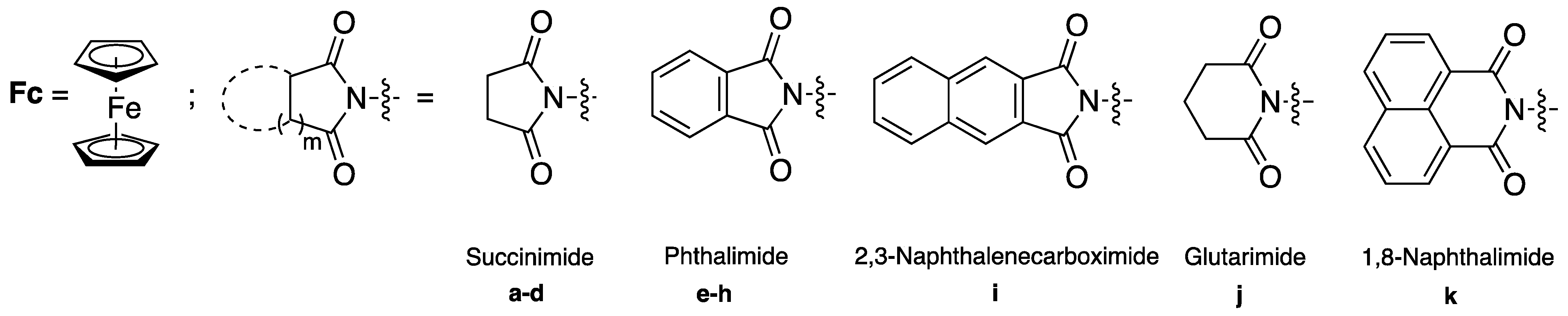
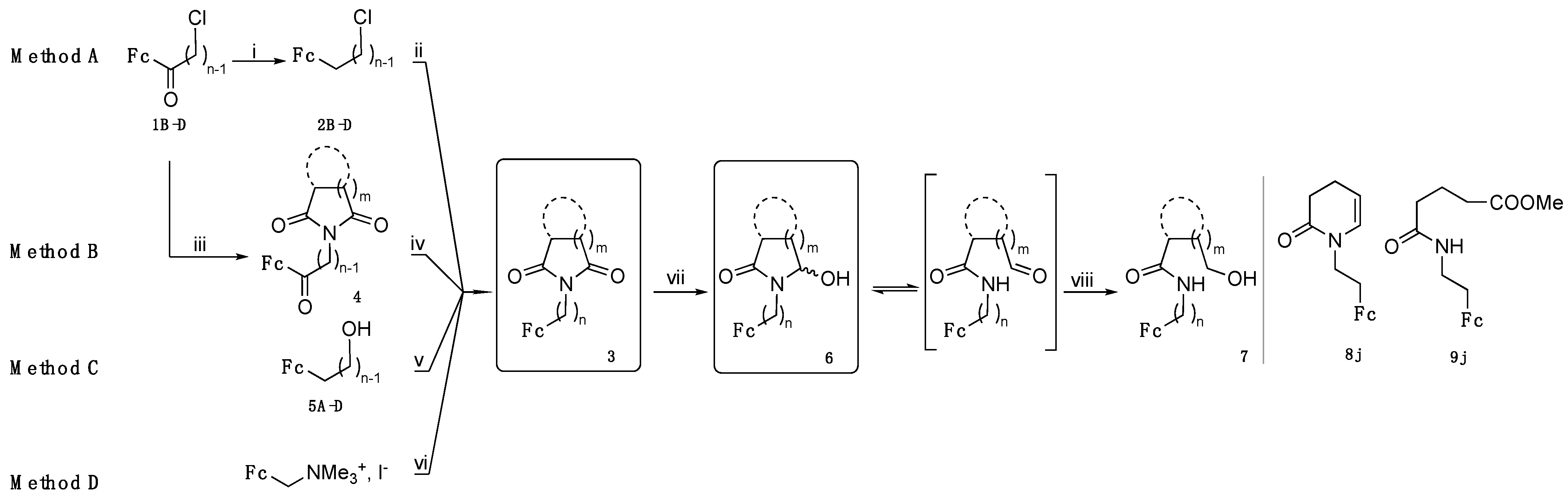
3.5. General Procedure for the Synthesis of Imides 3 by Method D
This method is adapted from a procedure described in reference [
24], replacing potassium phthalimide by a mixture of phthalimide and potassium carbonate (for
3e). To (ferrocenylmethyl) trimethylammonium iodide, potassium carbonate and phthalimide or succinimide, DMF was added (10 mL/mmol of iodide), and the mixture was heated to 80 °C overnight. After cooling to room temperature, the mixture was poured into water and was extracted three times with Et
2O. The combined organic layer was washed with water then dried with magnesium sulfate. After concentration under reduced pressure, the crude mixture was chromatographed on silica gel with a DCM/petroleum ether 9:1 mixture to afford pure imides.
3.5.1. N-(2-ferrocenyl-2-oxo-ethyl) Succinimide (4b)
Method B: From 2-chloro-1-ferrocenyl-1-ethanone 1B (4 g, 15.24 mmol), potassium carbonate (4.212 g, 30.5 mmol), succinimide (2.265 g, 22.9 mmol). Yield 33%. 1H NMR (CDCl3): δ 2.83 (s, 4H, succinimide), 4.32 (s, 5H, Cp), 4.56 (t, J = 2.0 Hz, 2H, C5H4), 4.68 (s, 2H, N-CH2-CO), 4.81 (t, J = 2.0 Hz, 2H, C5H4). 13C NMR (CDCl3): δ 28.4 (2CH2 succinimide), 45.2 (N-CH2-CO), 69.0 (2CH C5H4), 70.4 (5CH Cp), 72.9 (2CH C5H4), 75.5 (C C5H4), 177.0 (CO-N-CO), 194.3 (CO). IR (KBr, ν cm−1): 3096, 2965, 2931 (CH, CH2), 1703, 1684 (CO). MS (ESI) m/z: 326 [M + H]+. HRMS (ESI, C16H16FeNO3: [M + H]+) calcd: 326.0480, found: 326.0468.
3.5.2. N-(3-ferrocenyl-3-oxopropyl) Succinimide (4c)
Method B: from 3-chloro-1-ferrocenyl-propan-1-one 1C (4.148 g, 15 mmol), potassium carbonate (3.11 g, 22.5 mmol), succinimide (2.973 g, 30 mmol). Yield 33%. 1H NMR (CDCl3): δ 2.72 (s, 4H, succinimide), 3.04 (t, J = 7.5 Hz, 2H, CH2CO), 3.90 t, J = 7.5 Hz, 2H, CH2N), 4.20 (s, 5H, Cp), 4.51 (t, J = 2.0 Hz, 2H, C5H4), 4.76 (t, J = 2.0 Hz, 2H, C5H4). 13C NMR (CDCl3): δ 28.3 (2CH2 succinimide), 34.6 (CH2), 36.7 (CH2), 69.4 (2CH C5H4), 70.0 (5CH Cp), 72.7 (2CH C5H4), 78.5 (C C5H4), 177.2 (CO-N-CO), 201.4 (CO). IR (KBr, ν cm−1): 3094, 3080, 2978, 2952 (CH, CH2), 1699, 1658 (CO). MS (ESI) m/z: 340 [M + H]+. HRMS (ESI, C17H18FeNO3: [M + H]+) calcd: 340.0636, found: 340.0625.
3.5.3. N-(2-ferrocenyl-2-oxoethyl) Phthalimide (4f)
Method B: from 2-chloro-1-ferrocenyl-1-ethanone 1B (2.11 g, 8.04 mmol), potassium carbonate (1.111 g, 8 mmol), phthalimide (1.183 g, 8 mmol), yield 52%.
1H NMR (CDCl3): δ 4.38 (s, 5H, Cp), 4.60 (t, J = 2.0 Hz, 2H, C5H4), 4.87 (t, J = 2.0 Hz, 2H, C5H4), 4.89 (s, 2H, CH2), 7.75 (dd, J = 5.5 and 3.1 Hz, 2H, phthalimide), 7.90 (dd, J = 5.5 and 3.1 Hz, 2H, phthalimide). 13C NMR (CDCl3): δ 44.7 (CH2), 69.1 (2CH C5H4), 70.5 (5CH Cp), 72.9 (2CH C5H4), 75.5 (C C5H4), 123.6 (2CH phthalimide), 132.4 (2C phthalimide), 134.2 (2CH phthalimide), 168.2 (2CO), 195.0 (CO). MS (CI, NH3) m/z: 374 [M + H]+. IR (KBr, ν cm−1): 1713 (CO), 1684 (CO). HRMS (ESI, C20H16FeNO3: [M + H]+) calcd: 374.0480, found: 374.0490.
3.5.4. N-(3-ferrocenyl-3-oxopropyl) Phthalimide (4g)
Method B: from 3-chloro-1-ferrocenyl-propan-1-one 1C (1.95 g, 7.05 mmol), potassium carbonate (1.949 g, 14.1 mmol), phthalimide (1.556 g, 10.6 mmol), yield 45%. 1H NMR (CDCl3): δ 2.70 (t, J = 7.5 Hz, 2H, CH2), 4.11 (t, J = 7.5 Hz, 2H, CH2), 4.21 (s, 5H, Cp), 4.51 (t, J = 2.0 Hz, 2H, C5H4), 4.78 (t, J = 2.0 Hz, 2H, C5H4), 7.67–7.77 (m, 2H, phthalimide), 7.81–7.92 (m, 2H, phthalimide). 13C NMR (CDCl3): δ 33.7 (CH2), 37.7 (CH2), 69.4 (2CH C5H4), 70.0 (5CH Cp), 72.6 (2CH C5H4), 78.6 (C C5H4), 123.4 (2CH phthalimide), 132.2 (2C phthalimide), 134.1 (2CH phthalimide), 168.3 (2CO), 201.4 (CO). IR (ATR, ν cm−1): 1712 (CO), 1662 (CO). MS (CI, NH3) m/z: 388 [M + H]+, 405 [M + NH4]+. HRMS (ESI, C21H18FeNO3: [M + H]+) calcd: 388.0636, found: 388.0627. Anal. Calcd for C21H17FeNO3(H2O)0.2: C, 64.53; H, 4.48; N, 3.58. Found: C, 64.56; H, 4.49; N, 3.51.
