Halogenated Flavonoid Derivatives Display Antiangiogenic Activity
Abstract
1. Introduction
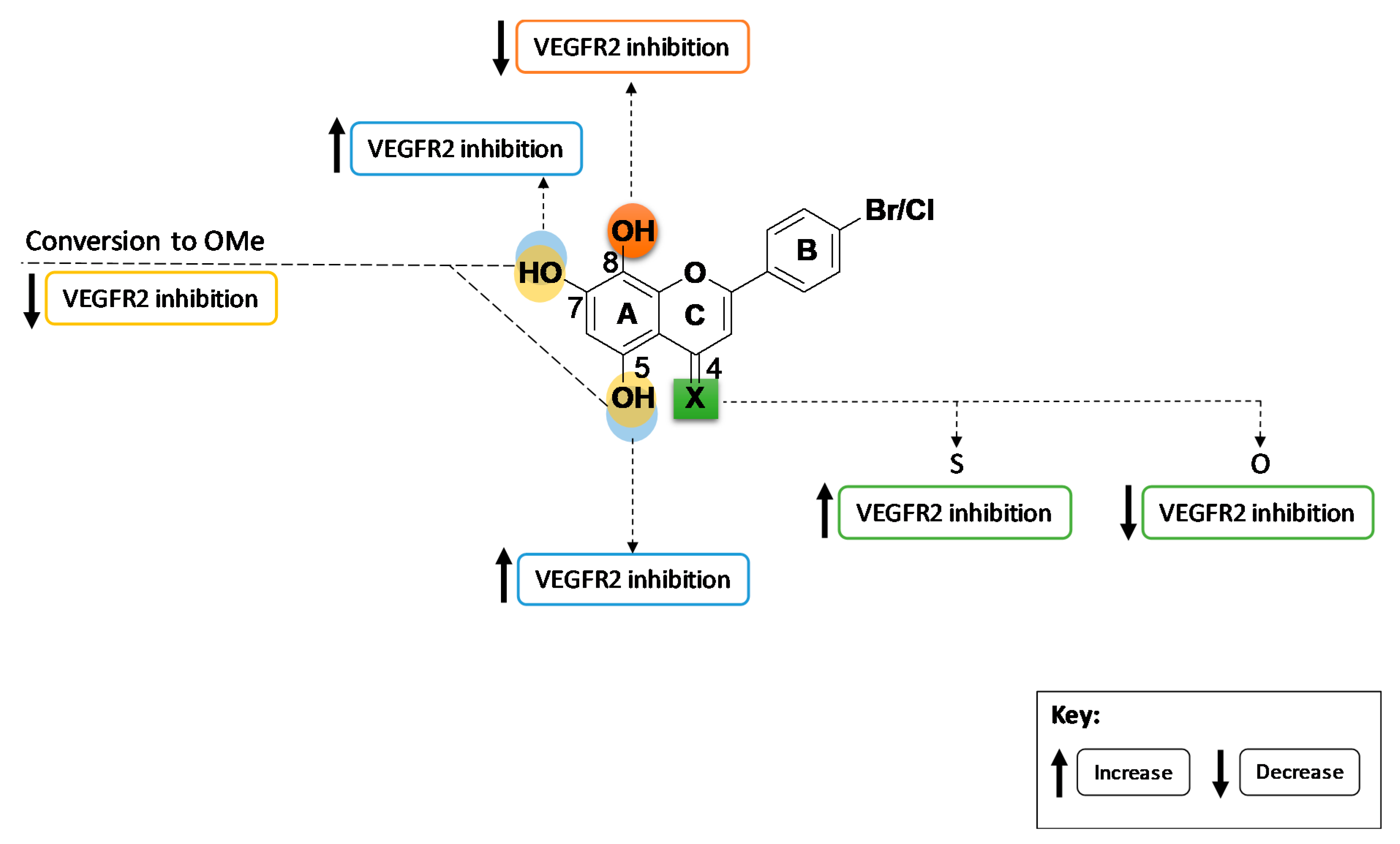
2. Results and Discussion
2.1. Synthesis
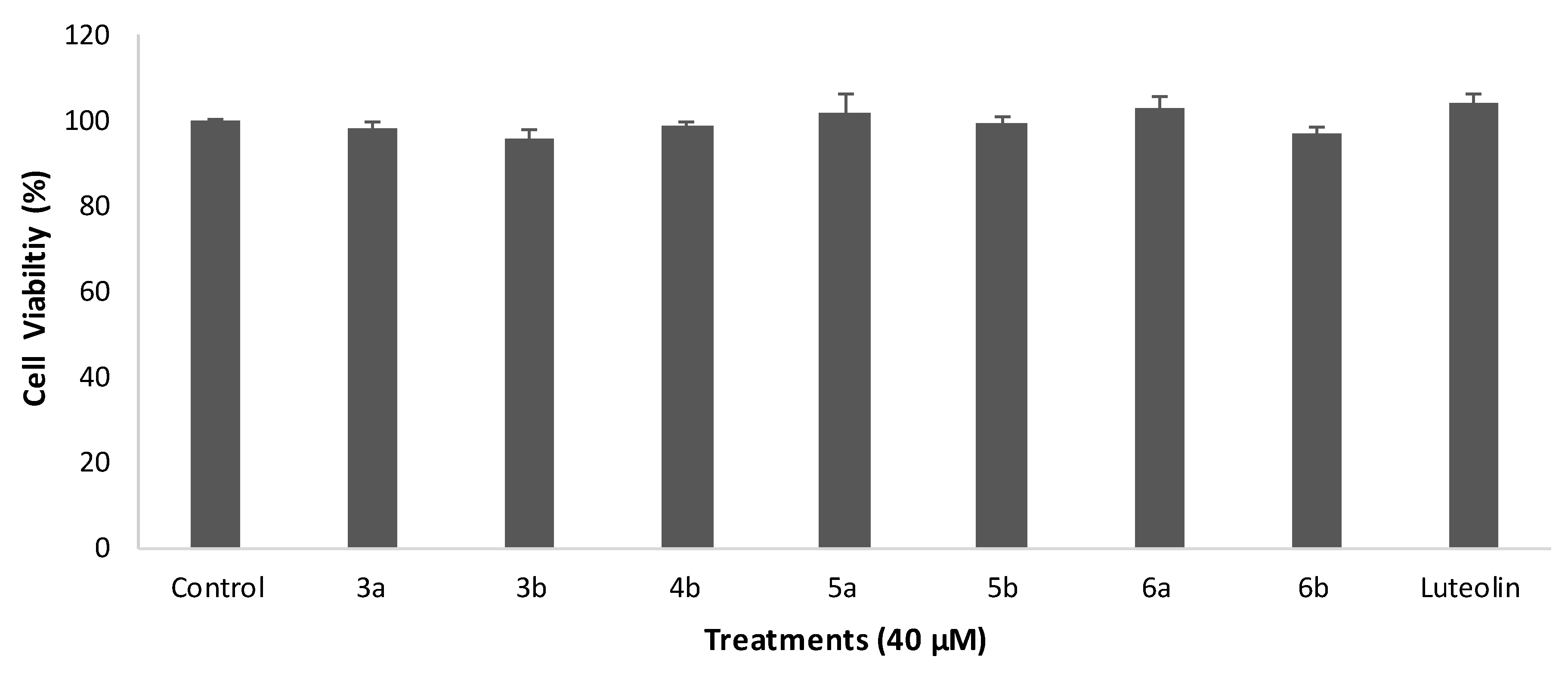
2.2. Cytotoxicity on HUVEC Cells
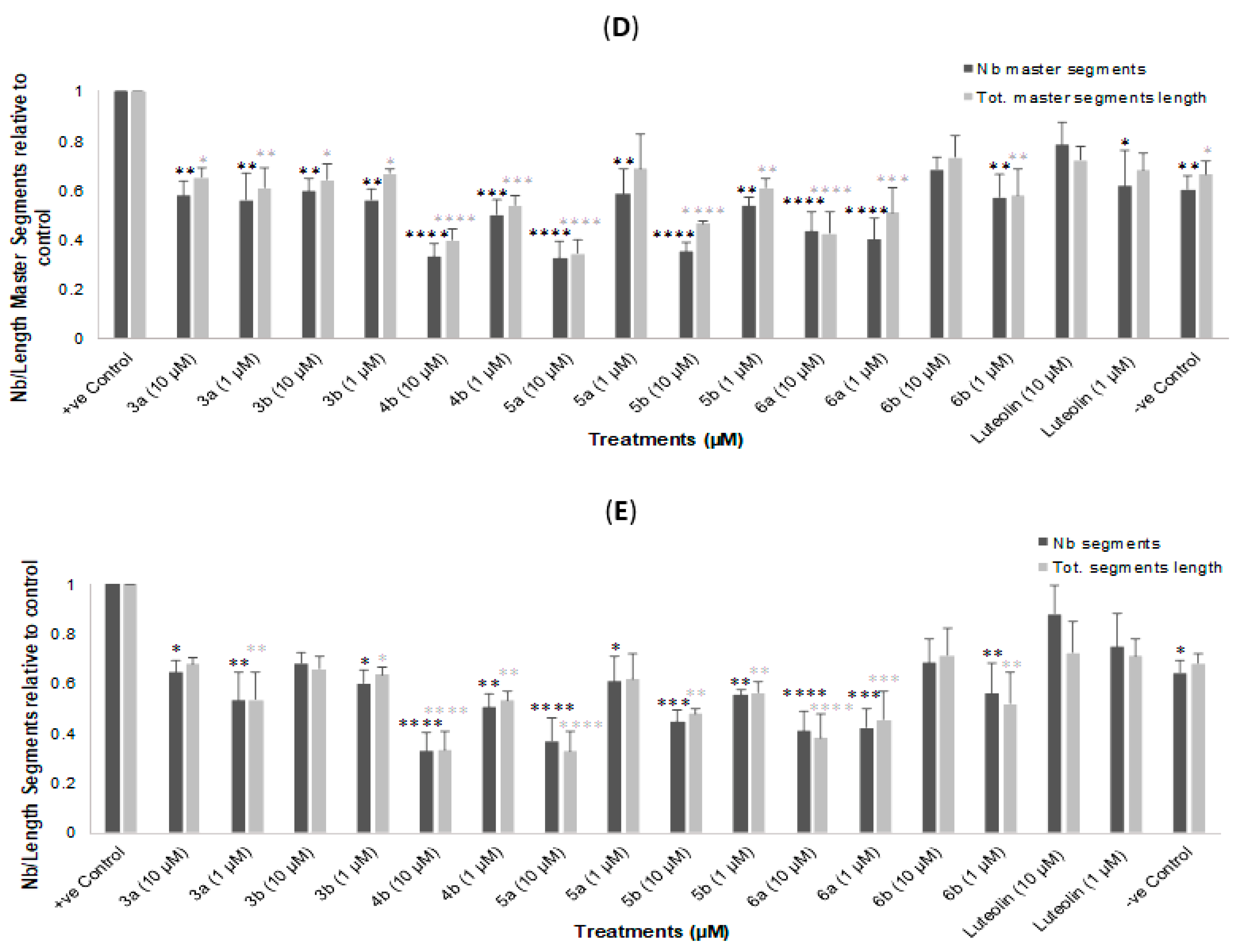
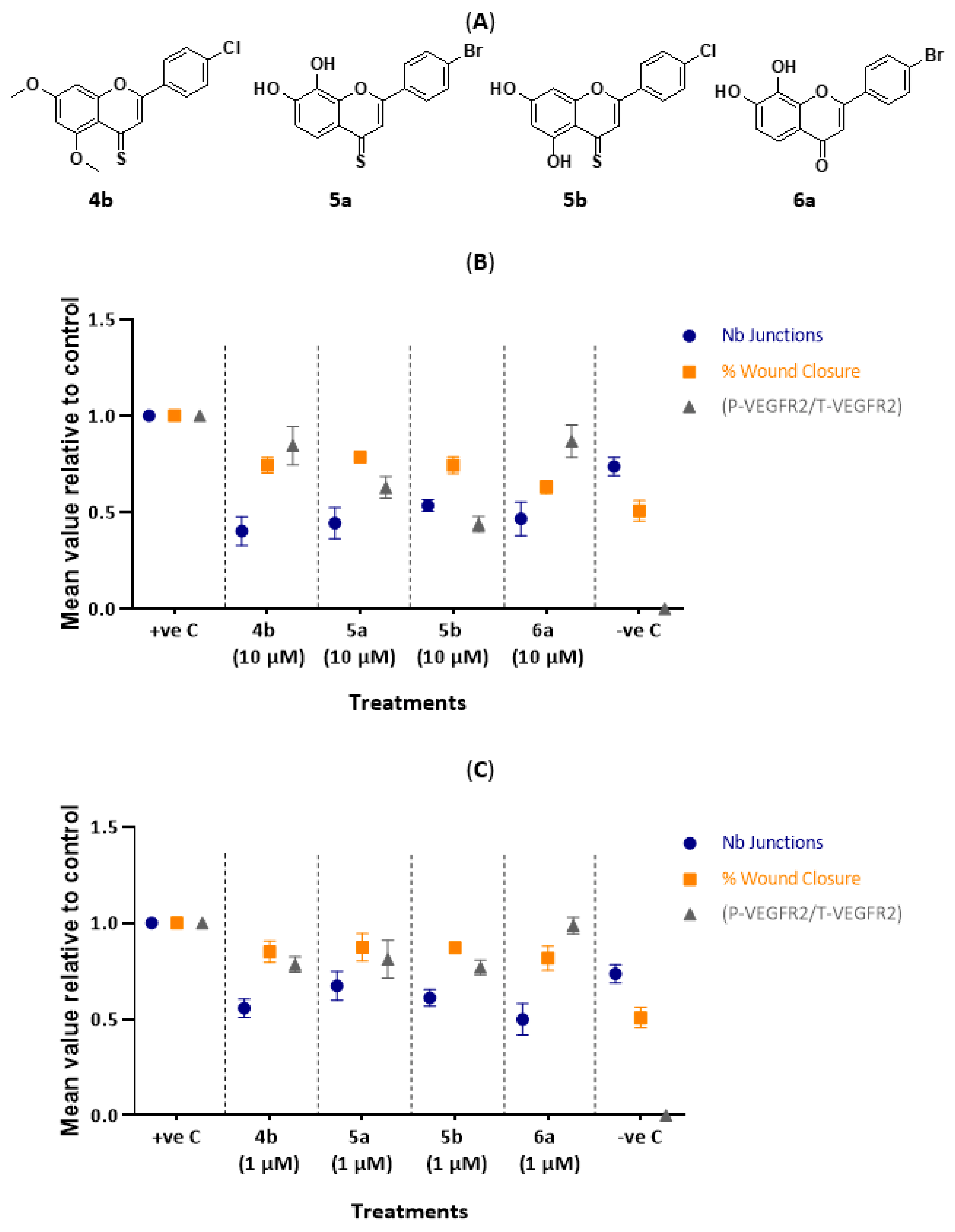
2.3. In Vitro Tube Formation Assay
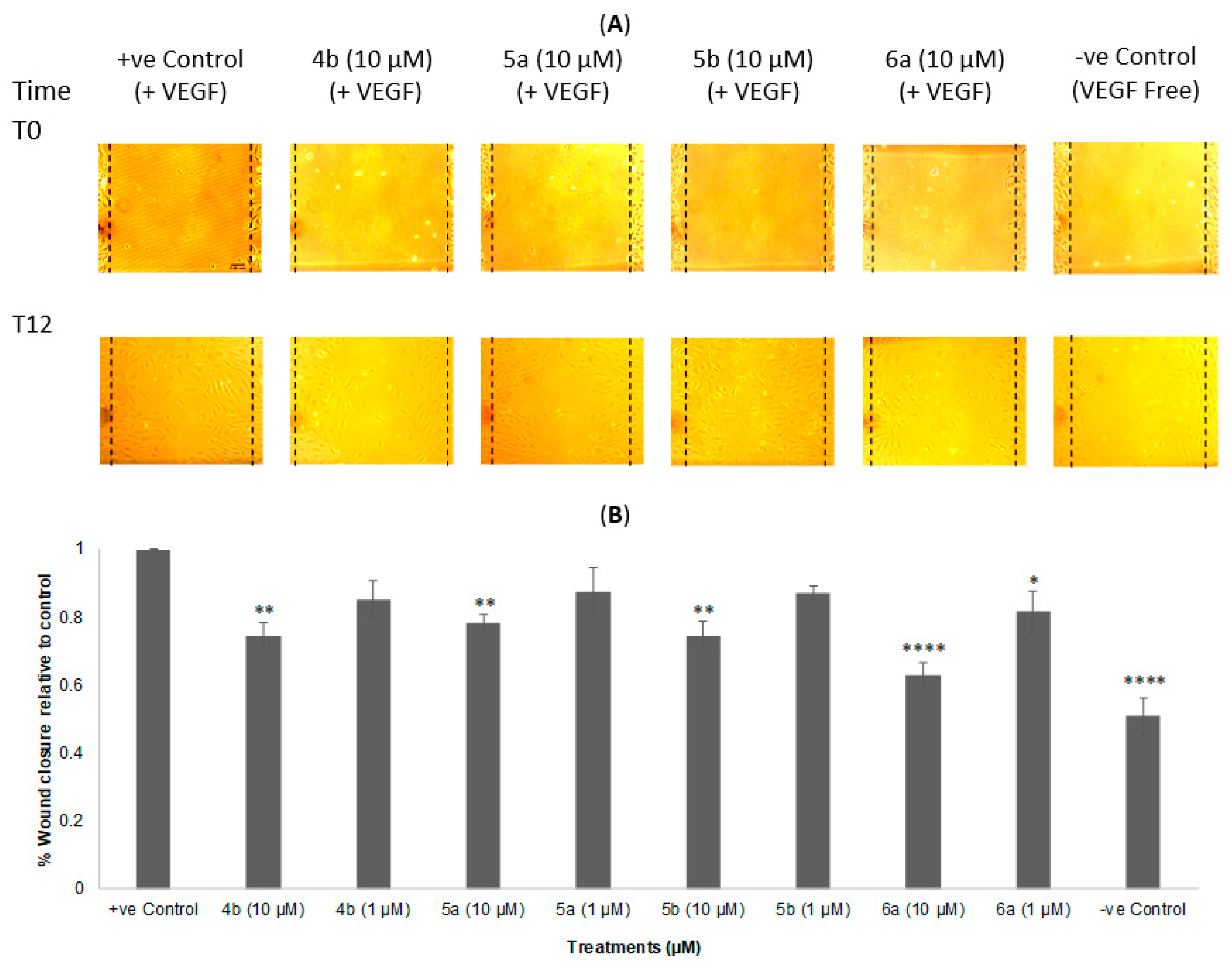
2.4. Wound Healing (Scratch) Assay
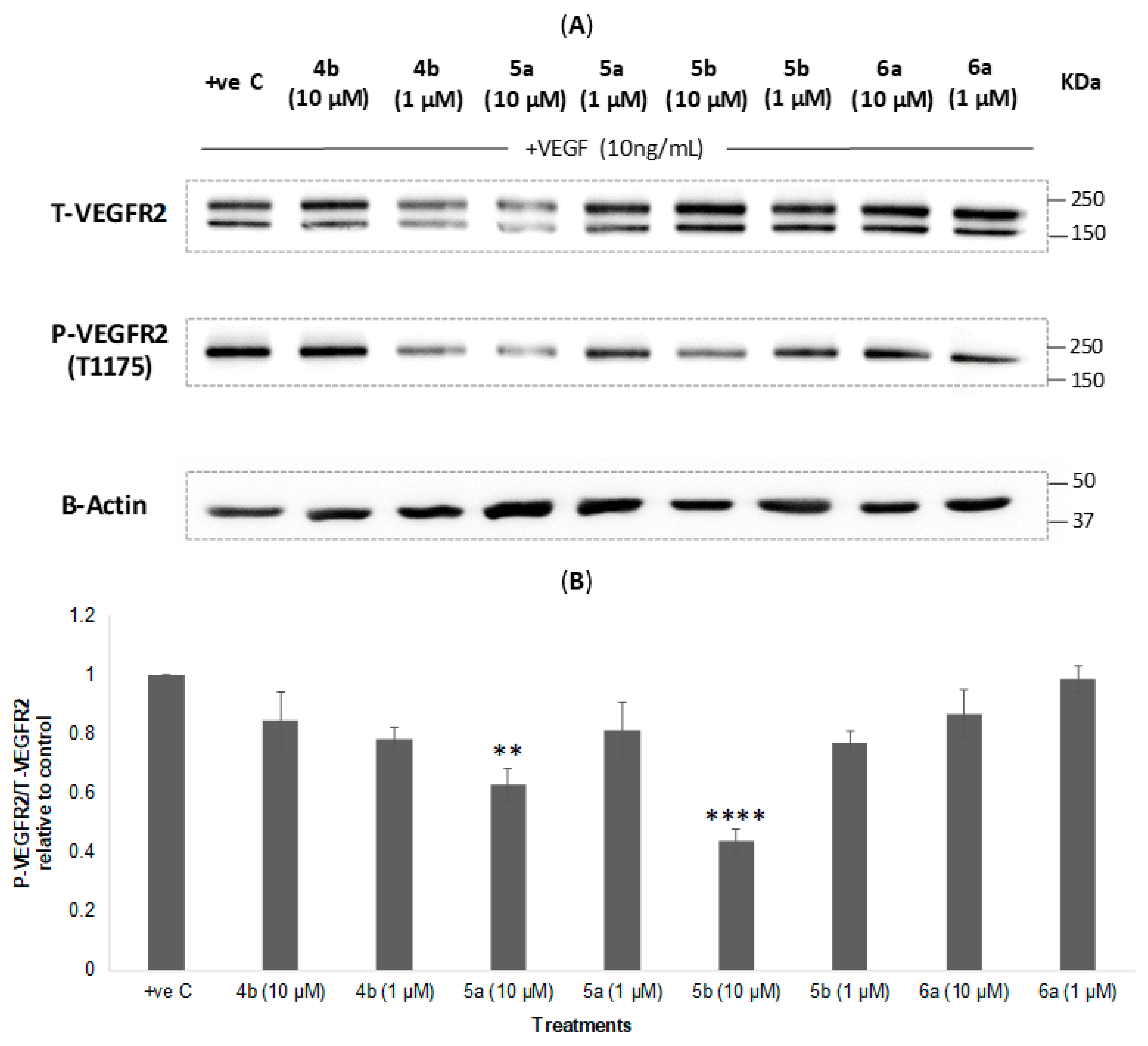
2.5. Western Blotting
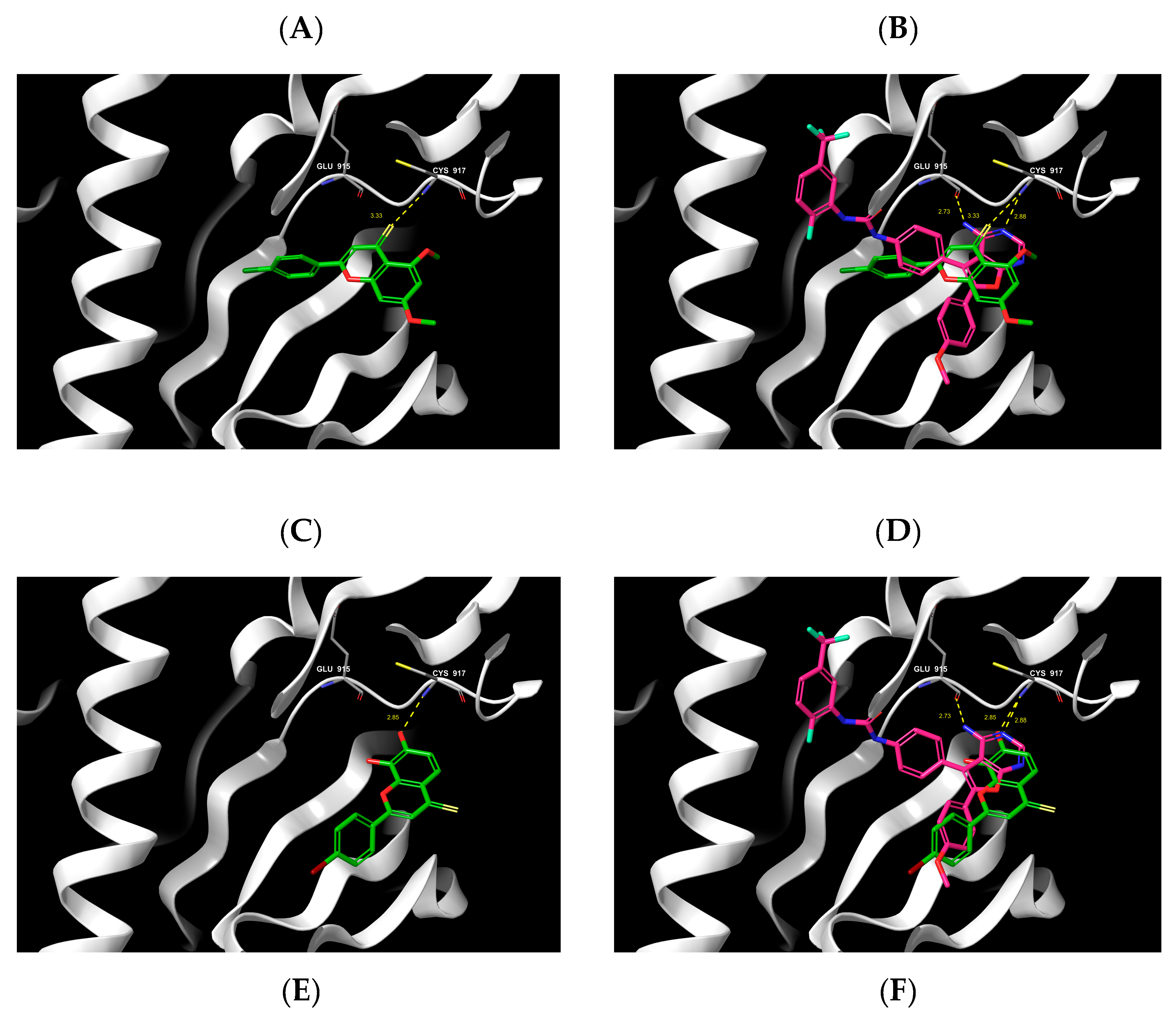
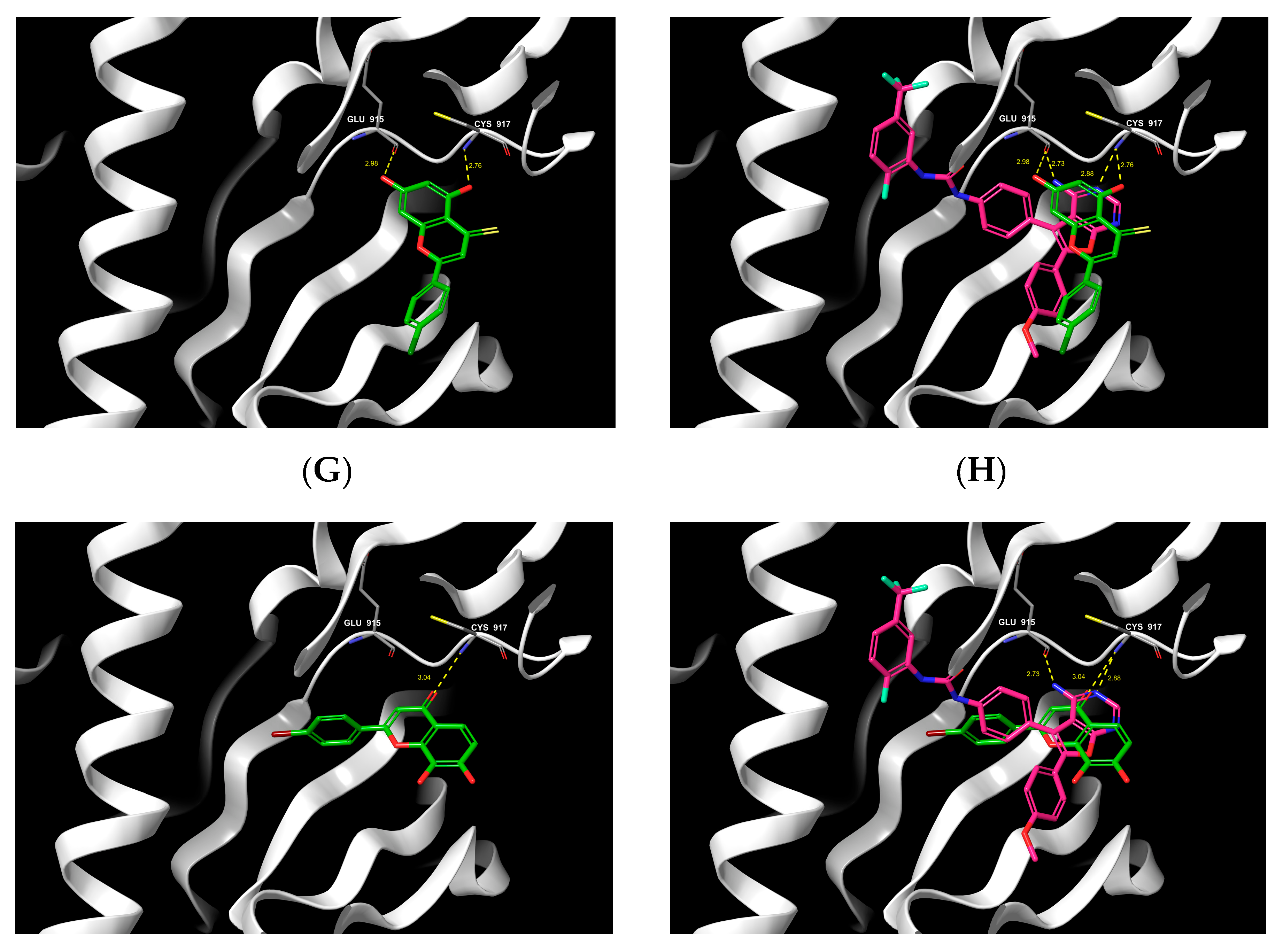
2.6. Molecular Docking
3. Materials and Methods
3.1. Synthesis
3.2. Biological Assays
3.2.1. Cytotoxicity on HUVECs
3.2.2. Endothelial Cell Tube Formation Assay
3.2.3. Scratch Assay
3.2.4. Western Blotting
3.2.5. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Carmeliet, P. Angiogenesis in Health and Disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Adair, T.H.; Montani, J.-P. Angiogenesis. In Colloquium Series on Integrated Systems Physiology from Molecule to Function; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar] [CrossRef]
- Geudens, I.; Gerhardt, O. Coordinating Cell Behaviour during Blood Vessel Formation. Development 2011, 138, 4569–4583. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dabrosin, C.; Yin, X.; Fuster, M.M.; Arreola, A.; Rathmell, W.K.; Generali, D.; Nagaraju, G.P.; El-Rayes, B.; Ribatti, D.; et al. Broad Targeting of Angiogenesis for Cancer Prevention and Therapy. Semin. Cancer Biol. 2015, 35, S224–S243. [Google Scholar] [CrossRef] [PubMed]
- Khater, M.; Greco, F.; Osborn, H.M.I. Antiangiogenic Activity of Flavonoids: A Systematic Review and Meta-Analysis. Molecules 2020, 25, 4712. [Google Scholar] [CrossRef] [PubMed]
