Influence of Nanocellulose Structure on Paper Reinforcement
Abstract
:1. Introduction
2. Results and Discussion
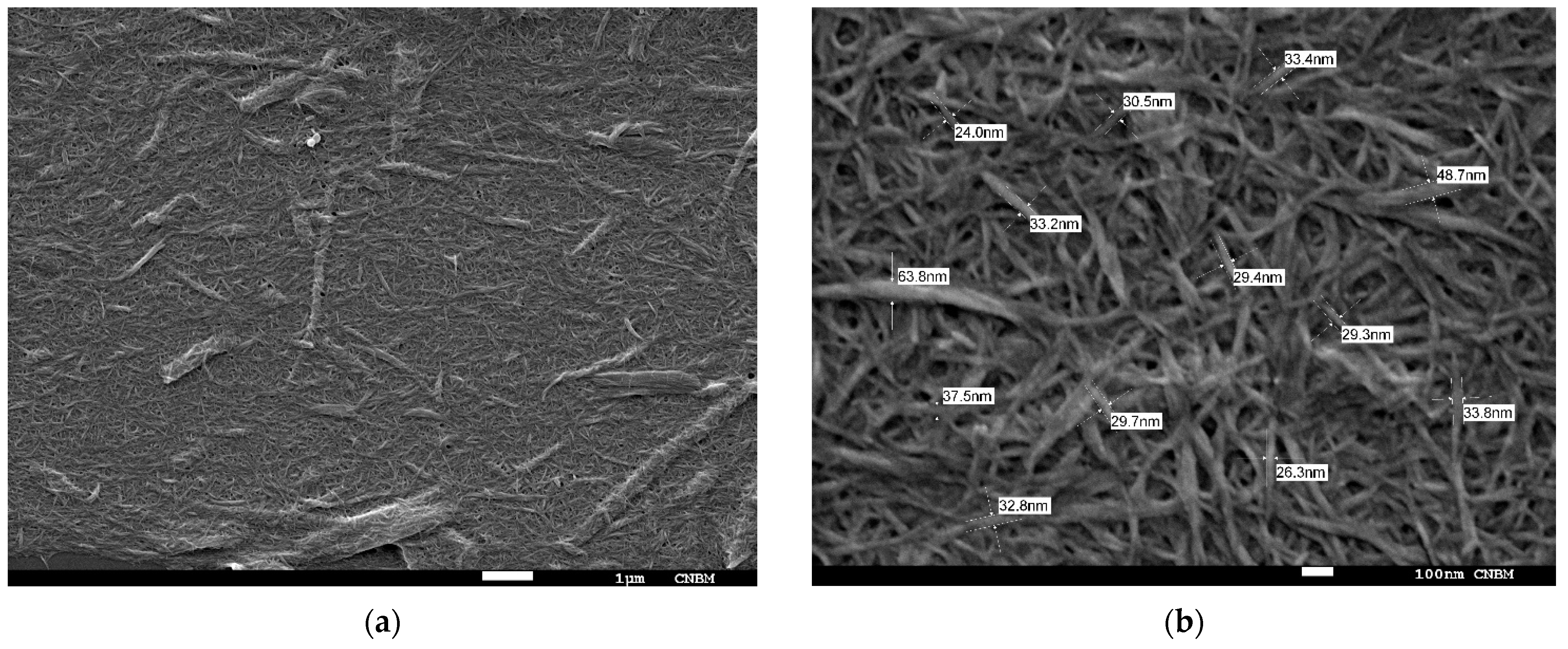
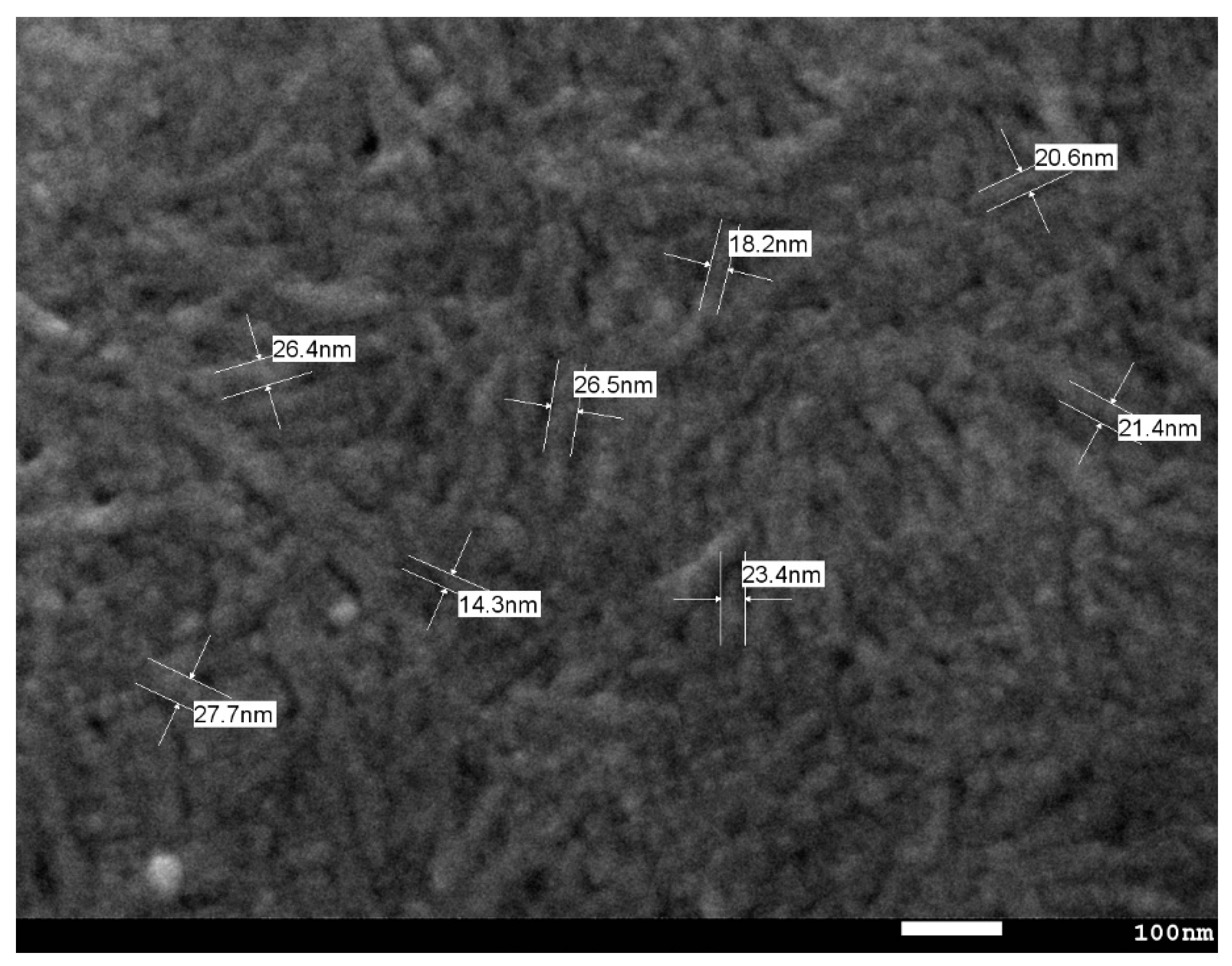
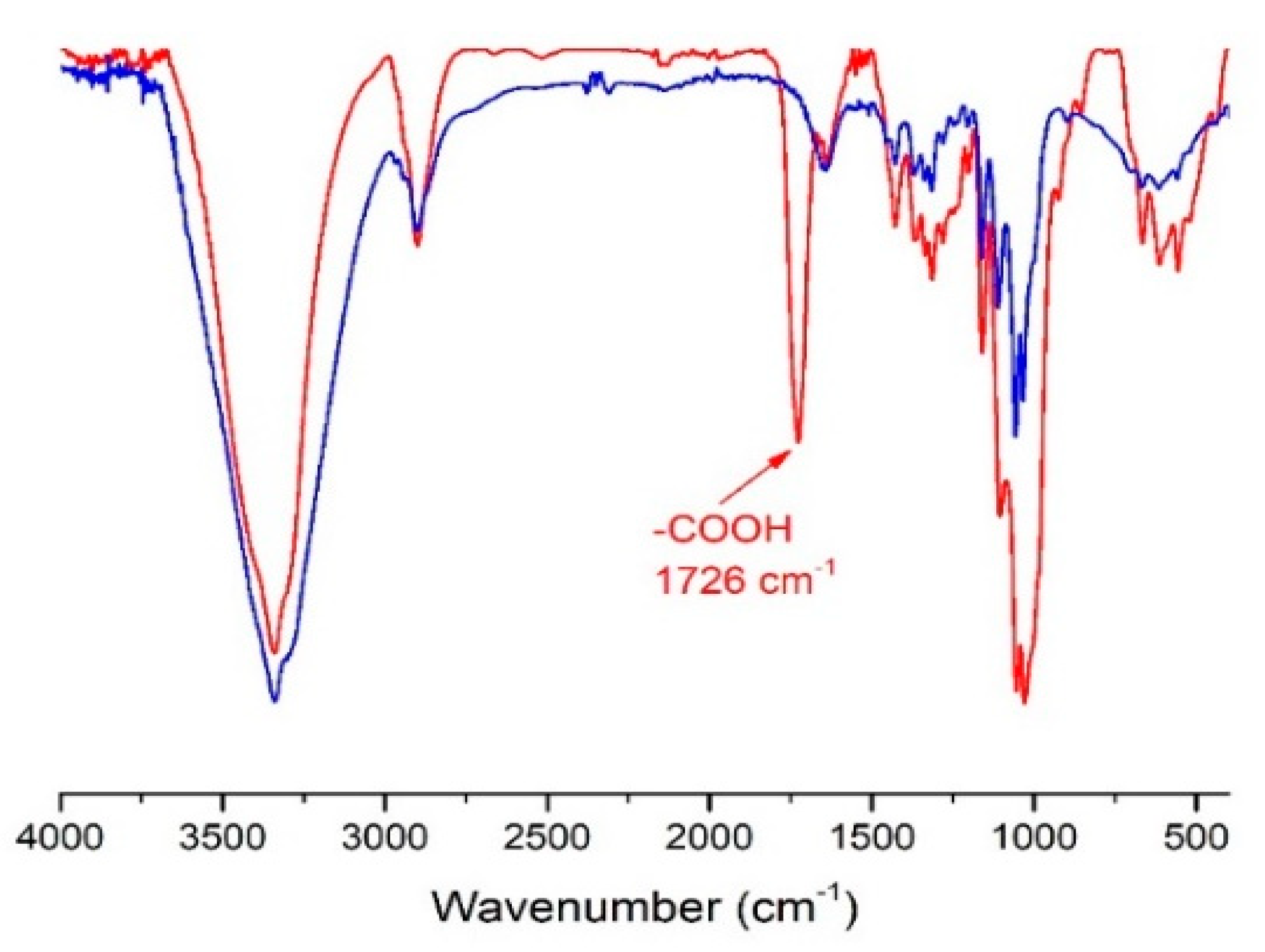
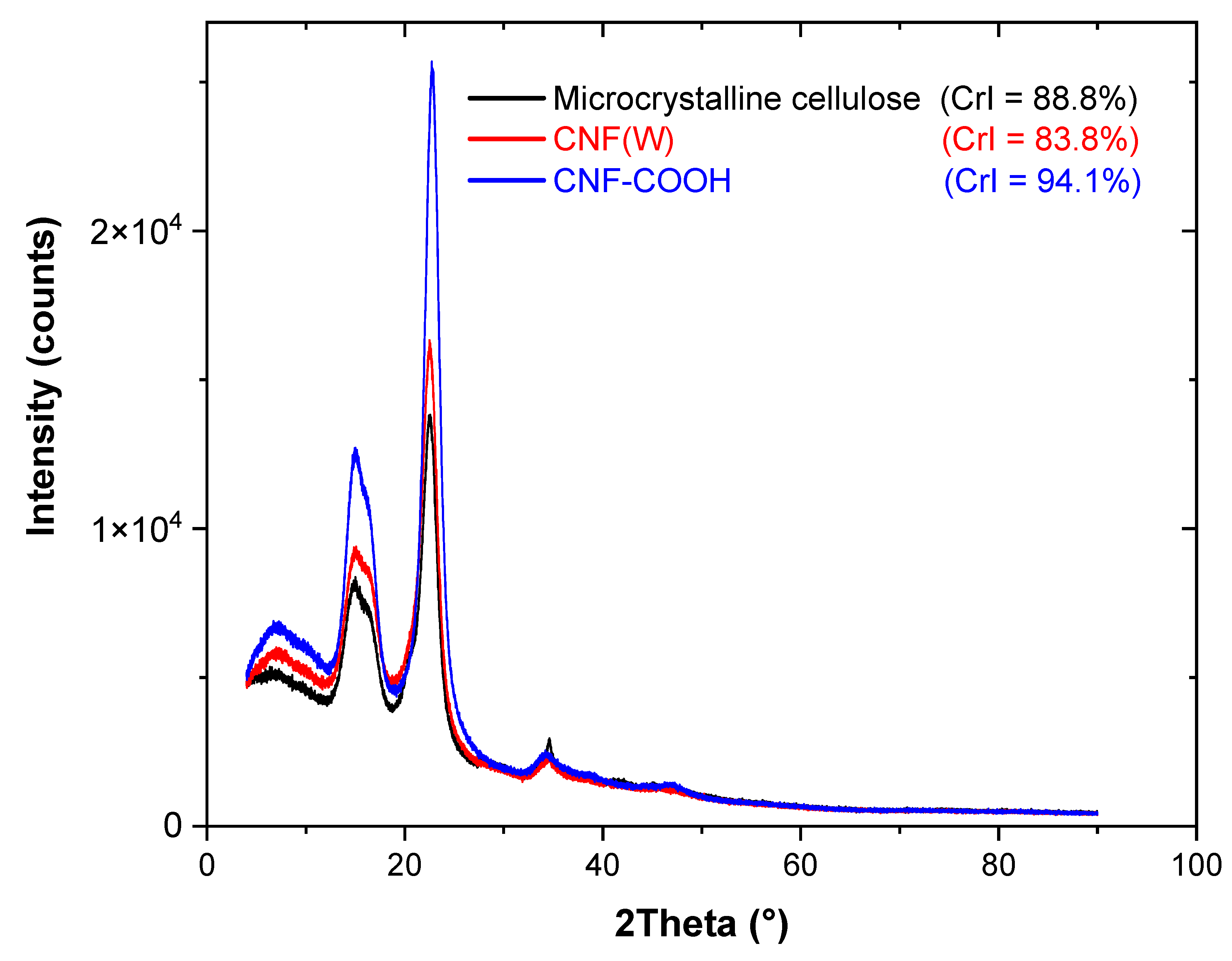
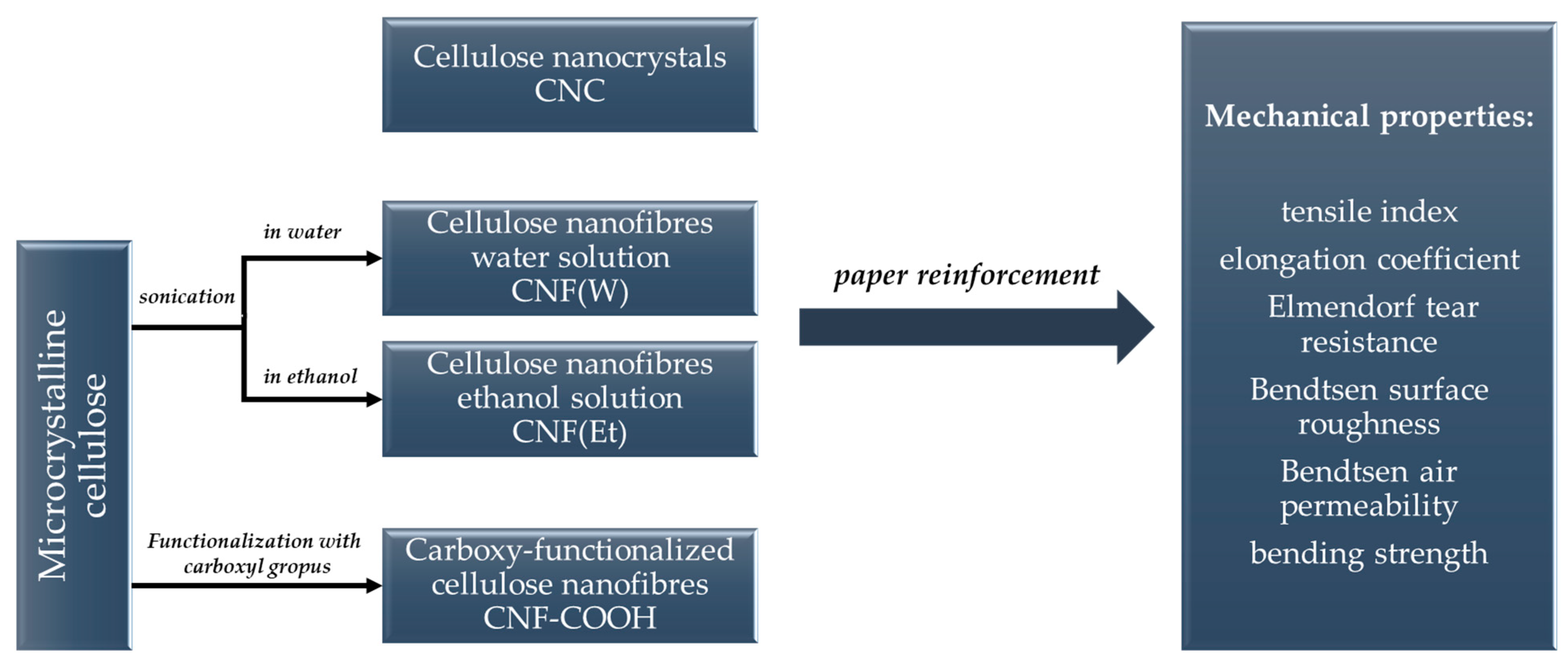
2.1. Nanocellulose Structure
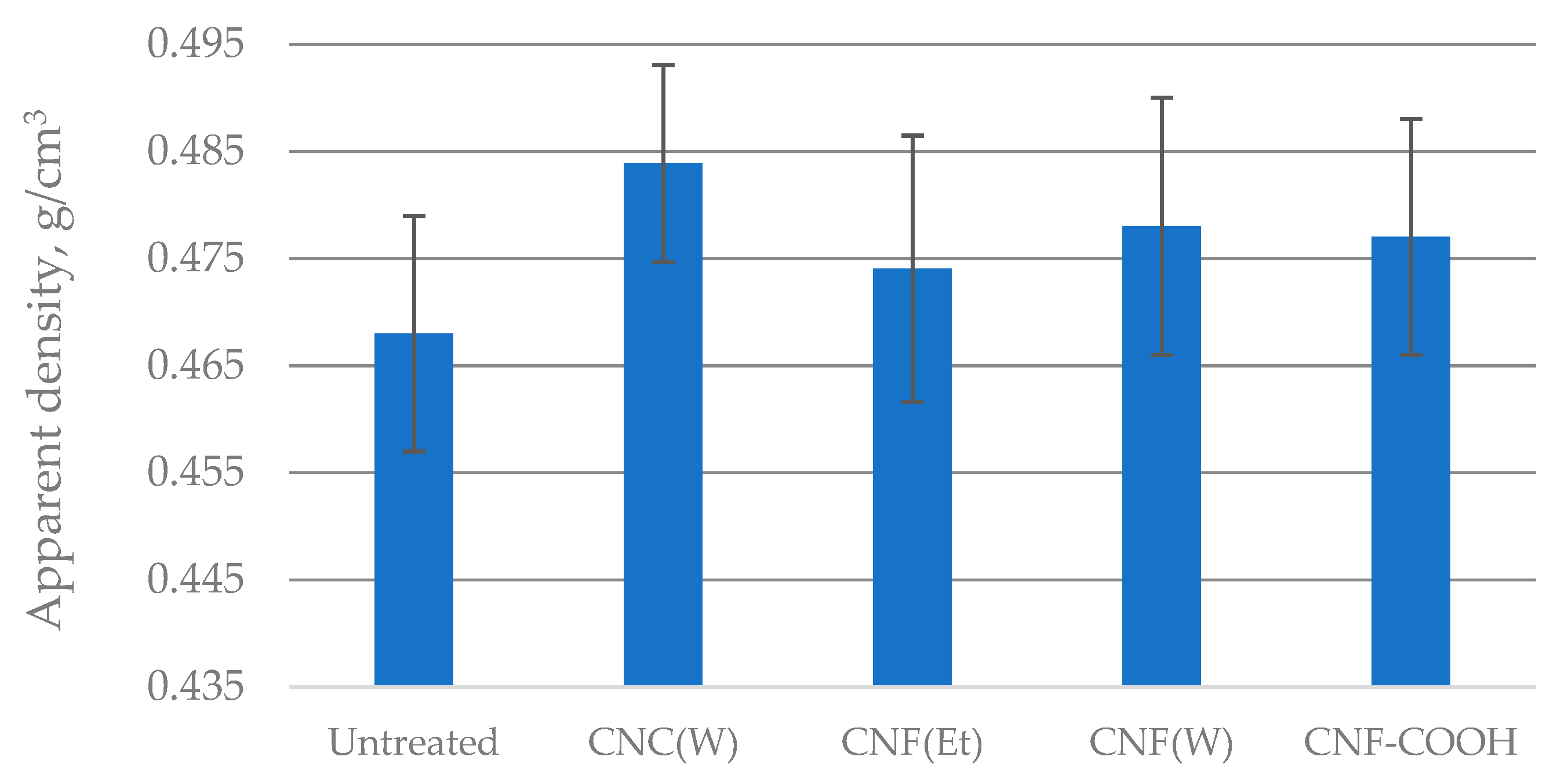
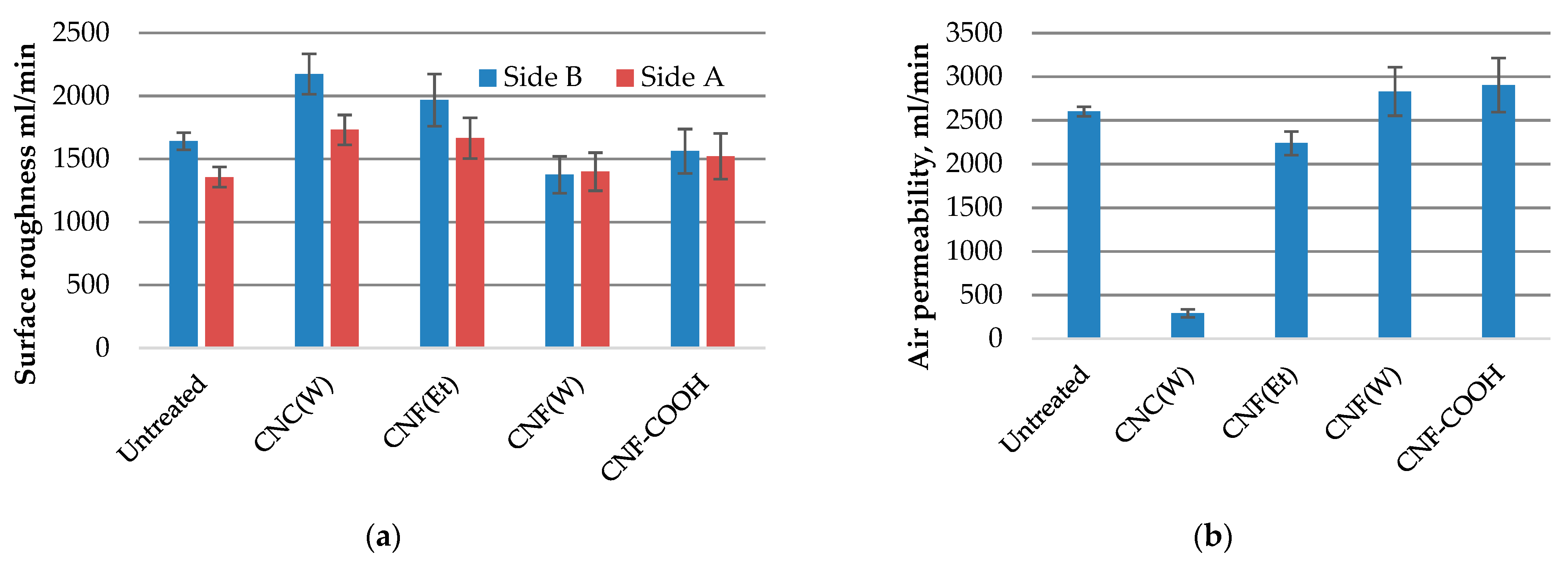
2.2. Structural Properties of Treated Paper
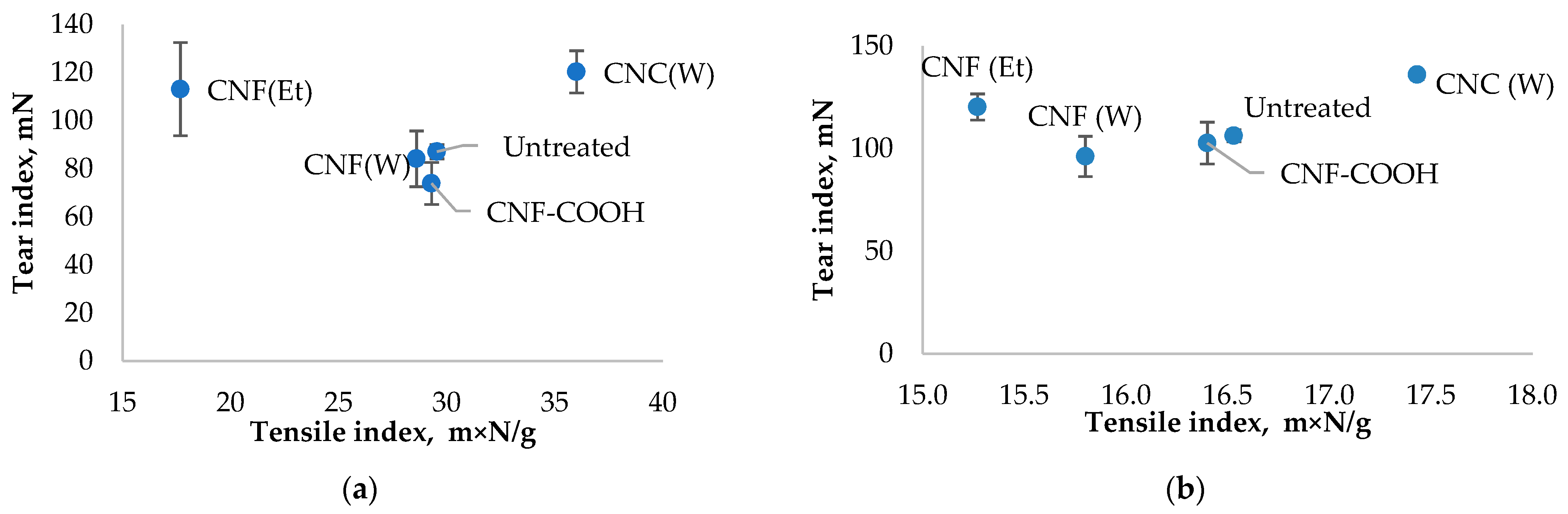
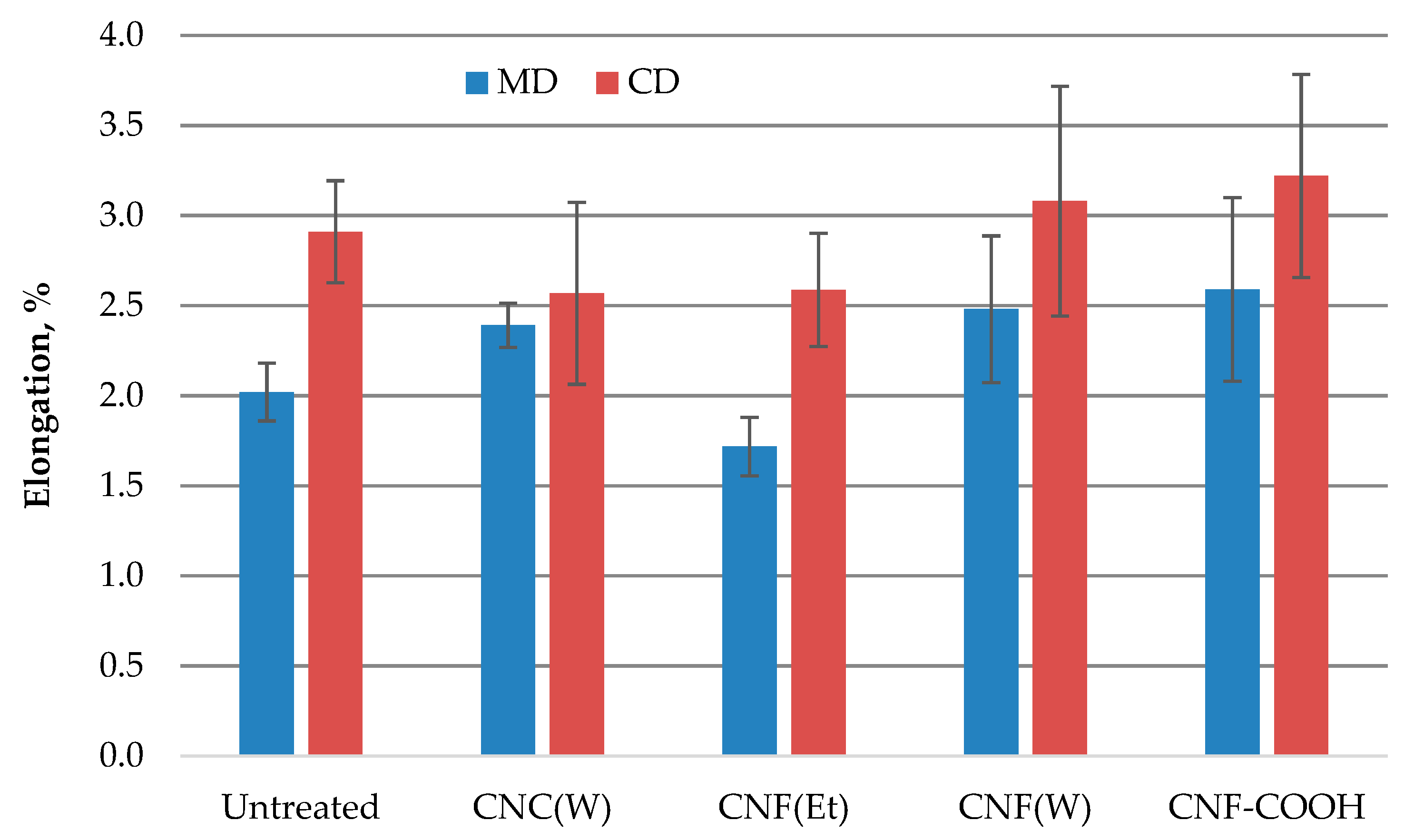
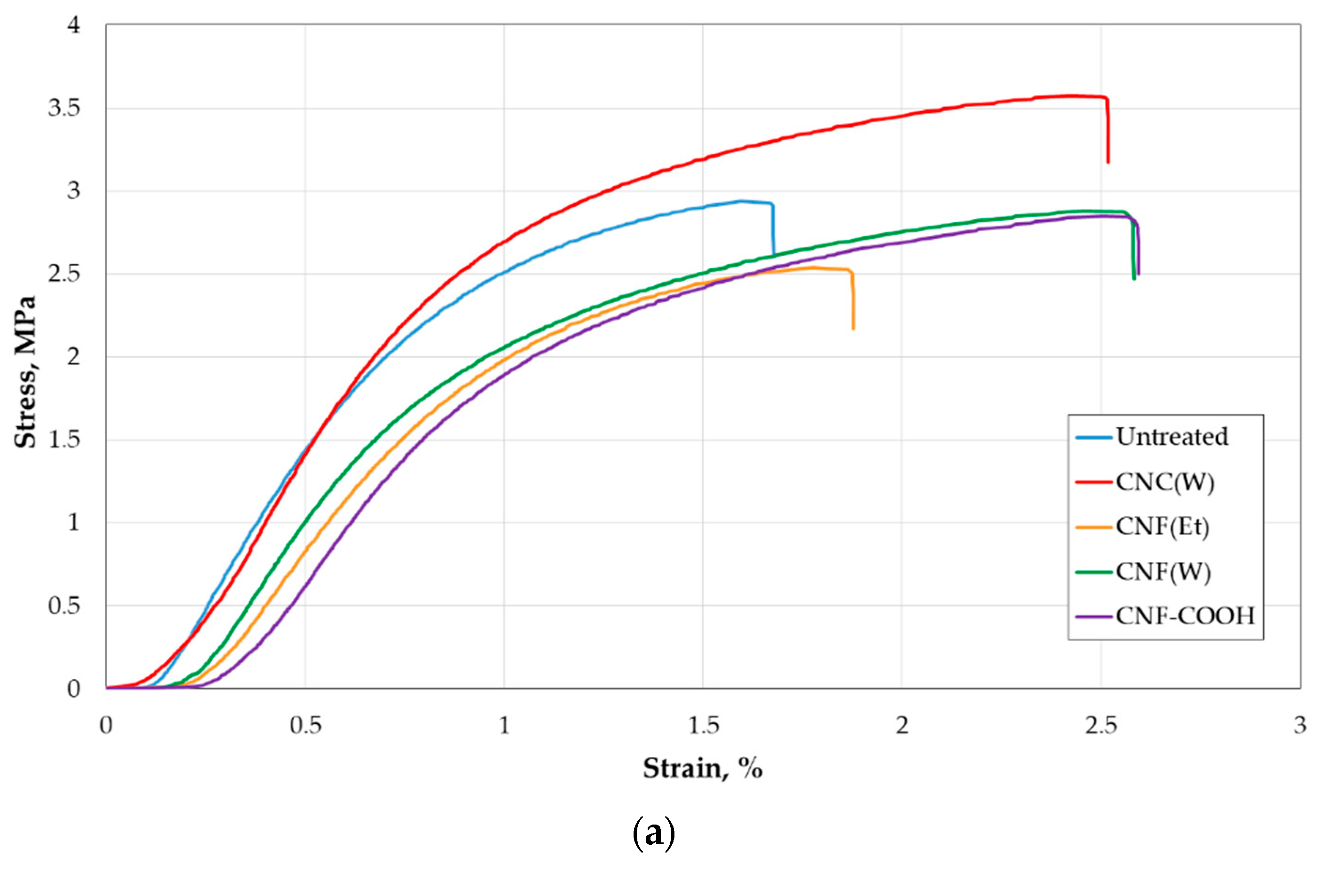
2.3. Mechanical Properties of Paper
3. Materials and Methods
3.1. Materials
3.1.1. CNF(W) and CNF(Et) Preparation
3.1.2. CNF-COOH(W) Preparation
3.2. Nanofiber Structure and Morphology
3.3. Sample Treatment
3.4. Paper Testing
- – Tensile index and elongation (ISO 1924-2:2008),
- – Elmendorf tear resistance (ISO 1974:2012),
- – Bendtsen surface roughness (ISO 8791-2:2013),
- – Bendtsen air permeance (ISO 5636-3:2013),
- – Folding endurance (Schopper device—ISO 5626:1993), and
- – Apparent density (ISO 534:2005).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- IFLA—Storing Digital Information for a Long Time. Available online: https://www.ifla.org/node/93091 (accessed on 17 March 2021).
- Lunt, B.M. How Long Is Long-Term Data Storage? In Proceedings of the Final Program and Proceedings of the Archiving Conference; Society for Imaging Science and Technology: Springfield, VA, USA, 2011; pp. 29–33. [Google Scholar]
- Liu, J.; Xing, H.; Wang, J.; Cao, J.; Chao, X.; Jia, Z.; Li, Y. A New Reinforcement Method for the Conservation of Fragile, Double-Sided, Printed Paper Cultural Relics. Herit. Sci. 2021, 9, 123. [Google Scholar] [CrossRef]
- Area, M.C.; Ceradame, H. Paper Aging and Degradation: Recent Findings and Research Methods. BioResources 2011, 6, 5307–5337. [Google Scholar]
