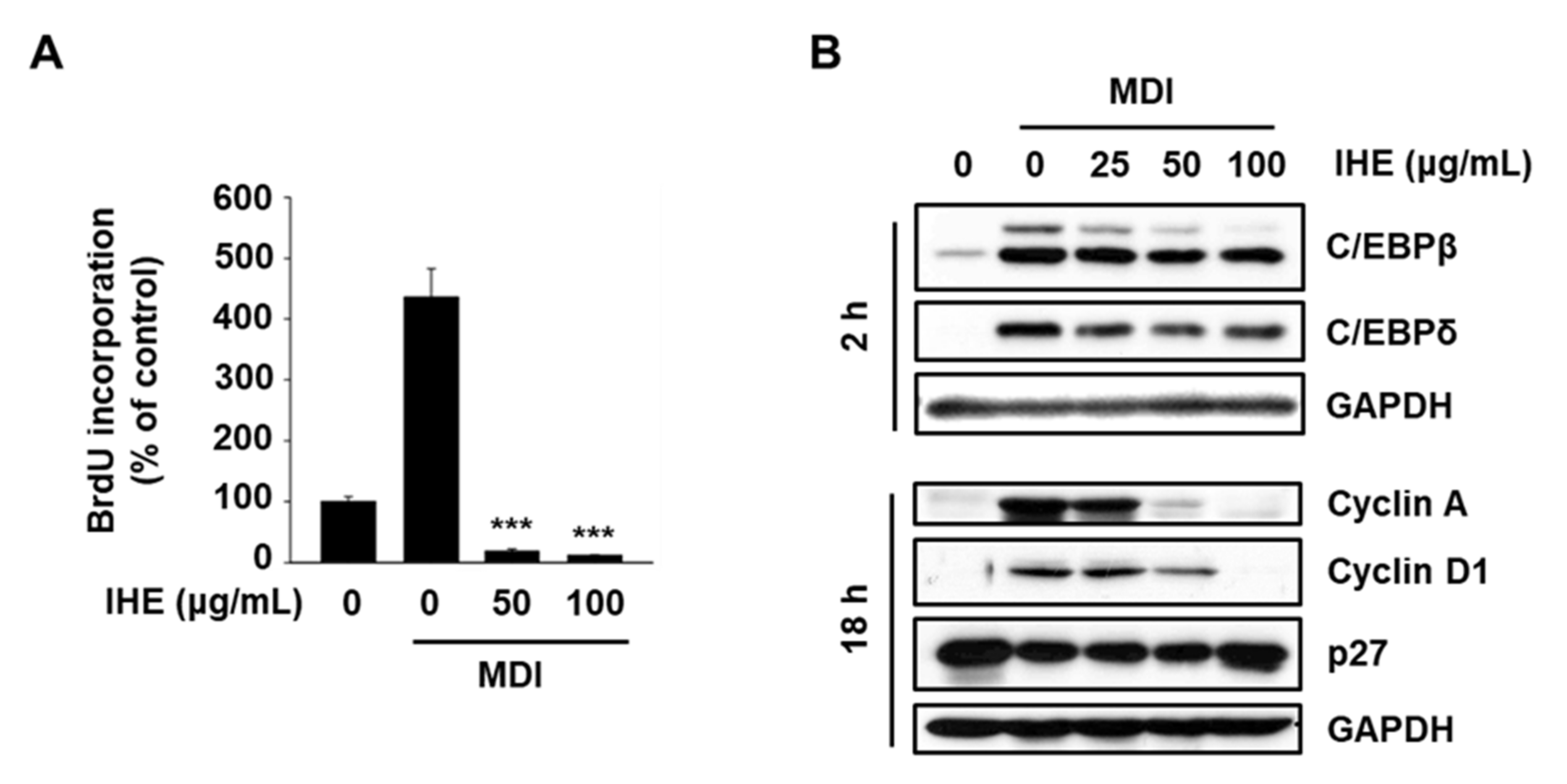
3.4. IHE Inhibits Adipogenesis in MDI-Induced 3T3-L1 Cells, in Part, through the Regulation of MCE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Fumoto, T.; Yamaguchi, T.; Hirose, F.; Osumi, T. Orphan nuclear receptor Nur77 accelerates the initial phase of adipocyte differentiation in 3T3-L1 cells by promoting mitotic clonal expansion. J. Biochem. 2006, 141, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Lee, H.S.; Cho, H.R.; Kim, K.J.; Kim, J.H.; Safe, S.; Lee, S.O. Dual targeting of Nur77 and AMPKα by isoalantolactone inhibits adipogenesis in vitro and decreases body fat mass in vivo. Int. J. Obes. 2018, 43, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Figarola, J.L.; Rahbar, S. Small-molecule COH-SR4 inhibits adipocyte differentiation via AMPK activation. Int. J. Mol. Med. 2013, 31, 1166–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Wang, Q.; Tian, X.H.; Li, H.L.; Shen, Y.H.; Xu, X.K.; Wu, G.Z.; Hu, Z.L.; Zhang, W.D. Total sesquiterpene lactones prepared from Inula helenium L. has potentials in prevention and therapy of rheumatoid arthritis. J. Ethnopharmacol. 2016, 196, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Y.M.; Zhang, M.L.; Shi, Q.W. Simultaneous determination of chlorogenic acid, caffeic acid, alantolactone and isoalantolactone in Inula helenium by HPLC. J. Chromatogr. Sci. 2014, 53, 526–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Zhang, G.; Zhang, Y.; Hua, P.; Fang, M.; Wu, M.; Liu, T. Isoalantolactone induces apoptosis through reactive oxygen species-dependent upregulation of death receptor 5 in human esophageal cancer cells. Toxicol. Appl. Pharmacol. 2018, 352, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Liu, X.; Wang, J.; Li, B.; Liu, Y.; Wang, J. Alantolactone enhances gemcitabine sensitivity of lung cancer cells through the reactive oxygen species-mediated endoplasmic reticulum stress and Akt/GSK3β pathway. Int. J. Mol. Med. 2019, 44, 1026–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedrick, E.; Lee, S.-O.; Doddapaneni, R.; Singh, M.; Safe, S. Nuclear receptor 4A1 as a drug target for breast cancer chemotheraphy. Endocr. Relat. Cancer 2015, 22, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Andey, T.; Jin, U.H.; Kim, K.; Sachdeva, M.; Safe, S. The nuclear receptor TR3 regulates mTORC1 signaling in lung cancer cells expressing wild-type p53. Oncogene 2011, 31, 3265–3276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhao, Y.M.; Tian, Y.T.; Yan, C.L.; Guo, C.Y. Ultrasound-assisted extraction of total phenolic compounds from Inula helenium. Sci. World J. 2013, 2013, 157527. [Google Scholar] [CrossRef] [Green Version]