3.5.5. N-(4-ferrocenyl-4-oxobutyl) Phthalimide (4h)
Method B: from 4-chloro-1-ferrocenyl-1-butanone 1D (1.19 g, 4.1 mmol), potassium carbonate (0.566 g, 4.1 mmol), phthalimide (0.904 g, 6.1 mmol). Yield 58%. Mp: 137 °C. 1H NMR (CDCl3): δ 2.00–2.19 (CH2), 2.79 (t, J = 7.4 Hz, 2H, CH2), 3.80 (t, J = 7.0 Hz, 2H, CH2), 4.18 (s, 5H, Cp), 4.48 (t, J = 1.9 Hz, 2H, C5H4), 4.76 (t, J = 1.9 Hz, 2H, C5H4), 7.66–7.77 (m, 2H, phthalimide), 7.80–7.90 (m, 2H, phthalimide). 13C NMR (CDCl3): δ 23.6 (CH2), 37.0 (CH2), 37.8 (CH2), 69.4 (2CH C5H4), 69.9 (5CH Cp), 72.3 (2CH C5H4), 78.9 (C C5H4), 123.4 (2CH phthalimide), 132.2 (2C phthalimide), 134.1 (2CH phthalimide), 168.5 (2CO), 203.2 (CO). IR (ATR, ν cm−1): 1706 (CO), 1666 (CO). MS (CI, NH3) m/z: 402 [M + H]+. HRMS (ESI, C22H20FeNO3: [M + H]+) calcd: 402.0793, found: 402.0789. Anal. Calcd for C22H19FeNO3: C, 65.85; H, 4.77; N, 3.49. Found: C, 65.82; H, 4.87; N, 3.49.
3.5.6. N-(ferrocenylmethyl) Succinimide (3a)
Method C: from ferrocenylmethanol 5A (1.09 g, 5.04 mmol), succinimide (0.5 g, 5 mmol), triphenylphosphine (1.323 g, 5 mmol) and diethyl azodicarboxylate (0.879 g, 0.93 mL, 5 mmol). Yield 73%. Method D: from (ferrocenylmethyl)trimethylammonium iodide (3 g, 7.8 mmol), succinimide (1.544 g, 15.6 mmol) and potassium carbonate (2.153 g, 15.6 mmol). Yield 30%. 1H NMR (CDCl3): δ 2.61 (s, 4H, succinimide), 4.08 (t, J = 1.9 Hz, 2H, C5H4), 4.15 (s, 5H, Cp), 4.31 (t, J = 1.9 Hz, 2H, C5H4), 4.41 (s, 2H, CH2). 13C NMR (CDCl3): δ 28.2 (2CH, succinimide), 38.1 (CH2N), 68.4 (2CH, C5H4), 68.7 (5CH, Cp), 69.9 (2CH, C5H4), 81.8 (C, C5H4), 176.8 (2CO). IR (ATR, ν cm−1): 1694 (CO). HRMS (ESI, C15H15FeNO2: [M]+.) calcd: 297.0447, found: 297.0448.
3.5.7. N-(2-ferrocenylethyl) Succinimide (3b)
Method A: from 2-chloroethylferrocene 5B (3 g, 12.07 mmol), potassium carbonate (5.005 g, 36.2 mmol), succinimide (3.588 g, 36.2 mmol), yield 92%. Method B: from compound 4b (1.429 g, 4.4 mmol), triethylsilane (1.84 g, 2.53 mL, 15.8 mmol), trifluoroacetic acid (15.535 g, 10.5 mL, 136.2 mmol), yield 18%. Method C: from 2-hydroxyethylferrocene (2.301 g, 10 mmol), triphenylphosphine (2.623 g, 10 mmol), diethyl azodicarboxylate (1.742 g, 1.83 mL, 10 mmol), succinimide (0.991 g, 10 mmol). Yield 66%. Mp: 169 °C. 1H NMR (CDCl3): δ 2.58 (t, J = 7.9 Hz, 2H, CH2), 2.67 (s, 4H, succinimide), 4.08 (t, J = 7.9 Hz, 2H, CH2N), 4.08 (s, 4H, C5H4), 4.13 (s, 5H, Cp). 13C NMR (CDCl3): δ 27.6 (CH2), 28.3 (2CH2 succinimide), 38.7 (CH2), 67.8 (2CH C5H4), 68.3 (2CH C5H4), 68.8 (5CH Cp), 84.5 (C C5H4), 177.2 (2CO). IR (ATR, ν cm−1): 1694 (CO). MS (ESI) m/z: 311 [M]+, 287, 177. HRMS (ESI, C16H17FeNO2: [M]+) calcd: 311.0609, found: 311.0622.
3.5.8. N-(3-ferrocenylpropyl) Succinimide (3c)
Method A: from 3-chloropropylferrocene 5B (0.583 g, 2.22 mmol), potassium carbonate (0.614 g, 4.4 mmol), succinimide (0.44 g, 4.4 mmol). Yield 86%. Method B: Failed (starting product 4c recovered). Method C: Failed. 1H NMR (CDCl3): δ 1.65–1.87 (m, 2H, CH2), 2.30 (t, J = 7.7 Hz, 2H, CH2), 2.60 (s, 4H, CH2-CH2 succinimide), 3.50 (t, J = 7.3 Hz, 2H, CH2N), 4.01 (s, 2H, C5H4), 4.06 (s, 7H, C5H4 + Cp). 13C NMR (CDCl3): δ 26.9 (CH2), 28.1 (CH2-CH2 succinimide), 28.6 (CH2), 38.8 (CH2), 67.2 (2CH C5H4), 68.0 (2CH C5H4), 68.6 (5CH Cp), 87.9 (C C5H4), 177.2 (2CO). IR (ATR, ν cm−1): 1688 (CO). HRMS (ESI, C17H19FeNO2: [M]+.) calcd: 325.0760, found: 325.076.
3.5.9. N-(ferrocenylmethyl) Phthalimide (3e)
The spectroscopic data were in agreement with reference [
24].
Method C: from ferrocenylmethanol 5A (1 g, 4.63 mmol), triphenylphosphine (1.214 g, 4.6 mmol), diethyl azodicarboxylate (0.806 g, 0.85 mL, 4.6 mmol), phthalimide (0.681 g, 4.6 mmol). Yield 59%. Method D: from (ferrocenylmethyl) trimethylammonium iodide (7.5 g, 19.5 mmol), phthalimide (1.91 g, 13 mmol) and potassium carbonate (1.795 g, 13 mmol), yield 77%. MS (EI, 70 eV) m/z: 345 [M]+., 280 [M − Cp]+, 202, 158, 121 [CpFe]+. IR (ATR, ν cm−1): 1705 (CO). Anal. Calcd for C19H15FeNO2: C, 66.11; H, 4.38; N, 4.05. Found: C, 65.77; H, 4.33; N, 4.08.
3.5.10. N-(2-ferrocenylethyl) Phthalimide (3f)
This compound has been reported in the literature using another pathway but NMR signals were not attributed [
26].
Method A: from 2-chloroethylferrocene 5B (3 g, 12.07 mmol), potassium carbonate (5.005 g, 36.2 mmol), phthalimide (5.328 g, 36.2 mmol). Yield 79%. Method B: from compound 4f (1.752 g, 4.69 mmol), triethylsilane (1.638 g, 2.25 mL, 14.1 mmol), trifluoroacetic acid (10.706 g, 7.23 mL, 93.9 mmol). Yield 59%. Method C: from 2-hydroxyethylferrocene (1.61 g, 7 mmol), triphenylphosphine (1.835 g, 7 mmol), diethyl azodicarboxylate (1.219 g, 1.28 mL, 7 mmol), phthalimide (1.03 g, 7 mmol). Yield 53%. 1H NMR (CDCl3): δ 2.70 (t, J = 7.8 Hz, 2H, CH2), 3.85 (t, J = 7.8 Hz, 2H, CH2), 4.07 (t, J = 1.7 Hz, 2H, C5H4), 4.11 (t, J = 1.7 Hz, 2H, C5H4), 4.14 (s, 5H, Cp), 7.66–7.77 (m, 2H, phthalimide), 7.80–7.88 (m, 2H, phthalimide). 13C NMR (CDCl3): δ 28.6 (CH2), 38.9 (CH2), 67.8 (2CH C5H4), 68.3 (2CH C5H4), 68.7 (5CH Cp), 84.7 (C C5H4), 123.3 (2CH phthalimide), 132.2 (2C phthalimide), 134.0 (2CH phthalimide), 168.3 (2CO). IR (ATR, ν cm−1): 1707 (CO). MS (EI, 70 eV) m/z: 359 [M]+, 294 [M - Cp]+, 199 [FcCH2]+, 121 [CpFe]+. HRMS (ESI, C20H17FeNO2: [M]+.) calcd: 359.0609, found: 359.0608. Anal. Calcd for C20H17FeNO2: C, 66.87; H, 4.77; N, 3.89. Found: C, 66.49; H, 4.78; N, 3.87.
3.5.11. N-(3-ferrocenylpropyl) Phthalimide (3g)
Method A: from 3-chloropropylferrocene 2C (0.583 g, 2.22 mmol), potassium carbonate (0.614 g, 4.4 mmol), phthalimide (0.653 g, 4.4 mmol), yield 88%. Method B: From compound 4g (4 g, 10.33 mmol), triethylsilane (4.204 g, 5.78 mL, 36.2 mmol), trifluoroacetic acid (35.336 g, 23.88 mL, 309.9 mmol), yield 39%. Method C: from 3-hydroxypropylferrocene (1.14 g, 4.67 mmol), triphenylphosphine (1.47 g, 5.6 mmol), diethyl azodicarboxylate (0.976 g, 1.03 mL, 5.6 mmol), phthalimide (0.825 g, 5.6 mmol). Yield 35%. Mp: 99–100 °C. 1H NMR (CDCl3): δ 1.84–2.00 (m, 2H, CH2), 2.40 (t, J = 7.4 Hz, 2H, CH2), 2.73 (t, J = 7.2 Hz, 2H, CH2), 4.03 (t, J = 1.8 Hz, 2H, C5H4), 4.05–4.11 (m, 7H, C5H4 + Cp), 7.66–7.76 (m, 2H, phthalimide), 7.80–7.90 (m, 2H, phthalimide). 13C NMR (CDCl3): δ 27.0 (CH2), 29.8 (CH2), 38.1 (CH2), 67.7 (2CH C5H4), 68.4 (2CH C5H4), 69.0 (5CH Cp), 88.6 (C C5H4), 123.3 (2CH phthalimide), 132.3 (2C phthalimide), 134.0 (2CH phthalimide), 168.5 (2CO). IR (ATR, ν cm−1): 1707 (CO). MS (CI, NH3) m/z: 374 [M + H]+, 391 [M + NH4]+. HRMS (ESI, C21H19FeNO2: [M]+.) calcd: 373.0765, found: 373.0777.
