Fluorescence, Absorption, Chromatography and Structural Transformation of Chelerythrine and Ethoxychelerythrine in Protic Solvents: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. General Procedure for Spectral Measurement
2.4. Measurement of Fluorescence Quantum Yield
2.5. HPLC Test
3. Results and Discussion
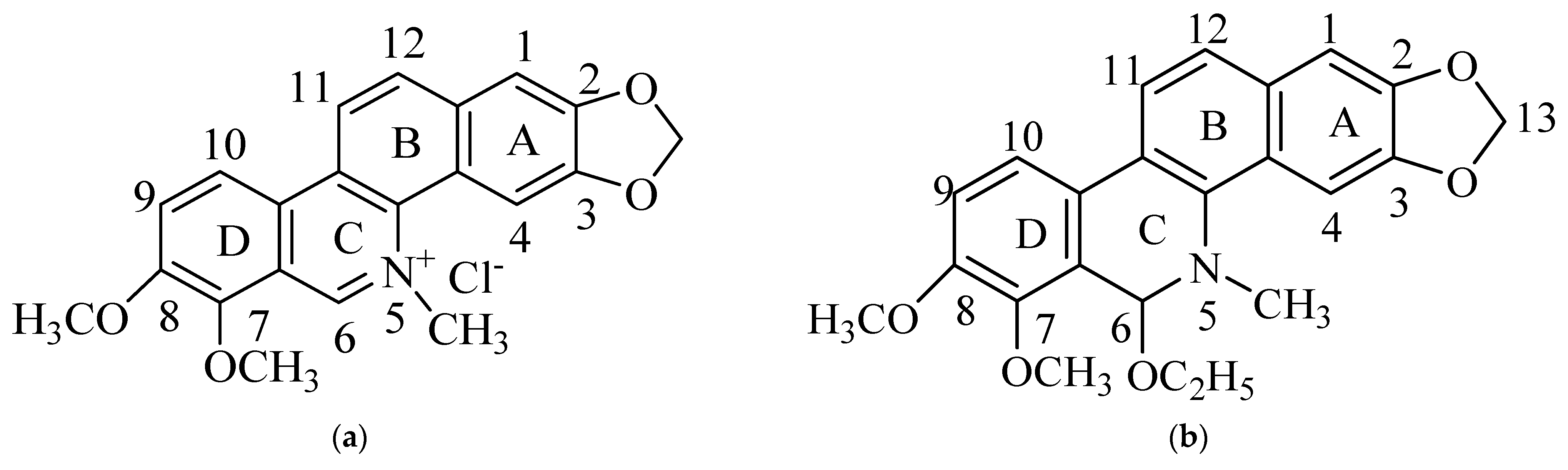
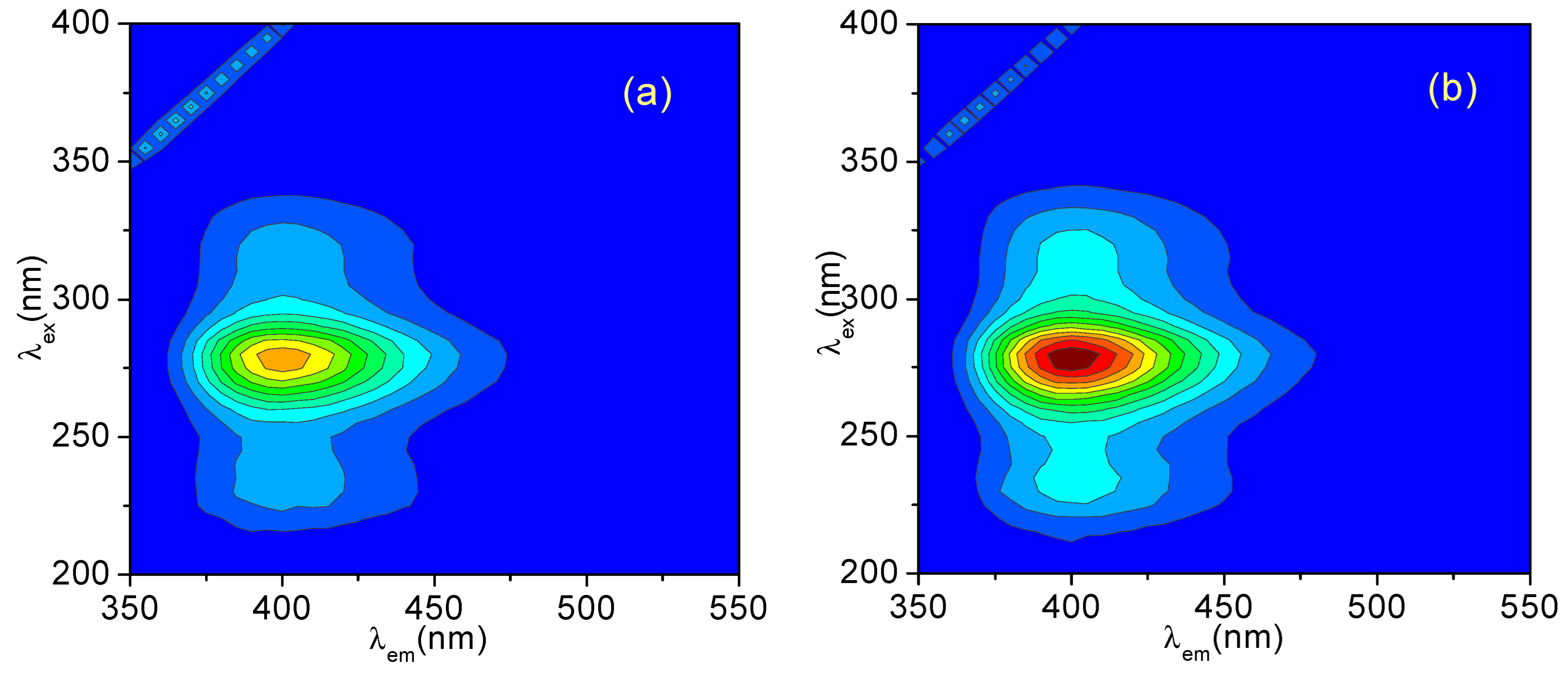
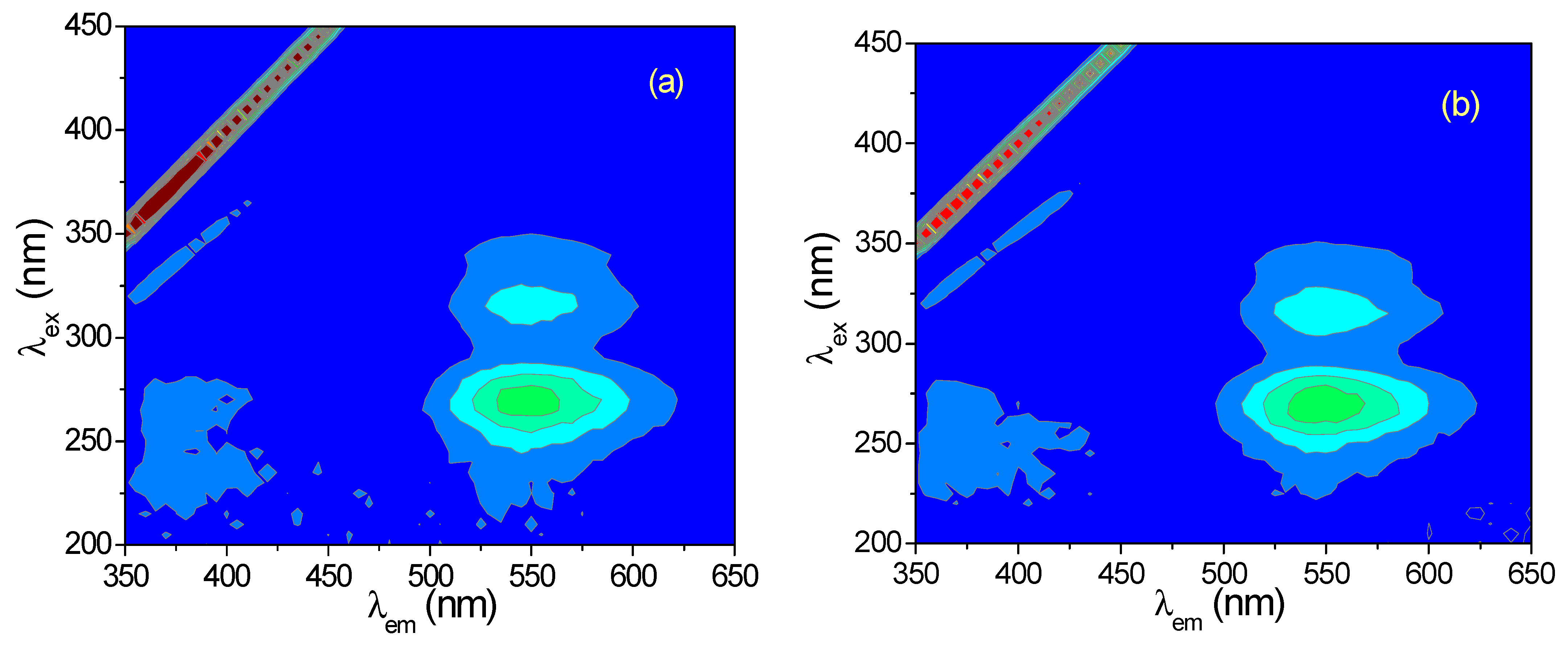
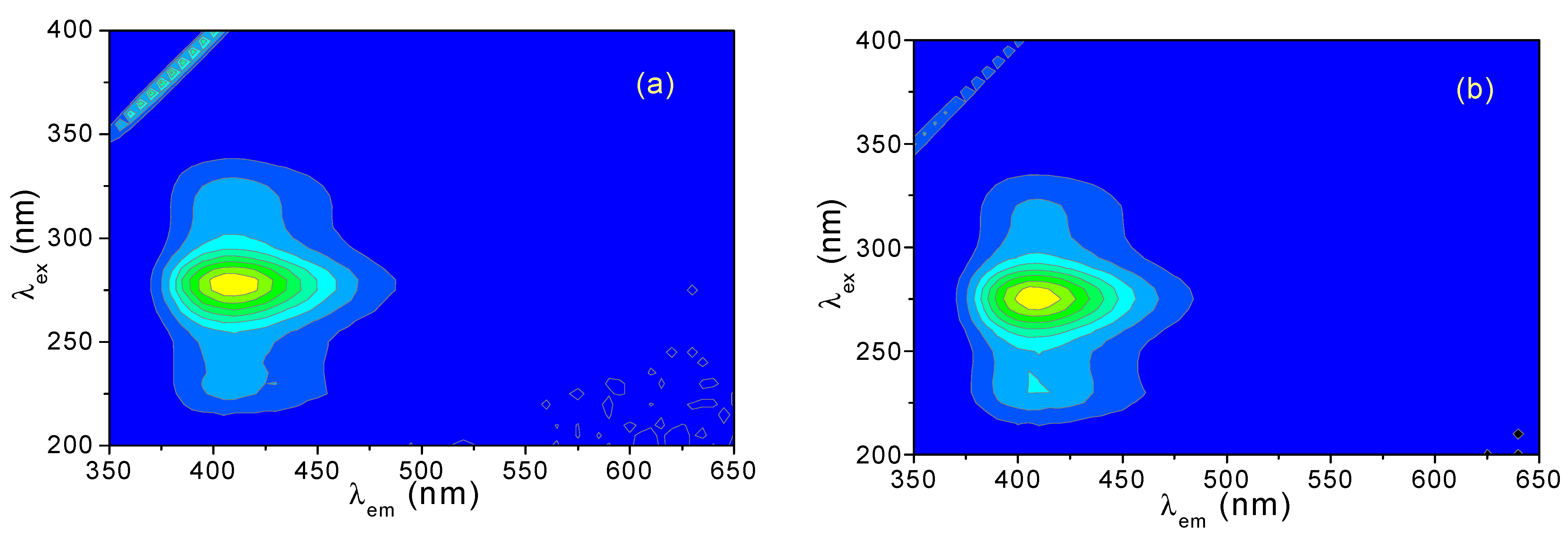
3.1. Three-Dimensional (3D) Fluorescence Spectra of CH and ECH in Methanol Solutions
3.2. 3D Fluorescence Spectra of CH and ECH in Acidic Methanol Solutions
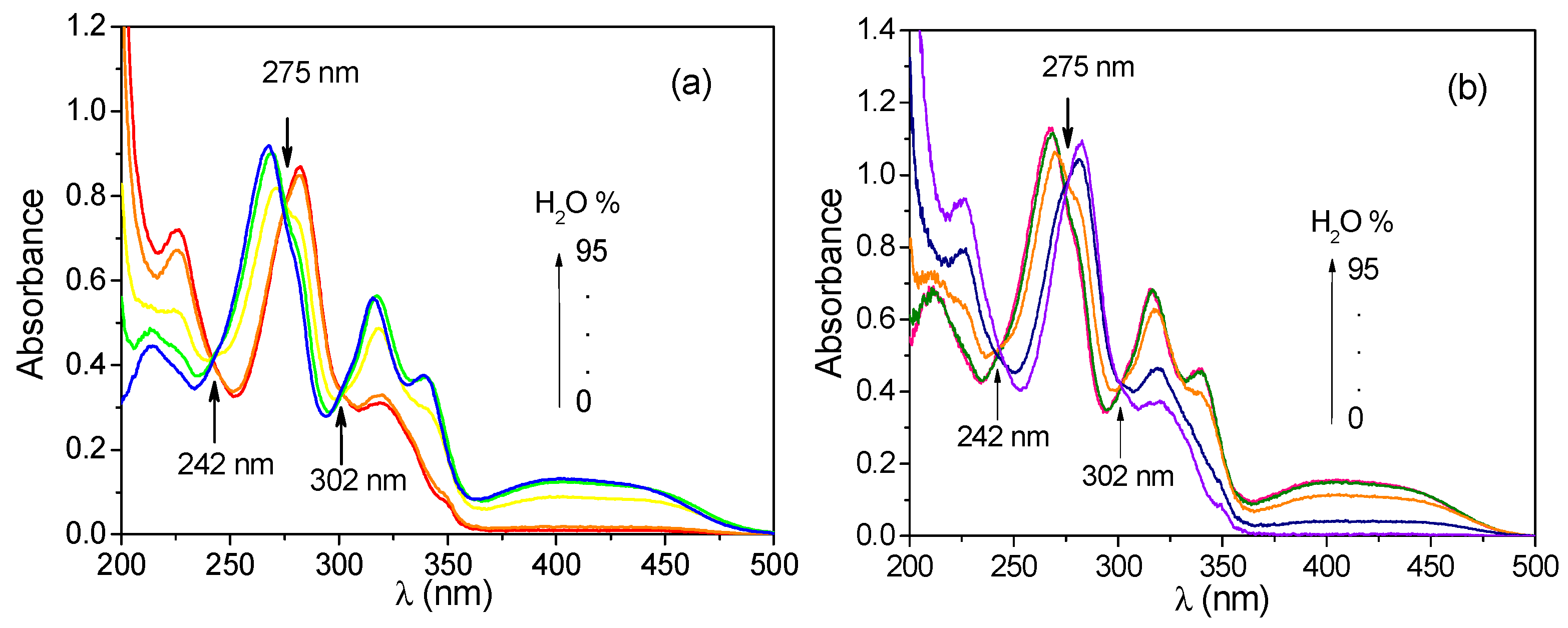
3.3. Absorbance Spectra of CH and ECH in Methanol–Water Solutions Containing Different Proportions of Water
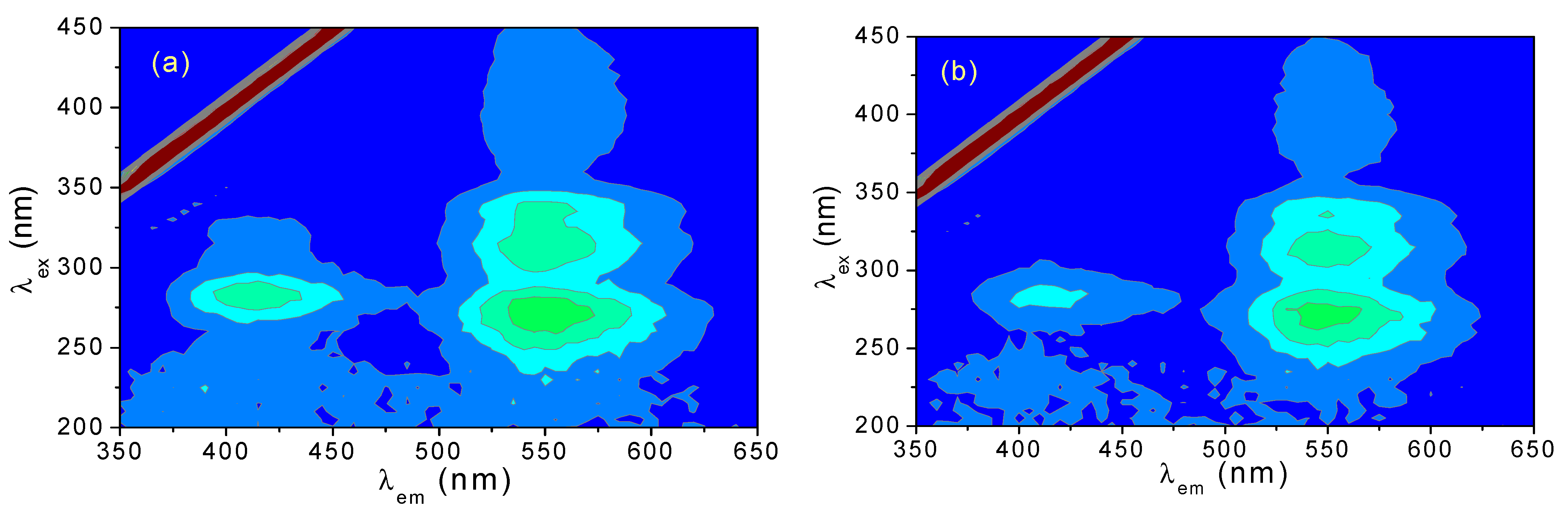
3.4. 3D Fluorescence Spectra of CH and ECH in Aqueous Solutions at Different pH Values
3.5. Absorbance Spectra of CH and ECH in Aqueous Solutions at Different pH Values
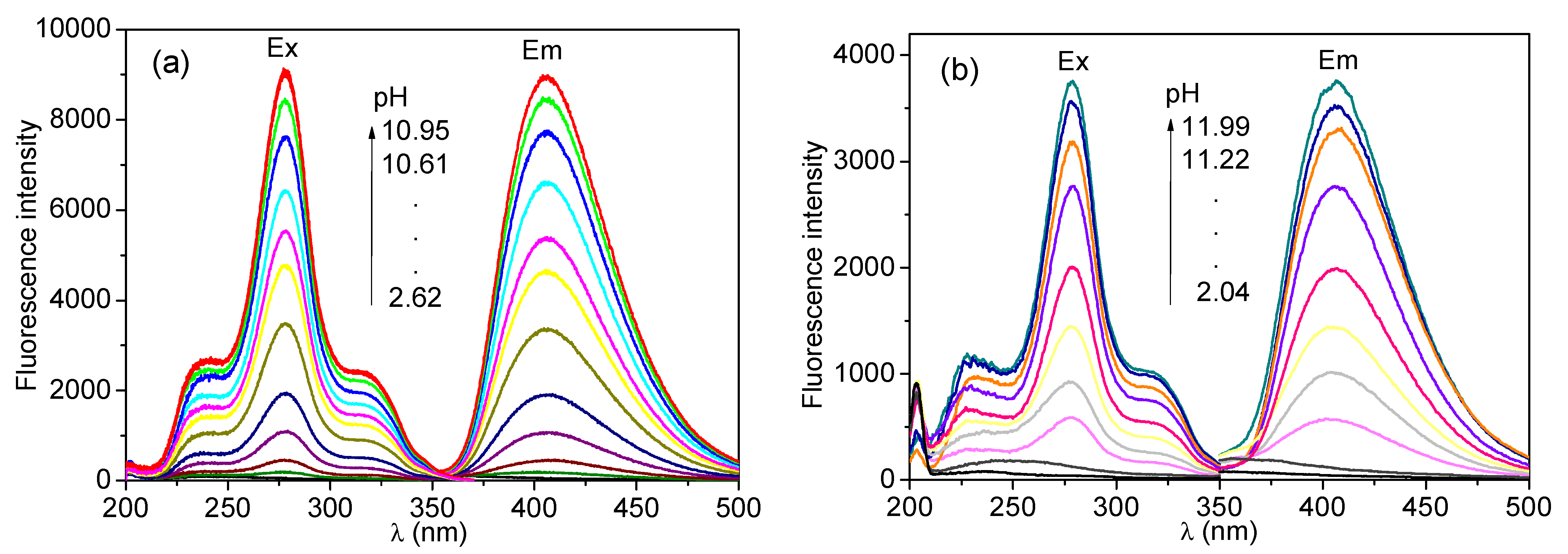
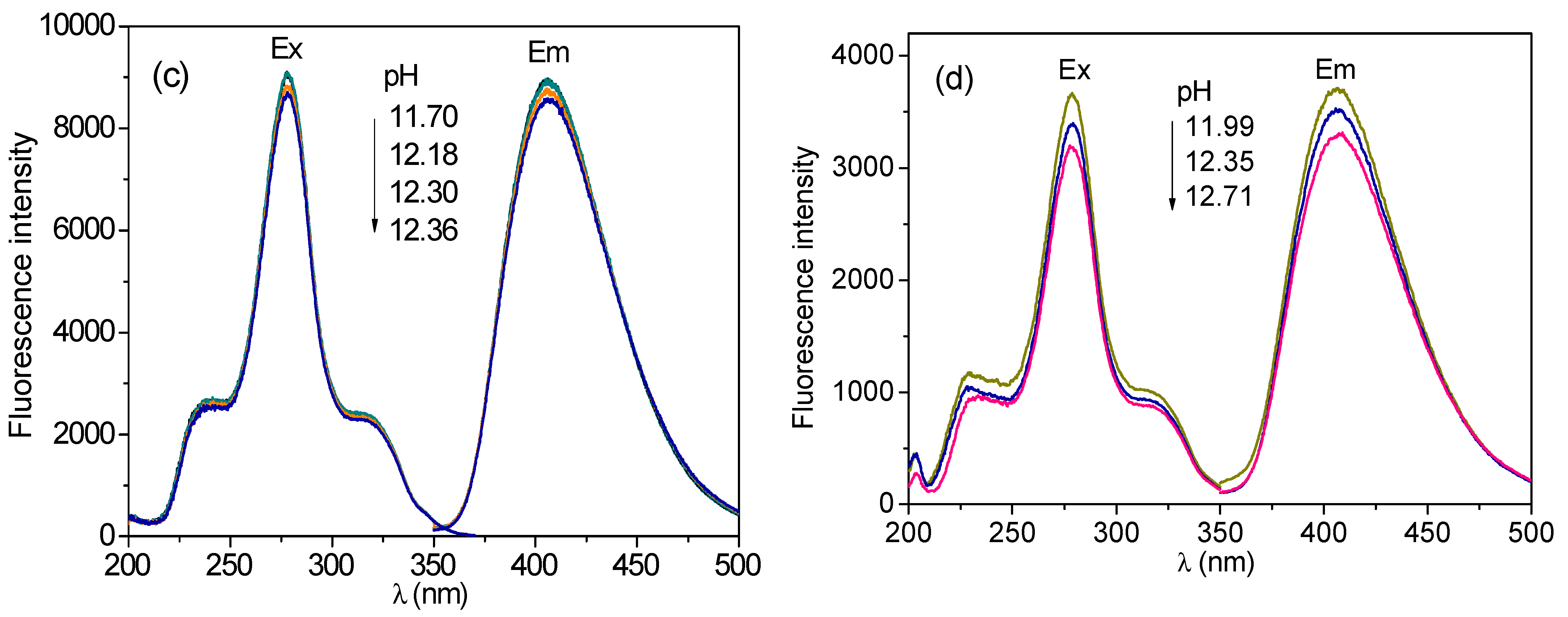
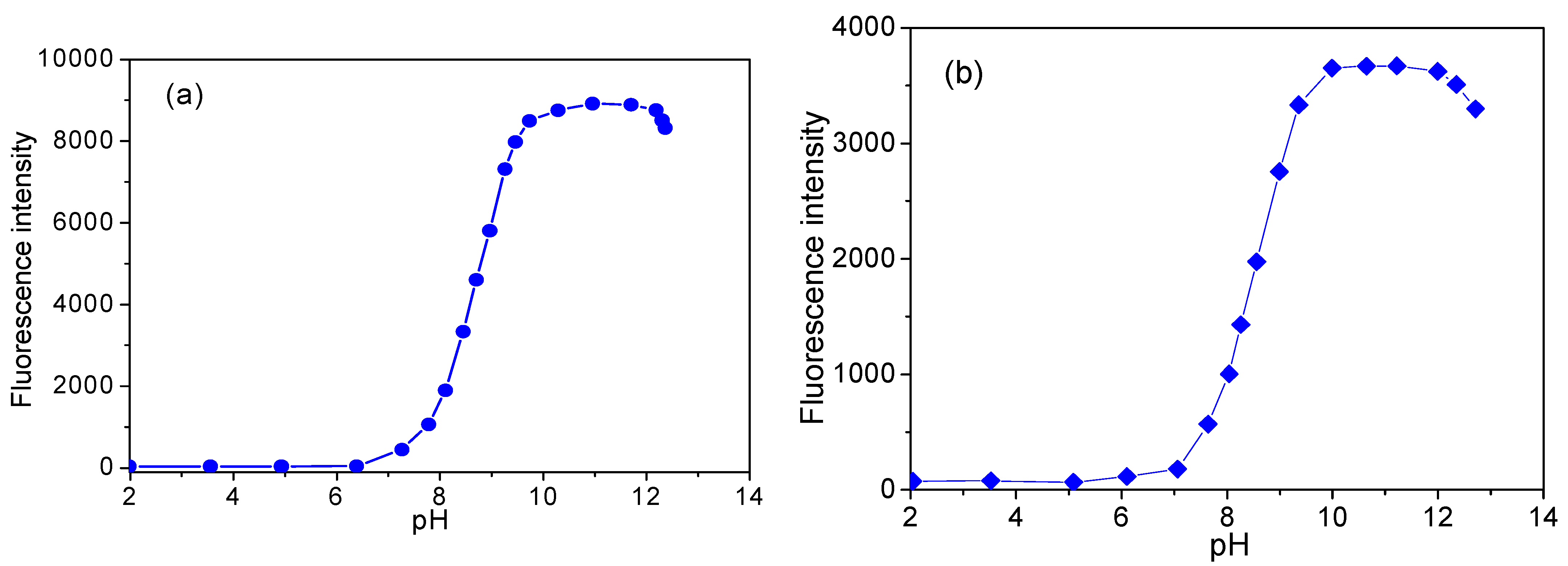
3.6. Effect of pH Value on Fluorescence Intensity of CH and ECH in Aqueous Solutions
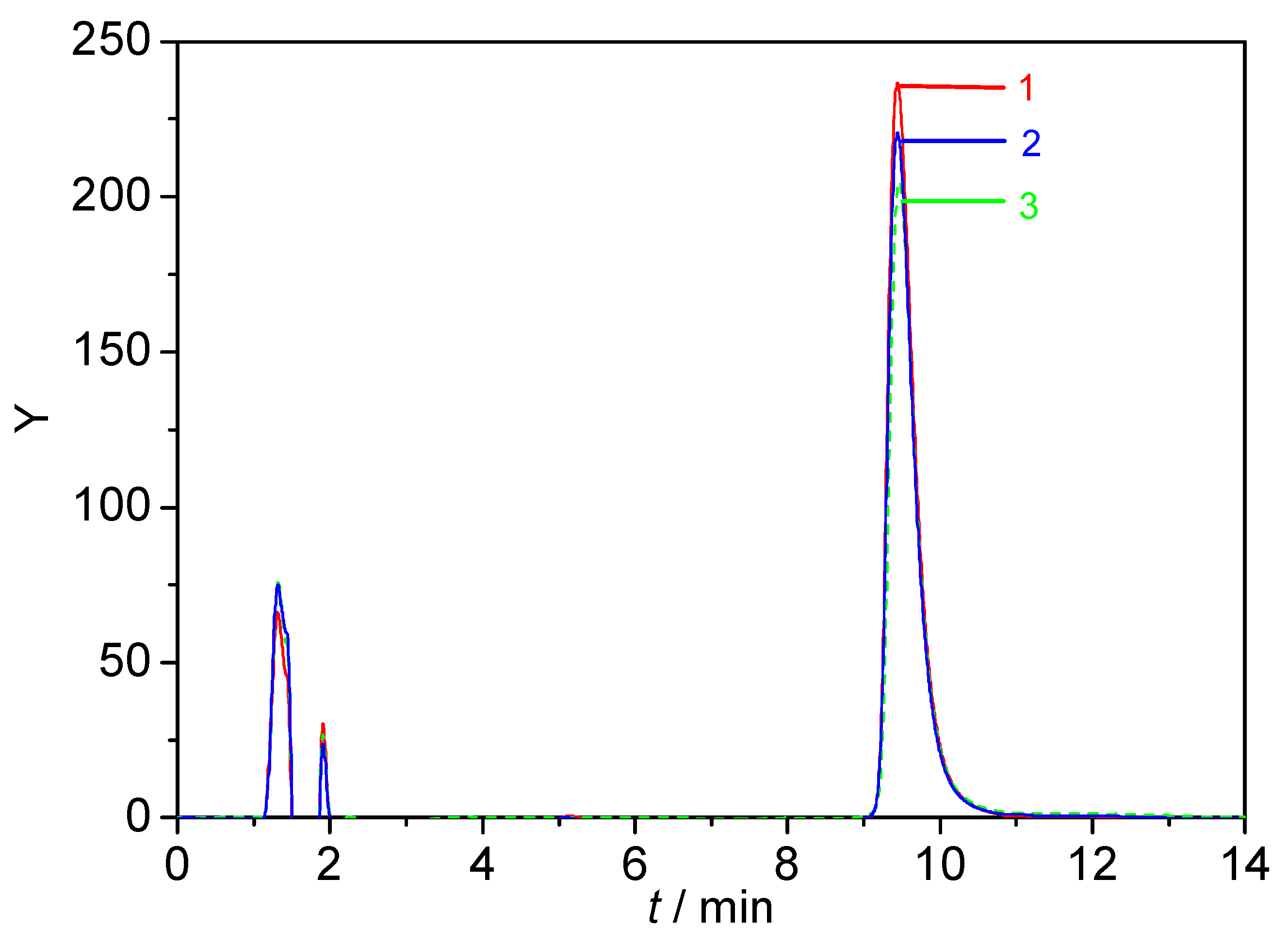
3.7. HPLC Chromatograms of CH and ECH in Acidic Aqueous MOBILE phase
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scheuer, P.J.; Chang, M.Y.; Swanholm, C.E. Hawaiian Plant Studies. VIII.1 Isolation of Chelerythrine and Dihydrochelerythrine from Fagara semiarticulata. J. Org. Chem. 1962, 27, 1472–1473. [Google Scholar] [CrossRef]