- Gacche, R.N.; Meshram, R.J.; Shegokar, H.D.; Gond, D.S.; Kamble, S.S.; Dhabadge, V.N.; Utage, B.G.; Patil, K.K.; More, R.A. Flavonoids as a Scaffold for Development of Novel Anti-Angiogenic Agents: An Experimental and Computational Enquiry. Arch. Biochem. Biophys. 2015, 577, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Diniz, C.; Suliburska, J.; Ferreira, I.M. New Insights into the Antiangiogenic and Proangiogenic Properties of Dietary Polyphenols. Mol. Nutr. Food Res. 2017, 61, 1600912. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.; Folkman, J. Clinical Translation of Angiogenesis Inhibitors. Nat. Rev. Cancer 2002, 2, 727–739. [Google Scholar] [CrossRef]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent Discoveries of Anticancer Flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular Mechanisms and Clinical Applications of Angiogenesis. Nature 2011, 473, 289–307. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in Cancer and Other Diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Ravishankar, D.; Watson, K.A.; Boateng, S.Y.; Green, R.J.; Greco, F.; Osborn, H.M.I.I. Exploring Quercetin and Luteolin Derivatives as Antiangiogenic Agents. Eur. J. Med. Chem. 2015, 97, 259–274. [Google Scholar] [CrossRef]
- Morbidelli, L. Polyphenol-Based Nutraceuticals for the Control of Angiogenesis: Analysis of the Critical Issues for Human Use. Pharmacol. Res. 2016, 111, 384–393. [Google Scholar] [CrossRef]
- Goodman, V.L.; Rock, E.P.; Dagher, R.; Ramchandani, R.P.; Abraham, S.; Gobburu, J.V.S.; Booth, B.P.; Verbois, S.L.; Morse, D.E.; Liang, C.Y.; et al. Approval Summary: Sunitinib for the Treatment of Imatinib Refractory or Intolerant Gastrointestinal Stromal Tumors and Advanced Renal Cell Carcinoma. Clin. Cancer Res. 2007, 13, 1367–1373. [Google Scholar] [CrossRef]
- Hao, Z.; Sadek, I. Sunitinib: The Antiangiogenic Effects and Beyond. Onco. Targets Ther. 2016, 9, 5495–5505. [Google Scholar] [CrossRef]
- Blumenthal, G.M.; Cortazar, P.; Zhang, J.J.; Tang, S.; Sridhara, R.; Murgo, A.; Justice, R.; Pazdur, R. FDA Approval Summary: Sunitinib for the Treatment of Progressive Well-Differentiated Locally Advanced or Metastatic Pancreatic Neuroendocrine Tumors. Oncologist 2012, 17, 1108–1113. [Google Scholar] [CrossRef]