3.5.12. N-(4-ferrocenylbutyl) Phthalimide (3h)
Method A: from 4-chlorobutylferrocene 2D (1.659 g, 6 mmol), potassium carbonate (1.659 g, 12 mmol), phthalimide (1.766 g, 12 mmol). Yield 12%. Method B: from compound 4h (2.711 g, 6.76 mmol), triethylsilane (2.846 g, 3.91 mL, 24.5 mmol), TFA (23.946 g, 16.18 mL, 210 mmol), yield 38%. Method C: from 4-hydroxybutylferrocene (1.427 g, 5.53 mmol), triphenylphosphine (1.74 g, 6.6 mmol), diethyl azodicarboxylate (1.156 g, 1.22 mL, 6.6 mmol), phthalimide (0.976 g, 6.6 mmol). Yield 66%. 1H NMR (CDCl3): δ 1.47–1.62 (m, 2H, CH2), 1.64–1.78 (m, 2H, CH2), 2.36 (t, J = 7.6 Hz, 2H, CH2), 3.69 (t, J = 7.2 Hz, 2H, CH2), 4.02 (t, J = 2.0 Hz, 2H, C5H4), 4.04 (t, J = 2.0 Hz, 2H, C5H4), 4.07 (s, 5H, Cp), 7.64–7.75 (m, 2H, phthalimide), 7.78–7.88 (m, 2H, phthalimide). 13C NMR (CDCl3): δ 28.4 (CH2), 28.5 (CH2), 29.2 (CH2), 37.9 (CH2), 67.8 (2CH C5H4), 68.8 (2CH C5H4), 69.3 (5CH Cp), 89.6 (C C5H4), 123.3 (2CH phthalimide), 132.2 (2C phthalimide), 134.0 (2CH phthalimide), 168.5 (2CO). IR (ATR, ν cm−1): 1706 (CO). MS (EI, 70 eV) m/z: 387 [M]+., 320, 202, 199, 158, 121 [CpFe]+, 56. HRMS (ESI, C22H21FeNO2: [M]+) calcd: 387.0922, found: 387.0914.
3.5.13. N-(2-ferrocenylethyl)-2,3-naphthalenedicarboximide (3i)
Method A: from 2-chloroethylferrocene 2B (0.5193 g, 2.09 mmol), potassium carbonate (0.578 g, 4.2 mmol), 2,3-naphthalenedicarboximide (0.412 g, 2.1 mmol), yield 68%. 1H NMR (CDCl3): δ 2.75 (t, J = 7.6 Hz, 2H, CH2), 3.92 (t, J = 7.6 Hz, 2H, CH2), 4.07 (t, J = 1.9 Hz, 2H, C5H4), 4.12 (t, J = 1.9 Hz, 2H, C5H4), 4.14 (s, 5H, Cp), 7.76–7.74 (m, 2H, naphthalene), 7.97–8.09 (m, 2H, naphthalene), 8.31 (s, 2H, naphthalene). 13C NMR (CDCl3): δ 28.5 (CH2), 39.2 (CH2), 67.7 (2CH C5H4), 68.3 (2CH C5H4), 68.7 (5CH Cp), 84.7 (C C5H4), 124.7 (2CH naphthalene), 128.0 (2C naphthalene), 129.2 (2CH naphthalene), 130.4 (2CH naphthalene), 135.6 (2C naphthalene), 168.0 (2CO). IR (ATR, ν cm−1): 1700 (CO). HRMS (ESI, C24H19FeNO2: [M]+) calcd: 409.0760, found: 409.076.
3.5.14. N-(2-ferrocenylethyl) Glutarimide (3j)
Method A: from 2-chloroethylferrocene 2B (3 g, 12.07 mmol), potassium carbonate (5.005 g, 36.2 mmol), glutarimide (4.096 g, 36.2 mmol). Yield 80%. Mp: 132 °C. 1H NMR (CDCl3): δ 1.91 (quin, J = 6.5 Hz, 2H, CH2 glutarimide), 2.52 (t, J = 7.9 Hz, 2H, CH2), 2.62 (d, t = 6.5 Hz, 4H, glutarimide), 3.93 (t, J = 7.9 Hz, 2H, CH2N), 4.05 (t, J = 1.8 Hz, 2H, C5H4), 4.08 (t, J = 1.8 Hz, 2H, C5H4), 4.13 (s, 5H, Cp). 13C NMR (CDCl3): δ 17.2 (CH2 glutarimide), 27.7 (CH2), 32.9 (2CH2 glutarimide), 40.2 (CH2N), 67.5 (2CH C5H4), 68.3 (2CH C5H4), 68.6 (5CH Cp), 85.1 (C C5H4), 172.3 (2CO). IR (ATR, ν cm−1): 1667 (CO). HRMS (ESI, C17H19FeNO2: [M]+) calcd: 325.0760, found: 325.076.
3.5.15. 2-(2-Ferrocenylethyl)-1H-Benzo[de]isoquinoline-1,3(2H)-dione (3k)
Method A: from 2-chloroethylferrocene 2B (1.343 g, 5.4 mmol), potassium carbonate (1.494 g, 10.8 mmol), 1,8-naphthalimide (2.131 g, 10.8 mmol), yield 70%. Mp: 193–194 °C. 1H NMR (CDCl3): δ 2.74 (t, J = 8.1 Hz, 2H, CH2), 4.09 (t, J = 1.7 Hz, 2H C5H4), 4.16–4.22 (m, 7H, Cp + C5H4), 4.36 (t, J = 8.1 Hz, 2H, CH2N), 7.76 (dd, J = 8.2 and 7.2 Hz, 2H, naphthalene), 8.21 (dd, J = 8.2 and 1.1 Hz, 2H, naphthalene), (dd, J = 7.2 and 1.1 Hz, 2H, naphthalene). 13C NMR (CDCl3): δ 27.9 (CH2), 41.2 (CH2), 67.6 (2CH C5H4), 68.4 (2CH C5H4), 68.8 (5CH Cp), 85.4 (C C5H4), 122.8 (2C naphthalene), 127.1 (2CH naphthalene), 128.3 (C naphthalene), 131.3 (2CH naphthalene), 131.7 (C naphthalene), 134.1 (2CH naphthalene), 164.2 (2CO). IR (ATR, ν cm−1): 1695, 1654 (CO). HRMS (ESI, C24H19FeNO2: [M]+) calcd: 409.0760, found: 409.0762.
3.6. General Procedure for the Reduction of Imides 3 into α-Hydroxylactams 6
In a flask, imides 3 were dissolved in a minimum quantity of THF, then methanol (approx. 50 mL/mmol of imide, the flask should not be filled more than 2/3 of its capacity because of gas emission) was added and stirring was started. Solid sodium borohydride was added portionwise (10–15 eq. each 10 min for 40 min, with control of the amount of emitted gas). The mixture was poured into a sodium hydrogen carbonate solution and extracted twice with DCM. The combined organic layer was washed with water and dried with magnesium sulfate. The solution was concentrated under reduced pressure and the residue was chromatographed on silica gel with dichloromethane as eluent, affording the α-hydroxylactams 6 as orange-yellow solids.
3.6.1. 1-(Ferrocenylmethyl)-5-hydroxy-2-pyrrolidone (6a)
From 3a, yield 66%. 1H NMR (DMSO-d6): δ 1.56–1.74 (m, 1H, CH2), 2.01–2.18 (m, 2H, CH2), 2.23–2.42 (m, 1H, CH2), 3.81 (d, J = 14.5 Hz, 1H, CH2N), 4.08 (s broad, 2H, C5H4), 4.15 (s broad, 1H, C5H4), 4.18 (s, 5H, Cp), 4.25 (s broad, 1H, C5H4), 4.39 (d, J = 14.5 Hz, 1H, CH2N), 5.00 (m, 1H, N-CH-O-), 6.02 (d, J = 7.2 Hz, 1H, OH). 13C NMR (DMSO-d6): δ 27.4 (CH2), 28.5 (CH2), 37.7 (CH2N), 67.4 (CH C5H4), 67.8 (CH C5H4), 68.4 (5CH Cp), 68.5 (CH C5H4), 68.9 (CH C5H4), 80.9 (>N-CH-O-), 83.4 (C C5H4), 172.7 (CO). IR (ATR, ν cm−1): 1646 (CO). HRMS (ESI, C15H17FeNO2: [M]+) calcd: 299.0603, found: 299.0604.
3.6.2. N-(ferrocenylmethyl)-4-hydroxy-butanamide (7a)
This compound was obtained as a by-product during the synthesis of 6a in 23% yield. 1H NMR (DMSO-d6): δ 1.67 (m, 2H, CH2), 2.13 (t, J = 7.5 Hz, 2H, CH2CO), 3.38 (m, 2H, CH2O), 3.97 (d, J = 5.8 Hz, 2H, CH2N), 4.07 (t, J = 1.8 Hz, 2H, C5H4), 4.15 (s broad, 7H, Cp + C5H4), 4.46 (t, J = 5.2 Hz, 1H, OH), 7.96 (t, J = 5.8 Hz, 1H, NH). 13C NMR (DMSO-d6): δ 28.7 (CH2), 32.1 (CH2-CO), 37.4 (CH2N), 60.4 (CH2O), 67.2 (2CH C5H4), 67.8 (2CH C5H4), 68.3 (5CH Cp), 86.4 (C C5H4), 171.6 (CO). IR (ATR, ν cm−1): 1642 (CO). HRMS (ESI, C15H19FeNNaO2: [M + Na]+) calcd: 324.065738, found: 324.0654.
3.6.3. 1-(2-Ferrocenylethyl)-5-hydroxy-2-pyrrolidone (6b)
From 3b, yield 33%. 1H NMR (DMSO-d6): δ 1.46–1.62 (m, 1H, CH2), 1.62–1.80 (m, 2H, CH2), 1.80–2.04 (m, 1H, CH2), 2.10–2.31 (m, 2H, CH2), 3.09–3.30 (m, 1H, N-CH2), 3.54–3.76 (m, 1H, N-CH2), 3.94–4.33 (m, 9H, CpFeC5H4), 4.62–4.84 (m, 1H, N-CH-O-), 5.85 (d, J = 6.4 Hz, 1H, OH). 13C NMR (DMSO-d6): δ 27.3 (CH2), 30.9 (CH2), 32.2 (CH2), 45.1 (CH2N), 66.9 (CH C5H4), 67.0 (CH C5H4), 67.5 (CH C5H4), 67.8 (CH C5H4), 68.3 (5CH Cp), 78.8 (C C5H4), 86.0 (>N-CH-O-), 168.7 (CO).
3.6.4. 1-(3-ferrocenylpropyl)-5-hydroxy-2-pyrrolidone (6c)
From 3c, yield 61%. 1H NMR (CDCl3): δ 1.60–1.84 (m, 2H, CH2), 1.84–1.98 (m, 1H, CH2), 1.98–2.16 (m, 1H, CH2), 2.17–2.37 (m, 3H, CH2), 2.38–2.57 (m, 1H, CH2), 3.02–3.19 (m, 1H, N-CH2), 3.32–3.56 (m, 1H, N-CH2), 3.94–4.19 (m, 9H, CpFeC5H4), 4.88 (ddd, J = 8.1, 6.3, 1.6 Hz, 1H, N-CH-O-). 13C NMR (CDCl3): δ 24.9 (CH2), 27.1 (CH2), 28.9 (CH2), 29.1 (CH2), 40.5 (CH2N), 67.2 (CH C5H4), 67.3 (CH C5H4), 68.0 (2CH C5H4), 68.6 (5CH Cp), 88.4 (C C5H4), 89.2 (>N-CH-O-), 175.0 (CO). IR (ATR, ν cm−1): 1650 (CO). HRMS (ESI, C17H21FeNNaO2: [M + Na]+) calcd: 350.081388, found: 350.0813.