- Hu, Z.B.; Xu, Y.; Feng, S.C.; Fan, G.J. Studies on the active principles of the fruits of Macleaya cordata (Willd.) R. Br. Acta Pharm. Sinic. 1979, 14, 535–540. [Google Scholar] [CrossRef]
- Huang, C.Y.; Huang, Y.J.; Zhang, Z.Y.; Liu, Y.S.; Liu, Z.Y. Metabolism and Tissue Distribution of Chelerythrine and Effects of Macleaya Cordata Extracts on Liver NAD(P)H Quinone Oxidoreductase. Front. Vet. Sci. 2021, 8, 659771. [Google Scholar] [CrossRef]
- Wei, Q.H.; Cui, D.Z.; Liu, X.F.; Chai, Y.Y.; Zhao, N.; Wang, J.Y.; Zhao, M. In vitro antifungal activity and possible mechanisms of action of chelerythrine. Pestic. Biochem. Physiol. 2020, 164, 140–148. [Google Scholar] [CrossRef]
- Czeszak, A.; Resztak, M.; Czyrski, A.; Nowak, I. Determination of partition coefficient of isoquinoline alkaloids from Chelidonium Majus by reversed phase thin layer chromatography. New J. Chem. 2020, 44, 7484–7489. [Google Scholar] [CrossRef]
- Lei, Q.F.; Zhao, X.L.; Xu, L.J.; Peng, Y.; Xiao, P.G. Chemical Constituents of Plants from Tribe Chelidonieae and their Bioactivities. Chin. Herb. Med. 2014, 6, 1–21. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Zeng, J.; Lu, Q.; Li, P.C. Microchip electrophoretic separation and fluorescence detection of chelerythrine and sanguinarine in medicinal plants. Talanta 2015, 142, 90–96. [Google Scholar] [CrossRef]