- Haibe, Y.; Kreidieh, M.; El Hajj, H.; Khalifeh, I.; Mukherji, D.; Temraz, S.; Shamseddine, A. Resistance Mechanisms to Anti-Angiogenic Therapies in Cancer. Front. Oncol. 2020, 10, 221–243. [Google Scholar] [CrossRef]
- Belotti, D.; Vergani, V.; Drudis, T.; Borsotti, P.; Pitelli, M.R.; Viale, G.; Giavazzi, R.; Taraboletti, G. The Microtubule-Affecting Drug Paclitaxel Has Antiangiogenic Activity. Clin. Cancer Res. 1996, 2, 1843–1849. [Google Scholar]
- Ge, H.; Luo, H. Overview of Advances in Vasculogenic Mimicry—A Potential Target for Tumor Therapy. Cancer Manag. Res. 2018, 10, 2429–2437. [Google Scholar] [CrossRef]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.G.; Pe’er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J.C. Vascular Channel Formation by Human Melanoma Cells in Vivo and in Vitro: Vasculogenic Mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef]
- Kerbel, R.S. Inhibition of Tumor Angiogenesis as a Strategy to Circumvent Acquired Resistance to Anti-Cancer Therapeutic Agents. BioEssays 1991, 13, 31–36. [Google Scholar] [CrossRef]
- Khater, M.; Ravishankar, D.; Greco, F.; Osborn, H.M.I. Metal Complexes of Flavonoids: Their Synthesis, Characterization and Enhanced Antioxidant and Anticancer Activities. Future Med. Chem. 2019, 11, 2845–2867. [Google Scholar] [CrossRef]
- Clinicaltrials.Gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=&type=&rslt=&recrs=b&recrs=a&recrs=f&recrs=d&recrs=g&age_v=&gndr=&intr=flavonoid&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s= (accessed on 13 January 2020).
- Zhao, D.; Qin, C.; Fan, X.; Li, Y.; Gu, B. Inhibitory Effects of Quercetin on Angiogenesis in Larval Zebra Fi Sh and Human Umbilical Vein Endothelial Cells. Eur. J. Pharmacol. 2014, 723, 360–367. [Google Scholar] [CrossRef]