3.6.5. 2,3-Dihydro-3-hydroxy-2-(ferrocenylmethyl)-1H-isoindol-1-one (6e)
From 3e, yield 99%. Mp: 246 °C. 1H NMR (DMSO-d6): δ 4.04–4.14 (m, 3H, CH2 + 2C5H4), 4.19–4.25 (m, 6H, Cp + C5H4), 4.29–4.34 (m, 1H, C5H4), 4.67 (d, J = 14.7 Hz, 1H, CH2), 5.69 (d, J = 8.9 Hz, 1H, CH-O), 6.69 (d, J = 8.9 Hz, 1H, OH), 7.46–7.67 (m, 4H, CHarom). 13C NMR (DMSO-d6): δ 37.6 (CH2), 67.6 (CH C5H4), 68.0 (CH C5H4), 68.5 (5CH Cp), 68.6 (CH C5H4), 69.0 (CH C5H4), 79.9 (CH-O), 83.7 (C C5H4), 122.4 (CH phthalimide), 123.7 (CH phthalimide), 129.4 (CH phthalimide), 131.5 (C phthalimide), 132.0 (CH phthalimide), 144.7 (CH phthalimide), 165.4 (CO). IR (ATR, ν cm−1): 1661 (CO). MS (CI, NH3) m/z: 348 [M + H]+, 199 [FcCH2]+, 365 [M + NH4]+. Anal. Calcd for C19H17FeNO2: C, 65.72; H, 4.93; N, 4.03. Found: C, 65.32; H, 4.77; N, 3.95.
3.6.6. 2,3-Dihydro-3-hydroxy-2-(2-ferrocenylethyl)-1H-isoindol-1-one (6f)
From
3f. This compound has been reported in the literature using another method, but NMR signals were not attributed [
26]. Yield 99%.
1H NMR (DMSO-d
6): δ 2.43–2.79 (m, 2H, CH
2), 3.82–3.60 (m, 1H, CH
2N), 3.70–3.88 (m, 1H, CH
2N), 4.02–4.22 (m, 9H, CpFeC
5H
4), 5.79 (d,
J = 9.0 Hz, 1H, N-CH-O), 6.62 (d,
J = 9.0 Hz, 1H, OH), 7.44–7.74 (m, 4H, isoindole).
13C NMR (DMSO-d
6): δ 27.7 (CH
2), 39.5 (CH
2), 67.1 (CH C
5H
4), 67.2 (CH C
5H
4), 67.7 (CH C
5H
4), 67.8 (CH C
5H
4), 68.4 (5CH Cp), 80.8 (N-CH-O), 85.6 (C C
5H
4), 122.4 (CH isoindole), 123.5 (CH isoindole), 129.3 (CH isoindole), 131.8 (CH isoindole), 131.9 (C isoindole), 144.9 (C isoindole), 165.8 (CO). IR (KBr, ν cm
−1): 1671 (CO). MS (EI, 70 eV)
m/
z: 361 [M]
+, 278, 212, 199, 121 [CpFe]
+. HRMS (ESI, C
20H
19FeNO
2: [M]
+) calcd: 361.0765, found: 361.0756.
3.6.7. 2,3-Dihydro-3-hydroxy-2-(3-ferrocenylpropyl)-1H-isoindol-1-one (6g)
From 3g, yield 90%. 1H NMR (DMSO-d6): δ 1.69–1.96 (m, 2H, CH2), 2.32 (t, J = 7.8 Hz, 2H, CH2), 3.27–3.42 (m, 1H, CH2N), 3.52–3.68 (m, 1H, CH2N), 3.98–4.19 (m, 9H, CpFeC5H4), 5.85 (d, J = 9.0 Hz, 1H, N-CH-O), 6.60 (d, J = 9.0 Hz, 1H, OH), 7.47–7.70 (m, 4H, isoindole). 13C NMR (DMSO-d6): δ 26.4 (CH2), 29.1 (CH2), 38.8 (CH2), 66.8 (2CH C5H4), 67.7 (2CH C5H4), 68.3 (5CH Cp), 80.8 (N-CH-O), 88.4 (C C5H4), 122.2 (CH isoindole), 123.5 (CH isoindole), 129.3 (CH isoindole), 131.8 (CH isoindole + C isoindole), 145.0 (C isoindole), 166.0 (CO). IR (ATR, ν cm−1): 1675 (CO). MS (EI, 70 eV) m/z: 375 [M]+, 310, 292, 266, 264, 121 [CpFe]+. HRMS (ESI, C21H21FeNO2: [M]+) calcd: 375.0922, found: 375.0925.
3.6.8. 2,3-Dihydro-3-hydroxy-2-(4-ferrocenylbutyl)-1H-isoindol-1-one (6h)
From 3h, yield 89%. 1H NMR (DMSO-d6): δ 1.39–1.74 (m, 4H, CH2-CH2), 2.24–2.44 (m, 2H, CH2), 3.22–3.75 (m, 1H, CH2N), 3.51–3.68 (m, 1H, CH2N), 3.98–4.12 (m, 9H, CpFeC5H4), 5.81 (d, J = 8.9 Hz, 1H, N-CH-O), 6.59 (d, J = 8.9 Hz, 1H, OH), 7.45–7.69 (m, 4H, isoindole). 13C NMR (DMSO-d6): δ 27.8 (CH2), 28.1 (CH2), 28.6 (CH2), 38.4 (CH2), 66.8 (2CH C5H4), 67.78 (CH C5H4), 67.83 (CH C5H4), 68.3 (5CH Cp), 80.6 (N-CH-O), 88.7 (C C5H4), 122.2 (CH isoindole), 123.5 (CH isoindole), 129.3 (CH isoindole), 131.8 (CH isoindole + C isoindole), 144.9 (C isoindole), 166.0 (CO). MS (EI, 70 eV) m/z: 389 [M]+, 324, 280, 121 [CpFe]+. HRMS (ESI, C22H23FeNO2: [M]+) calcd: 389.1078, found: 389.1080.
3.6.9. 2-(2-Ferrocenylethyl)-1H-3-hydroxy-benzo[f]isoindole-1(2H)-one (6i)
From 3i, yield 12%. 1H NMR (DMSO-d6): δ 2.54–2.67 (m, 1H, CH2), 2.67–2.80 (m, 1H, CH2), 3.47–3.62 (m, 1H, CH2N), 3.47–3.62 (m, 1H, CH2N), 3.78–3.91 (m, 1H, CH2N), 4.03–4.10 (m, 2H, C5H4), 4.13 (s, 1H, C5H4), 4.18 (s, 6H, Cp + C5H4), 5.95 (d, J = 9.0 Hz, 1H, O-CH-N), 6.71 (d, J = 9.0 Hz, 1H, OH), 7.54–7.70 (m, 2H, naphthalene), 8.03–8.20 (m, 3H, naphthalene), 8.28 (s, 1H, naphthalene). 13C NMR (DMSO-d6): δ 27.6 (CH2), 39.6 (CH2N), 67.10 (CH C5H4), 67.14 (CH C5H4), 67.70 (CH C5H4), 67.77 (CH C5H4), 68.4 (5CH Cp), 80.8 (O-CH-N), 85.6 (C C5H4), 122.3 (CH naphthalene), 122.6 (CH naphthalene), 126.7 (CH naphthalene), 127.7 (CH naphthalene), 128.4 (CH naphthalene), 129.4 (CH naphthalene), 129.8 (C naphthalene), 133.1 (CH naphthalene), 134.8 (C naphthalene), 140.8 (CH naphthalene), 165.6 (CO). IR (ATR, ν cm−1): 1661 (CO), 1644. HRMS (ESI, C24H21FeNO2: [M]+) calcd: 411.0916, found: 411.0912.
3.6.10. 1-(2-Ferrocenylethyl)-6-hydroxy-2-piperidone (6j)
From 3j. This compound was obtained with a yield up to 47% depending on the experiment. It is unstable and degraded before full characterization and only the 1H NMR could be obtained. 1H NMR (CDCl3): δ 1.55–1.71 (m, 1H, CH2), 1.71–1.86 (m, 2H, CH2), 1.88–2.09 (m, 1H, CH2), 2.18–2.51 (m, 2H, CH2), 2.62 (t, J = 7.3 Hz, 2H, CH2), 3.22–3.48 (m, 2H, CH2 + OH), 3.61–3.80 (m, 1H, CH2), 4.06 (s, 4H, C5H4), 4.11 (s, 5H, Cp), 4.68 (s broad, 1H, CH-O).
3.6.11. N-(2-ferrocenylethyl)-5-hydroxy-pentanamide (7j)
This compound was obtained as a by-product during the synthesis of 6j with a yield up to 46% depending on the experiment. 1H NMR (CDCl3): δ 1.48–1.65 (m, 2H, CH2), 1.65–1.82 (m, 2H, CH2), 2.04 (s broad, 1H, OH), 2.20 (t, J = 7.1 Hz, 2H, CH2), 2.53 (t, J = 7.0 Hz, 2H, CH2), 3.37 (q, J = 6.6 Hz, 1H, CH2), 3.64 (t, J = 6.1 Hz, 1H), 4.10 (s, 4H, C5H4), 4.14 (s, 5H, Cp), 5.66 (s broad, 1H, NH). 13C NMR (CDCl3): δ 21.8 (CH2), 29.8 (CH2), 32.1 (CH2), 36.2 (CH2), 40.7 (CH2), 62.1 (CH2), 67.9 (2CH C5H4), 68.5 (2CH C5H4), 69.0 (5CH Cp), 85.7 (C C5H4), 173.2 (CO). IR (ATR, ν cm-1): 1646 (CO). HRMS (ESI, C17H24FeNO2: [M + H]+) calcd: 330.1151, found: 330.1151.
3.6.12. 1-(2-Ferrocenylethyl)-5,6-ene-2-piperidone (8j)
This byproduct was obtained during the synthesis of 6j in a yield up to 23% depending on the experiment. 1H NMR (CDCl3): δ 2.21–2.32 (m, 2H, CH2), 2.39–2.52 (m, 2H, CH2), 2.56 (dd, J = 8.4, 6.6 Hz, 2H, CH2), 3.55 (t, J = 7.5 Hz, 2H, CH2N), 4.05 (s, 4H, C5H4), 4.11 (s, 5H, Cp), 5.05 (dt, J = 7.6, 4.4 Hz, 1H, =CH), 5.86 (dt, J = 7.6, 1.6 Hz, 1H, =CH). 13C NMR (CDCl3): δ 20.3 (CH2), 28.7 (CH2), 31.4 (CH2), 47.8 (CH2N), 67.6 (2CH C5H4), 68.4 (2CH C5H4), 68.7 (5CH Cp), 85.1 (C C5H4), 105.9 (-CH=), 130.3 (N-CH=), 169.4 (CO). IR (ATR, ν cm−1): 1622, 1606. HRMS (ESI, C17H20FeNO: [M + H]+) calcd: 310.0889, found: 310.0897.