- Wang, M. A study on the chemical constituents of Zanthoxylum nitidum (Lam) DC. 1. isolation of potential anticancer alkaloids and studies on the structure of alkalods C. Acta. Acad. Medi. Zhong Shan. 1980, 1, 341–402. [Google Scholar] [CrossRef]
- Wen, P.; Li, J.F.; Gao, Y.; Zhang, X.; Zhao, F. Research on the Anti-inflammatory Activity of Chelerythrine and Other Ingredients in Zanthoxylum nitidum (Roxb.) DC. Pharm. Today 2018, 28, 217–232. [Google Scholar]
- Fan, L.; Fan, Y.; Liu, L.; Tao, W.W.; Shan, X.; Dong, Y. Chelerythrine Attenuates the Inflammation of Lipopolysaccharide-Induced Acute Lung Inflammation Through NF-κB Signaling Pathway Mediated by Nrf2. Fitoterapia 2018, 26, 1047–1055. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; Ren, L.; Zhang, T.H. In vitro Anti-Inflammatory Potency of Sanguinarine and Chelerythrine via Interaction with Glucocorticoid Receptor. eFood 2020, 1, 392–398. [Google Scholar] [CrossRef]
- Qian, W.D.; Huang, J.; Zhang, J.N.; Li, X.C.; Kong, Y.; Wang, T.; Li, Y.D. Antimicrobial and Antibiofilm Activities and Mechanism of Action of Chelerythrine Against Carbapenem-Resistant Serratia marcescens In Vitro. Microb. Drug Resist. 2021, 27, 1105–1116. [Google Scholar] [CrossRef]
- Guo, W.H.; Lu, X.; Liu, B.; Yan, H.; Feng, J.T. Anti-TMV activity and mode of action of three alkaloids isolated from Chelidonium majus. Pest. Manag. Sci. 2021, 77, 510–517. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, Y.; Zhang, G.; Wu, Y.; Zhong, W.; Chu, C. Chelerythrine Inhibits Human Hepatocellular Carcinoma Metastasis in Vitro. Biol. Pharm. Bull. 2018, 41, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.H.; Cao, W.X.; Wang, Z.Y.; Lu, J.H.; Liu, B.; Chen, X. Induction of reactive oxygen species-stimulated distinctive autophagy by chelerythrine in non-small cell lung cancer cells. Redox. Biol. 2017, 12, 367–376. [Google Scholar] [CrossRef]
- Kumar, S.; Acharya, A. Chelerythrine induces reactive oxygen species-dependent mitochondrial apoptotic pathway in a murine T cell lymphoma. Tumour Biol. 2014, 35, 129–140. [Google Scholar] [CrossRef]
- He, H.C.; Zhou, R.; Dai, J.; Wang, X.J.; Huang, X.; Wang, H.F.; Xu, D.F. Chelerythrine induces apoptosis via ROS-mediated endoplasmic reticulum stress and STAT3 pathways in human renal cell carcinoma. J. Cell Mol. Med. 2020, 24, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.B.; Sun, H.X.; Zhang, W.D.; Zhang, Q.; Ma, J.; Li, Q.; Guo, X.M.; Xu, K.Y.; Tang, Y.L. Chelerythrine as a fluorescent light-up ligand for an i-motif DNA structure. New J. Chem. 2021, 45, 28–31. [Google Scholar] [CrossRef]
- Urbanová, J.; Lubal, P.; Slaninová, I.; Táborská, E.; Táborský, P. Fluorescence properties of selected benzo[c]phenantridine alkaloids and studies of their interaction with CT DNA. Anal. Bioanal. Chem. 2009, 394, 997–1002. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Wu, Y.; Shuang, S.; Dong, C.; Choi, M.M.F. Luminescence and binding properties of two isoquinoline alkaloids chelerythrine and sanguinarine with ctDNA. Spectrochim Acta A Mol. Biomol. Spectrosc. 2012, 95, 80–85. [Google Scholar] [CrossRef]