- Hwang, E.Y.; Yu, M.H.; Jung, Y.S.; Lee, S.P.; Shon, J.H.; Lee, S.O. Defatted safflower seed extract inhibits adipogenesis in 3T3-L1 preadipocytes and improves lipid profiles in C57BL/6J ob/ob mice fed a high-fat diet. Nutr. Res. 2016, 36, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Wang, J.; Liu, H.B.; Guo, C.Y.; Zhang, W.M. Microwave-assisted extraction of alantolactone and isoalantolactone from Inula helenium. Indian J. Pharm. Sci. 2015, 77, 116–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Park, J.E.; Song, S.B.; Cha, Y.S. Effects of black adzuki bean (Vigna angularis) extract on proliferation and differentiation of 3T3-L1 preadipocytes into mature adipocytes. Nutrients 2015, 7, 277–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 850–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.J.; Seo, H.A.; Go, Y.; Oh, C.J.; Jeoung, N.H.; Park, K.G.; Lee, I.K. Dimethylfumarate suppresses adipogenic differentiation in 3T3-L1 preadipocytes through inhibition of STAT3 activity. PLoS ONE 2013, 8, e61411. [Google Scholar] [CrossRef] [Green Version]
- Türküner, M.S.; Özcan, F. Monosodium glutamate restricts the adipogenic potential of 3T3-L1 preadipocytes through mitotic clonal expansion. Cell Biol. Int. 2019, 44, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, H.M.; Chung, H.C.; Lee, J.H. Licorice extract suppresses adipogenesis through regulation of mitotic clonal expansion and adenosine monophosphate-activated protein kinase in 3T3-L1 cells. J. Food Biochem. 2020, 44, e13528. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.; Gomez-Serrano, M.; Pieralisi, A.; Calvo, J.C.; Peral, B.; Guerra, L.N. N-acetylcysteine inhibits kinase phosphorylation during 3T3-L1 adipocyte differentiation. Redox Rep. 2016, 22, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kang, R.U.; Bae, S.; Yoon, Y. AICAR, an activator of AMPK, inhibits adipogenesis via the WNT/β-catenin pathway in 3T3-L1 adipocytes. Int. J. Mol. Med. 2011, 28, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.A.; Viollet, B.; Andreelli, F.; Kahn, A.; Vaulont, S.; Sul, H.S. Induced adiposity and adipocyte hypertrophy in mice lacking the AMP-activated protein kinase-α2 subunit. Diabetes 2004, 53, 2242–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.-S.; Jeong, Y.-J.; Kim, J.-H.; Jeon, C.-H.; Lee, S.-O. Elecampane (Inula helenium) Root Extract and Its Major Sesquiterpene Lactone, Alantolactone, Inhibit Adipogenesis of 3T3-L1 Preadipocytes. Molecules 2022, 27, 4765. https://doi.org/10.3390/molecules27154765
Jung Y-S, Jeong Y-J, Kim J-H, Jeon C-H, Lee S-O. Elecampane (Inula helenium) Root Extract and Its Major Sesquiterpene Lactone, Alantolactone, Inhibit Adipogenesis of 3T3-L1 Preadipocytes. Molecules. 2022; 27(15):4765. https://doi.org/10.3390/molecules27154765
Chicago/Turabian StyleJung, Yeon-Seop, Yun-Jeong Jeong, Joung-Hee Kim, Chang-Hwan Jeon, and Syng-Ook Lee. 2022. "Elecampane (Inula helenium) Root Extract and Its Major Sesquiterpene Lactone, Alantolactone, Inhibit Adipogenesis of 3T3-L1 Preadipocytes" Molecules 27, no. 15: 4765. https://doi.org/10.3390/molecules27154765
APA StyleJung, Y.-S., Jeong, Y.-J., Kim, J.-H., Jeon, C.-H., & Lee, S.-O. (2022). Elecampane (Inula helenium) Root Extract and Its Major Sesquiterpene Lactone, Alantolactone, Inhibit Adipogenesis of 3T3-L1 Preadipocytes. Molecules, 27(15), 4765. https://doi.org/10.3390/molecules27154765