- Noberini, R.; Koolpe, M.; Lamberto, I.; Pasquale, E.B. Inhibition of Eph Receptor–Ephrin Ligand Interaction by Tea Polyphenols. Pharmacol. Res. 2012, 66, 363–373. [Google Scholar] [CrossRef][Green Version]
- Cerezo, A.B.; Winterbone, M.S.; Moyle, C.W.A.; Needs, P.W.; Kroon, P.A. Molecular Structure-Function Relationship of Dietary Polyphenols for Inhibiting VEGF-Induced VEGFR-2 Activity. Mol. Nutr. Food Res. 2015, 59, 2119–2131. [Google Scholar] [CrossRef]
- Lamy, S.; Akla, N.; Ouanouki, A.; Lord-Dufour, S.; Béliveau, R. Diet-Derived Polyphenols Inhibit Angiogenesis by Modulating the Interleukin-6/STAT3 Pathway. Exp. Cell Res. 2012, 318, 1586–1596. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chan, C.-Y.; Chou, I.-T.; Lien, C.-H.; Hung, H.-C.; Lee, M.-F. Quercetin Induces Growth Arrest through Activation of FOXO1 Transcription Factor in EGFR-Overexpressing Oral Cancer Cells. J. Nutr. Biochem. 2013, 24, 1596–1603. [Google Scholar] [CrossRef]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean Diet Polyphenols Reduce Inflammatory Angiogenesis through MMP-9 and COX-2 Inhibition in Human Vascular Endothelial Cells: A Potentially Protective Mechanism in Atherosclerotic Vascular Disease and Cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef]
- Zgheib, A.; Lamy, S.; Annabi, B. Epigallocatechin Gallate Targeting of Membrane Type 1 Matrix Metalloproteinase-Mediated Src and Janus Kinase/Signal Transducers and Activators of Transcription 3 Signalling Inhibits Transcription of Colony-Stimulating Factors 2 and 3 in Mesenchymal Stroma. J. Biol. Chem. 2013, 288, 13378–13386. [Google Scholar] [CrossRef]
- Jo, H.; Jung, S.H.; Yim, H.B.; Lee, S.J.; Kang, K.D. The Effect of Baicalin in a Mouse Model of Retinopathy of Prematurity. BMB Rep. 2015, 48, 271–276. [Google Scholar] [CrossRef]
- Sarkar, J.; Nandy, S.K.; Chowdhury, A.; Chakraborti, T.; Chakraborti, S. Inhibition of MMP-9 by Green Tea Catechins and Prediction of Their Interaction by Molecular Docking Analysis. Biomed. Pharmacother. 2016, 84, 340–347. [Google Scholar] [CrossRef]