- Basu, P.; Bhowmik, D.; Kumar, G.S. The benzophenanthridine alkaloid chelerythrine binds to DNA by intercalation: Photophysical aspects and thermodynamic results of iminium versus alkanolamine interaction. J. Photoch. Photobio. B. 2013, 129, 57. [Google Scholar] [CrossRef] [PubMed]
- Haque, L.; Pradhan, A.B.; Bhuiya, S.; Das, S. Structural conversion of left handed protonated form of calf thymus DNA to right handed B-DNA by the alkaloid chelerythrine. J. Lumin. 2016, 173, 44–51. [Google Scholar] [CrossRef]
- Bhuiya, S.; Pradhan, A.B.; Haque, L.; Das, S. Molecular Aspects of the Interaction of Iminium and Alkanolamine Forms of the Anticancer Alkaloid Chelerythrine with Plasma Protein Bovine Serum Albumin. J. Phys. Chem. B. 2016, 120, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.H.; Lin, Y.; Chen, R.; Lu, H.M. Optimization of ultrasonic wave-enzymes extraction technology for chelerythrine and its 6-alkoxy derivatives from Zanthoxyli Radix. Chin. J. Exp. Trad. Med. Formulae 2016, 22, 23–26. [Google Scholar] [CrossRef]
- The State Pharmacopoeia Committee of China. The pharmacopoeia of the People’s Republic of China. China Med. Technol. Press. 2020, 112, 970. [Google Scholar]