3.6.13. N-(2-ferrocenylethyl)-3-methoxycarbonylbutanamide (9j)
This compound is an impurity that was identified during the synthesis of 6j and was not fully characterized. 1H NMR (CDCl3): δ 1.85–2.04 (m, 2H, CH2), 2.18 (t, J = 7.3 Hz, 2H, CH2), 2.35 (t, J = 7.1 Hz, 2H, CH2), 2.49 (t, J = 7.0 Hz, 2H, CH2), 3.33 (q, J = 6.6 Hz, 2H, CH2N), 3.65 (s, 3H, OMe), 3.98–4.21 (m, 9H, C5H4-Fe-Cp), 5.75 (s broad, 1H, NH). 13C NMR (CDCl3): δ 20.9 (CH2), 29.7 (CH2), 33.2 (CH2), 35.6 (CH2), 40.6 (CH2), 51.7 (OMe), 68.0 (2CH C5H4), 68.6 (2CH C5H4), 69.0 (5CH Cp), 85.9 (C C5H4), 172.0 (CO), 173.7 (CO). IR (ATR, ν cm−1): 1728, 1653 (CO).
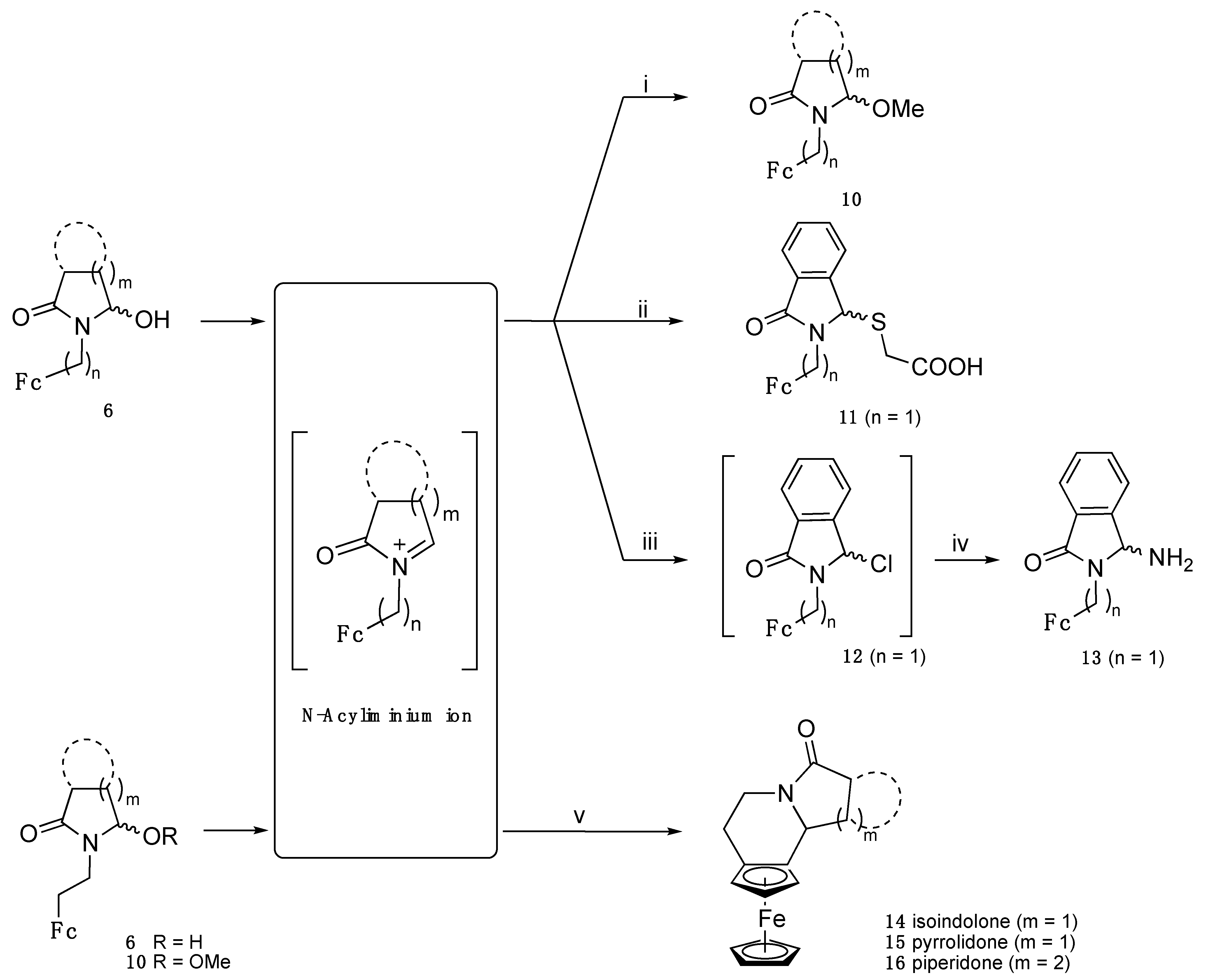
3.7. General Procedure for the Synthesis of α-Methoxylactams 10
In a flask, α-hydroxylactams 6 were dissolved into a minimum of THF. Methanol (10 mL/mmol of 6) and a spatula tip of TsOH were added. The mixture was stirred and monitored by TLC until substrate disappearance, then a solution of sodium hydrogen carbonate was added. The mixture was poured into water and extracted twice with dichloromethane. The combined organic layer was washed with water and dried with magnesium sulfate. The solution was concentrated under reduced pressure and the residue was chromatographed on silica gel with DCM/petroleum ether mixture (4:1), affording the α-methoxylactams 10 as orange-yellow solids.
3.7.1. 1-(2-Ferrocenylethyl)-5-methoxy-2-pyrrolidone (10b)
From 6b, yield of 89%. 1H NMR (CDCl3): δ 1.84–2.16 (m, 2H, -CO-CH2-CH2-CH-O), 2.19–2.36 (m, 1H, -CO-CH2-CH2-CH-O), 2.38–2.55 (m, 1H, -CO-CH2-CH2-CH-O), 2.50–2.71 (m, 2H, >N-CH2-CH2-Fc), 3.09–3.26 (m, 4H, >N-CH2-CH2-Fc + OMe), 3.57–3.74 (m, 1H, >N-CH2-CH2-C=), 3.98–4.16 (m, 9H, CpFeC5H4), 4.73 (dd, J = 6.3 and 1.6 Hz, 1H, >N-CH-O-). 13C NMR (CDCl3): δ 23.9 (-CO-CH2-CH2-CH-O), 27.9 (>N-CH2-CH2-Fc), 29.1 (-CO-CH2-CH2-CH-O), 41.9 (>N-CH2-CH2-Fc), 52.8 (OMe), 67.5 (CH C5H4), 67.6 (CH C5H4), 68.0 (CH C5H4), 68.4 (CH C5H4), 68.6 (5CH Cp), 85.5 (C C5H4), 90.5 (>N-CH-O-), 174.9 (CO). IR (ATR, ν cm−1): 1683 (CO). HRMS (ESI, C17H21FeNNaO2: [M + Na]+) calcd: 350.081388, found: 350.0809.
3.7.2. 2,3-Dihydro-3-methoxy-2-(ferrocenylmethyl)-1H-isoindol-1-one (10e)
From 6e, yield of 90%. 1H NMR (CDCl3): δ 2.86 (s, 3H, OMe), 3.99 (d, J = 13.4 Hz, 1H, CH2N), 4.05–4.52 (m, 9H, CpFeC5H4), 4.78 (d, J = 13.4 Hz, 1H, CH2N), 5.78 (s, 1H, >N-CH-O-), 7.37–7.62 (m, 3H, isoindole), 7.79 (d, J = 7.1 Hz, 1H, isoindole). 13C NMR (CDCl3): δ 38.8 (CH2N), 49.2 (OMe), 68.9 (CH C5H4), 69.4 (CH C5H4), 69.5 (5CH Cp), 70.1 (CH C5H4), 70.3 (CH C5H4), 83.4 (C C5H4), 85.6 (>N-CH-O-), 123.5 (CH isoindole), 123.6 (CH isoindole), 129.9 (CH isoindole), 132.0 (CH isoindole), 133.2 (C isoindole), 140.4 (C isoindole), 167.0 (CO). IR (ATR, ν cm−1): 1699 (CO). HRMS (ESI, C20H19FeNO2: [M]+) calcd: 361.0760, found: 361.0756.
3.7.3. 2,3-Dihydro-3-methoxy-2-(2-ferrocenylethyl)-1H-isoindol-1-one (10f)
From 6f, yield of 100%. 1H NMR (CDCl3): δ 2.55–2.80 (m, 2H, CH2-Fc), 2.85 (s, 3H, OMe), 3.25–3.42 (m, 1H, CH2N), 3.88–4.02 (m, 1H, CH2N), 4.16–4.24 (m, 9H, CpFeC5H4), 5.72 (s, 1H, >N-CH-O-), 7.44–7.62 (m, 3H, isoindole), 7.82 (d, J = 7.2 Hz, 1H, isoindole). 13C NMR (CDCl3): δ 28.2 (CH2-Fc), 40.7 (CH2N), 49.3 (OMe), 67.7 (CH C5H4), 67.8 (CH C5H4), 68.2 (CH C5H4), 68.5 (CH C5H4), 68.8 (5CH Cp), 85.5 (C C5H4), 86.6 (>N-CH-O-), 123.4 (2CH isoindole), 130.0 (CH isoindole), 132.0 (CH isoindole), 133.2 (C isoindole), 140.4 (C isoindole), 167.6 (CO). IR (ATR, ν cm−1): 1706 (CO). HRMS (ESI, C21H22FeNO2: [M + H]+) calcd: 376.0994, found: 376.0995.
3.7.4. 2,3-Dihydro-3-methoxy-2-(3-ferrocenylpropyl)-1H-isoindol-1-one (10g)
From 6g, yield of 94%. 1H NMR (CDCl3): δ 1.78–2.00 (m, 2H, >N-CH2-CH2-CH2-Fc), 2.31–2.48 (m, 2H, -CH2-Fc), 2.87 (s, 3H, OMe), 3.20–3.36 (m, 1H, CH2N), 3.71–3.90 (m, 1H, CH2N), 3.96–4.16 (m, 9H, CpFeC5H4), 5.87 (s, 1H, >N-CH-O-), 7.45–7.64 (m, 3H, isoindole), 7.78–7.89 (m, 1H, isoindole). 13C NMR (CDCl3): δ 27.1 (CH2), 29.6 (CH2-Fc), 39.5 (CH2N), 49.2 (OMe), 67.3 (2CH C5H4), 68.0 (CH C5H4), 68.1 (CH C5H4), 68.6 (5CH Cp), 86.3 (>N-CH-O-), 88.2 (C C5H4), 123.5 (2CH isoindole), 130.0 (CH isoindole), 132.0 (CH isoindole), 133.2 (C isoindole), 140.3 (C isoindole), 167.7 (CO). IR (ATR, ν cm−1): 1702 (CO). HRMS (ESI, C22H23FeNNaO2: [M + Na]+) calcd: 412.097038, found: 412.0971.