- Mojzis, J.; Varinska, L.; Mojzisova, G.; Kostova, I.; Mirossay, L. Antiangiogenic Effects of Flavonoids and Chalcones. Pharmacol. Res. 2008, 57, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Wang, S.; Hsin, I.; Lee, F.; Huang, H.; Huo, T.; Lee, W.; Lin, H.; Lee, S. Green Tea Polyphenol Decreases the Severity of Portosystemic Collaterals and Mesenteric Angiogenesis in Rats with Liver Cirrhosis. Clin. Sci. 2014, 126, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Warrier, S.; Kumar, A.P.; Sethi, G.; Arfuso, F. Potential Role of Natural Compounds as Anti-Angiogenic Agents in Cancer. Curr. Vasc. Pharmacol. 2017, 15, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Watson, K.A.; Greco, F.; Osborn, H.M.I. Novel Synthesised Flavone Derivatives Provide Significant Insight into the Structural Features Required for Enhanced Anti-Proliferative Activity. RSC Adv. 2016, 6, 64544–64556. [Google Scholar] [CrossRef]
- Staton, C.A.; Stribbling, S.M.; Tazzyman, S.; Hughes, R.; Brown, N.J.; Lewis, C.E. Current Methods for Assaying Angiogenesis in Vitro and in Vivo. Int. J. Exp. Pathol. 2004, 85, 233–248. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Son, Y.-O.; Budhraja, A.; Wang, X.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.-C.; Kim, D.; Divya, S.P.; et al. Luteolin Inhibits Human Prostate Tumor Growth by Suppressing Vascular Endothelial Growth Factor Receptor 2-Mediated Angiogenesis. PLoS ONE 2012, 7, 52279. [Google Scholar] [CrossRef]
- Afranie-Sakyi, J.A.; Klement, G.L. The Toxicity of Anti-VEGF Agents When Coupled with Standard Chemotherapeutics. Cancer Lett. 2015, 357, 1–7. [Google Scholar] [CrossRef]
- Angiogenesis Analyzer for ImageJ—Gilles Carpentier Research Web Site: Computer Image Analysis. Available online: http://image.bio.methods.free.fr/ImageJ/?Angiogenesis-Analyzer-for-ImageJ (accessed on 18 October 2021).