- Zou, X.; Gurnagul, N.; Uesaka, T.; Bouchard, J. Accelerated Aging of Papers of Pure Cellulose: Mechanism of Cellulose Degradation and Paper Embrittlement. Polym. Degrad. Stab. 1994, 43, 393–402. [Google Scholar] [CrossRef]
- Alexopoulou, I.; Zervos, S. Paper Conservation Methods: An International Survey. J. Cult. Herit. 2016, 21, 922–930. [Google Scholar] [CrossRef]
- Zervos, S.; Moropoulou, A. Methodology and Criteria for the Evaluation of Paper Conservation Interventions: A Literature Review. Restaurator 2006, 27, 219–274. [Google Scholar] [CrossRef]
- Jung, J.; Raghavendra, G.M.; Kim, D.; Seo, J. One-Step Synthesis of Starch-Silver Nanoparticle Solution and Its Application to Antibacterial Paper Coating. Int. J. Biol. Macromol. 2018, 107, 2285–2290. [Google Scholar] [CrossRef]
- Du, Y.; Liu, J.; Wang, B.; Li, H.; Su, Y. The Influence of Starch-Based Bio-Latex on Microstructure and Surface Properties of Paper Coating. Undefined 2018, 116, 51–56. [Google Scholar] [CrossRef]
- Lin, D.; Kuang, Y.; Chen, G.; Kuang, Q.; Wang, C.; Zhu, P.; Peng, C.; Fang, Z. Enhancing Moisture Resistance of Starch-Coated Paper by Improving the Film Forming Capability of Starch Film. Ind. Crops Prod. 2017, 100, 12–18. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, P.; Kuang, Y.; Liu, Y.; Lin, D.; Peng, C.; Wen, Z.; Fang, Z. Durable Superhydrophobic Paper Enabled by Surface Sizing of Starch-Based Composite Films. Appl. Surf. Sci. 2017, 409, 45–51. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lee, C.J.; Wang, L.W.; Wang, Y.H. Air-Stable Gelatin Composite Memory Devices on a Paper Substrate. Org. Electron. 2019, 65, 77–81. [Google Scholar] [CrossRef]