3.7.5. 2,3-Dihydro-3-methoxy-2-(4-ferrocenylbutyl)-1H-isoindol-1-one (10h)
From 6h, yield of 79%. 1H NMR (CDCl3): δ 1.48–1.78 (m, 2H, >N-CH2-CH2-CH2-CH2-Fc), 2.38 (t, J = 7.5 Hz, 2H, -CH2-Fc), 2.87 (s, 3H, OMe), 3.15–3.31 (m, 1H, CH2N), 3.72–3.88 (m, 1H, CH2N), 3.98–4.14 (m, 9H, CpFeC5H4), 5.86 (s, 1H, >N-CH-O-), 7.47–7.62 (m, 3H, isoindole), 7.83 (dd, J = 8.1 and 1.2 Hz, 1H, isoindole). 13C NMR (CDCl3): δ 28.0 (CH2), 28.7 (CH2), 29.4 (CH2-Fc), 39.4 (CH2N), 49.2 (OMe), 67.3 (2CH C5H4), 68.3 (2CH C5H4), 68.7 (5CH Cp), 86.3 (>N-CH-O-), 89.0 (C C5H4), 123.5 (CH isoindole), 123.6 (CH isoindole), 130.1 (CH isoindole), 132.0 (CH isoindole), 133.3 (C isoindole), 140.4 (C isoindole), 167.8 (CO). MS (EI, 70 eV) m/z: 403 [M]+, 338, 306, 199, 186, 121 [CpFe]+. IR (KBr, ν cm−1): 1705 (CO). HRMS (ESI, C23H25FeNO2: [M]+) calcd: 403.1235, found: 403.1223.
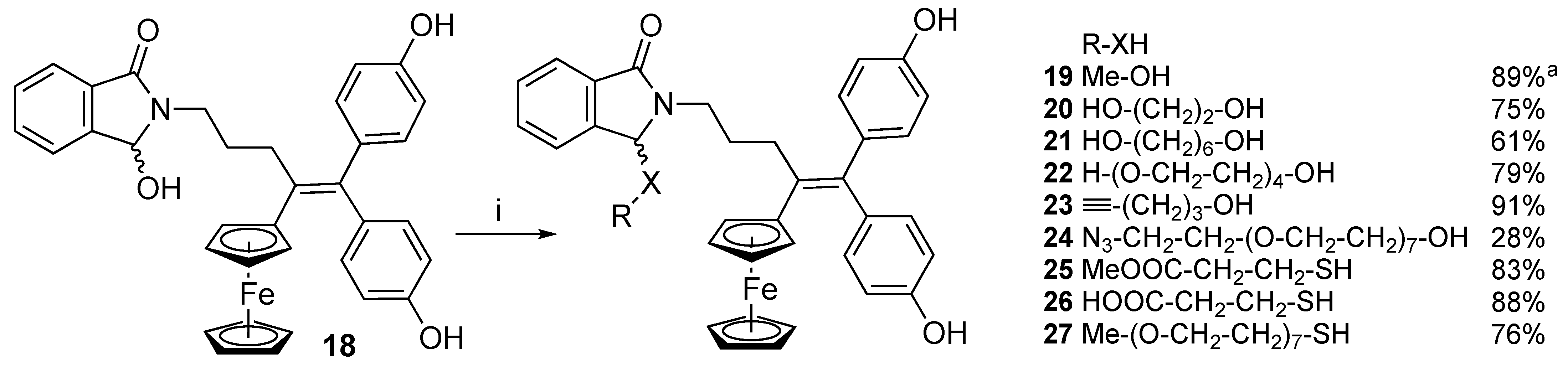
3.12. General Procedure for the Synthesis of Ferrocidiphenols 20–27
In a flask, α-hydroxylactam 18 was dissolved into a minimum of THF, and DCM was added. The alcohol or thiol and a spatula tip of TsOH were added. The solution was stirred at room temperature and the reaction was monitored by TLC. When the reaction was complete, the solution was poured into a solution of sodium hydrogen carbonate and extracted twice with DCM. The combined organic layer was washed with water and dried with magnesium sulfate. The solution was concentrated under reduced pressure and the residue was chromatographed on silica gel or precipitated, affording the pure compounds 20–27 as orange-yellow solids.
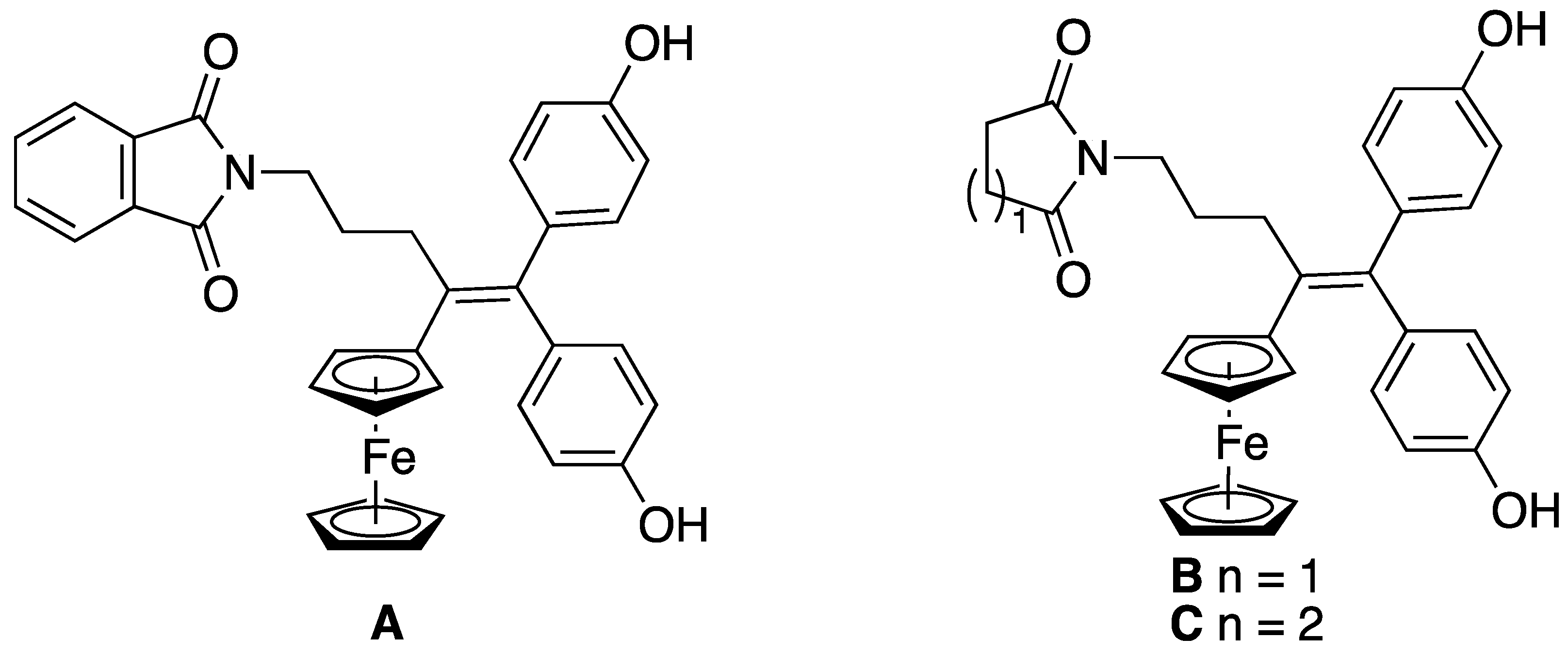
3.12.1. 2,3-Dihydro-3-(2-hydroxyethyl)-2-[4-ferrocenyl-5,5-bis-(4-hydroxyphenyl)-pent-4-enyl]-1H-isoindol-1-one (20)
From compound 18 (0.25 g, 0.427 mmol) and ethylene glycol (0.265 g, 4.3 mmol). Precipitation and filtration gave compound 20 in a yield of 75%. 1H NMR (DMSO-d6): δ 1.60–1.91 (m, 2H, CH2), 2.42–2.58 (m, 2H, CH2-C=C), 2.94–3.05 (m, 1H, CH2-O), 3.05–3.19 (m, 2H, CH2N + CH2-O), 3.38–3.51 (m, 2H, CH2-O), 3.56–3.69 (m, 1H, CH2N), 3.72 (s broad, 1H, C5H4), 3.95 (s broad, 1H, C5H4), 4.00–4.17 (m, 7H, Cp + 2C5H4), 5.51 (s, 1H, CH-O), 6.55–6.69 (m, 4H, C6H4), 6.77 (d, J = 8.0 Hz, 2H, C6H4), 6.94 (d, J = 8.0 Hz, 2H, C6H4), 7.49–7.75 (m, 4H, isoindole), 9.23 (s broad, 2H, OH). 13C NMR (DMSO-d6): δ 28.5 (CH2), 31.7 (CH2), 38.7 (CH2), 60.0 (CH2-O), 65.1 (CH2-O), 67.7 (CH C5H4), 67.8 (CH C5H4), 68.6 (CH C5H4), 68.7 (CH C5H4), 68.9 (5CH, Cp), 85.1 (CH-OH), 86.9 (C C5H4), 114.99 (2CH C6H4), 115.04 (2CH C6H4), 122.5 (CH isoindole), 123.8 (CH isoindole), 129.8 (CH isoindole), 129.9 (2CH C6H4), 130.4 (2CH C6H4), 131.99 (CH isoindole), 132.03 (C), 132.9 (C), 135.0 (C), 135.5 (C), 138.2 (C), 141.3 (C), 155.66 (C), 156.70 (C), 166.4 (CO). IR (ATR, ν cm−1): 1649 (CO). HRMS (ESI, C37H35FeNO5: [M]+.) calcd: 629.1859, found: 629.1864.