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A Comparative Morphometric Analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; Lei, X.; Huang, M.; Ye, W.; Zhang, R.; Zhang, D. Luteolin Inhibits Angiogenesis by Blocking Gas6/Axl Signaling Pathway. Int. J. Oncol. 2017, 51, 677–685. [Google Scholar] [CrossRef]
- Ferreira, A.K.; Freitas, V.M.; Levy, D.; Ruiz, J.L.M.; Bydlowski, S.P.; Rici, R.E.G.; Filho, O.M.R.; Chierice, G.O.; Maria, D.A. Anti-Angiogenic and Anti-Metastatic Activity of Synthetic Phosphoethanolamine. PLoS ONE 2013, 8, e57937. [Google Scholar] [CrossRef]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 1314–1325. [Google Scholar] [CrossRef]
- Li, X.; Claesson-Welsh, L.; Shibuya, M. Chapter 13 VEGF Receptor Signal Transduction. Methods Enzymol. 2008, 443, 261–284. [Google Scholar] [CrossRef]
- Sithisarn, P.; Michaelis, M.; Schubert-Zsilavecz, M.; Cinatl, J., Jr. Differential Antiviral and Anti-Inflammatory Mechanisms of the Flavonoids Biochanin A and Baicalein in H5N1 Influenza A Virus-Infected Cells. Antiviral Res. 2013, 97, 41–48. [Google Scholar] [CrossRef]
- Kothandan, G.; Gadhe, C.G.; Madhavan, T.; Choi, C.H.; Cho, S.J. Docking and 3D-QSAR (Quantitative Structure Activity Relationship) Studies of Flavones, the Potent Inhibitors of p-Glycoprotein Targeting the Nucleotide Binding Domain. Eur. J. Med. Chem. 2011, 46, 4078–4088. [Google Scholar] [CrossRef]
- Atrahimovich, D.; Vaya, J.; Khatib, S. The Effects and Mechanism of Flavonoid-RePON1 Interactions. Structure-Activity Relationship Study. Bioorg. Med. Chem. 2013, 21, 3348–3355. [Google Scholar] [CrossRef]
- Haraguchi, H.; Tanimoto, K.; Tamura, Y.; Mizutani, K.; Kinoshita, T. Mode of Antibacterial Action of Retrochalcones from Glycyrrhiza Inflata. Phytochemistry 1998, 48, 125–129. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Krych, J.; Gebicka, L. Catalase Is Inhibited by Flavonoids. Int. J. Biol. Macromol. 2013, 58, 148–153. [Google Scholar] [CrossRef]