- Battisti, R.; Fronza, N.; Vargas Júnior, Á.; da Silveira, S.M.; Damas, M.S.P.; Quadri, M.G.N. Gelatin-Coated Paper with Antimicrobial and Antioxidant Effect for Beef Packaging. Food Packag. Shelf Life 2017, 11, 115–124. [Google Scholar] [CrossRef]
- Khakalo, A.; Filpponen, I.; Johansson, L.S.; Vishtal, A.; Lokanathan, A.R.; Rojas, O.J.; Laine, J. Using Gelatin Protein to Facilitate Paper Thermoformability. React. Funct. Polym. 2014, 85, 175–184. [Google Scholar] [CrossRef]
- Ariafar, A.A.; Afsharpour, M.; Samanian, K. Use of TiO2/Chitosan Nanoparticles for Enhancing the Preservative Effects of Carboxymethyl Cellulose in Paper-Art-Works against Biodeterioration. Int. Biodeterior. Biodegrad. 2018, 131, 67–77. [Google Scholar] [CrossRef]
- Rahmaninia, M.; Rohi, M.; Hubbe, M.A.; Zabihzadeh, S.M.; Ramezani, O. The Performance of Chitosan with Bentonite Microparticles as Wet-End Additive System for Paper Reinforcement. Carbohydr. Polym. 2018, 179, 328–332. [Google Scholar] [CrossRef]
- Brodnjak, U.V. Experimental Investigation of Novel Curdlan/Chitosan Coatings on Packaging Paper. Prog. Org. Coat. 2017, 112, 86–92. [Google Scholar] [CrossRef]
- Raghavendra, G.M.; Jung, J.; Kim, D.; Seo, J. Effect of Chitosan Silver Nanoparticle Coating on Functional Properties of Korean Traditional Paper. Prog. Org. Coat. 2017, 110, 16–23. [Google Scholar] [CrossRef]
- Campano, C.; Merayo, N.; Balea, A.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Negro, C.; Blanco, Á. Mechanical and Chemical Dispersion of Nanocelluloses to Improve Their Reinforcing Effect on Recycled Paper. Cellulose 2018, 25, 269–280. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A New Ageless Bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose Properties and Applications in Colloids and Interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Ferrer, A.; Pal, L.; Hubbe, M. Nanocellulose in Packaging: Advances in Barrier Layer Technologies. Ind. Crops Prod. 2017, 95, 574–582. [Google Scholar] [CrossRef]