- Onda, M.; Abe, K.; Yonezawa, K.; Esumi, N.; Suzuki, T. Studies on the constituents of Bocconia cordata. II. Bocconine Chem. Pharm. Bull. 1970, 18, 1435–1439. [Google Scholar] [CrossRef] [Green Version]
- Vacek, J.; Papoušková, B.; Kosina, P.; Galandáková, A.; Ulrichová, J. Mass spectrometric investigation of chelerythrine and dihydrochelerythrine biotransformation patterns in human hepatocytes. J. Chromatogr. B. 2013, 941, 17–24. [Google Scholar] [CrossRef]
- Miskolczy, Z.; Megyesi, M.; Lendvay, G.; Biczók, L. Self-assembly of quaternary benzo[c]phenanthridine plant alkaloids into dimer in aqueous solution. J. Mol. Liq. 2021, 334, 116014-21. [Google Scholar] [CrossRef]
- Pradhan, A.B.; Bhuiya, S.; Haque, L.; Tiwari, R.; Das, S. Micelle assisted structural conversion with fluorescence modulation of benzophenanthridine alkaloids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 170, 89–96. [Google Scholar] [CrossRef]
- Valadan, M.; Pomarico, E.; Ventura, B.D.; Gesuele, F.; Velotta, R.; Amoresano, A.; Pinto, G.; Chergui, M.; Improta, R.; Altucci, C. A multi-scale time-resolved study of photoactivated dynamics in 5-benzyl uracil, a model for DNA/protein interactions. Phys. Chem. Chem. Phys. 2019, 21, 26301–26310. [Google Scholar] [CrossRef]
- Li, W.H.; Wang, D.Y.; Cao, J.J.; Wei, Y.J. Comparative study of absorption and fluorescence spectra of Glycitein and Glycitin. Chem. J. Chin. Univ. 2019, 40, 47–54. [Google Scholar]