- Giuliani, C.; Bucci, I.; Di Santo, S.; Rossi, C.; Grassadonia, A.; Piantelli, M.; Monaco, F.; Napolitano, G. The Flavonoid Quercetin Inhibits Thyroid-Restricted Genes Expression and Thyroid Function. Food Chem. Toxicol. 2014, 66, 23–29. [Google Scholar] [CrossRef]
- Wang, R.; Lu, Y.; Wang, S. Comparative Evaluation of 11 Scoring Functions for Molecular Docking. J. Med. Chem. 2003, 46, 2287–2303. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Matsunaga, S.; Tang, J.; Maeda, Y.; Nakano, M.; Philippe, R.J.; Shibahara, M.; Liu, W.; Sato, H.; Wang, L.; et al. Novel 4-Amino-Furo[2,3-d]Pyrimidines as Tie-2 and VEGFR2 Dual Inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 2203–2207. [Google Scholar] [CrossRef]
- El-Adl, K.; El-Helby, A.G.A.; Sakr, H.; El-Hddad, S.S.A. Design, Synthesis, Molecular Docking, and Anticancer Evaluations of 1-Benzylquinazoline-2,4(1H,3H)-Dione Bearing Different Moieties as VEGFR-2 Inhibitors. Arch. Pharm. 2020, 353, 2000068–2000081. [Google Scholar] [CrossRef]
- Okamoto, K.; Ikemori-Kawada, M.; Jestel, A.; Von König, K.; Funahashi, Y.; Matsushima, T.; Tsuruoka, A.; Inoue, A.; Matsui, J. Distinct Binding Mode of Multikinase Inhibitor Lenvatinib Revealed by Biochemical Characterization. ACS Med. Chem. Lett. 2015, 6, 89–94. [Google Scholar] [CrossRef]
- Acton, A.L.; Fante, C.; Flatley, B.; Burattini, S.; Hamley, I.W.; Wang, Z.; Greco, F.; Hayes, W. Janus PEG-Based Dendrimers for Use in Combination Therapy: Controlled Multi-Drug Loading and Sequential Release. Biomacromolecules 2013, 14, 564–574. [Google Scholar] [CrossRef]
- Endothelial Cell Tube Formation Assay|Thermo Fisher Scientific—UK. Available online: https://www.thermofisher.com/uk/en/home/references/protocols/cell-and-tissue-analysis/cell-profilteration-assay-protocols/angiogenesis-protocols/endothelial-cell-tube-formation-assay.html#prot4 (accessed on 18 October 2021).