- Chauhan, V.; Chakrabarti, S.K. Use of Nanotechnology for High Performance Cellulosic and Papermaking Products. Cellul. Chem. Technol. 2012, 46, 389–400. [Google Scholar]
- Mondal, S. Preparation, Properties and Applications of Nanocellulosic Materials. Carbohydr. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yao, Q.; Liu, J.; Sun, J.; Zhu, Q.; Chen, H. Processing Nanocellulose to Bulk Materials: A Review. Cellulose 2019, 26, 7585–7617. [Google Scholar] [CrossRef]
- Börjesson, M.; Westman, G. Crystalline Nanocellulose—Preparation, Modification, and Properties. In Cellulose—Fundamental Aspects and Current Trends; IntechOpen: London, UK, 2015; pp. 159–191. ISBN 978-953-51-2229-6. [Google Scholar]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Ioelovich, M. Cellulose Nanostructured Natural Polymer; LAP LAMBERT Academic Publishing: Sunnyvale, CA, USA, 2014; ISBN 3659174378. [Google Scholar]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. Handb. Nanocellulose Cellul. Nanocomposites 2017, 1, 1–51. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y. Lo Chemically and Mechanically Isolated Nanocellulose and Their Self-Assembled Structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different Preparation Methods and Properties of Nanostructured Cellulose from Various Natural Resources and Residues: A Review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a Natural Source for Groundbreaking Applications in Materials Science: Today’s State. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef] [Green Version]
- Robles, E.; Labidi, J.; Halász, K.; Csóka, L. Key Issues in Reinforcement Involving Nanocellulose. Cellul. -Reinf. Nanofibre Compos. Prod. Prop. Appl. 2017, 401–425. [Google Scholar] [CrossRef]