3.12.2. 2,3-Dihydro-3-(6-hydroxyhexyl)-2-[4-ferrocenyl-5,5-bis-(4-hydroxyphenyl)-pent-4-enyl]-1H-isoindol-1-one (21)
From compound 18 (0.2 g, 0.342 mmol) and 1,6-hexanediol (0.404 g, 3.4 mmol). Eluent: DCM; yield of 65%. 1H NMR (acetone-d6): δ 1.24–1.40 (m, 4H, CH2), 1.41–1.59 (m, 4H, CH2), 1.74–1.98 (m, 2H, CH2), 2.54–2.82 (m, 2H, CH2-C=C), 2.84–3.08 (m, 1H, CH2-O), 3.08–3.26 (m, 2H, CH2N + CH2-O), 3.45–3.59 (m, 3H, CH2-O + OH), 3.68–3.85 (m, 1H, CH2N), 3.79–3.85 (m, 1H, C5H4), 3.97–4.04 (m, 1H, C5H4), 4.04–4.15 (m, 7H, Cp + 2C5H4), 5.45 (s, 1H, CH-O), 6.69 (d, J = 8.4 Hz, 2H, C6H4), 6.73 (d, J = 8.4 Hz, 2H, C6H4), 6.86 (d, J = 8.4 Hz, 2H, C6H4), 7.03 (d, J = 8.4 Hz, 2H, C6H4), 7.50–7.68 (m, 3H, isoindole), 7.71 (d, J = 7.3 Hz, 1H, isoindole), 8.24 (s, 1H, OH), 8.31 (s, 1H, OH). 13C NMR (acetone-d6): δ 26.4 (CH2), 26.7 (CH2), 29.6 (CH2), 30.4 (CH2), 33.1 (CH2), 33.6 (CH2), 39.8 (CH2), 62.4 (CH2-O), 64.1 (CH2-O), 68.6 (CH C5H4), 68.7 (CH C5H4), 69.9 (5CH Cp + C5H4), 70.1 (CH C5H4), 86.3 (CH-O), 88.5 (C C5H4), 115.7 (2CH C6H4), 116.0 (2CH C6H4), 123.5 (CH isoindole), 124.6 (CH isoindole), 130.4 (CH isoindole), 131.3 (2CH C6H4), 131.8 (2CH C6H4), 132.7 (CH isoindole), 133.6 (C), 134.5 (C), 136.8 (C), 137.3 (C), 139.4 (C), 142.8 (C), 156.7 (C), 156.8 (C), 167.6 (CO). IR (ATR, ν cm−1): 1678 (CO). HRMS (ESI, C41H43FeNO5: [M]+) calcd: 685.2486, found: 685.2506.
3.12.3. O-{2,3-Dihydro-2-[4-ferrocenyl-5,5-bis-(4-hydroxyphenyl)-pent-4-enyl]-1H-isoindol-1-one-3-yl}-12-hydroxy-1,4,7,10-tetraoxadodecane (22)
From compound 18 (0.25 g, 0.427 mmol) and tetraethylene glycol (0.829 g, 4.3 mmol). Eluent: cyclohexane/ethyl acetate 1: 2; yield of 79%. 1H NMR (acetone-d6): δ 1.74–1.96 (m, 2H, CH2), 2.55–2.80 (m, 2H, CH2-C=C), 3.12–3.25 (m, 2H, CH2N + CH2-O), 3.26–3.37 (m, 1H, CH2-O), 3.48–3.68 (m, 14H, CH2-O), 3.68–3.80 (m, 1H, CH2N), 3.80–3.85 (m, 1H, C5H4), 3.99–4.04 (m, 1H, C5H4), 4.04–4.14 (m, 7H, Cp + 2C5H4), 5.49 (s, 1H, CH-O), 6.69 (d, J = 8.5 Hz, 2H, C6H4), 6.72 (d, J = 8.5 Hz, 2H, C6H4), 6.86 (d, J = 8.5 Hz, 2H, C6H4), 7.03 (d, J = 8.5 Hz, 2H, C6H4), 7.51–7.61 (m, 1H, isoindole), 7.61–7.67 (m, 2H, isoindole), 7.71 (d, J = 7.3 Hz, 1H, isoindole), 8.17 (s, 1H, OH), 8.20 (s, 1H, OH). 13C NMR (acetone-d6): δ 29.6 (CH2), 33.1 (CH2), 39.7 (CH2), 62.0 (CH2-O), 64.1 (CH2-O), 68.6 (CH C5H4), 68.7 (CH C5H4), 69.8 (5 CH Cp + 1 CH C5H4), 70.1 (CH C5H4), 70.9 (CH2-O), 71.1 (CH2-O), 71.2 (2CH2-O), 71.3 (CH2-O), 73.5 (CH2-O), 86.5 (CH-OH), 88.5 (C C5H4), 115.7 (2CH C6H4), 116.0 (2CH C6H4), 123.5 (CH isoindole), 124.8 (CH isoindole), 130.5 (CH isoindole), 131.3 (2CH C6H4), 131.8 (2CH C6H4), 132.7 (CH isoindole), 133.6 (C), 134.6 (C), 136.8 (C), 137.2 (C), 139.4 (C), 142.6 (C), 156.66 (C), 156.72 (C), 167.7 (CO). IR (ATR, ν cm−1): 1679 (CO). HRMS (ESI, C43H47FeNNaO8: [M + Na]+) calcd: 784.254328, found: 784.2544.
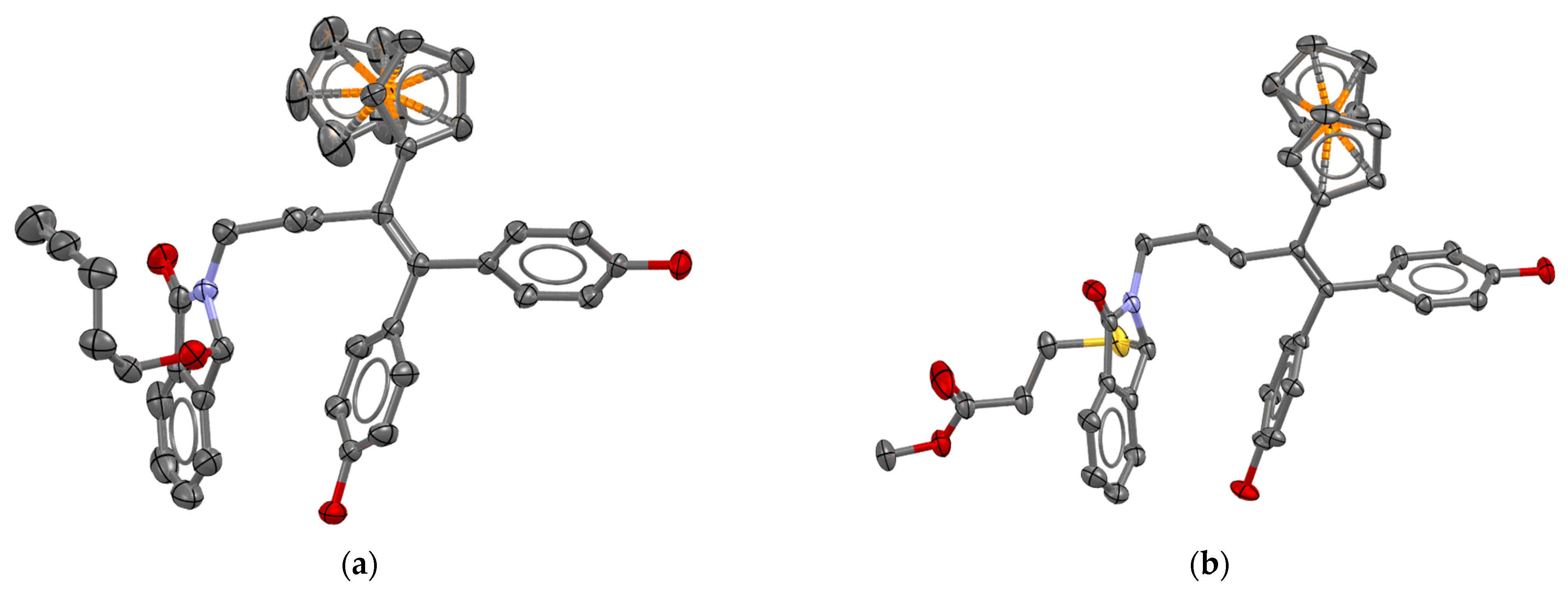
3.12.4. 2,3-Dihydro-3-(pent-4-ynyl)-2-[4-ferrocenyl-5,5-bis-(4-hydroxyphenyl)-pent-4-enyl]-1H-isoindol-1-one (23)
From compound 18 (0.25 g, 0.427 mmol) and 4-pentyn-1-ol (0.359 g, 4.3 mmol). Eluent: cyclohexane/ethyl acetate 2: 1. Yield of 91%. 1H NMR (acetone-d6): δ 1.61–1.76 (m, 2H, CH2), 1.77–1.96 (m, 2H, CH2), 2.19–2.32 (m, 3H, -CH2-alkyn-H), 2.54–2.82 (m, 2H, CH2-C=C), 3.04–3.22 (m, 2H, CH2N + CH2O), 3.23–3.36 (m, 1H, CH2O), 3.70–3.84 (m, 1H, CH2N), 3.81 (s, 1H, C5H4), 4.01 (s, 1H, C5H4), 4.09 (s, 7H, Cp + C5H4), 5.45 (s, 1H, N-CH-O), 6.69 (d, J = 8.5 Hz, 2H, C6H4), 6.73 (d, J = 8.5 Hz, 1H, C6H4), 6.86 (d, J = 8.5 Hz, 1H, C6H4), 7.03 (d, J = 8.5 Hz, 1H, C6H4), 7.51–7.68 (m, 3H, isoindole), 7.72 (dt, J = 7.3, 1.1 Hz, 1H, isoindole), 8.18 (s, 1H, OH), 8.22 (s, 1H, OH). 13C NMR (acetone-d6): δ 15.3 (CH2), 29.2 (CH2), 29.4 (CH2), 32.9 (CH2), 39.5 (CH2), 62.0 (CH2), 68.6 (CH C5H4), 68.7 (CH C5H4), 69.9 (CH C5H4 + 5CH Cp), 70.1 (CH C5H4), 70.3 (C alkyne), 83.9 (CH alkyne), 86.2 (N-CH-O), 88.5 (C C5H4), 115.7 (2CH C6H4), 116.0 (2CH C6H4), 123.5 (CH isoindole), 124.6 (CH isoindole), 130.5 (CH isoindole), 131.3 (2CH C6H4), 131.8 (2CH C6H4), 132.7 (CH isoindole), 133.6 (C), 134.6 (C), 136.9 (C), 137.3 (C), 139.4 (C), 142.6 (C), 156.67 (C), 156.73 (C), 167.6 (CO). IR (ATR, ν cm−1): 2118 (alkyne), 1670 (CO). HRMS (ESI, C40H38FeNO4: [M + H]+) calcd: 652.2145, found: 652.2143. Crystal data: C41.5H40FeNO4.5, monoclinic P 21/n, a = 9.8118(3) Å, b = 17.2893(6) Å, c = 20.2760(6) Å, α = γ = 90°, β = 91.557(2)°, V = 3438.33(19) Å3, Z = 4, orange prism 0.2 × 0.1 × 0.05 mm3, μ = 3.874 mm−1, min/max transmission = 0.65/0.90, T= 200(1) K, λ = 1.54178 Å, θ range = 4.37° to 66.47°, 26640 reflections measured, 6066 independent, Rint = 0.0505, completeness = 0.998, 455 parameters, 27 restraints, final R indices R1 [I > 2σ (I)] = 0.0382 and wR2 (all data) = 0.0982, GOF on F2 = 1.031, largest difference peak/hole = 0.27/−0.34 e·Å−3.