- Rasband, W.S. ImageJ. U.S.; National Institutes of Health: Bethesda, MD, USA, 1997–2015. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Western Blot Membrane Stripping for Restaining Protocol|Abcam. Available online: https://www.abcam.com/protocols/western-blot-membrane-stripping-for-restaining-protocol (accessed on 28 March 2022).
- Jain, A.N. Surflex: Fully Automatic Flexible Molecular Docking Using a Molecular Similarity-Based Search Engine. J. Med. Chem. 2003, 46, 499–511. [Google Scholar] [CrossRef]
- Schrödinger Release 2022-1: Maestro; Schrödinger, LLC: New York, NY, USA, 2021.
- Powell, M.J.D. Restart Procedures for the Conjugate Gradient Method. Math. Program. 1977, 12, 241–254. [Google Scholar] [CrossRef]

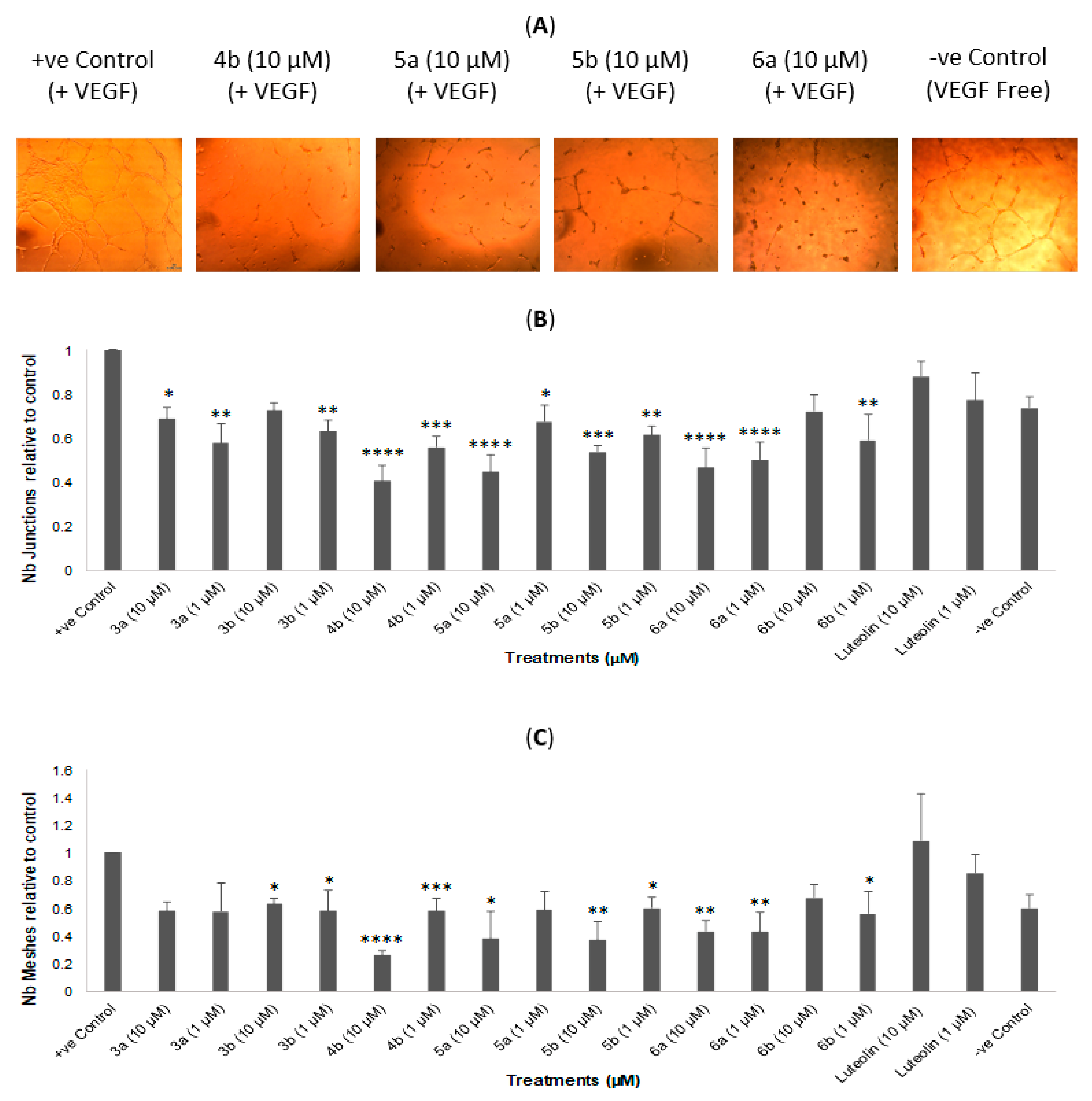



| Compound | Overall Tube Formation Inhibition at 10 µM (% of Control) |
|---|---|
| 3b (C=O) | 35% † |
| 4b (C=S) | 66% † |
| 6a (C=O) | 57% § |
| 5a (C=S) | 64% § |
| 6b (C=O) | 30% † |
| 5b (C=S) | 60% † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khater, M.; Watson, K.A.; Boateng, S.Y.; Greco, F.; Osborn, H.M.I. Halogenated Flavonoid Derivatives Display Antiangiogenic Activity. Molecules 2022, 27, 4757. https://doi.org/10.3390/molecules27154757
Khater M, Watson KA, Boateng SY, Greco F, Osborn HMI. Halogenated Flavonoid Derivatives Display Antiangiogenic Activity. Molecules. 2022; 27(15):4757. https://doi.org/10.3390/molecules27154757
Chicago/Turabian StyleKhater, Mai, Kimberly A. Watson, Samuel Y. Boateng, Francesca Greco, and Helen M. I. Osborn. 2022. "Halogenated Flavonoid Derivatives Display Antiangiogenic Activity" Molecules 27, no. 15: 4757. https://doi.org/10.3390/molecules27154757
APA StyleKhater, M., Watson, K. A., Boateng, S. Y., Greco, F., & Osborn, H. M. I. (2022). Halogenated Flavonoid Derivatives Display Antiangiogenic Activity. Molecules, 27(15), 4757. https://doi.org/10.3390/molecules27154757