- Rusmirović, J.D.; Ivanović, J.Z.; Pavlović, V.B.; Rakić, V.M.; Rančić, M.P.; Djokić, V.; Marinković, A.D. Novel Modified Nanocellulose Applicable as Reinforcement in High-Performance Nanocomposites. Carbohydr. Polym. 2017, 164, 64–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.Y.; Aitomäki, Y.; Berglund, L.A.; Oksman, K.; Bismarck, A. On the Use of Nanocellulose as Reinforcement in Polymer Matrix Composites. Compos. Sci. Technol. 2014, 105, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Fall, A.B.; Burman, A.; Wågberg, L. Cellulosic Nanofibrils from Eucalyptus, Acacia and Pine Fibers. Nord. Pulp Pap. Res. J. 2014, 29, 176–184. [Google Scholar] [CrossRef]
- Gumrah Dumanli, A. Nanocellulose and Its Composites for Biomedical Applications. Curr. Med. Chem. 2017, 24, 512–528. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in Biomedicine: Current Status and Future Prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Fneich, F.; Ville, J.; Seantier, B.; Aubry, T. Structure and Rheology of Aqueous Suspensions and Hydrogels of Cellulose Nanofibrils: Effect of Volume Fraction and Ionic Strength. Carbohydr. Polym. 2019, 211, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Lindström, T. Aspects on Nanofibrillated Cellulose (NFC) Processing, Rheology and NFC-Film Properties. Curr. Opin. Colloid Interface Sci. 2017, 29, 68–75. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in Bio-Based Food Packaging Applications. Ind. Crops Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Bharimalla, A.K.; Deshmukh, S.P.; Vigneshwaran, N.; Patil, P.G.; Prasad, V. Nanocellulose-Polymer Composites for Applications in Food Packaging: Current Status, Future Prospects and Challenges. Polym. Plast. Technol. Eng. 2017, 56, 805–823. [Google Scholar] [CrossRef]