- Micciarelli, M.; Curchod, B.F.E.; Bonella, S.; Altucci, C.; Valadan, M.; Rothlisberger, U.; Tavernelli, I. Characterization of the Photochemical Properties of 5-Benzyluracil via Time-Dependent Density Functional Theory. J. Phys. Chem. A 2017, 121, 3909–3917. [Google Scholar] [CrossRef]
- Micciarelli, M.; Valadan, M.; Ventura, B.D.; Fabio, G.D.; Napoli, L.D.; Bonella, S.; Röthlisberger, U.; Tavernelli, I.; Altucci, C.; Velotta, R. Photophysics and Photochemistry of a DNA−Protein Cross-Linking Model: A Synergistic Approach Combining Experiments and Theory. J. Phys. Chem. B 2014, 118, 4983–4992. [Google Scholar] [CrossRef]
- Eaton, D.F. Reference materials for fluorescence measurement. Pure Appl. Chem. 1988, 60, 1107–1114. [Google Scholar] [CrossRef]
- Yang, F.; Liu, C.G.; Wei, Y.J. Fluorimetric analysis of paeonol in Chinese herbal medicine Cynanchi Paniculati Radix by aluminum ion-sensitized fluorescence. Acta. Pharm. Sinic. 2012, 2, 294–299. [Google Scholar] [CrossRef] [Green Version]
- MotevichI, G.; Strekal’, N.D.; Nowicky, J.W.; Maskevich, S.A. Absorption, fluorescence, and SERS spectra of sanguinarine at different pH values. J. Appl. Spectrosc. 2007, 74, 666–672. [Google Scholar] [CrossRef]
- Huang, Z.X.; Li, Z.H. Studies on the antitumor constituents of Zanthoxylum nitidum (Roxb.) DC. Acta. Chim. Sinic. 1980, 38, 535–542. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Xu, B.J.; Yang, L.L.; Huang, Z.X. Crystal and molecular structure of 6-methoxy-5,6-dihydrochelerythrine. Chin. Sci. Bull. 1989, 34, 1182–1185. [Google Scholar]
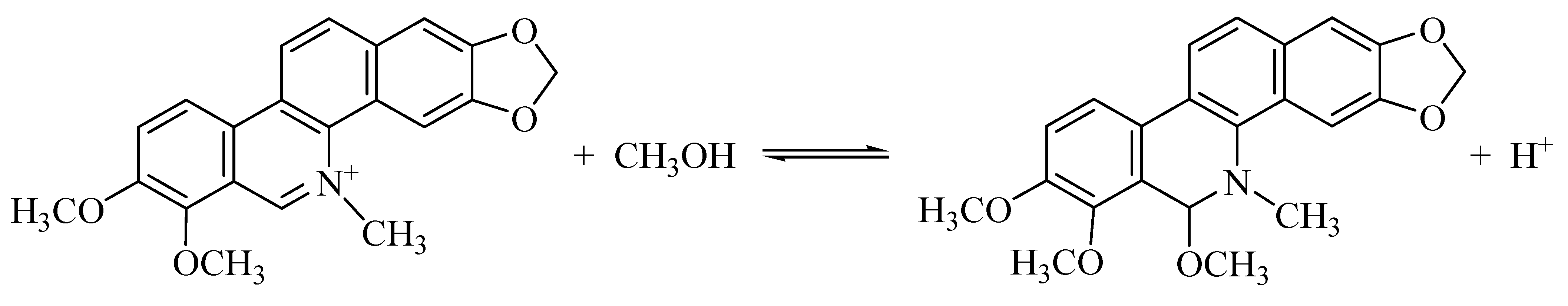