3.12.5. O-{2,3-Dihydro-2-[4-ferrocenyl-5,5-bis-(4-hydroxyphenyl)-pent-4-enyl]-1H-isoindol-1-one-3-yl}-24-azido-1,4,7,10,13,16,19,22-octaoxatetracosane (24)
From compound 18 (0.22 g, 0.376 mmol) and O-(2-azidoethyl)heptaethylene glycol (0.223 g, 0.564 mmol). Eluent: cyclohexane/ethyl acetate 1: 2. Yield of 28%. 1H NMR (acetone-d6): δ 1.76–1.95 (m, 2H, CH2), 2.53–2.78 (m, 2H, CH2-C=C), 2.82–3.01 (m, 1H, CH2N), 3.12–3.43 (m, 4H, CH2-O + CH2-N3), 3.48–3.72 (m, 28H, CH2-O), 3.68–3.81 (m, 1H, CH2N), 3.80–3.85 (m, 1H, C5H4), 3.98–4.04 (m, 1H, C5H4), 4.04–4.15 (m, 7H, Cp + 2C5H4), 5.49 (s, 1H, CH-O), 6.69 (d, J = 8.4 Hz, 2H, C6H4), 6.73 (d, J = 8.4 Hz, 2H, C6H4), 6.86 (d, J = 8.4 Hz, 2H, C6H4), 7.03 (d, J = 8.4 Hz, 2H, C6H4), 7.51–7.61 (m, 1H, isoindole), 7.61–7.68 (m, 2H, isoindole), 7.71 (d, J = 7.3 Hz, 1H, isoindole), 8.20 (s, 2H, OH). 13C NMR (CDCl3): δ 28.7 (CH2), 32.1 (CH2), 39.3 (CH2), 50.8 (CH2-N3), 63.1 (CH2-O), 68.2 (4CH C5H4), 69.4 (5CH Cp), 69.9–71.3 (14CH2-O), 85.9 (CH-OH), 87.8 (C C5H4), 115.3 (2CH C6H4), 115.7 (2CH C6H4), 123.4 (CH isoindole), 123.9 (CH isoindole), 130.0 (CH isoindole), 130.5 (2CH C6H4), 131.2 (2CH C6H4), 132.2 (CH isoindole), 132.5 (C), 133.7 (C), 136.2 (C), 136.7 (C), 138.6 (C), 141.0 (C), 154.9 (C), 155.2 (C), 167.9 (CO). IR (ATR, ν cm−1): 2105 (azide), 1678 (CO). HRMS (ESI, C51H62FeN4NaO11: [M + Na]+) calcd: 985.36567, found: 983.3664.
3.12.6. 2,3-Dihydro-3-(2-methoxycarbonylethylthio)-2-[4-ferrocenyl-5,5-bis-(4-hydroxyphenyl)-ent-4-enyl]-1H-isoindol-1-one (25)
From compound 18 (0.305 g, 0.52 mmol) and methyl 3-mercaptopropionate (0.219 g, 1.8 mmol), reaction time of 4 h. Eluent: cyclohexane/ethyl acetate 2: 1. Yield of 83%. 1H NMR (acetone-d6): δ 1.79–1.96 (m, 2H, CH2), 1.99–2.28 (m, 4H, SCH2CH2COO), 2.48–2.76 (m, 2H, CH2-C=C), 3.19–3.33 (m, 1H, CH2N), 3.56 (s, 3H, OMe), 3.74–3.78 (m, 1H, C5H4), 3.90–4.15 (m, 2H, CH2N + C5H4), 4.05–4.16 (m, 7H, 2C5H4 + Cp), 4.91 (CH-S), 6.68 (d, J = 8.5 Hz, 2H, C6H4), 6.72 (d, J = 8.5 Hz, 2H, C6H4), 6.85 (d, J = 8.5 Hz, 2H, C6H4), 7.01 (d, J = 8.5 Hz, 2H, C6H4), 7.49–7.59 (m, 1H, isoindole), 7.63–7.77 (m, 3H, isoindole), 8.23 (s, 1H, OH), 8.35 (s, 1H, OH). 13C NMR (acetone-d6): δ 22.2 (CH2), 29.4 (CH2), 32.8 (CH2), 34.5 (CH2), 39.4 (CH2), 51.8 (OCH3), 63.1 (CH-S), 68.6 (CH C5H4), 68.7 (CH C5H4), 69.80 (CH C5H4), 69.85 (5CH Cp), 70.1 (CH C5H4), 88.4 (C C5H4), 115.7 (2CH C6H4), 116.1 (2CH C6H4), 123.5 (CH isoindole), 124.8 (CH isoindole), 129.7 (CH isoindole), 131.3 (2CH C6H4), 131.8 (2CH C6H4), 132.8 (CH isoindole), 132.9 (C), 134.6 (C), 136.8 (C), 137.2 (C), 139.3 (C), 144.7 (C), 156.69 (C), 156.74 (C), 167.6 (CO), 172.2 (COO). IR (ATR, ν cm−1): 1714 (CO), 1660 (CO). HRMS (ESI, C39H37FeNO5S: [M]+) calcd: 687.1736, found: 687.1736. Crystal data: C42H43FeNO6S, triclinic P -1, a = 10.7220(5) Å, b = 13.2436(6) Å, c = 15.3329(6) Å, α = 97.196(3)°, β = 103.916(4)°, γ = 113.797(4)°, V = 1872.10(16) Å3, Z = 2, orange plate 0.3 × 0.2 × 0.1 mm3, μ = 0.507 mm−1, min/max transmission = 0.57/1.00, T = 200 K, λ = 0.71073 Å, θ range = 1.74° to 28.28°, 25312 reflections measured, 9219 independent, Rint = 0.0892, completeness = 0.997, 471 parameters, 0 restraints, final R indices R1 [I > 2σ (I)] = 0.0630 and wR2 (all data) = 0.1676, GOF on F2 = 1.102, largest difference peak/hole = 1.52/−0.59 e·Å−3.
3.12.7. 2,3-Dihydro-3-(2-carboxyethylthio)-2-[4-ferrocenyl-5,5-bis-(4-hydroxyphenyl)-pent-4-enyl]-1H-isoindol-1-one (26)
From compound 18 (0.3 g, 0.512 mmol) and 3-mercaptopropionic acid (0.19 g, 0.16 mL, 1.8 mmol), time overnight. Eluent: ethyl acetate. Yield of 88%. 1H NMR (DMSO-d6): δ 1.51–2.18 (m, 6H, CH2 + SCH2-CH2COO), 2.34–2.59 (m, 2H, CH2-C=C), 3.02–3.25 (m, 1H, CH2N), 3.63–3.69 (m, 1H, C5H4), 3.71–3.86 (m, 1H, CH2N), 3.95–4.16 (m, 8H, 3C5H4 + Cp), 5.04 (CH-S), 6.59 (d, J = 8.3 Hz, 2H, C6H4), 6.62 (d, J = 8.3 Hz, 2H, C6H4), 6.75 (d, J = 8.3 Hz, 2H, C6H4), 6.91 (d, J = 8.3 Hz, 2H, C6H4), 7.51–7.74 (m, 4H, isoindole). 13C NMR (DMSO-d6): δ 21.0 (CH2), 28.1 (CH2), 31.3 (CH2), 33.6 (CH2), 38.3 (CH2), 61.8 (CH-S), 67.6 (CH C5H4), 67.7 (CH C5H4), 68.5 (CH C5H4), 68.6 (CH C5H4), 68.9 (5CH Cp), 86.7 (C C5H4), 114.8 (2CH C6H4), 115.0 (2CH C6H4), 122.3 (CH isoindole), 123.7 (CH isoindole), 128.8 (CH isoindole), 129.8 (2CH C6H4), 130.2 (2CH C6H4), 131.1 (C), 132.0 (CH isoindole), 132.8 (C), 134.9 (C), 135.3 (C), 137.5 (C), 143.3 (C), 155.5 (2C), 166.2 (CO), 172.3 (COO). IR (ATR, ν cm−1): 1704 (CO), 1639 (CO). HRMS (ESI, C38H34FeNO5S: [M-H]-) calcd: 672.1513, found: 672.1511.
3.12.8. S-{2,3-Dihydro-2-[4-ferrocenyl-5,5-bis-(4-hydroxyphenyl)-pent-4-enyl]-1H-isoindol-1-one-3-yl}-1-thia-4,7,10,13,16,19,22-heptaoxatricosane (27)
From compound 18 (0.3422 g, 0.584 mmol) and O-(2-mercaptoethyl)-O′-methyl-hexa(ethylene glycol) (0.25 g, 0.701 mmol). Eluent: cyclohexane/ethyl acetate 1: 2. Yield of 76%. 1H NMR (acetone-d6): δ 1.72–1.96 (m, 2H, CH2), 1.98–2.16 (m, 2H, CH2-S), 2.48–2.78 (m, 2H, CH2-C=C), 3.16–3.69 (m, 30H, CH2N + CH2(OCH2CH2)6-OCH3), 3.74–3.81 (m, 1H, C5H4), 3.88–4.03 (m, 2H, CH2N + C5H4), 4.03–4.20 (m, 7H, Cp + 2C5H4), 4.96 (s, 1H, CH-S), 6.69 (d, J = 8.5 Hz, 2H, C6H4), 6.74 (d, J = 8.5 Hz, 2H, C6H4), 6.85 (d, J = 8.5 Hz, 2H, C6H4), 7.02 (d, J = 8.5 Hz, 2H, C6H4), 7.54 (td, J = 7.2, 1.6 Hz, 1H, isoindole), 7.61–7.77 (m, 3H, isoindole), 8.35 (s broad, 2H, OH). 13C NMR (acetone-d6): δ 27.3 (CH2), 29.4 (CH2), 32.8 (CH2), 39.5 (CH2), 58.8 (OCH3), 63.1 (CH-S), 68.6 (CH C5H4), 68.7 (CH C5H4), 69.79 (CH C5H4), 69.84 (5CH Cp), 70.1 (CH C5H4), 70.7 (CH2-O), 70.8 (CH2-O), 71.0 (2CH2-O), 71.2 (8CH2-O), 72.6 (CH2-O), 88.4 (C C5H4), 115.8 (2CH C6H4), 116.1 (2CH C6H4), 123.4 (CH isoindole), 124.9 (CH isoindole), 129.6 (CH isoindole), 131.3 (2CH C6H4), 131.8 (2CH C6H4), 132.76 (CH isoindole), 132.84 (C), 134.5 (C), 136.8 (C), 137.1 (C), 139.3 (C), 144.9 (C), 156.6 (C), 156.7 (C), 167.6 (CO). IR (ATR, ν cm−1): 1669 (CO). HRMS (ESI, C50H61FeNNaO10S: [M + Na]+) calcd: 946.32578, found: 946.3258.