- Santos, S.M.; Carbajo, J.M.; Gómez, N.; Quintana, E.; Ladero, M.; Sánchez, A.; Chinga-Carrasco, G.; Villar, J.C. Use of Bacterial Cellulose in Degraded Paper Restoration. Part II: Application on Real Samples. J. Mater. Sci. 2016, 51, 1553–1561. [Google Scholar] [CrossRef]
- Dreyfuss-Deseigne, R. Nanocellulose Films in Art Conservation: A New and Promising Mending Material for Translucent Paper Objects. J. Pap. Conserv. 2017, 18, 18–29. [Google Scholar] [CrossRef]
- Tajik, M.; Torshizi, H.J.; Resalati, H.; Hamzeh, Y. Effects of Cationic Starch in the Presence of Cellulose Nanofibrils on Structural, Optical and Strength Properties of Paper from Soda Bagasse Pulp. Carbohydr. Polym. 2018, 194, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.A.; Hassan, M.L.; Abou-zeid, R.E.; El-Wakil, N.A. Novel Nanofibrillated Cellulose/Chitosan Nanoparticles Nanocomposites Films and Their Use for Paper Coating. Ind. Crops Prod. 2016, 93, 219–226. [Google Scholar] [CrossRef]
- Beaumont, M.; Nypelö, T.; König, J.; Zirbs, R.; Opietnik, M.; Potthast, A.; Rosenau, T. Synthesis of Redispersible Spherical Cellulose II Nanoparticles Decorated with Carboxylate Groups. Green Chem. 2016, 18, 1465–1468. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Kolman, K.; Bridarolli, A.; Odlyha, M.; Bozec, L.; Oriola, M.; Campo-Francés, G.; Persson, M.; Holmberg, K.; Bordes, R. On the Potential of Using Nanocellulose for Consolidation of Painting Canvases. Carbohydr. Polym. 2018, 194, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Bridarolli, A.; Nechyporchuk, O.; Odlyha, M.; Oriola, M.; Bordes, R.; Holmberg, K.; Anders, M.; Chevalier, A.; Bozec, L. Nanocellulose-Based Materials for the Reinforcement of Modern Canvas-Supported Paintings. Stud. Conserv. 2018, 63, 332–334. [Google Scholar] [CrossRef] [Green Version]