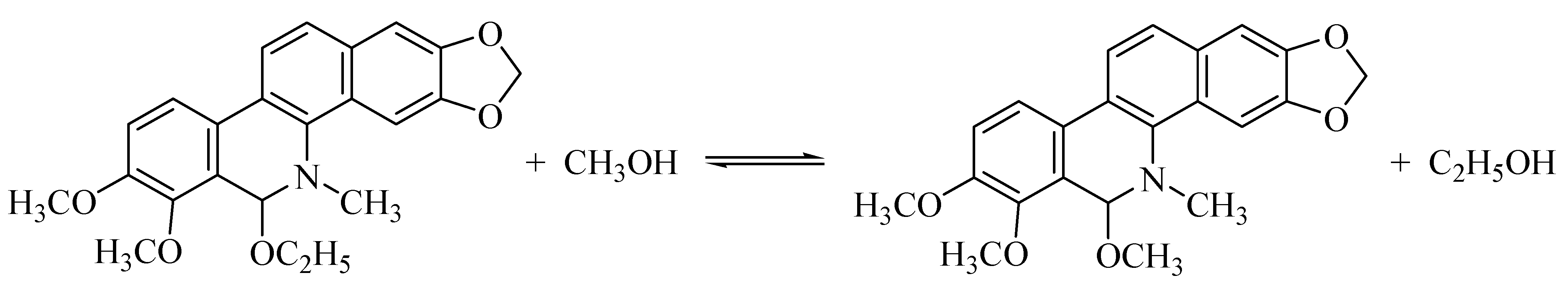
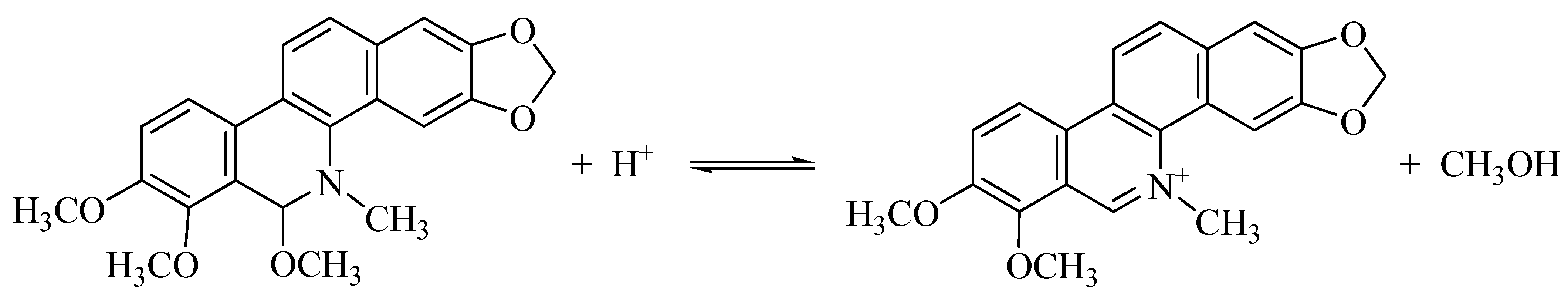

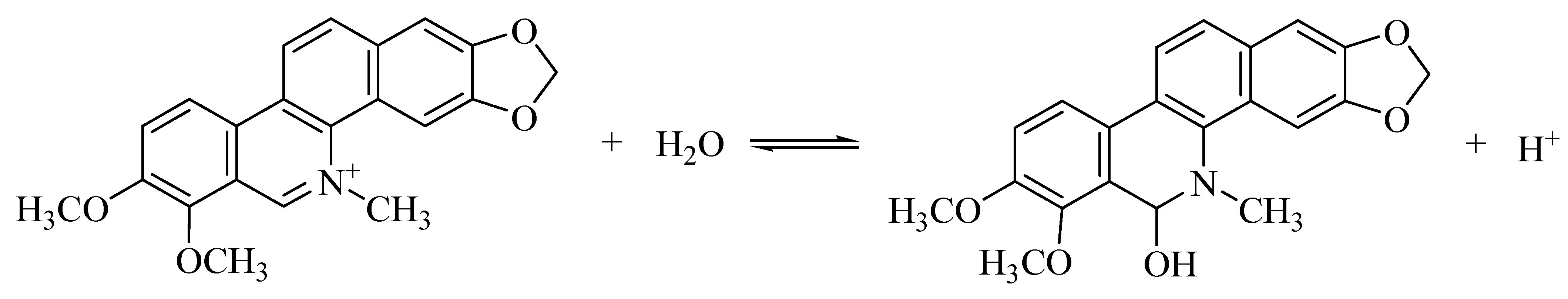

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Zheng, Y.; Liu, T.; Liu, J.; Liu, J.; Wang, J.; Sun, Q.; Li, W.; Wei, Y. Fluorescence, Absorption, Chromatography and Structural Transformation of Chelerythrine and Ethoxychelerythrine in Protic Solvents: A Comparative Study. Molecules 2022, 27, 4693. https://doi.org/10.3390/molecules27154693
Cao J, Zheng Y, Liu T, Liu J, Liu J, Wang J, Sun Q, Li W, Wei Y. Fluorescence, Absorption, Chromatography and Structural Transformation of Chelerythrine and Ethoxychelerythrine in Protic Solvents: A Comparative Study. Molecules. 2022; 27(15):4693. https://doi.org/10.3390/molecules27154693
Chicago/Turabian StyleCao, Jinjin, Yanhui Zheng, Ting Liu, Jiamiao Liu, Jinze Liu, Jing Wang, Qirui Sun, Wenhong Li, and Yongju Wei. 2022. "Fluorescence, Absorption, Chromatography and Structural Transformation of Chelerythrine and Ethoxychelerythrine in Protic Solvents: A Comparative Study" Molecules 27, no. 15: 4693. https://doi.org/10.3390/molecules27154693
APA StyleCao, J., Zheng, Y., Liu, T., Liu, J., Liu, J., Wang, J., Sun, Q., Li, W., & Wei, Y. (2022). Fluorescence, Absorption, Chromatography and Structural Transformation of Chelerythrine and Ethoxychelerythrine in Protic Solvents: A Comparative Study. Molecules, 27(15), 4693. https://doi.org/10.3390/molecules27154693