- Przybysz, P.; Dubowik, M.; Kucner, M.A.; Przybysz, K.; Buzała, K.P. Contribution of Hydrogen Bonds to Paper Strength Properties. PLoS ONE 2016, 11, e0155809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aquino, A.J.A.; Tunega, D.; Haberhauer, G.; Gerzabek, M.H.; Lischka, H. Solvent Effects on Hydrogen BondsA Theoretical Study. J. Phys. Chem. A 2002, 106, 1862–1871. [Google Scholar] [CrossRef]
- Urstöger, G.; Simoes, M.G.; Steinegger, A.; Schennach, R.; Hirn, U. Evaluating the Degree of Molecular Contact between Cellulose Fiber Surfaces Using FRET Microscopy. Cellulose 2019, 26, 7037–7050. [Google Scholar] [CrossRef] [Green Version]
- Hirn, U.; Schennach, R. Comprehensive Analysis of Individual Pulp Fiber Bonds Quantifies the Mechanisms of Fiber Bonding in Paper. Sci. Rep. 2015, 5, 10503. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.V.G.; Borsoi, C.; Lavoratti, A.; Zanini, M.; Zattera, A.J.; Santana, R.M.C. Drying Techniques Applied to Cellulose Nanofibers. J. Reinf. Plast. Compos. 2016, 35, 682–697. [Google Scholar] [CrossRef]
- Wang, L.; Lundahl, M.J.; Greca, L.G.; Papageorgiou, A.C.; Borghei, M.; Rojas, O.J. Effects of Non-Solvents and Electrolytes on the Formation and Properties of Cellulose I Filaments. Sci. Rep. 2019, 9, 16691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.R.; Zheng, B.; Sharma, S.K.; Zhan, C.; Wang, R.; Bhatia, S.R.; Hsiao, B.S. High Aspect Ratio Carboxycellulose Nanofibers Prepared by Nitro-Oxidation Method and Their Nanopaper Properties. ACS Appl. Nano Mater. 2018, 1, 3969–3980. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on Cellulose Nanocrystals Produced from Cellulose Sources with Various Polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Xiang, Z.; Qi, H.; Han, T.; Pranovich, A.; Song, T. Strategy towards One-Step Preparation of Carboxylic Cellulose Nanocrystals and Nanofibrils with High Yield, Carboxylation and Highly Stable Dispersibility Using Innocuous Citric Acid. Green Chem. 2019, 21, 1956–1964. [Google Scholar] [CrossRef]




| Parameter Tested/Sample Name | Apparent Density | Surface Roughness | Air Permeability | Tensile Index, MD/CD | Elongation, MD/CD | Tear Resistance, MD/CD | Double Folds Number, MD/CD |
|---|---|---|---|---|---|---|---|
| Untreated | 2.3% | 2.1% | 11.2% | 9.4%/7.5% | 9.6%/7.4% | 6.7%/7.2% | 15.6%/17.2% |
| CNC(W) | 1.9% | 7.3% | 10.6% | 6.8%/4.9% | 5.4%/9.6% | 7.7%/5.9% | 18.4%/22.7% |
| CNF(Et) | 2.6% | 10.5% | 6.6% | 5.2%/9.3% | 9.4%/12.1% | 9.9%/10.4% | 19.6%/23.9% |
| CNF(W) | 1.9% | 10.6% | 9.8% | 5.9%/9.6% | 10.4%/11.6% | 13.7%/10.2% | 18.5%/25.9% |
| CNF-COOH | 2.1% | 11.3% | 10.7% | 6.7%/8.9% | 10.4%/12.3% | 11.8%/9.9% | 12.6%/19.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perdoch, W.; Cao, Z.; Florczak, P.; Markiewicz, R.; Jarek, M.; Olejnik, K.; Mazela, B. Influence of Nanocellulose Structure on Paper Reinforcement. Molecules 2022, 27, 4696. https://doi.org/10.3390/molecules27154696
Perdoch W, Cao Z, Florczak P, Markiewicz R, Jarek M, Olejnik K, Mazela B. Influence of Nanocellulose Structure on Paper Reinforcement. Molecules. 2022; 27(15):4696. https://doi.org/10.3390/molecules27154696
Chicago/Turabian StylePerdoch, Waldemar, Zhuoran Cao, Patryk Florczak, Roksana Markiewicz, Marcin Jarek, Konrad Olejnik, and Bartłomiej Mazela. 2022. "Influence of Nanocellulose Structure on Paper Reinforcement" Molecules 27, no. 15: 4696. https://doi.org/10.3390/molecules27154696
APA StylePerdoch, W., Cao, Z., Florczak, P., Markiewicz, R., Jarek, M., Olejnik, K., & Mazela, B. (2022). Influence of Nanocellulose Structure on Paper Reinforcement. Molecules, 27(15), 4696. https://doi.org/10.3390/molecules27154696








